Abstract
Seven combinations of yogurt; C1 [yogurt starter culture (YSC)], T1, [YSC + Lactobacillus acidophilus (LA)], T2 [YSC + Bifidobacterium bifidum (BB)], T3 [YSC + Lactobacillus plantarum (LP)], T4 [YSC + Lactobacillus casei (LC)], T5 [YSC + LA + BB] and T6 [YSC + LP + LC] were developed. Nutritional [proximate and minerals], rheological [total soluble solids (TSS), pH, titratable acidity (TA), water holding capacity, synersis, viscosity] organoleptic and probiotic properties [viability, acid tolerance, bile salt tolerance] were assessed with standard methods. Nutritional composition differed significantly among samples except for the iron and zinc (P < 0.05). Yogurt containing LP as single or in combination with LC resulted in significantly higher ash, protein, calcium and phosphorous level. Probiotic combination also significantly affected the rheological properties of yogurts (P < 0.05). Yogurt with LP and LC as single or in combination lead to significantly higher TSS and viscosity while significantly low syneresis, whereas yogurt with LA as single or in combination resulted in low pH and high TA (P < 0.05). Interestingly, combination of LA and BB increased TSS, reduced pH and syneresis as compare to these bacteria as single probiotic source. Panel experts found yogurt with LP more flavourful. Combination of multi-strain and multi-species probiotic resulted in improved texture but we found no significant difference in overall acceptability. Combination of probiotic strains also resulted in better probiotic potential with multi-species combination found to be even more effective. BB seemed more stable than three other probiotic strains. The present study can be helpful to dairy industry in developing new probiotic products and may provide a rational for selecting a combination of probiotic strains.
Keywords: Probiotics, Nutritional properties, Strain combination, Rheological properties, Probiotic potential
Introduction
Probiotics are “live microorganisms, when administered in adequate amounts confer a health benefit on the host” (FAO/WHO 2001).
Different probiotics have been shown to exhibit certain health benefits common to most or all probiotic species known as “core benefits” includes regulation of intestinal transit, normalization of perturbed microbiota, turnover of enterocytes, competitive exclusion of pathogens, colonization resistance, and short-chain fatty acid production. Other health benefits are probiotic strain specific this includes neurological effects, immunological effects, endocrinological effects, and the production of bioactives (Scourboutakos et al. 2017).
The Joint FAO/WHO Working Party Report in 2002 suggested that microbes should have a minimum set of characteristics that could predict probiotic potential. These included the ability to resist passage through the stomach in the presence of acid and pepsin, and the ability to grow in the proximal small intestine in the presence of pancreatin and bile salts (FAO, WHO 2002). Traditional yogurt starters have nonhuman origins, and they (especially streptococci) are known to suffer from exposure to gastric acidic conditions (Elli et al. 2006).
Substantial amount of scientific research has been conducted in the past to assess the effectiveness of individual probiotic strain on improving various disease conditions, assessing knowledge of probiotics among health professionals (Soni et al. 2018) and understanding their mechanism of action. Probiotic research and development have traditionally focused on single strains for specific health application and little information exists on the influence of probiotic strain combination on nutritional, rheological properties and sensory characteristics of yogurts and fermented milks. Some studies have reported multi-strain probiotics as more efficient than single strain for gut and immune function (Chapman et al. 2011; Wu et al. 2013). Additionally, it has been suggested that multi-strain probiotic produces better texture and nutritional properties in cheese than mono-strain probiotic (Setyawardani et al. 2016). That being said, it should be noted that not all strain mixtures are beneficial, as strains can antagonize one another. Therefore, research is needed to verify if mixtures are synergistic or antagonistic (Scourboutakos et al. 2017). There is a lack of research on multi-strain probiotics because such research is more difficult to conduct and thus more expensive. Yogurt physical and sensory properties are important aspects for consumer acceptability and can be altered by the addition of some ingredients, acid production and microbial growth during fermentation and storage (Da Silva et al. 2017).
The main objectives of this study were to produce probiotic yogurt with combination of probiotic strain cultures and to investigate the effect of these combinations on some nutritional, rheological, sensory and probiotic properties during refrigerated storage.
Methods and materials
Procurement of cultures
Freeze dried bacterial cultures namely; Lactobacillus delbrueckiisubsp. bulgaricus (LB) (NCDC-253), Streptococcus thermophilus (ST) (NCDC-199), Lactobacillus acidophilus (LA) (NCDC-13), Bifidobacterium bifidum (NCDC-229) (BB), Lactobacillus casei (LC) (NCDC-17) and Lactobacillus plantarum (LP) (NCDC-20) were procured from National Dairy Research Institute, Karnal, India. All the reagents and glassware were sterilized either by autoclaving or hot air oven for each set of experiment.
Preparation of starter and mother culture
All NCDC cultures were maintained in sterilized reconstituted skimmed milk (RSM-11 g skimmed milk powder in 100 mL distilled water, pasteurized at 82 °C for 30 min and cooled to 37 °C). The sterilized skimmed milk bottles were inoculated with freeze dried cultures and incubated at 37 °C for 8 h. All the cultures were then subsequently inoculated in another set of sterilized RSM test tubes and incubated at 37 °C for 8 h, thereafter stored at 4 °C. The propagation of stock cultures (37 °C) was done once in 15 days to maintain their activity, whereas working cultures were freshly prepared in RSM as and when needed. Skimmed milk was medium heated before propagation. Cultures were stored at 4 °C between the transfers. Starter cultures were maintained and propagated individually and were mixed just before use.
Development of probiotic yogurt
Fresh toned milk (with 3% fat and 8.5% SNF) was heat treated at 90 °C for 15 min followed by immediate cooling at 37 °C. This milk was inoculated with a culture combination comprising yogurt starter culture (ST and LB at the ratio of 1:1 at 2% v/v) and probiotic cultures at the level of 2% v/v of final milk volume, filled in disposable cups and sealed with cup sealing machine followed by incubation at 37 °C for 10 h or until pH reached 4.5 then refrigerated at 4 °C. Total seven types of yogurts were developed T1 (YSC + LA), T2 (YSC + BB), T3 (YSC + LP), T4 (YSC + LC), T5 (YSC + LA + BB), T6 (YSC + LP + LC) and C1 (YSC only) served as control.
Assessment of nutritional composition
The yogurt samples were analyzed for proximate including moisture, ash, protein, fat, carbohydrate and minerals including calcium, phosphorous iron and zinc as described in the Association of Official Analytical Chemist procedures (AOAC 2002). Ash was assessed by taking the dried samples and using a muffle furnace to burn off all non-mineral matter. Protein content was analyzed using the Kjeldahl digestion method and a nitrogen conversion factor of 6.38. Fat was examined using Mojonnier method for fat analysis of dairy products. Carbohydrate content was then obtained by determining the variance in total solids to the other solid components. Calcium, Phosphorous, iron and zinc were analysed by using atomic absorption spectrophotometer after wet digestion (Lindsey and Norwal 1969). Three trials from each of the formulations were examined for chemical content.
Assessment of rheological properties
Total soluble solids
The total soluble solids (TSS) were determined as per method given by Mazumdar and Majumder (2003) using Digital-Bench Refrectometer. Instrument was cleaned with distilled water and adjusted to zero at 20 °C. Sample was placed on the prism plate of the refractometer with the help of the glass rod. The reading appeared on the screen was directly recorded as total soluble solids. For each sample instrument was calibrated using distilled water.
Assessment of pH
The pH of different yogurt samples was determined in duplicate by using M-tronics digital pH meter. The pH was determined by inserting a pH probe, directly into a homogenized sample.
Titratable acidity
5 mL of diluted yogurt samples were mixed with 100 mL of boiling water and titrated with 0.1 N (NaOH) until reaching the pale pink end point with phenolphthalein indicator.
Water holding capacity
Water-holding capacity (WHC) was determined using a procedure given by Guzman-Gonzalez et al. (1999). 20 g of yoghurt (Y) was centrifuged for 30 min at 1250× g at 20 °C. The whey expelled (WE) was removed and weighed. The WHC was determined as.
Syneresis
Syneresis of the yogurt was assessed through the centrifugation procedure given by Motoki and Seguro (1998). 20 g of yogurt was taken into a 50 mL glass tube and was centrifuged at 3500 rpm for 15 min at 20 °C. The syneresis was estimated as the percentage of the released whey over the initial gel weight.
Viscosity
The viscosity was measured using rotational viscometer (Brookfield model DV II, USA). Samples were put in a stainless measuring cylinder and viscosity readings taken on the viscometer at 600 rev/min. Viscosity measurements were carried out after yogurt production and during 10 days storage at temperature 4 °C using a Brookfield LV spindle no. 4 at 10 rpm.
