Abstract
Blueberry fruits are known for their high vitamin C, essential dietary fibre, antioxidant activity and anthocyanin pigments. Different blueberry varieties have been introduced in India but no attempt has been made for their nutritional profiling. Nutritional profiling of varieties helps us to know the unique varietal characters, which serves as a guideline for recommendation of a valuable variety for fresh consumption and/or processing. Therefore, the present study was conducted in eight different blueberry varieties such as ‘Misty’, ‘Sharp Blue’, ‘Biloxi’, ‘Jewel’, ‘Gulf Coast’, ‘Blue Crop’, ‘Star’, ‘Legacy’. The results of the study revealed that all tested varieties differed significantly in physical attributes (10-berry weight, fruit firmness, roundness index, moisture content) and biochemical and functional attributes (ascorbic acid, total anthocyanin, total phenolic content, antioxidant activity, total sugars, organic acids) and mineral content. Regression analysis and Principal Component Analysis showed that antioxidant potential of blueberries was mainly contributed by phenolics followed by anthocyanins and ascorbic acid content. However for taste perception, fructose among sugars and succinic acid among sugars were the most influencing factors (p ≤ 0.05). Total phenolics and anthocyanins content were responsible for overall difference in functional attributes among the varieties. The attributes such as high fruit firmness, sensorial score, and appropriate shape and weight make ‘Misty’, the best variety for marketability and fresh consumption among all tested varieties.
Keywords: Blueberry, Ascorbic acid, Total phenolics, Functional attributes, Fructose, Mineral content, PCA
Introduction
Now-a-days, consumers are becoming more and more health cautious. Hence, there is a great demand for antioxidant-rich fruits among the consumers. In this regard, it is worthwhile to mention that blueberry fruit is a future fruit because it is regarded as a store house of important antioxidant compounds such as ascorbic acid, anthocyanin content and phenolic compounds (Gunduz et al. 2015). Because of these functional attributes in blueberry, consumers are ready to pay high price for its fruits in the market. It is mainly consumed as fresh fruit but can be processed into several delicacies such as yogurt, milk shake, juice, jam and other processed products (Avram et al. 2017). Blueberry is a crop of North American origin, belonging to Ericaceae family. Blueberry consists of mainly 3 types of genotypes such as high bush blueberry (Vaccinium corymbosum), lowbush blueberry (Vaccinium angustifolium) and rabbit eye blueberry (Vaccinium ashei) (Saftner et al. 2008; Sharma and Krishna 2018). Blueberry crop is mainly cultivated in Canada, California and China. China has emerged as the leader in exporting the blueberries to entire Asian region. However, several blueberry varieties have also been introduced at the research stations of Himachal Pradesh, Jammu & Kashmir and Uttarakhand to explore its cultivation possibilities in India. Now its cultivation has started but at a slower speed because its requirements for soil (pH 4.5) are highly specific. Additionally, its propagation is very tough and availability of planting material in India is really a challenge (Sharma and Krishna 2018). However, due to health benefits and easy processing, farmers are interested to grow this valuable fruit at a commercial scale so as to get high price and benefits. Hence, it is pertinent to perform nutritional, functional and sensorial profiling of blueberry varieties grown in India so as to recommend the best variety for consumption and/or processing which will attract the growers and health cautious consumers.
Materials and methods
Experimental materials and site
For this study, eight blueberry varieties grown in Katrain valley of Himachal Pradesh, such as ‘Jewel’, ‘Gulf Coast’, ‘Misty’, ‘Blue Crop’, ‘Star’, ‘Biloxi’, ‘Sharp Blue’, and ‘Legacy’, were selected. All the selected blueberry varieties belonged to high-bush group. Blueberry bushes were eight-year-old, grown in an orchard located at 32.1297° N latitude, 77.1241° E longitude, and at an elevation of 1350 m above sea level. The orchard soil was sandy-loam, having a pH of 5.2. Fruits were harvested at full maturity i.e., purple colour stage. After harvesting, fruit were sorted, graded, packed in 500 g CFB boxes and brought to Division of Food Science and Postharvest Technology, ICAR-Indian Agricultural Research Institute, New Delhi-110 012 for observations on physical (10-berry fruit weight, fruit roundness index (RI), firmness and moisture content), quality (titratable acidity, sugars and organic acid and sensory evaluation) and functional attributes (ascorbic acid, total phenolics, total anthocyanin and antioxidant activity) and mineral content (major and minor) as described briefly hereunder.
