Abstract
The fortification of the processed cheese (PC) with bioactive compounds can increase their benefits for the health and also consumer acceptance. In the present study, rheological, chemical and sensory characteristics of the processed cheese containing different quantities of tomato powder (0%, 1%, 2% and 4% wt/wt) with the appropriate levels of bioactive components were evaluated during 90 days of storage. The results showed that the PC samples containing tomato powder (PCTs) had higher levels of proteolysis extent, phenolic and lycopene contents and antioxidant activity. However, the PCTs had lower levels of pH and lipolysis indexes. Generally, lycopene and phenolic contents and antioxidant activity decreased and pH, proteolysis and lipolysis index increased during storage. The results obtained from the frequency sweep test and temperature sweep test, indicated that, the samples had solid-type structure and tomato powder decreased the solid like behavior of the PC (G′ > G″). Sensory analysis showed that the processed cheese with the average level of tomato powder (2%) had maximum scores of flavor, color and total acceptance and also the PCTs were significantly (P < 0.05) less rigid and more spreadable than the control PC and these findings were according to the achievements obtained by dynamic oscillation rheometry. Finally overall results indicated that tomato powder could be helpful for the manufacturing of new product with different functional and sensory specifications.
Keywords: Processed cheese, Tomato, Proteolysis, Frequency sweep, Temperature sweep
Introduction
PCs are mixtures of cheese and other dairy and nondairy components which become homogenous by heating, mechanical shear and also by emulsifying salts (Kapoor and Metzger 2008). This product has low level of bioactive components and antioxidants and also its texture and properties allows to incorporate bioactive ingredients to this product. Therefore, functionality of processed cheeses could be improved by raising of its functional and bioactive compounds (Bachmann 2001). Effect of several functional materials such as extracts of different kinds of herbs and spices in cheese properties have been studied (Shan et al. 2011). Also substitution of the cheese fat with different oils including hazelnut oils, vegetable oils and fish oil emulsion (Fathi Achachlouei et al. 2015, 2013; Ye et al. 2009) have been investigated.
Tomato contains a high extent of functional ingredients such as lycopene, fiber, antioxidants, phenolic components, etc. and provides a remarkable quantity of antioxidants in the diet. Lycopene is a carotenoid which is causing the redness of tomato and tomato based products. Lycopene can act as an antioxidant and has many positive effects on prevention of many diseases (Georgé et al. 2011).
Lycopene has been added as a functional ingredient in several kinds of cheeses such as addition of powdered microcapsules of tomato extracts to Queso Blanco cheese (Jeong et al. 2017), and other foods such as extruded snacks fortified with lycopene (Da Costa et al. 2010).
One of the important determinants of processed cheese quality is its textural and rheological properties. Dynamic rheology testing is a useful and rapid technique for assessing the rheological features of viscoelastic materials like cheese which represents both solid (elastic) and liquid (viscous) specifications. Small deformation (dynamic oscillatory rheometry) techniques including stress or strain sweep, frequency sweep and temperature sweep are recommended to be used to evaluate the linear viscoelasticity of the PC (Gunasekaran and Ak 2003).
Characteristics of the processed cheese containing tomato powder (PCTs) have not been evaluated so far. The aim of this study is improving the functional and health attributes of the PC and also investigating the rheological, physicochemical and sensory traits of the PCTs.
Materials and methods
PC production
The processed cheese was produced by the method of Dimitreli and Thomareis (2008). Its ingredients were feta cheese, water (5%), butter (3%), emulsifier salts (2%) (Di sodium phosphate E339 (1%) and Tri sodium citrate E331 (1%). The processed cheese samples containing tomato powder prepared by mixing of the processed cheese with tomato powder (0% (the control sample), 1%, 2% and 4% wt/wt). The blending was done in a vertical vacuum mixer (Stephan Machinery Corp., Mundelein, Ill., U.S.A.).
Experiments
Effect of tomato powder on the processed cheese was investigated by compositional analysis of the cheese samples including dry matter, pH, fat and protein content, ripening indicators such as lipolysis index, proteolysis extent and stability of bioactive components including water-soluble phenolic content (WSPC), lycopene content and antioxidant activity (AOA) during storage of 3 month at days of (1, 20, 40, 60 and 90). Also the rheological and sensory properties were investigated after the production of the cheese samples.
