Abstract
We investigated the effects of at-harvest maturity of ‘YuLu’ peach fruit on soluble sugar metabolism and their relationship with chilling injury susceptibility. Peaches were sorted into four maturity groups at harvest by IAD (index of the absorbance difference between 670 and 720 nm) then stored at 5 °C for 28 days. Fruit quality parameters, flesh browning index, malondialdehyde (MDA) content, soluble sugar content, gene expression, and enzyme activities associated with sucrose metabolism were measured. The more mature fruit groups had significantly (p < 0.05) lower firmness, higher soluble solid content, a* values of background color, sorbitol and sucrose content at harvest. During the cold storage, the higher flesh browning index in the mature groups (M3 and M4) maybe due to the double stress of senescence and chilling injury because there was concomitant sharp increase in MDA content. However, the most immature at-harvest group (M1) had the significantly (p < 0.05) higher MDA content after 14 days of cold storage, and a flesh browning index significantly (p < 0.05) higher than the M2 group (the next more mature group), late in the storage period. Moreover, the M1 group had lower sucrose content at postharvest and higher activities and transcript levels of sucrose degrading enzymes and lower levels of sucrose synthesis enzymes, which was responsible for the lower sucrose levels than M2 group during storage. It was concluded that the more immature peach fruit with lower sucrose content, have a higher chilling susceptibility than more mature fruit.
Keywords: Prunus persica, Fruit maturity, Cold storage, Soluble sugar
Introduction
Peaches (Prunus persica L. Batsh) are climacteric and have a short shelf life at ambient temperature (Li et al. 2017; Yang et al. 2019). Although low-temperature prolongs storage time, chilling injury (CI) results within 1 or 2 weeks. The symptoms of CI include flesh or pit cavity browning (internal browning), flesh reddening (bleeding), lack of juiciness (mealiness), rubbery texture (leatheriness), gel breakdown, and woolliness (dry and mealy tissue) (Fernández-Trujillo et al. 1998; Lurie and Crisosto 2005). Fruit that ready for immediate consumption are not necessarily optimal for a successful long-term storage, an ideal peach fruit for long-term storage should be harvested at proper maturity.
Soluble sugar content contributes to the quality and taste of fruit, and affects its tolerance to stress (Der Agopian et al. 2011). Puig et al. (2015) posit that during cold storage, sugar partitioning and demand may play a role in the mechanism of chilling tolerance in peach fruit. Soluble sugars help support resistance to low-temperature and other types of stress by regulating osmotic pressure (Der Agopian et al. 2011), and controlling gene expression (Wang et al. 2013). The soluble sugars in peach fruit are sucrose, glucose, fructose, and sorbitol; the dominant sugar is sucrose, representing about 75% of the total (Aubert et al. 2014). Holland et al. (2002) have reported that higher sucrose content correlates with higher chilling tolerance in ‘Fortune’ mandarin fruit, and Abidi et al. (2015) hypothesize that high levels of sucrose and glucose alleviate CI symptoms in peaches. In our previous study (Wang et al. 2013), we reported that low temperature stimulates the activities and transcription levels of enzymes involved in sucrose cleavage, resulting in decreased sucrose content in cold stored peach fruit. Peach fruit treated with hot air, methyl jasmonate, or 1-methycyclopropene prior to cold storage, maintain higher levels of sucrose and have greater resistance to CI during cold storage than untreated peach fruit (Yu et al. 2015, 2016, 2017).
Numerous factors affect the susceptibility of CI in fruit, among them, cultivar, storage temperature, and their maturity at harvest have a significant effect (Crisosto et al. 1999; Aghdam et al. 2018). Lopresti et al. (2015) have reported that the more mature fruit at harvest the more susceptible and severe the CI, the study was done with ‘Fire Sweet’ nectarines and ‘Ryan sun’ peaches. Conversely, Fernández-Trujillo et al. (1998) and Gao et al. (2009) reported that immature fruit was more susceptible and showed more severe CI than riper fruit. These studies were done on ‘Paraguayo’ peaches and ‘Hujingmilu’ peaches respectively. These studies indicate that the effect of peach physiological maturity at harvest on severity of CI during cold storage has yet to be fully understood.
The index of absorbance difference (IAD) is related to the time course of ethylene production in on-tree ripening of peaches and nectarines (Ziosi et al. 2008), and the investigation on peach (Gonçalves et al. 2016) fruit have shown that this effective tool is very accurate for determining fruit maturity at harvest time. It is a fast, and nondestructive technique that, for apples, is more reliable than traditionally used parameters such as starch content, soluble solid content (SSC), and flesh firmness (FF) (Costamagna et al. 2013). The relationship between soluble sugar metabolism, severity of CI, and the at-harvest maturity of peaches during cold storage has not been explored. With the development of IAD to accurately measure fruit ripeness, we can now accurately sort a fruit population into maturity classes (Hale et al. 2012), therefore, the objective of our study was to determine the effect of fruit maturity at harvest on soluble sugar metabolism and CI during cold storage.
