Abstract
Modern research is directed towards finding naturally-occurring antioxidants of plant origin for improving nutrition and health benefit. Wild yam species are used as indigenous food and medicine by various tribal groups of Koraput, India. However, detailed analysis of health promoting bioactive compounds and antioxidant properties are lacking. The present study aims to evaluate the flavonoid, total antioxidant capacity and in vitro antioxidant activity of different wild and cultivated Dioscorea species of Koraput. The extract of these tuber was screened for its potential antioxidant activities by various tests, such as DPPH, nitric oxide, superoxide and ABTS radical cation assay. The phenol, flavonoid and total antioxidant capacity of the samples were ranged from 2.19 to 9.62 mg g−1 dry weight, 0.62–0.85 mg g−1 dry weight and 1.63–5.59%, respectively. The IC50 values were 77.9–1164, 101.2–1031.6, 27.0–1022.6 and 47.1–690 µg ml−1 for DPPH, ABTS, superoxide and nitric oxide scavenging activity, respectively. Taken together, some wild yam species such as D. bulbifera, D. pubera and D. pentaphylla had significantly higher flavonoids content and antioxidant capacity along with the lower IC50 values than the standards in compared to the other species. The present studies confirmed that some wild yam tubers have superior potential for scavenging deleterious free radicals effectively. Results indicated that these wild yam species should be promoted as natural source of antioxidants.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04470-x) contains supplementary material, which is available to authorized users.
Keywords: Antioxidant activity, Flavonoid, Radical scavenging activities, Wild yam species
Introduction
Naturally derived antioxidants are used as foods or medicinal products to replace synthetic antioxidants could help to protect from oxidative stress and to strengthen the defense system against degenerative diseases (Sylvie et al. 2014; Kathirvel and Sujatha 2012). Yams (Dioscorea spp.) are an integral part of food systems in the tropics, estimated to provide more than 200 dietary calories each day for over 60 million people (FAO 2015). It is also considered as a famine food for small and marginal rural families and forest-dwelling communities during the food scarcity periods (Otegbayo et al. 2018; Padhan et al. 2018). Apart from food, some yam species are commonly utilized in many pharmaceutical preparations owing to their special bioactive constituents (Wu et al. 2016; Padhan and Panda 2016). It is known to contain a good quantity of secondary metabolites such as alkaloids, tannins, flavonoids, saponins, glycoside steroids, anthraquinones and polyphenols (Bhandari and Kawabata 2004; Niu et al. 2010; Cornago et al. 2011). Some wild yam species have pharmacological properties which inhibit cell proliferation activities, antifungal, anticancer and hepato-protective properties (Wu et al. 2016). Despite their popular consumption and economic importance, there is an obscure knowledge of their antioxidant constituents has provided inadequate bases for pharmacological action. As a result, the risk of ineffective treatment has greatly increased in traditional medicinal practice (Wu et al. 2016). Yams are reported to having the 7th highest antioxidant concentration among 11 root and tuber crops (Halvorsen et al. 2002). The antioxidant properties was reported in different tubers crops such as potato (Reyes 2005; Rumbaoa et al. 2009), sweet potato (Teow et al. 2007; Rumbaoa et al. 2009), cassava and taro (Lako et al. 2007), however, there is dearth of information on the antioxidant properties of underutilized wild edible yam species.
The Koraput district of Odisha is one of the biodiversity hot spots in India, harbors abundant tropical root and tuberous plant resources (Padhan and Panda 2016). The diversity of wild edible yams in this region traditionally plays a significant role in meeting nutritional, minerals and antioxidant requirement of ethnic communities (Mishra et al. 2008; Padhan and Panda 2016; Kumar et al. 2017). However, the systematic investigation on natural antioxidant potential of the wild edibles yams of the region is meagre. Therefore, the present study aims to evaluate the health promoting antioxidant activity in different underutilized wild and cultivated yams from Koraput by using different in vitro assay. The findings of the study are of great help in determining suitable action in traditional medicine by the traditional healer of indigenous communities and will enrich the national food composition database for exploration of natural antioxidants.