Assessment of probiotic potential
Acid tolerance
Acid tolerance of the probiotic bacteria used to prepare yogurt was estimated via method given by Conway et al. (1987) and Sahadeva et al. (2011). 1 mL of yogurt sample was inoculated in MRS broth in tubes with varying pH 1, 1.5, 2, 3, 4 adjusted with 1 M HCl. The tubes were incubated 37 °C for 3 h. At 0 h (immediately after inoculation) 1 mL sample from each pH tube was inoculated in 9 mL broth and plated on MRS agar plates. After 1.5, 2, 2.5 and 3-h 1 mL sample was again inoculated in 9 mL broth form each tube and plated on MRS agar plates at 37 °C for 48 h. Broth with pH 6.2 served as a control for the study. Each assay was performed in duplicates. Acid tolerance was estimated by comparing the growth of viable cell counts in all the MRS agar plates after 48 h.
Bile salt tolerance
The effects of bile on the growth of probiotic strains were examined using methods given by Tsai et al. (2007). Bile salt tolerance was estimated at the end of the third hour of acid tolerance test. 5 mL sample from acid tolerance sample pH 1, 1.5, 2, 3 and 4 was taken in centrifuge tubes and centrifuged at 4000 rpm for 10 min at 25 °C. After that the supernatant was discarded and pallets were washed with PBS and centrifugation was repeated again at 4000 rpm for another 10 min at 25 °C, supernatant was again discarded and the remaining sample was re-suspended into three MRS broths with different bile salt concentrations (0.3, 0.5 and 2.0%), incubated aerobically/anaerobically at 37 °C for 24 h. Subsequently, 0.1 mL was pipetted out from each of the MRS broth and serial dilutions were performed for plating (duplicates). All the plates were incubated aerobically/anaerobically at 37 °C for 48 h. Bile tolerance was determined by comparing the viable cell counts on MRS agars with and without bile salt. Broth with 0% bile concentration serves as a control for the study.
Viability of probiotic bacteria during storage
The survival rate of the probiotic bacteria was investigated over 10 days of cold storage at 4 °C at day 0, and 10th. Yogurt sample was added to phosphate-buffered saline (PBS) and the appropriate serial dilutions were prepared. LA was enumerated selectively using deMan, Rogosa, Sharpe (MRS) agar, LB on MRS agar at pH (5.4) 1 M HCl was used to adjust the pH of the medium, STM-17 agar, LPSM (L. plantarum selective medium) was used to enumerate LP, MRS-clindamycin-ciprofloxacin (MRS-CC) agar for selective enumeration of LC and for BB 0.05% l-cysteine hydrochloride was added in the MRS medium. Enumeration was carried out using the pour plate technique. The plates were incubated at 37 °C for at least 72 h under both aerobiosis and anaerobiosis (Bujalance et al. 2006). After incubation, colonies were counted.
Assessment of storage stability
The packaged yogurt samples were stored at 4 °C for 10 days. Samples were monitored for rheological, microbial, organoleptic properties at 0 day, and at 10th day. Analysis of microbial count was done by estimating: total bacterial count (TBC), total mould count (TMC) and total yeast count (TYC), coliform count, staphylococcus count, salmonella and shigella count on nutrient agar, potato dextros agar, mannitol agar, MRS agar, MRS broth, MacConckey agar and Eosin-methylene blue agar respectively.
Assessment of sensory properties was done by a panel of 24 members including students and staff of the university. Yogurt samples were given two-digit codes and were served chilled. Panellists were provided with a glass of water and, instructed to rinse their palate with water and drink water between samples. They were given written instructions and asked to rate the coded samples on color, flavour, texture, aroma, consistency, appearance, mouthfeel, sourness, sweetness and overall acceptability, using a nine-point hedonic scale [1 = like extremely to 9 = dislike extremely].
Statistical analysis
The mean value of three measurements was taken for each parameter assessed in the study. Data obtained from the nutrient analysis, rheological properties of the samples were evaluated statistically using a variance analysis (ANOVA) and the Duncan’s new multiple range test. Survivability percentage was calculated to assess acid and bile salt tolerance and paired‘t’test was used to assess the effect of refrigerated storage on rheological properties, microbial quality, organoleptic properties and viability of probiotic bacteria in different yogurt formulations. All the statistical analysis was done using SPSS version 17.0 (Chicago, USA). The level of significance was set at P < 0.05.
Results
Effect on nutritional composition
The nutritional analysis of the developed probiotic dairy products revealed (Table 1) that the probiotic strain combination had significant effect on nutritional composition. There was approximately 86-88% moisture, 0.6–0.8 g ash, 3.19–3.93 g protein, 2.0 g fat, 5.0–8.0 g carbohydrate, 53–63 calories, 98–125 mg calcium, 75–93 mg phosphorous, 0.32–0.36 mg zinc and 0.2–0.23 mg iron per 100 g probiotic yogurts.
Table 1.
Nutrient composition and rheological properties of yogurt
| S. No. | Properties | C1 | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|---|---|
| Nutrient composition* | ||||||||
| 1. | Moisture (%) | 87.32 ± 4.40b | 87.46 ± 2.63b | 88.13 ± 2.61b | 85.81 ± 3.90a | 86.03 ± 2.34a | 88.52 ± 3.52b | 86.22 ± 2.99a |
| 2. | Ash (g) | 0.67 ± 0.07a | 0.71 ± 0.02a | 0.70 ± 0.21a | 0.85 ± 0.06c | 0.75 ± 0.04b | 0.70 ± 0.03a | 0.81 ± 0.01b |
| 3. | Protein (g) | 3.21 ± 0.98b | 3.10 ± 0.91b | 2.91 ± 0.87a | 3.93 ± 0.21c | 3.04 ± 0.73b | 3.19 ± 0.51b | 3.33 ± 0.63c |
| 4. | Fat (g) | 2.15 ± 0.02b | 2.03 ± 0.14a | 2.23 ± 0.26c | 2.07 ± 0.05b | 2.14 ± 0.31b | 2.02 ± 0.13a | 2.09 ± 0.17b |
| 5. | Carbohydrate (g) | 6.65 ± 0.08a | 6.70 ± 0.31a | 6.03 ± 0.82a | 7.34 ± 0.95b | 8.04 ± 1.83b | 5.57 ± 0.04a | 7.55 ± 0.16b |
| 6. | Energy (Kcal) | 59 ± 5.03b | 57 ± 4.51a | 55 ± 6.12a | 63 ± 6.1b | 63.58 ± 7.31b | 53 ± 4.31a | 62 ± 3.36b |
| 7. | Calcium (mg) | 110.07 ± 9.83c | 100.16 ± 5.31a | 99.56 ± 2031a | 125.61 ± 8.13b | 111.23 ± 7.41c | 98.77 ± 6.47a | 119.07 ± 6.14b |
| 8. | Phosphorous (mg) | 78.55 ± 14.82a | 70.61 ± 11.31a | 79.37 ± 9.15a | 85.31 ± 8.49b | 88.41 ± 7.41b | 75.44 ± 12.41a | 93.15 ± 10.21b |
| 9 | Zinc (mg) | 0.31 ± 0.06a | 0.35 ± 0.03a | 0.31 ± 0.09a | 0.33 ± 0.05a | 0.36 ± 0.06a | 0.33 ± 0.03a | 0.36 ± 0.01a |
| 10. | Iron (mg) | 0.21 ± 0.01a | 0.24 ± 0.17a | 0.29 ± 0.21a | 0.27 ± 0.03a | 0.29 ± 0.08a | 0.23 ± 0.16a | 0.20 ± 0.12a |
| Rheological properties | ||||||||
| 1. | Total soluble solids % | 12.11 ± 1.8b | 10.34 ± 0.62a | 10.31 ± 0.62a | 13.91 ± 0.91c | 13.10 ± 1.75c | 11.03 ± 1.77b | 13.11 ± 0.90c |
| 2. | pH | 4.43 ± 0.31b | 4.39 ± 0.01b | 4.44 ± 0.03b | 4.40 ± 0.31b | 4.49 ± 0.52b | 4.38 ± 0.28a | 4.40 ± 0.3b |
| 3. | Titratable acidity | 0.12 ± 0.06b | 0.14 ± 0.05a | 0.11 ± 0.03b | 0.13 ± 0.01b | 0.10 ± 0.02b | 0.16 ± 0.03a | 0.14 ± 0.01a |
| 4. | Water-holding capacity % | 56.5 ± 3.99a | 54.74 ± 3.44a | 55.91 ± 2.83a | 58.85 ± 1.86a | 56.91 ± 4.13a | 55.5 ± 5.11a | 57.0 ± 4.81a |
| 5. | Syneresis (mL) | 12.40 ± 1.36b | 13.91 ± 2.94c | 13.99 ± 1.83c | 10.31 ± 2.66a | 12.39 ± 3.65b | 12.58 ± 1.24b | 11.08 ± 2.11b |
| 6 | Viscosity (mPa s) | 816 ± 77b | 776 ± 71a | 811 ± 28a | 866 ± 41c | 823 ± 72b | 809 ± 82a | 831 ± 42c |
*Nutrient compositions are per 100 g of the yogurt (wet)
Values with different alphabet within the same row differs significantly according to Analysis of varience (ANOVA) and Duncun’s multiple range test
C1—containing yogurt starter culture (S. thermophilus + L. bulgaricus), T1—(yogurt starter culture + L. acidophilus), T2—(yogurt starter culture + Bifidobacterium bifidum), T3—(yogurt starter culture + L. plantarum), T4—(yogurt starter culture + L. casei), T5—(yogurt starter culture + L. acidophilus + Bifidobacterium bifidum), T6—(yogurt starter culture + L. plantarum + L. casei)
Nutritional composition differed significantly among samples except for the iron and zinc (P < 0.05). LA and BB as single probiotic strain or in combination had significantly higher moisture, and low carbohydrate level than LP and LC (P < 0.05). Yogurt containing LP as single (T3) or in combination with LC (T6) resulted in significantly higher ash, protein, calcium and phosphorous level in comparison with other probiotic yogurt samples (P < 0.05). Reeta et al. (2015) and Setyawardani et al. (2016) had also reported higher protein content in goat cheese produced with mixed culture (L. rhamnosus and L. plantarum) and varied nutritional composition of yogurt according to the starter culture used and length of the fermentation.