Materials and methods
Fruit material and maturity classification
‘Yulu’ peach fruit (Prunus persica L. Batsch) were harvested from the Fenghua Honey Peach Research Institute in Zhejiang Province, China (Latitude: 29° 65′ 47″ North and 121° 4′ 12″; Altitude: 26 m a.s.l.) on July 6 and July 13, 2017. All peaches were measured by a portable vis–NIR DA meter (Sinteleia, Bologna, Italy), and IAD was recorded. Based on the average of two IAD measurements per fruit, peaches were divided into four maturity levels: M1 (IAD = 0.81–1.0), M2 (IAD = 0.61–0.80), M3 (IAD = 0.41–0.60) and M4 (IAD = 0.21–0.40). The more mature the fruit the lower the index.
Cold storage experiment
A total of 150 fruit in M1 level and 150 fruit in M2 level were selected from fruit harvested on July 6, and 150 fruit in M3 level and 150 fruit in M4 level were selected from fruit picked on July 13, for cold storage at 5 °C for 28 days. In each maturity group, three replicates, consisting of 10 fruit per replicate were randomly sampled at 0, 7, 14, 21, and 28 days to detect fruit quality including color, fruit firmness, soluble solid content, titratable acid, and browning index. Slices of mesocarp (approximately 1 cm thick) from 10 fruit per replicate were combined, frozen in liquid nitrogen, and stored at − 80 °C for further analysis of malondialdehyde, soluble sugar content, enzyme activity, and RNA isolation for transcription analysis.
Determination of fruit quality
FF was determined using a digital fruit hardness tester (ZhiQu Precision Instruments Co., Ltd., China) fitted with an 8 mm diameter probe. The puncture depth was 10 mm. Firmness was recorded in Newtons (N).
Soluble solid content (SSC) was measured using a digital pocket refractometer (Atago, Japan). Results are expressed as %.
Titratable acid (TA) was determined by grinding 10 g of flesh pulp with 20 mL water to produce a homogenate. This mixture was incubated in a 75 °C water bath for 30 min, then filtered. The volume of filtrate was brought to 250 mL. 50 mL of the filtrate was titrated with 0.05 N NaOH to pH 8.1 and calculating the result as percent malic acid.
A Chroma Meter (CR-400, Konica Minolta, Japan) was used to determine the a* value of background color at the same sites on the fruit at which the IAD values were measured. + a* represents red, − a* green.
Determination of browning index and malondialdehyde (MDA) content
Browning index was estimated by visually accessing the brown area that was evident after cutting each fruit along its axial diameter. Browning levels were graded as described by Yu et al. (2017), and browning index was calculated as [(Browning level) × (the number of fruit at this Browning level)]/(4 × total number of fruit in each treatment).
MDA was extracted and measured using a plant MDA Kit (Jiancheng, Nanjing, China) according to manufacturer’s instructions. Briefly, 0.5 g of frozen peach tissue was added to 0.5 mL of extraction solution, this mixture was incubated in a 95 °C water bath for 40 min, then centrifuged at 4000g for 10 min at 4 °C. MDA content in the solution was measured at 532 nm using a UV-1600PC Type Visible Spectrophotometer (Shanghai MAPADA Spectrum Instruments Inc., China). MDA content is expressed as μmol/kg fresh weight (FW).
Measurement of soluble sugars
Soluble sugars content were measured as described by Yu et al. (2016), using an HPLC system (Model 2695, Waters, USA) with a refractive index (RI) detector (Model 2414, Waters, USA). Results are expressed as g/kg FW.
Extraction and assays of sucrose metabolism-related enzyme activities
Enzymes were extracted using the method of Yu et al. (2017). Frozen peach tissue (1 g) was homogenized on ice in 5 mL extraction buffer [100 mM sodium phosphate pH 7.5, 5 mM MgCl2, 1 mM EDTA, 2.5 mM dithiothreitol, and 0.1% (v/v) Triton X-100]. Homogenates were centrifuged at 10,000g for 20 min at 4 °C. Supernatants were collected and immediately dialyzed in a 10 × volume of extraction buffer (without Triton X-100) at 4 °C for 20 h. The dialysates were used for measuring enzyme activities according to the methods in Yu et al. (2017). One unit of activity for acid invertase (AI), neutral invertase (NI), and sucrose synthase-cleavage (SS-cleavage) is defined as the amount of enzyme that catalyzes the conversion of 1 μmol of sucrose into glucose per hour. One unit of SS-synthesis and SPS (sucrose phosphate synthase) activity is defined as the amount of sucrose that enzyme synthetized per hour. All activities are expressed as U/g FW.
