Abstract
In the present study protein was isolated from tamarind seed powder and was subjected to ultrasonication by varying the time (15 and 30 min) and intensity (100 and 200 W) of treatment. The effect of the ultrasound treatment on the various properties like solubility, emulsifying property, foaming property, water holding capacity, oil holding capacity, particle density and molecular weight was investigated. The solubility, emulsifying property, foaming property, water holding capacity and oil holding capacity of the ultrasonically treated tamarind seed protein isolates improved after treatment and was found to increase with time or intensity of the treatment. The particle density slightly decreased after ultrasonication, but significant differences could not be observed for the different treatment conditions. The SDS-PAGE profiling did not reveal any differences in the molecular weights of the treated and untreated proteins, implying that ultrasonication did not affect the primary structure of the proteins. It can be concluded from the study that ultrasonication can be used to improve the functional properties of tamarind seed protein isolates and therefore has potential for use in various food and non-food applications.
Keywords: Tamarind seed protein isolate, Ultrasonication, Time, Intensity, Properties
Introduction
Tamarind (Tamarindus indica) from the family of Fabaceae is a leguminous tree which is native to tropical Africa. In general, tamarind plays a central role in the cuisines of South East Asia, the Indian subcontinent and America mostly in Mexico (Chant 1993). It is primarily used for its fruit eaten either fresh or processed (Bagul et al. 2015). The pulp of the fruit is mainly used as a souring agent or acidulant in curries, sauces, gravies, chutneys and certain beverages (Kumar and Bhattacharya 2008). The seeds of the tamarind fruit are a by-product of the tamarind pulp industry. The seeds as a whole are not used for food or feed purposes due to presence of certain antinutritional factors like tannin, phytic acids, total free phenolics, etc. (Kumar and Bhattacharya 2008). Chambers of the tamarind seeds are covered with a parchment like a peel. The colours of the seeds are red to purple brown and hard in nature. Size of the seeds varies from 320 to 700 gm per kg of fruit. The seeds are dicotyledonous and are approximately 3 cm by 1.3 cm in size, flattened and glossy. The seed coat or testa consists of 20–30% of the weight and the endosperm or kernel consists of 70–75% of the weight of the tamarind seed. In tamarind seed the seed percentage is around 40% of the total mass (Bagul et al. 2015).
The powder of tamarind seed is having very high quantity of protein (18.4 to 26.9%) and rich in numerous and well balanced essential amino acids like valine, methionine, lysine, leucine, isoleucine, glutamic acid, aspartic acid and phenylalanine and has high level of in vitro digestibility of 71.3% (Bhattacharya et al. 1994). It is a by-product of the marketable or non-commercial application of the tamarind fruit for various purposes. This waste product mostly from commercial consumptions can serve as good source for tamarind seed (Bagul et al. 2015). As the tamarind seeds are rich in proteins and have a well-balanced amino acid profile, the protein can be isolated from the tamarind seeds for application in various food and non-food purposes.
Isolates are the distinguished form of proteins, which are without difficulty consumable and can be incorporated in different food products. The formulated foods that are to be developed of new class the protein isolates play the vital role for the development of product. It is an ideal raw component which is used in liquid refreshment, kids’ milk food and baby foods, textured protein foodstuffs and certain types of domain foods. Protein isolates are developing from various types of leguminous plant such as peanut, navy beans, soy bean sesame pinto, canola almonds and cashew nut.
Most of the native proteins do not possess the desired functional properties for application in various industries. Functional properties of the proteins can be altered by chemical, enzymatic and physical processes. Use of enzymes to change the functional properties of proteins is often costly and is not affordable. Chemical modification of protein may have undesirable and adverse health effects such as toxicity, allergy, impairment of nutrition etc. Physical methods like heating, freezing, extrusion etc. can be used to change the properties of proteins, but these processes are energy intensive (Mirmoghtadaie et al. 2016). The need for novel technologies which are environmental friendly, energy efficient and perceived safe by consumers are therefore important for modification of food products. Among this, ultrasound is one such technology which has wide range of applications.
In recent years, it has been seen that there is a rapid increase in the use of ultrasound for the modification of food products as well as for the analysis in the food industry. The sound waves of frequencies which are very high cannot be detected by the human ear i.e. above 16 kHz produces a form of sound energy i.e. ultrasound. When the ultrasound is propagated over a biological structure, a high total of energy is produced which induces compressions and depressions in the particles of the medium. The basis of analysis of ultrasound is the bond between that determines their physical properties (structure, physical state and composition) and the ultrasonic properties of foods (attenuations velocity, coefficient and impedance). Complementary to the classical techniques, in processing, the frequent use of ultrasound creates a novel and interesting methodologies in food. The recovery of extraction of organic compounds which are confined within the body of plants and seeds can be achieved by the significant practice of power ultrasound.
