Abstract
Though fresh-cut products save our time, but they are very much prone to enzymatic browning that drastically affects product’s quality and marketability. Drumstick pods are considered as super food due to high nutritional contents. However, the fresh-cut pods are prone to brown discoloration. The enzyme activities promote the softening and cut-surface browning of pods, thus deteriorates their texture, decreases consumer appeal and shortens the shelf life. So, we aimed to assess the effect of citric (1%) and ascorbic (1%) acid treatments on quality attributes of fresh-cut drumsticks at 3-d interval during storage (5 ± 1 °C). In general there was an increase in lignin and quinone contents, while phenolic content was decreased during storage. However, samples subjected to ascorbic acid dip had higher phenolic content, lower rate of lignin formation, and reduced membrane permeability. Enzyme activities (polyphenol oxidase and peroxidase) were found to increase during storage, however, samples treated with ascorbic acid showed lower activities than that of the control and citric acid treated samples. The reduced enzyme activities resulted in the reduced browning incidence and maintained the quality. Therefore, postharvest dip of fresh-cut drumstick in to ascorbic acid (1%) could be suggested to increase the shelf life with reduced browning during low temperature storage.
Keywords: Drumstick, Fresh-cut, Organic acids, Peroxidase, Polyphenol oxidase, Quality
Introduction
Fresh-cut fruit and vegetables offer convenience to consumers resulting in an increased demand over the past decade (Yousuf et al. 2018). Moreover, they provide a wide variety of safe and nutritious products. Fresh-cut vegetables refer to peeled, cut, chopped, sliced, diced or shredded forms of vegetables facilitating small serving-size portions e.g. broccoli, carrots, lettuce, spinach, sweetcorn, potatoes, etc. Though there is an increasing demand for such products, but the fresh-cut preparation steps may accelerate the deterioration in terms of increased microbial infection as well as physical damage to tissues (Qadri et al. 2015). The discoloration or browning due to enzymatic actions and loss of phytochemical constituents are some of the prevalent problems in fresh-cut products resulting in the reduced shelf life and triggered quality loss. Moreover, browning on the cut surface curtails the development and commercialization of many fresh-cut products (Jang and Moon 2011). In this line, optimum surface treatments with practical applicability such as edible coating and/or eco-friendly chemical treatments should be evaluated to control or minimize such damages in the fresh-cut fruit and vegetables.
Naturally occurring organic acids (OAs) are generally recognized as safe (GRAS) by USFDA. Organic acids are reported to retain quality and to prolong shelf-life of fresh-cut agricultural products (Rico 2007; Oms-Oliu et al. 2010; Gorny et al. 2002). OAs including ascorbic acid have been reported as antimicrobial and antioxidant agents. OAs also inhibit enzyme activity and thereby limit the browning reactions (Ventura-Aguilar et al. 2017; Azevedo et al. 2018).
Moringa oleifera, often known as drumstick, is widely grown in South Asia, Africa, and South America. Owing to be rich in health promoting contents, drumstick possesses substantial economic, industrial and medicinal importance. It is a multipurpose plant and almost all parts (leaves, fruit, flowers and green pods) are consumed in the form of vegetable across the world, particularly in India, Pakistan, Philippines, Hawaii and Africa (Anwar and Bhanger 2003; Anwar et al. 2005; Ghasi et al. 2000). The tender green pods are often used as a vegetable. These drumsticks can be minimally processed and used in various culinary preparations. However, pods needs to be peeled off before cooking, which is a cumbersome and time consuming process. Like other fresh-cut products, peeled pods lose their quality immediately after peeling and cutting due to enzymatic browning. The fresh-cut pods develop brown to dark-green discoloration resulting in rapid degradation of quality. The enzyme activities promote the softening and cut-surface browning of pods, thus deteriorates their texture, decreases consumer appeal and shortens the shelf life. So, there is a need to develop effective postharvest measures to maintain quality and enhance the potential of drumsticks as a new fresh-cut product with increased shelf life. Therefore, the aim of this investigation was to evaluate the influence of acidulants (ascorbic and citric acids) on browning metabolism and functional constituents of fresh-cut drumsticks.