Materials and methods
The antioxidant potential of eight wild edible yam tubers, namely Dioscorea oppositifolia L., D. hamiltonii Hook.f., D. bulbifera L., D. pubera Blume., D. pentaphylla L., D. wallichii Hook.f., D. glabra Roxb. and D. hispida Dennst. along with one cultivated species D. alata L. from Koraput, India were investigated in the current study. The details of yam tuber and their ethnomedicinal importance were reported in Table S1. These yam tubers were selected for the study for their abundant use in tribal food and ehno-medicine of this region. The mature tubers of different species were collected from the medicinal plant garden of Department of Biodiversity and Conservation of Natural Resources, Central University of Orissa, Koraput, India, which were grown under the same climatic and agronomic condition (Padhan and Panda 2018). Briefly, plants were grown in polythene bags filled with mixture of farm soil and farmyard manure with a 3:1 ratio and plants were irrigated regularly and subjected to natural solar radiation. The tubers were harvested after all the vines dried. After collection, the tubers were washed, peeled, made into small slices and shade dried for 4-6 days. The dried tuber samples were mechanically ground into powder and used for analysis.
The tuber flour of different yams (10 g) was macerating in 100 ml of methanol and keep at room temperature for 72 h. The solution obtained was filtered through a Whatmann filter paper No. 1 and stored at 4 °C for further analysis. The phenol content was determined using the method of Sadasivam and Manickam (2007) with some modifications. An aliquot was prepared with 1 ml extract, 0.5 ml Folin-Ciocalteu’s reagent (2:1) and 2 ml of 20% Na2CO3 and the mixture was incubated for 2 min. The absorbance was measured at 650 nm and the total phenol content was expressed as gallic acid equivalence (GAE) mg g−1 dw using gallic acid as a standard. Total flavonoid content was determined by Aluminium chloride colorimetric assay with some modification, according to Chang et al. (2002). The crude extracts (0.5 ml) was added with 0.1 ml AlCl3, 0.1 ml Pottasium acetate, 2.5 ml distilled water and allowed to stand for 30 min. The absorbance was measured at 415 nm with spectrophotometer. Results were expressed as mg flavonoid g−1 of dry samples using Quercitin as standard. The total antioxidant capacity of the extract was evaluated by the Phospho-molybdenum method with some modification of Prieto et al. (1999). An aliquot of 300 µl of tuber extract was mixed with 3 ml of the reagent 0.6 M sulphuric acid (H2SO4), 28 mM sodium dihydrophosphate dihydrate and 4 mM ammonium hepta-molybdate tetrahydrate) and allowed to incubate in water bath at 90 °C of 90 min. Then the absorbance was measured at 695 nm against blank after complete cooling. The percentage of antioxidant activity as calculated by following equation.
The free radical scavenging activity of different tuber extracts was evaluated by DPPH following Blois (1958) with some modifications. Briefly, the solution of DPPH (0.1 mM in methanol) of 1 ml was added to 3 ml of various concentrations (100 to 1000 µg/ml) of tuber extract. The mixtures were shaken vigorously and incubated at room temperature for 30 min in the dark. The reduction of the DPPH free radical was measured at 517 nm using UV- VIS spectrophotometer against a control contains DPPH and methanol. Ascorbic acid was used as the reference. The capability of the scavenging the DPPH radical was calculated by using the following formula
where Acontrol is the absorbance of the control and Atest is the absorbance of samples.
ABTS radical scavenging assay was carried out with slight modifications of Re et al. (1999). The stock solutions included 7 mM ABTS solution and 2.4 mM potassium per-sulfate solution. Various concentrations (100–1000 µg ml−1) of the tuber extracts (1 ml) were allowed to react with 2.5 ml of the ABTS solution and the absorbance was taken at 734 nm after 7 min. The ABTS scavenging capacity of the extract was compared with that of trolox and percentage inhibition was calculated as
where Acontrol is the absorbance of ABTS radical with methanol; Atest is the absorbance of ABTS radical with sample extract as standard.