Estimation of physical attributes
All physical attributes were estimated in each variety in 10 randomly selected fruits with three replications. 10-blueberry fruit weight was recorded by using electronic balance and represented as 10-berry weight. The dimensions of blueberry fruits were recorded by Vernier calliper and the roundness index (R.I) of fruits was calculated by dividing the length (polar) to the width (equatorial) of the fruits. Fruit firmness was determined using a texture analyzer (model: TA + Di, Stable micro systems, UK) (Yaman and Bayoundurlc 2002) and expressed in N (Newtons). Moisture content was estimated using oven dry method (50–55 °C) and expressed in percentage (%).
Estimation of quality attributes
All quality attributes were measured in each variety using 10 randomly selected fruits with three replications. The titratable acidity was estimated as per the method of Ranganna (1999) and expressed in (%). Samples for sugar and organic acid were prepared by grinding 5 g blueberry pulp in pestle and mortar, followed by centrifuging at 10,000×g at 2 °C for 15 min. Sugars and organic acids in aqueous phase were quantified using Aminex HPX-87H (Bio-Rad Laboratories, Hercules, CA) column operated with 5 mM H2SO4 as mobile phase and expressed in mg/ml of sample (Zeppa et al. 2001). The regression co-efficient for sugars was calculated as per the following equation.
| 1 |
The sensory evaluation was done on the basis of color, flavour, texture, and taste by a panel of ten semi-trained judges using 9 point Hedonic scale (Amerine et al. 1965).
Determination of functional attributes
All functional attributes of the eight blueberry varieties were estimated in 10 randomly selected fruits with three replications. Ascorbic acid was estimated as per the method of Ranganna (1999) and expressed in mg ascorbic acid/100 g of sample. The total phenolic content of the fruit extracts were determined using the method of Singleton and Rossi (1965) and expressed in mg gallic acid equivalents (GAE)/100 g of extract. The total monomeric anthocyanin content was determined, using pH differential method (Wrolstad et al. 2005) and expressed in mg/100 g of sample. Antioxidant capacity in the blueberry was determined by using CUPRAC (cupric reducing antioxidant capacity) method and expressed in µmol TE/g (Apak et al. 2004).
Estimation of minerals
The concentrations of major and minor minerals in the selected varieties estimated in 10 randomly selected fruits with three replications by acid digestion method (Drozdz et al. 2018). The major mineral content in blueberry fruits was expressed on percentage basis (%), and minor in ppm (parts per million).
Statistical design and analysis of data
The experiment was laid out in completely randomised design (CRD) with three replicates. The data of all the attributes were analyzed using one way analysis of variance (ANOVA) among different varieties of blueberry using SAS (9.4) software (Panse and Sukhatme 1984), and the results were compared by calculating critical difference (CD) at 5% level of significance, and also using Duncan’s multiple range test. Regression analysis was done to trace the impact of type of sugar to total sugars, total phenolics, ascorbates and total anthocyanins on total antioxidant activity as well as to trace the most prominent component among sugars and acids to decide overall taste perception of blueberry. Further, principal component analysis (PCA) was performed for clustering of different varieties on the basis of functional attributes and to validate findings of regression analysis.
Results and discussion
Physical attributes
The physical quality parameters are important for any fruit for easy identification by the consumers and farmers. So the different physical parameters were recorded in the selected blueberry varieties. Our results revealed that the 10-berry weight of blueberry varieties had shown significant variations and it ranged from 9.75 (‘Sharp Blue’) to 19.64 g (‘Jewel’). The roundness index of blueberry varieties was also significant which ranged from 0.72 (‘Legacy’) to 0.83 (‘Sharp Blue’) (Table 1). A perfect round shape is having roundness index of 1 and any value close to 1 would indicate a perfect round shape. Furthermore, there was a non-significant variation in moisture content of tested blueberry varieties but it ranged from 83.82 (‘Blue Crop’) to 87.64% (‘Jewel’). Similarly, the fruit firmness value differed significantly from 1.77 (‘Legacy’) to 3.36 N (‘Misty’) and the remaining varieties had revealed intermediate values for all physical attributes studied by us. The genetic variations among the different varieties might have contributed for such differences in physical attributes among the studied varieties of blueberry. In a similar study, Matiacevich et al. (2013) reported variability in roundness index and moisture content among six different cultivars of blueberry. Similarly, Mannozzi et al. (2017) reported difference in the fruit firmness of among blueberry varieties. Vaio et al. (2014) and Ellong et al. (2015) also reported significant variability in the fruit firmness of different mango and peach varieties, respectively, which they attributed this variability to genetic differences among the cultivars.
Table 1.
