Abstract
The aim of this study is to determine the effects of different drying methods, including freeze drying (FD), convective drying, sun drying, infrared drying and vacuum drying (VD), on the chemical composition and microstructure of maqui berries as well as their anti-inflammatory and antidiabetic activities. Results showed that all dried samples have high unsaturated fatty acids contents (up to 83%) and high total dietary fiber contents (above 50%). Also, one hundred grams of dried berries provide between 11 and 21% of the recommended daily intake of α-tocopherol. Moreover, all dried maqui extracts reduced topical inflammation in treated mice. The highest anti-inflammatory effect against phorbol 12-myristate 13-acetate was found for VD and FD samples. Also, all dried maqui extracts showed antidiabetic activity by inhibiting α-glucosidase activity. The highest α-glucosidase inhibition activity was found for FD samples. The different biological activities of the dried maqui berries were related to differences in the extractability of metabolites due to microstructural changes during drying. The results indicated the potential use of dried maqui as a food ingredient with high unsaturated fatty acids, dietary fiber and α-tocopherol or as a natural extract with therapeutic agents.
Keywords: Aristotelia chilensis (Mol.) Stuntz, Anti-inflammatory, α-Glucosidase, Functional food, Bioactive compounds
Introduction
Among the so-called “superfruits”, berries are attracting interest for their numerous beneficial health effects associated with their chemical composition. Maqui berries (Aristotelia chilensis (Mol.) Stuntz) is a tree or shrub species native to Chile. The plant yields small and edible purple-black berries about 5 mm in diameter that contain one to eight angular seeds (Brauch et al. 2017). These small berries contain high unsaturated fatty acids content (up to 86%) as well as micronutrients and minerals in its seeds (Brauch et al. 2016). The high concentration of phytochemicals, especially anthocyanins present in these berries has been associated with potential health benefits (Cespedes et al. 2010; Gironés-Vilaplana et al. 2014). Cespedes et al. (2010) suggested that the flavonoids, phenolic acids and anthocyanins present in maqui may be responsible of the excellent anti-inflammatory activities, including skin inflammatory attenuation by inhibiting the induced inflammation by an irritant agent. Gironés-Vilaplana et al. (2014) reported higher α-glucosidase and lipase inhibition activities in maqui fruits when compared with other berries such as açaí and cape gooseberry. In this context, maqui berries is considered a superfood with potential innovative food and pharmacological applications. Currently, the demand for maqui berries is satisfied by manual collection from the wild. However, fresh fruits are quickly degraded under ambient conditions. Therefore, fruit stabilization after harvest can increase the shelf-life of these berries. One of the most frequently used stabilization processes is dehydration, where a large amount of water is removed to increase stability against food degradation (Lucas et al. 2018). However, dehydration can have a negative impact on fruit appearance, chemical composition and biological activity. For example, anthocyanins present in maqui berries are easily degraded when exposed to high temperature, light or oxygen (Lucas et al. 2018). Thus, both drying methods and drying conditions should be carefully selected to ensure retention of the maximum quantity of bioactive compounds with health promoting properties.
There are previous studies on the effects of drying on maqui composition (Brauch et al. 2016; Quispe-Fuentes et al. 2018). However, there are no studies on the effects of different drying methods on the anti-inflammatory and anti-diabetic potential activity of maqui fruits. Therefore, the aim of this study was to evaluate the effects of different drying methods (freeze-, convective-, sun-, infrared- and vacuum-drying) on chemical composition, anti-inflammatory and α-glucosidase activities of maqui fruits.
Materials and methods
Maqui samples and drying process
Maqui (Aristotelia chilensis) fruits were harvested from forests close to Mulchen in the Bio-Bio Region of Chile. Fruits were selected to provide a homogeneous group by removing stems and leaves. Maqui fruits were spread in single layer for drying in different dryers. Five drying methods were employed to process maqui fruits until their weight became constant. The final moisture values were 8.77, 8.30, 4.70, 9.50 and 7.66% on the wet basis for freeze-, convective-, sun-, infrared- and vacuum-drying, respectively. Detailed procedures for each drying method are listed below:
Freeze drying (FD)—Samples were prior frozen at − 80 °C and then dried in a freeze dryer (Virtis, AdVantage Plus, Gardiner, NY, USA) at a vacuum pressure of 0.027 kPa for 40 h with a primary drying step at − 40 to 10 °C, a condenser set point at − 60 °C and a secondary drying step at 20 °C (final plate temperature).
Convective drying (CD)—Samples were dried for 4.5 h in a convective dryer constructed by the Department of Food Engineering at University of La Serena, Chile operated at 60 ± 2 °C with constant air circulation of 2.0 ± 0.2 m/s.
Sun drying (CD)—Samples were dried for 72 h in the Elqui Valley (Coquimbo Region, Chile) during February of 2015 on an open glass recipient (90 × 60 × 40 cm). The variable drying conditions, at the site of the assays with about 18 h sunshine, were recorded with a data logger (Lascar EL-USB-2, Whiteparish, England) with temperature ranging from 31.0 to 49.9 °C and air humidity from 20 to 45%.
Infrared drying (IR)—Samples were dried for 4.5 h in an IR-oven (TEKA, HT490, Velen, Germany) at 60 °C without air flow. The oven inside contained 900-Watt IR tubular heaters mounted on upper part. The distance between the IR source and the samples was fixed at 18 cm.
Vacuum drying (VD)—Samples were dried for 4 h in a vacuum oven (Memmert, VO 400, Schwabach, Germany) set at 60 °C and vacuum was maintained at 15 Kpa
The dried samples by the different methods were frozen with liquid nitrogen (1:2, w/v) and milled using a basic analytical mill (IKA® A-11, Wilmington, Delaware, USA). Samples were packaged in low-density polyethylene bags and stored at − 20 °C until analyzed.