Nitric oxide was generated from sodium nitroprusside and was measured by the Griess reagent using the method of Green et al. (1982). Sodium nitroprusside (10 mM) was mixed with different concentrations of the extract and incubated at 25 °C for 150 min. The samples were added to Griess reagent (1% sulphanilamide, 2% H3PO4 and 0.1% napthyl ethylene diamine dihydrochloride). The absorbance was read at 546 nm and referred to the absorbance of standard solutions of quercitine as a positive control. The percentage of inhibition was calculated by the following formula:
where A control is the absorbance of the control (without extract) and Atest is the absorbance in the presence of the extract/standard.
The superoxide anion scavenging activity was measured as described by Robak and Gryglewski (1988). The superoxide anion radicals were generated in 3.0 ml of Tris–HCl buffer (16 mM, PH 8.0), containing 0.5 ml of NBT (0.3 mM), 0.5 ml NADH (0.936 mM) solution, 1.0 ml extract of different concentration and 0.5 ml of Tris–HCl buffer (16 mM, PH 8.0) and the reaction was started by adding 0.5 ml PMS solution (0.12 mM) to the mixture and incubated at 25 °C for 5 min and the absorbance was measured at 560 nm against a blank sample and ascorbic acid. The percentage inhibition was calculated by using the following equation:
Where Acontrol is the absorbance of the control (without extract) and Atest is the absorbance in the presence of the extract/standard.
The measurements were replicated in three independent assays and mean value was presented. The antioxidant activity of each samples were expressed in terms of IC50 (µg ml−1) and calculated from the graph after plotting inhibition percentage against extract concentrations. Analysis of variance (ANOVA) was carried out for flavonid and total antioxidant capacity by using CROPSTAT (International Rice Research Institute, Philippines) software.
Results and discussion
Levels of flavonoid and total antioxidant capacity
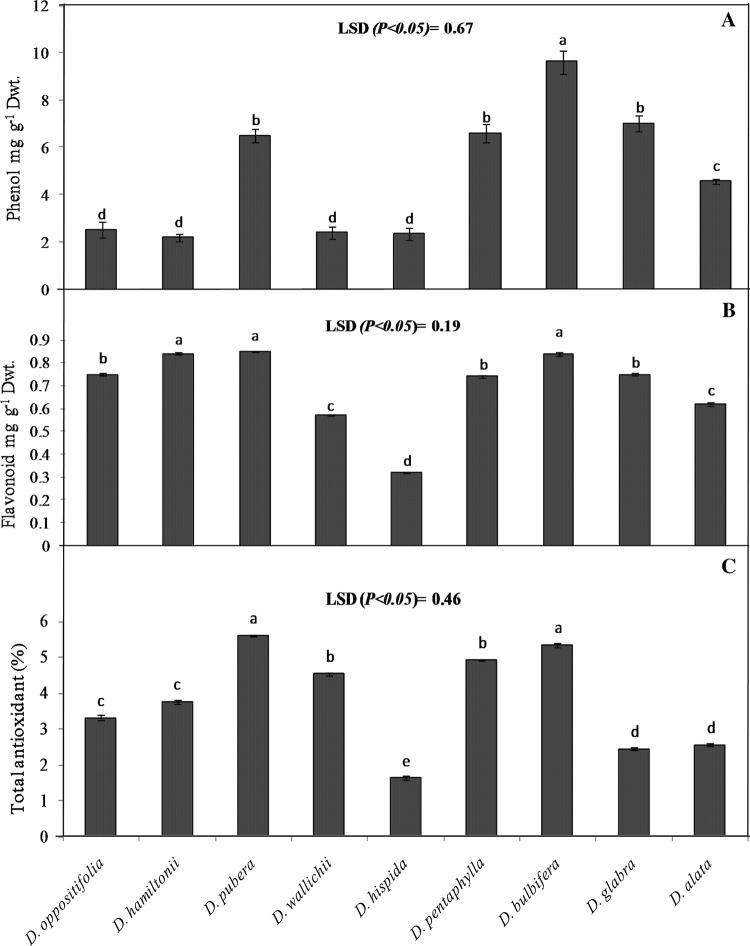
The level of phenol, flavonoid and total antioxidant capacity in the studied yam tubers are presented in Fig. 1. The phenol content varied significantly (P < 0.05) among the yam species and it was ranged from 2.19 to 9.62 mg g−1 Dwt. The phenol content of D. bulbifera was significantly (P < 0.05) higher than the other yam species. The flavonoid content was significantly (P < 0.05) varied among the yam species and the value was ranged from 0.62 to 0.85 mg g−1 Dwt. The level of flavonoid in all the wild Dioscorea species except D. hispida were significantly (P < 0.05) higher than the cultivated species (D. alata). The total antioxidant capacity (%) among different Dioscorea species varied from 1.63 to 5.59% and it was significantly (P < 0.05) higher in D. pubera and D. bulbifera as compared to other yam species. Such difference of antioxidant capacity among the Dioscorea species might be related to their genetic origin and geographical