Summary
A subtype of women with dysmenorrhea have a profile suggesting heightened risk for chronic pelvic pain including somatic and visceral hypersensitivity, and diminished pain modulation.
Introduction
Menstrual pain, known as dysmenorrhea, impairs quality of life in one-third of reproductive age women [45,55] and is among the leading risk factors for the development of chronic pelvic pain conditions [31]. A better understanding of the underlying mechanisms responsible for dysmenorrhea and the transition to chronic pelvic pain is needed because current treatments are often ineffective. Proposed factors for the chronification of pain include exaggerated peripheral organ inflammation [8], impaired central processing of noxious stimuli [28], and hypervigilance associated with psychological distress [47,59],
Several studies of dysmenorrhea have discussed impairments in nociceptive processing using quantitative sensory testing (QST: see Payne et al. [41] for a systematic review). There are, however, multiple limitations in these prior studies. For example, studies often have not accounted for potential confounders such as psychosocial dysregulation or menstrual cycle influences on pain sensitivity. Additionally, it is essential to use multiple modes of QST to establish whether somatic or visceral mechanisms are responsible for increased sensitivity in dysmenorrhea, given prior disparate results [6]. Although the large scale OPPERA study failed to identify meaningful relationships between alterations in QST and risk for the development of temporomandibular joint disorder (TMD), there was an association between widespread reductions in pressure pain sensitivity and the persistence of TMD[48], which the authors conclude points to a critical role for central nociceptive mechanisms. Recently, we have identified that a significant subset of dysmenorrhea sufferers harbor silent, comorbid bladder pain hypersensitivity (DYSB) [26], but we have not yet established whether hypersensitivity extends to somatic pelvic sites or affects the entire body. In patients with DYSB, QST impairments in select peripheral sites might suggest a focal pathology, whereas widespread alterations in QST may point more to the early emergence of central changes that have been implicated in the transition to chronic pelvic pain[58].
Based on prior studies with more severe pain phenotypes, we recruited a cohort of dysmenorrhea sufferers and controls to test the hypothesis that women with DYSB would have reductions in bodily pain pressure thresholds and impaired conditioned pain modulation, indicative of impaired descending inhibition [58]. Temporal summation was used to investigate hypersensitivity mediated by spinal sensitization [13] and pressure algometry was used to test for increased regional somatic pain sensitivity adjacent to the visceral site [27]. We conducted these assessments while also capturing key potential QST covariates (anxiety, age, catastrophizing, menstrual cycle status). To establish which specific nociceptive mechanisms observed in dysmenorrhea are also linked to the enhanced bladder sensitivity phenotype, we compared DYSB participants to participants with only dysmenorrhea or healthy controls. Finally, to establish the potential clinical relevance of QST, we examine the correlation between of these measures to self-report of urination-, bowel-, and sex-related pain.
Methods
Overview
The prospective observational study Chronic Pain Risk Associated with Menstrual Pelvic Pain (CRAMPP) was designed to characterize uterine cross-organ influences on bladder pain in reproductive-age women, and extend characterization of a group of women found to have both dysmenorrhea and silent bladder pain (DYSB). NorthShore University HealthSystem’s Institutional Review Board approved this study. Participants were recruited through advertisements posted on public transit and throughout the community, through the Illinois Women’s Health Registry, and by referral from NorthShore gynecology clinics. Trained research assistants telephone screened potential participants to determine eligibility for the screening visit. After eligibility was confirmed, participants signed a consent form, completed a screening visit, tracked a daily menstrual symptom diary for 1-2 months, and then participated in an assessment visit during their luteal phase.
Participants
All participants completed an initial phone screen before the screening visit. Those who rated their menstrual pain ≥5 on 0–10 numeric rating scale (NRS 0: no pain, 10: worst pain imaginable) without concurrent chronic pain diagnoses were defined as potentially eligible to participate in this study as a dysmenorrhea participant. This pain threshold was chosen initially because we have established in a different cohort that this intensity of menstrual pain is associated with a markedly higher likelihood of meeting criteria for DYSB (see below) [52]. Menstrual pain status was further confirmed with diary data (see the section on confirmation below).
A subset of dysmenorrhea participants who rated their bladder pain (>15 on 0-100 VAS) during a bladder filling test (see QST below) on the assessment visit were formally designated as dysmenorrhea with bladder hypersensitivity (DYSB). Since we met our target for DYS participants before DYSB participants after two years into the study, we declined assessment visits to 52 participants who did not report any provoked bladder pain hypersensitivity during the screening visit. The statistical basis of our selected bladder pain threshold and enrichment strategy is discussed in our earlier paper [26].
Participants who rated their average menstrual pain ≤3 on a 0–10 NRS without chronic pain diagnoses were designated as healthy controls (HC). Participants with overt BPS met the American Urological Association (AUA) diagnostic criteria, and had to report bladder pain ≥3 on a 0-10 NRS for more than 3 consecutive months[23]; these women often had comorbid pain conditions (e.g., fibromyalgia, irritable bowel syndrome, endometriosis). As a positive control group, a cohort of participants with clinically symptomatic chronic pain (but without BPS), were recruited to assess the validity of our conditioned pain modulation task (CPM), and were required to have general pain ≥5 on a 0-10 NRS for more than 3 consecutive months [23]. This group (n=23) was not included in the primary analyses, but we included their effect sizes to help validate our CPM measures, for reference, in a secondary analysis.
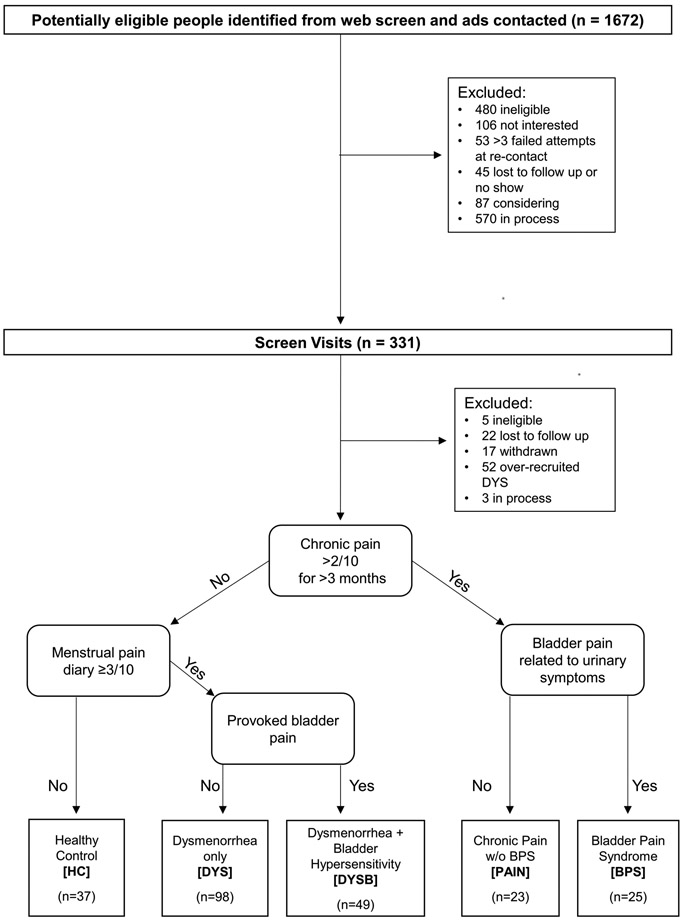
Participants were excluded for the presence of active pelvic or abdominal malignancies, absence of regular menses (except the chronic pain without BPS group), active genitourinary infection in the last four weeks, inability to read or comprehend the informed consent in English, refusal to undergo pelvic examination/testing, hypertension, or refusal to withdraw from oral contraceptives for two months prior to the study visit. An overview of study enrollment is shown in Figure 1.