Determination of fatty acid composition
The methyl ester preparation and analysis were carried out in accordance with International Union of Pure and Applied Chemistry method (IUPAC 1987) numbers 2.301 and 2.302 modified by Mendoza et al. (2000). Oil sample of maqui were extracted using the Bligh and Dyer (1959) method. The methyl esters of the fatty acids were prepared from the oil sample by saponification with KOH/methanol (2 N) and were agitated in vortex for 1 min. N-hexane was utilized for extraction of methyl fatty acid (300 µL) and was agitated for 3 min. Then the supernatant was injected into a gas chromatograph (Agilent Technologies 6890N model Network GC System) fitted with flame-ionization detector. The conditions, according to Aranda et al. (2006), include oven temperature of 50 °C for 1 min and an increase in temperature of 25°/min to 175 °C and then 4 °C/min to 230 °C during 5 min. Both the detector and the injector temperatures were fixed at 250 °C. The solution of methyl esters (1.0 µL) was injected into the DB-23 (60 m × 0.25 mm ID, 0.15 μm) column. A FAME-37 standard (Supelco, Sigma) was compared with the respective retention times.
Determination of dietary fiber
Dried maqui samples were analyzed for soluble and insoluble dietary fiber fractions according to a gravimetric enzymatic method (Lee et al. 1992) using a total dietary fiber assay kit (TDF100A; Sigma-Aldrich, St. Louis, MO) and an enzymatic digestion unit and filtration system (VELP Scientifica, GDE—CSF6, Usmate, Italy). Total dietary fiber was calculated as the sum of soluble and insoluble dietary fiber and expressed as g/100 g dry matter (d.m.). All measurements were performed in triplicate.
Determination of α-tocopherol
The content of tocopherols in fresh and dehydrated maqui was determined by mixing 20.0–30.0 g of sample with 150 mL of hexane. The mixture was treated with ultrasound for 15 min and shaken on a magnetic stirrer (OS-100, HiLab, Indonesia) at 280 rpm for 60 min. The mixture was allowed to stand for 24 h prior to centrifugation at 4193×g for 5 min. The supernatant was filtered (Whatman filter paper #1) and concentrated in a rotary evaporator (Büchi RE 121, Switzerland) at 40 °C. The extract was brought to 5 mL with methanol/BHT and passed through a 0.22 μm filter. The identification and quantification of the tocopherols was performed using HPLC-Fluorescence. The column was a Kromasil 100-5 C18 (250 × 4.6 mm), the mobile phase was a solution of methanol: acetonitrile (1: 1 v/v) at a flow of 1.2 mL/min and the wavelengths were 295 nm and 325 nm for excitation and emission, respectively. All measurements were determined in triplicate and the tocopherol content was expressed in mg α-tocopherol/kg d.m.
Maqui extracts for anti-inflammatory and α-glucosidase activities
The maqui extracts for anti-inflammatory and α-glucosidase analyses were carried out according to optimal extraction conditions described by Quispe-Fuentes et al. (2018). All extractions were performed in triplicate.
Determination of anti-inflammatory activity
The anti-inflammatory activity of maqui extracts was carried out according to Valenzuela-Barra et al. (2015). Adult male CF-1 mice (20–25 g) obtained from the stock at the Chilean Public Health Institute (ISP) were used. Dried maqui extracts (dissolved in acetone) were applied topically to the inner and outer surfaces of the right ear of each mouse (3 mg/ear). Likewise, 10 μL of a solution of phorbol 12-myristate 13-acetate (TPA; 5 μg in 20 μL acetone) or arachidonic acid (AA; 2 mg in 20 μL acetone) were applied immediately after maqui extracts application. Negative control mice received only TPA or AA at the same concentration (100% of inflammation). Left ear received only acetone. Indomethacin (0.5 mg/ear/20 μL) was used as reference drug for TPA and nimesulide (1.0 mg/ear/20 μL) for AA, respectively. After 6 h (TPA administration) or 1 h (AA administration), the animals were sacrificed by CO2 asphyxiation. Disks of 6 mm diameter were removed from each ear and the weight was determined. The edema was measured as the median values difference of the weights between the punches from right and left ears of the negative control and the treated animal groups, respectively. Experiments were performed in accordance with the current “Guidelines on the care and use of animals for scientific purpose” and approved by the Animal Care and Use Committee of the Facultad de Ciencias Químicas y Farmacéuticas of Universidad de Chile and ISP (code of approval CBE201707).
Determination of α-glucosidase activity
Effect of maqui extracts on α-glucosidase activity were measured in a range varying concentrations, according to method of Lordan et al. (2013), slightly modified. A volume of 50 μL of extract solution and 100 μL α-glucosidase from Saccharomyces cerevisiae (Sigma G5003, USA) solution (0.5 U/mL) in 0.1 M sodium phosphate buffer (pH 6.9) was mixed in a 96-well microplate and incubated at 25 °C for 10 min. Phosphate buffer containing 50 μl of 4-nitrophenyl α-d-glucopyranoside (Sigma N1377, Switzertand) was then added to each well. Absorbance at 405 nm was recorded every 30 s for 10 min using a Victor3 Multilabel Plate Reader set to 20 °C (Perkin–Elmer, Turku, Finland). α-Glucosidase activity was calculated as the percentage obtained through the slope of each exponential curve. Also, the inhibitory activity (%) against α-glucosidase versus maqui extracts was plotted. An exponential model was used to fit data so that IC50 was calculated as the concentration of seaweed extract required to produce a 50% of α-glucosidase inhibition.
Determination of microstructure
The microstructure of dried maqui berries was examined using scanning electron microscopy (SEM). Dried maqui berries were rehydrated overnight in distilled water at 5 °C to allow visualization of the parenchyma tissue. The maqui berries were removed from the rehydration solution, dabbed onto Kim wipes to remove excess moisture and mounted onto a copper sample holder with Tissue-Tek adhesive (Sakura Finetek USA Inc., Torrance, Cal., USA). The sample was then prepared for low temperature SEM using an Alto 2500 cryo system (Gatan Inc., Pleasanton, Cal., USA). The sample holder was attached to the rod of a vacuum transfer device and plunged into super-chilled liquid nitrogen. The specimen was evacuated, pulled up into the vacuum transfer device and transferred to a cryo preparation chamber. The specimen was fractured in the cryo preparation chamber at approximately − 180 °C, warmed to − 95 °C and held at that temperature for 5–10 min to remove excess surface water. The specimen was then cooled by shutting off the heater in the cryo system to less than − 135 °C, sputter coated with gold palladium and transferred to the cryo stage specimen chamber in the SEM. All samples were observed and photographed below − 135 °C at 2.0 kV using a Hitachi S-4700 field emission SEM (Hitachi High-Technologies Corp., Tokyo, Japan). Digital images were collected at 2180 × 960 pixels and were viewed under the scanning electron microscope.
