Abstract
In the present work, the effects of orange fibre (OF) supplementation at four different concentrations (0.5%, 1%, 1.5% and 2%) on some quality properties of probiotic set yogurt produced with ABT-2 culture (containing Streptococcus thermophilus ST-20Y, Lactobacillus acidophilus LA-5 and Bifidobacterium lactis BB-12) were evaluated, during 21 days of storage. The in vitro angiotensin converting enzyme (ACE)-inhibitory activity and antoxidant capacity as well as some physicochemical and microbiological analyses of probiotic yogurts were analysed and compared with plain yogurt. At the beginning of storage, yogurt samples had high survivability for probiotic bacteria leading to viable counts (> 107 CFU/g) higher than the minimum therapeutic threshold (106 CFU/g). A significant influence (p < 0.05) of OF supplementation on S. thermophilus ST-20Y and B. lactis BB-12 counts was observed. While OF addition enhanced the viability of B. lactis BB-12, it caused a decrease in S. thermophilus ST-20Y counts. OF addition up to 1.5% concentration affected positively the syneresis, apparent viscosity and consistency index values of yogurt samples. ACE-inhibitory activity showed an increasing trend throughout the storage. Control yogurt had the lowest ACE-inhibitory activity at 1 day of storage, and the highest activity was obtained in yogurt containing 1% OF at the end of the storage. Also, yogurts with OF had higher antioxidant activity than the control sample at all days of storage (p < 0.05). Antioxidant activity increased until 14 days of storage in all yogurt batches, while it was decreased 21th days of storage. Briefly, this study reflected the performance of using OF as an alternative hydrocolloid to improve the quality properties and bioactivity of bio-yogurt.
Keywords: Orange fibre, Probiotic yogurt, ACE-inhibitory activity, Antioxidant activity
Introduction
In recent years, a remarkable interest has occurred in the use of probiotic organisms [L. acidophilus and Bifidobacteria] in foods, particularly dairy products. Fermented dairy products containing probiotic bacteria especially yogurt and yogurt beverages are some of the most consumed fermented products in around the world. Probiotic yogurt is a popular functional food due to its health benefit properties such as stimulation of immune system, acting on prevention of allergies, reduction in cholesterol, treatment of diarrheas, and restoration of intestinal microbiata. Moreover, various strains of probiotic bacteria used in yogurt production may contribute the formation of bioactive peptides which have antioxidant, antihipertensive and anticancer potentials (Sah et al. 2014). ABT cultures which have L. acidophilus, Bifidobacteria and S. thermophilus as main fermenting organisms are generally used in the production of probiotic yogurt. As well as this, L. acidophilus, L. casei and L. paracasei have been studied extensively in the previous studies of probiotic yogurt. For the probiotic products, the most widely recommended concentration of viable cell counts of the probiotic bacteria is minimum 106 CFU per mililiter or gram (Lourens-Hattingh and Viljoen 2001). Many studies have focused on enrichment of milk some prebiotic ingredients to improve the growth of probiotic organisms. Adding fibre-rich sources like fruits, grains, and nut to milk is a perfect way for prebiotic fortification. As well as stimulating probiotic growth, fibre fortification may also provide to change in physical, textural, and rheological properties of yogurt (Espírito-Santo et al. 2013).
Fruit processing wastes are increasingly popular as food ingredients because of being sources of abundant dietary fibre, which has beneficial effects on human health. Dietary fibre (DF) is the edible plant components composed of carbohydrate polymers, oligosaccharides and polysaccharides, e.g., cellulose, hemicelluloses, pectic substances, resistant starch and gums. DFs have many health benefits because of their functional attributes which are the regulation of the intestinal transit, and the prevention or treatment of diabetes, cardiovascular diseases and colon cancer (Kaczmarczyk et al. 2012).
Many of DFs such as citrus (orange and lemon), carrot, date and oat have been used in yogurt manufacture for improving textural properties by increasing the viscosity and reducing syneresis (Sendra et al. 2010). Residues obtained citrus fruits are potentially perfect sources of DF owing to their rich pectin contents (Grigelmo-Miguel and Martín-Belloso 1998). Citrus fibres have better quality than other dietary fibres due to the presence of bioactive compounds, such as flavonoids, polyphenols and carotenes (Elleuch et al. 2011). Moreover, researchers noted that there were high growth and viability of probiotics in dairy products supplemented with citrus fibre and these products had more acceptability (Sendra et al. 2008).
In the present work, a new functional probiotic yogurt was developed due to the benefits of probiotic bacteria and dietary fibres on human health. Although there are many different studies on yogurt produced with some fibres and probiotic bacteria, this study is the first research to investigate the effects of orange fibre incorporation on ACE-inhibitory and antioxidant activities of bio-yogurts as well as physicochemical and microbiological characteristics. Hence, the aims of this study were to evaluate and compare the ACE-inhibitory and antioxidant activities, and some physicochemical and microbiological properties of probiotic yogurt having different orange fibre contents.
Materials and methods
Materials
Raw cow milk (total solids 11.25%, fat 3.70%, protein 3.03%, pH 6.55) was supplied from Dairy farm of Atatürk University in Erzurum province of Turkey. The oranges were purchased from local markets in Erzurum, Turkey. The ABT-2 culture consisted of S. thermophilus ST-20Y; L. acidophilus LA-5 and B. lactis BB-12 was obtained by Chr. Hansen-Peyma, Istanbul, Turkey.
Methods
Orange fibre preparation
OF was prepared according to the method offered by Fernández-Ginés et al. (2003). Dietary fibre obtained from orange was analyzed for determining some physicochemical and functional properties. Dry matter and protein contents of the fibre were detected. The pH was measured with a pH meter (Crison, pH meter, Basic 20 + , Spain) fitted with a combined glass electrode. Colour measurement of fibre was done using a Chroma Meter (CR-5, Konica Minolta, Osaka, Japan).
Water retention capacity (WRC) was determined using the method by Robertson et al. (2000). Firstly, 1 g of sample was placed in a graduated test tube and 30 mL of water was added. Then, it was homogenised using a vortex for 1 min and was hydrated for 18 h. the supernatant was removed by centrifugation (Hettich Rotina 420 R, Tuttlingen, Germany) at 3000 rpm for 20 min. The hydrated residue was weighed and it was dried 105 °C for 2 h to obtain the residual dry weight.
Oil-holding capacity (OHC) was also determined in OF by the same simple procedure given for the WRC; the only difference was to put sunflower oil instead of distilled water. For determining the swelling capacity (SC), 0.2 g of dry fibre was placed in graduated test tube; 10 mL of water was added and it was hydrated at 25 °C for 18 h. Then, the final volume was measured (Robertson et al. 2000).