Compositional analysis
Oven drying method at 102 °C was used for assessing of the moisture content of the PC samples according to the IDF (1982). Measurement of the fat content was carried out by Gerber method explained by Marshal (1992). The Protein content was estimated by the Kjeldahl procedure (IDF 1964). The pH of the PC samples was defined by insertion of a glass electrode of a digital pH meter (Hanna Instruments Pty. Ltd., Singapore) into the PC samples (Marshal 1992). The lycopene content of the samples was analyzed by the procedure of Javanmard et al. (2006) with slight modifications. WSPC was determined via the Folin–Ciocalteu technique described by Apostolidis et al. (2007).
Assessing of AOA
The AOA of the cheese samples was analyzed by DPPH (2, 2-diphenyl-1-picrylhydrazyl) radical scavenging assay according to the procedure explained by Apostolidis et al. (2007).
Ripening evaluations (lipolysis and proteolysis analyses)
The lipolysis extent was determined by the procedure explained by Nunez et al. (1996) which was de-emulsification and lipid extraction of the PC samples and titration by alcoholic KOH.
Proteolysis occurring during storage was monitored by measuring of the pH 4.6 soluble nitrogen (SN) fractions of the PC samples as described by Sousa and McSweeney (2001). Measurements of the pH 4.6-insoluble portion of the PC was carried out by Urea-polyacrylamide gel electrophoresis (PAGE) according to the method described by Shalabi and Fox (1987) which performed by Protean II XI vertical slab gel unit (Bio-Rad Laboratories Ltd., Watford, UK). Coomassie Brillant Blue G250 was used for staining of the gels, as explained by Blakesley and Boezi (1977).
Dynamic rheological measurements
Rheological measurements of the PC were performed using a controlled stress rheometer (Anton Paar, MCR301, Austria) via the technique described by Karami et al. (2009). The rheological factors including the storage modulus (G′), the loss modulus (G″) and the loss factor (tan d) were measured within the viscoelastic region. The limits of linear viscoelastic behavior of the PC were defined at room temperature with applying of frequency of 1 Hz and a strain sweep (0.1–100%).
Sensory analysis
A panel of 15 selected assessors evaluated the processed cheese samples via the method used by Macku et al. (2008). The PC samples were coded and cheese appearance (color), spreadability, rigidity, flavor and total acceptance evaluated by assessors at the room temperature, applying the five-point hedonic scale.
Statistical analysing
SPSS statistical software (IBM SPSS statistics 22) was used for data analyzing by one-way analysis of variance. The multiple comparison test on all main effect means was carried out by Duncan`s HST test at 5% significance level.
Results and discussion
Compositional measurements
The protein, fat and moisture contents of the PC were respectively about 12%, 23% and 56.67%. The fortified samples composition did not change significantly (P > 0.05) with tomato powder addition.
The pH of the PCTs was decreased by addition of the tomato powder. Also, the pH was not changed at the first stage of storage but there was an increase at the pH after 40 days of storage (Table 1). Bin Shan et al. (2011) reported that the pH raising during storage is hindered by herbal extracts due to their high quantity of phenolic composition.
Table 1.
Changes in pH, lycopene content (mg/Kg cheese), water soluble phenolic content (mg gallic acid/kg) and antioxidant activity (%) of control sample and cheese samples containing tomato powder during storage
| Cheese* | Storage time (day) | ||||
|---|---|---|---|---|---|
| 1 | 20 | 40 | 60 | 90 | |
| pH | |||||
| Control | 5.86b** | 5.86b | 5.86b | 5.83bc | 5.9a |
| PCT 1% | 5.75d | 5.75d | 5.75d | 5.71e | 5.8c |
| PCT 2% | 5.67f | 5.67f | 5.67f | 5.63g | 5.73de |
| PCT 4% | 5.52h | 5.52h | 5.53h | 5.49i | 5.61g |
| Lycopene content (mg/kg cheese) | |||||
| Control | 0.00j** | 0.00j | 0.00j | 0.00j | 0.00j |
| PCT 1% | 3.06e | 1.51gh | 1.73g | 1.37h | 0.58i |
| PCT 2% | 4.41d | 2.8e | 2.24f | 2.21f | 1.56gh |
| PCT 4% | 8.96a | 4.79c | 5.59b | 5.11c | 2.75e |
| Water soluble phenolic content (mg Gallic acid/kg) | |||||
| Control | 68.45f** | 97.15d | 99.67d | 87.35de | 64.42f |
| PCT 1% | 114.5bc | 78.52e | 68.98f | 60.54f | 42.26g |
| PCT 2% | 121.32ab | 129.88a | 30.18h | 40.62g | 41.25g |
| PCT 4% | 112.76c | 94.63d | 9.53i | 16.13i | 15.07i |
| Antioxidant activity (%) | |||||
| Control | 18g** | 12.8hi | 10.17i | 6.17j | 5.09j |
| PCT 1% | 37.05a | 18g | 16.08g | 14.52gh | 16.99g |
| PCT 2% | 35.29ab | 37.44a | 27.17de | 24.75ef | 21.35f |
| PCT 4% | 33.86abc | 36.62a | 30.43 cd | 32.12bc | 24.75ef |
*Control: process cheese. PCT 1%: process cheese with 1% wt/wt tomato powder. PCT 2%: process cheese with 2% wt/wt tomato powder. PCT 4%: process cheese with 4% wt/wt tomato powder
**The same letters show no significant difference (P > 0.05)
Lycopene content of the PCTs increased with the increasing of the tomato powder but lycopene content had a reduction during storage (Table 1). Generally, the lycopene levels of the fortified samples containing high amount of tomato powder (4% wt/wt) were decreased by 63% during 90 days of storage (Table 1). Lycopene can be degraded and converted to other compounds during storage to some extent, but the product with tomato powder had higher lycopene and bioactive content at the end which can have a positive effect on the consumer’s health. Since processed cheeses should be consumed within 2 months of storage, fortified cheeses still had higher content of lycopene after two months and could have beneficial effects for consumers.
