Abstract
Cell death is an essential feature of development in multicellular organisms, a critical driver of degenerative diseases, and can be harnessed for treating some cancers. Understanding the mechanisms governing cell death is critical for addressing its role in disease. Similarly, metabolism is essential for normal energy and biomolecule production, and goes awry in many diseases. Metabolism and cell death are tightly linked in the phenomenon of ferroptosis, a form of regulated cell death driven by peroxidation of phospholipids. Glutathione peroxidase 4 (GPX4) uses glutathione to protect cells from ferroptosis by eliminating phospholipid peroxides. Recent data have revealed glutathione/GPX4-independent axes for suppressing ferroptosis, and insight into the regulation of iron and mitochondria in ferroptosis. Ferroptosis has recently been implicated in multiple diseases, and functions as a tumor suppression mechanism. Ferroptosis induction is a promising approach in treating a number of conditions, including neoplastic diseases. Here, we summarize these recent advances.
Keywords: Ferroptosis, iron, lipid peroxidation, metabolism, ubiquinone, cancer
Ferroptosis is a recently discovered form of cell death that is controlled by numerous metabolic pathways
Cells are the fundamental organizing unit of biological systems. The mechanisms governing the division, growth, proliferation, and death of cells are central to understanding the logic underlying the functioning of life on Earth, the possibility of life elsewhere in the universe, and the processes by which disease develops. Ferroptosis is a recently described form of cell death involving iron-dependent damage to membrane lipids; numerous metabolic pathways involving iron, lipids, and amino acids control the sensitivity of cells to ferroptosis. In the last few years, numerous advances in understanding ferroptosis have shed light on the mechanisms by which ferroptosis is triggered, how cells protect themselves from ferroptosis, and the disease contexts in which ferroptosis is relevant. In this review, we summarize these recent advances and highlight future areas of ferroptosis research relevant to understanding the normal functions of ferroptosis in living systems, how ferroptosis contributes to disease, and how controlling ferroptosis may be harnessed to create new therapies. In particular, we discuss new advances in understanding how iron availability is regulated during ferroptosis, how membrane lipid oxidation is controlled, and how ferroptosis contributes to normal tumor suppression and ischemic organ injury.
Brief history of ferroptosis
Cell death is critical for normal development of multicellular organisms, and is aberrantly activated or suppressed in a large number of diseases [1]. Cell death was historically considered to be unregulated, until the 1950s, when the concept of “programmed cell death” emerged, which led in subsequent decades to the discovery of apoptosis as a form of programmed cell death, along with expanding insight into its mechanisms of operation. For a time, apoptosis was synonymous with programmed cell death, but in the early 2000s, the concept of programmed necrosis was established, leading to the emergence of cell death modalities that are molecularly controlled, and therefore regulated, regardless of whether they are developmentally programmed or not [2]. Hence, the term “regulated cell death” was coined to refer to such modes of cell death, including necroptosis and pyroptosis [2].
In parallel with these advances in understanding the regulation of cell death writ large, one of us reported in 2003 the discovery of a small molecule named erastin that could selectively kill engineered tumor cells through a non-apoptotic mechanism [3]. Over the next 9 years, it emerged that this compound, and several additional compounds discovered in the same screening system, could activate an iron-dependent form of cell death that showed the morphology of necrosis, but was distinct mechanistically from apoptosis and several well characterized forms of necrosis, including necroptosis and pyroptosis [4–6]. This form of cell death was termed ferroptosis, because of its iron-dependence. The initial pathway for inducing ferroptosis, which was worked out in 2012–2014, was that some cells basally accumulate lipid peroxides, and that turning off the defense system that eliminates these lipid peroxides would cause their accumulation to lethal levels. For example, starving cells of cysteine through inhibition of the cystine-glutamate antiporter, known as system xc−, depletes the antioxidant peptide glutathione from cells and result in failure of the glutathione-dependent peroxidase GPX4, resulting in the accumulation of lethal levels of peroxidized lipids [7]. Despite the powerful insights provided by these discoveries, several key questions remained unanswered—namely, which specific lipids undergo oxidation to drive ferroptosis, and what other mechanisms beyond GPX4 and glutathione might some cells use to prevent ferroptosis. Over the last several years, answers to these questions have emerged, yielding a more precise understanding of the pathways that execute and regulate ferroptosis; these advances in our understanding are described below.
Ferroptosis is regulated by numerous pathways and implicated in an increasing number of diseases
Ferroptosis is a form of cell death first reported in 2012 [4], although many of the processes involved in ferroptosis had been observed in isolation decades earlier, but not integrated into a unified process [8]. The mechanisms governing ferroptosis that were elucidated in the first few years after it was discovered centered around cysteine and glutathione metabolism, and the ability of the phospholipid peroxidase GPX4 to prevent accumulation of peroxidized lipids [9], which built on early work on the basic functions of these molecules. In the 1950s, Harry Eagle and his colleagues determined that cysteine was needed for the survival of many cell lines; cells deprived of cysteine died by a morphology that was different from that caused by deprivation of other amino acids, but similar to that caused by some viral infections [10, 11]. In the 1970s, necrotic cell death in the liver, dependent on cysteine and involving glutathione depletion, was reported [12–15]. In the 1970s, Shiro Bannai and colleagues reported that cell death induced by lack of glutathione and cysteine could be suppressed by a-tocopherol, an inhibitor of lipid peroxidation [16]. Then, in 1982 Ursini and colleagues isolated the enzyme that became known as GPX4, which could suppress iron-catalyzed lipid peroxidation in membranes [17], and in the following decade, GPX4 was shown to protect against cell death associated with lipid peroxidation [18] and oxidative stress [19].
The biological and pathological contexts in which ferroptosis might operate were at first poorly defined. In the last few years, numerous studies have revealed that the mechanisms by which ferroptosis can be induced and suppressed are more diverse than originally supposed, extending beyond the glutathione-GPX4 axis (Figure 1). In addition, several biological and pathological contexts in which ferroptosis operates have been clarified, including its role in tumor suppression and organ damage.
