Abstract
Melatonin is a neurohormone that regulates circadian rhythms in the human body. It can also be taken to alleviate insomnia and sleep disorders. Pasteurized milk is a good source of nutrients and some bioactive compounds. Recently, the growing trend of healthy foods has resulted in higher competition with regard to milk products. Functional milk has been developed with higher bioactive compounds to respond to consumer demand. High melatonin pasteurized milk was developed using selected edible grains and mulberry leaves to fortify melatonin in pasteurized milk. Melatonin and free tryptophan of fourteen edible grains and mulberry leaves were determined using HPLC-FD. Highest melatonin concentrations were observed in white sesame, sunflower and soybean (75.24, 67.45 and 56.49 ng/g dry weight (dw), with highest concentrations of free tryptophan in soybean, red bean and mung bean (2617.83, 1527.23 and 845.27 ng/g dw, respectively), while melatonin and free tryptophan contents in fresh mulberry leaves were 51.57 ng/g and 210.53 ng/g dw, respectively. Soymilk powder and mulberry leaf tea were supplemented to prepare high melatonin pasteurized milk. Results showed that chemical compositions, melatonin and free tryptophan contents significantly increased (P < 0.05) with increasing amounts of soymilk powder and mulberry leaf tea. Sensory evaluation gave highest overall liking score for the treatment consisting of mulberry leaf tea (4.00%), soymilk powder (4.00%) and milk (89.80%). Findings indicated that mulberry leaves and soybean are both good sources of melatonin and free tryptophan and can be applied to prepare high melatonin pasteurized milk.
Keywords: Melatonin, Tryptophan, Grain, Mulberry leaf tea, Pasteurized milk
Introduction
Melatonin (N-acetyl-5-methoxytryptamine), an endogenous neurohormone, is synthesized indirectly from tryptophan via serotonin. It was first discovered in the animal kingdom and is normally secreted from the pineal gland (Lerner et al. 1958). Rhythmic release of melatonin is regulated by the body’s circadian rhythm or sleep–wake cycle to ameliorate the effects of jet lag, enhance sleep and also act as an antioxidant (Cajochen et al. 2003). Due to its amphiphilic nature, melatonin permeates all cells and tissues and reacts with most free radicals to inhibit age-related diseases and cancer (Triantafillidis and Triantafillidis 2009). Melatonin is abundant in the plant kingdom and has been quantified in leaves, roots, shoots, bulbs and grains of most plant species ranging from picograms to micrograms per gram. High melatonin concentrations have been reported in Chinese herbs (Chen et al. 2003), edible grains (Manchester et al. 2000), mulberry leaves (Pothinuch and Tongchitpakdee 2011) and tropical fruits (Johns et al. 2013).
Melatonin has also been detected in food and beverage products including juices, beers, wines, bread and yogurt (Kocadağlı et al. 2014). However, melatonin concentration in plants depends on various factors including growth rates, cultivars, processing, and analysis techniques (Arnao and Hernández-Ruiz 2013; Pothinuch and Tongchitpakdee 2011) and environmental conditions such as high temperature and radiation. Evidence of melatonin uptake and antioxidant properties was demonstrated by chicks fed four kinds of cereals (corn, milo, beans and rice) or melatonin-rich food. Results showed increased melatonin levels in their blood (Hattori et al. 1995), while rats fed walnuts had significantly increased Trolox equivalent antioxidant capacity (TEAC) and ferric reducing antioxidant power assay (FRAP) in their serum (Reiter et al. 2005). Humans fed tropical fruit also recorded increased melatonin levels in plasma and decreased oxidative stress levels (Sae-Teaw et al. 2013).
Tryptophan is an essential amino acid and a precursor of melatonin synthesis via serotonin. Tryptophan is present in most protein-based foods and plays an important role as a sleep-inducing compound. Legumes and some cereals contain high quantities of protein as good sources of free and protein-bound tryptophan (Comai et al. 2007a, b). Increased melatonin synthesis may be influenced by ingesting foodstuffs containing tryptophan (Johns et al. 2013).
Milk is a natural food containing many different nutrients that are essential for human health (Alyaqoubi et al. 2014). With the growing trend of milk market value, milk factories must remain competitive through continuous innovation and production of new functional products that can respond to consumer demand for healthier food options. Numerous functional milk products have been developed that are high in nutrients and bioactive compounds with good characteristics and appearance as crucial factors when it comes to consumer preference (Corbo et al. 2014). Milk fortification with functional food ingredients can improve product characteristics, nutrients and bioactive compounds (Serafini et al. 2009) and melatonin has generated interest in this area. Milk contains melatonin in low concentrations at 0.004–0.056 ng/ml (Milagres et al. 2014; Valtonen et al. 2005) which may not be sufficient to promote relaxation and sleep quality; therefore, improvement or supplementation from other sources is needed. Melatonin has previously been reported in some edible plants but scant data exist concerning grains and leaves in tropical areas.
Here, melatonin and free tryptophan contents were determined in selected grains and mulberry leaf tea to develop high melatonin pasteurized milk. Suitable sources rich in melatonin and free tryptophan as grains and mulberry leaf tea were selected and prepared to supplement pasteurized milk. Chemical compositions, melatonin and free tryptophan contents of pasteurized milk were determined and different sensory attributes were evaluated. The most acceptable pasteurized milk treatment was selected for shelf life study.
Materials and methods
Chemicals and materials
Melatonin and tryptophan standards were purchased from Sigma-Aldrich Chemical Co., (St. Louis, MO, USA). Acetonitrile and ethanol (HPLC grade) were purchased from BDH (Poole, UK). Dichloromethane (analytical grade) was purchased from QRäC (New Zealand). Deionized water was obtained from Milli-Q Systems (Millipore Co., Bedford, MA, USA).
Mulberry leaves and grains have high nutritive values and contain bioactive compounds including melatonin and free tryptophan with high potential to improve milk products. Common grains as five rice, three corn, four legume, two oilseed cultivars and mulberry leaves were selected to determine melatonin and free tryptophan contents for high melatonin pasteurized milk development.