In order to improve the functional properties (gelation, viscosity, enhancement, foaming, and emulsifying etc., the ultrasound processing of proteins has the potential for the improvement and also added the exchange of current emulsification technologies. In the classification of food protein, the specific interest of proteins is as emulsifier because of their ability to form the interfacial films and to adsorb to oil water interfaces (Lam and Nickerson 2013). For the functional modification and lipids for emulsification in the food ingredients the ultrasound treatment mainly influences on protein. The ultrasound treatment of protein modification significantly compacts the aggregate size and hydrodynamic volume. The reduction in size of the protein was related to the cavitation produced by ultrasound and to the hydrodynamic shear forces.
Although there are studies on modification of proteins by ultrasound from various other sources like whey protein (Frydenberg et al. 2016; Jambrak et al. 2008), milk protein (Yanjun et al. 2014), soy protein (Jambrak et al. 2009; Hu et al. 2013a), wheat gluten (Zhang et al. 2011), squid ovary powder (Singh et al. 2018) and other proteins (Arzeni et al. 2012) or application of ultrasound for enzymolysis of walnut meal protein (Golly et al. 2019), there are no report on modification of tamarind seed protein using ultrasound. The present study was planned with the aim to modify the protein isolated from tamarind seeds using ultrasound treatment and to study the functional properties like solubility, emulsifying properties, foaming properties, particle density, molecular weights, water and oil holding capacities of untreated and treated proteins.
Materials and methods
Protein isolation
Tamarind seed powder was purchased from Akshar Exim Co. Pvt. Ltd. (Kolkata, India) for protein isolation and modification. All other chemicals used in the experiment were of analytical grade. Protein from tamarind seed powder was isolated by isoelectric point precipitation method as described by Smith and Circle (1939). For extraction of protein 100 g of tamarind seed powder was mixed with deionized water in the ratio of 1:10 and stirred properly in a vessel. The pH of the suspension was adjusted to 12 using 1 M NaOH and kept for 1 h. After 1 h the suspension was centrifuged at 7800×g for 15 min and the supernatant was collected. Afterwards, the pH of the collected supernatant was adjusted to 4 i.e. the isoelectric point of tamarind seed protein as determined previously using 1 M HCl, and kept for 1 h. Again after 1 h the suspension was centrifuged at 7800×g for 15 min and the precipitated protein was collected. The precipitate was then dried at 40 °C for 24 h and kept in air tight containers for further analysis. The moisture and protein content of the tamarind seed powder and tamarind seed protein isolate was determined by AOAC (1990) and Kjeldahl method (AACC 1990) respectively. The moisture content and protein content of the tamarind seed powder were 11.64 ± 0.23% (n = 5) and 13.78 ± 0.52% (n = 5) respectively, whereas that for untreated isolate was found to be 7.56 ± 0.23% (n = 5) and 68.32 ± 0.96% (n = 5) respectively for moisture and protein.
Modification of isolated protein by ultrasound
The modification of the isolated protein from the tamarind seed was carried out using ultrasonic processor (Ultrasonic Homogenizer Model U500 TAKASHI) with 6 mm diameter probe at a fixed frequency of 25 kHz. For modification, 10 g powder of the protein isolates was mixed with 100 mL distilled water in a 250 mL beaker and kept for ultrasonic treatment by varying the time and power of ultrasound. A 2 × 2 full factorial experiment was performed at two different levels of treatment time (15 and 30 min) and ultrasound power (100 and 200 W). The maximum time of treatment and power level were selected after preliminary experiments, so that there is no significant rise in temperature to cause thermal denaturation of the protein. After ultrasonication the isolated protein was separated from the solution by centrifugation at 7800×g for 15 min and the precipitated protein was collected. The precipitate was then dried at 40 °C for 24 h so that the isolated protein does not get denatured during drying. The dried powder was kept in air tight containers for further analysis.
Protein solubility
The solubility of tamarind seed protein isolate was determined as described by Butt and Batool (2010). Protein isolates of tamarind seed powder (0.25 g) was homogenized in 20 mL 0.1 M NaCl solution and was adjusted to pH of 7.0 and kept for 1 h. After 1 h centrifugation was carried out for 30 min at 10,000×g. The nitrogen content of the soluble fraction was determined by Kjeldhal method (AACC 1990) and finally solubility was calculated as the total nitrogen of the original sample to that of the soluble fraction in percentage.