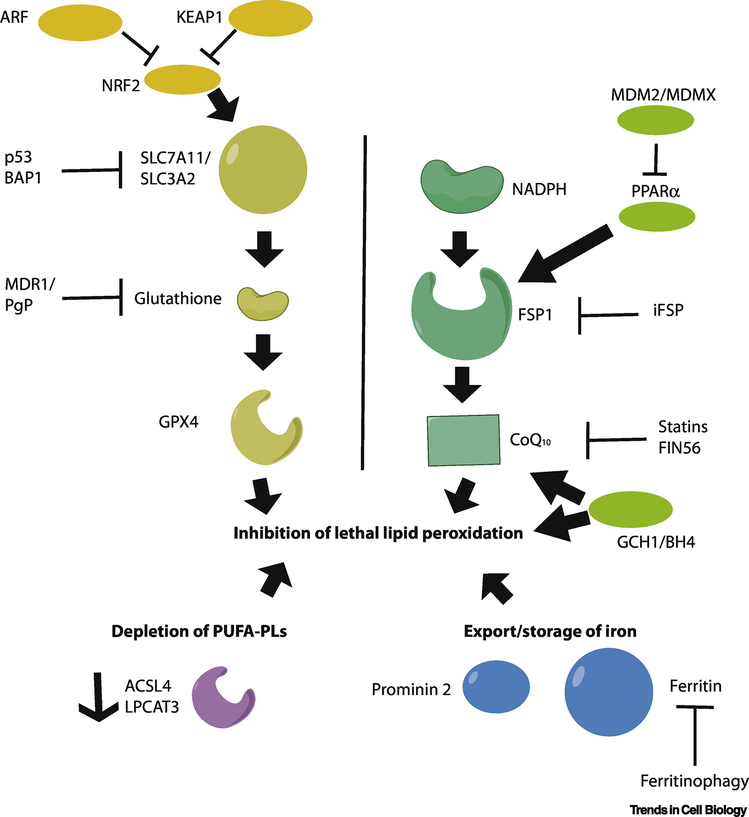
Figure 1. Key regulators of ferroptosis.
Three distinct processes can drive resistance to ferroptosis. Depletion of polyunsaturated fatty acyl phospholipids (PUFA-PLs, purple, lower left) depletes the key substrates needed for lethal lipid peroxidation. Depletion of iron by storage in ferritin or export driven by prominin2 blocks the iron-dependent peroxidation of PUFA-PLs. (blue, lower right). Finally, three parallel pathways act to suppress lipid peroxidation by intercepting intermediates in the process: the glutathione pathway (left), the ubiquinone pathway (middle, NADPH-FSP1-CoQ10), and the tetrahydrobiopterin (GCH1/BH4) pathway (right). These are further regulated by upstream genes and proteins as indicated.
Regulation of iron in ferroptosis
Despite incorporating iron in the name, the role of and regulation of iron in ferroptosis has only emerged recently. Initially, confusion emerged as to whether the lipid peroxidation that drives ferroptosis was caused by the labile iron pool reacting with lipid peroxides to propagate these species in membranes, or whether iron-dependent enzymes might solely drive this peroxidation process. Studies from several laboratories revealed that -dependent lipoxygenases often initiate ferroptosis by causing the appearance of lipid peroxides, and labile iron (not bound to enzymes) propagates these peroxides to drive overwhelming lipid peroxidation [20, 21]. It remains possible that other iron-dependent enzymes contribute to lipid peroxidation in some circumstances—recently, cytochrome P450 oxidoreductase was implicated as a driver of lipid peroxidation during ferroptosis [22].
Ferritin is used to store Fe(III) in an inert form, where it cannot contribute to lipid peroxidation. Hence, the abundance of ferritin is a key factor governing sensitivity to ferroptosis—more ferritin results in more iron(III) storage and greater resistance to ferroptosis, as the labile iron pool becomes scarce. Conversely, depletion of ferritin results in release of iron into the labile iron pool, resulting in greater sensitivity to ferroptosis. It has been demonstrated that ferritin-targeted autophagy, also known as ferritinophagy, results in the lysosomal degradation of ferritin and release of iron into the labile iron pool, thus causing increased sensitivity to ferroptosis [23, 24]. A recent report revealed another iron-regulating process, in which prominin2 drives ferroptosis resistance by promoting the formation of ferritin-containing multivesicular bodies and exosomes, which transport iron out of cells [25].
Lipid peroxide regulation
As noted above, the glutathione-dependent phospholipid peroxidase GPX4 was the first-discovered central inhibitor of ferroptosis [9]. GPX4 is a selenoprotein, implying that selenium availability impacts on sensitivity to ferroptosis. Indeed, delivery of selenium to cells or animals suppresses ferroptosis, including in a mouse model of intracerebral hemorrhage [26–28].
A genetic screen for regulators of ferroptosis sensitivity revealed the surprising finding that the multidrug resistance pump P-glycoprotein normally pumps glutathione out of the cell, resulting in collateral sensitivity to ferroptosis in MDR1/PgP-expressing cells that are otherwise multidrug resistant [29]; this is consistent with an emerging picture in which ferroptosis sensitivity and traditional chemoresistance often co-occur.
In 2016, a new chemical inducer of ferroptosis named FIN56 was reported [30]. FIN56 appeared to induce ferroptosis through a dual mechanism of depleting GPX4 protein and mevalonate-pathway-derived coenzyme Q10. CoQ10 is a critical part of the mitochondrial electron transport chain, but also functions outside of mitochondria to suppress lipid peroxidation by capturing radical intermediates in the process. Thus, depletion of CoQ10 sensitizes cells to ferroptosis. For example, statins, which inhibit mevalonate-derived CoQ10 through blocking the enzyme HMG CoA reductase, also sensitize cells to ferroptosis [30]. Two recent papers reported screens for genes that could suppress ferroptosis in the absence of GPX4, and discovered that AIFM2, now renamed ferroptosis suppressor protein 1 (FSP1), blocks lipid peroxidation and suppresses ferroptosis by regenerating reduced CoQ10, independently of any need for GPX4 or glutathione [31, 32]. This illuminated a new NADPH-FSP1-CoQ10 ferroptosis surveillance pathway that acts in parallel to glutathione-GPX4 to suppress ferroptosis. In addition to this pathway, a recent study identified another GPX4-independent ferroptosis-blocking pathway involving the gene GTP cyclohydrolase 1 (GCH1), which is the rate-limiting step in the production of the metabolite tetrahydrobiopterin (BH4) [33]. BH4 was found to suppress ferroptosis through aiding the formation of reduced CoQ10, and by blocking the peroxidation of specific lipids. This provides another independent mechanism by which some cells prevent death through ferroptosis.