Effect on rheological properties
Gel strength and viscosity are important quality indicators related to consistency and mouth feel of fermented dairy products and stability of viscosity during the storage is important qualitative characteristic of yoghurt (Stijepić et al. 2013). ST plays an important role in the production of exocellular texturing agents called exopolysaccharides that might interact with the protein content of milk and increase the viscosity and rheological quality of products.
Total soluble solids
Total soluble solids were found to be significantly higher in yogurt with LP and LC and their combination (T3, T4 and T6) as compare to LA and BB (P < 0.05). It was also observed that combination of LA and BB improved TSS. Purwandari et al. (2007) in his study reported that ST helps in the development of yoghurt texture through exopolysaccharide (EPS) production which tends to increase the total solids. Ahluwalia and Kumar (2013) have also reported ST and LB significantly increases total solids of the products as compare to LA and Bifidobacterium. These results are similar with the findings of Istikhar et al. (2009) that the natural yogurt contain higher total solids as compared to probiotic yogurt with LA and BB but less when compared with LP and LC yogurt.
pH
Table 1 shows the pH values of the seven yogurt formulations which lie within the range 4.38 to 4.49. Yogurt inoculated with LA and BB produced the lowest yogurt pH, followed by yogurt with LA as single probiotic culture. pH values of the yogurt with LP, LC, BB, their combinations and control yogurt (C1) were significantly higher (P < 0.05).
Titratable acidity
Titratable acidity shows opposite trends to pH. For yogurt T5 which showed lowest pH titratable acidity was highest. Lowest titratable acidity was noted in case of yogurt T4.
Water holding capacity and syneresis
Water holding capacity was found to be highest in case of yogurt T3 while it was lowest for yogurt T1. Highest syneresis was observed in case of T2 and lowest was in case of T3 (P < 0.05). Syneresis and water holding capacity were inversely proportional. The yogurt which had highest water holding capacity showed lowest syneresis. Ahluwalia and Kumar (2013) have also reported signified positive effect of LB and ST on syneresis while LA and Bifidobacterium had a negative effect.
Viscosity
Table 1 portrays the viscosity of the seven yogurt formulations which was in the range of 776 to 866 mPa s. According to Lee and Lucey (2010), gelation occurs when pH is just above the dairy milk isoelectric point (pH 5.2) and the observed viscosities are indicative of gelation. The addition of probiotic organisms, especially LP, resulted in a significantly (P < 0.05) higher viscosity compared to yoghurt with LA, BB, LC and starter culture only. The initial viscosity of yogurt containing combination of LP and LC was substantially higher than that containing LA and BB or control yogurt (C1). This may have been caused by the production of exopolysaccharide (EPS), although this was not measured in this study. Conversely LB seems to decrease yogurt viscosity which was also reported by Dahlan and Sani (2017). Similar to our findings, higher viscosity values were observed by Donkor et al. (2007). Our study also showed a substantial effect of the probiotic organisms on viscosity.
Overall it is stated that yogurt with LP and LC and their combination had significantly higher TSS, viscosity, pH and low synersis whereas, yogurt with LA and BB in alone or in combination had low pH.
Effect on probiotic potential
Acid tolerance
When seven yogurt formulations were subjected to different pH concentrations results are given in Table 2. At pH 1.0 no growth was observed after 1.5 h in any of the formulation. At pH 1.5 yogurts with LA and BB as single probiotic or in consortium showed growth after 2.5 h. Control yogurt failed to show any growth after 1.5 h and yogurt with LP and LC as single or in consortium also unsuccessful to grow after 2 h. Only test yogurts were capable of surviving pH 3.0 and 4.0 after 3 h and only LA and BB survived pH 1.5 till 2 h. Yogurt with LA and BB was found to be more acid tolerance with 66.1% survivability rate after 3 h at pH 3.0 as compare to yogurt with LP and LC with only 44.6% survivability rate. Multispecies combination resulted in better acid tolerance.
Table 2.
Total plate count for probiotic yogurt on MRS agar at different pH values over 3 h period
| pH value | Yogurt | Total plate count (log CFU/mL) | ||||
|---|---|---|---|---|---|---|
| 0 h | 1.5 h | 2 h | 2.5 h | 3 h | ||
| 1.0 | C1 | 3.01 ± 0.71 (37.1) | – | – | – | – |
| T1 | 3.51 ± 0.19 (47.9) | – | – | – | – | |
| T2 | 3.63 ± 1.81(44.3) | – | – | – | – | |
| T3 | 3.51 ± 1.0 (50.6) | – | – | – | ||
| T4 | 3.59 ± 0.71 (47.5) | – | – | – | ||
| T5 | 4.12 ± 0.01 (45.1) | – | – | – | ||
| T6 | 3.61 ± 0.18 (47.6) | – | – | – | ||
| 1.5 | C1 | 4.01 ± 2.03 (49.4) | – | – | – | – |
| T1 | 4.52 ± 0.15 (61.7) | 1.14 ± 1.34 (15.2) | 0.88 ± 0.61 (11.7) | 0.41 ± 0.04 (5.6) | – | |
| T2 | 4.03 ± 0.66 (49.2) | 0.93 ± 0.02 (11.6) | 0.63 ± 0.93 (7.7) | 0.21 ± 0.02 (2.6) | – | |
| T3 | 4.16 ± 0.28 (60.0) | 2.13 ± 1.11 (29.8) | 1.01 ± 0.63 (14.4) | – | – | |
| T4 | 4.44 ± 0.71(58.8) | 0.86 ± 0.23 (11.4) | 0.73 ± 0.20 (10.0) | – | – | |
| T5 | 5.24 ± 0.14 (57.4) | 2.68 ± 1.87 (29.6) | 2.01 ± 1.47 (26.2) | 1.01 ± 0.56 (11.1) | – | |
| T6 | 4.12 ± 0.64 (54.4) | 2.01 ± 0.13 (26.7) | 1.98 ± 0.67 (21.9) | – | – | |
| 2.0 | C1 | 5.91 ± 1.16 (72.8) | 2.35 ± 0.01 (27.1) | – | – | |
| T1 | 4.86 ± 1.05 (66.3) | 3.16 ± 0.07 (42.3) | 2.99 ± 0.82 (39.8) | 2.22 ± 0.17 (30.4) | 2.03 ± 0.68 (28.0) | |
| T2 | 4.01 ± 1.01 (48.9) | 2.94 ± 0.81 (36.7) | 2.16 ± 0.65 (26.6) | 1.98 ± 0.12 (24.9) | 1.72 ± 0.36 (21.4) | |
| T3 | 4.42 ± 0.93 (63.7) | 3.30 ± 0.51 (46.2) | 2.86 ± 0.16 (40.7) | 2.84 ± 1.03 (40.5) | 2.27 ± 0.49 (34.0) | |
| T4 | 4.47 ± 0.86 (59.2) | 3.56 ± 0.16 (47.4) | 2.55 ± 0.53 (35.0) | 2.01 ± 0.61 (28.4) | 1.96 ± 0.13 (26.6) | |
| T5 | 5.01 ± 0.51 (54.9) | 4.18 ± 0.85 (46.1) | 4.06 ± 0.16 (44.9) | 4.01 ± 0.09 (44.4) | 4.91 ± 0.25 (54.4) | |
| T6 | 4.49 ± 0.21 (59.3) | 3.31 ± 0.07 (44.0) | 3.10 ± 0.91 (41.5) | 3.17 ± 0.17 (42.6) | 3.19 ± 1.12 (44.6) | |