sources where they are grown. The antioxidant activities of plants are mainly contributed by the active compounds present in them. Antioxidant compounds help in inhibiting hydrogen abstraction, radical scavenging, and scavenging the peroxides radicals by donating electrons. Due the plant derived natural antioxidant compounds such as flavonoids and phenolic compounds known to possess antioxidant activities due to the presence of hydroxyl groups in their structures and their redox properties (Abbas et al. 2015). The values of phenolic content in the present study varied slightly compared to those in the literature (Shahidi and Naczk 2003). This may be due to the presence of different levels of sugars, carotenoids or ascorbic acid, or the duration, geographical variation or methods of extraction, which may alter the amount of phenolics (Bhandari and Kawabata 2004). Plant materials with high concentrations of the flavonoid and antioxidants will definitely play an important role in maintenance of human health as reported earlier by Bhatt et al. (2017) and Otegbayo et al. (2018).
Fig. 1.
Total phenol* (a), flavonoid (b) and total antioxidant capacity (c) of different wild and cultivated Dioscorea species from Koraput. Data are the mean of three replications. Means followed by a common letter are not significantly different at the 5% level by Fisher’s least significance difference (LSD) test (*Padhan et al. 2020)
In vitro antioxidant activity
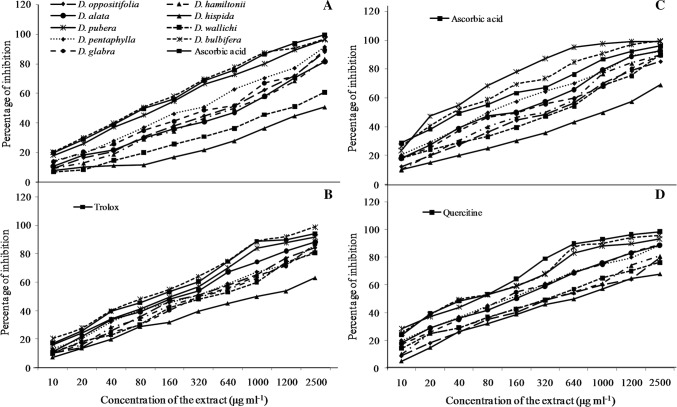
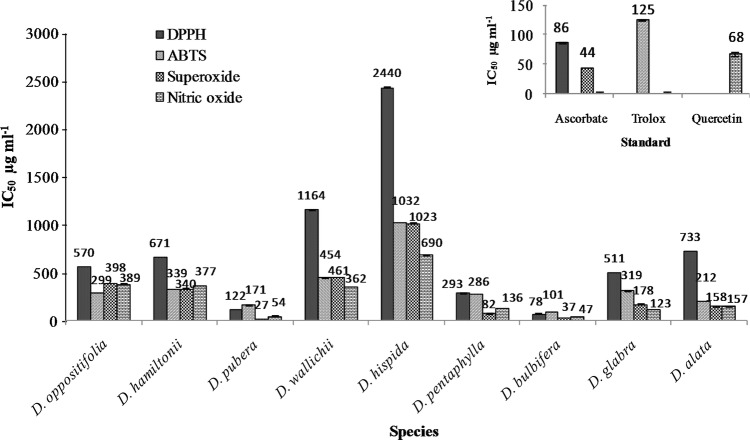
The radical scavenging activity of the yam tuber extract was measured by determining the ability to scavenge different free radicals such as DPPH, ABTS, superoxide and nitric oxide. The capacity to scavenge these free radicals were compared with the activity of standards such as ascorbic acid, trolox and quercetin (Fig. 2). The DPPH is a commercially available stable free radical and it is a highly reliable methods to quantify the antioxidant activities (Kathirvel and Sujatha 2012; Sakthidevi and Mohan 2013). The results of DPPH radical scavenging activities revealed that, all the tuber extract had lower scavenging activity than the standard ascorbic acid. Most of the yam extracts showed higher scavenging activity at the concentration between 80 to 800 µg ml−1 except D. hispida and D. wallichii (Fig. 2a). The scavenging effects of the yam species on the basis of IC50 value were in the following order: ascorbic acid > D. bulbifera > D. pubera > D. pentaphylla > D. glabra > D. oppositifolia > D.hamiltonii > D. alata > D. wallichii > D. hispida. The wild yam species D. bulbifera showed highest scavenging activity against DPPH radicals with IC50 value of 77.90 µg ml−1 whereas, lowest scavenging activity was observed in D. hispida with the IC50 of 1164 µg ml−1 (Fig. 3). In the present study, all the yam extracts were inhibited the DPPH radical in different manners which indicates potentiality of the yam extracts