Total phenolic content of tomato is 2.68 mg/g of DW (Jeong et al. 2017). Although, WSPC of the PCTs had a reduction during storage (Table 1), WSPC of the PCTs were higher than those in the control PC immediately after production and WSPC of the control PC did not have a significant (P > 0.05) change during 90 days of storage (Table 1). Rennet and starter in the cheese produce peptides during storage which may act as phenolic compounds and also some of these peptides which neutralize and inhibit the activity of the phenolic compounds existing in the cheese, could have interactions with the phenolic components of tomato. This could explain the decrease of WSPC of the PCTs during storage. These results are according to the findings of Fadavi and Beglaryan (2015). They reported that WSPC in the UF feta cheese containing peppermint were not as much as what expected, and also WSPC in the UF feta cheese without peppermint increased as the level of rennet increased. Several compositional and storage conditions can affect the interactions between proteins and phenolic compounds (Bartolome et al. 2000). According to the findings of Apostolidis et al. (2007), WSPC of the cheese supplemented with herbal extracts, did not have significant (P > 0.05) differences compared to those of the plain cheese. WSPC of the PCTs also could be influenced by Heat treatment. It is suggested to determine the WSPC accurately by HPLC rather than spectrophotometry.
Antioxidant activity
PCTs had significantly (P < 0.05) higher AOA levels than that in the control PC at all stages of storage (Table 1) and the AOA levels of the all cheese samples gradually diminished during storage (Table 1). This also could be due to the absorption of the phenolic molecules of tomato with active groups of proteins which might reduce the antioxidant effect of phenolic compounds. Starters presented in cheese produce enzymes which could turn the polyphenols to quinines. These quinines are very active and could react with proteins and change the physicochemical and nutritional specifications of proteins and the sensory characteristics of food materials (Shahidi and Naczk 2006). According to the findings of Apostolidis et al. (2007), AOA of the cheeses supplemented with herbal extracts were significantly (P < 0.05) higher than those in the nonenriched samples. Khalifa and Wahdan (2015), explained their research about the supplementation of the cheese with dehydrated cranberry fruit extract which enhanced the oxidation stability and decreased the acid value, proteolysis and lipolysis of the cheese. Although peppermint has a high antioxidant activity, the UF cheese supplemented with peppermint did not have significantly high AOA than those of plain samples (Fadavi and Beglaryan 2015).
Lipolysis and proteolysis assessments
The main agents responsible for the lipolysis and increasing of free fatty acid in cheese are thermoduric bacterial lipases which survive during pasteurization and also the intracellular bacterial lipases of cheese fat (Driessen 1989). Lipolysis has an important effect on the flavor of cheese during storage. The lipolysis index of the PCTs was lower than that in the control sample (Table 2). The lipolysis index of the cheese samples increased during storage. However, the increasing of the lipolysis index in the fortified cheeses was less than that in the control sample. This might be due to the heating process that was used in the production of the PCTs, and also tomato powder could influence the lipolysis of the PCTs. Driessen (1989) reported that thermization of milk (63 °C, 20 s) and also cooking of cheese curds (51 °C for 20 min) inactivated lipase enzymes.
Table 2.