| 3.0 | C1 | 5.11 ± 0.07 (63.0) | 2.37 ± 0.81 (27.4) | – | – | – |
| T1 | 5.28 ± 0.33 (72.1) | 4.81 ± 0.92 (65.0) | 4.03 ± 1.52 (53.7) | 3.63 ± 1.41 (49.7) | 3.52 ± 0.82 (48.5) | |
| T2 | 4.83 ± 0.36 (58.9) | 4.01 ± 1.25 (50.1) | 4.12 ± 1.83 (50.5) | 3.68 ± 1.30 (50.4) | 3.43 ± 0.77 (42.8) | |
| T3 | 4.62 ± 1.27 (66.6) | 4.42 ± 1.27 (61.9) | 4.16 ± 0.73 (59.3) | 3.81 ± 0.62 (54.4) | 3.53 ± 0.41 (53.0) | |
| T4 | 4.95 ± 1.61 (65.5) | 4.41 ± 1.31 (58.7) | 3.91 ± 1.22 (53.7) | 3.01 ± 0.22 (42.0) | 3.33 ± 0.63 (45.2) | |
| T5 | 6.86 ± 0.45 (75.2) | 6.17 ± 0.23 (68.1) | 6.19 ± 0.72 (68.4) | 5.87 ± 1.45 (65.0) | 5.96 ± 0.41 (66.1) | |
| T6 | 5.12 ± 0.02 (67.6) | 5.13 ± 0.72 (68.3) | 5.26 ± 0.91 (70.5) | 4.30 ± 0.81 (57.7) | 3.19 ± 0.05 (44.6) | |
| 4.0 | C1 | 5.49 ± 1.65 (67.6) | 3.35 ± 0.05 (38.7) | 2.61 ± 1.40 (34.0) | – | – |
| T1 | 5.91 ± 1.41 (80.7) | 5.80 ± 1.61 (77.7) | 5.61 ± 1.4 (74.8) | 5.71 ± 0.59 (78.3) | 5.31 ± 1.82 (73.2) | |
| T2 | 5.50 ± 1.21 (67.1) | 5.42 ± 1.73 (67.7) | 5.11 ± 0.86 (62.6) | 4.72 ± 1.02 (59.5) | 4.16 ± 1.05 (51.9) | |
| T3 | 5.27 ± 1.04 (76.0) | 5.21 ± 0.93 (73.0) | 4.93 ± 2.01 (70.3) | 4.41 ± 0.83 (63.0) | 4.00 ± 1.26 (60.6) | |
| T4 | 5.00 ± 1.22 (66.2) | 4.83 ± 0.85 (64.3) | 4.44 ± 1.71 (61.0) | 4.12 ± 0.66 (57.4) | 4.12 ± 0.74 (55.9) | |
| T5 | 6.71 ± 0.34 (73.5) | 6.49 ± 0.14 (71.7) | 6.32 ± 0.61 (69.9) | 6.04 ± 0.14 (66.8) | 5.81 ± 0.23 (64.4) | |
| T6 | 5.81 ± 0.81 (76.7) | 5.17 ± 0.77 (68.8) | 4.73 ± 0.49 (63.4) | 4.12 ± 0.51 (55.3) | 3.61 ± 1.12 (50.4) | |
|
Control (pH 6.2) |
C1 | 8.11 ± 0.26 (100) | 8.64 ± 0.41 (100) | 7.67 ± 0.96 (100) | 8.42 ± 0.99 (100) | 7.41 ± 1.30 (100) |
| T1 | 7.32 ± 0.06 (100) | 7.46 ± 0.82 (100) | 7.50 ± 0.71 (100) | 7.29 ± 0.94 (100) | 7.25 ± 0.68 (100) | |
| T2 | 8.19 ± 0.31 (100) | 8.00 ± 0.93 (100) | 8.15 ± 0.61 (100) | 7.93 ± 0.15 (100) | 8.01 ± 0.52 (100) | |
| T3 | 6.93 ± 0.26 (100) | 7.13 ± 0.64 (100) | 7.01 ± 0.18 (100) | 7.00 ± 0.74 (100) | 6.66 ± 0.29 (100) | |
| T4 | 7.55 ± 0.23 (100) | 7.51 ± 0.33 (100) | 7.27 ± 0.18 (100) | 7.16 ± 0.39 (100) | 7.36 ± 0.72 (100) | |
| T5 | 9.12 ± 0.08 (100) | 9.05 ± 0.07 (100) | 9.04 ± 0.06 (100) | 9.03 ± 0.12 (100) | 9.01 ± 0.71 (100) | |
| T6 | 7.57 ± 0.05 (100) | 7.51 ± 0.06 (100) | 7.46 ± 0.08 (100) | 7.44 ± 0.03 (100) | 7.15 ± 0.05 (100) | |
Values in parenthesis show percent survivability. C1—containing yogurt starter culture (S. thermophilus + L. bulgaricus), T1—(yogurt starter culture + L. acidophilus), T2—(yogurt starter culture + Bifidobacterium bifidum), T3—(yogurt starter culture + L. plantarum), T4—(yogurt starter culture + L. casei), T5—(yogurt starter culture + L. acidophilus + Bifidobacterium bifidum), T6—(yogurt starter culture + L. plantarum + L. casei)
Bile salt tolerance
Results were similar to the trend as acid tolerance. Bacteria that could not survive at low pH also failed to grow in subsequent bile test. No growth was observed in case of sample from pH 1.0 and 1.5 at different bile salt concentrations. Highest survivability (99.7) was observed in case of yogurt T5 at bile salt concentration 0.3% from the sample of pH 3.0 after that there was a gradual decline in viable count as bile concentration increased. Higher inhibition of growth seen as bile salt concentration increased (Table 3).
Table 3.
Total plate count for probiotic yogurt on MRS agar at different bile salt concentration
| S. No. | Bile concentration | Yogurt | Total plate count (log CFU/mL) at different pH value | ||||
|---|---|---|---|---|---|---|---|
| 1.0 | 1.5 | 2.0 | 3.0 | 4.0 | |||
| 1. | 0.0% | C1 | – | – | – | – | – |
| T1 | – | – | 1.47 ± 0.05 (100) | 2.91 ± 0.07 (100) | 3.69 ± 0.76 (100) | ||
| T2 | – | – | 1.03 ± 0.61 (100) | 2.83 ± 0.64 (100) | 3.33 ± 0.48 (100) | ||
| T3 | 1.61 ± 0.72 (100) | 2.22 ± 0.04 (100) | 3.69.0 ± 0.41 (100) | ||||
| T4 | 1.11 ± 0.41 (100) | 2.17 ± 0.04 (100) | 3.91 ± 0.62 (100) | ||||
| T5 | 2.19 ± 0.07 (100) | 3.61 ± 0.05 (100) | 4.29 ± 0.81(100) | ||||
| T6 | 1.87 ± 0.43 (100) | 2.61 ± 0.01 (100) | 3.99 ± 0.06 (100) | ||||
| 2. | 0.3% | C1 | – | – | – | – | – |
| T1 | – | – | 1.23 ± 0.12 (83.6) | 2.91 ± 0.65 (100) | 3.66 ± 0.19 (99.1) | ||
| T2 | – | – | 1.01 ± 0.88 (98.0) | 1.78 ± 0.41 (62.8) | 2.65 ± 0.48 (79.5) | ||
| T3 | 1.51 ± 0.58 (93.7) | 2.11 ± 0.06 (95.0) | 3.68 ± 0.83 (92.9) | ||||
| T4 | 1.11 ± 0.72 (100) | 1.99 ± 0.91 (91.7) | 3.83 ± 0.79 (97.9) | ||||
| T5 | 2.02 ± 0.14 (92.2) | 3.60 ± 0.45 (99.7) | 4.11 ± 0.21 (97.8) | ||||
| T6 | 1.81 ± 0.08 (96.7) | 2.58 ± 0.33 (98.8) | 3.91 ± 0.85 (97.9) | ||||
| 3. | 0.5% | C1 | – | – | – | – | – |
| T1 | – | – | 1.10 ± 0.72 (74.8) | 1.99 ± 0.60 (68.3) | 2.81 ± 0.82 (76.1) | ||
| T2 | – | – | 0.98 ± 0.04 (95.1) | 1.23 ± 0.32 (43.4) | 1.84 ± 0.11 (55.2) | ||
| T3 | 1.00 ± 0.06 (62.1) | 2.14 ± 0.58 (96.3) | 2.98 ± 0.62 (80.7) | ||||
| T4 | 1.01 ± 0.81 (90.9) | 1.83 ± 0.84 (84.3) | 3.13 ± 0.59 (80.0) | ||||
| T5 | 1.61 ± 0.86 (73.5) | 2.98 ± 0.49 (82.5) | 3.63 ± 0.14 (84.8) | ||||
| T6 | 1.12 ± 0.77 (59.8) | 2.15 ± 0.46 (82.3) | 3.13 ± 0.96 (78.4) | ||||
| 4. | 2.0% | C1 | – | – | – | – | – |
| T1 | – | – | 0.81 ± 0.28 (55.1) | 0.95 ± 0.41 (32.6) | 1.57 ± 0.41 (42.5) | ||
| T2 | – | – | 0.77 ± 0.22 (74.7) | 1.10 ± 0.42 (38.8) | 1.98 ± 0.37 (59.4) | ||
| T3 | 0.69 ± 0.29 (42.8) | 0.99 ± 0.81 (44.5) | 1.81 ± 0.27 (49.0) | ||||
| T4 | 0.72 ± 0.21 (64.8) | 1.10 ± 0.36 (50.6) | 1.86 ± 0.50 (47.5) | ||||
| T5 | 1.10 ± 0.37 (50.2) | 1.45 ± 0.07 (40.1) | 2.17 ± 0.21 (50.5) | ||||
| T6 | 0.78 ± 0.81 (41.7) | 1.11 ± 0.85 (42.5) | 1.94 ± 0.36 (48.6) | ||||
Values in parenthesis show percent survivability. C1—containing yogurt starter culture (S. thermophilus + L. bulgaricus), T1—(yogurt starter culture + L. acidophilus), T2—(yogurt starter culture + Bifidobacterium bifidum), T3—(yogurt starter culture + L. plantarum), T4—(yogurt starter culture + L. casei), T5—(yogurt starter culture + L. acidophilus + Bifidobacterium bifidum), T6—(yogurt starter culture + L. plantarum + L. casei)
Upon exposure to the bile acids cellular homeostasis disruption causes dissociation of lipid bilayer and integral protein of their cell membrane that result in leakage of bacterial content and cell death (Hassanzadazar et al. 2012).