Rice cultivars harvested in 2016 were purchased from a local market in Maha Sarakham Province, Thailand. Samples were dehulled using a milling machine to obtain brown rice. Five cultivars were selected, comprising two samples of Oryza sativa var. glutinosa as glutinous rice (RD6) and black glutinous rice, and three samples of non-glutinous rice (Oryza sativa L.) including khao dawk mali 105, red dawk mali, and riceberry. Three sweet and waxy corn ears with different grain pigments including sweet corn (yellow color) (F1 hybrid top sweet 1320) (Zea mays L. var. saccharata) and waxy corn (white color) (F1 hybrid top white 365) (Zea mays ceratina) were purchased from a local market in Maha Sarakham Province, and wax purple corn (F1 hybrid waxy corn) cultivar (Zea mays L.) was sourced from Khon Kaen Province, Thailand. Harvesting time of sweet corn was 75–80 days with other cultivars 60–65 days. Corn grains were shredded and freeze-dried before use. Legumes and oilseeds including soybean [Glycine max (L.) Merrill], peanut (Arachis hypogaea L.), mung bean [Vigna radiata (L.) Wilczek], red bean (Phaseolus vulgaris L.), white sesame (Sesamum indicum L.) and sunflower seed (Helianthus annuus L.) were purchased from a supermarket in Maha Sarakham Province.
Mulberry leaves are an excellent source of phytochemicals. Melatonin is an important compound that exists in high concentrations in mulberry leaves, especially the Buriram 60 cultivar (Pothinuch and Tongchitpakdee 2011). With the increasingly popular trend of functional drinks, flavor is an important factor for consumer preference. To produce acceptable high melatonin pasteurized milk, mulberry leaves were selected and prepared as a tea for milk supplementation. Adding mulberry leaf tea to pasteurized milk can enhance the flavor and increase melatonin and free tryptophan contents. Mulberry (Morus spp.) leaves from Buriram 60 cultivar were obtained from a local farm in Maha Sarakham Province, Thailand. Leaves were collected from the top down at leaf positions 4–10.
Raw milk was supplied by Kokko Milk Factory, Maha Sarakham Province, Thailand.
Sample preparation
Good quality raw milk was collected after product inspection and stored in clean packages at 4 °C until required for analysis. Grain samples were cleaned and 20 g aliquots were ground using a coffee grinder (Cuisinart, DBM-8HK, USA), with grinding halted every 30 s to avoid excessively heating the samples. Ground samples were sieved through Mesh No. 20 (841 μm aperture) and stored in zip-lock plastic bags at − 20 °C prior to extraction. Mulberry leaves were prepared as green tea by chopping into small pieces (1.5 × 1.5 cm2) before pan-firing with mild heating at 45–50 °C for 30 min. After rolling, the leaves were dried by three different methods including solar drying for 6–7 h following the process of the local milk factory, freeze drying, and drying in a hot air oven at 120 °C for 20 min (Pothinuch and Tongchitpakdee 2011). Dried mulberry leaf tea was then packed in airtight plastic bags and stored at − 20 °C prior to analysis.
Soymilk powder preparation
Soybeans were selected and prepared as soymilk powder before adding to milk following the method of Jiang et al. (2013) with some modifications. Briefly, whole soybeans were cleaned and then soaked in water (1:3 w/v) for 8 h. After rinsing, the soybeans were blended in two stages, each using a 1:1 w/v ratio of soaked soybeans to water for o1 min at the lowest speed (No. 1) using a household blender (QH-900B1, Joyoung Co., Ltd., Hangzhou, China). The soymilk slurry was filtered through two layers of cotton cloth, 1% of maltodextrin (DE10) food grade was added as an anticaking agent and the mixture was freeze-dried (Heto Power Dry PL3000, Czech Republic). Dried soymilk powder was packed in zip-lock plastic bags and stored at − 20 °C prior to use.
Pasteurized milk preparation
Grains with high melatonin content, free tryptophan and suitable physical appearance for improving functional milk color were selected to develop high melatonin pasteurized milk. Here, soybeans and mulberry leaves were selected and prepared as soymilk powder and mulberry leaf tea. Mulberry leaf tea provided an acceptable green color; therefore, we expected the consumer to readily accept the high melatonin pasteurized milk.
A preliminary study determined 12% as maximum added level of soymilk powder and mulberry leaf tea (sun dried). Therefore, nine milk formulations were generated by adding different quantities of ingredients. The three main ingredients used for pasteurized milk formulation included raw milk (85.80–93.80% RM), mulberry leaf tea (2–6% MLT) and soymilk powder (2–6% SMP), with the remaining ingredients as sugar (2%) and food grade color (0.2%). Pasteurized milk was prepared by brewing mulberry leaves in approximately one-quarter of the whole portion of raw milk at 73 ± 2 °C for 30 min. The brew was then filtered twice through cheesecloth, prior to mixing with the remaining three-quarters of milk, and soymilk powder and the remaining ingredients were added. The mixture was subjected to pasteurization at 65 °C for 30 min, cooled to 4 °C and then stored in sterilized bottles at 4 °C.
Melatonin and free tryptophan content analysis
Melatonin and free tryptophan content were determined according to Sae-Teaw et al. (2013) with some modifications. Samples (20 g) were dissolved in 100 ml of ethanol and mixed using a shaking incubator (Daihan Labtech, Model LSI-1005R, Korea) at 150 rpm, 27 ± 2 °C for 16 h. Mixed samples were then filtrated through Whatman (No. 1) filter paper and evaporated using a vacuum rotary evaporator (Büchi R-114, Switzerland) at 45 °C for 30 min. Dry samples were eluted with 2.5 ml of deionized water and dichloromethane. A sample of the extract (100 µl) was then pipetted into a test tube, 1 ml each of dichloromethane and deionized water were added and the mixture was sonicated for 10 min. After that, a further 2 ml of dichloromethane was added, mixed, and the dichloromethane layer was separated into a new test tube. The extracted dichloromethane layer was dried using an evaporator centrifuge (Savant SC210A SpeedVac Plus, USA) and 1 ml of HPLC-grade ethanol was added and mixed for 30 s using a vortex mixer (Harmony, Model VTX-3000L, Japan).
Melatonin and free tryptophan contents were analyzed using an HPLC-FD (Prominence HPLC, Shimadzu, Japan) equipped with a binary LC-20AD pumping system using an RF-20Axs detector at 290/330 nm excitation/emission for fluorescence detection. An RP-C18 column (4.6 × 250 mm, 5 µm), (GL Science, Japan) was used as the stationary phase and the mobile phase was 50 mM phosphate buffer at pH 7.2 (A) and acetonitrile (B). Gradient elution was controlled as follows: (time, solvent B), (0–5 min, 0–35%), (12 min, 40%), (20 min, 45%), (25 min, 50%), (30 min, 0%), and stopped at 40 min with flow rate of 1.0 ml/min and 20 μl injection volume. Accuracy (% recovery) and precision (repeatability and reproducibility, % RSD) of melatonin and free tryptophan contents were determined. Values were calculated as [recovery (%) = (recovered conc./injected conc.) × 100] and [RSD (%) = (standard deviation/mean) × 100].