Particle density
The particle density of the tamarind seed protein isolates were determined by using the gas (helium) pycnometer (Gas Pycnometer PYC-100A). Sample was placed in the sample cell and degassed by purging with a flow of dry gas (helium) by a series of pressurization cycles.
Water holding capacity
The water holding capacity of the tamarind seed protein isolate was determined as described by Sosulski et al. (1976). Protein isolate from tamarind seed (1 g) was taken and mixed with 10 mL of distilled water in the pre-weighed centrifuge tubes which were agitated for few seconds and kept for 30 min. After an interval of 30 min the contents were centrifuged at 3000×g for 25 min. Supernatant was removed by 25 min of drainage at 50 °C, then re-weighing the protein isolate.
Water holding capacity was calculated as:
Oil holding capacity
Oil holding capacity of the tamarind seed protein isolate was determined as described by Sarv et al. (2017). Tamarind seed protein isolate (0.5 g) was mixed with 6 mL corn oil in pre-weighed centrifuge tubes. The tubes were agitated for one min to get complete dispersion of the sample in oil. After 30 min holding time, the samples were centrifuged at 3000×g for 25 min. The supernatant was pipetted and the tubes were kept inverted for 25 min for the complete drainage of oil before re-weighing. The oil absorption was expressed as gram of oil absorbed per gram of tamarind seed protein isolate.
Oil absorption was calculated as:
Foaming properties
The foaming capacity of the tamarind seed protein isolate was determined by the method as described by Lin et al. (1974). A 50 mL of 3% (w/v) dispersions of tamarind seed protein isolate sample in distilled water was prepared and instantly transferred into a graduated cylinder, the volume of foam was recorded before and after whipping. The calculation of foaming capacity was done according to the following equation:
Foaming stability was calculated by change in volume of foam after 60 min of standing at 20 °C as follows:
Emulsifying properties
The emulsifying activity and stability of the tamarind seed protein isolate were determined as described by Naczk et al. (1985). Tamarind seed protein isolate (1.5 g) was homogenized in 25 mL water for 30 s at 10,000 rpm. Corn oil (12 mL) was added and the mixture was again homogenized for 30 s at 10,000 rpm. The emulsion was divided two equal volume aliquots for determination of emulsifying activity and emulsifying stability respectively. One aliquot was centrifuged for 5 min at 1100×g for determination of emulsifying activity. The other aliquot was heated for 15 min at 85 °C, cooled to room temperature and centrifuged for 5 min at 1100×g for determination of emulsifying stability. The ratio of height of emulsion to the height of layer of liquid was distinguished for the calculation of activity of emulsion. The emulsifying activity and stability were determined by using the following equations:
Sodium-dodecyl-sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
The configuration of protein for the TSP isolate solution was analyzed by means of sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) under reduced conditions. Newly prepared solution dissolved by the vortex at 4200×g at 20 °C was centrifuged for 20 min. Again with the SDS buffer (0.5 M Tris, 2.0% SDS, 0.05% b-mercapto ethanol, pH 6.8) (sample: sample buffer ¼ 50 mg:150 μL), the TSP isolate was mixed and heated to 95–100 °C for 15 min and was mixed again in the vortex for few minutes. The TSP isolate solution was also mixed with SDS buffer as specified above. SDS-PAGE was accepted out by loading 15 μL of samples on to the gels formerly prepared on a Mini-PROTEAN II system (Bio-Rad). The resolving gel contained 15.0% acrylamide and the stacking gel was made up of 10.0% acrylamide. After running, the gel was stained for 45 min with a Coomassie Brilliant Blue with water methanol and acetic acid in a ratio of 45:45:10 and it was retained in a shaker for 45 min. The gels were distained with the distaining solution which enclose the water methanol and acetic acid in the same ratio of 45:45:10 and was washed with running water and was kept overnight in water and was scanned using a Gel Doc system (Bio-Rad Chemi Doc XRS+) (Sarkar et al. 2016).
Statistical analysis
All the experiments were performed in triplicates and significant differences between the data were calculated by Duncan’s multiple range tests using SPSS 16 (IBM Analytics, USA).