Statistical analysis
The experiments in the present study were conducted in triplicate and the data points are mean values ± standard deviation (SD). When the statistical distribution was normal, one-way ANOVA followed by Tukey’s multiple range test were conducted. When the statistical distribution was not normal, the Kruskal–Wallis non-parametric test was applied. Mann–Whitney test was used for the individual comparisons. Significance of differences was defined at the 5% level (p < 0.05) in all tests. The software employed for statistical analysis was Statgraphics Centurion XVI (Statistical Graphics Corp., Herdon, USA).
Results and discussion
Fatty acid composition
The fatty acid profile (% weight of methyl esters), total saturated fatty acids (SFA), total monounsaturated fatty acids (MUFA), total polyunsaturated fatty acids (PUFA) and the PUFA/SFA ratios are presented in Table 1. Eleven different types of fatty acids, including six SFAs, two MUFAs and three PUFAs, were identified in the fresh and dried maqui regardless of the drying method. The major fatty acid in the fresh maqui sample was C18:2 (n-6), accounting for 45.41% of totals fatty acids, followed by C18:1 (n-9) (34.92%), C16:0 (9.49%), C18:0 (2.92%), and C18:3 (n-3) (2.12%). Other fatty acids were detected at percentages lower than 0.7%. The fatty acid composition of fresh maqui had been previously reported by Brauch et al. (2016), who also found that the major fatty acid was C18:2 (n-6) (46.00%), followed by C18:1 (n-9) (33.49%). After drying, the content of most individual fatty acids in the samples differed significantly from each other (Table 1). The total SFA contents ranged from 13.66 to 14.40%, with the highest percentage in CD- and SD-prepared maqui and the lowest in fresh maqui. Significant differences existed in the MUFA and PUFA contents of the dried maqui samples when compared with fresh maqui. The total MUFA content in dried maqui, regardless of the drying method, increased slightly, ranging from 35.38 to 36.10% of the total fatty acid content. In comparison, the total PUFA content decreased slightly, ranging from 46.72 to 47.66%. In summary, the lipid fraction from maqui was found to be highly unsaturated (up to 83%) with high PUFA/SFA ratios ranging from 3.24 to 3.50. Increased consumption of foods with a high PUFA/SFA ratio are associated with a lower risk of coronary heart diseases. Thus, the PUFA/SFA ratio is one of the parameters used to assess the nutritional quality of the lipid fraction in foods (Chan and Matanjun 2017). These results agreed quite well with those previously reported by Brauch et al. (2016), who found PUFA/SFA ratios ranging from 3.2 to 3.5 in maqui samples (fresh, juice, pomace and dried).
Table 1.
Composition of fatty acids in fresh and dry maqui
| Fatty acid (g/100 g of sample) | Drying methods | |||||
|---|---|---|---|---|---|---|
| Fresh | FD | CD | SD | IRD | VD | |
| Lauric acid C12:0 | 0.27 ± 0.01a | 0.29 ± 0.00b | 0.31 ± 0.00d | 0.29 ± 0.01b,c | 0.27 ± 0.00a | 0.30 ± 0.01c |
| Myristic acid C14:0 | 0.66 ± 0.01a,b | 0.68 ± 0.01b | 0.71 ± 0.00c | 0.68 ± 0.01b | 0.66 ± 0.01a | 0.68 ± 0.00b |
| Palmitic acid C16:0 | 9.49 ± 0.04a | 9.79 ± 0.16b | 9.89 ± 0.06b | 9.92 ± 0.05b | 9.48 ± 0.03a | 9.78 ± 0.02b |
| Stearic acid C18:0 | 2.92 ± 0.01a | 3.12 ± 0.03c | 3.07 ± 0.01b | 3.14 ± 0.01c | 2.94 ± 0.02a | 3.09 ± 0.01b |
| Arachidic acid C20:0 | 0.21 ± 0.01a | 0.22 ± 0.01a | 0.21 ± 0.01a | 0.25 ± 0.01b | 0.22 ± 0.01a | 0.22 ± 0.00a |
| Behenic acid C22:0 | 0.12 ± 0.08a | 0.11 ± 0.01a | 0.18 ± 0.09a | 0.13 ± 0.00a | 0.11 ± 0.02a | 0.13 ± 0.00a |
| Total SFA | 13.66 ± 0.08a | 14.09 ± 0.17b | 14.40 ± 0.14c | 14.40 ± 0.04c | 13.68 ± 0.04a | 14.20 ± 0.03b |
| Palmitoleic acid C16:1 (n-7) | 0.33 ± 0.01a | 0.33 ± 0.01a | 0.34 ± 0.00b | 0.33 ± 0.00a | 0.33 ± 0.01a,b | 0.33 ± 0.00a |
| Oleic acid C18:1 (n-9) | 34.92 ± 0.06a | 35.78 ± 0.16e | 35.34 ± 0.05c | 35.51 ± 0.06d | 35.04 ± 0.07b | 35.55 ± 0.06d |
| Total MUFA | 35.25 ± 0.07a | 36.10 ± 0.17d | 35.68 ± 0.05b | 35.84 ± 0.06b,c | 35.38 ± 0.08a | 35.88 ± 0.06c,d |
| Linoleic acid C18:2 (n-6) | 45.41 ± 0.05a | 44.63 ± 0.25b | 44.40 ± 0.07c | 44.25 ± 0.02c | 45.42 ± 0.03a | 44.43 ± 0.03c |
| Alpha-linolenic acid C18:3 (n-3) | 2.12 ± 0.03b | 2.24 ± 0.23c | 2.34 ± 0.04d | 2.12 ± 0.01b | 1.95 ± 0.06a | 2.15 ± 0.03b |
| Eicosapentaenoic acid C20:5 (n-3) | 0.26 ± 0.24a | 0.18 ± 0.16a | 0.31 ± 0.01a | 0.35 ± 0.02a | 0.29 ± 0.02a | 0.20 ± 0.17a |
