Abstract
The study reveals effect of pre-treatments, i.e., partial drying (PD), partial drying + freezing (PDF) and freezing (F) on physico-chemical, structural and sensory quality of vacuum fried papaya chips (100 °C for 28 min at 13.33 kPa). Pre-treatments significantly (p < 0.05) influenced the quality parameters of vacuum fried papaya chips. Partial drying pre-treated sample showed significantly (p < 0.05) higher reduction in oil uptake, i.e., 19.61% (d.b.) besides retaining significantly (p < 0.05) higher total phenolics content (394.07 mg/100 g gallic acid equivalent) as compared to other samples. Ascorbic acid content was retained significantly (p < 0.05) high in untreated control samples (209.81 mg/100 g). Freezing pre-treatment was found better in retaining total flavonoids, total carotenoids and antioxidant activity. The SEM micrographs of papaya chips showed dense and shriveled structure due to partial drying pre-treatment, however, more porous structure was observed in freezing pre-treated sample as compared to control. Freezing pre-treated samples were also rated high on hedonic scale for their sensory parameters, i.e., colour, crispness, taste, mouth feel, appearance and overall acceptability, which were in agreement with instrumental CIE colour values, firmness and microstructural characteristics of samples. The percent moisture, fat, protein, total carbohydrate, and ash content of freezing pre-treated vacuum fried papaya chips was found to be 1.51 ± 0.13%, 23.68 ± 0.12%, 3.42 ± 0.14%, 67.64 ± 1.78% and 3.75 ± 0.08%, respectively.
Keywords: Papaya, Vacuum frying, Drying, Freezing, Chips
Introduction
Papaya is the 3rd largest growing fruit in India with an annual production of 6108 MT, after banana and mango (NHB 2017). It is a rich source of nutrients such as carbohydrate, vitamins and minerals including antioxidants. However, unlike other fruits such as banana, mango, apple, strawberry etc., value added products of papaya are scarcely available in the market. Today, most of the papaya produce is consumed as such fresh and only few value added products like osmo-dehydrated papaya, papaya jam, candy and tutti-frutti are commercialized.
Frying is a traditional method used for preparation of food snacks. The major constraint in conventional deep fat frying is degradation of nutrients due to very high temperature (150–200 °C) used during frying (Ahmad Tarmizi and Niranjan 2013). Moreover, conventional deep fat frying of fruits and vegetables also leads to darkening of colour due to presence of high amount of sugar and ultimately formation of acrylamide (Diamante et al. 2015). According to European Union regular consumption of acrylamide for long time can cause cancer in humans (WHO 2002). Therefore, conventional method of deep fat frying is only limited to frying of low sugar containing few fruits and vegetables like potato and unripe banana. Alternatively, vacuum frying of fruits and vegetables have been tried and a number of studies have been conducted on vacuum frying of fruits such as banana, jackfruit, kiwifruit, apple, carrot, yam, sweet potato and apricot etc. (Diamante et al. 2015).
In the case of vacuum frying, fruits and vegetables are fried under vacuumized condition (< 1 atm) at low temperature (< 100 °C) (Dueik et al. 2010). Vacuum frying at lower temperatures results in significantly low oil uptake and reduce oxidative degradation of phyto-chemicals and nutrients. Earlier studies suggest that product obtained through vacuum frying show better physical characteristics in comparison to conventional deep fat fried products (Diamante et al. 2015). Moreover, pre-treatments such as blanching, hydrocolloids coating, and immersion in sugar or sodium chloride solution, drying and freezing prior frying had also shown significant improvement in physico-chemical attributes due to modification in cellular structure of fruit tissues such as jackfruit, kiwifruit, apple, pineapple etc. (Zhu et al. 2015; Diamante et al. 2015; Mariscal and Bouchon 2008).
As such literature is scarce with regards to vacuum frying of papaya as well as effect of pre-treatment on physico-chemical properties of vacuum fried papaya chips. Therefore, the present study was undertaken to see the effect of partial drying, freezing and their combination on physico-chemical, structural and sensory quality of vacuum fried papaya chips.
Materials and methods
Raw material
Papaya fruits (verity: Coorg honey dew) were procured from the local market of Mysore (India) and stored at room temperature (25 °C) for ripening. The fruits were shorted and graded manually on the basis of shape, size (length of 20–25 cm and weight 1.70–2.30 kg) and maturity based on skin color (yellow color strips on the half of their skin) for consistent raw material quality during frying (Pandey and Chauhan 2018). The total soluble solids, pH and acidity content of selected papaya fruits were ranging from 8.5 to 9.2°B, 5.4 to 5.6 and 0.28 to 0.35%, respectively.
Sample preparation and vacuum frying
Selected fruits were washed with 50 ppm sodium hypochlorite solution (SD fine chem Ltd, Mumbai, India) for sanitation, peeled using hand peeler and sliced to 3 mm thickness (Aerospace Hand Micrometer, 0–25 × 0.01 mm, Made in China) followed by dividing in four experimental groups. The first set of sample was stored for freezing pre-treatment in a deep freezer (Model no. URC-H-3402, Mfd. by Cryo Scientific Systems [P] Ltd., Chennai, India) at − 18 °C for 24 h. Whereas, the moisture content of second and third set of samples was reduced up to 45 ± 0.6% (wb) by partial dehydration in a cabinet hot air drier (Model no. 048T, Mfd. by Gansons Limited, Madras, India) at 70 °C for 3 h. The third set of sample after partial drying was kept in a deep freezer for freezing at − 18 °C for 24 h and fourth set of sliced papaya was used as such for vacuum frying and termed as control.