Results and discussion
Influence of ultrasound treatment on tamarind seeds protein isolates solubility
The solubilities of the untreated and ultrasonically treated tamarind seed protein isolates are presented in Table 1. There was significant increase in solubility of protein when power of treatment or treatment time was increased. Basically for the performance of protein isolates united in the food system, protein solubility is a beneficial indicator and also it extends the denaturation of protein because of treatment with heat and chemicals (Horax et al. 2004). During ultrasound treatment large number of cavitation bubbles can cause an increase in protein solubility which leads to unfolding of proteins due to large increase in local temperature and pressure in the neighboring area of collapsing bubbles, and during hydrolysis an infringement of peptide bonds. Protein solubility is increased by the high intensity ultrasound when the hydrophilic amino acids residues are oriented towards water by varying conformation and structure of proteins (Moulton and Wang 1982).
Table 1.
Solubility, particle density, water and oil holding capacity of untreated and treated tamarind seed protein isolates
| Sample no.1,2 | Code | Ultrasound treatment conditions | Solubility (%) | Particle density (g/cm3) | Water holding capacity (%) | Oil holding capacity (%) | |
|---|---|---|---|---|---|---|---|
| Treatment Time (min) | Treatment intensity (W) | ||||||
| 1 | N | 0 | 0 | 69.09 ± 0.47a | 1. 51 ± 0.03a | 108.7 ± 0.7a | 140.80 ± 0.54a |
| 2 | M1 | 15 | 100 | 72.38 ± 0.35b | 1.45 ± 0.02b | 132.06 ± 0.67b | 159.88 ± 0.36b |
| 3 | M2 | 15 | 200 | 77.12 ± 0.37c | 1.43 ± 0.03b | 138.27 ± 0.60c | 166.37 ± 0.42c |
| 4 | M3 | 30 | 100 | 80.75 ± 0.91d | 1.41 ± 0.01b | 142.30 ± 0.75d | 170.13 ± 0.26d |
| 5 | M4 | 30 | 200 | 81.02 ± 0.22d | 1.40 ± 0.04b | 145.54 ± 0.35e | 171.95 ± 0.31e |
1Values represented as mean ± SD of three replications
2Values followed by same superscript small letters within a column are not significantly different (p < 0.05)
Ultrasound treatment has exhibited high increase in solubility of protein of tamarind seed protein isolate when compared with untreated sample. Protein solubility was found to be higher after ultrasonication and was highest when power was 200 W and treatment time was 30 min, followed by the treatment with 100 W for 30 min. It shows that protein solubility increased with both time of treatment as well as intensity of treatment. Significant difference was not observed when the power was increased from 100 to 200 W at 30 min treatment time, implying that time of treatment is more important for unfolding of the protein to expose the amino or carboxylic groups of the protein. Ultrasound might disrupt the weaker bonds like hydrogen bonds present in the protein, thereby inducing conformational changes in the protein which might increase the solubility of the proteins. Ultrasonic treatment might also reduce the particle size of the protein aggregates, resulting in increased protein-water interactions thereby increasing the solubility (Arzeni et al. 2012). Similar results of increase in solubility with ultrasonication was observed by Hu et al. (2013a) for soy protein isolates, and Jambrak et al. (2009) for ultrasound treated soy protein.
Influence of ultrasound treatment on the particle density of tamarind seed protein isolates
The particle densities of the untreated and ultrasonically treated samples are presented in Table 1. The differences in the particle densities between the treated samples were not significantly different. Although, the particle densities of the treated samples were slightly lower than that of the untreated sample and the difference is statistically significant. The lowering of the particle density after ultrasonic treatment might be attributed to the unfolding and then rearrangement of the of the protein structure after ultrasound treatment. It has been reported that the particle density of most of the powdered foods is between 1.4 and 1.5 g/cm3 (Peleg 1983) and the particle densities obtained in the present study were found to be between 1.40 and 1.51 g/cm3.
Influence of ultrasound treatment on water holding capacity of tamarind seed protein isolates
Water holding capacity (WHC) signifies the interaction between protein molecules and water that occurs is various food systems and is one of the important properties of ingredients used for products like gravies or sauces. The water holding capacity for the untreated and treated tamarind seed protein isolates are presented in Table 1. Results from the present investigation shows that the WHC increased from 108.7% in the untreated sample to 132.06% for sample treated for 15 min at 100 W. When the treatment intensity or time was increased, further increase in the WHC was noticed. Highest WHC of 145.54% was obtained for the sample treated for 30 min at 200 W intensity. The increase in WHC for the protein isolates treated with ultrasound might be attributed to the unfolding of the polypeptide chain due to ultrasonic treatment which exposed the hydrophilic groups. Apart from the exposure of the hydrophilic groups due to ultrasound treatment hydrophilic–hydrophobic balance of amino acids in the molecules of proteins as well as carbohydrates lipids and tannins associated with proteins and several parameters such as the conformational characteristics, size, shape, steric factors are also responsible for change in water holding capacity. These results are consistent with the results obtained for solubility of the ultrasound treated proteins obtained in the present investigation. Improvement in WHC with increase in protein solubility was reported by Wu et al. (2011). Similar results were also obtained by Hu et al. (2013a) for soy protein isolate pre-treated with high intensity ultrasound.