to donate an electron or hydrogen to scavenge the DPPH radical. This variation observed between the scavenging activities of the different yam extract depends on the unequal distribution of the antioxidant molecules in the form of secondary metabolites such as polyphenol and flavonoids of the plant (Sylvie et al. 2014). The wild yam extract of D. bulbifera had the lowest IC50 and had superior scavenging activity. In this study, it is evident that the wild species, D. bulbifera tuber possess effective antioxidant activity. The results of the present findings in the studied wild yams agree with the findings of Rajalakshmi and Mohan (2013) on the DPPH radical scavenging of D. tomentosa and with the previous reports on Dioscorea esculenta of Thajunnisha et al. (2013).
Fig. 2.
Scavenging activities of different wild edible Dioscorea species. a DPPH assay; b ABTS radical scavenging activities; c Superoxide radical scavenging activities; d Nitric oxide radical scavenging activities
Fig. 3.
IC50 values of the tuber extract of different Dioscorea species
Similarly, the studied yam extract of D. bulbifera showed potent antioxidant activity against ABTS which is higher than standard trolox. The ABTS radical scavenging activity of D. bulbifera and D. pubera species increased up to 70% at the concentration of 320 to 640 µg ml−1 (Fig. 2b). The scavenging activity against ABTS radical cation was higher in D. bulbifera followed by > trolox > D. pubera > D. alata > D.pentaphylla > D. oppositifolia > D. glabra > D.hamiltonii > D. wallichii > D. hispida (Fig. 3). The flavonol glycosides and polyphenol constituents has been reported to be the major contributing factor to the antioxidant activity of ABTS radicals (Gao et al. 2002; Londhe et al. 2008). The results are also consistent with the other wild tuber crops and yam species (Bhandari and Kawabata 2004; Ghosh et al. 2013). This result indicates that these extracts could serve as a free radical inhibitors or scavengers using their proton-donating ability or could act as the primary antioxidants (Marxen et al. 2007).
The super oxide radical scavenging activity of D. pubera extracts increased up to 70% at the concentration of 80–160 µg ml−1 (Fig. 2c). The scavenging activity of yam species against superoxide radicals was higher in D. pubera followed by > D. bulbifera > ascorbic acid > D. pentaphylla > D. alata > D. glabra > D. hamiltonii > D. oppositifolia > D. wallichii > D. hispida. The IC50 values for D. pubera and D. bulbifera was 27.02 and 37.04 µg ml−1 respectively with higher scavenging activity than the standard (Fig. 3). The results clearly indicated that the most of the wild species had noticeable effect to scavenge superoxide radicals than the cultivated species. The tuber extract of D. pubera and D. bulbifera had superior radical scavenging activity than the other species as well as with the standard, which can be used as the dietary antioxidants (Naskar et al. 2010).