Ferroptosis as a tumor suppression mechanism
While illuminating the basic mechanisms by which ferroptosis operates is of value for understanding fundamental cell biology, determining the natural functions of ferroptosis will aid in understanding how and why this form of cell death emerged during evolution. Accumulating evidence indicates that ferroptotic cell death leads to tumor growth suppression. Nevertheless, it remains to be further defined whether ferroptosis indeed acts as a critical barrier to cancer development. Inactivation of the p53 tumor suppression pathway is a pivotal event in the formation of most human cancers [34]. Although it is established that many activities of p53, including cell-cycle arrest, senescence, and apoptosis, contribute to tumor suppression, accumulating evidence suggests that the combined loss of these activities of p53 is not sufficient to abrogate its tumor suppression activity [35]. Indeed, numerous studies indicate that p53-mediated metabolic regulation also promotes tumor suppression [36], but the mechanisms by which it does so remain unclear. Recent studies revealed that ferroptosis is one pathway connecting tumor suppression and metabolism. The tumor suppressor p53 plays an important role in modulating ferroptotic responses through its transcriptional targets [37–39]. Indeed, p533KR (3KR: K117R+K161R+K162R),an acetylation-defective mutant that fails to induce cell-cycle arrest, senescence and apoptosis, retains the ability to suppress tumor formation in vivo and to promote ferroptosis through metabolic regulation including downregulation of SLC7A11, a key component of the cystine-glutamate antiporter [39]. Loss of an additional acetylation site at K98 (4KR: K98R+117R+K161R+K162R) abrogates the ferroptosis activity of p53, as well as its remaining tumor suppression function [40]. Thus, it is likely that ferroptosis plays a key role in tumor suppression, particularly when cell-cycle arrest, senescence, and apoptosis are not operative (Figure 2).
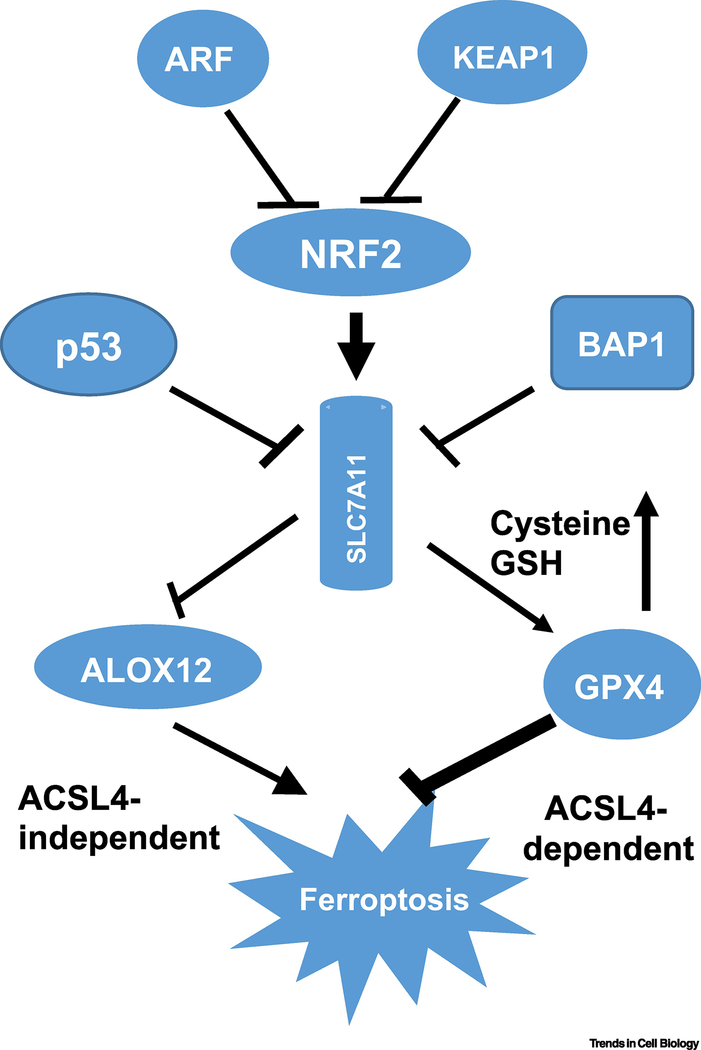
Figure 2. A schematic model for tumor suppressors regulating ferroptosis.
The expression of SLC7A11 is a key mechanism by which numerous tumor suppressor genes activate ferroptosis to prevent tumor formation. BAP1 and p53 repress SLC7A11, whereas KEAP1 and ARF prevent NRF1 from activating SLC7A11. Downstream of SLC7A11, ALOX12 and GPX4 act in parallel pathways to regulate lipid peroxidation.
Interestingly, p53 is not the only tumor suppressor involved in regulating ferroptotic responses. It was reported that the BRCA1-associated protein 1 (BAP1) tumor suppressor is also able to promote ferroptosis by repressing SLC7A11 [41]. Like p53, BAP1 is a tumor suppressor that is frequently deleted or mutated in human cancers, including clear cell renal cell carcinoma, uveal melanoma, cholangiocarcinoma, and mesothelioma. Inactivation of BAP1 in cancer cells results in SLC7A11 upregulation, ferroptosis resistance, and tumor development. Although the precise mechanism by which BAP1 induces SLC7A11 repression needs further elucidation, it was proposed that BAP1, a H2A deubiquitinase, represses SLC7A11 expression by regulating the levels of H2A ubiquitination (H2Aub) on the SLC7A11 promoter (Figure 2) [41].
The above studies not only support the role of ferroptosis in tumor suppression under physiological settings but also indicate the importance of SLC7A11 regulation in human cancers. SLC7A11 is a key component of a plasma membrane antiporter (the xc− system) that mediates Na+-independent cellular uptake of extracellular cystine in exchange for intracellular glutamate [42–45]. SLC7A11 overexpression is observed in many human cancers [46–48] and is induced under stress conditions, including oxidative stress. NRF2 (nuclear factor erythroid 2-related factor 2) is one of the key regulators of the pathway to defend against oxidative stress [49]. NRF2 is normally kept at low levels by tumor suppressor KEAP1-(Kelch ECH-associated protein 1)-mediated ubiquitination. Under oxidative stress, NRF2 is stabilized and activated by dissociation from KEAP1 [49]. NRF2 is able to recognize the antioxidant response element (ARE) and activate a number of antioxidant genes, including SLC7A11 [49].