Influence of ultrasound treatment oil absorption capacity of tamarind seed protein isolates
Knowledge of oil holding capacity (OHC) of proteins is important as it is directly related to the emulsifying properties of the proteins. Stability of the emulsion as well as the oxidative stability of the fats or oils present in a food system is dependent on the binding of the fats or oils to other components of the food system particularly protein (Menezes et al. 2015). The results obtained for the oil holding capacities in the present study shows that the OHC of the ultrasonically treated samples were much higher as compared to that of untreated tamarind seeds protein isolates (Table 1). Proteins which are composed of both hydrophilic and hydrophobic parts as the major chemical components affects the oil holding capacity, with the hydrocarbons chains of lipids the non-polar amino acids side chain forms the hydrophobic interactions (Jitngarmkusol et al. 2008). As discussed earlier the reason for increase in OHC of the protein isolates might be attributed to the unfolding of the polypeptide chains as well as conformational changes in the protein structure which are caused by the collapse of the cavitation bubbles near the protein molecules due to ultrasonication. These changes exposed the non-polar hydrophobic side chains of amino acids present in the protein molecule which increased binding of the oil molecules to the protein molecules. Since the oil holding capacity is of significance in improving the mouth feel of foods and acts as flavor retainer, it is considered very important property of proteins for incorporation in various food formulations. The variation in the OHC of the different ultrasonically treated samples might be due the different degrees of unfolding of the polypeptide chains, which was a function of both intensity of ultrasonication as well as time of treatment. Although, all the values of OHC are significantly different for the various treatments, it can be seen that the difference between the samples treated for 30 min at different intensity (100 and 200 W) was much less as compared to the other values. This implies that at higher treatment time changing the intensity may not affect much, and unfolding of polypeptide chain is time taking process.
Influence of ultrasound treatment on foaming properties of the tamarind seed protein isolates
Proteins are used as foaming agents in various food and non-food applications as proteins can be easily adsorbed in the air–water interface. The foaming capacity and stability of the of the untreated and ultrasonically treated tamarind protein isolates are given in Table 2. It was observed that the foaming capacities significantly increased for the ultrasonically treated samples. Highest foaming capacity of 36.41% was observed for the sample treated for 30 min at 200 W intensity as compared to 17.32% for the untreated sample. Significant increase in the foaming capacity was observed when both the time of treatment or intensity of treatment was increased. The increase in foaming capacity due to ultrasound treatment might be due to denaturation of the native protein and unfolding of the polypeptide chain. It is generally accepted that denaturation and unfolding of polypeptide chain exposes the hydrophobic regions which is essential for adsorption of the molecules to the air–water interface (Krešić et al. 2008). Similarly, foam stability was also found to increase with ultrasound treatment. The results of foaming properties in the present study are found to be consistent with the results obtained for solubility and OHC of the treated protein isolates as foam forming and foam stabilizing ability of protein is dependent on solubility of the protein as well as hydrophobicity of the protein (Barac et al. 2010). Similar increase in foaming capacity and stability was reported by Jambrak et al. (2009) for ultrasonically treated soy protein, Zhang et al. (2011) for wheat gluten treated with ultrasound, by Stefanović et al. (2014) for egg white protein and Jambrak et al. (2008) for ultrasound treated whey protein suspensions.
Table 2.