Materials and methods
Drumstick procurement and minimal processing
Fresh drumstick pods were purchased from the local market and immediately brought to the laboratory. Pods were properly washed, peeled and cut into desired length using sharp and sterile stainless steel knife.
Treatments and plan of experiment
Acid solutions of desired concentration were prepared by dissolving appropriate amount of the required chemical in distilled water. Prepared drumsticks were treated with water (control), ascorbic acid (AA; 1%) and citric acid (CA; 1%) for 10 min followed by packing in polypropylene trays and storage at 5 ± 1 °C for 9 days. On the basis of preliminary trials, this concentration was chosen for final experiment. During storage, different quality analyses including browning metabolism [total phenol, polyphenols oxidase (PPO), peroxidase (POD), and quinone content], and other quality factors (lignin and membrane permeability), were performed at 3 d intervals.
Determination of lignin content
Thioglycolic acid (TGA) method as described by An et al. (2007), was employed with slight modifications for determination of lignin content in the fresh-cut drumsticks. One gram of sample was subjected to homogenization along with 150 ml of 80% ethanol for 4 min. This homogenised blend was filtered with the help of vacuum filtration. The residue collected was washed using 20 mL of 80% ethanol and then subjected to drying at 50 °C for 24 h. The dried sample was treated with 15 mL of 2 M HCl and 1 mL of TGA. The mixture was subsequently exposed to heat at 100 °C while stirring for 4 h. the samples were cooled and centrifugation was done at 10,000 × g for 15 min. Supernatants obtained were discarded and the residues were suspended in 20 mL of 0.5 M NaOH, and stirred at 25 °C for 18 h to extract the lignin–thioglycolate complex. Samples were centrifuged and supernatants were recovered. The supernatant was then treated with 4 mL of concentrated HCl. Lignin thioglycolic acid was precipitated at 4 °C for 4 h. Thereafter, centrifugation was done at 10,000 × g for 15 min and the residue was dissolved in 10 mL of 0.5 M NaOH. Finally, its absorbance was recorded at 280 nm. Quantification was done with the help of standard coumaric acid curve.
Determination of quinone content
The quinone content of drumsticks was estimated by the method as explained by Degl’Innocenti et al. (2007). Ten gram of sample was homogenized with 20 mL of methanol. The homogenised material was filtered using a cheesecloth and then centrifugation was done for 15 min at 15,000 × g. The supernatant was collected and used as such to measure the soluble o-Quinones at a wavelength of 437 nm.
Determination of phenol content
The total phenolic content was determined according to Folin-Ciocalteu assay (Altisent et al. 2014). 5 g sample subjected to homogenisation with 20 mL of methanol followed by centrifugation at 10,000 × g for 15 min at 4 °C. The assay was performed by mixing 100 µL of extract, 0.5 mL of deionised H2O and 0.5 mL of Folin–Ciocalteu reagent. After 5 min, 0.4 mL of saturated sodium carbonate solution was mixed. The mixture was stirred well and left for incubation in dark up to 1 h. Finally, absorbance was recorded at 760 nm. The values obtained were expressed as mg GAE per 100 g of fresh weight.
Determination of membrane permeability
Membrane permeability was determined according to the method of Meng et al. (2012), with slight modifications. To assess the membrane permeability, 10 g of sample tissues was taken in a glass beaker containing 50 mL of deionised water and kept at 30 °C water bath for 15 min. The electric conductivity values (A0) of distilled water were measured by a conductivity meter. Electric conductivity values (A1) were measured again after boiling the water with samples for 15 min. Membrane permeability was calculated by the electrical conductivity ratio.
Measurement of polyphenol oxidase activity (PPO)
To determine the PPO activity, 5 g of sample tissue was homogenized in 20 mL of 0.05 mol/L sodium phosphate buffer (pH 7) followed by centrifugation at 15,000 × g for 10 min at 4 °C. Supernatants were recovered as enzyme extracts for determination of PPO activity. The rate of change (increase) in absorbance at 410 nm for catechol was noted for 3 min with the help of a spectrophotometer. Enzyme activities were explained as the change of 0.001 in absorbance at 410 nm and represented as U/g FW/min (Luo et al. 2015).