Yogurt production
Fresh raw cow milk was used for set-type yogurt manufacture. Firstly, milk was divided into five equal parts of 3.5 L. Then, OF was added into milk at ratios of 0.5%, 1%, 1.5% and 2% and the batches were homogenized by an Ultra-Turrax (IKA T25 digital, IKA Werke Staufen, Germany) at 20,000 rpm until the fibre completely dispersed in the milk. The yogurt formulations were coded as OF0.5%, OF1%, OF1.5% and OF2%, and the yogurt sample without OF was termed as C (control). The samples were pasteurized at 85 °C for 25 min and cooled down by a water bath to 37 ± 1 °C for the addition of the ABT-2 culture at the rate of 0.03% (w/w). Inoculated milks were distributed into 200-mL sterile glass cups and incubated at 37 ± 1 °C until the pH reached 4.6. After fermentation, yogurt samples were cooled immediately and stored in a refrigerator (4 ± 1 °C) for 21 days. They were analyzed at 1, 7, 14 and 21th days of cold storage.
Physicochemical analysis
After 1 day of the yogurt production, proximate analyses which are total solids (using oven drying), protein (using micro-Kjeldahl method) and fat contents (using Gerber method) of yogurt samples were determined (AOAC 2005). pH, titratable acidity, viscosity and syneresis determinations were performed at 1,7, 14 and 21 days of storage. The pH was measured with a calibrated pH meter (Crison, pH meter, Basic 20 + , Spain) fitted with a combined glass electrode. The titratable acidity was determined by titration with a 0.1 N sodium hydroxide solution and expressed as lactic acid percentage (AOAC 2005).
The viscosities of the yogurts were measured using a digital Brookfield Viscometer, (Brookfield model LVT, LV viscometer, Brookfield Engineering Laboratories, Inc., Middleborough, MA), equipped with V73 spindle. Measurements were taken at 20 rpm. Before measuring the viscosity, the yogurt samples were stirred softly and sample temperature was 4 °C. The readings were taken from instrument directly at the point of 30th s and were recorded in centipoise. Rheological models were used to fit the viscosity data of the yogurt samples. The rheological behavior of the samples was described by the power-law model described as follow:
where , K, γ and n are accepted as the apparent viscosity (mPa s−1), consistency coefficient (mPa s−1), shear rate (s−1), and flow behavior coefficient, respectively.
For the syneresis determination, twenty-five grams of yoghurt samples were weighed and filtered. After 120 min of drainage under refrigeration temperature, the amount of collected whey (mL) was recorded and expressed as an index of syneresis.
The color parameters of yogurt samples were also determined during storage period by using a CR-200 Minolta colorimeter (Minolta Camera Co., Osaka, Japan). The samples were put into an optically flat glass dish for measurements and the color parameters were measured as L* (brightness; 0: black, 100: white), a* (+: red; −: green), b* (+: yellow; −: blue).
Enumeration of viable bacteria
Viable counts of bacteria were determined in yogurt samples throughout the storage. Various selective media was used for enumeration of the microorganisms. S. thermophilus ST-20Y viability was monitored using pour-plating 1 mL of each dilution in M-17 agar (Oxoid), by incubating under aerobic conditions at 37 ± 1 °C for 48 h (Sah et al. 2014). MRS-nalidixic acid (150 mg/l), neomycin sulphate (100 mg/l), lithium chloride (3000 mg/l), and paramycin sulphate (200 mg/l) (NNLP) agar was used for enumeration of B. lactis BB-12 (Tharmaraj and Shah 2003). The viable counts of L. acidophilus LA-5 were enumerated on BL–MRS (agar supplemented with 1•5 g/l bile salts) (Lima et al. 2009). They were incubated at 37 °C for 72 h, anaerobically. Viable cell counts expressed as log CFU/mL.
ACE-inhibitory activity analysis
Water soluble extract (WSE) of yogurt samples was obtained by centrifugation at 10,000 × g for 10 min at 5 °C and by filtration through Whatman no. 40 filter. The ACE-inhibitory activity of the freeze-dried WSEs was measured using the colorimetric and spectrophotometric assay as described by Erkaya and Şengul (2015). The method was explained in the ACE activity assay section.
The percentage inhibition was calculated as follows:
where, A is the absorbance with ACE, HHL (Hip-His-Leu) and ACE-inhibitory sample, B is the absorbance with ACE and HHL without ACE-inhibitory sample, C is the absorbance with HHL and without ACE and D is the absorbance with HHL without ACE and ACE-inhibitory sample. ACE-inhibition was also expressed as IC50. The protein content of the samples was determined using the Foline Lowry method (Lowry et al. 1951). The IC50 was then determined from the linear regression as the protein concentration in the sample required to inhibit 50% of the ACE activity.
Antioxidant activity analysis by 2,2-diphenyl-1-picrylhydrazyl (DPPH)
DPPH radical-scavenging activity of the WSEs was determined as described previously (Apostolidis et al. 2007). 60 μM DPPH in ethanol was used for the analysis. 250 μL homogenised extract was mixed with 3 mL of the DPPH solution and then allowed to rest for 1 h. The absorbance was monitored at 517 nm and the % inhibition was calculated by:
DPPH scavenging activity was also expressed as IC50. The IC50 was then determined from the linear regression of DPPH radical scavenging activity against the protein concentration in the sample.
Statistical analysis
Data obtained from analysis of the yogurt samples were evaluated statistically using analysis of variance (ANOVA) and Duncan’s Multiple Comparison test was applied to determine significant differences using SPSS statistical software program version 17 (SPSS Inc., Chicago, IL, USA; SPSS 1999). Significance level of 95% (p < 0.05) was used for statistical differences, unless otherwise stated.
Results and discussion
Physicochemical characteristics of OF
The physicochemical characteristics of OF are shown in Table 1. Water retention and swelling capacity provide information about advantageous for fibre supplemented foods (Dhingra et al. 2012). Thus, hydration-related properties of fibres such as WRC, SC and OHC are important characteristics for them and fibres with high hydration capacity provide some functional benefits such as reducing calories, avoiding spoilage and improving textural properties of final product when added to foods. As shown in Table 1, OF had high levels of WRC (12.59 g water/g fibre), SC (12.50 mL water/g fibre) and OHC (3.48 g oil/g fibre). WRC values were higher than those obtained for some orange fibre products (Grigelmo-Miguel and Martín-Belloso 1998; Yi et al. 2014), but lower than those found by others (Nassar et al. 2008; Fernández-López et al. 2009). In the present work the solubility of OF was 16.45%. Soluble and insoluble fractions of dietary fibres are responsible for their physiological and physicochemical effects (Elleuch et al. 2011). Color values of OF (L*, a* and b*) obtained in this work showed similarity to the findings of micronized orange by-products dietary fibre by Yi et al. (2014). The researchers had emphasized that the brightness was higher in OF when the particle size is smaller.