Pre-treated and control papaya fruit slices were fried in a vacuum-fryer (Future Tech Foods Pvt. Ltd., Pune, India) using same procedure as described by Pandey and Chauhan (2018). Rice bran oil (120 L) was heated up to 100 ± 2 °C followed by loading of sample and the frying chamber was vacuumized up to 13.33 kPa prior frying. The sample to oil ratio was kept constant i.e. 1:60 for all experiments to avoid temperature fluctuation during frying. The samples were removed after 28 min of frying and centrifuged in a basket centrifugation system (Model no. 570078, Mfd. by MSE Limited, Made in England) at 750 RPM for 2 min followed by cooling (5 min) at room temperature (Fig. 1). Samples were packed in metalized polyester pouches and stored at ambient temperature (25 °C ± 4 °C) for further analysis.
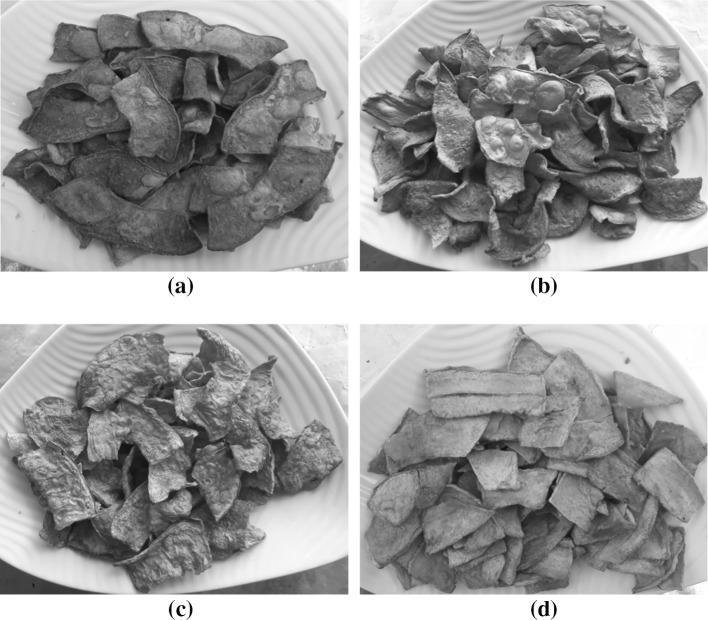
Fig. 1.
Effect of pre-treatments on visual appearance of vacuum fried papaya chips; a control, b partial drying, c partial drying + freezing and d freezing
Physico-chemical analysis
Proximate composition
The moisture and oil content of the sample was determined gravimetrically by following AOAC (2003) method. Water activity of the sample was analyzed using water activity meter (Aqua Lab, Decagon Device Inc., USA) at 25 °C. Protein content was determined through Kjeldahl method (AOAC 2003) using a nitrogen conversion factor of 6.25. The carbohydrate content was determined by the difference method. The ash content of sample was also analyzed by burning the sample at 550 °C in a muffle furnace. All experiments of the proximate analysis were performed in triplicates and the results were reported on % dry weight basis.
Colour
The colour of coarsely ground papaya fruit chips was measured using tri-stimulus colourimeter (Miniscan XE plus, Model number 45/0-S, Hunter Associates Laboratory Inc., Reston, VA, USA). Instrument was calibrated using white and black standard ceramic tiles before measurement and the total colour change was expressed in terms of L* value (lightness-darkness), a* value (redness-greenness), and b* value (yellowness-blueness). The total color difference of chips was calculated from fresh papaya slices using following equation:
| 1 |
where , and are the color values of the fresh papaya slices and L, a and b represented the individual values of pre treated and untreated papaya chips, to evaluate the total color difference of product after frying.
Hardness
Hardness of papaya chips was analyzed through Texture Analyzer (TA-Hdi, Stable Micro Systems™ Co, London, UK) using Texture Expert software (Version 1.22; Stable Microsystems). A Warner–Bratzler Blade (thickness 2.5 mm with flat edge) fixed in a 25 kg load cell was used to measure the hardness by cutting the sample at the geometric centre. The test speed of 1 mm/s was used whereas, pre- and post-test speed was set at 5 mm/s. Mean values were taken by taking peak force values of 15 samples.
Bulk density, true density, porosity and % shrinkage
Apparent bulk density (ρb) of papaya chips was analyzed as per the procedure described by Segnini et al. (2004) using Rye seeds (~ 1 mm). Whereas, true density (ρt) of papaya chips was analyzed as per the procedure described by Deshpande et al. (1993) using n-heptane as a displacement solvent. The relation between true density (ρt) and bulk density (ρb) was used to calculate porosity of vacuum fried papaya chips as described by Mohsenin (1970).
| 2 |
% Shrinkage of papaya chips was determined using following equation:
| 3 |
where Vo and Vt are the volume of fresh papaya slices and the volume of vacuum fried papaya chips at time (t), respectively.
Structural analysis
A horizontal section of sample was gold coated (20 nm) using a sputter coater (EMITECH, Model No. SC7620). Scanning Electron Microscope (SEM, Carl Zeiss, Model no. EVO LS 10, German make) was used to analyze the changes in microscopic structure of vacuum fried papaya chips due to pre-treatments.
Extraction of total phenolics and total flavonoids content
2 g of representative sample was taken from 50 g coarsely grinded papaya chips. Samples were crushed in pestal mortar and extraction was repeated thrice for each sample using 80% ethanol. The final volume of papaya chips extract was made up to 100 mL accurately using extraction solvent and filtered through Whatman No. 42 for further analysis.
Total phenolics
Total phenolic content was determined as the procedure described by Singleton and Rossi (1965). 1 mL of Folin–Ciocalteau reagent was added to 1 mL of sample aliquot, followed by addition of Na2CO3 solution (7%, 10 mL) after 6 min and the volume was made up to 25 mL using distilled water. The solution was incubated for 90 min in dark condition at room temperature and absorbance was measured at 750 nm in spectrophotometer (Shimadzu, model 1609, Tokyo, Japan) using gallic acid as standard and 80% ethanol as blank.