In addition, the nitric oxide radical scavenging activity was higher in D. bulbifera and D. pubera as compared to the standard. The order of the yam species in relation to IC50 values to inhibited the nitric oxide radicals were: D. bulbifera > D. pubera > quercetin > D. glabra > D. pentaphylla > D. alata > D. wallichii > D. hamiltonii > D. oppositifolia > D. hispida. The result revealed that two wild yam species, such as D. bulbifera and D. pubera had higher nitric oxide scavenging activity than the standard quercetin with IC50 values at 47.14 and 53.61 µg ml−1 respectively (Fig. 2 D). Based on the results, these tubers could significantly reduce the level of nitric oxide and also reported to play a crucial role in inflammation and other diseases (Naskar et al. 2010). Thus, the findings strongly rationalize the traditional use of D bulbifera in inflammations and wound healing (Nguelefack et al. 2010; Mbiantcha et al. 2011). The relationship of antioxidant compounds such as phenol and flavonoid with antioxidant activities were well proven by many researchers in other crops as well as in yam species (Bhandari and Kawabata 2004; Aryal et al. 2019). The species which showed higher phenols and flavonoid content having more scavenging activity with lower IC50 values (Aryal et al. 2019). The studied wild yam tubers such as D. bulbifera and D. pubera showed higher scavenging activities due to presence of higher phenol and flavonoids content than the other species.
In conclusion, the result provided a strong scientific evidence for considering the wild yam species as natural antioxidants. The wild yam species such as D. bulbifera and D. pubera contained significantly higher amounts of bioactive compounds and also showed superior radical scavenging activity over the other Dioscorea species. Hence, these understudied wild yam tubers can be used as a good dietary source of antioxidant for treatment of oxidative stress induced diseases and will enrich the national food composition database. Further research is aimed to isolate and identify the antioxidative components and validate their effects through in vitro studies to confirm their natural biological functions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are grateful to the Head, Department of Biodiversity and Conservation of Natural Resources for providing necessary facilities for the work and also grateful to University Grant Commission (UGC), New Delhi, India for providing Non-NET PhD Fellowship.
Authors contribution
BP and DP designed the experiments, collected the tuber. BP performed the laboratory analysis. DP and JKN analyzed the data and wrote the paper. All the authors read and provided helpful discussions for the manuscript.
Compliance with ethical standards
Conflict of interest
The authors report no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas ZK, Saggu S, Sakeran MI, Zidan N, Rehman H, Ansari AA. Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi J Biol Sci. 2015;22:322–326. doi: 10.1016/j.sjbs.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants. 2019;8(96):1–12. doi: 10.3390/plants8040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari MR, Kawabata J. Organic acid, phenolic content and antioxidant activity of wild yam (Dioscorea spp.) tubers of Nepal. Food Chem. 2004;88:163–168. doi: 10.1016/j.foodchem.2003.12.027. [DOI] [Google Scholar]
- Bhatt ID, Rawat S, Badhani A, Rawal RS. Nutraceutical potential of selected wild edible fruits of the Indian Himalayan region. Food Chem. 2017;215:84–91. doi: 10.1016/j.foodchem.2016.07.143. [DOI] [PubMed] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of stable free radicals. Nature. 1958;26:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Cornago DF, Rumbaoa RGO, Geronimo IM. Phillipine Yam (Dioscorea spp) Tubers phenolic content and antioxidant capacity. Phillip J Sci. 2011;140(2):145–152. [Google Scholar]