All the bacterial strains except LB and ST showed good probiotic potential. The survival at pH 3.0 was good in case of all four probiotic strains but decline in viable count was observed when samples were subjected to increased acidity. Four strains used in this study have met the criteria to be considered as good source of probiotics.
Effect on viability of probiotic bacteria during storage
Good viability is a prerequisite for the functionality of probiotics. It is very important that probiotic strains retain their viability and functional activity throughout the shelf life of product. The survival rate of the probiotic bacteria was assessed as viable counts (log CFU/mL) of all the bacterial strains used for the development of probiotic yogurts during storage at 4 °C over 10 days. The percent of viable bacteria at 10th day ranged 87.9 to 98.8. The viable counts of probiotics were decreased by less than 1 log cycle in all treatments during storage. Significant reduction was observed in the viability of all the probiotic bacteria during storage expect LA with highest viability of 98.80% (P < 0.05) LP had the poorest viability, recorded highest reduction and only 87% its initial viable population at the end of storage followed by LC and BB. This decline varied among probiotic species during storage because of different sensitivity to environmental stresses.
Effect on organoleptic properties
The probiotic combination had no significant effect on color, consistency, appearance, mouthfeel and overall acceptability of yogurt while flavour, texture, aroma, sourness and sweetness were significantly altered by the probiotic strain used (P > 0.05). Yogurt with LP was found to be significantly better in flavour and yogurt with LA was sourer (P > 0.05) (Table 4). Combination of probiotic strains resulted in better textural qualities. LP has minimum effect on textural qualities of fermented milk.
Table 4.
Organoleptic properties of developed yogurts
| S. No. | Organoleptic qualities | C1 | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|---|---|
| 1. | Color | 8.60 ± 1.47a | 8.66 ± 1.21a | 8.02 ± 1.0a | 8.36 ± 0.81a | 8.51 ± 1.4a | 8.40 ± 1.2a | 8.00 ± 0.34a |
| 2. | Flavour | 7.49 ± 0.88a | 8.51 ± 0.65b | 7.23 ± 1.40a | 9.0 ± 0.07c | 7.63 ± 0.04a | 8.60 ± 1.63b | 7.96 ± 1.58a |
| 3. | Texture | 7.30 ± 1.36a | 8.00 ± 1.41a | 7.98 ± 1.69a | 7.31 ± 1.31a | 7.51 ± 1.24a | 8.96 ± 1.24b | 8.77 ± 1.2b |
| 4. | Aroma | 7.67 ± 0.81b | 7.51 ± 0.52a | 7.20 ± 0.37a | 8.85 ± 0.38b | 7.60 ± 0.83b | 7.21 ± 1.31a | 8.16 ± 0.47b |
| 5. | Consistency | 8.27 ± 0.84a | 8.71 ± 0.36a | 8.54 ± 0.93a | 8.06 ± 1.41a | 8.22 ± 1.02a | 8.88 ± 1.61a | 8.0 ± 1.35a |
| 6. | Appearance | 8.0 ± 1.21a | 8.41 ± 0.63a | 8.37 ± 0.57a | 8.01 ± 0.83a | 8.13 ± 0.99a | 8.46 ± 0.81a | 8.28 ± 1.71a |
| 7. | Mouthfeel | 7.95 ± 0.92a | 8.23 ± 1.31a | 7.92 ± 1.11a | 7.58 ± 0.69a | 7.68 ± 1.03a | 8.13 ± 1.64a | 7.92 ± 0.81a |
| 8. | Sourness | 6.52 ± 0.34a | 8.58 ± 0.83c | 7.27 ± 1.36b | 7.51 ± 1.53b | 6.91 ± 1.41a | 7.91 ± 0.95b | 7.77 ± 0.89b |
| 9. | Sweetness | 8.26 ± 0.98b | 6.66 ± 1.05a | 7.53 ± 1.24b | 8.21 ± 1.48b | 8.51 ± 1.40b | 6.96 ± 1.31a | 8.81 ± 1.39b |
| 10 | Overall acceptability | 8.14 ± 0.34a | 7.91 ± 0.22a | 7.63 ± 0.89a | 8.51 ± 1.37a | 8.12 ± 1.56a | 8.77 ± 1.03a | 8.30 ± 1.54a |
Values with different alphabet within the same row differ significantly according to Analysis of varience (ANOVA) and Duncun’s multiple range test
Values in parenthesis show percent survivability. C1—containing yogurt starter culture (S. thermophilus + L. bulgaricus), T1—(yogurt starter culture + L. acidophilus), T2—(yogurt starter culture + Bifidobacterium bifidum), T3—(yogurt starter culture + L. plantarum), T4—(yogurt starter culture + L. casei), T5—(yogurt starter culture + L. acidophilus + Bifidobacterium bifidum), T6—(yogurt starter culture + L. plantarum + L. casei). 9-point hedonic scale was used to assess the organoleptic qualities of the developed probiotic products
Rating scale (9 = like extremely; 1 = dislike extremely)
Storage stability
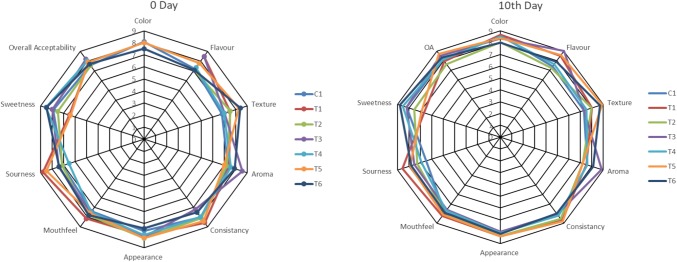
Table 5 and Fig. 1 shows the effect of refrigerated storage on rheological, microbial and organoleptic properties of developed yogurts. No significant change in TSS, total viable counts, flavour, aroma, appearance, mouthfeel was observed in all seven formulation during storage (P < 0.05). While, in yogurt with LA and BB, water holding capacity, sourness, and sweetness changed significantly (P < 0.05). Yogurt T3 and T6 with LP and LC noted significant reduction in viscosity and consistency (P < 0.05). No significant reduction in any parameters of rheological properties was observed in control yogurt while the sensory attributes like color, texture and sourness changed significantly (P < 0.05).
Table 5.