Total flavonoids
Total flavonoid content was determined as the procedure described by Zhishen et al. (1999). 5 mL of the sample aliquot was taken and 0.3 mL of NaNO2 (5%) was added, kept for 5 min. Thereafter, 0.3 mL of AlCl3 (10%) was added and kept for 6 min. Again 2 mL sodium hydroxide (1 N) solution was added to it and volume was made up to 10 mL using distilled water. The absorbance was measured at 510 nm in spectrophotometer (Shimadzu, model 1609, Tokyo, Japan) using catechin as standard and 80% ethanol as blank.
Total carotenoids
2 g of representative sample was taken from a coarsely grinded papaya chips (50 g) and extraction was done using a solvent mixture (40 mL acetone and 60 mL hexane) till the residue became colourless followed by filtration using Whatman No. 4 filter paper. The filtrate was transferred to a separating funnel and 50 mL of hexane was added. The acetone layer was separated from the extract by repeated washings with distilled water and NaCl (5%) solution. The hexane layer containing extracted pigment was passed through anhydrous sodium sulfate bed and volume was made up to 100 mL using n-hexane. The absorbance was measured using spectrophotometer (Shimadzu 1609, Tokyo, Japan) at 450 nm using hexane as blank (Kuti 2004). Following formula was used for calculation of total carotenoids:
| 4 |
Ascorbic acid
2 g papaya chip was washed with n-hexane thrice and dried using nitrogen. Fat free papaya sample was macerated using 3% meta-phosphoric acid followed by volume makeup to 100 mL using meta-phosphoric acid. Ascorbic acid content of vacuum fried papaya chips was estimated by titrating against 2,6-Dichlorophenol endophenol dye as procedure described by Ranganna (1999).
| 5 |
Antioxidant activity
The antioxidant activity of vacuum fried papaya chips was measured using DPPH assay as method described by Blois (1958). The reaction mixture consisted 0.5–3.0 mL methanolic extract of sample with 0.5 mL of methanolic DPPH (0.5 mM). After 30 min incubation period in the dark at room temperature the absorbance was measured against a blank at 517 nm using spectrophotometer (Shimadzu model 1609, Tokyo, Japan). The percent inhibition of DPPH was determined by comparing with methanol treated control group using following formula:
| 6 |
The potential of sample extract producing 50% inhibition (IC50 value) was calculated by plotting the graph between sample concentration and % of DPPH inhibition.
Sensory evaluation
Thirty semi-trained panelists were selected from the staff members of the laboratory to evaluate the sensory quality of vacuum fried papaya chips in terms of colour, crispness, taste, mouth feel, appearance and overall acceptability. These quality attributes were rated using a 9-point hedonic scale, where 9 represented to “extremely like” and 1 represented to “extremely dislike”. Samples were coded randomly and were served together to each sensory panelist separately.
Statistical analysis
Analysis of variance (ANOVA) was used to see the statistical differences among the data obtained for various physico-chemical parameters. All the measurements were taken in triplicates except firmness and density and average of them was used in the study. Statistica 7 software (Stat Soft, Tulsa, OK, USA) was used for conducting the ANOVA analysis at 5% level of confidence.
Result and discussion
The moisture content of selected papaya fruits was found to be 87.56 ± 0.17% (wb), whereas, fat, protein, total carbohydrate and ash content were found to be 0.20 ± 0.02%, 2.91 ± 0.17%, 93.12 ± 2.36% and 3.77 ± 0.28% (dry basis), respectively. The fresh papaya fruit slices were also found to have 544.45 ± 12.00 mg/100 g total phenolics, 271.31 ± 7.00 mg/100 g total flavonoids, 30.35 ± 2.40 mg/100 g total carotenoids and 397.30 ± 11.26 mg/100 g ascorbic acid content.
Effect of vacuum frying on physico-chemical characteristics
Moisture content, water activity (aw) and oil uptake
There was no significant (p < 0.05) difference observed in % moisture content of papaya chips among control, partial drying and partial drying + freezing except for freezing pre-treated samples at the same frying condition (100 °C/28 min) (Table 1). The low moisture content in freezing pre-treated sample was might be due to the fact that moisture evaporates rapidly from ice crystal state under vacuum condition. Moreover, formation of ice crystal might also increased the rate of heat transfer between frozen cells during frying and resulted in lower moisture content (Shyu et al. 2005). However, formation of surface crust and development of dense microstructure during partial drying pre-treatment might reduced the rate of moisture evaporation during frying and resulted in samples with high moisture content (Krokida et al. 2001). Pre-treatments also influenced the aw content of vacuum fried papaya chips which was ranging from 0.215 for freezing pre-treated sample to 0.235 for partially dehydrated samples. The aw content of partial drying + freezing pre-treated samples was found high as compared to controls sample however the difference was found to be non significant (p < 0.05).
Table 1.