Figure 1.
Recruitment Flow Chart
Screening Visit
Details regarding the screening visit for CRAMPP have already been published [26]. Participants completed questionnaires including medical, surgical, psychological, and gynecological history via REDCap [24]. Participants completed validated short-form PROMIS inventories for Pain Behavior, Pain Interference, Anxiety, and Depression [10]. Additionally, participants completed validated pain questionnaires such as the O’Leary-Sant Interstitial Cystitis Symptom Index, [40], the Pain Catastrophizing Scale [51], and the Brief Symptom Inventory subscale for somatic symptoms [12]. To identify potential secondary causes of dysmenorrhea, a gynecologist specializing in pelvic pain performed a pelvic exam on the first 98 dysmenorrhea participants. Ultrasonography was performed to follow up on participants with potential clinical exam findings to rule out secondary causes for dysmenorrhea. In this initial cohort, only three participants had small pelvic cysts (<3.0 cm), and one had subserosal and intramural leiomyomata (<2.5 cm). In order to reduce participant burden, these exams were discontinued once we confirmed that the screen rarely identified occult pathology in these women with only cyclical pain.
Participants also underwent a validated rapid bladder filling test at this visit to evaluate the likelihood of having dysmenorrhea with bladder pain hypersensitivity (DYSB). The detailed methods of this task have been published previously [26]. This task is similar to the bladder task employed during the assessment test (described below) except that participants were not monitored with ultrasound and did not have to reach maximum capacity.
Confirmation of menstrual pain and cycle status
To track pain intensity, all participants completed web-based daily diaries during the month preceding their assessment visit. They rated intensity of menstrual pain over the past 24 hours on a 0-10 NRS (0 = no pain and 10 = the worst pain imaginable) each day. All dysmenorrhea participants were required to have at least one menses pain score ≥3, while all healthy control participants were required to report pain <3. The lower threshold for dysmenorrhea was used for this specific prospective validation, because the use of medications and completion of the pain diary at the end of the day were associated with lower pain report in our pilot studies. Because the menstrual cycle phase may have some influence on experimental pain thresholds, the diary was also used to schedule the timing of the assessment visit (15–25 days after onset of menses). To confirm that participants were in their luteal phase, they were provided with luteinizing hormone (LH) urinary assay kits (Wondfo, Willowbrook, IL) and instructed to begin using them on day 10 of their cycle. The utilization of LH assays and cycle timing of visits follows prior guidelines for pain testing in women[20].
Assessment Visit
Participants were asked to avoid taking short-acting, over-the-counter analgesics (such as NSAIDs), opioids, and caffeine for at least six hours before the assessment visit. We also asked them to not consume longer-acting NSAIDs for twelve hours before the study visit. During this visit, participants underwent a noninvasive bladder filling test and completed additional QST measures described below.
Comprehensive validation and methods for the bladder assessment task have been published [26,52,54]. In brief, after voiding, participants had a transabdominal three-dimensional sonographic measurement of their bladder. Afterward, participants drank 20 oz of water within 5 minutes and were instructed to report when they reached three standard cystometric urgency thresholds: first sensation, first urge to void, and maximum capacity [2]. At each of these thresholds, the bladder volume was sonographically measured again, and participants rated their bladder pain and urgency on a 0–100 VAS using a tablet computer. On average, participants finished questionnaires 13 ± 5 minutes before rating “first urge” pain on the bladder task.
Pressure pain thresholds (PPT) were performed following our standardized, previously published protocol [54]. One examiner (FT) with over five years’ experience in performing both external and transvaginal pressure algometry trained two female examiners in the protocol, and testing on model patients yielded Cronbach’s α ≥ 0.9 across sites before the start of testing [53,54]. One female examiner (EG) conducted 224/232 (97%) of the exams. For all examiners, we calculated a final Cronbach’s α ≥ 0.89 at all sites. Our methods for pelvic PPTs have been previously published [27]. PPTs were measured at four transvaginal pelvic floor sites (right and left iliococcygeus, anteriorly against the bladder, and posterior anorectal raphe) using a 1 cm2 diameter force sensing resistor (Trossen Robotics, Downers Grove, IL) mounted inside the examiner’s glove with a ramp rate of 0.5 Newtons (N)/s with a computer generated visual guide. Body PPTs were evaluated with a digital algometer (Wagner Instruments, Greenwich, CT) with a 1 cm2 rubber tip applied at a ramp rate of 4 N/s with a computer generated visual guide. We assessed three external measurement sites corresponding to American College of Rheumatology guidelines for fibromyalgia tender point sites: the right trapezius, the right medial knee fat pad, and the right greater trochanter, as well as an additional location at the mid-forehead as a control [57]. Each site was measured in the same order with 10 seconds between each internal site, 15 seconds between each external site, and a two-minute break between repeat measurements. Two trials were performed at each site, and the average was used for analyses.
Conditioned pain modulation (CPM) testing involved repeating PPT testing in the presence of a heterotopic stimulus, as a measure of possible alterations in brainstem-mediated descending modulation of noxious stimulus awareness. We used ice water as a conditioning stimulus, similar to others [33-35]. Initially, baseline PPTs were determined for the left medial knee fat pad and trapezius. After a 2-minute break, the conditioning stimulus was presented by instructing the participant to insert their right hand up to their wrist into a bucket of water maintained between 0-6°C with a high-speed water circulation pump. After 10s of immersion, participants were asked to rate their hand pain on a 0-10 NRS scale. We used this value as a measure of cold pain sensitivity. After 20s of immersion, PPTs were measured at the knee while participants kept their hand in the water. Once the knee threshold was reached, participants removed their hand from the water and the trapezius site was tested. CPM was calculated by the standardized differences in pain threshold (Newtons) for each site between pre-immersion and post-immersion. Initial analysis of the data suggested that knee CPM was more variable even in healthy controls (knee SD: 11.1, trapezius SD: 9.1; knee range: −9.2 to 35.0 N, trapezius range: −7.4 to 26.4 N). Also, we noted the overall effect sizes of CPM across the groups was greater with knee CPM (knee x2 = 1.1, trapezius x2 = 9.5). Because trapezius CPM (which took place following removal of the hand) appeared to be less variable and more discriminative, we opted to include only trapezius CPM in these analyses.
Temporal summation [9] was then tested over the right medial knee fat pad. A software-based metronome was used to guide the application of a series of 10 pressure pulses at 4 N/s, 1 second apart. Each pulse was applied until the initial threshold for pain was achieved. Patients reported pain on a 0-10 NRS. Trials were also terminated early when participants reached a score of 6 or higher. The amount of temporal summation was determined by the tenth or final pain rating.