Table 1.
Physicochemical properties and color parameters of OF
| Parameters | |
|---|---|
| Dry matter (%) | 93.66 |
| Protein (%) | 5.22 |
| pH | 3.86 |
| Water retention capacity (g water/g fibre) | 12.59 |
| Oil holding capacity (g oil/g fibre) | 3.48 |
| Swelling capacity (mL water/g fibre) | 12.50 |
| Solubility (%) | 16.45 |
| L* | 81.79 |
| a* | − 1.77 |
| b* | 24.32 |
Data are the means of two replicates. Standard deviations were less than 5 (data not shown), OF orange fiber
Viable bacterial counts in yogurt samples
The results of bacterial counts obtained in yogurt samples during storage period are given in Table 2. Probiotic counts for L. acidophilus LA-5 and B. lactis BB-12 were generally higher than the minimum therapeutic threshold (106 CFU/g) up to 21 days of storage. While S. thermophilus ST-20Y and B. lactis BB-12 counts were significantly (p < 0.05) affected by fibre addition, however it had no effect (p > 0.05) on the viability of L. acidophilus LA-5. After 1 day of storage, the highest B. lactis BB-12 counts (7.17 and 7.18 log CFU/g) were observed in yogurt samples with 1% and %2 OF. Thus, it can be concluded that fibre addition significantly enhanced the viability of B. lactis BB-12. Similarly, researchers who were studied different supplements in yogurt found that counts of L. acidophilus or Bifidobacteria increased (Sendra et al. 2008; Espírito Santo et al. 2012). Santos et al. (2018) reviewed the prebiotic flours in dairy food processing and they also underlined that the addition of flours made from fruits or another sources present potential prebiotic effects by promoting the growth and enhancing the viability of probiotics, such as Lactobacillus and Bifidobacteria. During cold storage, there were minimal differences in the viability of the probiotic bacteria, but these differences were statistically significant (p < 0.05). The refrigerated storage may have affected the reduction of viable cell counts of probiotic bacteria. On the other hand, the numbers of S. thermophilus ST-20Y did not change significantly (p > 0.05) throughout the storage period. In the initial time of storage (1 and 7 days), it was observed that OF supplementation caused a significant (p < 0.05) decrease in S. thermophilus ST-20Y counts when compared to control yogurt (Table 2). After then, the counts in OF-yogurts slightly increased. At day 1, even though variation in S. thermophilus ST-20Y populations was below 0.5 log cfu/g in OF added yogurts upon 1.5% concentrations compared to yogurt without fibre, but when the fibre concentration was 2% this variation reached about 1.2 log cycle. This may be due to the pH drops of yogurts as the OF ratio increased. The same situation was observed by Vinderola et al. (2002) for the inhibition of S. thermophilus ST-20Y by some fruit juices and by Casarotti and Penna (2015) for the lower counts of S. thermophilus ST-20Y in fermented milks supplemented with apple, banana and grape flours compared with those of the fermented milk control.
Table 2.
Viable counts of probiotic bacteria in yogurt samples during 21 days of cold storage
| Microorganisms | Storage time (days) | Yogurt samples | ||||
|---|---|---|---|---|---|---|
| C | OF0.5% | OF1% | OF1.5% | OF2% | ||
| L. acidophilus | 1 | 6.32 ± 0.12ab,A | 6.47 ± 0.55a,A | 7.11 ± 0.64a,A | 6.61 ± 0.06b,A | 6.48 ± 0.06a,A |
| 7 | 6.63 ± 0.32b,A | 6.34 ± 0.09a,A | 6.49 ± 0.65a,A | 6.12 ± 0.17ab,A | 6.44 ± 0.19a,A | |
| 14 | 5.88 ± 0.08a,A | 5.91 ± 0.08a,A | 6.20 ± 0.06a,A | 5.76 ± 0.40a,A | 6.26 ± 0.31a,A | |
| 21 | 5.98 ± 0.21a,A | 6.06 ± 0.08a,A | 6.14 ± 0.06a,A | 5.84 ± 0.08a,A | 5.99 ± 0.13a,A | |
| S. thermophilus | 1 | 6.88 ± 0.09a,C | 6.16 ± 0.06a,B | 6.18 ± 0.01a,B | 6.59 ± 0.16a,C | 5.67 ± 0.26a,A |
| 7 | 7.34 ± 0.32a,B | 6.65 ± 0.07a,AB | 6.35 ± 0.16a,A | 6.36 ± 0.18a,A | 6.49 ± 0.62a,AB | |
| 14 | 6.66 ± 0.35a,AB | 6.80 ± 0.28a,AB | 7.07 ± 0.04c,B | 6.39 ± 0.30a,A | 6.42 ± 0.16a,AB | |
| 21 | 6.71 ± 0.18a,A | 6.62 ± 0.35a,A | 6.59 ± 0.01b,A | 6.49 ± 0.08a,A | 6.46 ± 0.13a,A | |
| B. lactis BB-12 | 1 | 6.58 ± 0.14b,A | 6.77 ± 0.19b,AB | 7.17 ± 0.03b,B | 6.65 ± 0.35b,A | 7.18 ± 0.01c,B |
| 7 | 6.68 ± 0.25b,A | 6.62 ± 0.03b,A | 6.39 ± 0.13a,A | 6.25 ± 0.20ab,A | 6.39 ± 0.37a,A | |
| 14 | 6.08 ± 0.16a,AB | 6.20 ± 0.35ab,AB | 6.50 ± 0.28a,B | 5.72 ± 0.03a,A | 6.63 ± 0.03ab,B | |
| 21 | 6.23 ± 0.04ab,C | 5.85 ± 0.07a,A | 6.24 ± 0.06a,C | 6.06 ± 0.01a,B | 6.95 ± 0.06bc,D | |
Data are expressed as mean ± standard deviation. C: Control yogurt without orange fiber; OF0.5%: 0.5% OF added probiotic yogurt, OF1%: 1% OF added probiotic yogurt, OF1.5%: 1.5% OF added probiotic yogurt, OF2%:2% OF added probiotic yogurt
a–cDifferent superscripts within the same column indicate that the means differ significantly (p < 0.05)
A–DDifferent superscripts within the same row indicate that the means differ significantly (p < 0.05)
Physicochemical properties of yogurts
Gross composition
The total solid contents of probiotic yogurt samples ranged from 11.34% to 13.08%, the fat from 2.70% to 3.90% and protein from 3.09% to 3.43% (data not shown). As expected, the total solid and protein contents increased with the increase of fibre concentration and conversely, fibre addition caused a decrease in fat contents of yogurts. The highest total solid and protein values and the lowest fat value were obtained in yogurt containing 2% OF.