Effect of pre-treatments on quality parameters of vacuum fried papaya chips
| Parameters | Pre-treatment | |||
|---|---|---|---|---|
| CON | PD | F | ||
| % Moisture (db) | 3.01 ± 0.18a | 3.32 ± 0.11a | 3.10 ± 0.09a | 2.51 ± 0.13b |
| Water activity (aw) | 0.228 ± 0.001b | 0.235 ± 0.001c | 0.230 ± 0.001b | 0.215 ± 0.001a |
| % Fat (db) | 25.45 ± 0.14d | 19.61 ± 0.08a | 21.49 ± 0.10b | 23.68 ± 0.12c |
| L* | 49.55 ± 0.90a | 52.81 ± 1.92ab | 53.28 ± 0.29b | 61.30 ± 0.79c |
| a* | 21.44 ± 1.29a | 21.91 ± 0.54a | 23.36 ± 0.42a | 22.35 ± 1.91a |
| b* | 39.00 ± 1.95a | 46.46 ± 3.12b | 45.07 ± 0.72b | 37.81 ± 7.23a |
| Crispiness (N) | 47.71 ± 5.38b | 65.06 ± 2.80c | 46.24 ± 4.69b | 36.97 ± 2.71a |
| Bulk density (ρb) (g/cm3) | 0.41 ± 0.03b | 0.48 ± 0.02c | 0.44 ± 0.04bc | 0.34 ± 0.02a |
| True density (ρt) (g/cm3) | 0.85 ± 0.06b | 0.90 ± 0.07c | 0.84 ± 0.09b | 0.79 ± 0.04a |
| Porosity (φ) | 0.51 ± 0.01b | 0.46 ± 0.01a | 0.45 ± 0.02a | 0.57 ± 0.02c |
| % Shrinkage | 57.05 ± 1.19b | 61.57 ± 1.21c | 60.11 ± 1.25c | 38.07 ± 1.02a |
CON Control, PD partial drying, PDF partial drying + freezing, F freezing
*Values with different superscripts in a same row differ significantly (p < 0.05)
Depending on the type of pre-treatment, level of oil uptake also changed significantly (p < 0.05) among the samples. The oil uptake was found highest in control sample (Table 1). High initial moisture content and uneven surface microstructure after frying might be the reason of highest oil uptake in untreated control sample (Shyu and Hwang 2001). Partial drying pre-treatment significantly (p < 0.05) reduced the oil content in papaya chips, i.e., 22.94%, 17.19% and 8.7% less compared to control followed by freezing and partial drying + freezing pre-treated samples, respectively. Low oil uptake in partial drying pre-treated sample was might be due to low initial moisture content and formation of outer crust on the surface of slices during pre-treatment (Krokida et al. 2001). Whereas, high initial moisture content and formation of ice crystals during freezing pre-treatment might be broken the cell wall of sample tissues which leads to formation of voids, porous and spongy structure during frying (Shyu et al. 2005). This spongy, porous structure of sample due freezing pre-treatment might be responsible for comparatively high oil uptake (Ren et al. 2018). However, the change in oil uptake behaviour of partial drying + freezing pre-treated sample might be due to combination effect of pre-treatments which changed the surface properties of the sample (Ren et al. 2018; Dueik et al. 2010, 2012).
CIE colour attributes and total color difference (TCD)
Table 1 shows the effect of pre-treatments on instrumental colour profile of vacuum fried papaya chips. The L* values differed significantly (p < 0.05) among the pre-treated and control chips. Freezing pre-treated samples had shown higher L* value followed by partial drying + freezing and partial drying pre-treated samples. The increase in L* value of freezing pre-treated sample might be due to less browning reaction and high carotenoid content that basically influenced the glossiness after frying (Dueik et al. 2010). The L* value of control sample was found 19.16%, 7.00% and 6.17% darker from freezing, partial drying + freezing and partial drying pre-treated samples, respectively. However, partial drying and partial drying + freezing pre-treated samples had significantly (p < 0.05) lower L* value than freezing pre-treated sample which might be due to browning reaction during partial drying and high concentration of phenolics (Krokida et al. 2001). Formation of maillard reaction products during partial drying pre-treatment of samples might be responsible for lower L* value (Nimmanpipug and Therdthai 2013). Moreover, Change in oil uptake during frying could be also responsible for increase or decrease in L* value. Ren et al. (2018) observed similar results in vacuum fried shiitake mushroom chips, where, L* value increased with decrease in oil content.
There was no significant (p < 0.05) difference observed in a* values of control, partial drying, partial drying + freezing and freezing pre-treated samples. However, a* value of partial drying + freezing pre-treated sample was found high as compared to other pre-treated and control samples. The a* value of partial drying pre-treated sample was almost similar to control one, whereas, freezing pre-treated sample resulted comparatively high a* value. The pre-treatments had significant (p < 0.05) effect on b* values of vacuum fried papaya chips which is related to yellow colour. However, there was no significant (p < 0.05) difference observed in b* values of partial drying and partial drying + freezing pre-treated samples and freezing pre-treated and control samples. The increase in b* value of partial drying and partial drying + freezing pre-treated sample was might be due to partial drying and less oil uptake as compared to freezing pre-treated and control samples (Krokida et al. 2001). Salehi (2018) also observed similar results in deep fat fried carrot chips, where, change in a* and b* value were due to browning reaction and oil uptake during frying.
Figure 2 shows the effect of pretreatment on total color difference of vacuum fried papaya chips. Total color difference was found significantly (p < 0.05) high in partial drying pre-treated sample, i.e., 19.25% and 16.51% higher than freezing pre-treated and control sample, respectively. Whereas, difference was found to be non significant (p > 0.05) between partial drying and partial drying + freezing pre-treated and freezing pre-treated and control samples. The change in surface properties and enzymatic and non-enzymatic browning due to partial drying pre-treatment may be the cause of high total color difference in partial drying and partial drying + freezing pre-treated samples. Zhu et al. (2015) also observed similar results where ∆E was increased significantly (p < 0.05) in vacuum fried peas due to partially drying pre-treatment.
Fig. 2.