Variations in physical attributes of different blueberry varieties
| Variety | 10-Berry weight (g) | Roundness index | Moisture content (%) | Fruit firmness (N) |
|---|---|---|---|---|
| Jewel | 19.64 ± 2.09d | 0.73 ± 1.12a | 87.64 ± 2.28a | 2.76 ± 0.57bc |
| Misty | 11.57 ± 1.05a | 0.81 ± 0.08b | 86.11 ± 0.96a | 3.36 ± 0.09c |
| Gulf Coast | 12.36 ± 0.83ab | 0.74 ± 0.06a | 86.72 ± 1.33a | 2.43 ± 0.59abc |
| Biloxi | 15.25 ± 1.42bc | 0.80 ± 0.15b | 85.47 ± 2.28a | 1.79 ± 0.29a |
| Star | 10.60 ± 1.29a | 0.79 ± 0.03b | 85.13 ± 2.13a | 1.85 ± 0.48a |
| Legacy | 16.47 ± 1.13c | 0.72 ± 0.16a | 83.91 ± 1.47a | 1.77 ± 0.34a |
| Blue Crop | 11.20 ± 0.72a | 0.78 ± 0.09ab | 83.82 ± 2.52a | 1.85 ± 0.06ab |
| Sharp Blue | 9.75 ± 1.43a | 0.83 ± 0.14b | 86.61 ± 2.58a | 2.64 ± 0.08abc |
| SEm± | 0.76 | 0.02 | 1.17 | 0.22 |
| CD (0.05) | 2.27* | 0.06 | NS | 0.65* |
*Significant at 5% level of significance
Means followed by different letters in a column differ significantly at 5% level of significance
Concentrations of sugars and organic acids
Sugars are responsible for the sweetness of fruits but their concentrations differs significantly by the proportions of different sugar molecules and are responsible for contributing a unique taste to fruits. Our results revealed that fruits of ‘Sharp Blue’ variety exhibited more sweetness due to highest concentrations of glucose (9.64 mg/ml), fructose (10.04 mg/ml) and total sugars (19.74 mg/ml) whereas the ‘Jewel’ variety exhibited the lowest sweetness due to the minimum concentrations of glucose (6.66 mg/ml), fructose (5.59 mg/ml) and/or total sugars (12.27 mg/ml) in the fruits (Table 2). Other varieties exhibited intermediate values for different sugars. Regression analysis showed that for total sugar content, glucose was the major contributing sugar than fructose (Eq. 1).
Table 2.
Concentrations of different sugars and organic acids in different blueberry varieties
| Variety | Sugars (mg/ml) | Organic acids (mg/ml) | Fructose: succinic acid ratio | Sensory score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Fructose | Total sugar content | Citric acid | Malic acid | Tartaric acid | Succinic acid | Total organic acid content | |||
| Jewel | 6.66 ± 0.12a | 5.59 ± 0.05a | 12.27 ± 0.03a | 3.66 ± 0.02f | 0.22 ± 0.03a | 0.00 ± 0.00a | 1.59 ± 0.02d | 6.06 ± 0.02h | 3.52 | 8.00 ± 0.06a |
| Misty | 7.53 ± 0.02b | 7.96 ± 0.06b | 15.52 ± 0.05b | 1.97 ± 0.12d | 0.23 ± 0.02a | 0.04 ± 0.01b | 2.46 ± 0.03f | 2.16 ± 0.03c | 3.24 | 8.50 ± 0.02e |
| Gulf Coast | 7.76 ± 0.17bc | 7.97 ± 0.17b | 15.67 ± 0.04bc | 2.85 ± 0.01e | 0.23 ± 0.01a | 0.00 ± 0.00a | 1.05 ± 0.01c | 3.05 ± 0.01e | 7.59 | 7.04 ± 0.06b |
| Biloxi | 8.10 ± 0.23 cd | 8.29 ± 0.13c | 16.36 ± 0.11d | 0.78 ± 0.03a | 0.31 ± 0.02b | 0.04 ± 0.01b | 0.74 ± 0.01a | 1.04 ± 0.02a | 11.2 | 7.02 ± 0.03b |
| Star | 7.68 ± 0.19b | 8.07 ± 0.16bc | 15.75 ± 0.11c | 1.65 ± 0.05c | 0.32 ± 0.02b | 0.05 ± 0.01b | 0.95 ± 0.01b | 1.93 ± 0.03b | 8.49 | 7.50 ± 0.01c |
| Legacy | 8.90 ± 0.09e | 8.94 ± 0.18d | 17.84 ± 0.09f | 1.64 ± 0.02c | 0.30 ± 0.01b | 0.00 ± 0.00a | 0.77 ± 0.02a | 2.64 ± 0.5d | 11.61 | 7.00 ± 0.01b |
| Blue Crop | 8.43 ± 0.09d | 8.83 ± 0.08d | 17.24 ± 0.12e | 1.85 ± 0.04d | 0.21 ± 0.01a | 0.07 ± 0.01c | 2.19 ± 0.07e | 4.52 ± 0.02g | 4.03 | 8.01 ± 0.01d |