- Food and Agriculture Organization of the United Nations (2015) Low-Income Food-Deficit Countries (LIFDC)—List for 2015. http://www.fao.org/countryprofiles/lifdc/en/. Accessed 10 may 2016
- Gao H, Kuroyanagi M, Wu L, Kawahara N, Yasuno T, et al. Antitumor-promoting constituents from D. bulbifera L. in JB6. mouse epidermal cells. Biol Pharm Bull. 2002;25:1241–1243. doi: 10.1248/bpb.25.1241. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Derle A, Ahire M, More P, et al. Phytochemical Analysis and Free Radical Scavenging activity of Medicinal Plants Gnidia glauca and Dioscorea bulbifera. PLoS One. 2013;8(12):1–18. doi: 10.1371/journal.pone.0082529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Godowsky J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite and [15 N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- Halvorsen BL, Holte K, Myhrstad MCW, Barikmo I, Hvattum E, Remberg SF, Wold A, Haffner K, Baugerod H, Anderse LF, Moskaug JO, Jacobs DR, Jr, Blomhoff R. A systematic screening of total antioxidants in dietary plants. J Nutrition. 2002;132:461–471. doi: 10.1093/jn/132.3.461. [DOI] [PubMed] [Google Scholar]
- Kathirvel A, Sujatha V. Phytochemical studies, antioxidant activities and identification of active compounds using GC–MS of Dryopteris cochleata leaves. Arab J Chem. 2012;9:S1435–S1442. doi: 10.1016/j.arabjc.2012.03.018. [DOI] [Google Scholar]
- Kumar S, Das G, Shin HS, Patra JK. Dioscorea spp (a wild edible tuber): a study on its ethnopharmacological potential and traditional use by the local people of similipal biosphere reserve, India. Front Pharmacol. 2017;8:52. doi: 10.3389/fphar.2017.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lako J, Trenerry VC, Wahlqvist M, Wattanapenpaiboon N, Sotheeswaran S, Prenier R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007;101:1727–1741. doi: 10.1016/j.foodchem.2006.01.031. [DOI] [Google Scholar]
- Londhe JS, Thomas PA, Devasagayam L, Yeap F, Saroj S, Ghaskadbi Antioxidant activity of some polyphenol constituents of the medicinal plant Phyllanthus amarus Linn. Redox Rep. 2008;13(5):199–207. doi: 10.1179/135100008X308984. [DOI] [PubMed] [Google Scholar]
- Marxen K, Vanselow KH, Lippemeier S, Hintze R, Ruser A, Hansen UP. Determination of DPPH radical oxidation caused by methanolic extracts of some microalgal species by linear regression analysis of spectrophotometric measurements. Sensors (Basel) 2007;7(10):2080–2095. doi: 10.3390/s7102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbiantcha M, Kamanyi A, Teponno RB, Tapondjou AL, Watcho P, Nguelefack TB. Analgesic and anti-inflammatory properties of extracts from the bulbils of Dioscorea bulbifera L. var sativa (Dioscoreaceae) in mice and rats. Evid Based Complement Alternat Med. 2011;1:912–935. doi: 10.1155/2011/912935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Swain S, Chaudhary S, Ray S. Wild edible tubers (Dioscorea spp.) and their contribution to the food security of tribes of Jeypore tract, Orissa, India. PGR Newsl. 2008;56:63–67. [Google Scholar]
- Naskar S, Islam A, Mazumder UK, Saha P, Haldar PK, Gupta M. In vitro and in vivo antioxidant potential of hydro methanolic extract of Phoenix dactylifera fruits. J Sci Res. 2010;2(1):144–157. doi: 10.3329/jsr.v2i1.2643. [DOI] [Google Scholar]
- Nguelefack TB, Dutra RC, Paszcuk AF, Andrade EL, Tapondjou LA, et al. Antinociceptive activities of the methanol extract of the bulbs of Dioscorea bulbifera L. var sativa in mice is dependent of NO–cGMP–ATP-sensitive-K + channel activation. J Ethnopharmacol. 2010;128:567–574. doi: 10.1016/j.jep.2010.01.061. [DOI] [PubMed] [Google Scholar]