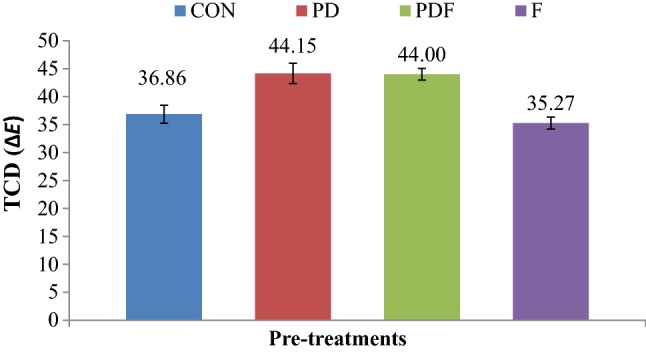
Effect of pre-treatments on total color difference (TCD) of vacuum fried papaya chips; a control, b partial drying, c partial drying + freezing and d freezing
Crispiness
Crispiness is considered as one of the most important quality parameter for fried products. The value of crispiness is inversely proportional to breaking force, therefore, higher breaking force results low crispiness of product. Pre-treatments were significantly (p < 0.05) affected crispiness of vacuum fried papaya chips. Freezing pre-treatment of sample prior to frying significantly (p < 0.05) increased the crispiness followed by partial drying + freezing and partial drying pre-treated samples. The high crispness value of sample was might be due to formation of ice crystals during freezing pre-treatment which resulted in formation of porous, spongy microstructure of chips during frying (Shyu et al. 2005). However, there was no significant (p < 0.05) difference observed between crispiness of control and partial drying + freezing pre-treated samples. Crust formation on the surface of papaya slices as well as reduction in ice crystal formation due to low moisture content might be resulted in high breaking force in partial drying + freezing pre-treated sample as compared to sample treated with freezing alone. Shyu and Hwang (2001) also reported increased breaking strength of pre-dehydrated apple chips due to casehardening which resulted in low crispiness in the product. Partial drying pre-treatment leads to low porosity, dense and comparatively harder texture in papaya. Diffusion of moisture from inner tissue to the surface along with movement of solutes during partial drying pre-treatment might be responsible for hard crust of papaya chips. The loss of crispiness in fried sweet potato was also observed due to drying pre-treatment (Taiwo and Baik 2007).
Bulk density, true density, porosity and shrinkage
Table 1 shows the effect of pre-treatments on density of vacuum fried papaya chips where apparent bulk density was ranging from 0.34 to 0.48 kg m−3 and true density was ranging from 0.79 to 0.9 kg m−3.
The apparent bulk density of partial drying pre-treated sample was found significantly (p < 0.05) higher as compared to freezing pre-treated samples. Whereas, non significant (p > 0.05) difference was observed in apparent bulk density of partial drying and partial drying + freezing pre-treated and partial drying + freezing pre-treated and control papaya chips. The increase in apparent bulk density of partial drying and partial drying + freezing pre-treated samples was might be due to irregular shape and formation of bubbles after frying. Because, control and freezing pre-treated samples were resulted flat regular shaped chips after frying and might be the reason of low apparent bulk density. Crust formation on the surface of papaya slices during partial drying pre-treatment might have caused to accumulation of vapour within the cells which resulted in formation of bubbles and bursting of cell due to high vapour pressure at the time of frying. Whereas, freezing pre-treatment caused breakdown of cell wall of papaya slices due to increased volume of ice crystals which leading to easy evaporation of moisture during frying. Krokida et al. (2000) reported that change in rate of moisture vaporization and formation of air pores during frying significantly (p < 0.05) influenced the apparent density of fried potatoes. Garayo and Moreira (2002) also observed change in surface structure where formation of air bubbles in vacuum fried potato chips due to gelatinization of starch producing barrier for the saturated vapour to escape.
The true density of freezing pre-treated sample was found significantly (p < 0.05) lower as compared to partial drying pre-treated and control samples. However, non significant (p > 0.05) difference was observed in true density of control and partial drying + freezing pre-treated samples. Moreover, partial drying pre-treated sample was retained high true density after frying. The increase in true density of partial drying and partial drying + freezing pre-treated samples might be due to crust formation and less porous structure as compared to freezing pre-treated samples. Moreover, visual observations indicated that pre-drying treatment lead formation of bubbles which resulted more displacement of solvent as compared to freezing pre-treated sample. Krokida et al. (2001) also observed similar changes in true density of pre-dried French fries.
Porosity is nothing but the ratio of bulk density and true density of the sample, therefore, change in those parameters resulted in change in porosity. Table 1 shows that porosity of vacuum fried papaya chips, where porosity of freezing pre-treated sample was observed significantly (p < 0.05) high i.e. 21.05, 19.29 and 10.53% higher as compared to partial drying, partial drying + freezing and CON samples, respectively. However, the difference was non significant (p > 0.05) in porosity of PD and PDF pre-treated samples. Kawas and Moreira (2001) also observed similar changes in tortilla chips where porosity was influenced significantly due to decreased sample thickness, bulk density and crust formation.
Shrinkage generally occurs due to change in volume of a sample during frying. Table 1 shows that pre-frying treatment significantly (p < 0.05) influenced the shrinkage of vacuum fried papaya chips. Freezing pre-treatment resulted in minimum shrinkage, i.e., 33.27, 36.67 and 38.17% less as compared to control, partial drying + freezing and partial drying pre-treated samples, respectively. However, shrinkage in PD and PDF pre-treated samples did not differ significantly (p < 0.05). Formation of outer crust on the surface and change in microstructural properties during partial drying pre-treatment might be responsible for high shrinkage. However, evaporation of moisture formed voids in freezing pre-treated and control samples which might be filled with air after frying and resulted in less shrinkage or collapse. Taiwo and Baik (2007) also observed similar changes in shrinkage of sweet potato chips due to partial drying and freezing pre-treatments.