Power analysis
Our overall recruitment was guided by a primary power analysis (http://clinicaltrials.gov/ct2/show/NCT02214550) for the central study objectives which indicated a need for 255 reproductive age women across 5 different groups to demonstrate hormonal suppression for menstrual pain reduces bladder pain sensitivity. To show the effect sizes for QST typically seen in pain studies (Cohen’s d=1 or higher), for this planned subanalysis, we calculated a minimum sample size of 25 per group for ANOVA accounting for 4 multiple comparisons (DYS vs Healthy Control [HC], DYSB vs HC, BPS vs HC, DYSB vs DYS: α=0.05 /4; β=1 - 0.8). In practice, we over-recruited dysmenorrhea participants to ensure adequate sample for the overarching interventional aim of the study, which targeted 60 DYSB participants, and these participants cannot be identified until they are formally assessed for bladder sensitivity. Ultimately, our effect sizes were lower and the QST measures had skewed distributions necessitating use of nonparametric tests. Therefore, we confirmed we had 80% power to detect a minimal effect of d=.55 for HC vs DYS, d=.63 for HC vs DYSB, d=.51 for DYS vs DYSB, and d=.75 for BPS vs HC using Kruskal-Wallis tests.
Statistical Analyses
Data was analyzed in Stata 13.1 (College Station, TX) and graphics were generated in R 3.4.2 [44]. Complete data was obtained for all participants, except for vaginal PPT measurements (n=3 DYSB, 2 DYS, 1 HC, 1 BPS) because they had opted out of participating in this aspect of the study, and CPM (n=11 DYSB, 31 DYS, 13 HC, 9 BPS) because of delayed implementation. Differences in characteristics across the groups were evaluated with ANOVA when normally distributed, or the Kruskal-Wallis test with Sidak adjustments for multiple comparisons. To identify correlations between confounding variables, QST measurements, and pain outcomes, we used Spearman correlations with Sidak correction for multiple comparisons because many of the variables were skewed or heteroskedastic. Ordered logistic regression was used to adjust for potential confounders when confounders were correlated to outcome variables. Proportional odds ratios (pOR ± standard error of the mean) were reported for confounder analyses.
Results
Demographics, pain, and psychological characteristics
There were no significant differences in age or race between HC, DYS, or DYSB participants (Table 1). Consistent with the literature, participants with BPS were older, by a decade roughly, and more likely to be white (p's <0.01). DYSB participants shared some symptoms of BPS, such as pain on urination and reduced bladder capacity. However, DYSB participants rarely reported bladder symptoms outside of menses (8% DYSB vs. 88% BPS). DYSB participants also reported less pain on urination than BPS participants {median 13 [interquartile range (IQR) 5 −32] vs 32 [IQR 16 - 66] on 0-100 VAS, respectively}. Anxiety and somatic symptoms were significantly greater in all symptomatic groups (DYS, DYSB and BPS), whereas depression scores were elevated only in DYSB and BPS participants.
Table 1: Demographic factors and pain characteristics of study cohort.
BOLD - Results significantly different (p<0.05) than healthy controls. Tabulated results indicate count (percent) or median (25th, 75th percentile). DYSB: DYS + Bladder pain sensitivity, BPS: Bladder Pain Syndrome.
| Group: |
Healthy Control |
Dysmenorrhea | DYSB | BPS | p-value |
|---|---|---|---|---|---|
| N | 37 | 98 | 49 | 25 | |
| Age | 21 (19, 28) | 21 (20, 29) | 22 (20, 26) | 30 (25, 32) | 0.004 |
| Race | 0.006 | ||||
| Black | 2 (5%) | 22 (22%) | 9 (18%) | 3 (12%) | |
| Other | 12 (32%) | 14 (14%) | 12 (24%) | 0 (0%) | |
| White | 23 (62%) | 63 (64%) | 28 (57%) | 22 (88%) | |
| Hispanic | 3 (8%) | 13 (13%) | 8 (16%) | 0 (0%) | 0.170 |
| ≥1 vaginal delivery | 1 (3%) | 6 (6%) | 3 (6%) | 3 (12%) | 0.528 |
| Cycle Day | 20 (17, 23) | 20 (18, 23) | 19 (17, 22) | 20 (18, 24) | 0.540 |
| Pain & Bladder | |||||
| Menstrual Pain (0-100 VAS) | 13 (5, 22) | 73 (66, 80) | 70 (64.5, 85) | 70 (60, 92) | 0.001 |
| Urination Pain (0-100 VAS) | 1 (1, 3) | 2 (1, 5) | 13 (5, 32) | 32 (16, 66) | 0.001 |
| Bladder Pain Off Period | 0 (0%) | 1 (1%) | 4 (8%) | 22 (88%) | 0.001 |
| IC Symptom Index (0-19) | 2 (1, 4) | 4 (2, 6) | 6 (4, 9) | 11 (9, 13) | 0.001 |
| Bladder Capacity (mL) | 641 (501, 763) | 487 (323, 626) | 459 (343, 597) | 363 (204, 538) | 0.001 |
| Pain Behavior (T-score) | 37 (37, 55) | 54 (37, 57) | 56 (54, 59) | 59 (56, 61) | 0.001 |
| Pain Interference (T-score) | 41 (41, 51) | 49 (41, 55) | 54 (51, 58) | 61 (55, 64) | 0.001 |
| Somatic Symptoms (0-24) | 1 (0, 2) | 2 (0, 3) | 2 (1, 5) | 4 (2, 7) | 0.001 |
| Pain Catastrophizing (0-52) | 9 (6, 16) | 13 (7, 21) | 19 (7, 26) | 18 (6, 27) | 0.016 |
| Psychological | |||||
| Anxiety (T-score) | 53 (48, 58) | 56 (50, 60) | 56 (54, 61) | 60 (55, 65) | 0.002 |
| Depression (T-score) | 50 (43, 56) | 51 (46, 55) | 53 (50, 59) | 55 (48, 59) | 0.016 |
QST differences across groups
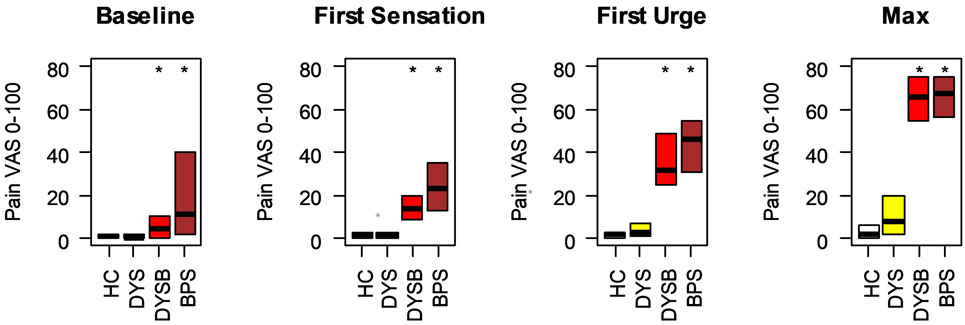
These analyses add 24 DYS, 25 DYSB and 25 BPS participants on top of our earlier published study of experimental bladder sensitivity in this cohort [26]. This permitted us to reconfirm whether levels of bladder sensitivity in DYSB were heightened and compare them for the first time to a BPS cohort (Fig 2). Provoked bladder pain in DYS participants was minimal and comparable to healthy controls (Figure 2). In contrast, DYSB participants reported significantly greater provoked bladder pain than healthy controls at all cystometric thresholds (Figure 2; p’s < 0.01). Their pain intensity notably was comparable to women with BPS.