RNA isolation and real-time PCR analysis
Total RNA was isolated from 1 g of frozen tissue from each replicate in each maturity group using the RNA plant Plus Reagent kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. RNA was quantified using a NanoDrop 2000 spectrophotometer (Thermo, USA) and accessed for integrity by agarose gel electrophoresis. cDNA was synthesized using HiScript® Q RT SuperMix for QPCR containing gDNA wiper (Vazyme, Nanjing, China). All qPCR reactions were normalized by the cycle threshold (Ct) value compared to a housekeeping gene TEF2 (GenBank Accession: JQ732180) following the 2−ΔΔCt method for relative quantification (Livak and Schmittgen 2001). Different cDNAs synthesized from three replicates in each maturity group and each sampling time were used for RT-qPCR analysis. qPCR primer sequences were as follow: PpVIN2 (an AI family gene) (forward: 5′-ACAAGGTCTTCCGTGGCAAA-3′; reverse: 5′-AGCAGCCCCATAAATTGCCT-3′), SPS/1 (forward: 5′-TTGAGGCTACAGGAAAGGAAAG-3′; reverse: 5′-GGA CGCTCCTCTGAATGAATAG-3′) and SPS/2 (forward: 5′-CTTCCCTTTGTGG TGGATTTAG-3′; reverse: 5′-GAGTTCCTTA ACAGGGGGAATC-3′).
Statistical analysis
Experiments were performed using a randomized design. All statistical analyses were performed with SPSS software (version 22.0, IBM, New York, USA).Mean separations were compared using Tukey’s test, and the level of significance difference was p < 0.05. A regression analysis of the trend of soluble sugar content and the Pearson correlation analysis between MDA content and browning index were also analyzed by SPSS22.0. Figures were prepared using Origin Pro 9.0 G (Microcal Software Inc., Northampton, MA, USA).
Results
Fruit quality
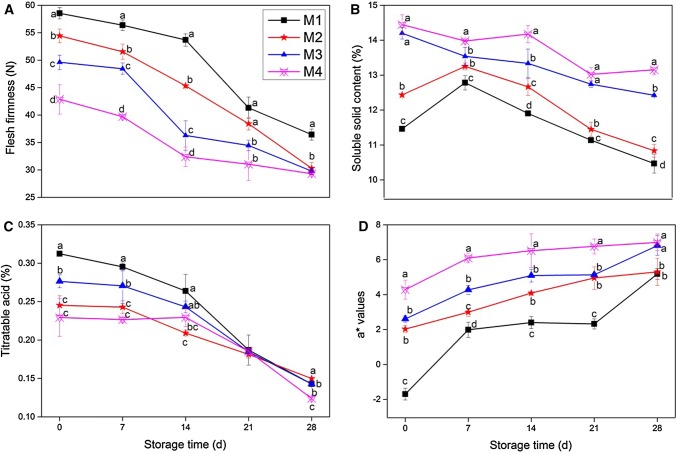
As can be seen in the photo accompanying Table 1, there are obvious visible differences among the maturity groups; these differences are reflected in the IAD index, which decrease with increased maturity. FF and a* values of background color were significantly (p < 0.05) different among the groups immediately postharvest; fruit with higher IAD index had greater FF, and lower SSC and a* values. There was no obvious correlation between IAD index and TA. As shown in Fig. 1, for all groups, FF and TA decreased, and a* values increased as storage time increased. SSC initially increased in the M1 and M2 groups then decreased over the remaining time in storage. SSC in the M3 and M4 groups decreased over storage time but remained significantly higher during storage than the M1 and M2 peaches.
Table 1.
Flesh firmness (FF), soluble solid content (SSC), titratable acidity (TA), a* values of background color of ‘YuLu’ fruit graded according to the IAD at postharvest
| FF (N) | SSC (%) | TA (%) | a* values | ||
|---|---|---|---|---|---|
 |
M1 (IAD: 0.8–1.0) | 58.55 ± 1.04 a | 11.46 ± 0.06 c | 0.31 ± 0.01 a | − 1.7 ± 0.34 c |
| M2 (IAD: 0.6–0.8) | 54.43 ± 1.23 b | 12.43 ± 0.03 b | 0.25 ± 0.01 c | 2.03 ± 0.21 b | |
| M3 (IAD: 0.4–0.6) | 49.62 ± 1.33 c | 14.20 ± 0.07 a | 0.28 ± 0.01 b | 2.61 ± 0.18 b | |
| M4 (IAD: 0.2–0.4) | 42.87 ± 2.70 d | 14.45 ± 0.12 a | 0.23 ± 0.02 c | 4.29 ± 0.54 a |
The values represent mean ± standard error (SE) of 3 replicates derived from 10 fruits in each maturity. Different letters indicate significant differences at p < 0.05 according to the Tukey’s test
Fig. 1.
Effect of fruit maturity at harvest on FF (A), SSC (B), TA (C), and a* values (D) during storage at 5 °C for 28 days. Values are the mean ± SE of triplicate assays. Different lowercase letters indicate significant difference at p < 0.05 according to the Tukey’s test
Browning index and MDA content
Flesh browning was visibly detectable in M1, M2, M3, and M4 fruit on 28 days, 28 days, 21 days and 21 days of storage respectively. The browning trend at day 21 of storage was M4 > M3 > M2 = M1, at day 28 M4 > M3 > M1 > M2. The differences were significant (p < 0.05) (Fig. 2A).