Foaming and emulsifying properties of untreated and treated tamarind seed protein isolates
| Sample no.1,2 | Code | Foaming capacity (%) | Foaming stability (%) | Emulsifying capacity (%) | Emulsifying stability (%) |
|---|---|---|---|---|---|
| 1 | N | 17.32 ± 0.26a | 9.21 ± 0.37a | 62.34 ± 0.29a | 67.65 ± 0.34a |
| 2 | M1 | 21.47 ± 0.29b | 9.29 ± 0.22a | 75.17 ± 0.36b | 71.48 ± 0.36b |
| 3 | M2 | 26.15 ± 0.30c | 14.63 ± 0.59b | 75.85 ± 0.28b | 73.64 ± 0.27c |
| 4 | M3 | 28.84 ± 0.56d | 15.69 ± 0.48c | 76.59 ± 0.48c | 81.58 ± 0.39d |
| 5 | M4 | 36.41 ± 0.35e | 17.23 ± 0.43d | 79.41 ± 0.46d | 82.53 ± 0.39e |
1Values represented as mean ± SD of three replications
2Values followed by same superscript small letters within a column are not significantly different (p < 0.05)
Influence of ultrasound treatment on emulsifying properties of the tamarind seed protein isolates
Properties of emulsion are significant for the functional properties of protein which affects the performance of food products. Emulsifying properties characterizes the ability of a protein to get adsorbed into the oil-water interface and expresses the interfacial area stabilized per unit weight of protein. The emulsifying activity and stability of the untreated and ultarsound treated tamarind seed protein isolates are presented in Table 2. The results show that emulsifying activity as well as stability improved with ultrasonication and was more when time of treatment or the intensity of treatment was more. Highest emulsifying activity and stability of 79.41% and 82.53% respectively was obtained for protein isolate treated for 30 min at 200 W intensity. The change in emulsifying activity or stability was much higher for all the ultrasonically treated samples as compared to the untreated one, but within the treated samples the differences were less. This shows that ultrasound has a profound effect in improving the emulsifying property of proteins. The emulsifying property of protein is attributed to (1) protein solubility (2) surface charge (3) surface hydrophobicity and (4) molecular flexibility. Ultrasound can modify the functional properties of proteins with some secondary structure changes as reported by Krešić et al. (2008). The effects of ultrasound are a combination of thermal, mechanical and chemical effects as mentioned by Hu et al. (2013b). The instantaneous extreme temperatures and pressures generated by ultrasound which is able to change the structure of the protein molecules might be the major force that improves the emulsifying properties of the tamarind seed protein isolates. As with foaming capacity, emulsifying ability is also a function of solubility and hydrophobicity of the proteins. The results of the present study are in agreement with the results obtained by Jambrak et al. (2009), Zhang et al. (2011), Yanjun et al. (2014) and Stefanović et al. (2014) for ultrasonically treated soy proteins, wheat gluten, reconstituted milk protein and egg white protein respectively.
SDS-PAGE profiles of tamarind seed protein isolates
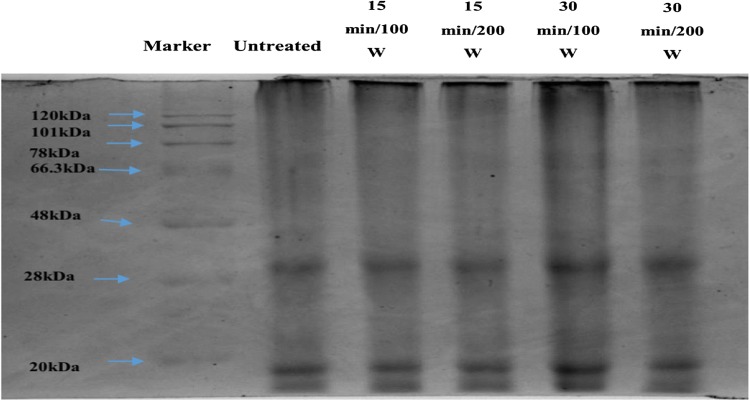
SDS-PAGE was used to observe the changes in the protein molecular weight after ultrasonication (Fig. 1). Two bands at 33 kDa and 14 kDa were seen for all the treated and untreated samples showing that ultrasound did not affect the molecular weight of the proteins or the primary structure of the proteins. Similar results were reported by Hu et al. (2013a) for soy protein isolates where no change was observed between the ultrasonically treated and untreated samples. Zhang et al. (2011) showed that ultrasound treatment did not induce major changes on the protein electrophoretic patterns of wheat gluten samples. Similar findings were also reported by Yanjun et al. (2014) for milk protein, Jiang et al. (2014) for black bean protein isolate and O’Sullivan et al. (2016) for various animal and plant proteins after ultrasound treatment. The results suggest that ultrasound may not affect the primary structure or the molecular weight of the protein, but has definite effect on the conformation of the protein aggregates as can be observed from the changes in other properties after ultrasound treatment.
Fig. 1.