Changes in lipolysis index (ml KOH 0.1 N/g fat) and level of pH 4.6-soluble nitrogen as % of total nitrogen (% SN/TN) of control sample and cheese samples containing tomato powder during storage
| Cheese* | Storage time (day) | ||||
|---|---|---|---|---|---|
| 1 | 20 | 40 | 60 | 90 | |
| Lipolysis index (ml KOH 0.1 N/g fat) | |||||
| Control | 4.73h** | 5.1g | 5.78f | 7d | 24.28a |
| PCT 1% | 4.4hi | 4.5hi | 4.4hi | 5.6f | 12b |
| PCT 2% | 3.6j | 4.3i | 5.2g | 6.5e | 10.23c |
| PCT 4% | 2.5l | 2.6l | 2.5l | 3k | 6.72de |
| % SN/TN | |||||
| Control | 0.75l** | 1jkl | 1.08jkl | 1.25jk | 1.33j |
| PCT 1% | 0.91kl | 3.25i | 4.41g | 5.91e | 7.41d |
| PCT 2% | 0.91kl | 3.75h | 5.5f | 7.33d | 9b |
| PCT 4% | 1jkl | 4.66g | 5.75ef | 8.25c | 10.16a |
*See Table 1 for the treatments
**The same letters show no significant difference (P > 0.05)
SN (soluble nitrogen at pH 4.6) is an index of proteolysis and includes peptides, whey proteins, proteoso peptones, and free amino acids. The main reasons for the production of these nitrogen compounds are Rennet and plasmin proteolytic activity and/or microorganism peptidases (O’keefe et al. 1978). There was no significant (P > 0.05) enhancement in the quantity of the soluble nitrogen in the control PC during 90 days (Table 2). On the other hand, the SN levels of the PCTs had a significant (P > 0.05) intensifications during 90 days. These results denoted that tomato powder caused an enhancement in the proteolysis of the processed cheese during storage.
Electrophoretic patterns of the samples demonstrated that the hydrolysis of β-casein in the PCTs was more than that in the control sample and the accumulation of γ-caseins as the primary yield of β-casein breakdown was somewhat evident (Fig. 1). Also, β-casein was hydrolyzed faster than αs1-casein thus, the intense bands of γ-caseins were accumulated and there was no evidence of the aggregation of breakdown yields of αs1-casein (Fig. 1). These results were similar to the results of Mulvihil and McCarthy (1994). According to their findings, caseins are mainly the reason of remaining proteolytic activity of processed cheeses that arise during storage, since processed cheeses are produced from rennet casein. Commonly, the proteolytic factor in processed cheeses prepared from rennet casein is plasmin which incorporates in the substantial quantity of rennet caseins (Mulvihil and McCarthy 1994).
Fig. 1.
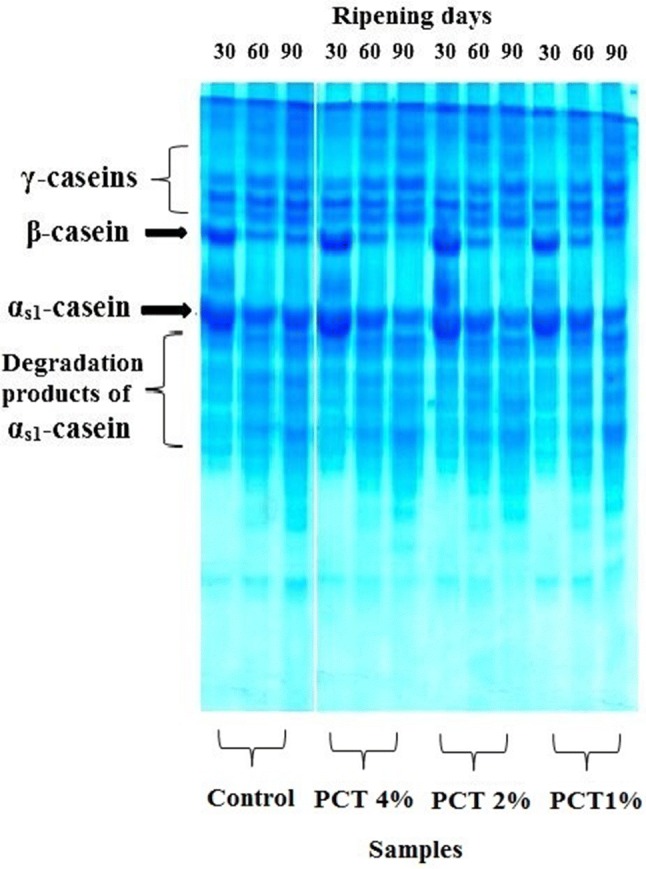
Electrophoretic patterns of cheese samples. Control: process cheese. PCT 1%: process cheese with 1% wt/wt tomato powder. PCT 2%: process cheese with 2% wt/wt tomato powder. PCT 4%: process cheese with 4% wt/wt tomato powder
According to the SN levels and electrophoretic patterns of the PCTs, tomato powder, made an enhancement in the proteolytic activity of processed cheese owing to thermoduric micro flora or indigenous proteolytic enzymes of tomato powder.