Fig. 2.
Effect of fruit maturity at harvest on browning index (A) and MDA content (B) in peaches stored at 5 °C for 28 days. Values are the mean ± SE of triplicate assays. Different lowercase letters indicate significant difference at p < 0.05 according to the Tukey’s test
At the end of storage, MDA content of M1, M3 and M4 groups were higher than those at the beginning of the storage period (day 0), while that of M2 group was lower. During the cold storage, the MDA content of M3 and M4 fruit was increased on the whole, and increased sharply after day 14, while in M1 and M2 fruit decreased after 14 days and then increased after 21 days until to the end of storage. The MDA content at day 21 and 28 were similar to flesh browning: M4 > M3 > M1 > M2, the differences between M1 and M2 were significant (p < 0.05) (Fig. 2B).
Changes in sucrose, glucose, fructose, and sorbitol content
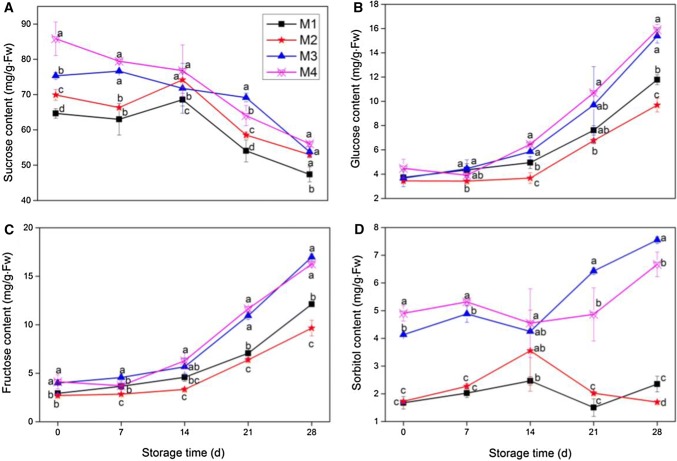
Immediately postharvest, the mature groups (M3 and M4) had significantly (p < 0.05) higher sucrose, fructose and sorbitol levels than the immature groups (M1 and M2), M2 fruit had significantly (p < 0.05) higher sucrose than M1 fruit, while glucose content did not show statistically significant differences in all groups. For all fruit groups sucrose levels decreased during the storage period, while glucose and fructose levels increased, sorbitol levels in mature group increased significantly after 14 days, while in immature group sorbitol levels increased before 14 days then decreased to nearly day 0 levels. Throughout the storage period, the higher the ripeness of the fruit group, the higher the sucrose and sorbitol levels of the fruit, and the M2 fruit had higher sucrose and sorbitol levels and lower glucose and fructose levels than M1 fruit (Fig. 3). While there was no significant difference between the glucose and fructose levels in M3 fruit and M4 fruit.
Fig. 3.
Effect of fruit maturity at harvest on sucrose (A), glucose (B), fructose (C), and sorbitol (D) content in peaches stored at 5 °C for 28 days. Values are the mean ± SE of triplicate assays. Different lowercase letters indicate significant difference at p < 0.05 according to the Tukey’s test
Activities of AI, NI, SS-cleavage, SS-synthesis and SPS
As shown in Fig. 4, the activities of these enzymes, which are involved in sucrose metabolism, were altered in peaches during cold storage. For all groups AI activity was higher after 28 days in cold storage than at 0 day. Throughout the storage period, the more mature at-harvest peaches (M3 and M4) had AI activity levels significantly (p < 0.05) higher than the immature at-harvest peaches (M1 and M2), the immature at harvest peaches M1 and M2 had similar AI activity, except at 28 days when the AI activity of M2 peaches was significantly (p < 0.05) lower than M1 peaches (Fig. 4A).
Fig. 4.
Effect of fruit maturity at harvest on AI (A), NI (B), SS-cleavage (C), SS-synthesis (D), and SPS (E) activity in peaches stored at 5 °C for 28 days. Values are the mean ± SE of triplicate assays. Different lowercase letters indicate significant difference at p < 0.05 according to the Tukey’s test
The profile of NI activity for all groups was the same, initially decreasing, then increasing afterwards. Between 0 and 28 days of storage, NI activity in groups M1, M2, and M4 fluctuated more than in group M3. Overall the mature groups (M3 and M4) had higher NI activity, and the M1 fruit had higher NI activity than M2 fruit (Fig. 4B) on the average, and a peak of activity at day 21.