Rheological and microbial properties of developed yogurt during storage
| Sample | Storage days | pH | Titratable acidity | Total Solids | WHC % | Syneresis (mL) | Viscosity |
|---|---|---|---|---|---|---|---|
| Rheological properties | |||||||
| C1 | 0 day | 4.43 ± 0.31b | 0.21 ± 0.41a | 12.11 ± 1.80a | 56.5 ± 3.99a | 12.40 ± 1.36a | 816 ± 77a |
| 10th day | 4.41 ± 0.91b | 0.19 ± 0.62a | 11.99 ± 1.35a | 54.31 ± 4.11a | 12.41 ± 1.20a | 811 ± 68a | |
| T1 | 0 day | 4.39 ± 0.01b | 0.14 ± 0.05a | 10.34 ± 0.62a | 54.74 ± 3.44a | 13.91 ± 2.94b | 776 ± 71a |
| 10th day | 4.25 ± 0.05a | 0.29 ± 0.06b | 10.38 ± 0.05a | 52.11 ± 2.82a | 14.81 ± 1.98c | 788 ± 69a | |
| T2 | 0 day | 4.44 ± 0.03b | 0.11 ± 0.03a | 10.31 ± 0.62a | 55.91 ± 2.83a | 13.99 ± 1.83b | 811 ± 28a |
| 10th day | 4.41 ± 0.06b | 0.12 ± 0.01a | 10.22 ± 0.31a | 54.21 ± 3.21a | 14.21 ± 1.20c | 801 ± 39a | |
| T3 | 0 day | 4.40 ± 0.31b | 0.13 ± 0.01a | 13.91 ± 0.91a | 58.85 ± 1.86a | 10.31 ± 2.66a | 866 ± 41b |
| 10th day | 4.35 ± 0.22a | 0.16 ± 0.02a | 13.71 ± 0.82a | 58.21 ± 1.72a | 10.79 ± 1.42a | 801 ± 72a | |
| T4 | 0 day | 4.49 ± 0.52b | 0.10 ± 0.02a | 13.10 ± 1.75a | 56.91 ± 4.13a | 12.39 ± 3.65a | 823 ± 72b |
| 10th day | 4.46 ± 0.71b | 0.11 ± 0.07a | 13.11 ± 0.84a | 56.62 ± 3.82a | 12.60 ± 2.14a | 800 ± 68a | |
| T5 | 0 day | 4.38 ± 0.28a | 0.16 ± 0.01a | 11.03 ± 1.77a | 55.5 ± 5.11a | 12.58 ± 1.24a | 809 ± 82a |
| 10th day | 4.31 ± 0.34a | 0.18 ± 0.03a | 11.01 ± 0.99a | 52.1 ± 4.21b | 13.47 ± 1.11b | 798 ± 92a | |
| T6 | 0 day | 4.40 ± 0.3b | 0.17 ± 0.01a | 13.11 ± 0.90a | 57.0 ± 4.81a | 11.08 ± 2.11a | 831 ± 42b |
| 10th day | 4.37 ± 0.19b | 0.15 ± 0.34a | 12.58 ± 0.85a | 54.05 ± 4.27a | 12.12 ± 1.81b | 811 ± 63a | |
| Sample | Storage days | Total viable count | Mould count | Yeast Count | Coliform count | Salmonella count | Shigella count |
|---|---|---|---|---|---|---|---|
| Microbial quality (log cfu/mL) | |||||||
| C1 | 0 day | 6.41 ± 0.30a | Nil | Nil | Nil | Nil | Nil |
| 10th day | 6.29 ± 0.26a | Nil | Nil | Nil | Nil | Nil | |
| T1 | 0 day | 5.46 ± 0.41a | Nil | Nil | Nil | Nil | Nil |
| 10th day | 5.11 ± 0.25a | Nil | Nil | Nil | Nil | Nil | |
| T2 | 0 day | 6.77 ± 0.57a | Nil | Nil | Nil | Nil | Nil |
| 10th day | 5.93 ± 0.31a | Nil | Nil | Nil | Nil | Nil | |
| T3 | 0 day | 5.33 ± 0.28a | Nil | Nil | Nil | Nil | Nil |
| 10th day | 4.91 ± 0.29a | Nil | Nil | Nil | Nil | Nil | |
| T4 | 0 day | 5.44 ± 0.31a | Nil | Nil | Nil | Nil | Nil |
| 10th day | 5.41 ± 0.28a | Nil | Nil | Nil | Nil | Nil | |
| T5 | 0 day | 6.81 ± 0.26b | Nil | Nil | Nil | Nil | Nil |
| 10th day | 6.48 ± 0.26a | Nil | Nil | Nil | Nil | Nil | |
| T6 | 0 day | 5.98 ± 0.91a | Nil | Nil | Nil | Nil | Nil |
| 10th day | 5.61 ± 0.53a | Nil | Nil | Nil | Nil | Nil | |
The packaged yoghurt samples were stored at 4 °C for 10 days. Values in parenthesis show percent survivability. C1—containing yogurt starter culture (S. thermophilus + L. bulgaricus), T1—(yogurt starter culture + L. acidophilus), T2—(yogurt starter culture + Bifidobacterium bifidum), T3—(yogurt starter culture + L. plantarum), T4—(yogurt starter culture + L. casei), T5—(yogurt starter culture + L. acidophilus + Bifidobacterium bifidum), T6—(yogurt starter culture + L. plantarum + L. casei). Values with different alphabet within the same cell differs significantly according to paired ‘t’ test
Fig. 1.
Representation of quantitative organoleptic parameters of developed yogurts during storage. C1—containing yogurt starter culture (S. thermophilus + L. bulgaricus), T1—(yogurt starter culture + L. acidophilus), T2—(yogurt starter culture + Bifidobacterium bifidum), T3—(yogurt starter culture + L. plantarum), T4—(yogurt starter culture + L. casei), T5—(yogurt starter culture + L. acidophilus + Bifidobacterium bifidum), T6—(yogurt starter culture + L. plantarum + L. casei). 9-point hedonic scale was used to assess the organoleptic qualities of the developed probiotic products. Rating scale (9 = like extremely; 1 = dislike extremely) was used for sensory evaluation
Overall it can be concluded that probiotic bacteria have more significant changes during storage rather than yogurt starter culture.
Discussion
Yogurt has been a part of human diet in many parts of the world because of acceptance of its taste (along with remarkable beneficial effects). Textural properties of yogurt, such as viscosity, smoothness, thickness acquiring natural flavours and structural resistance to stress are important attributes to determine its consumer acceptance, and these attributes nowadays are accompanied with certain health benefits (Han et al. 2016).
Different probiotic strain (single or in combination) can produce yogurts with varied nutritional, rheological and sensory behaviour according to starter and probiotic culture used, therefore interactive behaviour amongst probiotic and yogurt cultures must be evaluated prior to their commercial application.
Probiotic strains combination had significant effect on nutritional composition of the probiotic yogurts. Yogurt with LA and BB produced yogurt with higher moisture and low calorie while fermentation of yogurt LP and LC resulted in higher ash, protein, carbohydrate, energy, calcium and phosphorous content.
The rheological and physical characteristics of non-fat or low-fat yogurt are key parameters for assessing its quality because reducing the fat content of yogurt results in alteration in its physico-chemical and sensory properties.
It was observed in this study that addition of probiotic strain with starter culture exhibited significant alteration in rheological properties. Addition of LA and BB produced yogurt with lowest pH and water holding capacity and highest syneresis and acidity, On the other hand addition of LP and LC produced yogurt with more soluble solids, highest water holding capacity and viscosity and lowest syneresis.
Syneresis could be influenced by solids content and the type of starter culture used for the preparation of yogurt. The increased total solid increases yogurt gel strength and thereby increases the density and reduces the pore size, with the result water is bound more firmly which increases the firmness of the yogurt. Other researchers (Ahluwalia and Kumar 2013; Dahlan and Sani 2017; Donkor et al. 2007; Istikhar et al. 2009) have also reported the varied rheological properties in yogurt fermented with different probiotic strains.
In order to survive passage through the gastrointestinal tract, resistance to low pH is important. Acid and bile have separate and combine effects on the growth of bacteria. As bile stress takes place after pH stress in the stomach. Sub lethally injured microorganisms may have a different and unpredictable resistance to new stress factor. The probiotic strain must be able to overcome the extremely low pH and the emulsifying effect of bile salts, and reach the site of action in a feasible physiological state. The pH in human stomach ranged from 1 during fasting to 4.5 after a meal (Soliman et al. 2015) and food ingestion can take up to 3 h.