Chemical composition analysis
Pasteurized milk compositions consisting of fat, solids not fat (SNF), lactose and protein were determined using an Ultrasonic Milk Analyzer (Milkotronic, Stara Zagora, Bulgaria). Total solids (TS) were calculated by SNF and fat content, while pH values were measured using a pH meter (Mettler Toledo, USA). Total microbial count was determined according to the method of Saxena and Rai (2013).
Color measurement
Values of L*, a* and b* were measured using a Minolta Chroma Meter CR-300 (Konica Minolta, Japan). L* value expresses light (+) and black (−), a* value refers to red (+) and green (−), and b* value is yellow (+) and blue (−).
Sensory evaluation
The study was reviewed and approved by the Ethics Committee for Research Involving Human Subjects, Mahasarakham University, Certificate of Approval No. 048/2016. Thirty untrained panelists were enrolled and all provided signed informed consent. Different attributes of pasteurized milk samples were evaluated as color, flavor, taste, texture and overall liking using a 9-point hedonic scale following the method of Marsanasco et al. (2015). Liking score of each attribute was represented as a number between 1 and 9 as follows; 9 = like extremely, 8 = like very much, 7 = like moderately, 6 = like slightly, 5 = neither like nor dislike, 4 = dislike slightly, 3 = dislike moderately, 2 = dislike very much, and 1 = dislike extremely.
Shelf life study
Pasteurized milk with the highest score of overall liking was selected for stability study. The sample was stored at 4 °C and sampling procedures to determine chemical composition, melatonin, tryptophan, total plate count and other parameters were conducted every second day for 8 days.
Statistical analysis
All experiments were performed in triplicate, with results expressed as mean and standard deviation for each treatment. One-way ANOVA in a completely randomized design was employed for data analyses, including F-test with multiple comparisons to test for significant differences between treatments using Duncan’s Multiple Range Test. Sensory score was evaluated by two-way ANOVA in randomized block design (RBD) using SPSS software version 19.0 (trial version) (IBM Corporation, USA).
Results and discussion
Melatonin and free tryptophan contents
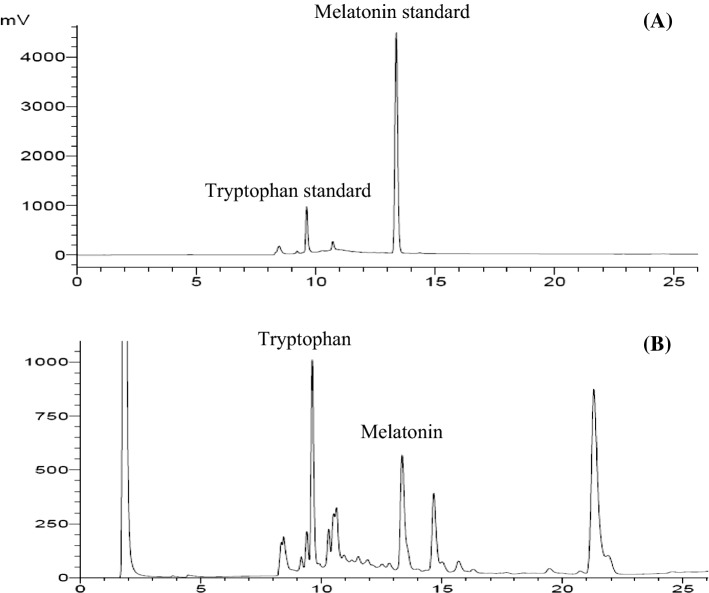
This is the first report on melatonin and tryptophan content in selected grains and leaf to prepare high melatonin pasteurized milk. Accuracy and precision were determined by validation of method parameters. Linear equations and correlation curves of melatonin standard were evaluated. Correlation coefficients for the calibration curves of melatonin and free tryptophan were 0.9992 and 0.9993 (data not shown). Typical HPLC chromatograms of the standards and samples are shown in Fig. 1. Retention times of free tryptophan and melatonin were roughly 9.6 and 13.3 min, respectively.
Fig. 1.
HPLC chromatograms; a melatonin (31.25 ng/ml) and free tryptophan standards (156.25 ng/ml), b melatonin and free tryptophan in soybean extract at concentration of 400 mg/ml
Melatonin contents of seed cultivars are shown in Table 1. High concentrations of melatonin were recorded in oilseeds and legumes with highest in white sesame (75.24 ng/g dw) followed by sunflower (67.45 ng/g dw) and soybean (56.49 ng/g dw) and lowest in red dawk mali rice (17.50 ng/g dw). For corn and rice cultivars, highest concentrations were shown in sweet corn and riceberry rice at 34.88 and 36.26 ng/g dw, respectively. Our results concurred with Manchester et al. (2000) who reported melatonin concentration of 15 edible seeds ranging from 2 to 189 ng/g dw, with highest levels found in white mustard (189 ng/g dw) and sunflower seeds at 29 ng/g dw. Melatonin in rice grain varieties ranging 16.0–264.0 ng/g was reported by Setyaningsih et al. (2012). After synthesis in plants, melatonin is transported to germ and bran layer tissues for cell protection against oxidative damage; this enhances survival and protects the next plant generation (Arnao and Hernández-Ruiz 2013).
Table 1.