- Niu CS, Chen W, Wu HT, Cheng KC, Wen YJ, Lin KC, Cheng JT. Decrease of plasma glucose by allantoin, an active principle of Yam (Dioscorea spp.) in spteptozotocin-induced diabetics rats. J Agric Food Chem. 2010;22:12031–12035. doi: 10.1021/jf103234d. [DOI] [PubMed] [Google Scholar]
- Otegbayo BO, Oguniyan DJ, Olunlade BA, Oroniran OO, Atobatele OE. Characterizing genotypic variation in biochemical composition, anti-nutritional and mineral bioavailability of some Nigerian yam (Dioscorea spp.) land races. J Food Sci Technol. 2018;55(1):205–216. doi: 10.1007/s13197-017-2913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhan B, Panda D. Wild tuber species diversity and its ethno-medicinal use by tribal people of Koraput district of Odisha, India. J Nat Prod Resour. 2016;2(1):33–36. [Google Scholar]
- Padhan B, Panda D. Variation of photosynthetic characteristics and yield in wild and cultivated species of yams (Dioscorea spp.) from Koraput, India. Photosynthetica. 2018;56:1–9. doi: 10.1007/s11099-018-0823-7. [DOI] [Google Scholar]
- Padhan B, Biswas M, Dhal NK, Panda D. Evaluation of mineral bioavailability and heavy metal content in indigenous food plant wild yams (Dioscorea spp) from Koraput, India. J Food Sci Technol. 2018;55(11):4681–4686. doi: 10.1007/s13197-018-3388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhan B, Biswas M, Panda D. Nutritional, anti-nutritional and physico-functional properties of wild edible yam (Dioscorea spp.) tubers from Koraput, India. Food Biosci. 2020;34:100527. doi: 10.1016/j.fbio.2020.100527. [DOI] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Rajalakshmi K, Mohan VR. Antioxidant properties of Polygala chinensis L: whole plant on alloxan induced diabetic rats. Int J Pharm Sci Res. 2013;4(1):330–334. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reyes LF. Antioxidant capacity, anthocyanins and total phenolics in purple- and red-fleshed potato (Solanum tuberosum, L.) genotypes. Am J Potato Res. 2005;82:271–277. doi: 10.1007/BF02871956. [DOI] [Google Scholar]
- Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988;36:317–322. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- Rumbaoa RGO, Cornago DF, Geronimo IM. Phenolic content and antioxidant capacity of Philippine potato (Solanum tuberosum) tubers. J Food Composit Anal. 2009;22:546–550. doi: 10.1016/j.jfca.2008.11.004. [DOI] [Google Scholar]
- Sadasivam S, Manickam A. Biochemical Methods. New Delhi, India: New Age International Pvt. Ltd; 2007. p. 284. [Google Scholar]
- Sakthidevi G, Mohan VR. Total phenolic, flavonoid contents and in vitro antioxidant activity of Dioscorea alata tuber. Int J Pharm Sci Res. 2013;5(5):115–119. [Google Scholar]
- Shahidi F, Naczk M. Phenolics in food and neutraceuticals. Boca Raton: CRC Press; 2003. [Google Scholar]
- Sylvie DD, Anatole PC, Cabral BP, Veronique PB. Comparison of in vitro antioxidant properties of extracts from three plants used for medical purpose in Cameroon: acalypha racemosa, Garcinia lucida and Hymenocardia lyrata. Asian Pac J Trop Biomed. 2014;4(2):625–632. doi: 10.12980/APJTB.4.201414B168. [DOI] [Google Scholar]
- Teow CC, Truong VD, Mcfeeters RF, Thompson RL, Pecota KV, Yencho GC. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007;103:829–838. doi: 10.1016/j.foodchem.2006.09.033. [DOI] [Google Scholar]
- Wu ZG, Wu J, Nitin M, Bao XQ, Chen SL, Tao ZM. Characterizing diversity based on nutritional and bioactive compositions of yam germplasm (Dioscorea spp.) commonly cultivated in China. J Food Drug Anal. 2016;24:367–375. doi: 10.1016/j.jfda.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.