Abstract
Inflammatory bowel disease, a typical chronic inflammatory disease of the gastrointestinal tract, make up a growing share of the global disease burden. This study firstly evaluated the anti-inflammatory effects of corn protein hydrolysate (CPH) using a cell model of tumor cell necrosis factor-α (TNF-α)-induced inflammation and a mouse model of colitis induced by dextran sodium sulfate. CPH digests significantly inhibited the expression of cyclooxygenase-2 and inducible nitric oxide synthase, and reduced the secretion of interleukin-8 in TNF-α-induced inflammation in Caco-2 cells. In mice, CPH digests significantly improved the body weight loss, clinical scores, shortening of the colon and histological symptoms, and decreased the myeloperoxidase activity, and down regulated the expression of TNF-α, and interleukin-6 in the colon. The above results indicate that the CPH can potentially be used as a health food/nutraceutical for the treatment/management of intestinal inflammation.
Keywords: Corn protein hydrolysate, Inflammatory bowel disease, Anti-inflammatory activity, Caco-2 cells, Mice
Introduction
Inflammation is a defensive response of the body to stimuli such as injury, tissue damage, and infectious pathogens; it is an adaptive immune response that includes chronic inflammation and acute inflammation. Chronic inflammation-related diseases make up a growing share of the global disease burden (Majumder et al. 2016). Inflammatory bowel disease (IBD) is a typical chronic inflammatory disease of the gastrointestinal tract, the main types of which are Crohn's disease (CD) and ulcerative colitis (UC) (Mine and Zhang 2015). The UC is limited to the large intestine, and CD occurs anywhere in the gastrointestinal tract (Wada et al. 2013). The prevalence of IBD in developed countries continues to rise, and poses a significant economic burden on the life quality of IBD patients as well as on the healthcare system (Gismera and Aladrén 2008; Maria et al. 2008).
A large number of studies have reported that IBD is caused by a variety of complex factors, including genetic susceptibility, immunology, intestinal microbes and external environmental factors (Zhao et al. 2017). Although the pathogenesis of IBD is not fully understood, numerous studies have shown that it is associated with a wide range of immune imbalances characterized by extensive infiltration of macrophages, T cells and neutrophils into intestinal epithelial cells, and excessive production of pro-inflammatory cytokines (e.g. interleukin 8, IL-8; interleukin 6, IL-6; tumor necrosis factor-α, TNF-α) (Sobczak et al. 2014). Some inflammation-related enzymes such as cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) have been proved to play an important role in the progression of colorectal cancer (CRC), which is deteriorated by IBD (Rodriguez-Ramiro et al. 2013). Therefore, controlling the over-expression of inflammatory cytokines such as IL-6, IL-8 and TNF-α, as well as inhibiting the abnormal up-regulation of iNOS and COX-2, are critical for the treatment and prevention of IBD. The current available treatment for IBD mainly use corticosteroids and immunosuppressive agents that target inflammatory molecules and surgical procedures. However, these treatments are often associated with side effects, increased cost, and low efficacy (Young et al. 2012; Salaga et al. 2018). Therefore, there is growing interest to develop/identify new natural products that are safe and effective in preventing and treating IBD.
It has been scientifically demonstrated that many food-derived proteins and protein hydrolysate have anti-inflammatory activity against IBD. Lee et al. have reported that treatment with hen egg white peptides effectively inhibited the production of pro-inflammatory cytokines (IL-6, TNF-a, IL-17, IL-1β, IFN-r and IL-8) in the rat models of dextran sodium sulfate (DSS)-induced colitis (Lee et al. 2009). Soy-derived dipeptides and tripeptides could significantly alleviate colon and ileum inflammation of dextran sodium sulfate (TNBS)-induced colitis in pigs (Young et al. 2012). Furthermore, a novel tripeptide, Tyr-Pro-D-Ala-NH2 attenuated acute, semi-chronic and relapsing TNBS- as well as DSS-induced colitis in mice after topical administration (Salaga et al. 2018). Corn, one of the three widely cultivated cereals in the world, has the advantages of high yield, wide planting area and high nutritional value. Corn wet milling process generates large quantities of protein as a main byproduct with a high proportion of hydrophobic amino acids (leucine, phenylalanine and alanine, etc.) and branched-chain amino acids (Ren et al. 2018), making it a potential source of bioactive peptides. A large amount of researchers have reported that corn protein hydrolysate (CPH) and its derived peptides exhibit various biological activities such as antioxidant activity (Liang et al. 2017), promotion of alcohol metabolism (Yamaguchi et al. 1997a), inhibition of breast tumor progression (Yamaguchi et al. 1997b), and angiotensin-converting enzyme inhibitory activity (Kim et al. 2004). Recently, our group reported that zein-derived hydrolysates and its simulated gastrointestinal digests exhibited strong anti-inflammatory activity in endothelial EA.hy926 cells (Liang et al. 2018). Although the anti-inflammatory effects of corn gluten hydrolysate were preliminarily studied using a rat model of TNBS-colitis (Mochizuki et al. 2010). The studies related to the anti-inflammatory effects of CPH are not comprehensive; hence, this study used an in vitro Caco-2 cells model of inflammation and a mouse model of colitis to study the anti-inflammatory activity of simulated gastrointestinal digests from CPH.