Figure 2: Participants with dysmenorrhea and bladder pain hypersensitivity report pain comparable to participants with bladder pain syndrome.
Participants in each of the 4 groups rated their bladder pain across cystometric thresholds. Box plots indicate median, 25 th and 75th percentile. * indicates worse compared to healthy controls (p < 0.05). HC: Healthy Control, DYS: Dysmenorrhea only, DYSB: DYS + Bladder pain sensitivity, BPS: Bladder Pain Syndrome, VAS: Visual Analogue Scale.
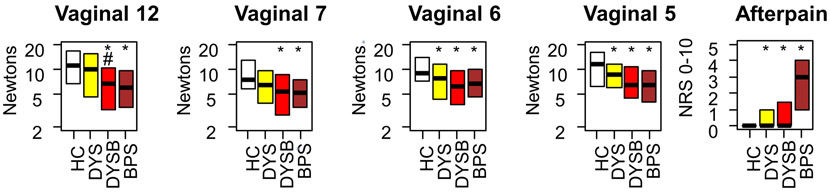
Next, we assessed somatic pain sensitivity. DYS participants had lower vaginal PPTs at 2 sites (p's < 0.05) and more after-pain sensation (p = 0.031) than healthy controls (Figure 3). Compared to healthy controls, DYSB and BPS participants had significantly lower vaginal PPTs at all sites and significantly worse after-pain sensation (p's < 0.01).
Figure 3: Dysmenorrhea (DYS and DYSB) and BPS are associated with decreased vaginal pressure pain thresholds (PPTs) and more after-pain.
Numbers designate vaginal positions relative to a clockface (12, under bladder; 7 and 5, iliococcygeus; 6 anorectal raphe). Five minutes after PPTs, after-pain was rated on a 0-10 NRS. * indicates worse compared to healthy controls (p < 0.05). # indicates worse compared to dysmenorrhea-only participants (p < 0.05). HC: Healthy Control, DYS: Dysmenorrhea only, DYSB: DYS + Bladder pain sensitivity, BPS: Bladder Pain Syndrome.
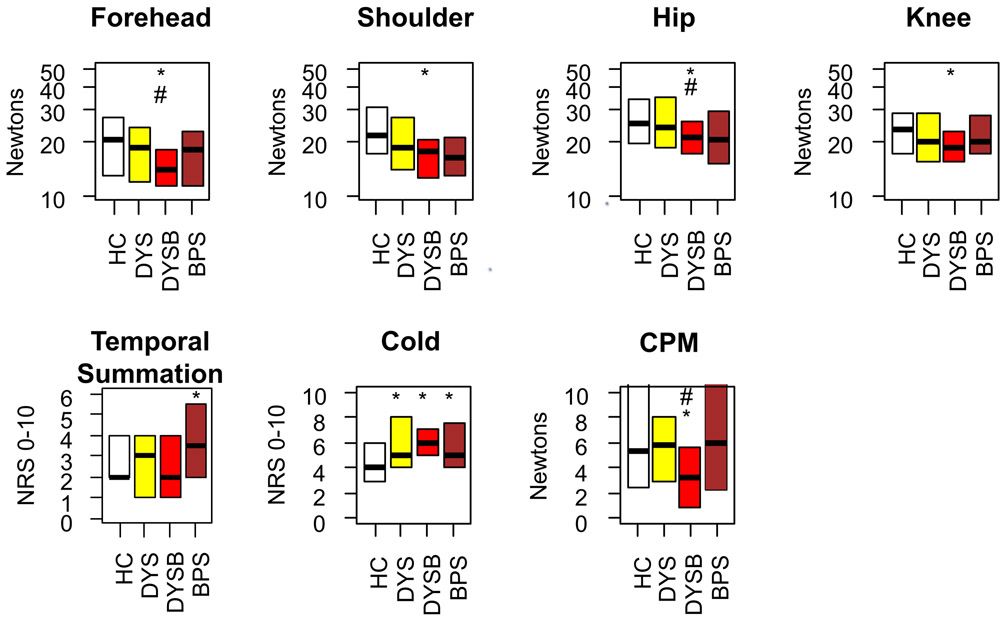
Following the pelvic assessments, we examined body PPTs, temporal summation, cold pain (10 s of hand immersion in ice water, obtained when applied as the conditioning stimulus for CPM), and CPM (Figure 4). Although DYS participants reported greater cold pain than healthy controls (p = 0.026), there were no significant differences from healthy controls in body PPTs (p = 0.180), temporal summation (p = 0.290), or CPM (p = 0.700). In contrast, DYSB participants had significantly lower body PPTs (p = 0.002) and increased cold pain report (p = 0.002). DYSB participants also had reduced CPM compared to healthy controls (p = 0.045), suggesting less efficient pain modulation. Notably, PPTs at forehead and hip (p’s <0.05) and CPM magnitude (p = 0.005) were lower in DYSB participants even when compared to DYS participants. Compared to healthy controls, cold pain was increased in BPS participants (p = 0.025), but body PPT and CPM contrasts were nonsignificant. Interestingly temporal summation was elevated in BPS compared to healthy controls (p = 0.044).
Figure 4: Dysmenorrhea with provoked bladder pain hypersensitivity is associated with widespread alterations in bodily somatic sensitivity.
PPTs were obtained twice at each of the four locations above. * indicates worse compared to healthy controls (p < 0.05). # indicates worse compared to dysmenorrhea-only participants (p < 0.05). HC: Healthy Control, DYS: Dysmenorrhea only, DYSB: DYS + Bladder pain sensitivity, BPS: Bladder Pain Syndrome. NRS: Numeric Rating Scale, CPM: Conditioned Pain Modulation.
To better understand these discrepant CPM findings in the two enhanced pain groups (DYSB and BPS), we analyzed results from a positive control group (a mixed diagnosis chronic pain cohort; see Figure 1 recruitment diagram and Supplemental Table 1). This group of 23 women [median age 26 (IQR 22 – 31), 9% minority race] had a mixed pattern of pain presentations (e.g., back pain, fibromyalgia, endometriosis) except BPS, but all had chronic pain [median pain intensity 4 (IQR 3-6) on a 0-10 NRS, median duration 5 (IQR 2-9) years]. Although the strength of CPM in participants with chronic pain was numerically lower than all other groups [1.2 (IQR −2.8 – 8.4 N)], the smaller number of both BPS, and chronic pain patients did not provide sufficient power to test this contrast adequately (p = 0.070; Supplemental Table 1). Nevertheless, the effect size of CPM impairment in this chronic pain cohort was d = −0.54 (95% CI −0.10 - 0.98). Thus, although we may have been underpowered to detect impairment of CPM in BPS [d = 0.30 (95% CI −0.20 - 0.80); n=16], the magnitude of the effect in the mixed chronic pain cohort was consistent with the observed CPM deficit in DYSB [d = −0.52 (95% CI −0.18 - −0.86); n=38].