Melatonin and free tryptophan contents of grain cultivars, raw milk and mulberry leaf
| Sample | Melatonin (ng/g dw) | Free tryptophan (ng/g dw) |
|---|---|---|
| Red dawk mali rice | 17.50 ± 0.40f | 347.99 ± 9.65e |
| Khao dawk mali rice 105 | 18.56 ± 0.57f | 201.75 ± 2.71gh |
| Black glutinous rice | 21.15 ± 0.65f | 183.75 ± 6.69gh |
| Glutinous rice (RD 6) | 21.84 ± 1.52f | 145.86 ± 13.03h |
| Waxy corn | 27.04 ± 0.12e | 137.20 ± 5.82h |
| Wax berry corn | 27.97 ± 0.12e | 230.18 ± 12.80fg |
| Mung bean | 30.25 ± 0.03e | 845.27 ± 14.59c |
| Sweet corn | 34.88 ± 1.02d | 171.65 ± 5.41gh |
| Riceberry rice | 36.26 ± 0.80d | 743.53 ± 26.36d |
| Peanut | 39.43 ± 1.61d | 205.97 ± 13.67gh |
| Red bean | 54.79 ± 0.79c | 1527.23 ± 31.66b |
| Soybean | 56.49 ± 1.45c | 2617.83 ± 143.06a |
| Sunflower | 67.45 ± 0.96b | 298.11 ± 26.66ef |
| White sesame | 75.24 ± 9.66a | 356.20 ± 19.50e |
| Fresh mulberry leaves (ML) | 51.57 ± 2.41a | 210.53 ± 9.26a |
| ML-freeze drying (F) | 42.15 ± 3.89b | 124.99 ± 5.73b |
| ML-solar drying (S) | 40.18 ± 2.39b | 101.07 ± 1.59c |
| ML-hot air oven drying (H) | 31.57 ± 0.26c | 82.63 ± 0.43d |
| Soymilk powder | 46.40 ± 4.45 | 3807.48 ± 156.67 |
| Raw milk* | 0.03 ± 0.00 | 1896.87 ± 35.79 |
Raw milk (*) is expressed per ml, dw refers to dry weight. Values within a column represent mean ± standard deviation of triplicate experiments (n = 3) with different letters indicating significant difference (P < 0.05)
Melatonin content in grains is affected by a number of factors including cultivar, harvesting time, location and growth conditions (Arnao and Hernández-Ruiz 2013; Pothinuch and Tongchitpakdee 2011). Melatonin in fresh mulberry leaves was determined as 51.57 ng/g dw and mulberry leaf tea produced by different drying methods contained melatonin ranging from 31.57 to 42.15 ng/g dw. Melatonin concentration was significantly different (P < 0.05) in mulberry leaves dried using diverse methods. Pothinuch and Tongchitpakdee (2011) reported that melatonin concentration of mulberry leaf cultivars ranged from 40.8 to 279.6 ng/g dw while melatonin contents in mulberry leaf green and black tea brews were 46.5 and 40.6 ng/g dw, respectively. Here, melatonin content decreased in dried mulberry leaves, especially after oven drying. This can be explained because high temperature influenced melatonin losses. Moussaoui and Bendriss (2014) reported noticeable melatonin degradation through heat treatment of a melatonin solution stored at 50 °C compared to solutions stored at lower temperatures, while de la Puerta et al. (2007) recorded melatonin concentration in olive oil produced without heat-treatment as higher than oil extracted using heat. In soymilk powder, melatonin concentration was 46.40 ng/g dw whereas raw milk recorded 0.03 ng/ml. Milagres et al. (2014) reported melatonin concentration in milk milked at night time (02:00 a.m.) as 0.039 ng/ml, higher than milking at day time (03:00 p.m.) (0.004 ng/ml).
Free tryptophan concentration in seed cultivars ranged from 137.20 to 2617.83 ng/g dw (Table 1). Highest concentration was found in soybean (2617.83 ng/g dw), followed by red bean (1527.23 ng/g dw) and mung bean (845.27 ng/g dw), with lowest in waxy corn (137.20 ng/g dw). High free tryptophan levels in legumes, especially soybean and red bean, render them good sources of melatonin. High free tryptophan content was also found in pigmented rice and oilseeds. Comai et al. (2007a, b) reported free tryptophan concentration of cereal flours ranging from 3000 to 77,900 ng/g dw, whereas corn recorded 6600 ng/g dw and legumes ranged from 22,400 to 582,000 ng/g dw with highest in chickpeas and lowest in groundnuts. Free tryptophan in fresh mulberry leaves was 210.53 ng/g dw, while mulberry leaf tea ranged from 82.63 to 124.99 ng/g dw. Highest value was found in freeze-dried mulberry leaves and lowest in leaves dried in a hot air oven. Free tryptophan in raw milk was 1896.87 ng/ml. This result was supported by Biasiolo et al. (1995) who reported free tryptophan in cow’s milk (UHT) at 350 ng/g. Differences in tryptophan concentration between our results and previous research may be due to diverse extraction and purification conditions, and also to disparities in dietary sources.
Chemical composition of pasteurized milk
Nine different pasteurized milk treatments were formulated and their compositions were evaluated (Table 2). The pH values varied from 6.76 to 6.91. Fat ranged from 3.89 to 4.64%, solids not fat (SNF) 9.85–11.67%, lactose 5.72–6.07%, protein 4.32–5.59%, and total solids (TS) 13.82–16.31%. Highest composition was found in the sample consisting of milk (85.80%), mulberry leaf tea (6.00%) and soymilk powder (6.00%), whereas lowest composition was recorded in the treatment consisting of milk (91.80–93.80%), mulberry leaf tea (2.00–4.00%) and soymilk powder (2.00%). These results indicated that high levels of protein, fat, SNF and TS in pasteurized milk treatments resulted from addition of soymilk powder. Soybean is abundant in protein, carbohydrate and oil content; it improves nutrients and physiochemical properties of milk. Kpodo et al. (2013) found that protein, fat, carbohydrate and total solids content increased with increasing soy, peanut and cow milk in the formulation.
Table 2.