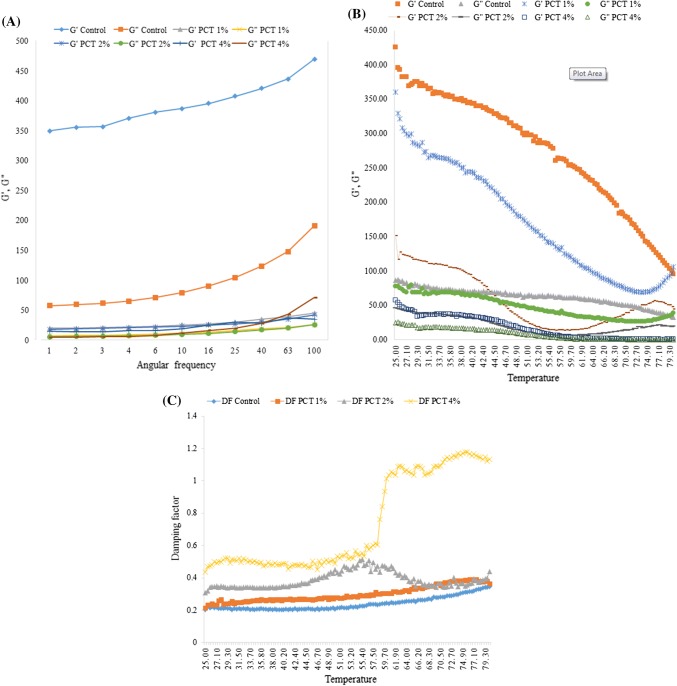
Dynamic rheological behavior of the processed cheeses
Several Compositional and processing Factors are reported to influence the rheological characteristics of processed cheeses (Dimitreli and Thomareis 2008). Dynamic rheological measurements were carried out by using of the linear viscoelastic area of the PC samples. The frequency sweep test showed that G′ in the all samples was higher than G″, and also both of the factors (G′ and G″) increased as frequency increased (Fig. 2a) indicating that the processed cheese structure is solid type. G′ and G″ levels of the PCTs were less than those in the control sample. However, G′ and G″ values among the PCTs did not have any significant (P > 0.05) differences (Fig. 2a). These results showed that tomato powder decreased the solid like behavior of the processed cheese. One of the reasons for this effect of tomato powder could be the effect of stirring and heating of the processed cheese while making the samples. According to the research of Jeong et al. (2017) about the Queso Blanco cheese showed that gumminess, chewiness, and hardness of the cheese samples containing powdered microcapsules of tomato extract which had a different production method, were higher than those in the control sample. They explained that, this effect could be due to lower pH levels of the supplemented Queso Blanco cheese.
Fig. 2.
a Changes in storage modulus (G′) and loss modulus (G″) as function of angular frequency, measured at strain of 0.2% and temperature of 5 °C for processed cheeses. b Storage modulus and loss modulus, during temperature sweep (25–80 °C) at frequency of 10 1/s and strain of 1% for the processed cheeses. c The changes in damping factor (tan d) during temperature sweep (25–80 °C) at frequency of 0.1 Hz and strain amplitude of 0.5% for cheese samples. See Fig. 1 for the treatments
Dynamic rheology measurements about the heating effects on PC viscoelasticity could obtain helpful outcomes. The temperature sweep test showed that the values of G′ for the all products at 25 °C, were significantly (P < 0.05) higher than the values of G″ (Fig. 2b), denoting the overcoming elastic structure of the processed cheese. G′ values of the cheese samples decreased as temperature increased from 25 to 80 °C. According to the findings of Rosenberg et al. (1995) these specifications has already been observed in the natural cheddar cheese and also the same results has been achieved about the imitation Mozzarella cheese (Mounsey and O’Riordan 1999) which showed that the elasticity of the cheese structure diminished as the temperature increased. Hennelly et al. (2006) explained about this behavior of the cheese that the heating of the PC could make its fat globules to melt and deform followed by plasticizing and loosening of the interactions between proteins which induce the protein structure to flow. G′ and G″ of the PCTs at 25 °C was lower than those in the control sample and the samples with higher concentration of tomato powder, had lower G′ and G″ (Fig. 2b). These results showed that adding tomato powder, decreased the firmness of the processed cheese. G′ of the fortified samples slightly increased or became liner at more than about 60 °C. It means that after this temperature some kind of solidification occurred in the samples. Inulin also had similar effect on the imitation cheese. According to the results of Hennelly et al. (2006), using inulin instead of fat in the PC increased the G′ and G″ values above 55 °C. Liu et al. (2008) and Cernıkova et al. (2008) reported that the raising concentrations of pectin gel, ι-carrageenan and k-carrageenan in the formulation of cheese increased the intensive interactions between their fiber chains causing the establishment of a denser network structure of the cheese.