Contribution of potential confounders
We also sought to confirm if any confounding might explain the observed QST differences. There were not significant differences between individual examiners (p’s > 0.05) and QST group differences reported earlier were still significant after re-analysis within a single examiner who conducted most of the exams (p’s <0.05). To establish whether demographic or psychological factors contributed to QST we generated a correlation matrix (Table 2). Among all psychological covariates, only the relationship between catastrophizing and vaginal PPTs (r = −0.24) was significant. However, ordered logistic regression confirmed that DYSB participants had lower vaginal PPTs (proportional odds ratio (pOR) = 0.3 ± 0.1; p = 0.003) even accounting for catastrophizing (pOR = 1.0 ± 0.1; p = 0.003).
Table 2: Confounder assessment of quantitative sensory testing and dysmenorrhea.
A matrix of Spearman correlation coefficients was generated to identify potential confounding relationships between clinical characteristics and QST. Coefficients were calculated only from healthy control, dysmenorrhea and dysmenorrhea with bladder hypersensitivity participants to avoid long-term effects of bladder pain syndrome. BOLD - significant correlations after corrections for multiple comparisons (p < 0.05). PPT: Pain Pressure Threshold, CPM: Conditioned Pain Modulation.
| Experimental Bladder Pain |
Average Body PPT |
Average Vaginal PPT |
Vaginal Afterpain |
Cold Pain |
Temporal Summation |
CPM | |
|---|---|---|---|---|---|---|---|
| Age | −.05 | 0.18 | 0.10 | −0.08 | 0.16 | 0.12 | −0.05 |
| Anxiety | .16 | 0.10 | 0.02 | 0.11 | −0.07 | −0.09 | 0.09 |
| Depression | .17 | 0.02 | −0.05 | 0.08 | −0.04 | −0.09 | 0.04 |
| Catastrophizing | .22 | −0.18 | —0.24 | 0.14 | 0.11 | −0.06 | −0.12 |
| Cycle Day | −.05 | −0.07 | −0.08 | 0.17 | 0.25 | 0.07 | 0.00 |
Even though pain testing was only conducted during the luteal phase, we did additional testing to ensure that cycle phase effects on pain sensitivity could not explain our group differences. We assessed for confounding both by exact cycle day (between 15-25) and by the presence of an LH surge. Although there was a correlation between cycle day and cold pain (r = 0.25), there was no difference in average cycle day tested across the study groups. Using the interpretable urine dipstick data available from 61% of the cohort (125/209), there was no difference in the presence of LH surge across the groups (p = 0.12) and no difference in QST outcomes (Supplemental Table 2). Some participants did not document performing an LH test. Participants missing LH data had increased after-pain (p = 0.009) and cold pain (p = 0.036). To ensure that misclassification of cycle phase did not bias results and because DYS participants were more likely to miss documenting LH (Supplemental Table 2: p<0.001), we confirmed DYSB participants still had more after-pain (pOR = 4.6 ± 2.4 ;p = 0.004) than DYS or healthy controls even after adjusting for missing LH status (pOR = 0.5 ± 0.2; p = 0.036). Similarly, DYSB participants had more cold pain (pOR = 3.3 ± 1.3; p = 0.002) than DYS or healthy controls after adjusting for missing LH status (pOR = 0.5 ± 0.2; p = 0.036; pOR = 0.4 ± 0.1; p = 0.001). Thus, all assessed QST covariates did not cause confounding and had little influence on pain sensitivity.
Provoked bladder pain hypersensitivity is robustly associated with multiple pelvic pain symptoms
To explore how sensory mechanisms influence clinical pelvic pain report, we compared how much psychosocial factors vs. QST responses each predicted VAS ratings of menstrual, overall pelvic, bowel, urinary, and coital pain. Initially, we only analyzed healthy controls, women with DYS, and DYSB participants because the inclusion of BPS participants would largely skew correlation coefficients, reflective of demographic differences (the BPS group is older than the other groups) rather than uncover potential factors for increased pain in an at-risk cohort. After corrections for multiple comparisons, psychological factors were not correlated with menstrual, pelvic, urination, or intercourse pain. However, significant correlations were observed between bowel pain and anxiety (r = 0.31) and depression (r = 0.28).
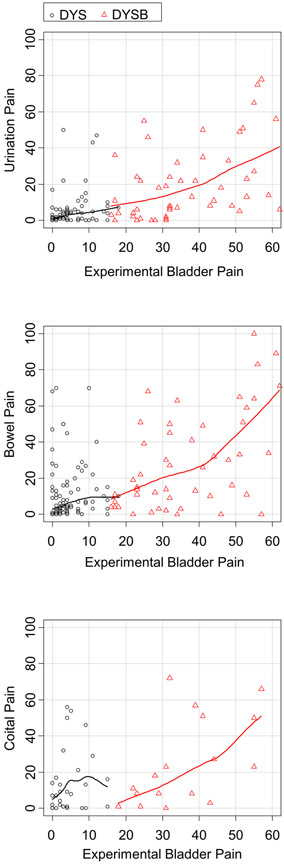
Similarly, we looked at the relationship between QST and self-report of pelvic pain. Provoked bladder pain hypersensitivity was a robust predictor of all reports of pelvic pain (Table 3, Figure 5). Even among participants with DYSB, participants with worse provoked bladder pain had worse urination, bowel, and coital pain (Figure 5). Afterpain (pain after vaginal PPT testing) was correlated with coital pain (r=0.31) and bowel pain (r=0.33). However, no PPT measures significantly correlated with clinical outcome variables other than bowel pain (Table 3). Afterpain (pain after vaginal PPT testing) was correlated with coital pain (r=0.31). Thus, comparing psychological profiles with QST responses, provoked bladder pain sensitivity was the most robust factor associated with worse pelvic pain symptoms.
Table 3: Comparative relationships between psychosocial factors, QST findings and clinical self-report of pelvic pain.
Spearman correlation coefficients are shown for factors and pain self-report. Analysis was limited to healthy control, dysmenorrhea and dysmenorrhea with bladder pain sensitivity participants, to avoid long-term effects of bladder pain syndrome. BOLD - significant correlations (p < 0.05) after corrections for multiple comparisons. QST: Quantitative Sensory Testing, PPT: Pain Pressure Threshold.
| Menstrual Pain |
Pelvic Pain |
Urination Pain |
Bowel Pain |
Sex Pain |
General Pain |
|
|---|---|---|---|---|---|---|
| Covariates | ||||||
| Anxiety | 0.11 | 0.21 | 0.14 | 0.31 | 0.07 | 0.22 |
| Depression | −0.02 | 0.17 | 0.14 | 0.28 | −0.03 | 0.24 |
| Catastrophizing | 0.24 | 0.25 | 0.16 | 0.22 | −0.05 | 0.11 |
| QST | ||||||
| Provoked Bladder Pain | 0.26 | 0.44 | 0.57 | 0.45 | 0.39 | 0.36 |
| Body PPT | −0.18 | −0.20 | −0.13 | −0.26 | 0.01 | −0.09 |
| Pelvic PPT | −0.19 | −0.12 | −0.10 | −0.27 | −0.07 | −0.11 |
| Afterpain | 0.14 | 0.14 | 0.19 | 0.33 | 0.31 | 0.13 |
| Cold | 0.23 | 0.15 | 0.12 | 0.17 | −0.01 | 0.04 |
| Temporal Summation | −0.10 | 0.01 | −0.02 | −0.05 | −0.04 | −0.03 |
| Conditioned Pain Modulation | −0.11 | −0.23 | −0.17 | −0.08 | −0.14 | −0.13 |
Figure 5: Provoked bladder pain ratings correlate with self-reported pelvic pain over the past week.