Materials and methods
Materials and chemicals
Corn gluten meal was bought from Yishui Earth Corn Development Co., Ltd. (Shandong, China) with a protein content of 652 mg/Kg. Corn protein with 92.5% purity was extracted based on our previous studies (Guo et al. 2009). Pepsin and pancreatin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Alcalase 2.4L (23,400 U/mL enzyme activity) was bought from Novozymes Co., Ltd. (Tianjin, China). Simulated gastric and intestinal fluids were prepared as described in United States Pharmacopeia (United States Pharmacopeial Convention Council of Experts 2007). The dextran-sulfate sodium salt (DSS) was purchased from MP Biomedicals (Solon, OH, USA). Recombinant human TNF-α was bought from R&D System (Minneapolis, MN, USA). Dulbecco’s modified Eagle medium (DMEM) and Fetal bovine serum (FBS) were purchased from Gibco/Invitrogen (Carlsbad, CA, U.S.A.). The antibodies of iNOS, COX-2 and α-tubulin were procured from Abcam (Cambridge, UK). Donkey anti-mouse and goat anti-rabbit fluorochrome-conjugated secondary antibodies were obtained from Licor (Licor Biosciences, Lincoln, NE, USA). IL-6, TNF-α and myeloperoxidase (MPO) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Enzyme-linked immunosorbent assay (ELISA) kits for IL-8 were procured from Invitrogen Life Technologies Corp. (Frederick, MD, USA). All other chemicals and reagents were of analytical grade.
Preparation of simulated gastrointestinal digests from CPH
The 2% (w/w) of corn protein suspension was preheated at 50 °C for 10 min, pH of the protein suspension was adjusted to pH 9.0, and alcalase (E/S = 2500 U/g) was added to initiate the reaction. The pH of the enzymatic hydrolysis system was stabilized at 9.0 by continuously adding 1 M NaOH. After 1 h of hydrolysis, the reaction was stopped by boiling for 15 min. Then the mixture was centrifuged at 5030 g for 20 min to get the supernatant, ie. CPH. The supernatant was concentrated and lyophilized for subsequent preparation of CPH digests. Simulated gastrointestinal digestion of CPH was performed according to our previously reported method (Liang et al. 2018).
Caco-2 cells experimental design
The human colon adenocarcinoma cell line, Caco-2 (HTB-37™), was purchased from American-type culture collection (ATCC, Manassas, VA, USA). The growth medium of Caco-2 cells were prepared by supplementing 10% FBS, 2.5% HEPES, 1% antibiotics (Penicillin–Streptomycin) and 1% non-essential amino acids to the DMEM medium. The cells were cultured in a humidified incubator containing 5% CO2 at 37 °C. The media was replaced every two days, and 0.25% trypsin–EDTA was used to treat the cells for cell subculture.
The Caco-2 cells at passage number 20–50 were seeded at 105 cells/cm2 in 48-well plates and allowed to grow to confluence. When the cells were cultured for 4–5 days and reached 80–90% confluence, the cells were treated with various concentrations (500, 1500, 2500 µg/mL) of the CPH digests for 6 h. Subsequently, TNF-α at a concentration of 40 ng/mL was added and incubated together for 18 h to induce inflammation. After 24 h of treatment, the supernatant of the culture was collected for measurement of the concentration of IL-8.
Determination of IL-8 by ELISA
The IL-8 levels by Caco-2 cells was determined using a solid phase sandwich ELISA kit according to the manufacturer's instructions. The standard curve of IL-8 is generated by plotting the IL-8 concentration of the standard well versus the corresponding absorbance value.
Western blot analysis of iNOS and COX-2 protein expression
The levels of iNOS and COX-2 were determined by Western blot analysis according to our previous study (Meram and Wu 2017). After removing the cell culture medium, Caco-2 cells were lysed by adding the boiled Laemmli containing 50 mM DTT (a reducing agent) and 0.2% Triton-X-100 for 5 min. The cell lysates were then run on 9% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto nitrocellulose membranes, and immunoblotted with the corresponding antibodies. The expression of iNOS and COX-2 were normalized using the control α-tubulin. The concentration of antibody used was 0.1–0.4 μg/mL. The protein bands were detected using the fluorochrome-conjugated secondary antibodies. The protein bands were scanned using Licor Odyssey BioImager (Licor Biosciences, Lincoln, NE, USA) and quantified by densitometry using Image Studio Lite 5.2. All the data were expressed as the percentage change of the corresponding positive control (cells treated with TNF-α alone).
Animal study design and colitis scoring
The present animal study was approved by the Laboratory Animal Management Committee of Jiangsu University (Zhenjiang, China). All the experimental procedures were reviewed in advance by the Laboratory Animal Management Committee of Jiangsu University; they were in accordance with the National Institutes of Health Laboratory Animal Care and Use Guidelines. 40 Healthy female Balb/c mices (15 ± 2 g) were provided by the Laboratory Animal Research Center of Jiangsu University; they were allowed free access to food and water. After one week of acclimatization, the 40 mices were randomly divided into 4 groups (n = 10). Group I (blank control group, BC): normal diet and the mices received only drinking water; Group II (colitis model group, DSS group): the mices received drinking water for the first seven days, and 3% DSS (w/v) was added into the drinking water for another seven days. Group III: low-dose (LD, 3% DSS + 300 mg/Kg/d CPH digests) group, and Group IV: high-dose (HD, 3%DSS + 300 mg/Kg/d CPH digests) group. To verify the anti-inflammatory effect of CPH digests in DSS-induced colitis model, LD and HD group mice received an intragastric administration of 100 mg/Kg/d and 300 mg/Kg/d CPH digests for the whole 14 days, respectively. After 14 days, the mice were subjected to cervical dislocation, and the colons were taken (from cecum to anus) for further analysis.