Chemical compositions of nine pasteurized milk formulations
| Treatment | % SMP | % MLT | % RM | pH | % Fat | % SNF | % Lactose | % Protein | % TS | Color values | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||||||
| Raw milk | – | – | 100.00 | 6.72 ± 0.01 | 3.35 ± 0.02 | 8.37 ± 0.01 | 5.38 ± 0.03 | 3.05 ± 0.09 | 11.72 ± 0.03 | 82.35 ± 0.19 | − 4.47 ± 0.04 | 3.57 ± 0.02 |
| 1 | 2.00 | 2.00 | 93.80 | 6.85 ± 0.01cd | 3.89 ± 0.08c | 9.93 ± 0.07c | 5.81 ± 0.02b | 4.32 ± 0.03f | 13.82 ± 0.15d | 74.72 ± 028a | − 14.86 ± 0.38e | 22.63 ± 0.30a |
| 2 | 2.00 | 4.00 | 91.80 | 6.76 ± 0.03e | 3.94 ± 0.12c | 9.97 ± 0.11c | 5.72 ± 0.04b | 4.48 ± 0.04de | 13.91 ± 0.08d | 66.15 ± 0.59e | − 11.20 ± 0.33c | 19.20 ± 0.32d |
| 3 | 2.00 | 6.00 | 89.80 | 6.85 ± 0.02cd | 4.01 ± 0.10c | 9.85 ± 0.13c | 5.96 ± 0.12a | 4.35 ± 0.04ef | 13.87 ± 0.13d | 61.88 ± 0.18g | − 9.87 ± 0.24a | 18.04 ± 0.23f |
| 4 | 4.00 | 2.00 | 91.80 | 6.89 ± 0.02ab | 4.19 ± 0.08b | 10.33 ± 0.08b | 5.81 ± 0.05b | 4.50 ± 0.05d | 14.52 ± 0.16c | 73.05 ± 0.19b | − 14.45 ± 0.12d | 22.36 ± 0.16ab |
| 5 | 4.00 | 4.00 | 89.80 | 6.83 ± 0.02d | 4.26 ± 0.07b | 10.38 ± 0.09b | 5.82 ± 0.02b | 4.87 ± 0.03c | 14.64 ± 0.16c | 66.79 ± 0.07e | − 11.04 ± 0.04c | 19.75 ± 0.03c |
| 6 | 4.00 | 6.00 | 87.80 | 6.85 ± 0.01cd | 4.31 ± 0.06b | 10.47 ± 0.10b | 5.95 ± 0.09a | 4.76 ± 0.23c | 14.78 ± 0.14c | 64.37 ± 0.43f | − 9.77 ± 0.04a | 18.77 ± 0.76e |
| 7 | 6.00 | 2.00 | 89.80 | 6.91 ± 0.02a | 4.53 ± 0.08a | 11.52 ± 0.07a | 6.07 ± 0.07a | 5.27 ± 0.06b | 16.04 ± 0.15b | 72.09 ± 0.13c | − 14.10 ± 0.23d | 22.01 ± 0.28b |
| 8 | 6.00 | 4.00 | 87.80 | 6.90 ± 0.02a | 4.60 ± 0.08a | 11.64 ± 0.02a | 5.98 ± 0.05a | 5.49 ± 0.04a | 16.24 ± 0.10ab | 69.14 ± 0.58d | − 10.41 ± 0.19b | 19.65 ± 0.34c |
| 9 | 6.00 | 6.00 | 85.80 | 6.87 ± 0.02bc | 4.64 ± 0.09a | 11.67 ± 0.12a | 6.00 ± 0.08a | 5.59 ± 0.02a | 16.31 ± 0.20a | 65.04 ± 0.64f | − 9.52 ± 0.09a | 18.57 ± 0.18e |
SMP refers to soymilk powder, MLT refers to mulberry leaf tea and RM refers to raw milk. SNF refers to solids not fat, TS refers to total solids, L* refers to light (+) and black (−), a* refers to red (+) and green (−), b* refers to yellow (+) and blue (−). Values within a column are expressed as mean ± standard deviation of triplicate experiments (n = 3) and different letters indicate significant difference (P < 0.05)
Color parameters of pasteurized milk
Color parameter results of all treatments indicated that lightness (L*) value was highest in treatments with least amounts of mulberry leaf tea added, similar to green (a*) and yellow (b*) color values (Table 2). Lightness decreased when mulberry leaf tea increased, while addition of soymilk powder enhanced yellow color. Green color increased due to chlorophyll pigment released by the leaf during the brewing process. These results concurred with Shokery et al. (2017) who reported that L* values of yogurt decreased with addition of extracted leaves, while a* values increased and became redder with addition of green tea, and greenish with addition of moringa leaves. Sawale et al. (2015) found that decreasing lightness and increasing yellow and redness in milk infused with herbs were due to anthocyanins.
Melatonin and free tryptophan contents in pasteurized milk
Melatonin and free tryptophan contents in pasteurized milk were significantly different (P < 0.05) for all treatments (Table 3). Concentrations of melatonin ranged from 0.53 to 4.34 ng/ml and free tryptophan content ranged 36.72–474.82 ng/ml. Highest concentrations were found in milk mixed with 6.00% mulberry leaf tea and 6.00% soymilk powder (4.34 ng/ml and 474.82 ng/ml, whereas lowest concentrations were found in milk added with 2.00% of each ingredient. Previous studies regarding melatonin content in beverages reported that variation of melatonin concentration in red wines ranged from 0 to 129.5 ng/ml (Rodriguez-Naranjo et al. 2011) and 0.5 ng/ml in grape juice (Mercolini et al. 2012). Higher concentrations of melatonin and free tryptophan in milk gave a better product because consumers ingested higher amounts of beneficial compounds which promote health and prevent diseases by scavenging various radical species. Sae-Teaw et al. (2013) reported that intake of tropical fruits containing melatonin improved melatonin plasma with increased antioxidant activity. However, too high quantities of soymilk powder and mulberry leaf tea affected total solids in milk and taste acceptability. Here, increasing amounts of mulberry leaf tea and soymilk powder in milk improved melatonin and free tryptophan contents.
Table 3.