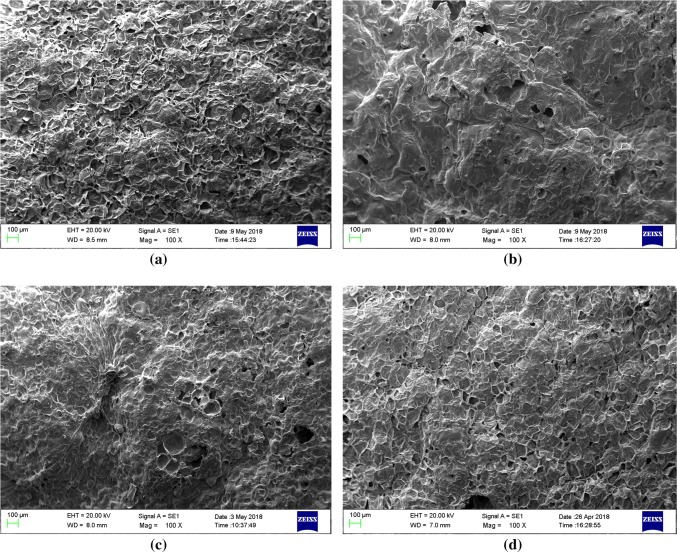
Structural changes
The sample pre-treated with freezing resulted in porous structure as compared to other samples (Fig. 3). Whereas, microstructural changes of partial drying and partial drying + freezing pre-treated samples were found similar. Evaporation of moisture during drying forms surface crust on sample which might resulted in similar microstructure characteristics in both partial drying and partial drying + freezing pre-treated samples after frying. Moreover, adsorption of oil at the surface might also be responsible for smooth surface and similar microstructural characteristics in partial drying and partial drying + freezing pre-treated samples. Whereas, development of ice crystals in sample during freezing pre-treatment might formed channels for easy evaporation of moisture during frying and resulted in more frequent and uniform surface characteristics of chips (Shyu et al. 2005). Dueik et al. (2012) reported that changes in surface properties attributed to oil uptake of the sample. Yagua and Moreira (2011) reported that rate of heat transfer and moisture loss significantly affected microstructure of potato chips during frying. The higher visibility of surface microstructure in freezing pre-treated and control samples were might be due to porous structure and low surface oil as compared to partial drying and partial drying + freezing pre-treated samples.
Fig. 3.
Effect of pre-treatments on microscopic structure of vacuum fried papaya chips at 200 × ; a control, b partial drying, c partial drying + freezing and d freezing
Total phenolics, flavonoids and carotenoids
Table 2 shows that partial drying pre-treatment resulted in significantly (p < 0.05) higher retention of total phenolics as compared to freezing pre-treated followed by control and partial drying + freezing pre-treated samples, i.e., 25.95%, 22.15% and 3.35%, respectively. The higher retention of total phenolics in partial drying pre-treated papaya chips might be due to formation of surface crust and development of compact micro structure of sample during pre-treatment. Formation of Maillard reaction products during partial drying pre-treatment might also be responsible for higher total phenolics content (Sharma et al. 2015). The loss of total phenolics content in control sample was found to be 43.80%, whereas, the loss in freezing pre-treated sample was found to be 46.82% of fresh papaya. The increased volume of ice-crystals as compared to water in sample might be ruptured cell structure during freezing pre-treatment and increased the exposure of bound phenolics to heat during frying. Shyu and Hwang (2001) also reported changes in microstructure of vacuum fried apple chips due to freezing pre-treatment. However, Fang et al. (2011) reported up to 57% loss in total phenolics content during vacuum frying of pre-frozen Chinese purple yam.
Table 2.
Effect of pre-treatments on chemical characteristics of vacuum fried papaya chips
| Parameters | Pre-treatment | |||
|---|---|---|---|---|
| CON | PD | F | ||
| Total phenolics (mg GAE/100 g) (db) | 306.81 ± 2.94b | 394.07 ± 3.04d | 380.86 ± 3.13c | 291.81 ± 2.47a |
| Total flavonoids (mg Catechin/100 g) (db) | 196.69 ± 1.56c | 158.66 ± 1.38b | 155.27 ± 2.18a | 226.01 ± 1.32d |
| Total carotenoids content (mg/100 g) (db) | 09.79 ± 0.11b | 08.74 ± 0.12a | 09.42 ± 0.10ab | 11.23 ± 0.21c |
| Vitamin C (mg/100 g) (db) | 209.81 ± 3.42c | 165.58 ± 2.30a | 172.74 ± 2.18a | 186.49 ± 1.76b |
| IC50 (mg/mL) (db) | 60.30 ± 0.29b | 64.16 ± 0.20d | 60.52 ± 0.71bc | 59.03 ± 0.23a |
CON Control, PD partial drying, PDF partial drying + freezing, F freezing
*Values with different superscripts in a same row differ significantly (p < 0.05)
The retention of total flavonoids content was significantly (p < 0.05) low in partial drying + freezing pre-treated sample (Table 3). However, freezing pre-treatment has shown significantly (p < 0.05) higher retention of total flavonoids content of fresh papaya followed by control, partial drying and partial drying + freezing pre-treated samples i.e., 83.30%, 72.60%, 58.48% and 57.22%, respectively. The higher total flavonoids in freezing pre-treated sample was might be due breakdown of complex phenolics compounds to simple flavonoids due to preventive effect of freezing during frying. Al-Sanabani et al. (2016) also observed similar results where flavonoids were retained to a higher extent as compared to phenolics during freeze drying of pomegranate seeds. Untreated control sample has shown 19.33% and 21.05% more retention of total flavonoids when compared to partial drying and partial drying + freezing pre-treated samples. The less flavonoid retention in partial drying pre-treated samples was might be due to long exposure of sample to heat during pre-treatment. Sharma et al. (2015) also reported that the degradation of total flavonoids increases with increasing drying temperature and exposure time.
Table 3.
Effect of pre-treatments on palatability characterization of vacuum fried papaya chips on the basis of sensory score
| Parameters | Pre-treatment | |||
|---|---|---|---|---|
| CON | PD | F | ||
| Color | 7.2 ± 0.2a | 7.5 ± 0.3b | 8.2 ± 0.2c | 8.6 ± 0.1d |
| Crispness | 8.2 ± 0.1a | 7.8 ± 0.2b | 8.3 ± 0.2ac | 8.7 ± 0.3c |
| Taste | 8.2 ± 0.3ab | 8.0 ± 0.3a | 8.4 ± 0.1ab | 8.5 ± 0.1b |
| Mouth feel | 7.9 ± 0.1b | 7.6 ± 0.1a | 8.3 ± 0.3c | 8.7 ± 0.2c |
| Appearance | 8.0 ± 0.2a | 8.2 ± 0.4a | 8.6 ± 0.3ab | 8.8 ± 0.1b |
| Overall acceptability | 8.0 ± 0.3a | 7.6 ± 0.2a | 8.4 ± 0.1b | 8.7 ± 0.1c |
CON Control, PD partial drying, PDF partial drying + freezing, F freezing
*Values with different superscripts in a same row differ significantly (p < 0.05)
Table 3 shows that retention of total carotenoids content was found to be high in freezing pre-treated sample, however, partial drying pre-treatment resulted in higher losses. There was non-significant (p > 0.05) difference observed in retention of total carotenoids content between partial drying + freezing pre-treated and control samples and between partial drying + freezing and partial drying pre-treated samples. Yan et al. (2011) reported up to 60% loss of carotene content of sweet potato due to hot air drying, whereas, carotene content was retained up to 80% during vacuum freeze drying. However, the loss of total carotenoids content of fresh papaya after vacuum frying was found to be 71.20%, 68.92%, 67.74% and 63.00% for partial drying, partial drying + freezing, control and freezing pre-treated samples, respectively. Da Silva and Moreira (2008) also reported loss of carotenoids during vacuum frying, i.e., 80% in green beans, 71% in mango and 56% in sweet potato.