Moreover, cancer cells rewire their cellular metabolism to meet the energetic and substrate demands of tumor development, generally accompanied by increased ROS production. Early studies showed that antioxidants may protect against cancer, as ROS and other free radicals may induce DNA damage and promote tumorigenesis [50, 51]. However, large randomized clinical trials have produced inconsistent results, and suggest that antioxidants may even increase cancer risk, although the precise mechanism remains unclear [52, 53]. Notably, recent studies showed that the levels of NRF2 are induced upon oncogenic stress, and that several oncoproteins, including c-Myc, K-RAS and B-Raf, promote tumor cell proliferation in part by stimulating NRF2-mediated expression of endogenous antioxidants and reducing ROS levels [54].
Moreover, genomic analyses of human cancers have also uncovered a high frequency of mutations associated with the NRF2/Keap1axis, validating the bona fide oncogenic role of NRF2 in vivo [55]. Thus, it is important to know how the NRF2-mediatetd effect on ferroptosis is regulated in human cancers. ARF was initially identified as the product of an alternative reading frame within the Ink4a/ARF tumor suppressor locus [56]. ARF acts as a major regulator of p53 function in response to oncogenic stress by inhibiting the enzymatic activity of ubiquitin E3 ligases (i.e., Mdm2 and ARF-BP1) that target p53 for proteasomal degradation [57]. Surprisingly, through biochemical purification, ARF was found to be a key regulator of NRF2 [58]. ARF does not modulate NRF2 protein levels by interfering with KEAP1-mediated ubiquitination—ARF inhibits CBP-dependent NRF2 acetylation and therefore significantly represses NRF2 transcriptional activity. As a consequence, ARF expression sensitizes cells to ferroptosis in a p53-independent manner, while ARF depletion induces NRF2 activation and promotes cancer cell survival in response to oxidative stress, suggesting that NRF2 is an important target of p53-independent tumor suppression by ARF. Together, these studies indicate that SLCA711 expression is tightly regulated by both oncoproteins (e.g., NRF2) and tumor suppressors (e.g., p53, BAP1 and ARF) during stress responses (Figure 2), and that SLC7A11 might be useful as a biomarker for ferroptosis in vivo.
Similar to p53 and BAP1, the tumor suppressor enzyme fumarase sensitizes cells to ferroptosis [59]. As an enzyme in the mitochondrial TCA cycle, fumarase catalyzes the conversion of fumarate to malate. Loss of function of fumarase can cause an increase in fumarate, which, through the inhibition of prolyl hydroxylases, enhances the stabilization of the proto-oncogenic hypoxia-inducible factor 1 alpha (HIF1α), which is involved in sensing and responding to oxygen concentrations. However, it has been shown that HIF1α and HIF2α are dispensable for the tumor suppressive function of fumarase, leaving the mechanism of its tumor suppressive function an enigma. More recently, it has been demonstrated that the normal metabolic function of mitochondria, including the TCA cycle in which fumarase is a key component, facilitates ferroptotic cell death in response to cysteine starvation (Figure 3) [59]. This finding provides a mechanistic explanation for the tumor suppressive nature of fumarase (Figure 3). Intriguingly and much like that of p53, the role of fumarase in ferroptosis appears to be context-dependent; while loss of fumarase mitigates ferroptosis via interference with the TCA cycle, the resulting accumulation of fumarate has been reported to enhance succination and subsequent degradation of GPX4 [60], thus sensitizing cells to ferroptosis.
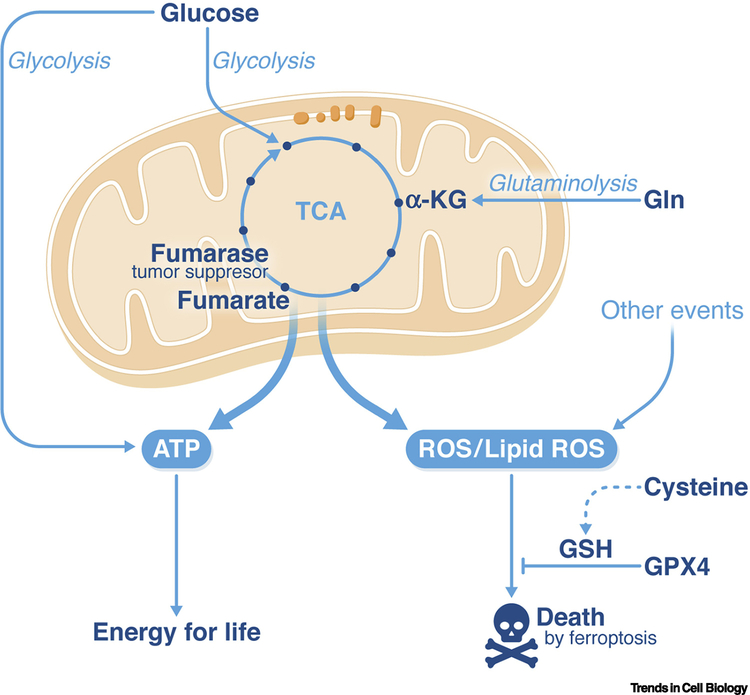
Figure 3. The role of mitochondria in ferroptosis.
Mitochondria, through the citric acid cycle and electron transport chain activity, are the major source of cellular energy production. A side effect accompanying this oxidative process is the generation of reactive oxygen species, including ferroptosis-inducing lipid peroxides. Such a role for mitochondria in ferroptosis can explain the tumor-suppressive function of the citric acid cycle enzyme fumarase. The blue arrows indicate the flow of reactions in metabolism, with glucose providing intermediates that enter the citric acid (TCA) cycle, glutamine (Gln) providing alpha-ketoglutarate (αKG) via glutaminolysis, and then ATP and reactive oxygen species (ROS) flowing out of the TCA cycle.
The precise and diverse mechanisms by which ferroptosis is induced under physiological settings remain to be elucidated. As noted above, it is established that ferroptosis is controlled by glutathione peroxidase 4 (GPX4) [9].The physiological significance of GPX4 in regulating ferroptosis is illustrated by the severe phenotypes associated with GPX4 mutant mice. For example, while Gpx4-null mice undergo embryonic lethality at 7.5 dpc, conditional-Gpx4-mutant mice revealed that GPX4 loss not only induces ferroptosis, but also results in neurodegeneration [19], loss of antiviral immunity [61], infertility, and ischemia/reperfusion injury in kidney and liver [27, 28, 62, 63].