Each data point represents one participant divided by group status (DYS vs DYSB). Locally weighted scatterplot smoothed lines demonstrate the relationship between provoked bladder pain (0-100 VAS) and self-reported urination pain (top), bowel pain (middle) or coital pain (bottom) over the past week (0-100 VAS). DYS: Dysmenorrhea only, DYSB: DYS + Bladder pain sensitivity, VAS: Visual Analog Scale.
Discussion
We found that dysmenorrheic women with provoked bladder pain hypersensitivity exhibit increased pelvic and external body site mechanical sensitivity, and inefficient conditioned pain modulation. The group differences in pain sensitivity in both dysmenorrhea and DYSB participants were not explained by psychological factors, ovulatory status or age. Notably, among all of the forms of QST we evaluated, provoked bladder pain hypersensitivity had the strongest and most consistent associations to patient-reported pelvic pain symptoms among those without chronic pain. Although we have previously established that a subset of women with dysmenorrhea have silent bladder pain hypersensitivity [26,52], these analyses directly show that they have provoked bladder pain intensity approaching that of full blown BPS (Figure 2).
By using a broad array of QST methods and a significantly larger sample of dysmenorrhea sufferers, this study clarifies some inconsistencies in the earlier uterine pain literature. Both the presence and absence of mechanical, thermal or visceral hypersensitivity have been reported in women with dysmenorrhea [1,4,5,15,17,32,42,49]. Adding to a systematic review that highlights key potential confounders (ovulatory status, psychological factors, etc.) of QST findings [41], our results suggest future mechanistic studies should also account for comorbid visceral sensitivity. In the limited prior work, one study reported that women with dysmenorrhea have increased sensitivity to rectal distension (n=21) [7], but another study failed to show this (n=39) [6]. We should also be cautious drawing strong conclusions from studies with smaller sample sizes, which may yield cohorts with skewed proportions of women with comorbid pelvic sensitivity.
The differences in experimental sensitivity in isolated cases of dysmenorrhea versus the enhanced phenotype with bladder sensitivity raise intriguing implications. Although reductions in vaginal PPTs and increases in vaginal after-pain were observed in both DYS and DYSB participants, only DYSB participants show hypersensitivity at extrapelvic sites. This suggests that a regional sensory sensitivity is already progressing to a more widespread dysregulation in DYSB. The reduction in CPM we observed in DYSB along with the absence of exaggerated temporal summation, suggests that aberrant descending modulation is a key feature of altered central nociceptive processing in these women [58]. Mixtures of peripheral and central components for pain have similarly been reported for related conditions - urologic chronic pelvic pain syndromes [25] and irritable bowel syndrome [56]. This is distinct from other somatic pain conditions which may have predominately peripheral (e.g. neuropathic pain) or central components (e.g. fibromyalgia) as suggested by QST [18,50]. Conversely, since DYSB appears to exhibit dysregulated central and peripheral pain mechanisms, treatment could have reciprocal effects. For example, treating dysmenorrhea has been shown to improve fibromyalgia [11]. Further investigation of the DYSB cohort would help establish the relevant mechanisms underlying these treatment effects.
We did have some inconsistent findings regarding evidence of descending inhibition across other pain states, notably, no significant finding in BPS patients. First, we recognize our sample size of BPS participants was not necessarily planned to assess all QST differences. Similarly, the sample size in the chronic pain control cohort we recruited to validate our CPM methodology was insufficient for formal contrast. Using a different CPM technique with a thermal stimulus, Ness and colleagues were able to identify CPM impairments (effect size = 0.99) in a small pilot study of 28 women [38]. Using this same technique, Grinberg et al. replicated these findings (effect size = 1.01) in a slightly larger cohort (n=41) [22]. Notably, they further identified a similar effect in participants with vestibulodynia (n= 18). A possible explanation for these CPM discrepancies is that the BPS cohorts in Grinberg and Ness et al. had more severe form of BPS and less CPM. Whereas in Grinberg et al. BPS participants had an average pain catastrophizing score of 28.7, our BPS participants only had a median score of 18. In Ness et al. 67% of BPS participants were on daily opioids, but only 24% of our participants took opioids even occasionally. Since CPM is correlated with severity of the pain phenotype [14,22,37], our sampled BPS phenotype may not have been severe enough to manifest a deficit in CPM.
The effect size for CPM is also known to vary across chronic pain states. For example, meta-analyses suggest an effect size of 1.36 for chronic orofacial pain [36], 0.89 for irritable bowel syndrome [3] , and 0.57 for fibromyalgia [39]. If the actual effect size for the CPM methodology used here is lower than that for a thermal-based paradigm, we are likely underpowered for studying this particular contrast. Given that the observed effect size in our chronic pain controls was comparable to women with DYSB participants, we conclude that our CPM methodology (which is less resource intensive) is generally robust for identifying significantly symptomatic chronic pain patients vs. healthy controls. However, this approach for assessing CPM may have limitations for assessing some cohorts of BPS participants, a syndrome which is not only defined in part by pain symptoms, but also by bladder irritative symptoms (urgency, frequency) [21,46]. Our smaller BPS cohort, compared to these other studies may have met diagnostic criteria moreso through irritation-dominant symptoms, which likely reflect a different set of neurological mechanisms. More importantly, the overall difference in magnitude of CPM in our primary target condition, DYSB, is consistent with the estimates noted above for chronic pain conditions.
Key strengths of our study include ethnic diversity, the use of positive control groups (i.e. BPS, chronic pain) and the use of multiple blinded QST assessments. Our study extends a recent study of 32 women with dysmenorrhea that found increased thermal pain sensitivity in women with dysmenorrhea compared to healthy controls [42], by further demonstrating group differences in mechanical sensitivity in 147 dysmenorrheic women. Importantly, our study also addresses potential confounders (anxiety, depression, catastrophizing, ovulatory status, cycle day of testing) that have been concerns in prior studies [41]. The relative ease of measuring provoked bladder sensitivity pain should make these findings more easily reproducible and translatable for future research studies. Although we and others have identified reduced vaginal pressure thresholds in pelvic pain conditions [27,43], the current data set extends those findings with a larger number of healthy controls and women with dysmenorrhea.
We should note some key limitations. The naturalistic bladder filling test represents a stimulus of increasing intensity, unlike balloon distension of hollow organs (e.g. colon) in which stimulus intensity can be varied randomly. In the absence of bladder hypervigilance, however, the bladder filling test should not be heavily susceptible to bias since it reflects a routine physiological process. We could not confirm if any dysmenorrhea participants had occult endometriosis which could contribute to reduced response thresholds, but a structured physical examination by a trained gynecological surgeon minimizes the chance of missing meaningful deep infiltrating endometriosis [26]. This is a cross-sectional study of mostly younger women, with very few having had a prior vaginal delivery, or having exposure to a sexually transmitted disease, so future work is needed to establish the long-term trajectory of QST profiles in general, and in populations with more gynecological issues.