pH, acidity and syneresis
The changes in some physicochemical characteristics of probiotic yogurts containing OF during storage period are presented in Table 3. After 1 day of refrigerated storage, pH varied from 4.23 to 4.49 among the yogurt samples. The yogurts containing OF had lower pH values when compared to the control sample. During storage, the pH values in all probiotic yogurts decreased over time and all pH of yogurt samples with OF were significantly (p < 0.05) lower from control sample (Table 3). However, at the end of the storage the highest pH value (4.15) was obtained in OF2% sample. Some researchers have observed no significant differences in pH due to fibre addition to the yogurt (Staffolo et al. 2004; Espírito Santo et al. 2012). However, García-Pérez et al. (2005) found that pH values in yogurt lowered as OF content increased. Similarly, Bakirci et al. (2017) reported that the addition of pumpkin fibre to yogurt contributed the development of pH. OF addition at different ratios significantly (p < 0.05) affected titratable acidity values of the probiotic yogurts and the highest titratable acidity values were obtained in yogurt sample containing 1% OF at all days of storage (Table 3). Issar et al. (2017) pointed out that the acidity of acidophilus yogurt decreased with increasing levels (2.5%, 5.0%, 7.5%, 10%) of apple pomace. Titratable acidity values of the yogurts increased throughout the storage period and this increase was found to be significant (p < 0.05).
Table 3.
The changes in some physicochemical and color characteristics of probiotic yogurts during storage
| Physicochemical parameters | Storage time (days) | Yogurt samples | ||||
|---|---|---|---|---|---|---|
| C | OF0.5% | OF1% | OF1.5% | OF2% | ||
| Titratable acidity (%) | 1 | 0.57 ± 0.01a,A | 0.62 ± 0.04a,AB | 0.66 ± 0.02a,B | 0.58 ± 0.01a,A | 0.60 ± 0.01a,A |
| 7 | 0.62 ± 0.01b,A | 0.73 ± 0.07a,B | 0.73 ± 0.02b,B | 0.69 ± 0.04b,AB | 0.70 ± 0.02b,AB | |
| 14 | 0.70 ± 0.02c,A | 0.77 ± 0.08a,A | 0.76 ± 0.01b,A | 0.72 ± 0.01bc,A | 0.71 ± 0.01b,A | |
| 21 | 0.75 ± 0.01d,AB | 0.78 ± 0.02a,B | 0.78 ± 0.03b,B | 0.75 ± 0.02c,AB | 0.72 ± 0.02b,A | |
| pH | 1 | 4.49 ± 0.04c,B | 4.42 ± 0.09b,B | 4.23 ± 0.03d,A | 4.25 ± 0.02c,A | 4.24 ± 0.06a,A |
| 7 | 4.40 ± 0.03bc,C | 4.21 ± 0.07a,B | 4.18 ± 0.01c,AB | 4.16 ± 0.04b,AB | 4.09 ± 0.01a,A | |
| 14 | 4.28 ± 0.11ab,B | 4.15 ± 0.04a,AB | 4.09 ± 0.01b,AB | 4.08 ± 0.03a,A | 4.13 ± 0.11a,AB | |
| 21 | 4.13 ± 0.03a,B | 4.04 ± 0.03a,A | 4.04 ± 0.02a,A | 4.04 ± 0.01a,A | 4.15 ± 0.06a,B | |
| Syneresis (mL/25 g) | 1 | 9.75 ± 0.35b,B | 9.38 ± 0.88b,B | 8.63 ± 0.53b,AB | 7.25 ± 0.35a,A | 11.75 ± 0.35a,C |
| 7 | 8.63 ± 0.18a,C | 7.88 ± 0.18ab,BC | 7.25 ± 0.35a,AB | 6.63 ± 0.88a,A | 11.88 ± 0.18ab,D | |
| 14 | 8.38 ± 0.18a,B | 7.75 ± 0.35b,B | 6.63 ± 0.18a,A | 6.00 ± 0.01a,A | 12.75 ± 0.35b,C | |
| 21 | 8.25 ± 0.35a,C | 7.13 ± 0.53b,B | 6.50 ± 0.01a,AB | 5.88 ± 0.18a,A | 13.75 ± 0.35c,D | |
| Apparent viscosity (20 rpm) | 1 | 1427 ± 408a,A | 1996 ± 388a,AB | 3240 ± 368a,C | 8733 ± 170a,D | 2622 ± 76.66b,BC |
| 7 | 6741 ± 453b,B | 7953 ± 1766b,BC | 8431 ± 1936b,BC | 10,969 ± 1359b,C | 308 ± 94.54a,A | |
| 14 | 7082 ± 127b,B | 7125 ± 122b,B | 6139 ± 284ab,B | 7530 ± 170a,B | 227 ± 94.61a,A | |
| 21 | 8105 ± 567b,B | 8351 ± 788b,B | 8141 ± 1069b,B | 9269 ± 397ab,B | 174 ± 56.78a,A | |
| L | 1 | 87.01 ± 0.03a,B | 86.97 ± 0.22a,B | 85.89 ± 1.03aAB | 86.01 ± 0.44a,AB | 85.02 ± 0.06a,A |
| 7 | 87,34 ± 0.28a,B | 87.14 ± 0.57a,B | 86.95 ± 0.03a,B | 86.59 ± 0.25a,B | 85.41 ± 0.03a,A | |
| 14 | 87,13 ± 0.13a,C | 86.16 ± 0.23a,BC | 85.84 ± 0.82a,AB | 86.26 ± 0.05a,BC | 84.98 ± 0.31a,A | |
| 21 | 86,85 ± 0.35a,B | 86.97 ± 0.39a,B | 86.38 ± 0.11a,B | 86.35 ± 0.42a,B | 85.15 ± 0.45a,A | |
| a | 1 | − 3.71 ± 0.06a,A | − 3.37 ± 0.05a,AB | − 3.45 ± 0.04a,BC | − 3.32 ± 0.06ab,BC | − 3.23 ± 0.14a,C |
| 7 | − 3.60 ± 0.09ab,A | − 3.48 ± 0.15a,AB | − 3.36 ± 0.13a,AB | − 3.22 ± 0.02ab,B | − 3.15 ± 0.19a,B | |
| 14 | − 3.50 ± 0.05bc,A | − 3.35 ± 0.04a,AB | − 3.13 ± 0.24a,B | − 3.10 ± 0.13b,B | − 3.30 ± 0.03a,AB | |
| 21 | − 3.38 ± 0.05c,A | − 3.41 ± 0.01a,A | − 3.34 ± 0.06a,A | − 3.38 ± 0.06a,A | − 3.51 ± 0.13a,A | |
| b | 1 | 9.60 ± 0.16a,A | 10.09 ± 0.13a,A | 11.27 ± 0.01a,B | 10.98 ± 0.14a,B | 12.99 ± 0.46a,C |
| 7 | 9.85 ± 0.07a,A | 10.21 ± 0.12a,A | 10.74 ± 0.42a,AB | 11.40 ± 0.45a,B | 12.84 ± 0.47a,C | |
| 14 | 9.92 ± 0.08a,A | 10.25 ± 0.11a,A | 11.38 ± 0.47a,B | 11.54 ± 0.13a,B | 13.27 ± 0.04a,C | |