In addition to GPX4-mediated neutralization of lipid peroxidation, the levels of cellular lipid peroxides can be enhanced enzymatically by lipoxygenases. Lipoxygenases and GPX4 thus have opposite functions in ferroptosis: lipoxygenases cause the formation of lipid peroxides, while GPX4 eliminates them. Mice deficient for most of the lipoxygenase family members display a variety of physiological impairments, including defects in inflammation, ischemic cardioprotection, and airway epithelial injury in asthma. Both Aloxe3-deficient mice and Alox12b-deficient mice suffer postnatal death characterized by impaired barrier function of the skin, while Alox15b-deficient mice also display ichthyotic skin conditions similar to those of Alox12b- and Aloxe3-deficient mice [63–67]. Alox5-deficient mice exhibit a suppressed response to inflammation, and reduced organ injury including pancreases, lung, and liver, as well as amelioration in an Alzheimer’s-disease-like phenotype [67–71]. Together, these studies reveal the physiological significance of both activation and loss of ferroptotic responses in vivo.
Finally, the precise role of p53 in modulating ferroptotic responses is complex and clearly requires further investigation. Initially, p53-dependent ferroptosis was thought to occur through prevention of cystine uptake and glutathione synthesis, which are required for GPX4 function, similar to the effect induced by erastin [39]. A recent report showed that ALOX12 is critical for p53-mediated ferroptosis. SLC7A11 interacts directly with ALOX12, but not other ALOX family members, resulting in inhibition of ALOX12 activity [72]. Accordingly, p53 promotes ferroptosis through transcriptional repression of SLC7A11, which releases ALOX12 from SLC7A11-mediated inhibition (Figure 2).
Notably, in contrast to GPX4 and the other lipoxygenase family members, deletion of ALOX12 does not elicit major developmental defects in mice [72, 73]. Indeed, ALOX12+/− and ALOX12–/– mice are fertile, healthy, and develop normally. Moreover, the ALOX12 gene resides on human chromosome 17p13.1 at a position close to the TP53 gene. Loss of one ALOX12 allele is sufficient to accelerate c-Myc-induced tumorigenesis in a haploinsufficient manner even when the classic functions of the p53 pathway (cell cycle arrest, apoptosis, and senescence) remain intact [72]. These studies provide genetic evidence that ferroptosis acts as a tumor suppression mechanism independent of classic tumor suppression mechanisms.
Recent studies show that the ferroptotic responses induced by either erastin or GPX4 inhibitors are often dependent on acyl-CoA synthetase long-chain family member 4 (ACSL4) [74–76]. ACSL4 catalyzes synthesis of long-chain polyunsaturated CoAs with a preference for arachidonic acid, thus facilitating their esterification into phospholipids. Consistent with these observations, further analyses showed that oxidized polyunsaturated fatty acid (PUFA)-containing phospholipids (PL-PUFA-OOH), but not free oxidized PUFAs (PUFA-OOH), act as the major executioners of ferroptosis [76]. It remains unclear why ACSL4 is specifically required for ferroptosis upon GPX4 inhibition, as other acyl-CoA synthetase long-chain family members are also able to induce esterification of PUFAs into phospholipids. Moreover, ferroptosis can occur in an ACSL4-independent manner, since ACSL4 is dispensable for the ferroptosis induced by the p53/ALOX12 axis [72]. Future investigations are required to examine whether other ACSL family members are involved in esterification of phospholipids targeted by ALOX12.
Ferroptosis modulation as a therapeutic avenue
Although a definitive physiological function for ferroptosis has yet to be unambiguously demonstrated, the role of ferroptosis in human diseases has been established. A wealth of studies suggests that pharmacological modulation of this unique cell death modality, by either inhibiting it or stimulating it, may yield significant clinical benefit for certain diseases.
Ischemic organ injuries and degenerative diseases
Ischemia is a major cause of a variety of devastating diseases, including ischemic heart disease, stroke, kidney failure, and liver damage. Notably, ischemic heart disease, for which effective therapies are lacking, results in more mortality annually than any other disease worldwide [77].
Pathologically, all ischemic organ injuries (IOI) share a similar symptom: massive cell death in affected organs. However, therapies that function by preventing IOI-associated cell death have not been developed, mainly due to the elusive mechanisms underlying ischemia-induced cell death. Over the last several years, mounting evidence has demonstrated that ferroptosis is a major contributor to IOI-associated cell death, and inhibition of ferroptosis significantly mitigates IOI in a cohort of experimental models. For example, in an ex vivo mouse heart model mimicking ischemia/reperfusion (IR), pharmacological inhibition of ferroptosis by an iron chelator and a glutaminase-2 inhibitor both reduced heart damage significantly, establishing the crucial role of ferroptosis in ischemic heart disease [38]. This conclusion was confirmed by a more recent in vivo study [78]. Conversely, induction of ferroptosis by conditional deletion of the GPX4 gene in mouse kidney caused kidney failure with pathological features similar to that in patients [62, 79]. Taken together, these pharmacological and genetic studies indicate that ferroptosis inhibition is a promising approach for the treatment of IOI. It should be noted that studies have shown that another type of regulated necrosis, necroptosis, may also contribute to IOI-associated cell death. However, many of these studies used a pharmacological inhibitor of necroptosis, necrostatin-1, which was found to be able to inhibit ferroptosis as well [62].
Ferroptosis is also implicated in other forms of organ injuries and degenerative diseases. Recent studies suggest a role for lung epithelial cell ferroptosis in cigarette-smoking-associated chronic obstructive pulmonary disease [80, 81]. Additionally, studies using lipid-peroxide-trapping agents and iron chelators support a role for ferroptosis on neurodegeneration [82]. Ferroptosis may also contribute to glutamate excitotoxicity of neurons: by inhibiting the activity of system xc− cystine/glutamate antiporter, high dose of glutamate can prevent cellular cystine import, thus leading to the induction of ferroptosis [4].
Therapeutic potential of ferroptosis induction in cancer
A prominent role for ferroptosis in cancer development and treatment is emerging. We discussed earlier the potential physiological function of ferroptosis in tumor suppression and how cancer cells may bypass this tumor suppressive mechanism by genetic mutations. Strikingly, it has been demonstrated that numerous types of therapy-resistant cancer cells, especially those with mesenchymal and de-differentiated characteristics, are more susceptible to ferroptosis [83–85]. These findings suggest that induction of ferroptosis might be a promising cancer therapeutic approach, especially for the treatment of mesenchymal and metastatic cancers, which are often resistant to all available therapies.