Measurement of peroxidase activity (POD)
POD activity was estimated as per Lurie et al. (1997) and following the modification of Luo et al. (2015). Five gram of sample was ground in 20 mL sodium phosphate buffer (0.05 mol/L), containing 1% polyvinylpyrrolidone (PVPP). It was then centrifugated at 12,000 × g for 10 min at 4 °C. The supernatant (enzyme extract) was employed to calculate POD activity. The assay mixture consisted of a final volume of 3 mL (2.8 mL substrate solution and 0.2 mL crude enzyme mixture). The substrate solution contained 25 mmol/L guaiacol and 25 mmol/L hydrogen peroxide dispersed in 0.05 mol/L sodium phosphate buffer. Increase in absorbance values at 470 nm was noted for 3 min. Enzyme (POD) activity was described as a change of 0.001 in absorbance at 470 nm and expressed as U/g FW/min.
Statistical analysis
Experiment was conducted in a completely randomized design with three replicates. IBM SPSS Statistic 20 software was used to normalize the data and then analysed by the help of analysis of variance (ANOVA) test. The means were discriminated by Duncan Multiple Range Test (DMRT) at p < 0.05.
Results and discussion
Lignin content
Lignins are polymers of phenolic compounds and are insoluble in all solvents. From a functional standpoint, lignin provides support and strength to cell wall, favours water transportation and protects cell wall polysaccharides from degrading thereby serving as a defence mechanism against pathogen or insect attack. Changes in lignin content of fresh-cut drumsticks were observed during storage at 5 °C (Fig. 1). The initial lignin content was 0.27%. During storage, the lignin content increased and reached to 0.63, 0.53, and 0.45% in control, citric acid treated and ascorbic acid treated samples, respectively. Acidulant treatments were found to delay the lignin formation in fresh-cut drumsticks, in which the AA treatment was the best followed by citric acid and control. Sothornvit and Kiatchanapaibul (2009) found an increase in lignin content in fresh-cut asparagus on storage at 10 °C. An increasing trend in lignin content was also found by in fresh-cut Jicama (Aquino-Bolanos and Mercado-Silva 2004), fresh-cut celery (Vina and Chaves 2003), fresh-cut asparagus (An et al. 2007; Sothornvit and Kiatchanapaibul 2009). This increase in lignin content may be due to the response to abiotic stresses such as physical damage caused by the cutting of tissue. Since, lignins have reddish-brown color, browning of some fresh cut products may be because of the presence of such components.
Fig. 1.
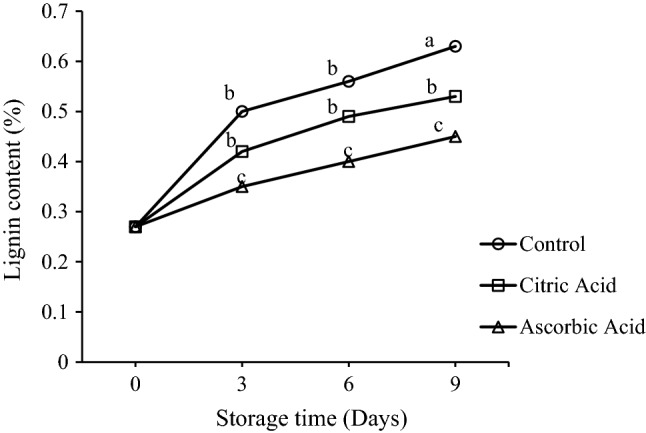
Effect of citric acid and ascorbic acid on lignin content of fresh-cut drumsticks stored at 5 °C
Quinone content
Quinones are a highly reactive class of organic compounds. In fruits and vegetables, oxidation of phenolic compounds to quinones occurs by PPO enzyme. The spontaneous polymerization of quinines results in to accumulation of brown pigments, which could be considered the most responsible culprit for tissue browning (Deng et al. 2005). In this study, quinone content increased during storage in all the samples (Fig. 2). The highest quinone content was recorded in the control sample followed by CA and AA treated samples throughout storage. Initial quinone content was found to be 0.11 OD 437/g FW. The control sample showed a sharp increase from day 1 to 3 and thereafter a gradual increase was seen. However, the treated samples indicated comparatively lesser increase. At the end of storage, significant differences were observed between quinone contents of control and treated samples with values reaching to 0.32, 0.25, and 0.23 OD 437/g FW, for control, CA and AA treated samples, respectively. The lower quinone contents in treated samples might be due to the lower PPO and POD activities as a result of such treatments. Ascorbic acid is a known reducing substance and might be responsible to prevent formation of melanin because of its ability to reduce o-quinones to o-diphenol precursors (Cantos et al. 2002).