Tan d (G″/G′) could be a helpful index for meltability of the PC. the modulus value at tan d = 1, is crossover modulus, where the sample has elastic and viscose characteristics at the same time in a way that if tan d is minor 1, the sample is more solid like, and if it is more than 1 the sample is more liquid like (Mounsey and O’Riordan 1999). In the temperature sweep test, the G′ and G″ curves of the PCTs with high concentrations of tomato powder crossed each other (tan d = 1) at 60 °C and above this temperature, viscose behavior of the PC prevailed the elastic one (Fig. 2c). These results showed that the viscose behavior of PC above 60 °C increased followed by weakening of the network structure, denoting that the reduction of G′ overcame to the reduction of G″. On the other hand, raising of the temperature above 85 °C might increase the interactions between proteins and also between proteins and polysaccharides that decreased the liquid like behavior of the PCTs. Hosseini-Parvar et al. (2014) reported the same achievements about the processed cheese supplemented with basil seed gum.
Sensory analysis
Analyzing of the sensory properties of the samples showed that the processed cheese samples containing tomato powder had higher scores of total acceptance, flavor and color than those in the control samples (Fig. 3). Maximum scores of flavor, color and total acceptance belonged to the PC with 2% wt/wt of tomato powder and as tomato powder increased, the rigidity decreased and the spreadability increased (P < 0.05) (Fig. 3). These findings were similar to the findings achieved by dynamic oscillation rheometry.
Fig. 3.
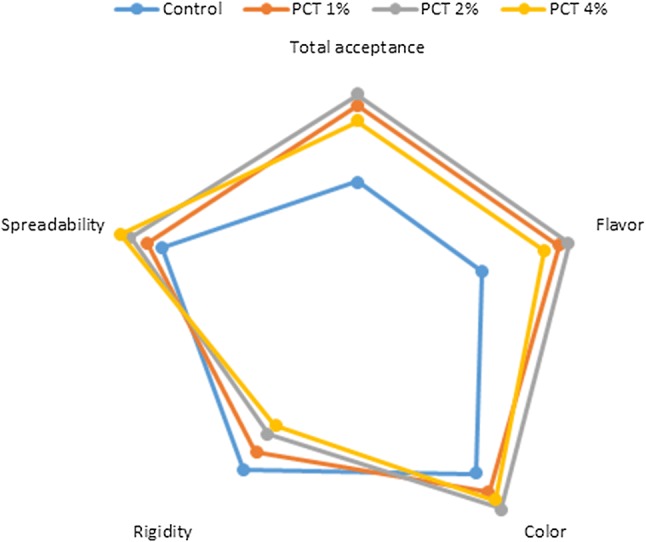
Results (expressed as median) of the sensory analysis of tested processed cheese samples. Used hedonic scales: Total acceptance: 1—unacceptable to 5—excellent, flavor: 1—very poor to 5—excellent, color: 1—very poor to 5—excellent. Rigidity: 1—very soft to 5—very stiff. Spreadability: 1—spreadability is impossible to 5—optimal spreadability. See Fig. 1 for the treatments
Conclusion
The processed cheese samples containing tomato powder did not have significantly (P < 0.05) different values of protein, fat and moisture content. The PCTs generally had higher quantities of lycopene, WSPC and AOA, which are important from the nutritional point of view. The processed cheese containing tomato powder had higher proteolysis and lower lipolysis and pH than those in the control sample. Generally proteolysis, lipolysis and pH of the samples increased during storage. Sensory analysis showed that the maximum scores of flavor, color and total acceptance belonged to the PC with 2% wt/wt of tomato powder and also tomato powder decreased the rigidity and increased the spreadability of the processed cheese which are positive attributes in the processed cheese and corresponded to the results obtained by rheological measurements. The results indicated that tomato powder could be useful for the producing of a new variety of processed cheese with novel sensory and functional properties.