| 21 | 10.08 ± 0.28a,A | 11.15 ± 0.13b,B | 11.22 ± 0.19a,B | 11.45 ± 0.23a,B | 13.28 ± 0.04a,C | |
Data are expressed as mean ± standard deviation. C: Control yogurt without orange fiber; OF0.5%: 0.5% OF added probiotic yogurt, OF1%: 1% OF added probiotic yogurt, OF1.5%: 1.5% OF added probiotic yogurt, OF2%:2% OF added probiotic yogurt
a–cDifferent superscripts within the same column indicate that the means differ significantly (p < 0.05)
A–DDifferent superscripts within the same row indicate that the means differ significantly (p < 0.05)
Syneresis is the major visible defect resulting whey separation on the surface of yogurt gel and can negatively influence the consumer acceptance of the product. The measured syneresis values of all yogurts ranged from 5.88 ml/25 g to 13.75 ml/25 g during storage period. The syneresis significantly (p < 0.05) increased only in 2% OF added yogurt, while it decreased in all other samples during 21 days of storage. Moreover, OF2% sample had the highest syneresis values in all storage days. On the other hand, the lowest syneresis values were obtained in OF1.5% sample, and hence the ratio of OF up to 1.5% showed a proportional decrease in the syneresis of yogurt samples (Table 3). Previous studies have showed that fibre supplementation level is critical because of rearrangement of yogurt gel matrix by the protein in fibre (Sendra et al. 2010; Tseng and Zhao 2013). In the present work, for the elimination of syneresis defect 1.5% OF was more appropriate content in the preparation of OF yogurt. Similarly, Staffolo et al. (2004) reported that no syneresis was observed when yogurt was supplemented with 1.3% of wheat, bamboo, inulin and apple fibres during 21 days of storage.
Color
The color parameters L*, a* and b* were significantly affected by the OF addition to the yogurt (Table 3). The OF samples had lower lightness (L*) values than the values obtained in control sample. The lowest L value (85.02 ± 0.06) was obtained in OF2% sample on first day of storage. This result should be expected considering the yellowish color of OF (Table 1). García-Pérez et al. (2005) also found similar results in OF added yogurt and pointed out that fibre exhibited a darkening effect, probably due to its absorption of water. A slight increase in a* values (green-redness) and a significant increase in b* values (blue-yellowness) were monitored in yogurt samples with the increment of OF percentage. When compared to OF-free yogurt, a* values were closer to red wavelength and b* values were closer to yellow wavelength in OF-yogurts. Nevertheless, cold storage during 21 days had no significant effect (p > 0.05) on L*, a* and b* values of the yogurt samples.
Rheological behavior
Table 3 shows the changes in apparent viscosity values of probiotic yogurts during storage. Fibre addition and storage time had significant effect (p < 0.05) on the viscosity values of all yogurt samples. The increment of the OF concentration up to 1.5% the viscosity values increased linearly. As reported before (Staffolo et al. 2004), fibre addition improved the rheological properties of the yogurt. However, yogurt sample with 2% OF had the lowest viscosity values. This was similar to the changes in syneresis values of the yogurts (Table 3). Sendra et al. (2010) explained the estimated reason of the decrease of viscosity values in fibre added yogurt as the formation of fibre aggregates that interfered with yogurt structure. Meanwhile, regarding the citrus fibre in dairy products, Dervisoglu and Yazici (2006) reported that the addition of citrus fibre had a negative effect on the viscosity values of ice cream mixes. In the present study, for the rheological properties of yogurts, there was a positive impact of OF supplementation up to 1.5% level.
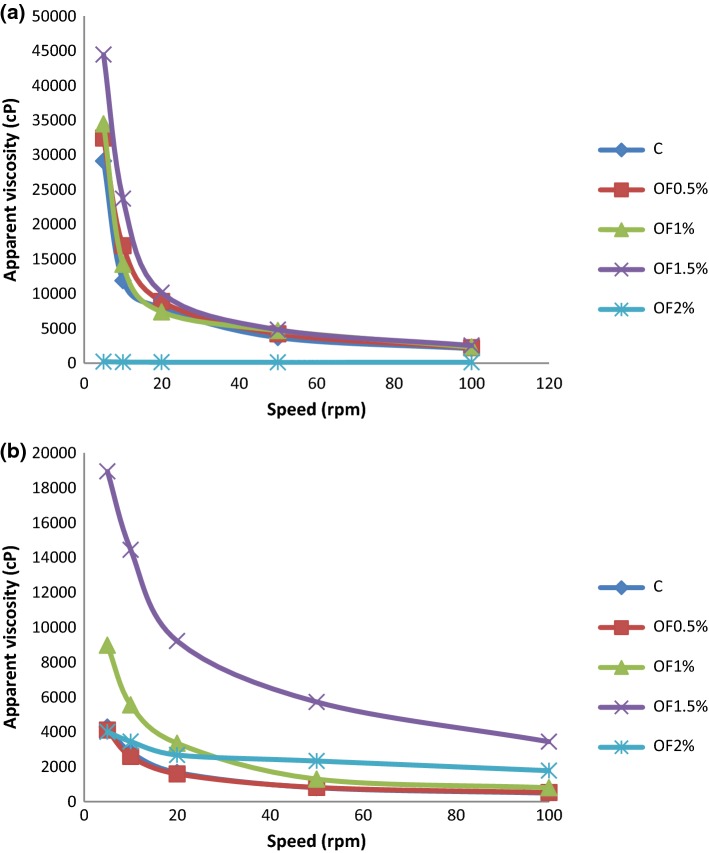
All yogurt samples showed non-Newtonian flow behavior (n < 1; Fig. 1) and Table 4 power-law model well described the flow behavior of the yogurts. The consistency index values ranged from 0.257 to 204.22 Pa.sn throughout the storage period, with lower values for the yogurt sample with 2% OF compared to the control and other OF-yogurts (Table 4.). As expected, this result was parallel to the apparent viscosity results. Similar results were reported previously in several studies with lower values of apparent viscosity in inulin or fibre containing yogurts were compared with control yogurt (Paseephol et al. 2008; Sah et al. 2016).