Clinical evaluation of colitis
Mice were weighed and recorded daily. Pictures of the fresh colons were taken and their length were also recorded. Colons were inspected for stool consistency, presence of blood in stool or bleeding, and general appearance. Diarrhea, fecal bleeding and appearance were checked and scored daily with some modification based on the previous method (Shi et al. 2014). Diarrhea score: 0, normal; 1, wet/sticky stool; 2, soft stool; 3, diarrhea. Fecal bleeding score: 0, negative; 1, light bleeding; 2, mild bleeding; and 3, severe bleeding. Appearance score: 0, normal; 1, ruffled fur or altered gait; 2, lethargic; 3, moribund. Clinical scoring is the superposition of the above three scores.
Histological analysis of colitis
The distal colons were fixed in 10% (w/v) buffered formalin. Paraffin-embedded Sects. (5 μm) were sectioned, stained with hematoxylin and eosin, and observed by light microscopy (Nikon EPIPHOT 300, Tokyo, Japan). The objective of the microscope used was Objective CF Plan 100X A, BD.
Determination of myeloperoxidase (MPO) activity, TNF-α and IL-6 concentration in colon tissues
The MPO activity, TNF-α and IL-6 concentration in colon tissues were measured according to the method reported by Shiet al. with a slight modification (Shi et al. 2014). Colon tissues were rinsed with PBS containing 1 mM phenylmethylsulfonyl fluoride (PMSF), and flash frozen using liquid nitrogen and stored at -80 °C until further use. Tissues were homogenized in a mixture of 10 μg/mL aprotinin, 10 μg /mL leupeptin, and 10 μg/mL pepstatin A in PBS. Homogenates were separated by centrifugation at 10,000 rpm for 15 min at 4 °C. The above supernatants of colon tissues were collected for the assays with test kits according to the manufacturer’s instructions. Protein concentration was measured by Coomassie Brilliant Blue G 250 method (Sedmak and Grossberg 1977). The TNF-α and IL-6 concentrations are respectively expressed as pg cytokine per mg protein. The MPO activity are respectively expressed as mU MPO per mg protein.
Statistical analysis
All data were presented as means and standard deviation of 4–6 independent experiments. Statistical analysis was performed using one-way analysis of variance. A level of P < 0.05 was considered to be with significant difference. And the calculations and graphs were performed using PRISM 5 statistical software (Graph Pad Software, San Diego, CA).
Results
Effect of CPH digests on TNF-α-induced iNOS, COX-2 protein expression and IL-8 production in Caco-2 cells
Bioactive peptides are expected to be taken orally, while gastrointestinal digestion will carry out the secondary enzymatic hydrolysis of the bioactive peptides prepared by enzymatic hydrolysis. Thus, CPH digests were prepared by simulated gastrointestinal digestion and used in this study. As depicted in Fig. 1, the expression of iNOS, COX-2 and the content of IL-8 in normal Caco-2 cells (Control) was very low; whereas the 18 h TNF-α stimulation induced a significant increase indicating confirmation of inflammation model of Caco-2 cells. This model was used to study the anti-inflammatory activity of CPH digests at 500, 1500, and 2500 µg/mL. The results showed that three concentrations of CPH digests could significantly (P < 0.05) inhibit iNOS, COX-2 expression and IL-8 release by 18.72–46.91%, 37.77–56.61% and 15.77–54.95%, respectively. Furthermore, the inhibitory effects of CPH digests on COX-2 expression and IL-8 secretion was significantly (P < 0.05) better at 2500 µg/mL than that of 500 µg/mL. The above results indicated that CPH digests exhibited good anti-inflammatory activity by inhibiting iNOS, COX-2 expression and IL-8 secretion in TNF-α-stimulated Caco-2 cells.
Fig. 1.