A recent study revealed a novel, non-cell autonomous mechanism for the regulation of ferroptosis that provides mechanistic insights into the basis for the enhanced sensitivity of drug-resistant cancer cells to ferroptosis [86]. In epithelial cells, E-cadherin-mediated intercellular interactions, signaling through the intracellular Merlin/NF2-Hippo pathway, inhibit the transcription co-activator YAP and its ferroptosis-potentiating activity (the study showed that two ferroptosis-promoting factors, ACSL4 [74] and transferrin receptor [38], are transcriptional targets of the YAP-TEAD4 complex). In epithelial cancer cells, decreased E cadherin or NF2 expression, reduced Hippo pathway activity, and enhanced YAP activation can promote epithelial-mesenchymal transition (EMT) and metastasis [87, 88]. Therefore, this finding explains why EMT/metastasis-prone cancer cells are highly susceptible to ferroptosis [83]. Further, since the E-cadherin, NF2, and Hippo signaling components Lats1 and Lats2 are tumor suppressors frequently mutated in cancers that promote cancer progression, these malignant mutations might be Achilles’ heels and could potentially be used as biomarkers to predict cancer cell responsiveness to ferroptosis. In xenograft mouse models for mesothelioma (loss of function of NF2 mutation occurs in > 35% of mesothelioma patients), although loss of NF2 function increases mesothelioma tumor growth and metastasis, it renders tumor cells more sensitive to GPX4 inactivation [86]. Consistent with this study, it was reported in a separate study that TAZ, a homolog of YAP often upregulated in renal cancer, is also able to sensitize renal cancer to ferroptosis induction [89].
Regulation of ferroptosis by a non-cell autonomous mechanism also has intriguing implications beyond cancer biology. Ferroptosis, like other programmed cell death mechanisms, employs cell-intrinsic machinery for execution. Remarkably, in the context of ferroptotic cell death, neighboring cells can have a significant impact on decision making, via the E-cadherin-NF2-Hippo-YAP signaling axis. Such intercellular communication appears to be mutually beneficial, as it increases the resistance to ferroptosis of all involved cells. The consequence of this intercellular communication is in stark contrast to that of death-receptor-mediated apoptosis [90, 91], in which one cell (bearing the death receptor ligand) induces apoptotic death of the other (bearing the death receptor). Considering that multicellular organisms are under frequent insult from oxidative stress, this intercellular anti-ferroptotic mechanism might be another layer of crucial defense that cells use to protect themselves from ferroptosis, a terminal and irreversible consequence of oxidative stress.
GPX4 and the system xc− cystine-glutamate antiporter are two of the validated targets for inducing ferroptosis. While genetic deletion of GPX4 causes lethality in mice [92], indicating potential toxicity of its inhibition, there may still be a therapeutic window, as suggested by studies revealing differential sensitivity of cancer cells to GPX4 inhibition [9, 83, 84, 86].
Inhibition of the system xc− cystine-glutamate antiporter will most likely be more tolerable, as mice with genetic deletion of SLC7A11, which encodes an essential subunit of the antiporter, are viable without obvious developmental defects [93, 94]. Indeed, tumor xenograft experiments in mice using an erastin derivative with better pharmacological properties, imidazole ketone erastin (IKE), showed a clear therapeutic benefit without causing significant weight loss of mice, which is a general sign of toxicity [86, 95]; this suggests that IKE will be well tolerated as a therapeutic agent. In addition, the newly discovered ferroptosis-inhibitory protein FSP1 provides another potential therapeutic target, considering that this protein is expressed in many cancer cells [31, 32].
A combination of ferroptosis induction and other therapeutic approaches is also promising. It was reported recently that anti-PDL1 immune checkpoint blockade can elicit a cancer cell ferroptotic response through the downregulation of SLC7A11 expression in cancer cells, which is a consequence of CD8+-T-cell-secreted IFNγ. These T cells are key components of the natural mechanism by which the immune system eliminates developing cancers. As such, a combination of anti-PDL1 treatment with ferroptosis induction was shown to have a synergistic anticancer effect in mouse models [96–98]. Ferroptosis has also been suggested to be partially responsible for the anticancer effect of radiation therapy [97]. All these recent advances underscore the cancer therapeutic potential of ferroptosis induction as a monotherapy and as a component of combination therapies.
Concluding Remarks and Future Perspectives
The rapid expansion of our understanding of ferroptosis is due to the growing number of laboratories that are exploring the mechanisms and functions of this form of cell death. Given the recent advances, we know that there are at least three major pathways that control sensitivity of cells to ferroptosis — the glutathione-GPX4, NADPH-FSP1-CoQ10 and GCH1-BH4 pathways. Moreover, it seems clear that ferroptosis contributes to both degenerative disease pathology and tumor suppression, and that inhibiting and inducing ferroptosis has the potential to be therapeutic in disease settings. We now know that cells have evolved a complex set of pathways for controlling when and how ferroptosis is activated, and harnessed this mode of cell death to prevent tumorigenesis. Numerous targets have been identified for inducing and inhibiting ferroptosis, but which of these provide the best therapeutic index, are translatable to animal models and patients, and are chemically tractable is not clear.
Future research may shed light on other natural functions of ferroptosis, such as its role in preventing infections or in the normal development of tissues and organs. In addition, ferroptosis more conserved throughout the diversity of life on earth than any other form of cell death, so it will be interesting to compare how ferroptosis is regulated and used in diverse species.
Finally, it is still unknown exactly why and how lipid peroxidation leads to the death of cells in ferroptosis—this is a key area of future research (see Outstanding Questions). We know that accumulation of oxidized phospholipids is a death signal, but we do not know how this causes cell death and whether it is possible to intervene in this process after these species have accumulated.
Outstanding Questions.
What are the best mechanisms for therapeutically modulating ferroptosis?
What are the mechanism by which ferroptosis is executed downstream of phospholipid peroxidation?
Has nature co-opted ferroptosis for normal physiological functions in other species?
Key remaining questions for the field include: (i) what are the best scaffolds and mechanisms for therapeutically modulating ferroptosis, (ii) what are the mechanisms by which ferroptosis is actually executed downstream of phospholipid peroxidation, and (iii) has nature co-opted ferroptosis for normal physiological functions in any species? Given that new discoveries continue to emerge even regarding the mechanisms and function of apoptosis, the first-discovered form of regulated cell death that has been extensively studied over the past 50 years, there appears to be a vast untapped terrain of ferroptosis biology awaiting study.
Highlights.