Melatonin and free tryptophan contents of nine pasteurized milk treatments
| Treatment | % SMP | % MLT | % RM | Melatonin (ng/ml) | Free tryptophan (ng/ml) |
|---|---|---|---|---|---|
| 1 | 2.00 | 2.00 | 93.80 | 0.53 ± 0.08g | 36.72 ± 0.95f |
| 2 | 2.00 | 4.00 | 91.80 | 0.99 ± 0.49f | 59.82 ± 2.42f |
| 3 | 2.00 | 6.00 | 89.80 | 1.11 ± 0.10f | 70.94 ± 1.84f |
| 4 | 4.00 | 2.00 | 91.80 | 1.44 ± 0.11e | 151.78 ± 4.57e |
| 5 | 4.00 | 4.00 | 89.80 | 1.60 ± 0.06e | 260.66 ± 20.41d |
| 6 | 4.00 | 6.00 | 87.80 | 1.99 ± 0.09d | 341.54 ± 27.35c |
| 7 | 6.00 | 2.00 | 89.80 | 2.70 ± 0.14c | 374.86 ± 34.13bc |
| 8 | 6.00 | 4.00 | 87.80 | 3.26 ± 0.10b | 410.98 ± 34.07b |
| 9 | 6.00 | 6.00 | 85.80 | 4.34 ± 0.37a | 474.82 ± 29.59a |
Values within a column are expressed as mean ± standard deviation of triplicate experiments (n = 3) with different letters indicating significant difference (P < 0.05)
Sensory evaluation
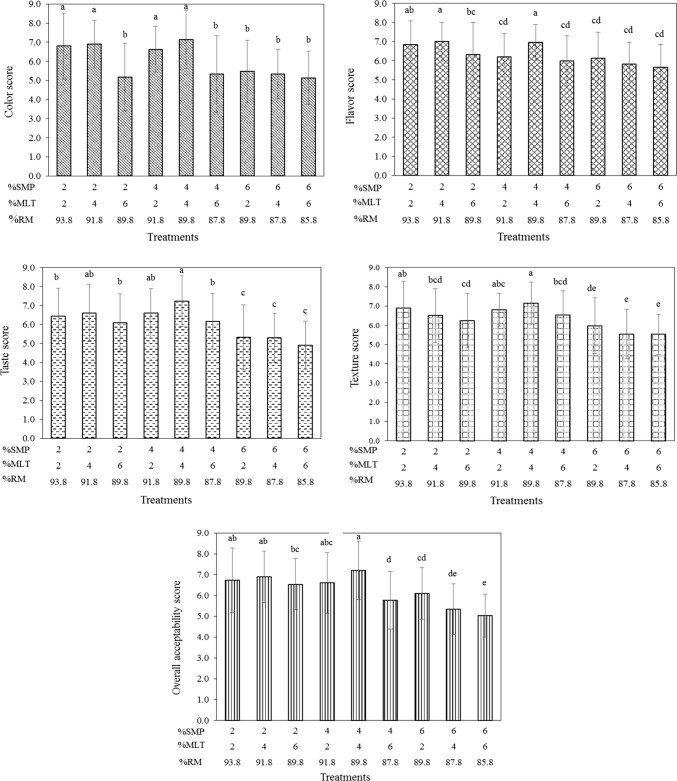
Sensory score was evaluated using a 9-point hedonic scale with five different attributes including color, flavor, taste, texture and overall liking. Results showed that all treatments were significantly different (P < 0.05) in all attributes (Fig. 2). Highest scores of each attribute were found in the treatment added with mulberry leaf tea (4.00%), soymilk powder (4.00%) and milk (89.80%), whereas the lowest were found in milk treatment added with soymilk powder (6.00%), mulberry leaf tea (6.00%) and milk (85.80%). Highest recorded score by panelists was 7 (like moderately) for the treatment added with mulberry leaf tea (4.00%), soymilk powder (4.00%) and milk (89.80%) while panelists mostly liked treatments containing 2.00–4.00% soymilk powder. Treatments with high levels of soymilk powder at 6.00% were neither liked nor disliked. Results indicated that increasing amounts of mulberry leaf tea and soymilk powder affected product appearance and consumer acceptability. Previous studies by Sawale et al. (2015) reported that adding 0.2–0.5% of the herb (Pueraria tuberosa) in milk resulted in a significant decrease in color and appearance, flavor, mouthfeel and overall acceptability. Shokery et al. (2017) found that addition of different plant extracts (green tea and moringa leaves) to yogurt affected product appearance. Thus, supplementation of different materials or levels of ingredients in milk-based products influenced product characteristics and consumer satisfaction. Our pasteurized milk treatment with the highest score of overall liking was chosen to study shelf life at different storage times.
Fig. 2.
Sensory score of pasteurized milk treatment using a 9-point hedonic scale. Different letters on top of columns indicate significant differences (P < 0.05). For sensory score, 1 = dislike extremely, 2 = dislike very much, 3 = dislike moderately, 4 = dislike slightly, 5 = neither like nor dislike, 6 = like slightly, 7 = like moderately, 8 = like very much, and 9 = like extremely
Shelf life study
Accepted pasteurized milk [mulberry leaf tea (4.00%), soymilk powder (4.00%) and milk (89.80%)] was prepared and kept in sterilized bottles and refrigerated at 4 °C. Sampling was performed every 2 days to evaluate melatonin, free tryptophan and milk composition. Chemical compositions and pH values of pasteurized milk during storage for 8 days showed no significant variation differences (Table 4). Color parameters of lightness (L*) did not significantly decrease, similar to b* value (yellow color) and a* (green color) over the 8 days of storage. Changes in color were due to increase in microbial count in accordance with Paul-Sadhu (2016) who found that bacterial count correlated with color values. Increasing bacterial count slightly influenced color parameters L*, a*, and b* over 30 days of storage time due to bacterial consumption of oxygen for growth. Increase in a* value was influenced by riboflavin in the milk which absorbed visible light and converted this energy into oxygen (Chandan et al. 2009). Popov-Raljić et al. (2008) observed that L* and b* values of UHT milk stored at ambient temperature decreased, while a* value increased from negative to positive over storage for 90 days. Our results indicated that L* and b* values decreased inversely to a* value due to the mixed ingredients. Green color was derived from the pigments of mulberry leaf tea with yellow color from soymilk powder. Thus, variations in color can be influenced by several factors including pigments of the ingredients, survival of bacterial population, milk composition and heat treatment processes.
Table 4.