Effects of corn protein hydrolysate (CPH) digests on TNF-α-induced iNOS (a), COX-2 (b) protein expression and IL-8 secretion (c) in Caco-2 cells. Caco-2 monolayers were pretreated for 6 h with CPH digests prior to 18 h of incubation with 40 ng/mL of TNF-α. iNOS, COX-2 protein and IL-8 secretion levels were expressed as % TNF-α. Bars represent mean ± SEM, n = 5. Different letters indicate significant differences (P < 0.05)
Effect of CPH digests on body weight loss and clinical symptoms of mice with DSS-induced colitis
In addition to in vitro cell culture experiments, the anti-inflammatory effects of CPH digests were further examined using DSS-induced colitis model. It is well known that the DSS can destroy mice colonic epithelial cells, damage the intestinal barrier and results in colitis, the severity of which is closely related to changes in body weight and clinical symptoms in mice. As shown in Fig. 2a, during the first 7 days of the experiment, there were no significant differences in the body weight and clinical scores between BC and CPH digests-treated groups, indicating that the CPH digests had little effects on the body weight and clinical symptoms of the normal mice. The addition of 3% DSS induced the significant (P < 0.05) decrease of body weight of the DSS group from the 11th day. While, CPH digests significantly (P < 0.05) prevented the decrease of body weight, although there was no significant difference between LD group and HD group. After the DSS treatment for 2 days, the mice began to show obvious clinical signs including diarrhea, lethargy and blood in stool; and as the DSS administration time increases, the symptoms became more and more serious. As shown in Fig. 2b, the DSS-induced clinical symptoms was significantly improved after treatment with two doses of CPH digests in the last three days. Preserved egg white digests (Zhao et al. 2017), hydrolysate from eggshell membrane (Shi et al. 2014) were also reported to significantly improve the weight loss and clinical symptoms of DSS-induced inflammation in mice.
Fig. 2.

Effects of corn protein hydrolysate (CPH) digests on weight loss (a) and clinical signs (b) in DSS-induced colitis mice. BC blank control group; DSS colitis model group; LD low-dose group; HD high-dose group. Mice were given 100 or 300 mg/kg d CPH digests for 14 days. On day 7, colitis was induced with 3% DSS water. Weight of the mice were recorded daily. Mice were scored daily for clinical signs. Date are represented as mean ± SD (n = 10). Different letters indicate significant differences (P < 0.05)
Effects of CPH digests on IL-6 and TNF-α production, and MPO activity in the colons of mice with DSS-induced colitis
MPO activity and pro-inflammatory cytokines (IL-6, TNF-α) play an important role in the inflammatory cascade and are involved in the immunopathology of IBD (Sartor 1994). BC-group as described in the methods section, the MPO activity, IL-6 and TNF-α levels in the colon tissue of the DSS group were significantly increased. (Fig. 3). There was little effect on the MPO activity at low dose of CPH digests, but high dose induced significant decrease of MPO activity as compared with DSS group (Fig. 3a). In addition, both doses of CPH digestion treatment could significantly (P < 0.05) inhibit the DSS-induced TNF-α, IL-6 secretion, while for IL-6, the inhibitory effect of HD group was significantly better than LD group (Fig. 3b, c).
Fig. 3.
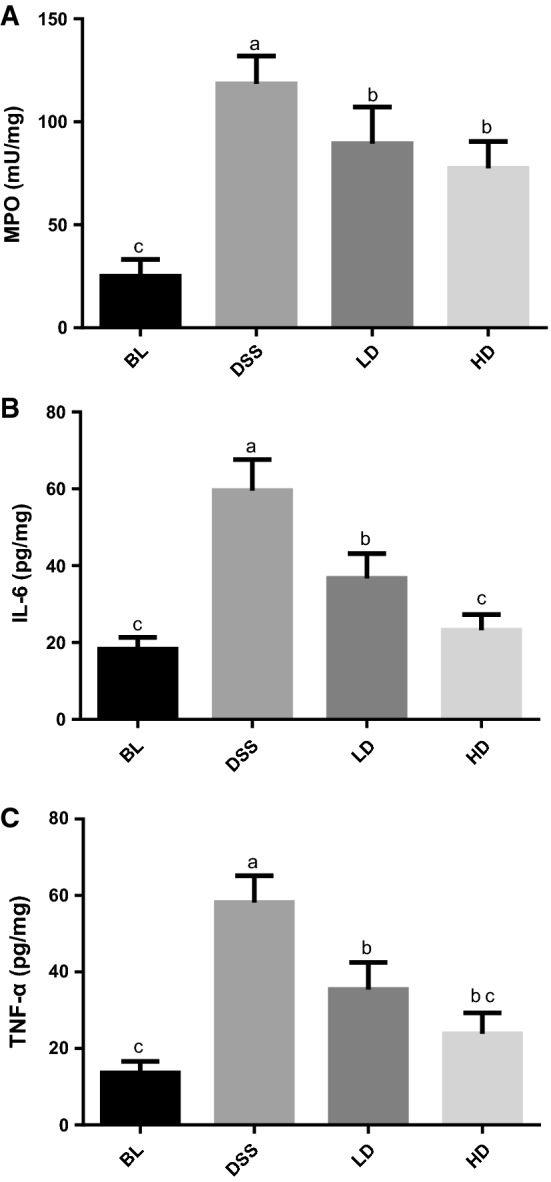
Effects of corn protein hydrolysate (CPH) digests on the IL-6 and TNF-α production, MPO activity in colon. BC blank control group; DSS colitis model group; LD low-dose group; HD high-dose group. The IL-6, TNF-α expression and MPO activity were determined by test kits and respectively expressed as pg cytokine, mU MPO per mg protein. Different letters indicate significant differences (P < 0.05)
Effect of CPH digests on morphological changes in the colons of mice with DSS-induced colitis
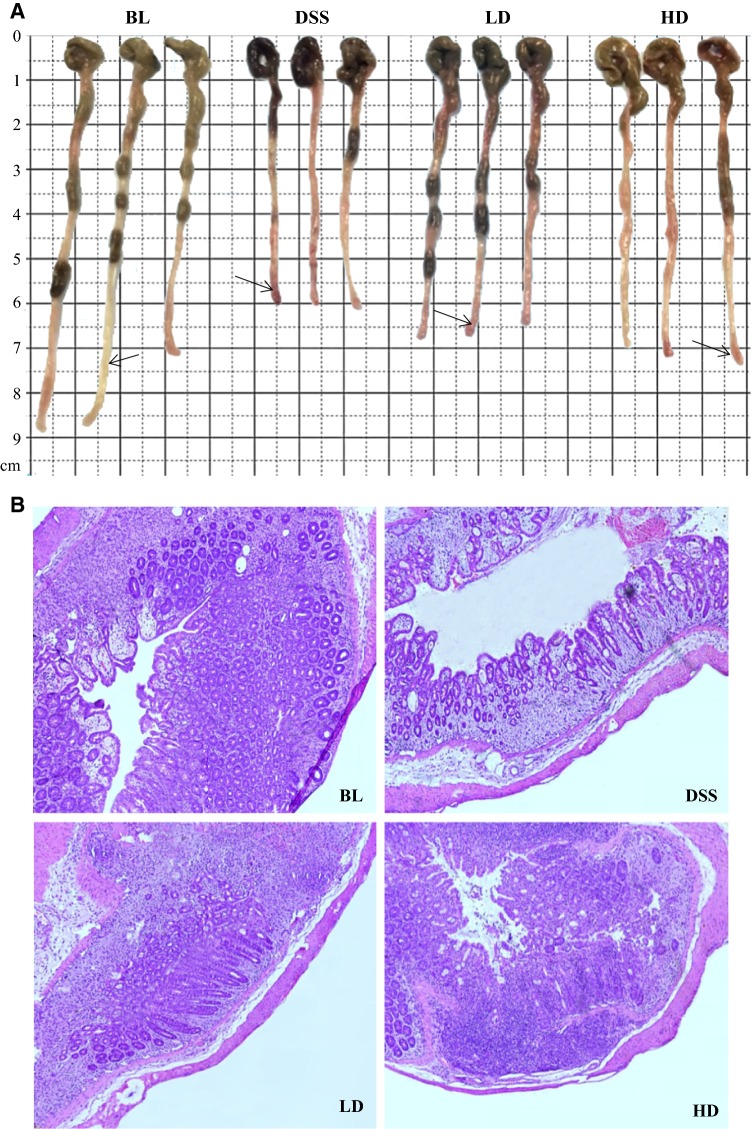