Fig. 2.
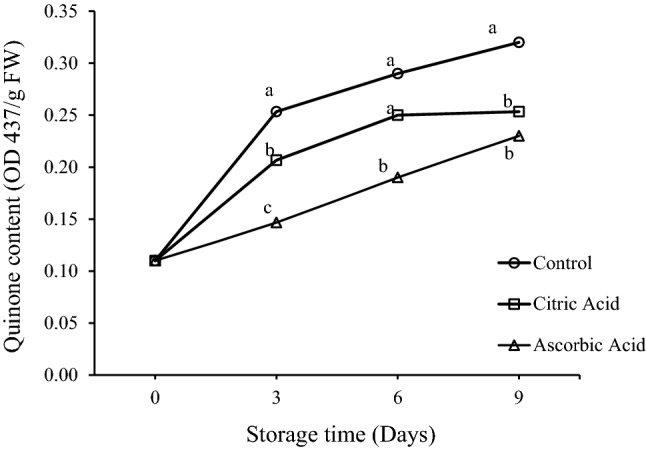
Effect of citric acid and ascorbic acid on quinone content of fresh-cut drumsticks stored at 5 °C
Phenol content
Mechanical damage due to minimal processing triggers physical stresses, resulting in altered phenolic metabolism in fresh cut tissues. Figure 3 represents the variations in phenol content of control and treated fresh-cut drumsticks stored at 5 °C. The initial phenolic content in drumsticks was 163.34 mg GAE/100 g FW, which declined over time. However, the decreasing trend was not similar in all the samples. From day 1 to day 3, the phenolic content decreased in all the samples. It kept on decreasing in control and CA treated samples, but the AA treated sample showed an increase trend up to day 6 and declined thereafter. At the end of storage, phenolic content corresponding to control, citric acid treated and ascorbic acid treated samples were 115.63, 121.66 and 141.02 mg CE/100 g FW, respectively. It is evident that ascorbic acid treatment was more effective in retaining phenolic compounds. In contrast, CA treatment was not able to retain phenols and showed comparable results with that of the control sample. Decrease in phenolic content with time was also reported by Aquino-Bolanos and Mercado-Silva (2004) in cut jicama. Higher phenolic content in AA treated samples may be because of the ability of ascorbic acid to reduce quinones to phenolic compounds. This reduction of quinones to phenols has been reported to inhibit browning of the tissue. This can also be correlated with (Fig. 6), which represents the fresh-cut drumsticks at day 0 and effect of treatments at day 9. Nonetheless the extent of potential for browning inhibition could be affected by the amount and nature of phenolic compounds (Altisent et al. 2014).
Fig. 3.
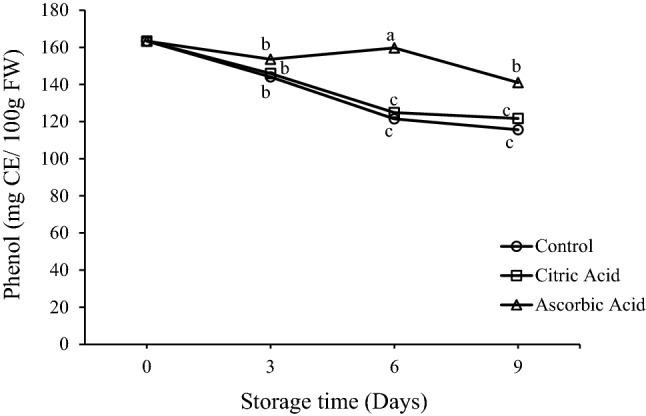
Effect of citric acid and ascorbic acid on phenol content of fresh-cut drumsticks stored at 5 °C
Fig. 6.