| Total PUFA | 47.78 ± 0.32a | 47.06 ± 0.64b,c | 47.05 ± 0.12b | 46.72 ± 0.05d | 47.66 ± 0.11a | 46.78 ± 0.23c,d |
| PUFA/SFA | 3.50 | 3.34 | 3.27 | 3.24 | 3.49 | 3.29 |
Values are expressed as mean ± standard deviation
FD freeze drying, CD convective drying, SD solar drying, IRD infrared drying, VD vacuum drying, SFA saturated fatty acids, MUFA monounsaturated fatty acids, PUFA polyunsaturated fatty acids
Different letters in the same row indicate significant differences (p < 0.05) according to multiple range test (MRT). Standard deviation was calculated on three replicates
Dietary fiber content in dried maqui
Table 2 shows the dietary fiber fractions (TDF, IDF and SDF) of dried maqui berries. The TDF values ranged from 53.3 to 64.5 g/100 g d.m. with IDF (50.8–58.2 g/100 g d.m.) values making up to 90% of the TDF values. The high IDF content in the samples might be due to the high fruit pulp:seed ratio of 1:1 (w/w) in fresh berries. The TDF contents in maqui berries are similar to those of gooseberry, Royal apple and Eureka lemon (Figuerola et al. 2005; Vega-Gálvez et al. 2015).
Table 2.
Dietary fiber contents of dried maqui
| Parameters (g/100 g d.m.) | Drying methods | ||||
|---|---|---|---|---|---|
| FD | CD | SD | IRD | VD | |
| IDF | 50.85 ± 1.33b | 58.25 ± 4.41a | 54.59 ± 2.84a,b | 54.70 ± 0.10a,b | 57.66 ± 0.06a |
| SDF | 2.46 ± 0.60b | 6.22 ± 0.29a | 6.26 ± 0.12a | 5.93 ± 0.73a | 5.96 ± 0.65a |
| TDF | 53.31 ± 0.73b | 64.48 ± 4.12a | 60.86 ± 2.72a | 60.60 ± 0.83a | 63.63 ± 0.71a |
Values are expressed as mean ± standard deviation
FD freeze drying, CD convective drying, SD solar drying, IRD infrared drying, VD vacuum drying, IDF insoluble dietary fiber, SDF soluble dietary fiber, TDF total dietary fiber
Different letters in the same row indicate significant differences (p < 0.05) according to multiple range test (MRT). Standard deviation was calculated on three replicates
The drying method had no significant effect on the dietary fiber fractions except for FD samples, which showed lower values for all dietary fiber fractions (Table 2).
The effects of drying temperature on dietary fiber content and composition are not clear. Garcia-Amezquita et al. (2018) reported that high-temperature processing may hydrolyze structural pectin or protopectin and convert them into soluble pectins, leading to an increase in SDF values. High temperature processing may also cause a partial degradation of cellulose and hemicellulose into simpler carbohydrates, thereby decreasing the IDF values. Dietary fiber food ingredients should contain more than 50% TDF, have low moisture and lipid contents and have low caloric value (Larrauri 1999). Therefore, dried maqui berries might be a potentially interesting dietary fiber source.
α-Tocopherols content in maqui
The levels of α-tocopherol in fresh and dried maqui extracts are shown in Fig. 1. A small amount of α-tocopherol was detected in the fresh fruits extracts (4.5 ± 0.1 μg/g d.m.). Similar values were reported in several varieties of grape seeds (Tangolar et al. 2011). In comparison, raspberries, blackberries and some grape varieties had higher α-tocopherol contents than fresh maqui (Adhikari et al. 2008; Tangolar et al. 2011; Xu et al. 2006). All the drying methods significantly increased α-tocopherol content. Freeze drying resulted in a 6.1-fold increase in α-tocopherol content (31.7 ± 0.5 μg/g d.m.) compared to the fresh fruits, followed by CD (24.8 ± 0.6 μg/g d.m.), VD (23.7 ± 0.4 μg/g d.m.), IRD (23.4 ± 0.3 μg/g d.m.) and SD (17.1 ± 0.8 μg/g d.m.). Gao et al. (2012) also found that α-tocopherol content increased significantly in both freeze-dried and oven-dried jujubes. Similarly, Garcia-Martinez et al. (2012) found that α-tocopherol content in apricot significantly increased after hot air drying at both 40 and 60 °C. Heating had been shown to enhance the release of bound compounds, resulting in higher contents after processing (Leong and Oey 2012). The results in this study also suggested that heating to the experimental temperatures did not degrade α-tocopherol. The effects of drying might be related with changes to the maqui microstructure after drying (Fig. 3), which is discussed later in this paper. FD, VD and CD resulted in a porous structure with higher surface area, which might have improved the yield of extractable α-tocopherol.
Fig. 1.