The effects of CPH digests administration on the severity of tissue damage were assessed by analyzing histological and morphological changes in the colons. As observed from the appearance’s arrow marks of the colon (Fig. 4a), the DSS treated mice group exhibited severe colitis clinical symptoms such as severe congestion, bloody stools and significant atrophy compared to BL group. While, the remarkable improvement in DSS-induced severe clinical symptoms of the colon was achieved by administration of CPH digests. The colons of the mice were sectioned and stained for further observation (Fig. 4b). The colon sections of the BL group showed typical characteristic features of a well-defined structure, normal glands, abundant goblet cells in the epithelium and a small quantity of infiltrating immune cells. However, DSS treatment caused a series of changes in the colon tissue; the mucosal structure was destructed; the thickness of the colon muscle became thinner; and vast inflammatory cells infiltrated into the mucosa and submucosa. Both doses of CPH digests showed great improvement on the DSS-induced morphological changes; the improvements of the HD group were significantly better than that of the LD group.
Fig. 4.
Effects of CPH digests on morphological changes in the colons of mice with DSS-induced colitis. The macro photos of the mice colons (a); the microscope photo of H&E-stained colon sections (b). BC blank control group; DSS colitis model group; LD low-dose group; HD high-dose group
Discussion
Although IBDs have diverse etiologies, they share many potential pathological mechanisms including abnormalities in inflammatory responses. In recent years, targeting to common pathological pathways has been increasingly used to prevent and treat chronic diseases. Furthermore, previous study reported CPH contained considerable amounts of hydrophobic amino acids (429.95 µg/mL) (Liang et al. 2017). The considerable amounts of hydrophobic amino acids contrubuting to its excellent antioxidant activity might increase its anti-inflammatory activity (Liang et al. 2018). Caco-2 cells are derived from colon (large intestine) cancer, which is similar in morphology, function, and phenotype to intestinal cells in the small intestine. Caco-2 cells is recognized as a well-established model of intestinal inflammation (Fernández-Tomé et al. 2017). Numerous studies have reported that both undifferentiated and fully differentiated Caco-2 cells can be used for the study of intestinal inflammation, and there is few literatures comparing the differences between these two states of cells to inflammation stress (Chen et al. 2018; Huang et al. 2015). In this study, we investigated the effect of CPH digests on TNF-α induced inflammatory in undifferentiated Caco-2 cells. TNF-α is a pro-inflammatory cytokine involved in the pathogenesis of colitis, secreted by macrophages and monocytes (Lee et al. 2009).TNF-α is generally used as an inducer of inflammation in Caco-2 cells. COX-2, iNOS and IL-8 are important pro-inflammatory proteins, whose expressions are rapidly increasing in TNF-α-induced inflammation in Caco-2 cells. The COX-2 is widely distributed in immune cells, epithelial cells and endothelial cells, directly regulates the secretion of prostaglandin E2, and participates in inflammatory reactions through multiple signals (Tsatsanis et al. 2006). Numerous studies reported the high levels of COX-2 in local tissues and organs of patients with IBD (Singer et al. 1998). iNOS and its downstream products mediate NO production that plays a key role in the development of chronic inflammatory diseases including colitis and colon cancer. iNOS and its related signaling molecules have become important targets for anti-inflammatory and anti-cancer drugs (Rao 2004). IL-8 is an α-chemokine that increases the number of neutrophils and promotes the activation of transcription factors such as kappa-light-chain-enhancer of activated B cells (NF-κB) or activator protein-1 (AP-1) pathways on epithelial cells to aggravate inflammation and even change cell growth (Huang et al. 2015). High expression of IL-8 protein are observed in the colonic mucosa of IBD patients (Daig et al. 1996). The present study found that CPH digests effectively prevented increase in iNOS and COX-2 expression and IL-8 secretion, suggesting CPH digests exhibited excellent anti-inflammatory activity by down-regulating the TNF-α-induced overexpression of pro-inflammatory proteins in Caco-2 cells.