It was observed in our study that combination of LA and BB survived at pH 1.5 during an incubation period of 1.5 h and then the growth was delayed till 2.5 h and after 3 h no growth was observed in any of the yogurt sample. At pH 2.0, 3.0 and 4.0 the survivability rate for of LA and BB was noted to be 54, 66 and 64% respectively. This is due to the fact that Bifidobacterium stimulates the growth of acidophilus due to the production of acetate (Gomes et al. 1998). Similarly, survivability rate for LP and LC at pH 2.0, 3.0 and 4.0 was found to be 44, 44 and 50%. In contrast exposure to pH 2.0 and 3.0 eliminated more than 73% of LB and ST during an incubation period of 2 h. All four of our probiotic strains were able to survive pH 3.0 for 3 h and exhibit good probiotic potential. Good acid survivable abilities of selected lactic acid bacteria (LP, LC, LA) was also reported by Srinu et al. (2013) and Soliman et al. (2015).
Bile salt tolerance is the most crucial property as it determines the ability of bacteria to survive in the small intestine, and consequently their capacity to play their functional role as probiotics. A concentration of 0.3% of bile salt closely appropriates the bile salt level found in the gastro intestinal tract (Soliman et al. 2015). All strains showed considerable difference with regards to growth in different bile salt concentrations. Highest survivability was in case of LA and BB combination at 0.3% bile salt concentration while LP, LC also showed good survivability at 0.3% level. As the bile salt concentration increased the survival rate declined. Survival rate reported in the study at 0.3 and 0.5% bile salt concentration are similar to the rates reported by other researchers (Jamaly et al. 2011; soliman et al. 2015). These researchers also reported higher bile salt tolerance by LA as compare to LP and LC. This can be due to the fact that acidophilus and bifidobacterium display a variety of proteins devoted to the efflux of bile salts or protons to modify sugar metabolism or to prevent protein misfolding. Exopolysaccharides produced by lactic acid bacteria is also thought to play an important role in the protection of microbial cells against low pH and bile salts (Ruiz et al. 2013).
Overall it can be said that the bile salt did not inhibit the growth of bacteria completely as when subjected to 2% bile salt there was still a high number of bacterial count. May be because of stress adaptation mechanism explains the increased growth with longer incubation hours after pre exposure to acid stress. Other reason could be enhanced survival capabilities appeared to be due to acclimatization of bacteria to the low pH environment therefore, minimizing the relative toxicity to glycoconjugates in the intestine. The probiotic strains proved to exhibit an excellent quality of bile salt tolerance.
For dairy products, the sensory properties depend largely on the relative balance of flavour compounds derived from carbohydrate, protein or fat in the milk and specific compounds produced from milk fermentation. Starter cultures make key contributions to the formation of the flavour compounds in yogurt (Chen et al., 2017).
In case of traditional yogurt starter cultures ST and LB, these two strains have a symbiotic interaction called “proto-cooperation” in mixed cultures, which means that they are mutually beneficial during fermentation. It has been suggested that the level of flavour compounds is much greater in mixed cultures than single culture due to their associative growth and mutual stimulation (Tamime and Robinson 1999). In our study the probiotic combination had no significant effect on color, consistency, appearance, mouthfeel and overall acceptability while flavour, aroma, sourness and sweetness were significantly affected.
These results suggest that in co-fermentation with traditional starters, the probiotic strains do not influence the physical appearance of yogurt, but produce different amount of metabolic products and key aroma-forming volatile metabolites that results in varied flavour, aroma and taste.
Other researchers have reported the presence of unique volatile flavour compounds (2,3 Pentanedione, acetaldehyde, acetate) in yogurt supplemented with LP. These compounds are result of LP metabolism and were absent in control yogurt (Cheng 2010, Changkun et al. 2017).
As also reported by other researchers that bifidobacteria often exhibit a characteristic aroma and a slightly acidic flavour. Fermentation of dairy products with LC alone resulted in formation of acetic acid, acetoin, butyric acid, caproic acid, 2-pentanone, and 2-butanone, while the volatile compounds typical of yogurt were absent. Fermentation with LC and yogurt cultures resulted in greatly increased levels of 3-hydroxy-2-butanone and hexanoic acid (Zhuang et al. 2010; Prasanna et al. 2014).
In order to provide the claimed health benefits to humans, the minimum viable count of probiotic bacteria in the fermented milks should be ≥ 106 colony forming units (CFU)/g at the end of the shelf-life of the product. The international standard FIL/IDF describe that the probiotic products should contained minimum of 106 viable probiotic bacteria per gram of product at the time of consumption (Daneshi et al. 2013). In the present study the count of viable cells was in the range of 5.61 to 6.64 log CFU/mL which was more than minimum count required to provide probiotic benefit. In addition, the decline in viability was dependent on the strain of probiotic. All six strains showed an acceptable viability with less than one log CFU/mL reduction at refrigerator temperature for 10 days with highest viability was reported for LA (98.80%) and lowest for LP (87.98%).
Yogurt bacteria can suppress probiotics during yogurt storage via ‘post-acidification’ process which is noticeably intensified in temperatures of more than 5 °C. Ferdousi et al. (2013) and Mani-López et al. (2014) had also reported strain specific survivability during storage with highest survivability reported in case of LA.
Furthermore, like many other dairy products, yogurt is prone to deterioration, especially under improper storage conditions. Generation of volatile by-products leads to off-flavours and makes the product unsatisfactory for consumers (Chen et al. 2017). It was observed that most of the sensory and rheological properties were maintained during refrigerated storage of 10 days among all seven yogurt formulations. While in LA and BB combination sourness increased but sweetness and WHC decreased. In contrast the combination of LP and LC resulted in decreased viscosity and consistency. Control yogurt retained its rheological and sensory properties throughout the storage period and the changes were less significant.
Conclusion
So, after considering all the results it can be concluded that different bacterial combination significantly affects the nutritional, rheological, organoleptic and probiotic properties. LA and BB in combination or alone produces yogurt with higher moisture low calorie, more acidic and more syneresis, exhibited higher acid tolerance and consumer acceptability with more sourness and less sweetness. Whereas, the consortium of LP and LC produced yogurt with more protein, carbohydrate, calcium, higher viscosity and lower syneresis. It was also observed that probiotic yogurt exhibits more changes in rheological and sensory characteristics than traditional yogurt during storage. Strain combination affects nutritional, rheological and organoleptic properties more than the viability and probiotic potential.
Combination of multi-strain and multi-species probiotic resulted in improved texture and better probiotic potential with multi-species combination found to be even more effective. Therefore, the selection of mono or multi-strain probiotics should be based on the required rheological and organoleptic properties in final product. The present study can be helpful to dairy industry in developing new probiotic products and may provide a rational for selecting a combination of probiotic strains.