| Sharp Blue | 9.64 ± 0.26f | 10.04 ± 0.08e | 19.74 ± 0.06g | 1.24 ± 0.02b | 0.41 ± 0.01c | 0.00 ± 0.00a | 0.97 ± 0.04b | 3.78 ± 0.02f | 10.35 | 7.50 ± 0.02c |
| SEm± | 8.09 | 0.07 | 0.05 | 0.03 | 0.26 | 0.02 | 0.02 | 0.01 | – | 0.02 |
|
CD (0.05) |
0.10* | 0.21* | 0.14* | 0.09* | 0.05* | 0.04* | 0.06* | 0.04* | – | 0.05* |
*Significant at 5% level of significance
Means followed by different letters in a column differ significantly at 5% level of significance
The acid content in the fruits impart sourness but they also impart delicacy to fruits when intermingled with sugars in a right proportions. In this study, it was observed that different blueberry varieties exhibited variations in the amount of citric acid, malic acid, tartaric acid, succinic acid, total organic acid content and titratable acidity. It is evident from Table 3 that the citric acid content in the tested varieties ranged from 0.78 (‘Biloxi’) to 3.66 mg/ml (‘Jewel’), malic acid from 0.21 (‘Blue Crop’) to 0.41 mg/ml (‘Sharp Blue’), tartaric acid from 0.0 (‘Jewel’, ‘Gulf Coast’, ‘Legacy’ and ‘Sharp Blue’) to 0.07 mg/ml (‘Blue Crop’), succinic acid from 0.74 (‘Biloxi’) to 2.46 mg/ml (‘Misty’) and the total organic acid content from 4.52 (‘Blue Crop’) to 6.06 mg/ml (‘Jewel’). Such differences in organic acid profile among different blueberry varieties may be due to differences in the genetic makeup of blueberry varieties. In a similar study, Gunduz et al. (2015) reported variability in the organic acids among different highbush and rabbiteye blueberry varieties. Interestingly, Reig et al. (2013) observed a 5-fold time differences in malic acid concentration in different cultivars of peach grown in Spain.
Table 3.
Variations in functional attributes of different blueberry varieties
| Variety | Ascorbic acid content (mg/100 g) | Total phenolic content (mg GAE/100 g) | Total anthocyanin content (mg/100 g) | Total antioxidant activity (µmol TE/g) |
|---|---|---|---|---|
| Jewel | 22.38 ± 2.12ab | 157.70 ± 0.3c | 135.42 ± 0.03c | 35.83 ± 0.42d |
| Misty | 23.27 ± 2.03abc | 154.60 ± 0.3b | 138.70 ± 0.3d | 22.86 ± 1.19b |
| Gulf Coast | 24.79 ± 1.95abc | 178.57 ± 0.35d | 126.22 ± 0.71b | 27.05 ± 1.63c |
| Biloxi | 26.76 ± 1.31bc | 113.80 ± 0.3a | 135.82 ± 0.03c | 25.55 ± 1.04c |
| Star | 20.70 ± 1.48a | 203.40 ± 0.3g | 190.54 ± 0.05g | 15.71 ± 1.80a |
| Legacy | 24.14 ± 1.88abc | 344.20 ± 0.3h | 150.82 ± 0.03f | 35.73 ± 0.51d |
| Blue Crop | 25.77 ± 3.73abc | 185.00 ± 0.3e | 146.47 ± 0.35e | 22.85 ± 0.82b |
| Sharp Blue | 28.65 ± 1.48c | 186.30 ± 0.3f | 120.60 ± 0.3a | 36.89 ± 1.19d |
| SEm± | 1.23 | 0.18 | 0.19 | 0.68 |
| CD (0.05) | 3.67* | 0.53* | 0.56* | 2.03* |
*Significant at 5% level of significance
Means followed by different letters in a column differ significantly at 5% level of significance
Functional attributes
Functional activity of fruits is mainly due to presence of different compounds such as ascorbic acid content, total phenolics and total anthocyanin content. Such compounds scavenge free radicals from our body un stress conditions thereby provides immunity against dreaded diseases such as cancer. After analysing different components in the 8-tested blueberry varieties, it is evident the data presented in Table 3 that the ascorbic acid content ranged from 20.70 (‘Star’) to 28.65 mg/100 g (‘Sharp Blue’), total phenolic content from 113.80 mg (‘Biloxi’) to 344.20 GAE/100 g (‘Legacy’) total anthocyanin content from 126.22 (‘Gulf Coast’) to 190.54 mg/100 g (‘Star’) to and total antioxidant activity from 15.71 (‘Star’) to 36.89 (‘Sharp Blue’) which may be attributed to genetic differences among the varieties. Statistical analysis showed that total antioxidant potential in blueberry was mainly contributed by phenolics (regression coefficient 0.064), followed by total anthocyanins (regression coefficient − 0.37) and ascorbic acid (regression coefficient − 0.32). It is well established in the literature that varietal inheritance played a significant role in the variations in different functional attributes of blueberry varieties. For instance, Gil et al. (2002) had reported wider variability in antioxidant activity of 25 cultivars of peach and nectarines grown in California. Wu et al. (2007) had reported that there is a significant variation in phenolic acid content among apple cultivars. Sharma et al. (2014) recorded variability in total phenolics and antioxidant activity among five different kiwifruit varieties grown in India., Similarly, Lia et al. (2017) did profiling for anthocyanin, polyphenols and flavonoids content in 13 blueberry cultivars and reported that all cultivars contained similar types of anthocyanins but their concentrations were cultivar-dependent. Recently, Kumar et al. (2018) reported varietal diversity in ascorbic acid content in different apple varieties which significantly contribute to AOX activity.
Concentrations of major and minor minerals
Fruits have been designated as ‘protective foods’ by medical experts, mainly because they are rich source of vitamins and minerals, which protect us from several ailments and disorders. In this study, we recorded a wider variability for most of the major and minor minerals in the tested blueberry varieties. The sodium content in the varieties varied from 1.84 (‘Jewel’) to 3.09% (‘Star’), phosphorus from 0.09 (‘Sharp Blue’) to 0.16% (‘Star’), potassium from 0.42 (‘Gulf Coast’) to 0.62% (‘Bluecrop’), calcium t from 0.11% (‘Blue Crop’) to 0.33 (‘Star’) and magnesium from 0.07% (‘Blue Crop’) to 0.25 (‘Star’) (Table 4). This kind of variability in mineral content among different blueberry varieties may be due to their genetical differences amongst them as reported by Drozdz et al. (2018) who observed significant variability in 13 elements among wild and cultivated blueberry varieties. They further reported that wild blueberry varieties contained higher concentrations of calcium, magnesium and sodium and reported that this variation in concentrations of different minor mineral elements may be due to genetic and edaphic factors.
Table 4.
Concentrations of major and minor minerals in fruits of different blueberry varieties
| Variety | Major mineral content (%) | Minor mineral content (ppm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Na | P | K | Ca | Mg | Mn | Zn | Cu | |
| Jewel | 1.84 ± 0.05a | 0.12 ± 0.03ab | 0.47 ± 0.05a | 0.19 ± 0.02b | 0.13 ± 0.02b | 22.22 ± 2.52a | 12.24 ± 1.00b | 5.56 ± 0.08a |
| Misty | 2.57 ± 0.03d | 0.12 ± 0.02ab | 0.44 ± 0.03a | 0.25 ± 0.03c | 0.16 ± 0.03bc | 43.54 ± 1.75c | 13.06 ± 0.75b | 7.26 ± 0.27bc |
| Gulf Coast | 2.06 ± 0.07bc | 0.14 ± 0.03ab | 0.41 ± 0.03a | 0.17 ± 0.02b | 0.17 ± 0.04bc | 29.99 ± 0.75b | 11.99 ± 0.96b | 7.19 ± 0.48bc |