There are three parallel pathways for defending against cell death by ferroptosis
Iron availability is controlled by cellular iron import, storage, and efflux mechanisms
Several tumor suppressor genes normally prevent tumor development by activating ferroptosis in developing tumor cells
As cancers evolve to a more aggressive state, they become increasingly sensitive to ferroptosis
The immune system uses ferroptosis as a means of eliminating cancer cells
Acknowledgements
The research of the authors is supported by the National Institutes of Health (NIH) R01CA204232 (to X.J.), a Geoffrey Beene Cancer Research fund (to X.J.), a Functional Genomic Initiative fund (to X.J), and the National Cancer Institute R35CA209896, P01CA087497 (to B.R.S.), and R01CA085533 and RO1CA224272 (to W.G), and the National institute of Neurological Disorders and Stroke R61NS109407 (to B.R.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fuchs Y and Steller H (2011) Programmed cell death in animal development and disease. Cell 147 (4), 742–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galluzzi L et al. (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25 (3), 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolma S et al. (2003) Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3 (3), 285–96. [DOI] [PubMed] [Google Scholar]
- 4.Dixon SJ et al. (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149 (5), 1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang WS and Stockwell BR (2008) Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 15 (3), 234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yagoda N et al. (2007) RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447 (7146), 864–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon SJ et al. (2014) Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife (May 20), doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirschhorn T and Stockwell BR (2019) The development of the concept of ferroptosis. Free Radic Biol Med 133, 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang WS et al. (2014) Regulation of ferroptotic cancer cell death by Gpx4. Cell 156 (1–2), 317–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eagle H (1955) Nutrition needs of mammalian cells in tissue culture. Science 122 (3168), 501–14. [DOI] [PubMed] [Google Scholar]
- 11.Eagle H (1955) The specific amino acid requirements of a human carcinoma cell (Stain HeLa) in tissue culture. J Exp Med 102 (1), 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potter WZ et al. (1973) Acetaminophen-induced hepatic necrosis. 3. Cytochrome P-450-mediated covalent binding in vitro. J Pharmacol Exp Ther 187 (1), 203–10. [PubMed] [Google Scholar]
- 13.Mitchell JR et al. (1973) Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 187 (1), 211–7. [PubMed] [Google Scholar]
- 14.Mitchell JR et al. (1973) Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther 187 (1), 185–94. [PubMed] [Google Scholar]
- 15.Jollow DJ et al. (1973) Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther 187 (1), 195–202. [PubMed] [Google Scholar]
- 16.Bannai S et al. (1977) Effect of antioxidants on cultured human diploid fibroblasts exposed to cystine-free medium. Biochem Biophys Res Commun 74 (4), 1582–8. [DOI] [PubMed] [Google Scholar]
- 17.Ursini F et al. (1982) Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta 710 (2), 197–211. [DOI] [PubMed] [Google Scholar]
- 18.Geiger PG et al. (1991) Lethal damage to murine L1210 cells by exogenous lipid hydroperoxides: protective role of glutathione-dependent selenoperoxidases. Arch Biochem Biophys 288 (2), 671–80. [DOI] [PubMed] [Google Scholar]
- 19.Seiler A et al. (2008) Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab 8 (3), 237–48. [DOI] [PubMed] [Google Scholar]
- 20.Shah R et al. (2018) Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent Sci 4 (3), 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wenzel SE et al. (2017) PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou Y et al. (2020) Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol 16 (March), 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou W et al. (2016) Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12 (8), 1425–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao M et al. (2016) Ferroptosis is an autophagic cell death process. Cell Res 26 (9), 1021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown CW et al. (2019) Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export. Dev Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alim I et al. (2019) Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell 177 (5), 1262–1279 e25. [DOI] [PubMed] [Google Scholar]
- 27.Ingold I et al. (2018) Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 172 (3), 409–422 e21. [DOI] [PubMed] [Google Scholar]
- 28.Friedmann Angeli JP and Conrad M (2018) Selenium and GPX4, a vital symbiosis. Free Radic Biol Med 127, 153–159. [DOI] [PubMed] [Google Scholar]
- 29.Cao JY et al. (2019) A Genome-wide Haploid Genetic Screen Identifies Regulators of Glutathione Abundance and Ferroptosis Sensitivity. Cell Rep 26 (6), 1544–1556 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimada K et al. (2016) Global Survey of Cell Death Mechanisms Reveals Metabolic Regulation of Ferroptosis. Nat Chem Biol 12 (7), 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doll S et al. (2019) FSP1 is a glutathione-independent ferroptosis suppressor. Nature. [DOI] [PubMed] [Google Scholar]
- 32.Bersuker K et al. (2019) The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraft VAN et al. (2020) GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci 6 (1), 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kastenhuber ER and Lowe SW (2017) Putting p53 in Context. Cell 170 (6), 1062–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiser AM and Attardi LD (2018) Deconstructing networks of p53-mediated tumor suppression in vivo. Cell Death Differ 25 (1), 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kung CP and Murphy ME (2016) The role of the p53 tumor suppressor in metabolism and diabetes. J Endocrinol 231 (2), R61–R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jennis M et al. (2016) An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev 30 (8), 918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao M et al. (2015) Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell 59 (2), 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang L et al. (2015) Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520 (7545), 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang SJ et al. (2016) Acetylation Is Crucial for p53-Mediated Ferroptosis and Tumor Suppression. Cell Rep 17 (2), 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y et al. (2018) BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol 20 (10), 1181–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo M et al. (2008) The xc- cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. British journal of cancer 99 (3), 464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conrad M and Sato H (2012) The oxidative stress-inducible cystine/glutamate antiporter, system x (c) (−) : cystine supplier and beyond. Amino Acids 42 (1), 231–46. [DOI] [PubMed] [Google Scholar]
- 44.Ishimoto T et al. (2011) CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell 19 (3), 387–400. [DOI] [PubMed] [Google Scholar]
- 45.Sato H et al. (2000) Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc. Antioxid Redox Signal 2 (4), 665–71. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y et al. (2005) Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res 65 (16), 7446–54. [DOI] [PubMed] [Google Scholar]
- 47.Liu XX et al. (2011) MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 585 (9), 1363–7. [DOI] [PubMed] [Google Scholar]
- 48.Guo W et al. (2011) Disruption of xCT inhibits cell growth via the ROS/autophagy pathway in hepatocellular carcinoma. Cancer Lett 312 (1), 55–61. [DOI] [PubMed] [Google Scholar]