Acknowledgements
Authors are thankful to the University Grants Commission for providing financial assistance for this research work [Grant no.F.15-1/2011-12/PDFWM-2011-12-O B-RAJ-11103(SA-II)]. We are also grateful to the Department of Foods and Nutrition and Department of Fisheries, Maharana Pratap University of Agriculture and Technology, Udaipur for providing technical assistance in estimation of nutrients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahluwalia S, Kumar P. Effect of yoghurt cultures and probiotic cultures on physicochemical and sensory properties of mango soy fortified probiotic yoghurt (Msfpy) J Food Process Technol. 2013 doi: 10.4172/2157-7110.1000239. [DOI] [Google Scholar]
- AOAC . Official methods of analysis association of official analytical chemists. Washington: AOAC International; 2002. [Google Scholar]
- Bujalance C, Jiménez-Valera M, Moreno E, Ruiz-Bravo A. Elective differential medium for Lactobacillus plantarum. J Micro Methods. 2006;66:572–575. doi: 10.1016/j.mimet.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Changkun L, Song J, Kwok LY, Wang J, Dong Y, Yu H, Qiangchuan H, Zhang H, Chen Y. Influence of Lactobacillus plantarum on yogurt fermentation properties and subsequent changes during post fermentation storage. J Dairy Sci. 2017;100:2512–2525. doi: 10.3168/jds.2016-11864. [DOI] [PubMed] [Google Scholar]
- Chapman CMC, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr. 2011;50:1. doi: 10.1007/s00394-010-0166-z. [DOI] [PubMed] [Google Scholar]
- Chen C, Shanshan Z, Guangfei H, Haiyan Y, Huaixiang T, Guozhong Z. Role of lactic acid bacteria on the yogurt flavour: a review. Int J Food Prop. 2017 doi: 10.1080/10942912.2017.1295988. [DOI] [Google Scholar]
- Cheng H. Volatile flavor compounds in yogurt: a review. Crit Rev Food Sci Nutr. 2010;50:938–950. doi: 10.1080/10408390903044081. [DOI] [PubMed] [Google Scholar]
- Conway PL, Gorbach SL, Goldin BR. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J Dairy Sci. 1987;70:1–12. doi: 10.3168/jds.S0022-0302(87)79974-3. [DOI] [PubMed] [Google Scholar]
- Da Silva DF, Tenorio NN, Gomes RG, Pozza MSDS, Britten M, Printo PTM. Physical, microbiological, rheological properties of probiotic yogurt supplemented with grape extract. JFST. 2017;54:1608–1615. doi: 10.1007/s13197-017-2592-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlan HA, Sani NA. The interaction effect of mixing starter cultures on homemade natural yogurt’s pH and viscosity. IJFS. 2017;6:152–158. doi: 10.7455/ijfs/6.2.2017.a3. [DOI] [Google Scholar]
- Daneshi M, Ehsani MR, Razavi SH, Labbafi M. Effect of refrigerated storage on the probiotic survival and sensory properties of milk/carrot juice mix drink. EJB. 2013 doi: 10.2225/vol16-issue5-fulltext-2. [DOI] [Google Scholar]
- Donkor ON, Nilmini SLI, Stolic P, Vasiljevic T, Shah NP. Survival and activity of selected probiotic organisms in set-type yoghurt during cold storage. Int Dairy J. 2007;17:657–665. doi: 10.1016/j.idairyj.2006.08.006. [DOI] [Google Scholar]
- Elli M, Callegari ML, Ferrari S, Bessi E, Cattivelli D, Soldi S, Morelli L, Feuillerat NG, Antoine JM. Survival of yogurt bacteria in the human gut. Appl Environ Microbiol. 2006;72:5113–5117. doi: 10.1128/AEM.02950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO (2001) Expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. FAO/WHO, Argentina. http://www.fao.org/3/a-a0512e.pdf
- FAO, WHO . Guidelines for the evaluation of probiotics in food. London: Food and Agriculture Organization and World Health Organization; 2002. [Google Scholar]
- Ferdousi R, Rouhi M, Mohammadi R, Mortazavian AM, Darani KK, Rad HA. Evaluation of probiotic survivability in yogurt exposed to cold chain interruption. IJPR. 2013;12:139–144. [PMC free article] [PubMed] [Google Scholar]
- Gomes AM, Matcata FX, Klaver FA. Growth enhancement of Bifidobacterium lactis Bo and Lactobacillus acidophilus Ki by milk hydrolyzates. J Dairy Sci. 1998;81:2817–2825. doi: 10.3168/jds.S0022-0302(98)75840-0. [DOI] [PubMed] [Google Scholar]
- Guzman-Gonzalez M, Morais F, Ramos M, Amigo L. Influence of skimmed milk concentrate replacement by dry dairy products in a low fat set-type yoghurt model system: use of whey protein concentrates. Milk protein concentrates and skimmed milk powder. J Sci Food Agric. 1999;79:1117–1122. doi: 10.1002/(SICI)1097-0010(199906)79:8<1117::AID-JSFA335>3.0.CO;2-F. [DOI] [Google Scholar]
- Han X, Yang Z, Jing X, Yu P, Zhang Y, Yi H, Zhang L. Improvement of the texture of yogurt by use of Exopolysaccharide producing lactic acid bacteria. Bio Med Res Int. 2016 doi: 10.1155/2016/7945675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanzadazar H, Ehsani A, Mardani K, Hesari J. Investigation of antibacterial, acid and bile tolerance properties of lactobacilli isolated from Koozeh cheese. Vet Res Forum. 2012;3:181–185. [PMC free article] [PubMed] [Google Scholar]
- Istikhar H, Attiq R, Nigel A. Quality comparison of probiotic and natural yogurt. Pak J Nutr. 2009;8:9–12. [Google Scholar]
- Jamaly N, Benjouad A, Bouksaim M. Probiotic potential of Lactobacillus strains isolated from known popular traditional Moroccan dairy products. Br Microbiol Res J. 2011;1:79–94. doi: 10.9734/BMRJ/2011/438. [DOI] [Google Scholar]
- Lee WJ, Lucey JA. Formation and physical properties of yogurt. Asian Aust J Ani Sci. 2010;23:1127–1136. doi: 10.5713/ajas.2010.r.05. [DOI] [Google Scholar]
- Lindsey WL, Norwal MA. Anew DPTA-Tea soil test for zinc and iron. Agron. 1969;61:84. [Google Scholar]
- Mani-López E, Palou E, López-Malo A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J Dairy Sci. 2014;97:2578–2590. doi: 10.3168/jds.2013-7551. [DOI] [PubMed] [Google Scholar]
- Mazumdar BC, Majumder K (2003) Methods on physico-chemical analysis of fruits. Practical Manual Book. Metropolitan New Delhi.
- Motoki M, Seguro K. Transglutaminase and its use for food processing. Trends Food Sci Technol. 1998;89:204–210. doi: 10.1016/S0924-2244(98)00038-7. [DOI] [Google Scholar]
- Prasanna PHP, Grandison AS, Charalampopoulos D. Bifidobacteria in milk products: an overview of physiological and biochemical properties, exopolysaccharide production, selection criteria of milk products and health benefits. Food Res Int. 2014;55:247–262. doi: 10.1016/j.foodres.2013.11.013. [DOI] [Google Scholar]
- Purwandari U, Shah NP, Vasiljevic T. Effects of exopolysaccharide-producing strains of Streptococcus thermophilus on technological and rheological properties of set-type yoghurt. Int Dairy. 2007;J17:1344–1352. doi: 10.1016/j.idairyj.2007.01.018. [DOI] [Google Scholar]
- Reeta Kumar S, Ankita J, Ramadevi N. Fortification of yoghurt with health-promoting additives: a review. Res Rev JFPDT. 2015;3:9–17. [Google Scholar]
- Ruiz L, Margolles A, Sánchez A. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front Microbiol Microbial Physiol Metab. 2013;4:1–8. doi: 10.3389/fmicb.2013.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahadeva RPK, Leong SF, Chua KH, Tan CH, Chan HY, Tong EV, Wong SYW, Chan HK. Survival of commercial probiotic strains to pH and bile. Int Food Res. 2011;J18:1515–1522. [Google Scholar]
- Scourboutakos MJ, Franco-Arellano B, Murphy SA, Norsen S, Comelli EM, L’Abbé MR. Mismatch between probiotic benefits in trials versus food products. Nutrients. 2017 doi: 10.3390/nu9040400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setyawardani T, Rahayu WP, Palupi NS. Physicochemical and stability of goat cheese with mono and mixed culture of Lactobacillus plantarum and Lactobacillus rhamnosus. Anim Prod. 2016;18:36–42. doi: 10.20884/1.anprod.2016.18.1.533. [DOI] [Google Scholar]
- Soliman AHS, Sharoba AM, Bahlol HEM, Soliman AS, Radi OMM. Evaluation of Lactobacillus acidophilus, Lactobacillus casei and Lactobacillus plantarum for probiotic characteristics. Middle East J Appl Sci. 2015;5:10–18. [Google Scholar]
- Soni R, Tank K, Jain NK. Knowledge, attitude and practice of health professionals about probiotic use in Ahmedabad, India. Nutr Food Sci. 2018;48:125–135. doi: 10.1108/NFS-02-2017-0032. [DOI] [Google Scholar]
- Srinu B, Madhava T, Rao PV, Reddy M, Reddy KK. Evaluation of different lactic acid bacterial strains for probiotic characteristics. Vet World EISSN. 2013;6:785–788. doi: 10.14202/vetworld.2013.785-788. [DOI] [Google Scholar]
- Stijepić M, Glušac J, Durđević-milošević D, Pešić-mikulec D. Physicochemical characteristics of soy probiotic yoghurt with inulin additon during the refrigerated storage. Rom Biotech Lett. 2013;18:8077–8085. [Google Scholar]
- Tamime AY, Robinson RK. Biochemistry of fermentation. In: Tamime AY, Robinson RK, editors. Yoghurt: science and technology. Cambridge, England: CRC Press; 1999. [Google Scholar]
- Tsai CC, Lin PP, Hsieh YM. Three Lactobacillus strains from healthy infant stool inhibit entero-toxigenic Escherichia coli grown in vitro. Anaerobe. 2007;14:1–7. doi: 10.1016/j.anaerobe.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Wu SF, Chiu HY, Chen AC, Lin HY, Lin HC, Caplan M. Efficacy of different probiotic combinations on death and nacrotizing enterocolitis in a premature rat model. J Pediatr Gastroenterol Nutr. 2013;57:23–28. doi: 10.1097/MPG.0b013e3182929210. [DOI] [PubMed] [Google Scholar]
- Zhuang G, Wang J, Yan L, Wei C, Liu XM, Zhang HP. In vitro comparison of probiotic properties of lactobacillus casei zhang, a potential new probiotic, with selected probiotic strains. LWT Food Sci Technol. 2010;42:1640–1646. [Google Scholar]