The profile of SS-cleavage activity was similar to that for NI. SS-cleavage activity in the mature fruit was significantly (p < 0.05) higher throughout the time in storage than in immature fruit. Also in the mature fruit groups, SS-cleavage activity fluctuated during storage and was higher at the end of storage than at the beginning. Whereas in the immature groups (M1 and M2) SS-cleavage activity fluctuated very little during storage and was essentially unchanged at the end of the storage period (Fig. 4C).
SS-synthesis activity among the four peach groups varied narrowly and fluctuated irregularly during storage. In general SS-synthesis activity for each group had increased by the end of the storage period. Activity in M2 group rose sharply after day 7, peaked significantly (p < 0.05) higher than other groups at day 14, then decreased sharply by day 21 (Fig. 4D).
The immature fruit groups (M1 and M2) had significantly (p < 0.05) higher SPS activity throughout the storage period, and all groups had SPS activity that was essentially at the same level at the end of the storage period as at the beginning of storage. Activity in all groups except M2 declined in the first 7–14 days before rising again, and activity in all groups peaked at day 21 days (Fig. 4E).
Transcription of genes involved in soluble sugar metabolism
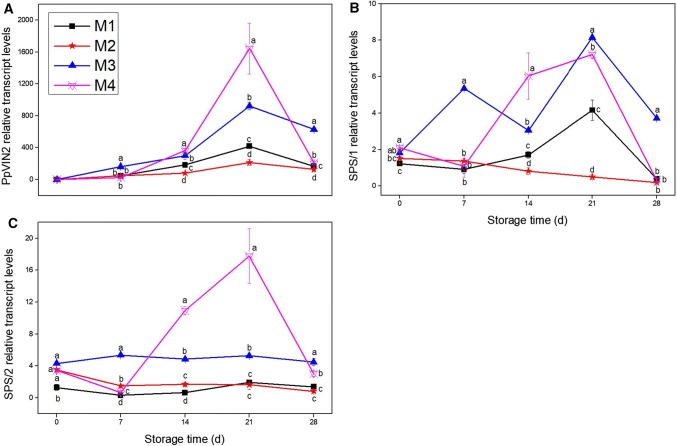
As shown in Fig. 5A, PpVIN2 transcript levels increased rapidly after 14 days of cold storage, peaked at day 21, and then decreased sharply back to pre-storage levels, except in M3 fruit where the transcripts levels were still elevated over pre-storage levels. The peak PpVIN2 levels in M3 and M4 groups were over two and four times higher respectively than levels observed at M1 and M2 groups.
Fig. 5.
Effect of fruit maturity at harvest on PpVIN2 (A), SPS/1 (B) and SPS/2 (C) transcript levels in peaches stored at 5 °C for 28 days. Values are the mean ± SE of triplicate assays. Different lowercase letters indicate significant difference at p < 0.05 according to the Tukey’s test
The transcript levels of SPS/1 in the mature fruit groups increased irregularly and for both groups peaked at day 21 before decreasing. Levels in the M1 group also rose until day 21 but were significantly lower than in the M3 and M4 fruit. The transcript levels of SPS/1 in the M2 group declined slowly during storage (Fig. 5B). The SPS/2 transcript levels in the M4 group increased from day 7, peaking at day 21 then decreased to pre-storage levels. Transcript levels of other three groups were similar and did not change significantly during storage (Fig. 5C). The results indicate that mature fruit has higher levels of gene transcription of sucrose metabolizing enzymes than immature fruit (Fig. 5).
Discussion
IAD has been used in many flesh fruit for a number of purposes: to estimate shelf-life potential (Lurie et al. 2013), to assess ripening stage (Ziosi et al. 2008), to compare with destructive harvest indices (Gonçalves et al. 2016), and to study factors that affect fruit quality and bioactive compound accumulation (Gasic et al. 2016). It has been confirmed that the IAD index predicts apple maturity more reliably than other parameters traditionally used such as starch, SSC, and FF (Costamagna et al. 2013). In this study, ‘YuLu’ peaches were sorted by IAD index into four maturity groups: M1–M4, M1 being the least mature, M4 being the most mature. As expected, the fruit in these groups had visible differences, and we found that mature fruit had higher a* values, sucrose, and sorbitol content and lower FF at harvest, the difference between four groups were significant (p < 0.05) (Table 1 and Fig. 3A, D). So the IAD can accurately indicate ‘YuLu’ fruit ripeness and allows fruit to be sorted into different maturity classes.