Melatonin and free tryptophan contents of accepted pasteurized milk at different storage times
| Day | pH (ns) | % Fat (ns) | % SNF (ns) | % Lactose (ns) | % Protein (ns) | % TS (ns) | Colony (cfu/ml) | Color values (ns) | Melatonin (ng/ml) (ns) | Free tryptophan (ng/ml) (ns) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||||||
| 0 | 6.74 ± 0.05 | 4.34 ± 0.05 | 10.62 ± 0.14 | 5.75 ± 0.13 | 4.77 ± 0.13 | 14.96 ± 0.18 | 3.89 × 10a | 67.05 ± 0.06 | − 10.86 ± 0.07 | 20.00 ± 0.07 | 1.83 ± 0.18 | 284.37 ± 21.11 |
| 2 | 6.75 ± 0.05 | 4.34 ± 0.10 | 10.63 ± 0.06 | 5.76 ± 0.08 | 4.79 ± 0.08 | 14.97 ± 0.15 | 2.78 × 102b | 67.03 ± 0.23 | − 10.83 ± 0.06 | 19.95 ± 0.01 | 1.79 ± 0.07 | 289.39 ± 19.13 |
| 4 | 6.74 ± 0.05 | 4.33 ± 0.07 | 10.59 ± 0.10 | 5.73 ± 0.09 | 4.76 ± 0.09 | 14.92 ± 0.18 | 1.69 × 103c | 67.00 ± 0.02 | − 10.84 ± 0.01 | 19.92 ± 0.04 | 1.66 ± 0.11 | 302.60 ± 25.20 |
| 6 | 6.72 ± 0.09 | 4.31 ± 0.09 | 10.60 ± 0.05 | 5.70 ± 0.09 | 4.77 ± 0.10 | 14.91 ± 0.14 | 5.32 × 103d | 67.02 ± 0.07 | − 10.82 ± 0.05 | 19.96 ± 0.06 | 1.69 ± 0.04 | 267.76 ± 11.88 |
| 8 | 6.70 ± 0.10 | 4.32 ± 0.08 | 10.61 ± 0.10 | 5.71 ± 0.11 | 4.75 ± 0.07 | 14.93 ± 0.17 | 9.78 × 103d | 66.97 ± 0.07 | − 10.79 ± 0.07 | 19.94 ± 0.07 | 1.55 ± 0.11 | 276.85 ± 12.13 |
SNF refers to solids not fat, TS refers to total solids, L* refers to light (+) and black (−), a* refers to red (+) and green (−), b* refers to yellow (+) and blue (−) and ns means no significant difference. Values within a column are expressed as mean ± standard deviation of triplicate experiments (n = 3)
Total microbial count significantly increased (P < 0.05) throughout 8 days of storage from 3.9 × 10 to 9.8 × 103 cfu/ml but the count was still within acceptable limits of 1 × 105 cfu/ml. Gnan et al. (2013) reported that total microbial count in pasteurized milk increased from day 1 (7.9 × 102 cfu/ml) to day 7 (9.0 × 103 cfu/ml). However, differences in total microbial populations detected depend on total microbial counts in materials, hygiene, aseptic techniques and processes used.
Melatonin and free tryptophan content of accepted pasteurized milk showed no significant difference in storage time (Table 4). Pasteurized milk samples were kept in closed bottles which delayed oxidation, and stored at low temperature (4 °C) to suppress microbial growth. Previous studies determined that melatonin stored in aqueous solution in pyrogen-free glass vacuums at room temperature, 4 °C and − 70 °C was more stable over 6 months (Cavallo and Hassan 1995). Moussaoui and Bendriss (2014) indicated that melatonin was stable over 6 days and did not significantly decrease until day 13 on exposure to light and dark conditions at 32 °C, 4 °C, 25 °C and 50 °C with no air. They concluded that oxygen exposure combined with other factors resulted in high degradation.
Conclusion
Highest melatonin concentrations were found in white sesame, sunflower and soybean while highest concentrations of free tryptophan were detected in soybean, red bean and mung bean. To produce high melatonin pasteurized milk, mulberry leaf tea and soymilk powder were selected, prepared and supplemented in milk. All parameters including milk compositions, and melatonin and free tryptophan contents, significantly increased with increasing amounts of soymilk powder and mulberry leaf tea. Sensory evaluation of pasteurized milk gave highest score of overall liking for milk containing mulberry leaf tea 4.00%, soymilk powder 4.00% and milk 89.80% which contained melatonin and tryptophan at 1.83 ng/ml and 284.37 ng/ml, respectively. Results suggested that supplementing mulberry leaf tea and soymilk powder in milk can increase nutrients and bioactive compounds. However, benefits of high melatonin pasteurized milk and other functional food products on human health will be studied in depth before commercial application.
Acknowledgements
This research was supported by the Research and Researchers for Industry Program (RRI) of the Thailand Research Fund (TRF) (Grant No. PHD58010086, 2015) and Mahasarakham University (Graduate Scholarship, 2018). We are grateful to the Unit of Nutrition for Health, Department of Food Technology and Nutrition, Mahasarakham University, and the Faculty of Pharmaceutical Sciences and Melatonin Research Group, Khon Kaen University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alyaqoubi S, Abdullah A, Samudi M, Abdullah N, Addai ZR, Al-Ghazali M. Effect of different factors on goat milk antioxidant activity. Int J Chemtech Res. 2014;6:3091–3196. [Google Scholar]
- Arnao MB, Hernández-Ruiz J. Growth conditions influence the melatonin content of tomato plants. Food Chem. 2013;138(2):1212–1214. doi: 10.1016/j.foodchem.2012.10.077. [DOI] [PubMed] [Google Scholar]
- Biasiolo M, Bertazzo A, Costa C, Beghetto A, Allegri G. Determination of nonprotein tryptophan in yoghurts by selective fluorescence and HPLC. Food Chem. 1995;52(1):87–92. doi: 10.1016/0308-8146(94)P4186-J. [DOI] [Google Scholar]
- Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15(4):432–437. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- Cavallo A, Hassan M. Stability of melatonin in aqueous solution. J Pineal Res. 1995;18(2):90–92. doi: 10.1111/j.1600-079X.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Chandan RC, Kilara A, Shah NP. Dairy processing and quality assurance. London: Wiley; 2009. [Google Scholar]
- Chen G, Huo Y, Tan DX, Liang Z, Zhang W, Zhang Y. Melatonin in Chinese medicinal herbs. Life Sci. 2003;73(1):19–26. doi: 10.1016/S0024-3205(03)00252-2. [DOI] [PubMed] [Google Scholar]
- Comai S, Bertazzo A, Bailoni L, Zancato M, Costa CV, Allegri G. Protein and non-protein (free and protein-bound) tryptophan in legume seeds. Food Chem. 2007;103(2):657–661. doi: 10.1016/j.foodchem.2006.07.045. [DOI] [Google Scholar]
- Comai S, Bertazzo A, Bailoni L, Zancato M, Costa CV, Allegri G. The content of proteic and nonproteic (free and protein-bound) tryptophan in quinoa and cereal flours. Food Chem. 2007;100(4):1350–1355. doi: 10.1016/j.foodchem.2005.10.072. [DOI] [Google Scholar]