Fig. 1.
The flow behavior of the yogurt samples at 1 (a), and 21 (b) days of storage period
Table 4.
Viscosity parameters of the probiotic yogurt samples
| Yogurt samples | K (Pa.Sn) | n | R2 |
|---|---|---|---|
| C | |||
| 1 | 14.73 | 0.263 | 0.998 |
| 7 | 40.99 | 0.383 | 0.998 |
| 14 | 85.48 | 0.180 | 0.995 |
| 21 | 99.41 | 0.155 | 0.988 |
| OF0.5% | |||
| 1 | 23.06 | 0.241 | 0.994 |
| 7 | 82.79 | 0.194 | 0.993 |
| 14 | 83.87 | 0.167 | 0.981 |
| 21 | 131.53 | 0.114 | 0.999 |
| OF1% | |||
| 1 | 36.31 | 0.169 | 0.994 |
| 7 | 92.69 | 0.230 | 0.997 |
| 14 | 108.12 | 0.114 | 0.982 |
| 21 | 114.01 | 0.151 | 0.977 |
| OF1.5% | |||
| 1 | 50.56 | 0.429 | 0.992 |
| 7 | 130.48 | 0.172 | 0.998 |
| 14 | 159.85 | 0.038 | 0.975 |
| 21 | 204.22 | 0.040 | 0.996 |
| OF2% | |||
| 1 | 6.296 | 0.731 | 0.969 |
| 7 | 0.527 | 0.939 | 0.577 |
| 14 | 1.795 | 0.437 | 0.965 |
| 21 | 0.257 | 0.816 | 0.822 |
C: Control yogurt without orange fiber; OF0.5%: 0.5% OF added probiotic yogurt, OF1%: 1% OF added probiotic yogurt, OF1.5%: 1.5% OF added probiotic yogurt, OF2%:2% OF added probiotic yogurt
ACE-inhibitory activity
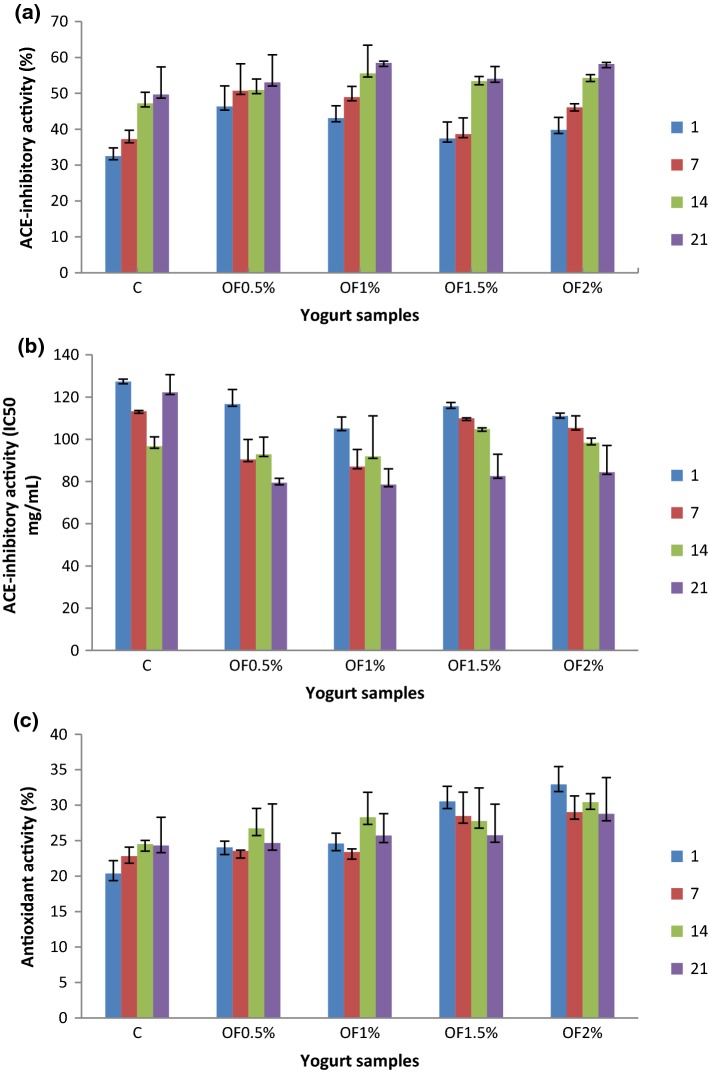
The ACE-inhibitory activity results of freeze-dried WSEs of the probiotic yogurts during 21 days of storage are shown in Fig. 2a, b. Significant differences (p < 0.05) were determined in terms of ACE-inhibitory activities of the yogurt samples, which increased throughout the refrigerated storage. Researchers have demonstrated that yogurt produced with probiotic bacteria had greater ACE-inhibitory activity than the yogurt produced with only yogurt cultures (Donkor et al. 2005). The percentage inhibition values of the yogurt samples ranged from 32.52% to 58.50%. ACE-inhibitory activity displayed an increasing trend throughout the storage. This result was in consistent with research by Hrnjez et al. (2014). Donkor et al. (2005) found that ACE-inhibitory activity increased up to 28 days of cold storage and then it was decreased. They also reported that the extent of ACE inhibition might only partially depend on the extent of proteolytic activity. Regarding the control and OF yogurts, control yogurt had the lowest ACE-inhibitory activity at 1 day of storage, and the highest activity was obtained in yogurt containing 1% OF at the end of the storage. The higher ACE-inhibitory activity presented in OF yogurts at all the storage days could be related to a possible contribution of the dietary fibre to this activity. However, ACE-inhibitory activity values obtained in yogurt with 1% OF were grater than the yogurts with 1.5% and 2% OF (p < 0.05). Therefore, the supplementation of 1% OF would be sufficient to provide the high inhibiton of ACE. This might be interpreted as binding interaction between phenolic compounds and milk proteins which caused a decrease in the bioavailability of polyphenols in yogurt as ACE-I inhibitors (Baba et al. 2014). Habibi et al. (2017) found that the addition of prebiotic compounds (inulin and wheat fibres) significantly increased ACE-inhibition of all treatments. The ACE-inhibitory activity was determined as IC50 which was the concentration of the protein required to inhibit 50% of ACE activity. As shown in Fig. 2b, IC50 values ranged from 78.55 μg/ml (determined in OF1% yogurt at day 21 of storage) to 127.31 μg/ml (in control yogurt at the beginning of storage). The calculated IC50 values are in line with the results obtained by other studies (Donkor et al. 2005; Papadimitriou et al. 2007). Calculation of ACE-inhibitory activity as IC50 showed that an increase in OF-yogurts throughout the storage period similar to the results of percentage ACE-inhibition. Papadimitriou et al. (2007) also indicated that an increase in ACE-I activity and peptide content in water soluble extract of sheep milk yogurt at 4 °C for 26 days. However, a significant decrease was observed in ACE-inhibitory activity of control sample after 14 days of storage (Fig. 2b).