To further study the anti-inflammatory activity of CPH digests in vivo, a DSS-induced experimental colitis mice model was used as a model of intestinal inflammation. The use of DSS to induce colitis in mice is a well-established and classical method to produce an experimental model of colitis (Olamilosoye et al. 2018). Typical clinical symptoms of DSS-induced colitis are weight loss, diarrhea and bloody diarrhea, colon shortening, neutrophil infiltration, intestinal epithelial cell inflammation and mucosal ulceration (Puneet Kaur et al. 2014), which have been proved again in this study. We found that CPH digests with two doses (100 mg/Kg d and 300 mg/Kg d) can relieve clinical symptoms of DSS induced-colitis and alleviate inflammation of colitis by significantly inhibiting MPO activity and secretion of TNF-α and IL-6. MPO is an enzyme mainly present in the azurophilic granules of neutrophils (Olamilosoye et al. 2018). Accumulation of granulocytes in the colon could result in granulocyte-mediated mucosal tissue damage, mucosal barrier destruction and subsequent inflammatory responses (Mantovani et al. 2001). Measurement of MPO activity has been used as an index of neutrophil infiltration into inflamed intestinal tissue and aggravated inflammation (Kim et al. 2004). Current research found that the colonic MPO activity vastly increased in DSS-induced colitis in mice, indicating recruitment of neutrophils and subsequent inflammation. Administration of CPH digests significantly decreased MPO activity in the colon, indicating indirectly that CPH digests inhibit neutrophil infiltration in intestinal tissues, showing its protective effect on inflammation-related tissue damage. Leukocytes (macrophages and neutrophils) infiltrate the intestinal mucosa in IBD and spread to the thickened mucosa and submucosa, which strongly express activated NF-κB and secrete pro-inflammatory cytokines such as TNF-α, and IL-6 (Kühl et al. 2015; Stanislava Stanojevića et al. 2018). The over-expression of these cytokines then activate and recruits the leukocytes to the site of inflammation, thereby promoting the progression of the IBD (Majumder et al. 2016). Therefore, cytokines represented by TNF-α and IL-6 play a key role in the development and progression of IBD.
In conclusion, the present study demonstrated that CPH digests possess potent anti-inflammatory effects on the colonic tissue in vitro and in vivo. The current results suggest that the CPH digests can inhibit iNOS, COX-2 expression and IL-8 secretion of TNF-α-stimulated Caco-2 cells. CPH digests ameliorated clinical symptoms and alleviated inflammation of colitis by significantly inhibiting MPO activity and secretion of TNF-α and IL-6. The CPH digests with anti-inflammatory effects could be useful in the preparation of health foods/nutraceuticals for the management of chronic intestinal inflammatory diseases. Pathogenetic causes of the DSS-induced colitis model have not yet been clear. However, as one of the causes, intestinal epithelium was damaged by DSS, resulting in production of inflammatory cytokines and chemokines such as IL-8, TNF-α and IL-6. Different transcription factors such as NF-κB and AP-1 are the major factors regulating these inflammatory diseases (Chakrabarti et al. 2014). CPH might directly suppress the activation of NF-κB and the janus kinase/signal transducer and activator of tran-ions (JAK/STAT) signal by inhibiting or reducing the expression of these inflammatory biomarkers and/or by modulating the activity of these transcription factors (Majumder et al. 2016). Further studies would need to focus on the isolation of monomeric peptides from CPH digests and elucidate their molecular mechanisms of anti-inflammatory effects in intestinal inflammatory diseases.
Acknowledgements
The authors wish to express their appreciation for the support obtained from Grant of the Project of National 863 Plan of China (2013AA102203), National Natural Science Foundation of China (31801552), Jiangsu Province Key Research and Development Program (BE2018368), China Postdoctoral Science Foundation Special Funding Project (2018T110459) and Jiangsu Province Postdoctoral Science Foundation Special Funding Project (2019K114).