| Biloxi | 2.73 ± 0.04e | 0.12 ± 0.01ab | 0.46 ± 0.03a | 0.20 ± 0.02bc | 0.23 ± 0.03d | 60.65 ± 1.30e | 12.74 ± 1.20b | 7.97 ± 0.32 cd |
| Star | 3.09 ± 0.07f | 0.16 ± 0.01b | 0.59 ± 0.03b | 0.33 ± 0.02d | 0.25 ± 0.03d | 55.36 ± 1.13d | 8.94 ± 0.72a | 7.13 ± 0.20b |
| Legacy | 1.94 ± 0.06ab | 0.11 ± 0.00ab | 0.43 ± 0.03a | 0.18 ± 0.04b | 0.14 ± 0.02bc | 23.42 ± 1.23a | 22.07 ± 1.96c | 8.22 ± 0.32d |
| Blue Crop | 2.15 ± 0.06c | 0.14 ± 0.02ab | 0.62 ± 0.03b | 0.11 ± 0.02a | 0.07 ± 0.00a | 42.92 ± 0.80c | 14.36 ± 070b | 5.97 ± 0.29a |
| Sharp Blue | 1.89 ± 0.02a | 0.09 ± 0.0a | 0.46 ± 0.04a | 0.15 ± 0.02ab | 0.21 ± 0.01cd | 24.76 ± 2.52a | 14.34 ± 0.31b | 5.66 ± 0.49a |
| SEm± | 0.03 | 0.01 | 0.02 | 0.01 | 0.17 | 0.95 | 0.61 | 0.19 |
| CD (0.05) | 0.10* | 0.03* | 0.06* | 0.04* | 0.01* | 2.84* | 1.82* | 0.57* |
*Significant at 5% level of significance
Means followed by different letters in a column differ significantly at 5% level of significance
Like major minerals, we observed significant variability in the concentrations of minor elements as well. Among minor minerals, manganese content ranged from 22.22 (‘Jewel’) to 60.65 ppm (‘Biloxi’), zinc from 8.94 ppm (‘Star’) to 22.07 ppm (‘Legacy’), copper from 5.56 ppm (‘Jewel’) to 8.22 ppm (‘Legacy’) and the remaining varieties revealed intermediate concentrations for minor mineral content. In a similar study, Drozdz et al. (2018) reported significant variability in minor mineral elements among wild and cultivated blueberry varieties. And also, Fazli and Fazli (2014) studied the mineral constituents of various fruits and found that fruits differ in their mineral composition.
Principal component analysis (PCA)
PCA is a powerful dimension reduction technique that explains the variance of larger inter-correlated variables by transforming them into a smaller set of independent uncorrelated principal components. In this study, first two PCs explained 67% of the variance in the biochemical attributes such as organic acids, sugar molecules, total anthocyanin content, total antioxidant content, total phenolic content and ascorbic acid content studied in eight different blueberry varieties, i.e., ‘Jewel’, ‘Gulf Coast’, ‘Misty’, ‘Blue Crop’, ‘Star’, ‘Biloxi’, ‘Sharp Blue’, and ‘Legacy’ (Fig. 1). The higher component loadings were seen for tartaric acid, succinic acid and total anthocyanin content in varieties such as ‘Blue Crop’, ‘Misty’, ‘Star’, respectively whereas for total organic acids and citric acid content, loadings were recorded to be maximum in ‘Jewel’ and ‘Gulf Coast’ varieties, respectively. ‘Legacy’ and ‘Sharp Blue’ varieties showed the presence of maximum concentration of biochemical components such as ascorbic acid, total antioxidant activity, glucose and total phenolics. These observations are inclined with the table values for each biochemical attribute in a particular variety (Tables 2, 3). The variety ‘Biloxi’ invariably contained higher amount of fructose and malic acid content (Fig. 1). Total ascorbic acid followed by total phenolic content, after which total anthocyanins are responsible for total antioxidants activity. Therefore, while screening a large number of germplasm of blueberry for the antioxidant potential; screening can predominantly be done on the basis of ascorbic acid content. Regression analysis also supported this finding on antioxidants.
Fig. 1.