In the aggregate, our present results extend previous work showing broad bladder hypersensitivity and aberrant psychosocial profiles [26,52] in this potential subphenotype, dysmenorrhea associated with provoked bladder pain hypersensitivity. This phenotype exhibits distinctive patterns of PPTs not only showing the vaginal hypersensitivity seen in dysmenorrhea, but also widespread body pressure hypersensitivity as seen in other chronic pain syndromes. This is consistent with a broader finding in the NIDDK-funded Multidisciplinary Approach to Pelvic Pain study suggesting that both peripheral and central pain phenotypes exist, and that the latter has overall worsened clinical presentation [29]. Peripheral sensitization as seen in dysmenorrhea, is widely accepted to underlie emergence of many cases of broader, central sensitization [19,30]. Our study suggests that readily identifiable reductions in pelvic PPTs could precede BPS, since dysmenorrhea generally has an earlier onset than these conditions. The DYSB phenotype is characterized by a level of severity between healthy controls and women with bladder pain syndrome, but further work is needed to identify whether they represent a transitional subgroup. If this group is prospectively found to harbor greater risk for developing chronic pain, an urgent clinical research question will be to test if such enhanced sensory sensitivity can be reversed by suppression of cyclical menstrual pain. Indeed, prior studies suggest that hormonal and surgical amelioration of dysmenorrhea improves other visceral pain conditions [16] and fibromyalgia [11].
Supplementary Material
Acknowledgements:
We would like to acknowledge G.F. Gebhart and Arielle Shlobin at NorthShore University HealthSystem/Pritzker School of Medicine for valuable scientific and editorial advice. This research was supported by NIDDK DK100368 and NorthShore University Health System. The authors have no conflicts in interest to report.
Footnotes
The authors have no conflicts in interest to report.
References
- [1].Aberger EW, Denney DR, Hutchings DF. Pain sensitivity and coping strategies among dysmenorrheic women: Much ado about nothing. Behaviour Research and Therapy 1983;21:119–127. [DOI] [PubMed] [Google Scholar]
- [2].Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 2002;187:116–126. [DOI] [PubMed] [Google Scholar]
- [3].Albusoda A, Ruffle JK, Friis KA, Gysan MR, Drewes AM, Aziz Q, Farmer AD. Systematic review with meta-analysis: conditioned pain modulation in patients with the irritable bowel syndrome. Aliment Pharmacol Ther 2018;48:797–806. [DOI] [PubMed] [Google Scholar]
- [4].Amodei N, Nelson-Gray RO. Reactions of dysmenorrheic and nondysmenorrheic women to experimentally induced pain throughout the menstrual cycle. J Behav Med 1989;12:373–385. [DOI] [PubMed] [Google Scholar]
- [5].Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L. A comparison of modality-specific somatosensory changes during menstruation in dysmenorrheic and nondysmenorrheic women. Clin J Pain 2002;18:180–190. [DOI] [PubMed] [Google Scholar]
- [6].Böttcher B, Gizewski ER, Siedentopf C, Steiger R, Verius M, Riedl D, Ischebeck A, Schmid J, Wildt L, Elsenbruch S. Behavioural and neural responses to aversive visceral stimuli in women with primary dysmenorrhoea. Eur J Pain 2019;23:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brinkert W, Dimcevski G, Arendt-Nielsen L, Drewes AM, Wilder-Smith OHG. Dysmenorrhoea is associated with hypersensitivity in the sigmoid colon and rectum. Pain 2007;132 Suppl 1:S46–51. [DOI] [PubMed] [Google Scholar]
- [8].Brumovsky PR, Gebhart GF. Visceral organ cross-sensitization - an integrated perspective. Auton Neurosci 2010;153:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cathcart S, Winefield AH, Rolan P, Lushington K. Reliability of temporal summation and diffuse noxious inhibitory control. Pain Res Manag 2009;14:433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care 2007;45:S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Costantini R, Affaitati G, Wesselmann U, Czakanski P, Giamberardino MA. Visceral pain as a triggering factor for fibromyalgia symptoms in comorbid patients. Pain 2017;158:1925–1937. [DOI] [PubMed] [Google Scholar]
- [12].Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med 1983;13:595–605. [PubMed] [Google Scholar]
- [13].Eisenberg E, Burstein Y, Suzan E, Treister R, Aviram J. Spinal cord stimulation attenuates temporal summation in patients with neuropathic pain. Pain 2015;156:381–385. [DOI] [PubMed] [Google Scholar]
- [14].Ferrer-Peña R, Muñoz-García D, Calvo-Lobo C, Fernández-Carnero J. Pain Expansion and Severity Reflect Central Sensitization in Primary Care Patients with Greater Trochanteric Pain Syndrome. Pain Med 2019;20:961–970. [DOI] [PubMed] [Google Scholar]
- [15].Giamberardino MA, Berkley KJ, Iezzi S, de Bigontina P, Vecchiet L. Pain threshold variations in somatic wall tissues as a function of menstrual cycle, segmental site and tissue depth in non-dysmenorrheic women, dysmenorrheic women and men. Pain 1997;71:187–197. [DOI] [PubMed] [Google Scholar]
- [16].Giamberardino MA, Costantini R, Affaitati G, Fabrizio A, Lapenna D, Tafuri E, Mezzetti A. Viscero-visceral hyperalgesia: characterization in different clinical models. Pain 2010;151:307–322. [DOI] [PubMed] [Google Scholar]
- [17].Goolkasian P An ROC analysis of pain reactions in dysmenorrheic and nondysmenorrheic women. Percept Psychophys 1983;34:381–386. [DOI] [PubMed] [Google Scholar]
- [18].Gormsen L, Bach FW, Rosenberg R, Jensen TS. Differential pain modulation in patients with peripheral neuropathic pain and fibromyalgia. Scand J Pain 2012;3:116–123. [DOI] [PubMed] [Google Scholar]
- [19].Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol 2010;6:599–606. [DOI] [PubMed] [Google Scholar]
- [20].Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ, Consensus Working Group of the Sex, Gender, and Pain SIG of the IASP. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 2007;132 Suppl 1:S26–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Griffith JW, Stephens-Shields AJ, Hou X, Naliboff BD, Pontari M, Edwards TC, Williams DA, Clemens JQ, Afari N, Tu F, Lloyd RB, Patrick DL, Mullins C, Kusek JW, Sutcliffe S, Hong BA, Lai HH, Krieger JN, Bradley CS, Kim J, Landis JR. Pain and Urinary Symptoms Should Not be Combined into a Single Score: Psychometric Findings from the MAPP Research Network. J Urol 2016;195:949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grinberg K, Granot M, Lowenstein L, Abramov L, Weissman-Fogel I. A common pronociceptive pain modulation profile typifying subgroups of chronic pelvic pain syndromes is interrelated with enhanced clinical pain. Pain 2017;158:1021–1029. [DOI] [PubMed] [Google Scholar]