Funding
Funding was provided by University of Tabri (Grant No. 2432).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Apostolidis E, Kwon YI, Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov Food Sci Emerg Technol. 2007;8:46–54. doi: 10.1016/j.ifset.2006.06.001. [DOI] [Google Scholar]
- Bachmann P. Cheese analogues: a review. Int Dairy J. 2001;11:505–515. doi: 10.1016/S0958-6946(01)00073-5. [DOI] [Google Scholar]
- Bartolome B, Estrella I, Hernandez MT. Interaction of low molecular weight phenolics with proteins (BSA) J Food Sci. 2000;65:617–621. doi: 10.1111/j.13652621.2000.tb16060.x. [DOI] [Google Scholar]
- Blakesley RW, Boezi JA. A new staining technique for proteins in polyacrylamide gels using coomassie brilliant blue G250. Anal Biochem. 1977;82:580–581. doi: 10.1016/0003-2697(77)90197-X. [DOI] [PubMed] [Google Scholar]
- Cernıkova M, Bunka F, Pavlınek V, Brezina P, Hrabe J, Valasek P. Effect of carrageenan type on viscoelastic properties of processed cheese. Food Hydrocoll. 2008;22:1054–1061. doi: 10.1016/j.foodhyd.2007.05.020. [DOI] [Google Scholar]
- Da Costa PFP, Ferraz MBM, Ros-Polski V, Quast E, Collares Queiroz FP, Steel CJ. Functional extruded snacks with lycopene and soy protein. Ciênc Tecnol Aliment. 2010;30:101. doi: 10.1590/S0101-20612010005000017. [DOI] [Google Scholar]
- Dimitreli G, Thomareis AS. Effect of chemical composition on the linear viscoelastic properties of spreadable-type processed cheese. J Food Eng. 2008;84:368–374. doi: 10.1016/j.jfoodeng.2007.05.030. [DOI] [Google Scholar]
- Driessen FM (1989) Inactivation of lipases and proteinases (indigenous and bacterial). In: Heat-induced changes in milk; Bulletin 238; International Dairy Federation: Brussels, Belgium
- Fadavi A, Beglaryan R. Optimization of UF-Feta cheese preparation, enriched by peppermint extract. J Food Sci Technol. 2015;52:952–959. doi: 10.1007/s13197-013-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi Achachlouei B, Hesari J, Azadmard Damirchi S, Peighambardoust SH, Esmaiili M, Alijani S. Production and characterization of a functional Iranian white brined cheese by replacement of dairy fat with vegetable oils. Food Sci Technol Int. 2013;19:389–398. doi: 10.1177/1082013212455341. [DOI] [PubMed] [Google Scholar]
- Fathi Achachlouei B, Hesari J, Azadmard Damirchi S. Physicochemical, sensory properties and proteolysis index of produced cheese by replacement of milk fat with hazelnut oils. J Food Process Preserv. 2015;7:77–89. [Google Scholar]
- Georgé S, Tourniaire F, Gautier H, Goupy P, Rock E, Caris-Veyrat C. Changes in the contents of carotenoids, phenolic compounds and vitamin C during technical processing and lyophilisation of red and yellow tomatoes. Food Chem. 2011;124:1603–1611. doi: 10.1016/j.foodchem.2010.08.024. [DOI] [Google Scholar]
- Gunasekaran S, Ak MM (2003) Cheese rheology and texture. CRC Press LLC, Boca Raton. eBook ISBN: 9781420031942
- Hennelly PJ, Dunne PG, OSullivan M, ORiordan ED. Textural, rheological and microstructural properties of imitation cheese containing inulin. J Food Eng. 2006;75:388–395. doi: 10.1016/j.jfoodeng.2005.04.023. [DOI] [Google Scholar]
- Hosseini-Parvar SH, Matia-Merino L, Golding M (2014) Characterization of gum extracted from basil seed (Ocimum basilicum L.): a physicochemical and rheological study. Submitted for publication in Carbohydr Polym Food Hydrocoll 1–11. 10.1016/j.foodhyd.2014.07.015
- IDF (1964) Determination of the protein content of processed cheese products. Standard No. 25, International Dairy Federation, Brussels
- IDF (1982) Determination of the total solid content: (cheese and processed cheese). Standard No. 4a, International Dairy Federation, Brussels, Belgium