Fig. 2.
Angiotension-converting enzyme (ACE)-inhibitory activity and antioxidant activity of the yogurt samples during 21 days of storage presented as: a IC50 (concentration of the protein required to inhibit 50% of ACE activity) values of WSEs, b Percentage ACE inhibition of WSEs. c Percentage antioxidant activity of WSEs. The values presented as mean ± standard error of two replicate trials means; all data analysed in duplicate. (refer to Table 2 for abbreviations)
Antioxidant activity
The percent DPPH-scavenging as a criterion of antioxidant activities of water soluble extracts obtained from control and OF-yogurts during cold storage are presented in Fig. 2c. Results showed that antioxidant activity increased until 14 days of storage in all yogurt batches, while it decreased 21th days of storage. This finding was accordance with those reported several authors who found a similar trend in the antioxidant activity of yogurt during cold storage up to 14 days (Perna et al. 2013). Yogurts incorporated with OF had higher antioxidant activity (23.56–32.94%) than the control sample (20.36–24.51%) at all days of storage (p < 0.05).
Conclusion
Orange fibre addition to the probiotic yogurt had no negative effect on the bacterial counts. Conversely, it enhanced the survivability of the bacteria used, especially S. thermophilus ST-20Y and B. lactis BB-12 (p < 0.05). OF, as a possible stabilizer, showed practicable characteristics by improving viscosity and decreasing syneresis of yogurt. Viscosity and consistency index values were higher in yogurts containing 0.5%, 1% and 1.5% OF than control yogurt. But the addition of 2% OF affected negatively the viscosity and syneresis of yogurt. Thus, these results suggest that 2% and above levels of OF incorporation into yogurt was not suitable for the textural properties of yogurt. On the other hand, ACE-inhibitory and antioxidant activities of WSEs increased with the increment of OF levels. Hovewer, the highest ACE-inhibitory activity was observed in yogurt with 1% OF. The addition of OF decreased lightness (L*) values and increased a* and b* values. Based on all of these results, OF could be a suitable natural supplement for probiotic yogurt to enhance nutritional quality and to improve physicochemical properties, due to its nutritive value and beneficical effects on yogurt.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AOAC . Official methods of analysis of the Association of Analytical Chemists International. 18. Gaithersburg: AOAC International; 2005. [Google Scholar]
- Apostolidis E, Kwon YI, Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov Food Sci Emerg Technol. 2007;8:46–54. doi: 10.1016/j.ifset.2006.06.001. [DOI] [Google Scholar]
- Baba AS, Najarian A, Shori AB, Lit KW, Keng GA. Viability of lactic acid bacteria, antioxidant activity and in vitro inhibition of angiotensin-I-converting enzyme of Lycium barbarum yogurt. Arab J Sci Eng. 2014;39:5355–5362. doi: 10.1007/s13369-014-1127-2. [DOI] [Google Scholar]
- Bakirci S, Dagdemir E, Boran OS, Hayaloglu AA. The effect of pumpkin fibre on quality and storage stability of reduced-fat set-type yogurt. Int J Food Sci Technol. 2017;52:180–187. doi: 10.1111/ijfs.13264. [DOI] [Google Scholar]
- Casarotti SN, Penna ALB. Acidification profile, probiotic in vitro gastrointestinal tolerance and viability in fermented milk with fruit flours. Int Dairy J. 2015;41:1–6. doi: 10.1016/j.idairyj.2014.08.021. [DOI] [Google Scholar]
- Dervisoglu M, Yazici F. The effect of citrus fibre on the physical, chemical and sensory properties of ice cream. Food Sci Technol Int. 2006;12:159–164. doi: 10.1177/1082013206064005. [DOI] [Google Scholar]
- Dhingra D, Michael M, Rajput H, Patil RT. Dietary fiber in foods: a review. J Food Sci Technol. 2012;49:255–266. doi: 10.1007/s13197-011-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkor ON, Henriksson A, Vasiljevic T, Shah NP. Probiotic strains as starter cultures improve angiotensin-converting enzyme inhibitory activity in soy yogurt. J Food Sci. 2005;70:375–381. doi: 10.1111/j.1365-2621.2005.tb11522.x. [DOI] [Google Scholar]
- Elleuch M, Bedigian D, Roiseux O, Besbes S, Blecker C, Attia H. Dietary fiber and fiber-rich by-products of food processing: characterisation, technological functionality and commercial applications: a review. Food Chem. 2011;124:411–421. doi: 10.1016/j.foodchem.2010.06.077. [DOI] [Google Scholar]
- Erkaya T, Şengul M. Bioactivity of water soluble extracts and some characteristics of white cheese during the ripening period as effected by packaging type and probiotic adjunct cultures. J Dairy Res. 2015;82:47–55. doi: 10.1017/S0022029914000703. [DOI] [PubMed] [Google Scholar]
- Espírito Santo AP, Cartolano NS, Silva TF, Soares FA, Gioielli LA, Perego P, Oliveira MN. Fibers from fruit by-products enhance probiotic viability and fatty acid profile and increase CLA content in yoghurts. Int J Food Microbiol. 2012;154:135–144. doi: 10.1016/j.ijfoodmicro.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Espírito-Santo AP, Lagazzo A, Sousa ALOP, Perego P, Converti A, Oliveira MN. Rheology, spontaneous whey separation, microstructure and sensorial characteristics of probiotic yoghurts enriched with passion fruit fiber. Food Res Int. 2013;50:224–231. doi: 10.1016/j.foodres.2012.09.012. [DOI] [Google Scholar]
- Fernández-Ginés JM, Fernández-López J, Sayas-Barberá E, Sendra E, Pérez-Alvarez JA. Effect of storage conditions on quality characteristics of bologna sausages made with citrus fiber. J Food Sci. 2003;68:710–714. doi: 10.1111/j.1365-2621.2003.tb05737.x. [DOI] [Google Scholar]