- [23].Hanno PM, Burks DA, Clemens JQ, Dmochowski RR, Erickson D, Fitzgerald MP, Forrest JB, Gordon B, Gray M, Mayer RD, Newman D, Nyberg L Jr, Payne CK, Wesselmann U, Faraday MM. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol 2011;185:2162–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Harte SE, Schrepf A, Gallop R, Kruger GH, Lai HHH, Sutcliffe S, Halvorson M, Ichesco E, Naliboff BD, Afari N, Harris RE, Farrar JT, Tu F, Landis JR, Clauw DJ, MAPP Research Network. Quantitative assessment of nonpelvic pressure pain sensitivity in urologic chronic pelvic pain syndrome: a MAPP Research Network study. Pain 2019;160:1270–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hellman KM, Datta A, Steiner ND, Kane Morlock JN, Garrison EF, Clauw DJ, Tu FF. Identification of experimental bladder sensitivity among dysmenorrhea sufferers. Am J Obstet Gynecol 2018;219:84.e1–84.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hellman KM, Patanwala IY, Pozolo KE, Tu FF. Multimodal nociceptive mechanisms underlying chronic pelvic pain. Am J Obstet Gynecol 2015;213:827.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update 2015;21:762–778. [DOI] [PubMed] [Google Scholar]
- [29].Lai HH, Thu JHL, Moh FV, Paradis A, Vetter J. Clustering of Patients with Interstitial Cystitis/Bladder Pain Syndrome and Chronic Prostatitis/Chronic Pelvic Pain Syndrome. J Urol 2019;202:546–551. [DOI] [PubMed] [Google Scholar]
- [30].Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Latthe P, Mignini L, Gray R, Hills R, Khan K. Factors predisposing women to chronic pelvic pain: systematic review. BMJ 2006;332:749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee L-C, Tu C-H, Chen L-F, Shen H-D, Chao H-T, Lin M-W, Hsieh J-C. Association of brain-derived neurotrophic factor gene Val66Met polymorphism with primary dysmenorrhea. PLoS ONE 2014;9:e112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee YC, Lu B, Edwards RR, Wasan AD, Nassikas NJ, Clauw DJ, Solomon DH, Karlson EW. The role of sleep problems in central pain processing in rheumatoid arthritis. Arthritis Rheum 2013;65:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lewis GN, Heales L, Rice DA, Rome K, McNair PJ. Reliability of the conditioned pain modulation paradigm to assess endogenous inhibitory pain pathways. Pain Res Manag 2012;17:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Martel MO, Wasan AD, Edwards RR. Sex differences in the stability of conditioned pain modulation (CPM) among patients with chronic pain. Pain Med 2013;14:1757–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moana-Filho EJ, Herrero Babiloni A, Theis-Mahon NR. Endogenous pain modulation in chronic orofacial pain: a systematic review and meta-analysis. Pain 2018;159:1441–1455. [DOI] [PubMed] [Google Scholar]
- [37].Morris MC, Walker LS, Bruehl S, Stone AL, Mielock AS, Rao U. Impaired conditioned pain modulation in youth with functional abdominal pain. Pain 2016;157:2375–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ness TJ, Lloyd LK, Fillingim RB. An endogenous pain control system is altered in subjects with interstitial cystitis. J Urol 2014;191:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].O’Brien AT, Deitos A, Triñanes Pego Y, Fregni F, Carrillo-de-la-Peña MT. Defective Endogenous Pain Modulation in Fibromyalgia: A Meta-Analysis of Temporal Summation and Conditioned Pain Modulation Paradigms. J Pain 2018;19:819–836. [DOI] [PubMed] [Google Scholar]
- [40].O’Leary MP, Sant GR, Fowler FJ Jr, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology 1997;49:58–63. [DOI] [PubMed] [Google Scholar]
- [41].Payne LA, Rapkin AJ, Seidman LC, Zeltzer LK, Tsao JC. Experimental and procedural pain responses in primary dysmenorrhea: a systematic review. J Pain Res 2017;10:2233–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Payne LA, Seidman LC, Sim M- S, Rapkin AJ, Naliboff BD, Zeltzer LK. Experimental evaluation of central pain processes in young women with primary dysmenorrhea. Pain 2019;160:1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Phillips N, Brown C, Bachmann G, Wan J, Wood R, Ulrich D, Bachour C, Foster D. Relationship between nongenital tender point tenderness and intravaginal muscle pain intensity: ratings in women with provoked vestibulodynia and implications for treatment. Am J Obstet Gynecol 2016;215:751.e1–751.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].R Development Team. R: A Language and Environment for Statistical Computing . Vienna, Austria, 2008. [Google Scholar]
- [45].Schoep ME, Nieboer TE, van der Zanden M, Braat DDM, Nap AW. The impact of menstrual symptoms on everyday life: a survey among 42,879 women. Am J Obstet Gynecol 2019;220:569.e1–569.e7. [DOI] [PubMed] [Google Scholar]
- [46].Schrepf A, Williams DA, Gallop R, Naliboff BD, Basu N, Kaplan C, Harper DE, Landis JR, Clemens JQ, Strachan E, Griffith JW, Afari N, Hassett A, Pontari MA, Clauw DJ, Harte SE, MAPP Research Network. Sensory sensitivity and symptom severity represent unique dimensions of chronic pain: a MAPP Research Network study. Pain 2018;159:2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Silberg JL, Martin NG, Heath AC. Genetic and environmental factors in primary dysmenorrhea and its relationship to anxiety, depression, and neuroticism. Behav Genet 1987;17:363–383. [DOI] [PubMed] [Google Scholar]
- [48].Slade GD, Sanders AE, Ohrbach R, Fillingim RB, Dubner R, Gracely RH, Bair E, Maixner W, Greenspan JD. Pressure pain thresholds fluctuate with, but do not usefully predict, the clinical course of painful temporomandibular disorder. Pain 2014;155:2134–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Slater H, Paananen M, Smith AJ, O’Sullivan P, Briggs AM, Hickey M, Mountain J, Karppinen J, Beales D. Heightened cold pain and pressure pain sensitivity in young female adults with moderate-to-severe menstrual pain. Pain 2015;156:2468–2478. [DOI] [PubMed] [Google Scholar]
- [50].Sluka KA. Peripheral and central mechanisms of chronic musculoskeletal pain. Pain Manag 2013;3:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment 1995;7:524–532. [Google Scholar]
- [52].Tu FF, Epstein AE, Pozolo KE, Sexton DL, Melnyk AI, Hellman KM. A noninvasive bladder sensory test supports a role for dysmenorrhea increasing bladder noxious mechanosensitivity. Clin J Pain 2013;29:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tu FF, Fitzgerald CM, Kuiken T, Farrell T, Harden RN. Vaginal pressure-pain thresholds: initial validation and reliability assessment in healthy women. Clin J Pain 2008;24:45–50. [DOI] [PubMed] [Google Scholar]
- [54].Tu FF, Kane JN, Hellman KM. Noninvasive experimental bladder pain assessment in painful bladder syndrome. BJOG 2017;124:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Westling AM, Tu FF, Griffith JW, Hellman KM. The association of dysmenorrhea with noncyclic pelvic pain accounting for psychological factors. Am J Obstet Gynecol 2013;209:422e.1–422.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroenterol 2007;13:3699–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160–172. [DOI] [PubMed] [Google Scholar]
- [58].Yarnitsky D Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Current Opinion in Anesthesiology 2010;23:611–615. [DOI] [PubMed] [Google Scholar]
- [59].Zuckerman RM, Silton RL, Tu FF, Eng JS, Hellman KM. Somatic symptoms in women with dysmenorrhea and noncyclic pelvic pain. Arch Womens Ment Health 2018;21:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.