- Corbo MR, Bevilacqua A, Petruzzi L, Casanova FP, Sinigaglia M. Functional beverages: the emerging side of functional foods: commercial trends, research, and health implications. Compr Rev Food Sci Food Saf. 2014;13(6):1192–1206. doi: 10.1111/1541-4337.12109. [DOI] [Google Scholar]
- de la Puerta C, Carrascosa-Salmoral MP, García-Luna PP, Lardone PJ, Herrera JL, Fernández-Montesinos R, Guerrero JM, Pozo D. Melatonin is a phytochemical in olive oil. Food Chem. 2007;104(2):609–612. doi: 10.1016/j.foodchem.2006.12.010. [DOI] [Google Scholar]
- Gnan SO, Gebali MK, Eshelli MS (2013) Microbial quality and shelf life of pasteurized camel milk. In: Proceedings of the international scientific conference of camel research and production (ISCCRP) Khartoum-Sudan, pp 54–58
- Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int. 1995;35(3):627–634. [PubMed] [Google Scholar]
- Jiang S, Cai W, Xu B. Food quality improvement of soy milk made from short-time germinated soybeans. Foods. 2013;2(2):198–212. doi: 10.3390/foods2020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns NP, Johns J, Porasuphatana S, Plaimee P, Sae-Teaw M. Dietary intake of melatonin from tropical fruit altered urinary excretion of 6-sulfatoxymelatonin in healthy volunteers. J Agric Food Chem. 2013;61(4):913–919. doi: 10.1021/jf300359a. [DOI] [PubMed] [Google Scholar]
- Kocadağlı T, Yılmaz C, Gökmen V. Determination of melatonin and its isomer in foods by liquid chromatography tandem mass spectrometry. Food Chem. 2014;153:151–156. doi: 10.1016/j.foodchem.2013.12.036. [DOI] [PubMed] [Google Scholar]
- Kpodo FM, Afoakwa EO, Amoa BB, Saalia FKS, Budu AS. Application of multiple component constraint mixture design for studying the effect of ingredient variations on the chemical composition and physico-chemical properties of soy–peanut–cow milk. Int Food Res J. 2013;20(2):811. [Google Scholar]
- Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. J Am Chem Soc. 1958;80(10):2587–2587. doi: 10.1021/ja01543a060. [DOI] [Google Scholar]
- Manchester LC, Tan DX, Reiter RJ, Park W, Monis K, Qi W. High levels of melatonin in the seeds of edible plants: possible function in germ tissue protection. Life Sci. 2000;67(25):3023–3029. doi: 10.1016/S0024-3205(00)00896-1. [DOI] [PubMed] [Google Scholar]
- Marsanasco M, Márquez AL, Wagner JR, Chiaramoni NS, Alonso SDV. Bioactive compounds as functional food ingredients: characterization in model system and sensory evaluation in chocolate milk. J Food Eng. 2015;166:55–63. doi: 10.1016/j.jfoodeng.2015.05.007. [DOI] [Google Scholar]
- Mercolini L, Mandrioli R, Raggi MA. Content of melatonin and other antioxidants in grape-related foodstuffs: measurement using a MEPS-HPLC-F method. J Pineal Res. 2012;53(1):21–28. doi: 10.1111/j.1600-079X.2011.00967.x. [DOI] [PubMed] [Google Scholar]
- Milagres MP, Minim VP, Minim LA, Simiqueli AA, Moraes LE, Martino HS. Night milking adds value to cow’s milk. J Sci Food Agr. 2014;94(8):1688–1692. doi: 10.1002/jsfa.6480. [DOI] [PubMed] [Google Scholar]
- Moussaoui NE, Bendriss A. The influence of storage conditions on melatonin stability. Int J Eng Res Technol. 2014;3:2243–2246. [Google Scholar]
- Paul-Sadhu S. Impact of low refrigeration temperature on colour of milk. Acta Aliment. 2016;45(3):390–397. doi: 10.1556/066.2016.45.3.10. [DOI] [Google Scholar]
- Popov-Raljić JV, Lakić NS, Laličić-Petronijević JG, Barać MB, Sikimić VM. Color changes of UHT milk during storage. Sensors. 2008;8(9):5961–5974. doi: 10.3390/s8095961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothinuch P, Tongchitpakdee S. Melatonin contents in mulberry (Morus spp.) leaves: effects of sample preparation, cultivar, leaf age and tea processing. Food Chem. 2011;128(2):415–419. doi: 10.1016/j.foodchem.2011.03.045. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Manchester LC, Tan DX. Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood. Nutrition. 2005;21(9):920–924. doi: 10.1016/j.nut.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Naranjo MI, Gil-Izquierdo A, Troncoso AM, Cantos E, Garcia-Parrilla MC. Melatonin: a new bioactive compound in wine. J Food Compos Anal. 2011;24(4–5):603–608. doi: 10.1016/j.jfca.2010.12.009. [DOI] [Google Scholar]
- Sae-Teaw M, Johns J, Johns NP, Subongkot S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J Pineal Res. 2013;55(1):58–64. doi: 10.1111/jpi.12025. [DOI] [PubMed] [Google Scholar]
- Sawale PD, Singh RRB, Arora S. Stability and quality of herb (Pueraria tuberosa)-milk model system. J Food Sci Technol. 2015;52(2):1089–1095. doi: 10.1007/s13197-013-1067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena M, Rai P. Microbiological and chemical analysis of raw, pasteurized and UHT milk during preservation in India. Int J Chemtech Res. 2013;5(6):2804–2809. [Google Scholar]
- Serafini M, Testa MF, Villaño D, Pecorari M, Van Wieren K, Azzini E, Brambilla A, Maiani G. Antioxidant activity of blueberry fruit is impaired by association with milk. Free Radic Biol Med. 2009;46(6):769–774. doi: 10.1016/j.freeradbiomed.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Setyaningsih W, Palma M, Barroso CG. A new microwave-assisted extraction method for melatonin determination in rice grains. J Cereal Sci. 2012;56(2):340–346. doi: 10.1016/j.jcs.2012.02.012. [DOI] [Google Scholar]
- Shokery ES, El-Ziney MG, Yossef AH, Mashaly RI. Effect of green tea and moringa leave extracts fortification on the physicochemical, rheological, sensory and antioxidant properties of set-type yoghurt. J Adv Dairy Res. 2017;5(179):2. doi: 10.4172/2329-888X.1000179. [DOI] [Google Scholar]
- Triantafillidis A, Triantafillidis JK. Melatonin: a potent antioxidant agent with anti-inflammatory and anti-apoptotic effects that might be useful in the treatment of IBD patients. Ann Gastroenterol. 2009;22(1):10–12. [Google Scholar]
- Valtonen MAIJA, Niskanen L, Kangas AP, Koskinen TEUVO. Effect of melatonin-rich night-time milk on sleep and activity in elderly institutionalized subjects. Nord J Psychiatr. 2005;59(3):217–221. doi: 10.1080/08039480510023034. [DOI] [PubMed] [Google Scholar]