Ascorbic acid content
Table 3 shows that pre-treatment of papaya slices significantly (p < 0.05) influenced the retention of ascorbic acid content in vacuum fried chips. Partial drying pre-treated sample resulted in higher losses of ascorbic acid followed by partial drying + freezing and freezing pre-treated samples i.e. 58.32%, 56.52% and 52.06%, respectively. However, retention of ascorbic acid in control sample was up to 52.81% of fresh papaya. The oxidation of ascorbic acid during cabinet drying might be the reason of low ascorbic acid content in partial drying pre-treated sample (Zhu et al. 2015). Whereas, in freezing pre-treated sample high moisture content and quick sublimation of ice crystals during frying leads to oxidation of ascorbic acid. However, the ascorbic acid content was retained to a better extent in partial drying + freezing pre-treated sample when compared to partial drying pre-treated sample which might be due to combination pre-treatment.
Antioxidant activity
The antioxidant activity of vacuum fried papaya chips was found to be associated with retained natural pigments and ascorbic acid content after frying (Sivakumar and Wall 2013). The antioxidant property was influenced significantly (p < 0.05) due to pre-treatments. Table 3 shows that freezing pre-treatment of sample prior vacuum frying resulted in lowest IC50 value i.e., 59.03 ± 0.23 mg/mL. The loss of antioxidant activity in partial drying and partial drying + freezing pre-treated samples resulted in high IC50 value, which was found to be 6.02% and 0.36% high, respectively, as compared to control sample. There is an inverse relation between IC50 value and antioxidant activity, smaller the IC50 value higher the antioxidant activity. The sample pre-treated with freezing had 2.1% less IC50 value as compared to control. High total carotenoids as well as flavonoids and ascorbic acid contents might be responsible for higher antioxidant activity in freezing pre-treated samples (Dueik et al. 2010).
Sensory evaluation
The hedonic scale rating for sensory colour values differed significantly (p < 0.05) among the pre-treated and control samples. Table 3 shows that pre-treatment significantly (p < 0.05) affected sensory parameters of vacuum fried papaya chips. Freezing pre-treated sample was significantly (p < 0.05) rated high on hedonic scale for overall sensory acceptability followed by partial drying + freezing and control samples. Whereas, partial drying pre-treated sample was rated low on hedonic scale by the panellists for all the sensory parameters except for appearance. High overall acceptability of freezing pre-treated sample was might be due to light colour, crisp texture and good appearance. Darkening of colour due to drying might have resulted in low sensory rating for the partial drying and partial drying + freezing pre-treated samples. Chauhan et al. (2019) also reported higher sensory acceptability in terms of color, flavor, texture and overall acceptability of freezing pre-treated vacuum fried strawberry chips. Retention of natural color, porous and crisp structure and moderate oil uptake might be the reason of higher sensory acceptability of freezing pre-treated vacuum frying products.
Conclusion
Partial drying prior to vacuum frying reduced oil uptake and caused significant (p < 0.05) retention of total phenolics to a higher extent in papaya chips. Untreated control sample was found good in retaining ascorbic acid content as compared to pre-treated samples. Whereas, freezing pre-treatment was found better in retention of phyto-chemicals such as total flavonoid and total carotenoids contents as well as antioxidant activity besides having good colour, firmness and overall sensory acceptability. Freezing prior vacuum frying could be an effective pre-treatment for production of good quality papaya chips.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad Tarmizi AH, Niranjan K. Post-frying oil drainage from potato chips and French fries: a comparative study of atmospheric and vacuum drainage. Food Bioprocess Technol. 2013;6:489–497. doi: 10.1007/s11947-011-0685-5. [DOI] [Google Scholar]
- Al-Sanabani AS, Youssef KM, Shatta AA, El-Samahy SK. Impact of freezing and freeze drying processes on color, phytochemical contents and antioxidant capacity of pomegranate seeds. J Food Sci. 2016;3:27–34. [Google Scholar]
- AOAC . Official methods of analysis. Washington: Association of Official Analytical Chemists; 2003. [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Chauhan OP, Pandey AK, Ravi N, Patki PE, Semwal AD. Use of vacuum frying technology for the development of strawberry chips. Int J Trop Agric. 2019;37:147–156. [Google Scholar]
- Da Silva PF, Moreira RG. Vacuum frying of high-quality fruit and vegetable-based snacks. LWT Food Sci Technol. 2008;41:1758–1767. doi: 10.1016/j.lwt.2008.01.016. [DOI] [Google Scholar]
- Deshpande SD, Bal S, Ojha TP. Physical properties of soybean. J Agric Eng Res. 1993;56:89–98. doi: 10.1006/jaer.1993.1063. [DOI] [Google Scholar]
- Diamante LM, Shi S, Hellmann A, Busch J. Vacuum frying foods: products, process and optimization. Int Food Res J. 2015;22:15–22. [Google Scholar]