Internal browning of fruit is a common symptom of CI and is often observed when peach and nectarine fruit are kept in cold storage for long periods (Cáceres et al. 2016). MDA is a product of the oxidation of the lipids in the cell membrane. Its content in fruit has been used to evaluate the development of CI under the chilling stress. In our study, flesh browning was manifested earlier by the more mature peaches (M3 and M4), and reached significant (p < 0.05) higher levels by the end of storage than the more immature fruit (M1 and M2). Pearson correlation analysis showed that there was extremely significant correlation between MDA content and browning index (r = 0.561, p < 0.01). MDA levels in these fruit matched these results well. Peach fruit harvested at different maturity can affect change of flesh color and MDA content when stored at shelf life, the more mature fruit the more browning and the higher MDA content, due to rapid senescence in the more mature fruit (Zhou et al. 2010). So we supposed that the higher flesh browning index and MDA content of mature fruit during cold storage was due to the double stress of chilling and rapid senescence. Chanasut et al. (2018) found that early-season ‘Sai Nam Peung’ tangerine fruits had higher MDA concentration than mid-season, the susceptibility to CI of it was more related to fruit maturity at harvest and loss of membrane integrity. Gao et al. (2009) showed that in more mature ‘Hujingmilu’ fruit activities of superoxide dismutase and peroxidase, which reduce accumulation of free radical and can alleviate CI, where higher than in less mature peaches kept at 1 °C. In our study, the M2 fruit had a lower browning index and MDA content than M1 fruit during cold storage, so we conclude that the M1 fruit had a higher CI susceptibility than M2 fruit.
Soluble sugars play a role in protecting plants from CI (Couée et al. 2006). Higher sucrose content improves CI tolerance in peaches (Wang et al. 2013) and mandarins (Holland et al. 2002). We have previously found that peaches with higher sucrose levels had a greater chilling tolerance during cold storage (Yu et al. 2015, 2016, 2017). Sucrose enhances the anti-oxidization system to eliminate free radicals (Nishizawa et al. 2008), and stabilizes cell membrane structure (Oliver et al. 2002). Although in other fruit such as loquat (Shao et al. 2013), enhanced CI tolerance was related to reducing sugar content, rather than to sucrose. In our research, a regression analysis of the trend of soluble sugar content showed there were significant differences between soluble sugar content in four maturity classes during the storage, while M1 fruit had a higher MDA content and lower levels of sucrose than M2 fruit during cold storage. The results suggest that higher sucrose content protects peach fruit from CI during cold stress, and is consistent with our previous conclusions. In fact, different storage temperatures (Wang et al. 2013; Yu et al. 2015) or postharvest treatments (Yu et al. 2016, 2017) that result in higher CI tolerance, all decrease the reduction of sucrose in peach fruit.
Among sucrose metabolizing enzymes, invertases and SS-cleavage are involved in transforming sucrose into reducing sugars, while SS-synthesis and SPS are involved in catalyzing sucrose biosynthesis. AI and NI invertases have the decisive roles in sucrose hydrolysis and regulation in peach fruit (Lara et al. 2009). We have reported previously that PpVIN2 transcript levels initially increase sharply during storage at 5 °C, with peak PpVIN2 levels as much as one thousand times higher than those observed in peach fruit immediately postharvest (Wang et al. 2013; Yu et al. 2015, 2016). He et al. (2018) reported that the higher expression of PpVIN2, which occurs during chilling stress, improves vacuolar invertase activity and promotes the degradation of sucrose indirectly. Similar results have been reported for potato tubers subjected to cold storage (Xun et al. 2011). When bananas are ripened under natural conditions, the changes in SPS and AI activity are significantly (p < 0.05) faster in mature fruit than in immature fruit (Li and Chen 2011). In our research, we found that in the end of cold storage, peach fruit in M4 with higher maturity had the higher AI, NI and SS-cleavage activity, lower SS-syntheses and SPS activity. When comparing peach in M1 with M2, it is likely that lower PpVIN2 expression and AI activity, combined with higher SS-synthesis and SPS activity, is responsible for the higher content of sucrose in M2 peaches than in M1. However, among peach fruit in the 4 maturity classes, it is difficult to make a clear distinction between chilling injury and senescence, we cannot draw a similar conclusion as the previous report in banana (Li and Chen 2011).