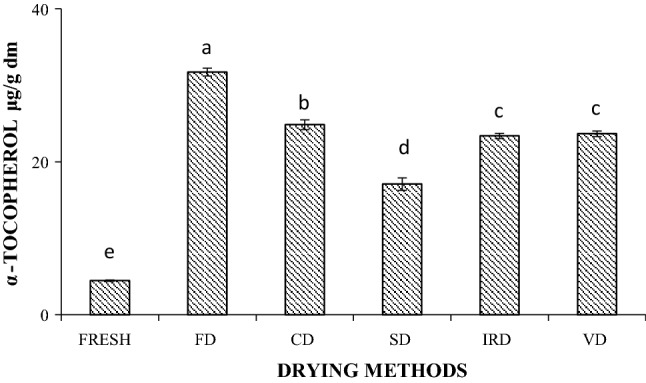
α-Tocopherol content in fresh and dry maqui. FD freeze drying, CD convective drying, SD sun drying, IRD infrared drying, VD vacuum drying. Values are averages (n = 3), error bars are standard deviation
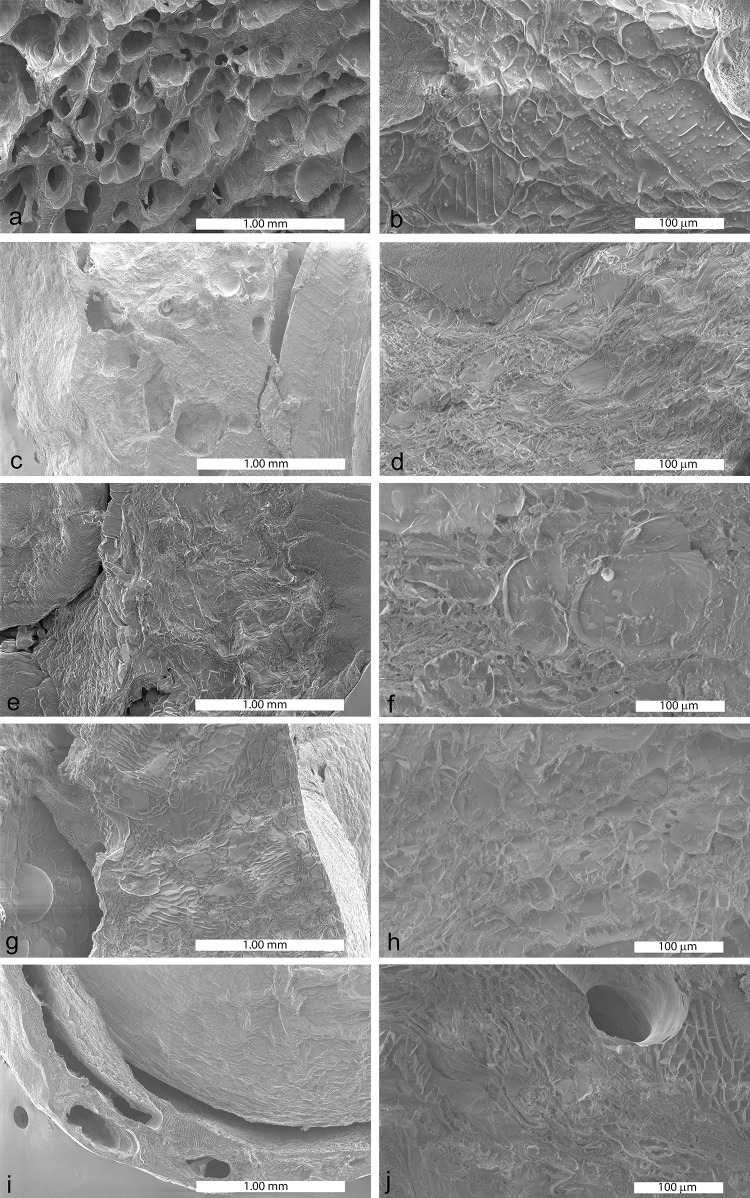
Fig. 3.
Representative microscopic images of dried maqui by different methods. a and b freeze drying; c and d convective drying; e and f sun drying; g and h infrared drying; i and j vacuum drying
Anti-inflammatory activity of maqui extracts
Table 3 shows the anti-inflammatory effect of extracts obtained from fresh and dried maqui by different drying methods on ear edema in mice induced by topical administration of two phlogistic agents, phorbol 12-myristate 13-acetate (TPA) and arachidonic acid (AA). Topical applications of both phlogistic agents to mice ears provide a chronic inflammation model suitable for evaluation of anti-inflammatory agents (Xian et al. 2012). TPA induces a longer-lasting skin edema associated to protein kinase C activation with the subsequent nuclear factor kappa B (NF-κB) activation (Miño et al. 2004; Ban et al. 2009). This transcription factor promotes the over production of inflammatory mediators such as tumor necrosis factor-alpha (TNF-α), interleukins (IL-1b and IL-6) and cyclooxygenase 2 (COX-2) (Xian et al. 2012). In contrast, inflammation induced by AA irritant agent is related to an increased activity of myeloperoxidase and elastase (Ban et al. 2009). It has been established that this agent generates a rapid onset short-lived edema associated to increases in prostaglandins (PGs), thromboxane TXB2 and leukotriene LTB4 with leukocyte extravasation (Griswold et al. 1991). In this present study, the fresh maqui extract at a dose of 3 mg/ear was able to produce a moderate anti-inflammatory activity in TPA-induced mice ear edema (20.2% inhibition) while was ineffective in the ear edema induced by AA suggesting that the extracts act previous to metabolization of the AA (Miño et al. 2004). However, drying treatment (except with CD) significantly increased (p < 0.05) the anti-inflammatory effectiveness of extracts compared to the fresh maqui extract. As result, the mice treated with dried maqui extracts obtained from VD and FD methods produced the highest effect anti-inflammatory against TPA (54.4 and 51.8% inhibition, respectively). On the other hand, dried maqui extracts by the different methods achieved only a moderate but significant effect anti-inflammatory in the AA test (among 11.3 and 26.3% inhibition). In comparison, indomethacin (0.5 mg/ear; positive control to TPA) and nimesulide (1.0 mg/ear; positive control to AA) exhibited the maximum effect anti-inflammatory (93 and 49% inhibition, respectively) on their respective inflammatory model.
Table 3.