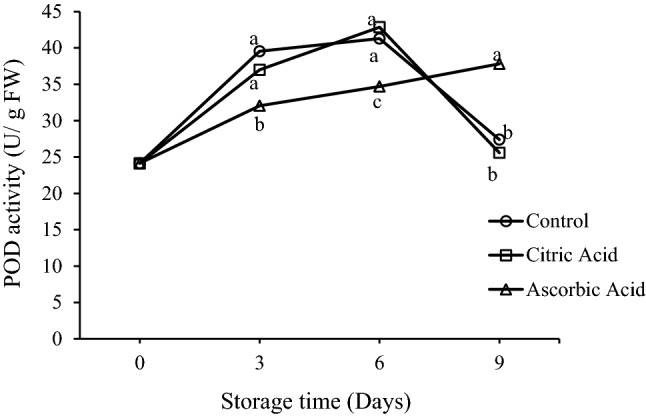
Effect of citric acid and ascorbic acid on peroxidase (POD) activity of fresh-cut drumsticks stored at 5 °C
Membrane permeability
Membrane permeability is an indicator of membrane stability and integrity. Fresh-cut preparation is essentially associated with the rupturing of surface cells and stress of underlying tissues. Enzyme activity increases as a result of the elevated permeability because of tissue disruption and mixing of enzyme and substrate which are otherwise isolated within vacuoles. Membrane permeability is an essential factor affecting the quality of fresh-cut products as browning has been correlated with the electrolyte leakage and integrity of the membrane (Chung and Moon 2009; Cantos et al. 2002). Examination of the effect of organic acid treatments on membrane permeability of drumsticks showed significant differences between the treatments towards the end of storage (Fig. 4). Initially the membrane permeability was recorded as 6%. During storage, it gradually increased to 14.2% in control sample, which was significantly higher that recorded in both the AA and CA treated drumsticks. Among the organic acid treatments, AA treated drumsticks were found to have little variations in membrane permeability with time. Therefore, AA was found to be effective in preventing the increase in membrane permeability. Li-Qin et al. (2009), also observed an elevation in membrane permeability of peach chunks treated with AA, but at a remarkably less rate as compared to that of the control sample. They suggested that cells of peach slices treated with AA retained the integrity, while as the plasmalemma of cells in case of control peach slices was disrupted, the cell wall had started to disintegrate, and the mitochondrion and vacuole had vanished. Elevation in membrane permeability with time was also reported in fresh-cut green peppers (Meng et al. 2012) cut green peppers (Raymond et al. 2013), and fresh-cut green bell pepper (Chen et al. 2018).
Fig. 4.
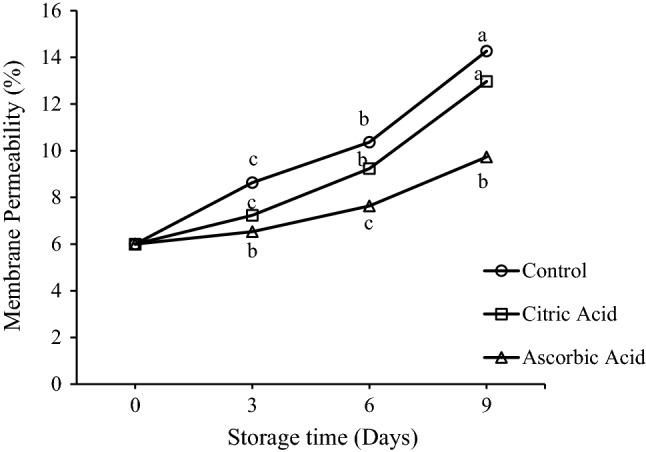
Effect of citric acid and ascorbic acid on membrane permeability of fresh-cut drumsticks stored at 5 °C
Polyphenol oxidase activity (PPO)
Enzymatic activities result in the impairment of nutritive and sensory quality of fresh-cut produce (He and Luo 2007). As soon as the fruits and vegetables are subjected to minimal processing operations, deteriorative changes are initiated and compartmentalization of the cells starts to fail. As a result of this, mixing of polyphenol substrates with deteriorative enzymes such as PPO and POD takes place. PPO is a copper-containing oxido-reductase enzyme. Oxidation of phenolic compounds due to PPO in the presence of oxygen is the main step in enzymatic browning. AA and CA act as inhibitors of PPO activity (Altunkaya et al. 2008). Effect of organic acid treatments on PPO activity of fresh-cut drumsticks is shown in Fig. 5. Significant differences does not occurred in the initial PPO activities among all the samples. Polyphenol oxidase activity at day 0 was 12.97, which was increased over the time. The highest increase throughout the storage was observed in control sample as compared to organic acid treated samples. At the end of storage, the highest values 25.27 U/g FW/min was found in control, and 21.58 and 18.25 for CA and AA treated samples, respectively. The results indicate the reduction of PPO activity by the organic acid treatments. However, the extent of reduction/inhibition may vary with the type and concentration of the organic acid used. Organic acids including citric acid and ascorbic acids have been reported to exhibit inhibitory effect on PPO and its antibrowning activity in minimally processed agricultural produce (Ahvenaien 1996).