Conclusion
IAD accurately measured ripeness in ‘YuLu’ fruit. The higher flesh browning index in the mature fruit during the cold storage may be due to senescence and CI. Among the four maturity classes, fruit in M2 group showed the lowest browning index and MDA content in the end of storage at 5 °C for 28 days. Thus, we suggest that peach fruit harvested in M2 (IAD = 0.61–0.80) is best for cold storage.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 31671903; 31972124), the Agricultural Research Project of Ningbo City (No. 2019C10069) and the K. C. Wong Magna Fund in Ningbo University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abidi W, Cantín CM, Jiménez S, Giménez R, Ángeles Moreno M, Gogorcena Y. Influence of antioxidant compounds, total sugars and genetic background on the chilling injury susceptibility of a non-melting peach (Prunus persica (L.) Batsch) progeny. J Sci Food Agric. 2015;95:351–358. doi: 10.1002/jsfa.6727. [DOI] [PubMed] [Google Scholar]
- Aghdam MS, Jannatizadeh A, Luo Z, Paliyath G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends Food Sci Technol. 2018;76:67–81. doi: 10.1016/j.tifs.2018.04.003. [DOI] [Google Scholar]
- Aubert C, Bony P, Chalot G, Landry P, Lurol S. Effects of storage temperature, storage duration, and subsequent ripening on the physicochemical characteristics, volatile compounds, and phytochemicals of Western Red nectarine (Prunus persica L. Batsch) J Agric Food Chem. 2014;62:4707–4724. doi: 10.1021/jf4057555. [DOI] [PubMed] [Google Scholar]
- Cáceres D, Díaz M, Shinya P, Infante R. Assessment of peach internal flesh browning through colorimetric measures. Postharvest Biol Technol. 2016;111:48–52. doi: 10.1016/j.postharvbio.2015.07.007. [DOI] [Google Scholar]
- Chanasut U, Rattanpanone N, Boonyakiat D, Kampoun W. Chilling injury susceptibility of early-season “Sai Nam Peung” tangerine fruit and alteration of alpha-farnesene and conjugated trienols during low temperature storage. Chiang Mai J Sci. 2018;45:147–153. [Google Scholar]
- Costamagna F, Giordani L, Costa G, Noferini M. Use of AD index to define harvest time and characterize ripening variability at harvest in ‘Gala’ apple. Acta Hortic. 2013;998:117–124. doi: 10.17660/ActaHortic.2013.998.12. [DOI] [Google Scholar]
- Couée I, Sulmon C, Gouesbet G, El Amrani A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J Exp Bot. 2006;57:449–459. doi: 10.1093/jxb/erj027. [DOI] [PubMed] [Google Scholar]
- Crisosto CH, Mitchell FG, Ju Z. Susceptibility to chilling injury of peach, nectarine, and plum cultivars grown in California. HortScience. 1999;34:1116–1118. doi: 10.21273/HORTSCI.34.6.1116. [DOI] [Google Scholar]
- Der Agopian RG, Goncalves Perono-Okita FH, Soares CA, Mainardi JA, OLiveira do Nascim ento JR, Cordenunsi BR, Lajolo FM, Purgatto E. Low temperature induced changes in activity and protein levels of the enzymes associated to conversion of starch to sucrose in banana fruit. Postharvest Biol Technol. 2011;62:133–140. doi: 10.1016/j.postharvbio.2011.05.008. [DOI] [Google Scholar]
- Fernández-Trujillo JP, Cano A, Artés F. Physiological changes in peaches related to chilling injury and ripening. Postharvest Biol Technol. 1998;13:109–119. doi: 10.1016/S0925-5214(98)00006-4. [DOI] [Google Scholar]
- Gao HY, Chen HJ, Chen WX, Song LL, Mao JL, Zhou YJ, Zheng YH. Effects of harvest maturity on quality and chilling injury of juicy peaches during low temperature storage. Sci Agric Sin. 2009;42(2):612–618. [Google Scholar]
- Gasic K, Abdelghafar A, Gregory R, Windham J, Ognjanov M. Fruit maturity affects fruit quality and bioactive compound accumulation in peach. Acta Hortic. 2016;1119:197–202. doi: 10.17660/ActaHortic.2016.1119.27. [DOI] [Google Scholar]
- Gonçalves RG, Couto J, Almeida DPF. On-tree maturity control of peach cultivars: comparison between destructive and nondestructive harvest indices. Sci Hortic. 2016;209:293–299. doi: 10.1016/j.scienta.2016.06.040. [DOI] [Google Scholar]
- Hale G, Lopresti J, Stefanelli D, Jones R, Bonora L. Using non-destructive methods to correlate chilling injury in nectarines with fruit maturity. Acta Hortic. 2012;1012:83–89. [Google Scholar]
- He X, Wei Y, Kou J, Xu F, Chen Z, Shao X. PpVIN2, an acid invertase gene family member, is sensitive to chilling temperature and affects sucrose metabolism in postharvest peach fruit. Plant Growth Regul. 2018;86:169–180. doi: 10.1007/s10725-018-0419-z. [DOI] [Google Scholar]
- Holland N, Menezes HC, Lafuente MAT. Carbohydrates as related to the heat-induced chilling tolerance and respiratory rate of ‘Fortune’ mandarin fruit harvested at different maturity stages. Postharvest Biol Technol. 2002;25:181–191. doi: 10.1016/S0925-5214(01)00182-X. [DOI] [Google Scholar]