- 49.Jaramillo MC and Zhang DD (2013) The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev 27 (20), 2179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chio IIC and Tuveson DA (2017) ROS in Cancer: The Burning Question. Trends Mol Med 23 (5), 411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willcox JK et al. (2004) Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr 44 (4), 275–95. [DOI] [PubMed] [Google Scholar]
- 52.Alpha-Tocopherol, B.C.C.P.S.G. (1994) The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 330 (15), 1029–35. [DOI] [PubMed] [Google Scholar]
- 53.Klein EA et al. (2011) Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 306 (14), 1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeNicola GM et al. (2011) Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475 (7354), 106–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayes JD and McMahon M (2009) NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci 34 (4), 176–88. [DOI] [PubMed] [Google Scholar]
- 56.Sherr CJ (2006) Divorcing ARF and p53: an unsettled case. Nat Rev Cancer 6 (9), 663–73. [DOI] [PubMed] [Google Scholar]
- 57.Kruse JP and Gu W (2009) Modes of p53 regulation. Cell 137 (4), 609–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen D et al. (2017) NRF2 Is a Major Target of ARF in p53-Independent Tumor Suppression. Mol Cell 68 (1), 224–232 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao M et al. (2019) Role of Mitochondria in Ferroptosis. Mol Cell 73 (2), 354–363 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kerins MJ et al. (2018) Fumarate hydratase inactivation in hereditary leiomyomatosis and renal cell cancer is synthetic lethal with ferroptosis induction. Cancer Sci 109 (9), 2757–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsushita M et al. (2015) T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med 212 (4), 555–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friedmann Angeli JP et al. (2014) Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16 (12), 1180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imai H et al. (2017) Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis. Curr Top Microbiol Immunol 403, 143–170. [DOI] [PubMed] [Google Scholar]
- 64.Moran JL et al. (2007) A mouse mutation in the 12R-lipoxygenase, Alox12b, disrupts formation of the epidermal permeability barrier. J Invest Dermatol 127 (8), 1893–7. [DOI] [PubMed] [Google Scholar]
- 65.Yu Z et al. (2005) Mutations associated with a congenital form of ichthyosis (NCIE) inactivate the epidermal lipoxygenases 12R-LOX and eLOX3. Biochim Biophys Acta 1686 (3), 238–47. [DOI] [PubMed] [Google Scholar]
- 66.Cuzzocrea S et al. (2003) 5-lipoxygenase knockout mice exhibit a resistance to acute pancreatitis induced by cerulein. Immunology 110 (1), 120–30. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Joshi YB and Pratico D (2011) Knockout of 5-lipoxygenase results in age-dependent anxiety-like behavior in female mice. PLoS One 6 (12), e29448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Magnusson LU et al. (2012) Arachidonate 15-lipoxygenase type B knockdown leads to reduced lipid accumulation and inflammation in atherosclerosis. PLoS One 7 (8), e43142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kinder M et al. (2010) Interferon regulatory factor-8-driven myeloid differentiation is regulated by 12/15-lipoxygenase-mediated redox signaling. Exp Hematol 38 (11), 1036–1046 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reddy MA et al. (2003) Reduced growth factor responses in vascular smooth muscle cells derived from 12/15-lipoxygenase-deficient mice. Hypertension 41 (6), 1294–300. [DOI] [PubMed] [Google Scholar]
- 71.Krieg P et al. (2013) Aloxe3 knockout mice reveal a function of epidermal lipoxygenase-3 as hepoxilin synthase and its pivotal role in barrier formation. J Invest Dermatol 133 (1), 172–80. [DOI] [PubMed] [Google Scholar]
- 72.Chu B et al. (2019) ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol 21 (5), 579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson EN et al. (1998) Increased platelet sensitivity to ADP in mice lacking platelet-type 12-lipoxygenase. Proc Natl Acad Sci U S A 95 (6), 3100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doll S et al. (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13 (1), 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dixon SJ et al. (2015) Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem Biol 10 (7), 1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kagan VE et al. (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13 (1), 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Writing Group M et al. (2016) Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 133 (4), e38–360. [DOI] [PubMed] [Google Scholar]
- 78.Fang X et al. (2019) Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A 116 (7), 2672–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Linkermann A et al. (2014) Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A 111 (47), 16836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoshida M et al. (2019) Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat Commun 10 (1), 3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park EJ et al. (2019) Whole cigarette smoke condensates induce ferroptosis in human bronchial epithelial cells. Toxicol Lett 303, 55–66. [DOI] [PubMed] [Google Scholar]
- 82.Devos D et al. (2014) Targeting Chelatable Iron as a Therapeutic Modality in Parkinson’s Disease. Antioxidants &Redox Signaling 21 (2), 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Viswanathan VS et al. (2017) Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547 (7664), 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hangauer MJ et al. (2017) Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551 (7679), 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsoi J et al. (2018) Multi-stage Differentiation Defines Melanoma Subtypes with Differential Vulnerability to Drug-Induced Iron-Dependent Oxidative Stress. Cancer Cell 33 (5), 890–904 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu J et al. (2019) Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 572 (7769), 402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson R and Halder G (2014) The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov 13 (1), 63–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Overholtzer M et al. (2006) Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A 103 (33), 12405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang WH et al. (2019) The Hippo Pathway Effector TAZ Regulates Ferroptosis in Renal Cell Carcinoma. Cell Rep 28 (10), 2501–2508 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ashkenazi A and Dixit VM (1998) Death receptors: signaling and modulation. Science 281 (5381), 1305–8. [DOI] [PubMed] [Google Scholar]
- 91.Jiang XJ and Wang XD (2004) Cytochrome C-mediated apoptosis. Annual Review of Biochemistry 73, 87–106. [DOI] [PubMed] [Google Scholar]
- 92.Yant LJ et al. (2003) The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med 34 (4), 496–502. [DOI] [PubMed] [Google Scholar]
- 93.McCullagh EA and Featherstone DE (2014) Behavioral characterization of system xc- mutant mice. Behav Brain Res 265, 1–11. [DOI] [PubMed] [Google Scholar]
- 94.Sato H et al. (2005) Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem 280 (45), 37423–9. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y et al. (2019) Imidazole Ketone Erastin Induces Ferroptosis and Slows Tumor Growth in a Mouse Lymphoma Model. Cell Chem Biol 26 (5), 623–633 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang W et al. (2019) CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569 (7755), 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lang X et al. (2019) Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stockwell BR and Jiang X (2019) A Physiological Function for Ferroptosis in Tumor Suppression by the Immune System. Cell Metab 30 (1), 14–15. [DOI] [PMC free article] [PubMed] [Google Scholar]