Biplot based on principal component analysis (PCA) for biochemical attributes in eight blueberry varieties
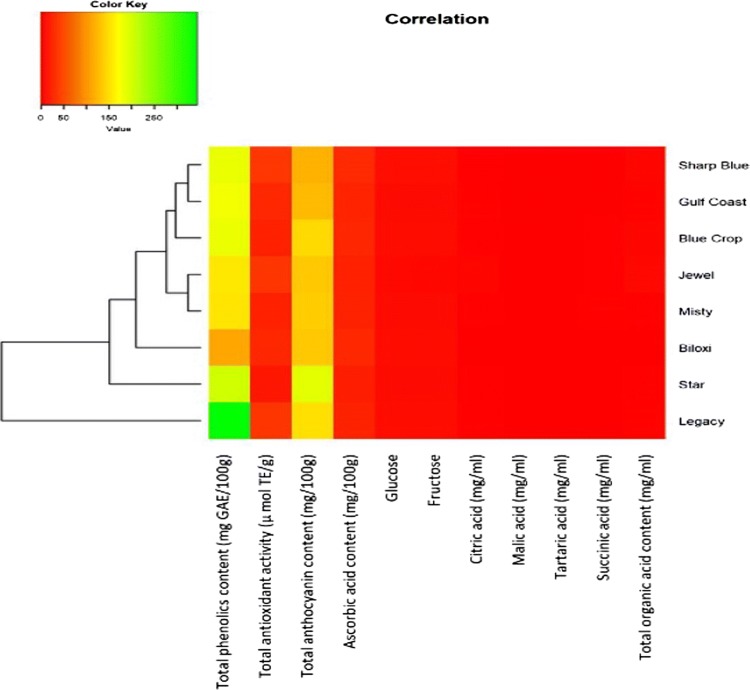
The cluster heat map of biochemical attributes in eight blueberry varieties is represented in Fig. 2. All the tested blueberry varieties differed significantly according to their total phenolic content, then followed by anthocyanin content. This shows the inherent difference among eight varieties with respect to total phenolics and total anthocyanins, however total antioxidant activity, concentrations of different organic acids and sugar molecules did not play a significant role in the variations among tested blueberry varieties. Abundance of total phenolics and total anthocyanins was observed in ‘Legacy’ variety, followed by ‘Star’ and ‘Biloxi’ which formulated one group. Based on other biochemical parameters such as glucose, fructose, citric acid, malic acid, tartaric acid, succinic acid, total organic acid, and ascorbic acid content, a larger cluster of varieties such as ‘Sharp Blue’, ‘Gulf Coast’, ‘Blue Crop’, ‘Jewel’ and ‘Misty’ was formed as second group (Fig. 2).
Fig. 2.
Cluster heat map of biochemical attributes in eight blueberry varieties. The color scale indicates the degree of correlation (red: low correlation; green: high correlation) (colour figure online)
Sensory evaluation
Regression analysis showed that for sensorial properties of blueberry, concentration of fructose among sugars, and succinic acid among acids, played the most significant role (Eq. 2), and perhaps because of this sweetness (fructore) and acid (succinic acid) blend of ‘Misty’, ‘Blue Crop’ and ‘Jewel’ varieties was the most liked by the semi-trained penalists (Fig. 1, Table 2). The ratio of fructose and succinic acid which ranged between 3 and 4 appeared to be the best for taste perception. Sensory scores revealed that among acids, succinic acid was the major contributing factor for overall taste perception among eight studied blueberry varieties. The best sensory score (8.5) was observed in ‘Misty’, followed by ‘Blue Crop’ and ‘Jewel’ (Table 2).
| 2 |
Conclusion
The eight tested blueberry varieties such as ‘Jewel’, ‘Gulf Coast’, ‘Misty’, ‘Blue Crop’, ‘Star’, ‘Biloxi’, ‘Sharp Blue’, and ‘Legacy’ had shown a significant variability with respect to physical, functional attributes and mineral content mainly due to the strong influence of genetical behaviour of different blueberry cultivars. Among functional attributes, total phenolics played major role for antioxidant potential of blueberries, followed by total anthocyanins and ascorbic acid. Acceptable sensory scores were mainly contributed by fructose sugar and succinic acid, and total phenolics and anthcyanins content were responsible for overall difference among the tested blueberry varieties. The highest firmness, sensorial scores, appropriate shape and weight dimensions make ‘Misty’, the best variety for fresh consumption and marketability among all tested varieties of blueberry. However, ‘Blue Crop’ and ‘Jewel’ were equally competitive.