- Lara MV, Borsani J, Budde CO, Lauxmann MA, Lombardo VA, Murray R, Andreo CS, Drincovich MF. Biochemical and proteomic analysis of ‘Dixiland’ peach fruit (Prunus persica) upon heat treatment. J Exp Bot. 2009;60:4315–4333. doi: 10.1093/jxb/erp267. [DOI] [PubMed] [Google Scholar]
- Li W, Chen W. The effects of harvest maturity on storage quality and sucrose-metabolizing enzymes during banana ripening. Food Bioprocess Technol. 2011;4:1273–1280. doi: 10.1007/s11947-009-0221-z. [DOI] [Google Scholar]
- Li D, Li L, Luo Z, Lu H, Yue Y. Effect of nano-ZnO-packaging on chilling tolerance and pectin metabolism of peaches during cold storage. Sci Hortic. 2017;225:128–133. doi: 10.1016/j.scienta.2017.07.003. [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopresti J, Hale G, Brady S, Stefanelli D. Effect of fruit maturity and cool storage on chilling injury in peach and nectarine. Acta Hortic. 2015;1084:741–749. doi: 10.17660/ActaHortic.2015.1084.99. [DOI] [Google Scholar]
- Lurie S, Crisosto CH. Chilling injury in peach and nectarine. Postharvest Biol Technol. 2005;37:195–208. doi: 10.1016/j.postharvbio.2005.04.012. [DOI] [Google Scholar]
- Lurie S, Friedman H, Weksler A, Dagar A, Eccher Zerbini P. Maturity assessment at harvest and prediction of softening in an early and late season melting peach. Postharvest Biol Technol. 2013;76:10–16. doi: 10.1016/j.postharvbio.2012.08.007. [DOI] [Google Scholar]
- Nishizawa A, Yabuta Y, Shigeoka S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008;147:1251–1263. doi: 10.1104/pp.108.122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver AE, Hincha DK, Crowe JH. Looking beyond sugars: the role of amphiphilic solutes in preventing adventitious reactions in anhydrobiotes at low water contents. A Mol Integr Physiol. 2002;131:515–525. doi: 10.1016/S1095-6433(01)00514-1. [DOI] [PubMed] [Google Scholar]
- Puig CP, Dagar A, Ibanez CM, Singh V, Crisosto CH, Friedman H, Lurie S, Granell A. Pre-symptomatic transcriptome changes during cold storage of chilling sensitive and resistant peach cultivars to elucidate chilling injury mechanisms. BMC Genom. 2015;16(245):1–35. doi: 10.1186/s12864-015-1395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Zhu Y, Cao S, Wang H, Song Y. Soluble sugar content and metabolism as related to the heat-induced chilling tolerance of loquat fruit during cold storage. Food Bioprocess Technol. 2013;6:3490–3498. doi: 10.1007/s11947-012-1011-6. [DOI] [Google Scholar]
- Wang K, Shao X, Gong Y, Zhu Y, Wang H, Zhang X, Yu D, Yu F, Qiu Z, Lu H. The metabolism of soluble carbohydrates related to chilling injury in peach fruit exposed to cold stress. Postharvest Biol Technol. 2013;86:53–61. doi: 10.1016/j.postharvbio.2013.06.020. [DOI] [Google Scholar]
- Xun L, Chi Z, Yong BO, Yuan L, Botao S, Cong HX, Jun L, Xiu QL. Systematic analysis of potato acid invertase genes reveals that a cold-responsive member, StvacINV1, regulates cold-induced sweetening of tubers. Mol Genet Genom. 2011;286:109–118. doi: 10.1007/s00438-011-0632-1. [DOI] [PubMed] [Google Scholar]
- Yang W, Wang L, Ban Z, Yan J, Lu H, Zhang X, Wu Q, Aghdam MS, Luo Z, Li L. Efficient microencapsulation of Syringa essential oil; the valuable potential on quality maintenance and storage behavior of peach. Food Hydrocoll. 2019;95:177–185. doi: 10.1016/j.foodhyd.2019.04.033. [DOI] [Google Scholar]
- Yu F, Shao X, Yu L, Xu F, Wang H. Proteomic analysis of postharvest peach fruit subjected to chilling stress or non-chilling stress temperatures during storage. Sci Hortic. 2015;197:72–89. doi: 10.1016/j.scienta.2015.10.045. [DOI] [Google Scholar]
- Yu L, Liu H, Shao X, Yu F, Wei Y, Ni Z, Xu F, Wang H. Effects of hot air and methyl jasmonate treatment on the metabolism of soluble sugars in peach fruit during cold storage. Postharvest Biol Technol. 2016;113:8–16. doi: 10.1016/j.postharvbio.2015.10.013. [DOI] [Google Scholar]
- Yu L, Shao X, Wei Y, Xu F, Wang H. Sucrose degradation is regulated by 1-methycyclopropene treatment and is related to chilling tolerance in two peach cultivars. Postharvest Biol Technol. 2017;124:25–34. doi: 10.1016/j.postharvbio.2016.09.002. [DOI] [Google Scholar]
- Zhou H, Qiao Y, Zhang S, Wang H, Chen Z. Study on the effects of different maturity on the fruit shelf-qualities and difference in metabolism of Datuanmilu honey peach cultivar. J Fruit Sci. 2010;27:244–250. [Google Scholar]
- Ziosi V, Noferini M, Fiori G, Tadiello A, Trainotti L, Casadoro G, Costa G. A new index based on vis spectroscopy to characterize the progression of ripening in peach fruit. Postharvest Biol Technol. 2008;49:319–329. doi: 10.1016/j.postharvbio.2008.01.017. [DOI] [Google Scholar]