Topical anti-inflammatory effects of maqui fruit extracts against phorbol 12-myristate 13-acetate (TPA)- and arachidonic acid (AA)-induced inflammation of mice ear oedema
| Drying methods | Topical anti-inflammatory effects | ||
|---|---|---|---|
| Dose (mg/ear) | %EATPA ± SEM | %EAAA ± SEM | |
| Fresh | 3 | 20.2 ± 3.8*,d,α | 8.3 ± 4.0e,α |
| FD | 3 | 51.8 ± 5.9*,b,α | 18.3 ± 3.8*,b,c,α |
| CD | 3 | 17.7 ± 4.2*,d,α | 15.0 ± 3.6*,c,α |
| SD | 3 | 28.2 ± 4.5*,c,α | 26.3 ± 4.6*,b,α |
| IRD | 3 | 33.1 ± 6.0*,c,α | 11.3 ± 4.0c,e,α |
| VD | 3 | 54.4 ± 5.3*,b,α | 18.8 ± 3.8*,b,α |
| IND | 0.5 | ↑92.9 ± 10.2*,a | n.d |
| NM | 1 | n.d | ↑48.8 ± 4.0*,a |
↑maximum effect of reference drugs; FD freeze drying, VD vacuum drying, IRD infrared radiation drying, CD convective drying, SD sun drying, EATPA topical anti-inflammatory effect against phorbol 12-myristate 13-acetate, EAAA topical anti-inflammatory effect against arachidonic acid, IND indomethacin, NIM nimesulide; n.d not determined
An asterisk (*) on a column denotes significant differences (p ≤ 0.05) between samples respect to the negative control (100% of inflammation); a–d values in the same column with different superscripts indicate significant differences (p < 0.05) for each sample. An alpha (α) on a column represents significant differences between the samples and the reference drug (NIM or IND)
Overall, the anti-inflammatory effect observed with the dried maqui extract by different methods may be attributable to their appreciable amounts of flavonoids, particularly anthocyanins. In our previous study (Quispe-Fuentes et al. 2018), we identified in the maqui extract the presence of eight anthocyanins, where delphinidin derivatives predominated in all dried samples by different methods (especially in VD- and FD-samples). Cespedes et al. (2010) reported that all extracts, fractions and purified sub-fractions from maqui produced a significant inhibition in TPA-induced mice ear edema in a dose dependent manner. For instance, they showed that the effect anti-inflammatory of the sub-fraction SF21-SF25 (delphinidin derivatives) was comparable even to used reference drug (indomethacin). In other study on anthocyanin-rich berries such as blueberries, chokeberries, black raspberries, bilberries, cranberry and strawberries indicated that consuming of these berries in different forms (i.e. fresh, juice, extracts, powder) reduce inflammatory stress in humans (Joseph et al. 2014).
α-Glucosidase activity of dried maqui extracts
Bioactive substances that retard the absorption of glucose by inhibiting carbohydrate-hydrolyzing enzymes, such as α-glucosidase, had been widely employed to treat type 2 diabetes (Lordan et al. 2013). The α-glucosidase activity of dried maqui extracts is presented in Fig. 2. Dried maqui extracts exhibited a dose-dependent inhibitory effect on α-glucosidase that ranged from 0.5 to 7.0 mg/mL. Also, the drying method had a significant effect on α-glucosidase activity. For example, 3 mg/ml of FD maqui extract completely inhibited α-glucosidase, whereas 7 mg/mL of SD maqui was required to completely inhibit α-glucosidase.
Fig. 2.

Each curve is a representative exponential curve for each drying method at different concentrations. FD freeze drying, VD vacuum drying, SD sun drying, CD convective drying, IRD infrared drying. Values are averages (n = 3), error bars are standard deviation
The FD samples had the highest α-glucosidase inhibiting activity, which was expressed as the lowest IC50 values (0.315 ± 0.046 mg/mL). In comparison, SD maqui extracts had the highest IC50 value (0.961 ± 0.148 mg/mL). These results might be due to differences in microstructural changes during drying that affected extractability of metabolites involved in α-glucosidase inhibition activity (Fig. 3). Voda et al. (2012) suggested that ice crystals formed during freezing drying can rupture, push and compress the cells, which aids in releasing phenolic compounds. Also, FD samples had the highest delphinidin and cyanidin derivatives (73% and 64% respectively) when compared with samples dried using other methods (Quispe-Fuentes et al. 2018). These polyphenolic compounds have been reported as the best α-glucosidase inhibitors among the flavonoids (Romanucci et al. 2016). Rojo et al. (2012) reported that anthocyanins from Aristotelia chilensis improve fasting blood glucose levels and glucose tolerance in obese diabetic mice. Also, Karaman et al. (2014) found that polyphenolic compounds inhibited α-glucosidase activity due to the formation of hydrogen bonds between phenolic groups and enzymatic polar groups. In conclusion, extracts that possess α-glucosidase inhibitory activities could be prepared from dried maqui berries, with the most effective prepared using freeze drying.
Microstructural analysis
Figure 3 shows cross-sectional SEM images of maqui berries using different drying methods.
In general, the microstructural changes in the berries developed during moisture migration from the cells. This led to loss of cell turgor, shrinkage and structural collapse at different levels. From Fig. 3, the freeze-dried samples had the highest porosity. The cellular structure had a honeycomb-like appearance with many small pores. These pores were formed after ice crystals sublimated during the freeze drying process. Also, the cellular structure of FD samples was better preserved when compared with the other samples. VD resulted in the formation of large voids in the parenchyma tissue of the berries. These large pores were created during vapor expansion under vacuum and an increased evaporation rate due to the lower boiling point (Ngamwonglumlert and Devahastin 2018). Samples dried by CD showed smaller intracellular spaces than the FD and VD samples. This was due to the low drying temperature (60 °C) that led to uniform shrinkage and a more homogenous appearance of the parenchyma tissue. In comparison, samples dried by IRD and SD showed a compact and collapsed structure with dense areas and lower porosity. SD, IRD and VD samples did not have distinct cells and intercellular spaces since drying might have caused the flow of the vacuolar sap from the cells outwards. Water and solutes then mix together to form the continuous tissue observed in these micrographs. Link et al. (2017) also found that FD mango had a spongy structure with many small pores, whereas CD mango had a compact structure with smaller intracellular spaces due to uniform and pronounced shrinkage. In addition, Yi et al. (2016) observed homogenous honeycomb structures in FD pears, but dense and non-porous structures in CD pears.