SDS-PAGE profiles of untreated and treated tamarind seed protein isolates
Conclusion
The present study reveals that application of ultrasound treatment improves the functional properties of proteins like solubility, emulsifying, foaming, water and oil holding capacities. Solubility, emulsifying activity, foaming capacity water holding capacity and oil holding capacity was found to be highest when both time of treatment as well as intensity of treatment were high. Therefore, it can be concluded that ultrasound can be used to modify the properties of tamarind seed proteins as it consumes less energy and was found to be more time effective when compared to other traditional technologies for protein modification. This study also present a way of utilizing tamarind seeds which is considered to be a waste product in various food and non-food applications.
Acknowledgements
The authors greatly acknowledge the help received from Dr. Laxmikant S. Badwaik, Associate Professor, Department of Food Engineering and Technology, Tezpur University, Assam, India for allowing us to use his laboratory and equipment for carrying out the work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AACC American Association of Cereal Chemists . Approved methods of the AACC. 7. St. Paul: AACC American Association of Cereal Chemists; 1990. [Google Scholar]
- AOAC Official Methods of Analysis (1990) Association of Official Analytical Chemists, 15th edn. Washington, DC, USA
- Arzeni C, Martínez K, Zema P, Arias A, Pérez OE, Pilosof AMR. Comparative study of high intensity ultrasound effects on food proteins functionality. J Food Eng. 2012;108(3):463–472. [Google Scholar]
- Bagul Mayuri, Sonawane Sachin K, Arya Shalini S. Tamarind seeds: chemistry, technology, applications and health benefits: a review. Indian Food Ind Mag. 2015;34(3):28–35. [Google Scholar]
- Barac M, Cabrilo S, Pesic M, Stanojevic S, Zilic S, Macej O, Ristic N. Profile and functional properties of seed proteins from six pea (Pisum sativum) genotypes. Int J Mol Sci. 2010;11(12):4973–4990. doi: 10.3390/ijms11124973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Bal S, Mukherjee RK, Bhattacharya S. Functional and nutritional properties of tamarind (Tamarindus indica) kernel protein. Food Chem. 1994;49(1):1–9. [Google Scholar]
- Butt MS, Batool R. Nutritional and functional properties of some promising legumes protein isolates. Pak J Nutr. 2010;9(4):373–379. [Google Scholar]
- Chant SR. Fables. In: Heywood VH, editor. Flowering plants of the world. London: B.T. Batsford Ltd.; 1993. [Google Scholar]
- Frydenberg RP, Hammershøj M, Andersen U, Greve MT, Wiking L. Protein denaturation of whey protein isolates (WPIs) induced by high intensity ultrasound during heat gelation. Food Chem. 2016;192:415–423. doi: 10.1016/j.foodchem.2015.07.037. [DOI] [PubMed] [Google Scholar]
- Golly MK, Ma H, Yuqing D, Wu P, Dabbour M, Sarpong F, Farooq M. Enzymolysis of walnut (Juglans regia L.) meal protein: ultrasonication-assisted alkaline pretreatment impact on kinetics and thermodynamics. J Food Biochem. 2019;43(8):e12948. doi: 10.1111/jfbc.12948. [DOI] [PubMed] [Google Scholar]
- Horax R, Hettiarachchy NS, Chen P, Jalaluddin M. Functional properties of protein isolate from cowpea (Vigna unguiculata L. Walp.) J Food Sci. 2004;69(2):fct119–fct121. [Google Scholar]
- Hu H, Li-Chan EC, Wan L, Tian M, Pan S. The effect of high intensity ultrasonic pre-treatment on the properties of soybean protein isolate gel induced by calcium sulfate. Food Hydrocoll. 2013;32(2):303–311. [Google Scholar]
- Hu H, Wu J, Li-Chan EC, Zhu L, Zhang F, Xu X, Fan G, Wang L, Huang X, Pan S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013;30(2):647–655. [Google Scholar]
- Jambrak AR, Mason TJ, Lelas V, Herceg Z, Herceg IL. Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J Food Eng. 2008;86(2):281–287. [Google Scholar]
- Jambrak AR, Lelas V, Mason TJ, Krešić G, Badanjak M. Physical properties of ultrasound treated soy proteins. J Food Eng. 2009;93(4):386–393. [Google Scholar]