Abbreviations
- RI
Roundness index
- AOX activity
Antioxidant activity
- GAE
Gallic acid equivalents
- TE
Trolox equivalent
- N
Newton
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amerine MA, Pangborn RM, Roessler EB. Principles of sensory evaluation of food. 1. New York: Academic Press; 1965. [Google Scholar]
- Apak R, Guclu K, Ozyurek M, Karademir SE. Novel total antioxidants capacity index for dietary polyphenol and vitamins C and E using their cupric ion reducing capability in the presence of neocuprine: CUPRAC method. J Agric Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- Avram AM, Morin P, Brownmiller C, Howard LR, Sengupta A, Wickramasinghe SR. Concentrations of polyphenols from blueberry pomace extract using nanofiltration. Food Bioprod Process. 2017;106:91–101. doi: 10.1016/j.fbp.2017.07.006. [DOI] [Google Scholar]
- Drozdz P, Seziene V, Pyrzynska K. Mineral composition of wild and cultivated blueberries. Biol Trace Elem Res. 2018;181:173–177. doi: 10.1007/s12011-017-1033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellong EN, Adenet S, Rochefort K. Physicochemical, nutritional, organoleptic characteristics and food applications of four mango (Mangifera indica) varieties. Food Nutr Sci. 2015;6:242–253. [Google Scholar]
- Fazli AF, Fazli NA. Evaluation and determination of mineral contents in fruits. Int J Plant Environ Sci. 2014;4(2):160–166. [Google Scholar]
- Gil MI, Tomas F, Betty BA, Pierce H, Kader AA. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J Agric Food Chem. 2002;50:4976–4982. doi: 10.1021/jf020136b. [DOI] [PubMed] [Google Scholar]
- Gunduz K, Serce S, Hancock JH. Variation among highbush and rabbiteye cultivars of blueberry for fruit quality and phytochemical characteristics. J Food Compos Anal. 2015;38:69–79. doi: 10.1016/j.jfca.2014.09.007. [DOI] [Google Scholar]
- Kumar P, Sethi S, Sharma RR, Singh S, Saha S, Sharma VK, Verma MK, Sharma SK. Nutritional characterization of apple as a function of genotype. J Food Sci Technol. 2018;7:2729–2738. doi: 10.1007/s13197-018-3195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lia D, Lia B, Mab Y, Suna X, Lina Y, Menga X. Polyphenols, anthocyanins, and flavonoids contents and the antioxidant capacity of various cultivars of highbush and half-high blueberries. J Food Compos Anal. 2017;62:84–93. doi: 10.1016/j.jfca.2017.03.006. [DOI] [Google Scholar]
- Mannozzi C, Cecchini JP, Tylewicz U, Siroli L, Patrignani F, Lanciotti R, Rocculi P, Dalla Rosa M, Romani S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT-Food Sci Technol. 2017;85:440–444. doi: 10.1016/j.lwt.2016.12.056. [DOI] [Google Scholar]
- Matiacevich S, Cofre DC, Silva P, Enrione J, Osorio F. Quality parameters of six cultivars of blueberry using computer vision. Int J Food Sci. 2013;2013:1–8. doi: 10.1155/2013/419535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panse VG, Sukhatme PV. Statistical methods for agricultural workers. 3. New Delhi: Indian Council of Agricultural Research; 1984. [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruits and vegetable products. 3. New Delhi: Tata McGraw-Hill Publishing Company Ltd; 1999. [Google Scholar]
- Reig G, Iglesias I, Gatius F, Algere S. Antioxidant capacity, quality, anthochyanins and nutrients content of several peach cultivars [Prunus persica (L.) Batsch] grown in Spain. J Agric Food Chem. 2013;61(26):6344–6357. doi: 10.1021/jf401183d. [DOI] [PubMed] [Google Scholar]
- Saftner R, Polashock J, Ehlenfeldt M, Vinyard B. Instrumental and sensory quality characteristics of blueberry fruit from twelve cultivars. Postharvest Biol Technol. 2008;49:19–26. doi: 10.1016/j.postharvbio.2008.01.008. [DOI] [Google Scholar]
- Sharma RR, Krishna H. A textbook of temperate fruits. New Delhi: CBS Publishers; 2018. [Google Scholar]
- Sharma RR, Jhalegar MJ, Jha SK, Rana V. Genotypic variation in total phenolics, antioxidant activity, enzymatic activity and quality attributes among kiwifruit cultivars. J Plant Biochem Biotechnol. 2014;24:114–119. doi: 10.1007/s13562-013-0242-6. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Vaio CD, Marallo N, Graziani G, Ritieni A, Matteio AD. Evaluation of fruit quality, bioactive compounds of flat peach cultivars. J Sci Food Agric. 2014;95(10):2124–2131. doi: 10.1002/jsfa.6929. [DOI] [PubMed] [Google Scholar]
- Wrolstad RE, Durst RW, Lee J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci Technol. 2005;16:423–428. doi: 10.1016/j.tifs.2005.03.019. [DOI] [Google Scholar]
- Wu J, Ga RZL, Lia X, Chan F, Wang Z, Xiasng H. Chemical compositional characterization of some apple cultivars. Food Chem. 2007;103:88–93. doi: 10.1016/j.foodchem.2006.07.030. [DOI] [Google Scholar]
- Yaman O, Bayoundurlc L. Effect of an edible coating and cold storage on shelf life and quality of cherries. LWT Food Sci Technol. 2002;35:146–150. doi: 10.1006/fstl.2001.0827. [DOI] [Google Scholar]
- Zeppa G, Conterno L, Gerbi V. Determination of organic acids, sugars, diacetyl and acetoin in cheese by high-performance liquid chromatography. J Agric Food Chem. 2001;49(6):2722–2726. doi: 10.1021/jf0009403. [DOI] [PubMed] [Google Scholar]