The more open, spongy structure in FD samples as well as the presence of voids and pores in VD and CD samples might have led to easier extraction of bioactive compounds and the subsequent higher bioactivities (Tables 2, 3). In contrast, the compact structures of the SD and IRD samples might have hindered the extraction of bioactive compounds.
Conclusion
The present study provided useful information on the enormous potential of dried maqui as a “superfruit” that is rich in unsaturated fatty acids, dietary fiber and α-tocopherol and their use as a natural drug with therapeutic agents. However, the drying method will affect the composition and biological activities of the dried maqui. Overall, freeze drying was found to be the most desirable drying method. FD maqui berries have 53% dietary fiber and a lipid fraction with high unsaturation (up to 83%) and high PUFA/SFA ratio (3.34). It also had the highest α-tocopherol content (21% of the RDI in 100 grams). FD maqui extracts also showed high anti-inflammatory activity against phorbol 12-myristate 13-acetate (TPA) and arachidonic acid (AA). In addition, FD maqui extracts exhibited the highest inhibition of α-glucosidase activity.
Acknowledgements
The authors appreciate financial support from the Food Engineering Department of University of La Serena and CONICYT-PCHA/National Doctorate/2016-21161653.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adhikari P, Hwang KT, Shin MK, Lee BK, Kim SK, Kim SY, Lee KT, Kim SZ. Tocols in caneberry seed oils. Food Chem. 2008;111:687–690. doi: 10.1016/j.foodchem.2008.04.038. [DOI] [Google Scholar]
- Aranda M, Mendoza N, Villegas R. Lipid damage during frozen storage of whole jack mackerel (Trachurus symmetricus murphyi) J Food Lipids. 2006;13(2):155–166. doi: 10.1111/j.1745-4522.2006.00041.x. [DOI] [Google Scholar]
- Ban J, Oh JH, Kim DJ, Jeong H-S, Han SB, Hong JT. Antiinflammatory and arthritic effects of thiacremonone, a novel sulfur compound isolated from garlic via inhibition of NF-κB. Arthritis Res Ther. 2009;11:1–13. doi: 10.1186/ar2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh E, Dyer W. A rapid method of total extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brauch JE, Buchweitz M, Schweiggert RM, Carle R. Detailed analyses of fresh and dried maqui (Aristotelia chilensis (Mol.) Stuntz) berries and juice. Food Chem. 2016;190:308–316. doi: 10.1016/j.foodchem.2015.05.097. [DOI] [PubMed] [Google Scholar]
- Brauch JE, Reuter L, Conrad J, Vogel H, Schweiggert RM, Carle R. Characterization of anthocyanins in novel Chilean maqui berry clones by HPLC–DAD ESI/MSn and NMR-spectroscopy. J Food Compost Anal. 2017;58:16–22. doi: 10.1016/j.jfca.2017.01.003. [DOI] [Google Scholar]
- Cespedes CL, Alarcon J, Avila JG, Nieto A. Anti-inflammatory activity of Aristotelia chilensis Mol. (Stuntz) (Elaeocarpaceae) Bol Latinoam Caribe Plant Med Aromat. 2010;9:91–99. [Google Scholar]
- Chan PT, Matanjun P. Chemical composition and physicochemical properties of tropical seaweed, Gracilaria changii. Food Chem. 2017;221:302–310. doi: 10.1016/j.foodchem.2016.10.066. [DOI] [PubMed] [Google Scholar]
- Figuerola F, Hurtado ML, Estévez AM, Chiffelle I, Asenjo F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005;91(3):395–401. doi: 10.1016/j.foodchem.2004.04.036. [DOI] [Google Scholar]
- Gao QH, Wu CS, Wang M, Xu BN, Du LJ. Effect of drying of jujubes (Ziziphus jujuba Mill.) on the contents of sugars, organic acids, α-tocopherol, β-carotene, and phenolic compounds. J Agric Food Chem. 2012;60:9642–9648. doi: 10.1021/jf3026524. [DOI] [PubMed] [Google Scholar]
- Garcia-Amezquita LE, Tejada-Ortigoza V, Serna-Saldivar SO, Welti-Chanes J. Dietary fiber concentrates from fruit and vegetable by-products: processing, modification, and application as functional ingredients. Food Bioprocess Technol. 2018;11:1439–1463. doi: 10.1007/s11947-018-2117-2. [DOI] [Google Scholar]
- Garcia-Martinez E, Igual M, Martin-Esparza ME, Martinez-Navarrete N. Assessment of the bioactive compounds, color, and mechanical properties of apricots as affected by drying treatment. Food Bioprocess Technol. 2012;6:3247–3255. doi: 10.1007/s11947-012-0988-1. [DOI] [Google Scholar]
- Gironés-Vilaplana A, Baenas N, Villaño D, Speisky H, García-Viguera C, Moreno DA. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J Funct Foods. 2014;7:599–608. doi: 10.1016/j.jff.2013.12.025. [DOI] [Google Scholar]
- Griswold DE, Marshall PJ, Lee JC, Webb EF, Hillegass LM, Wartell J, Newton J, Jr, Hanna N. Pharmacology of the pyrroloimidazole, SK&F 105809—II: anti-inflammatory activity and inhibition of mediator production in vivo. Biochem Pharmacol. 1991;42:825–831. doi: 10.1016/0006-2952(91)90042-4. [DOI] [PubMed] [Google Scholar]
- IUPAC . Preparation of fatty acid methyl ester In standard methods for analysis of oils, fats and derivatives. 7. Oxford: Blackwell; 1987. [Google Scholar]
- Joseph SV, Edirisinghe I, Burton-Freeman BM. Berries: anti-inflammatory effects in humans. J Agric Food Chem. 2014;62:3886–3903. doi: 10.1021/jf4044056. [DOI] [PubMed] [Google Scholar]