- Jiang L, Wang J, Li Y, Wang Z, Liang J, Wang R, Chen Y, Ma W, Qi B, Zhang M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res Int. 2014;62:595–601. [Google Scholar]
- Jitngarmkusol S, Hongsuwankul J, Tananuwong K. Chemical compositions, functional properties, and microstructure of defatted macadamia flours. Food Chem. 2008;110(1):23–30. doi: 10.1016/j.foodchem.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Krešić G, Lelas V, Jambrak AR, Herceg Z, Brnčić SR. Influence of novel food processing technologies on the rheological and thermophysical properties of whey proteins. J Food Eng. 2008;87(1):64–73. [Google Scholar]
- Kumar CS, Bhattacharya S. Tamarind seed: properties, processing and utilization. Crit Rev Food Sci Nutr. 2008;48(1):1–20. doi: 10.1080/10408390600948600. [DOI] [PubMed] [Google Scholar]
- Lam RS, Nickerson MT. Food proteins: a review on their emulsifying properties using a structure–function approach. Food Chem. 2013;141(2):975–984. doi: 10.1016/j.foodchem.2013.04.038. [DOI] [PubMed] [Google Scholar]
- Lin MJY, Humbert ES, Sosulski FW. Certain functional properties of sunflower meal products. J Food Sci. 1974;39(2):368–370. [Google Scholar]
- Menezes BS, Zanette B, Souza JTA, Cortez-Vega WR, Prentice C. Comparison of physicochemical and functional properties of surimi and protein isolate obtained from mechanically deboned meat of chicken. Int Food Res J. 2015;22(4):1374–1379. [Google Scholar]
- Mirmoghtadaie L, Aliabadi SS, Hosseini SM. Recent approaches in physical modification of protein functionality. Food Chem. 2016;199:619–627. doi: 10.1016/j.foodchem.2015.12.067. [DOI] [PubMed] [Google Scholar]
- Moulton KJ, Wang LC. A pilot-plant study of continuous ultrasonic extraction of soybean protein. J Food Sci. 1982;47(4):1127–1129. [Google Scholar]
- Naczk M, Diosady LL, Rubin LJ. Functional properties of canola meals produced by a two-phase solvent extraction system. J Food Sci. 1985;50(6):1685–1688. [Google Scholar]
- O’Sullivan J, Murray B, Flynn C, Norton I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016;53:141–154. [Google Scholar]
- Peleg M. Physical properties of foods. In: Peleg M, Bagley EB, editors. IFT basic symposium series (USA) West-Port: AVI Publishing Co., Inc.; 1983. pp. 293–321. [Google Scholar]
- Sarkar A, Kamaruddin H, Bentley A, Wang S. Emulsion stabilization by tomato seed protein isolate: influence of pH, ionic strength and thermal treatment. Food Hydrocoll. 2016;57:160–168. [Google Scholar]
- Sarv V, Trass O, Diosady LL. Preparation and characterization of Camelina sativa protein isolates and mucilage. J Am Oil Chem Soc. 2017;94(10):1279–1285. [Google Scholar]
- Singh A, Benjakul S, Kijroongrojana K. Effect of ultrasonication on physicochemical and foaming properties of squid ovary powder. Food Hydrocoll. 2018;77:286–296. [Google Scholar]
- Smith AK, Circle CJ. Soy bean protein precipitation from water and alkaline dispersions by acids and by electrodialysis. Ind Eng Chem. 1939;31:1284–1288. [Google Scholar]
- Sosulski F, Humbert ES, Bui K, Jones JD. Functional propreties of rapeseed flours, concentrates and isolate. J Food Sci. 1976;41(6):1349–1352. [Google Scholar]
- Stefanović A, Jovanović J, Dojčinović M, Lević S, Žuža M, Nedović V, Knežević-Jugović Z. Impact of high-intensity ultrasound probe on the functionality of egg white proteins. J Hyg Eng Des. 2014;6:215–224. [Google Scholar]
- Wu W, Hua Y, Lin Q, Xiao H. Effects of oxidative modification on thermal aggregation and gel properties of soy protein by peroxyl radicals. Int J Food Sci Technol. 2011;46(9):1891–1897. doi: 10.1007/s13197-011-0533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanjun S, Jianhang C, Shuwen Z, Hongjuan L, Jing L, Lu L, Uluko H, Yanling S, Wenming C, Wupeng G, Jiaping L. Effect of power ultrasound pre-treatment on the physical and functional properties of reconstituted milk protein concentrate. J Food Eng. 2014;124:11–18. [Google Scholar]
- Zhang H, Claver IP, Zhu KX, Zhou H. The effect of ultrasound on the functional properties of wheat gluten. Molecules. 2011;16(5):4231–4240. [Google Scholar]