Compliance with ethical standards
Conflicts of interest
There are no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Chakrabarti S, Jahandideh F, Wu J. Food-derived bioactive peptides on inflammation and oxidative stress. Biomed Res Int. 2014 doi: 10.1155/2014/608979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Patel A, Meier PP, Fantuzzi G. Digested early preterm human milk suppresses tumor necrosis factor–induced inflammation and cytotoxicity in intestinal epithelial cells. J Pediatr Gastroenterol Nutr. 2018;66(6):1. doi: 10.1097/MPG.0000000000001932. [DOI] [PubMed] [Google Scholar]
- Daig R, Andus T, Aschenbrenner E, Falk W, SchöLmerich J, Gross V. Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut. 1996;38(2):216–222. doi: 10.1136/gut.38.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Tomé S, Sanchón J, Recio I, Hernández-Ledesma B. Transepithelial transport of lunasin and derived peptides: inhibitory effects on the gastrointestinal cancer cells viability. J Food Compos Anal. 2017 doi: 10.1016/j.jfca.2017.01.011. [DOI] [Google Scholar]
- Gismera CS, Aladrén BS. Inflammatory bowel diseases: a disease(s) of modern times? Is incidence still increasing? World J Gastroenterol. 2008;14(36):5491–5498. doi: 10.3748/wjg.14.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Sun J, He H, Yu G-C, Du J. Antihepatotoxic effect of corn peptides against Bacillus Calmette-Guerin/lipopolysaccharide-induced liver injury in mice. Food Chem Toxicol. 2009;47(10):2431–2435. doi: 10.1016/j.fct.2009.06.041. [DOI] [PubMed] [Google Scholar]
- Huang H-L, Liu C-T, Chou M-C, Ko C-H, Wang C-K. Noni (Morinda citrifolia L.) fruit extracts improve colon microflora and exert anti-inflammatory activities in Caco-2 cells. J Med Food. 2015;18(6):663–676. doi: 10.1089/jmf.2014.3213. [DOI] [PubMed] [Google Scholar]
- Kim JM, Whang JH, Kim KM, Koh JH, Suh HJ. Preparation of corn gluten hydrolysate with angiotensin I converting enzyme inhibitory activity and its solubility and moisture sorption. Process Biochem. 2004;39(8):989–994. doi: 10.1016/S0032-9592(03)00205-X. [DOI] [Google Scholar]
- Kühl AA, Erben U, Kredel LI, Siegmund B. Diversity of intestinal macrophages in inflammatory bowel diseases. Front Immunol. 2015;6:613. doi: 10.3389/fimmu.2015.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Kovacs-Nolan J, Archbold T, Fan MZ, Juneja LR, Okubo T, Mine Y. Therapeutic potential of hen egg white peptides for the treatment of intestinal inflammation. J Funct Foods. 2009;1(2):161–169. doi: 10.1016/j.jff.2009.01.005. [DOI] [Google Scholar]
- Liang QF, Ren XF, Ma HL, Li SY, Xu KK, Oladejo AO. Effect of low-frequency ultrasonic-assisted enzymolysis on the physicochemical and antioxidant properties of corn protein hydrolysates. J Food Qual. 2017 doi: 10.1155/2017/2784146. [DOI] [Google Scholar]
- Liang Q, Chalamaiah M, Ren X, Ma H, Wu J. Identification of new anti-inflammatory peptides from zein hydrolysate after simulated gastrointestinal digestion and transport in caco-2 cells. J Agric Food Chem. 2018;66(5):1114. doi: 10.1021/acs.jafc.7b04562. [DOI] [PubMed] [Google Scholar]
- Majumder K, Mine Y, Wu J. The potential of food protein-derived anti-inflammatory peptides against various chronic inflammatory diseases. J Sci Food Agric. 2016;96(7):2303–2311. doi: 10.1002/jsfa.7600. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Muzio M, Garlanda C, Sozzani S, Allavena P. Macrophage control of inflammation: negative pathways of regulation of inflammatory cytokines. New York: Wiley; 2001. [DOI] [PubMed] [Google Scholar]
- Etchevers JM, Aceituno M, Sans M. Are we giving azathioprine too late? The case for early immunomodulation in inflammatory bowel disease. World J Gastroenterol. 2008;14(36):5512. doi: 10.3748/wjg.14.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meram C, Wu J. Anti-inflammatory effects of egg yolk livetins (alpha, beta, and gamma-livetin) fraction and its enzymatic hydrolysates in lipopolysaccharide-induced RAW 264.7 macrophages. Food Res Int. 2017;100(Pt 1):449–459. doi: 10.1016/j.foodres.2017.07.032. [DOI] [PubMed] [Google Scholar]
- Mine Y, Zhang H. Anti-inflammatory effects of poly-l-lysine in intestinal mucosal system mediated by calcium-sensing receptor activation. J Agric Food Chem. 2015;63(48):10437–10447. doi: 10.1021/acs.jafc.5b03812. [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Shigemura H, Hasegawa N. Anti-inflammatory effect of enzymatic hydrolysate of corn gluten in an experimental model of colitis. J Pharm Pharmacol. 2010;62(3):389–392. doi: 10.1211/jpp.62.03.0015. [DOI] [PubMed] [Google Scholar]