- Javanmard M, Rokni N, Bokai S, Shahhosseini G. Effects of gamma irradiation and frozen storage on microbial, chemical and sensory quality of chicken meat in Iran. Food Control. 2006;17:469–473. doi: 10.1016/j.foodcont.2005.02.008. [DOI] [Google Scholar]
- Jeong HJ, Lee YK, Ganesan P, Kwak HS, Chang YH. Physicochemical, microbial, and sensory properties of queso blanco cheese supplemented with powdered microcapsules of tomato extracts. Korean J Food Sci An. 2017;37(3):342–350. doi: 10.5851/kosfa.2017.37.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor R, Metzger LE. Process cheese: scientific and technological aspects: a review. Compr Rev Food Sci Food Saf. 2008;7:194–214. doi: 10.1111/j.1541-4337.2008.00040. [DOI] [Google Scholar]
- Karami M, Ehsani MR, Mousavi SM, Rezaei K, Safari M. Changes in the rheological properties of Iranian UF-Feta cheese during ripening. Food Chem. 2009;112:539–544. doi: 10.1016/j.foodchem.2008.06.003. [DOI] [Google Scholar]
- Khalifa SA, Wahdan KM (2015) Improving the quality characteristics of white soft cheese using cranberry (Vaccinium macrocarpon) fruit extract. Int Food Res J 22: 2203–2211. http://www.ifrj.upm.edu.my
- Liu H, Xu XM, Guo SD. Comparison of full-fat and low-fat cheese analogues with or without pectin gel through microstructure, texture, rheology, thermal and sensory analysis. Food Sci Technol Int J. 2008;43:1581–1592. doi: 10.1111/j.13652621.2007.01616.x. [DOI] [Google Scholar]
- Macku I, Bun Ka F, Pavlınek V, Lecianova P, Hrabe J. The effect of pectin concentration on viscoelastic and sensory properties of processed cheese. Int J Food Sci Technol. 2008;43:1663–1670. doi: 10.1111/j.13652621.2008.01734.x. [DOI] [Google Scholar]
- Marshal RT (1992) Standard methods for the examination of dairy products. Am J Public Health. Washington, DC http://www.nal.usda.gov/
- Mounsey JS, O’Riordan ED. Empirical and dynamic rheological data correlation to characterize melt characteristics of imitation cheese. J Food Sci. 1999;64:701–703. doi: 10.1111/j.1365-2621.1999.tb15114.x. [DOI] [Google Scholar]
- Mulvihil DM, McCarthy A. Proteolitic and rheological changes during aging of cheese analogues made from rennet casein. Int Dairy J. 1994;4:15–23. doi: 10.1016/09586946(94)90046-9. [DOI] [Google Scholar]
- Nunez F, Rodriguez MM, Cordoba JJ, Bermudez ME, Asensiov MA. Yeast population during storage of dry-cured Iberian ham. Int J Food Microbiol. 1996;29:271–280. doi: 10.1016/0168-1605(95)00037-2. [DOI] [PubMed] [Google Scholar]
- O’keefe AM, Fox PF, Daly C. Proteolysis in cheddar cheese: role of coagulants and starter bacteria. J Dairy Res. 1978;45:465–477. doi: 10.1017/S002202990001668X. [DOI] [Google Scholar]
- Rosenberg Z, Chuang SL, Shoemaker CF. Viscoelastic property changes in Cheddar cheese during storage. J Food Sci. 1995;60:640–664. doi: 10.1111/j.13652621.1995.tb09846.x. [DOI] [Google Scholar]
- Shahidi F, Naczk M (2006) Phenolics in food and nutraceuticals. CRC Press LLC, Boca Raton, pp 439–478
- Shalabi SI, Fox PF (1987) Electrophoretic analysis of cheese: comparison of methods. Irish J Food Sci Technol 11:135–151. https://www.jstor.org/stable/25558164
- Shan B, Cai YZ, Brooks JD, Croke H. Potential application of spice and herb extracts as natural preservatives in cheese. J Med Food. 2011;14:284–290. doi: 10.1089/jmf.2010.0009. [DOI] [PubMed] [Google Scholar]
- Sousa MJ, McSweeney PLH (2001) Studies on the storage of cooleeney an Irish farmhouse camembert-type cheese. Irish J Agric Food Res 40:83–95. https://www.jstor.org/stable/25562424
- Ye A, Cui J, Taneja A, Zhu X, Singh H. Evaluation of processed cheese fortified with fish oil emulsion. Food Res Int. 2009;42:1093–1098. doi: 10.1016/j.foodres.2009.05.006. [DOI] [Google Scholar]