- Dueik V, Robert P, Bouchon P. Vacuum frying reduces oil uptake and improves the quality parameters of carrot crisps. Food Chem. 2010;199:1143–1149. doi: 10.1016/j.foodchem.2009.08.027. [DOI] [Google Scholar]
- Dueik V, Moreno MC, Bouchon P. Microstructural approach to understand oil absorption during vacuum and atmospheric frying. J Food Eng. 2012;111:528–536. doi: 10.1016/j.jfoodeng.2012.02.027. [DOI] [Google Scholar]
- Fang Z, Wu D, Yu D, Ye X, Liu D, Chen J. Phenolic compounds in Chinese purple yam and changes during vacuum frying. Food Chem. 2011;128:943–948. doi: 10.1016/j.foodchem.2011.03.123. [DOI] [Google Scholar]
- Garayo J, Moreira R. Vacuum frying of potato chips. J Food Eng. 2002;55:181–191. doi: 10.1016/S0260-8774(02)00062-6. [DOI] [Google Scholar]
- Kawas ML, Moreira RG. Characterisation of product quality attributes of tortilla chips during the frying process. J Food Eng. 2001;47:97–107. doi: 10.1016/S0260-8774(00)00104-7. [DOI] [Google Scholar]
- Krokida MK, Oreopoulou V, Maroulis ZB. Effect of frying conditions on shrinkage and porosity of fried potatoes. J Food Sci. 2000;66:300–306. [Google Scholar]
- Krokida MK, Oreopoulou V, Maroulis ZB, Marinos-Kouris D. Effect of pre-drying on quality of French fries. J Food Eng. 2001;49:347–354. doi: 10.1016/S0260-8774(00)00233-8. [DOI] [Google Scholar]
- Kuti JO. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004;85:527–533. doi: 10.1016/S0308-8146(03)00184-5. [DOI] [Google Scholar]
- Mariscal M, Bouchon P. Comparison between atmospheric and vacuum frying of apple slices. Food Chem. 2008;107:1561–1569. doi: 10.1016/j.foodchem.2007.09.031. [DOI] [Google Scholar]
- Mohsenin NN. Physical properties of plant and animal materials. New York: Gordon and Breach Science Publishers; 1970. [Google Scholar]
- NHB (2017) Horticultural statistics at a glance. Horticulture Statistics Division, Department of Agriculture, Cooperation and Farmers Welfare, Ministry of Agriculture and Farmers Welfare, Government of India. Oxford University Press, New Delhi
- Nimmanpipug N, Therdthai N. Effect of osmotic dehydration time on hot air drying and microwave vacuum drying of papaya. Food Appl Biosci J. 2013;1:1–10. doi: 10.1016/j.fbio.2013.04.003. [DOI] [Google Scholar]
- Pandey AK, Chauhan OP. Process optimization for development of vacuum fried papaya (Carica papaya) chips using response surface methodology. Agric Res. 2018;8:364–373. doi: 10.1007/s40003-018-0375-x. [DOI] [Google Scholar]
- Ranganna S. Handbook of analysis and quality for fruit and vegetable products. New Delhi: Tata McGraw-Hill Publishing Company Limited; 1999. pp. 105–106. [Google Scholar]
- Ren A, Pan S, Li W, Chen G, Duan X. Effect of various pretreatments on quality attributes of vacuum-fried shiitake mushroom chips. J Food Qual. 2018 doi: 10.1155/2018/4510126. [DOI] [Google Scholar]
- Salehi F. Color changes kinetics during deep fat frying of carrot slice. Heat Mass Trans. 2018;54:3421–3426. doi: 10.1007/s00231-018-2382-7. [DOI] [Google Scholar]
- Segnini S, Pedreschi F, Dejmek P. Volume measurement method of potato chips. Int J Food Prop. 2004;7:37–44. doi: 10.1081/JFP-120022494. [DOI] [Google Scholar]
- Sharma K, Ko EY, Assefa AD, Ha S, Nile SH, Lee ET, Park SW. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J Food Drug Anal. 2015;23:243–252. doi: 10.1016/j.jfda.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu SL, Hwang LS. Effects of processing conditions on the quality of vacuum fried apple chips. Food Res Int. 2001;34:133–142. doi: 10.1016/S0963-9969(00)00141-1. [DOI] [Google Scholar]
- Shyu SL, Hau LB, Hwang LS. Effect of processing conditions on the quality of vacuum-fried carrot chips. J Sci Food Agric. 2005;85:1903–1908. doi: 10.1002/jsfa.2195. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colourimetry of total phenolics with phosphomolybdic-phosphotungustic acid reagent. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Sivakumar D, Wall MM. Papaya fruit quality management during the postharvest supply chain. Food Rev Int. 2013;29:24–48. doi: 10.1080/87559129.2012.692138. [DOI] [Google Scholar]
- Taiwo KA, Baik OD. Effects of pre-treatments on the shrinkage and textural properties of fried sweet potatoes. LWT. 2007;40:661–668. doi: 10.1016/j.lwt.2006.03.005. [DOI] [Google Scholar]
- WHO (2002) Health implication of acrylamide in food. In: Report of a joint FAO/WHO consultation WHO headquarters. Geneva, Switzerland. Issued by the World Health Organization in collaboration with the Food and Agriculture Organization of the United Nations
- Yagua CV, Moreira RG. Physical and thermal properties of potato chips during vacuum frying. J Food Eng. 2011;104:272–283. doi: 10.1016/j.jfoodeng.2010.12.018. [DOI] [Google Scholar]
- Yan WQ, Zhang M, Sun JC, Huang LL, Mujumdar AS, Tang J (2011) Influence of microwave drying method on the characterists of the sweet potato dices. In: European drying conference—EuroDrying. Palma, Balearic Island, 26–28 October 2011
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoids content in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;54:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- Zhu YY, Zhang M, Wang YQ. Vacuum frying of peas: effect of coating and pre-drying. J Food Sci Technol. 2015;52:3105–3110. doi: 10.1007/s13197-014-1314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]