- Karaman S, Toker OS, Çam M, Hayta M, Doğan M, Kayacier A. Bioactive and physicochemical properties of persimmon as affected by drying methods. Dry Technol. 2014;32:258–267. doi: 10.1080/07373937.2013.821480. [DOI] [Google Scholar]
- Larrauri JA. New approaches in the preparation of high dietary fibre powders from fruit by-products. Trends Food Sci Technol. 1999;10(1):3–8. doi: 10.1016/S0924-2244(99)00016-3. [DOI] [Google Scholar]
- Lee S, Prosky L, De Vries J. Determination of total, soluble, and insoluble dietary fiber in foods: enzymatic–gravimetric method, MES-TRIS buffer: collaborative study. J Assoc Off Anal Chem. 1992;75:395–416. [Google Scholar]
- Leong SY, Oey I. Effects of processing on anthocyanins, carotenoids and vitamin C in summer fruits and vegetables. Food Chem. 2012;133(4):1577–1587. doi: 10.1016/j.foodchem.2012.02.052. [DOI] [Google Scholar]
- Link JV, Tribuzi G, Laurindo JB. Improving quality of dried fruits: a comparison between conductive, multi-flash and traditional drying methods. LWT Food Sci Technol. 2017;84:717–725. doi: 10.1016/j.lwt.2017.06.045. [DOI] [Google Scholar]
- Lordan S, Smyth TJ, Soler-Vila A, Stanton C, Ross RP. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013;141:2170–2176. doi: 10.1016/j.foodchem.2013.04.123. [DOI] [PubMed] [Google Scholar]
- Lucas BF, Zambiazi RC, Costa JAV. Biocompounds and physical properties of açaí pulp dried by different methods. LWT Food Sci Technol. 2018;98:335–340. doi: 10.1016/j.lwt.2018.08.058. [DOI] [Google Scholar]
- Mendoza N, Villegas R, Valladares J, Inzunza R. Modificaciones producidas a nivel de ácidos grasos durante el proceso de conservación del jurel. Revista Chile Pesquero. 2000;1:23–26. [Google Scholar]
- Miño J, Moscatelli V, Hnatyszyn O, Gorzalczany S, Acevedo C, Ferraro G. Antinociceptive and antiinflammatory activities of Artemisia copa extracts. Pharmacol Res. 2004;50:59–63. doi: 10.1016/j.phrs.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Ngamwonglumlert L, Devahastin S. 8-Microstructure and its relationship with quality and storage stability of dried foods. In: Devahastin S, editor. Woodhead Publishing series in food science, technology and nutrition. Cambridge: Woodhead Publishing; 2018. pp. 139–159. [Google Scholar]
- Quispe-Fuentes I, Vega-Gálvez A, Aranda M. Evaluation of phenolic profiles and antioxidant capacity of maqui (Aristotelia chilensis) berries and their relationships to drying methods. J Sci Food Agric. 2018;98:4168–4176. doi: 10.1002/jsfa.8938. [DOI] [PubMed] [Google Scholar]
- Rojo LE, Ribnicky D, Logendra S, Poulev A, Rojas-Silva P, Kuhn P, Dorn R, Grace MH, Lila MA, Raskin I. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis) Food Chem. 2012;131:387–396. doi: 10.1016/j.foodchem.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanucci V, D’Alonzo D, Guaragna A, Di Marino C, Davinelli S, Scapagnini G, Di Fabio G, Zarrelli A. Bioactive compounds of Aristotelia chilensis stuntz and their pharmacological effects. Curr Pharm Biotechnol. 2016;17(6):513–523. doi: 10.2174/1389201017666160114095246. [DOI] [PubMed] [Google Scholar]
- Tangolar S, Ozogul F, Tangolar S, Yagmur C. Tocopherol content in fifteen grape varieties obtained using a rapid HPLC method. J Food Compost Anal. 2011;24:481–486. doi: 10.1016/j.jfca.2010.08.003. [DOI] [Google Scholar]
- Valenzuela-Barra G, Castro C, Figueroa C, Barriga A, Silva X, de las Heras B, Hortelano S, Delporte C. Anti-inflammatory activity and phenolic profile of propolis from two locations in Región Metropolitana de Santiago, Chile. J Ethnopharmacol. 2015;168:37–44. doi: 10.1016/j.jep.2015.03.050. [DOI] [PubMed] [Google Scholar]
- Vega-Gálvez A, Zura-Bravo L, Lemus-Mondaca R, Martinez-Monzó J, Quispe-Fuentes I, Puente L, Di Scala K. Influence of drying temperature on dietary fibre, rehydration properties, texture and microstructure of Cape gooseberry (Physalis peruviana L.) J Food Sci Technol. 2015;52(4):2304–2311. doi: 10.1007/s13197-013-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voda A, Homan N, Witek M, Duijster A, van Dalen G, van der Sman R, Nijsse J, van Vliet L, Van As H, van Duynhoven J. The impact of freeze-drying on microstructure and rehydration properties of carrot. Food Res Int. 2012;49(2):687–693. doi: 10.1016/j.foodres.2012.08.019. [DOI] [Google Scholar]
- Xian Y-F, Lin Z-X, Xu X-Y, Su Z-R, Chen J-N, Lai X-P, Ip S-P. Effect of Rhizoma Polygonati on 12-O-tetradecanoylphorbol-acetate-induced ear edema in mice. J Ethnopharmacol. 2012;142:851–856. doi: 10.1016/j.jep.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhang Y, Chen M, Tu P. Fatty acids, tocopherols and proanthocyanidins in bramble seeds. Food Chem. 2006;99:586–590. doi: 10.1016/j.foodchem.2005.08.059. [DOI] [Google Scholar]
- Yi J, Zhou L, Bi J, Chen Q, Liu X, Wu X. Impacts of pre-drying methods on physicochemical characteristics, color, texture, volume ratio, microstructure and rehydration of explosion puffing dried pear chips. J Food Process Preserv. 2016;40:863–873. doi: 10.1111/jfpp.12664. [DOI] [Google Scholar]