- Fernández-López J, Sendra-Nadal E, Navarro C, Sayas E, Viuda-Martos M, Alvarez JAP. Storage stability of a high dietary fiber powder from orange by-products. Int J Food Sci Technol. 2009;44:748–756. doi: 10.1111/j.1365-2621.2008.01892.x. [DOI] [Google Scholar]
- García-Pérez FJ, Lario Y, Fernández-López J, Sayas E, Pérez-Alvarez JA, Sendra E. Effect of orange fiber addition on yogurt color during fermentation and cold storage. Color Res Appl. 2005;30:457–463. doi: 10.1002/col.20158. [DOI] [Google Scholar]
- Grigelmo-Miguel N, Martín-Belloso O. Characterization of dietary fiber from orange juice extraction. Food Res Int. 1998;31:355–361. doi: 10.1016/S0963-9969(98)00087-8. [DOI] [Google Scholar]
- Habibi NMB, Tavakoli M, Fatemizadeh SS. Investigation on the effect of fat content and prebiotic compound on proteolysis rate, ace-inhibitory and antioxidant activity of probiotic yogurt. J Appl Microbiol Food Ind. 2017;3:16–29. [Google Scholar]
- Hrnjez D, Vaštag Ž, Milanović S, Vukić V, Iličić M, Popović L, Kanurić K. The biological activity of fermented dairy products obtained by kombucha and conventional starter cultures during storage. J Funct Foods. 2014;10:336–345. doi: 10.1016/j.jff.2014.06.016. [DOI] [Google Scholar]
- Issar K, Sharma PC, Gupta A (2017) Utilization of apple pomace in the preparation of fiber-enriched acidophilus yogurt. J Food Process Preserv 41: n/a. 10.1111/jfpp.13098
- Kaczmarczyk MM, Miller MJ, Freund GG. The health benefits of dietary fiber: beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism. 2012;61:1058–1066. doi: 10.1016/j.metabol.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima KGDC, Kruger MF, Behrens J, Destro MT, Landgraf M, de Melo Gombossy, Franco BD. Evaluation of culture media for enumeration of Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium animalis in the presence of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus. LWT-Food Sci Technol. 2009;42:491–495. doi: 10.1016/j.lwt.2008.08.011. [DOI] [Google Scholar]
- Lourens-Hattingh A, Viljoen BC. Yogurt as probiotic carrier food. Int Dairy J. 2001;11:1–17. doi: 10.1016/S0958-6946(01)00036-X. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Nassar AG, AbdEl-Hamied AA, El-Naggar EA. Effect of citrus by-products flour incorporation on chemical, rheological and organolepic characteristics of biscuits. WJAS. 2008;4:612–616. [Google Scholar]
- Papadimitriou CG, Vafopoulou-Mastrojiannaki A, Silva SV, Gomes AM, Malcata FX, Alichanidis E. Identification of peptides in traditional and probiotic sheep milk yoghurt with angiotensin I-converting enzyme (ACE)-inhibitory activity. Food Chem. 2007;105:647–656. doi: 10.1016/j.foodchem.2007.04.028. [DOI] [Google Scholar]
- Paseephol T, Small DM, Sherkat F. Rheology and texture of set yogurt as affected by inulin addition. J Texture Stud. 2008;39:617–634. doi: 10.1111/j.1745-4603.2008.00161.x. [DOI] [Google Scholar]
- Perna A, Intaglietta I, Simonetti A, Gambacorta E. Effect of genetic type and casein haplotype on antioxidant activity of yogurts during storage. J Dairy Sci. 2013;96:3435–3441. doi: 10.3168/jds.2012-5859. [DOI] [PubMed] [Google Scholar]
- Robertson JA, de Monredon FD, Dysseler P, Guillon F, Amado R, Thibault JF. Hydration properties of dietary fiber and resistant starch: a European collaborative study. LWT-Food Sci Technol. 2000;33:72–79. doi: 10.1006/fstl.1999.0595. [DOI] [Google Scholar]
- Sah BNP, Vasiljevic T, McKechnie S, Donkor ON. Effect of probiotics on antioxidant and antimutagenic activities of crude peptide extract from yogurt. Food Chem. 2014;156:264–270. doi: 10.1016/j.foodchem.2014.01.105. [DOI] [PubMed] [Google Scholar]
- Sah BNP, Vasiljevic T, McKechnie S, Donkor ON. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT-Food Sci Technol. 2016;65:978–986. doi: 10.1016/j.lwt.2015.09.027. [DOI] [Google Scholar]
- Santos RO, Silva MVF, Nascimento KO, Batista AL, Moraes J, Andrade MM, Silva MC. Prebiotic flours in dairy food processing: technological and sensory implications. Int J Dairy Technol. 2018;71:1–10. doi: 10.1111/1471-0307.12394. [DOI] [Google Scholar]
- Sendra E, Fayos P, Lario Y, Fernandez-Lopez J, Sayas-Barbera E, Perez-Alvarez JA. Incorporation of citrus fibers in fermented milk containing probiotic bacteria. Food Microbiol. 2008;25:13–21. doi: 10.1016/j.fm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Sendra E, Kuri V, Fernandez-Lopez J, Sayas-Barbera E, Navarro C, Perez-Alvarez JA (2010) Viscoelastic properties of orange fiber enriched yogurt as a function of fiber dose, size and thermal treatment. LWT – Food Sci Technol 43:708–714
- Staffolo MD, Bertola N, Martino M. Influence of dietary fiber addition on sensory and rheological properties of yogurt. Int Dairy J. 2004;14:263–268. doi: 10.1016/j.idairyj.2003.08.004. [DOI] [Google Scholar]
- Tharmaraj N, Shah NP. Selective enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus, and Propionibacteria. J Dairy Sci. 2003;86:2288–2296. doi: 10.3168/jds.S0022-0302(03)73821-1. [DOI] [PubMed] [Google Scholar]
- Tseng A, Zhao Y. Wine grape pomace as antioxidant dietary fiber for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013;138:356–365. doi: 10.1016/j.foodchem.2012.09.148. [DOI] [PubMed] [Google Scholar]
- Vinderola CG, Mocchiutti P, Reinheimer JA. Interactions among lactic acid starter and probiotic bacteria used for fermented dairy products. J Dairy Sci. 2002;85:721–729. doi: 10.3168/jds.S0022-0302(02)74129-5. [DOI] [PubMed] [Google Scholar]
- Yi T, Huang X, Pan S, Wang L. Physicochemical and functional properties of micronized jincheng orange by-products (Citrus sinensis Osbeck) dietary fiber and its application as a fat replacer in yogurt. Int J Food Sci Nutr. 2014;65:565–572. doi: 10.3109/09637486.2014.898252. [DOI] [PubMed] [Google Scholar]