- Olamilosoye KP, Akomolafe RO, Akinsomisoye OS, Adefisayo MA, Alabi QK. The aqueous extract of Ocimum gratissimum leaves ameliorates acetic acid-induced colitis via improving antioxidant status and hematological parameters in male Wistar rats. Egypt J Basic Appl Sci. 2018 doi: 10.1016/j.ejbas.2018.05.006. [DOI] [Google Scholar]
- Puneet Kaur R, Kavinder S, Nirmal S, Amteshwar Singh J. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol Off J Korean Physiol Soc Korean Soc Pharmacol. 2014;18(4):279. doi: 10.4196/kjpp.2014.18.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao CV. Nitric oxide signalling in colon cancer chemoprevention. Mutat ResFundam Mol Mech Mutagenesis. 2004;555(1):107–119. doi: 10.1016/j.mrfmmm.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Ren X, Liang Q, Zhang X, Hou T, Li S, Ma H. Stability and antioxidant activities of corn protein hydrolysates under simulated gastrointestinal digestion. Cereal Chem. 2018;95(6):760–769. doi: 10.1002/cche.10092. [DOI] [Google Scholar]
- Rodriguez-Ramiro I, Ramos S, Lopez-Oliva E, Agis-Torres A, Bravo L, Goya L, Martin MA. Cocoa polyphenols prevent inflammation in the colon of azoxymethane-treated rats and in TNF-alpha-stimulated Caco-2 cells. Br J Nutr. 2013;110(2):206–215. doi: 10.1017/S0007114512004862. [DOI] [PubMed] [Google Scholar]
- Salaga M, Binienda A, Draczkowski P, Kosson P, Kordek R, Jozwiak K, Fichna J. Novel peptide inhibitor of dipeptidyl peptidase IV (Tyr-Pro-D-Ala-NH2) with anti-inflammatory activity in the mouse models of colitis. Peptides. 2018;108:34–45. doi: 10.1016/j.peptides.2018.08.011. [DOI] [PubMed] [Google Scholar]
- Sartor RB. Cytokines in intestinal inflammation: Pathophysiological and clinical considerations. Gastroenterology. 1994;106(2):533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Sedmak JJ, Grossberg SE. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977;79(1):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Shi Y, Rupa P, Jiang B, Mine Y. Hydrolysate from eggshell membrane ameliorates intestinal inflammation in mice. Int J Mol Sci. 2014;15(12):22728–22742. doi: 10.3390/ijms151222728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115(2):297–306. doi: 10.1016/S0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- Sobczak M, Fabisiak A, Murawska N, Wesołowska E, Wierzbicka P, Wlazłowski M, Wójcikowska M, Zatorski H, Zwolińska M, Fichna J. Current overview of extrinsic and intrinsic factors in etiology and progression of inflammatory bowel diseases. Pharmacol Rep. 2014;66(5):766–775. doi: 10.1016/j.pharep.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Stanojevića S, Blagojevića V, Ćuruvijaa I, Veljovićb K, Bajićb SS, Kotur-Stevuljevićc J, Bogdanovićd A, Petrovića R, Vujnovića I, Kovačević-Jovanoviće V. Oral treatment with Lactobacillus rhamnosus 64 during the early postnatal period improves the health of adult rats with TNBS-induced colitis. J Funct Foods. 2018;48:92–105. doi: 10.1016/j.jff.2018.07.014. [DOI] [Google Scholar]
- Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38(10):1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- United States Pharmacopeial Convention Council of Experts (2007) Simulated gastric and intestinal fluids. In: TS Board of Trustees (Ed.) United States pharmacopeial convention and national formulary, United States Pharmacopeial Convention, Inc, Rockville, p 2728
- Wada S, Sato K, Ohta R, Wada E, Bou Y, Fujiwara M, Kiyono T, Park EY, Aoi W, Takagi T, Naito Y, Yoshikawa T. Ingestion of low dose pyroglutamyl leucine improves dextran sulfate sodium-induced colitis and intestinal microbiota in mice. J Agric Food Chem. 2013;61(37):8807–8813. doi: 10.1021/jf402515a. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Nishikiori F, Ito M, Furukawa Y. The effects of corn peptide ingestion on facilitating alcohol metabolism in healthy men. Biosci Biotechnol Biochem. 1997;61(9):1474–1481. doi: 10.1271/bbb.61.1474. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Takeuchi M, Ebihara K. Inhibitory effect of peptide prepared from corn gluten meal on 7, 12-dimethylbenz [a] anthracene-induced mammary tumor progression in rats. Nutr Res. 1997;17(7):1121–1130. doi: 10.1016/s0271-5317(97)00083-3. [DOI] [Google Scholar]
- Young D, Ibuki M, Nakamori T, Fan M, Mine Y. Soy-derived di- and tripeptides alleviate colon and ileum inflammation in pigs with dextran sodium sulfate-induced colitis. J Nutr. 2012;142(2):363–368. doi: 10.3945/jn.111.149104. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yao Y, Xu M, Wang S, Wang X, Tu Y. Simulated gastrointestinal digest from preserved egg white exerts anti-inflammatory effects on Caco-2 cells and a mouse model of DSS-induced colitis. J Funct Foods. 2017;35:655–665. doi: 10.1016/j.jff.2017.06.028. [DOI] [Google Scholar]