Fig. 5.

Effect of citric acid and ascorbic acid on polyphenol oxidase (PPO) activity of fresh-cut drumsticks stored at 5 °C
Peroxidase activity (POD)
Peroxidases are the enzymes involved in several metabolic processes of plant tissue. Peroxidase activity was affected by the organic acid treatments. The effect of organic acid treatments on POD activity of fresh-cut drumsticks is indicated in Fig. 6. Soon after the treatments, the POD activity of control and treated samples was identical. Peroxidase activity of control and CA treated samples increased rapidly up to day 6, but showed a sudden decrease from day 6 to day 9. A similar behavior of elevation in POD activity during the starting storage and a decrease during the latter part of storage of control peach slices was reported by Li-Qin et al. (2009). Moreover, there were no significant differences between POD activities of control and CA treated drumsticks at any point during the storage. In contrast, the AA treated sample showed a gradual increase in POD activity throughout the storage and differed significantly from the control sample. Therefore, the pattern of changes in POD activities of CA and AA treated samples was not similar. Nevertheless, the maximum activity at the end of the storage was observed in AA treated samples. The overall analyses of the results suggest that the AA was somehow effective in controlling the POD activity. Similar results of checking the POD activities by AA were reported by Lamikanra and Watson (2001) and Kuwar et al. (2015) in case of fresh-cut cantaloupe and fresh-cut papaya respectively. POD enzyme is generally linked with wound-healing mechanisms such as lignification. Both PPO and POD activities are essential factors that have influence on quality of tissue as they affect polyphenol concentration, and the rate and intensity of enzymatic browning. In view of the increased activities of enzyme in fresh-cut substances corresponding to the intact produce, the characteristics of enzymes for example POD and PPO that affect fruit quality attributes can remarkably affect storage quality of fresh-cut products.
Conclusion
Considering health issues and consumer acceptance, organic acids are perceived as natural additives and can be used at industrial level. The use of organic acids is a feasible and convenient method of preservation for fresh-cut drumsticks. On the basis of overall enzyme activities and effect on functional constituents of drumsticks, 1% AA was found to be the best to maintain the quality during storage. It was found to be more effective than citric acid in preventing lignin formation. Acidulant treatments reduced the browning of pods due to the retardation in the production of quinones, which later participate in browning reactions. More retention of phenolic compounds was observed after the treatments. The acidulant treatments also caused reduction in both polyphenol oxidase and peroxidase activities. Further research should be performed with commercial conditions and distribution. A comparison study with other treatments on the quality of fresh-cut drumstick would also be valuable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahvenaien R. New approaches in improving the shelf life of minimally processed fruits and vegetables. Trends Food Sci Technol. 1996;7(6):179–187. doi: 10.1016/0924-2244(96)10022-4. [DOI] [Google Scholar]
- Altisent R, Plaza L, Alegre I, Viñas I, Abadias M. Comparative study of improved vs. traditional apple cultivars and their aptitude to be minimally processed as ‘ready to eat’ apple wedges. LWT-Food Sci Technol. 2014;58:541–549. doi: 10.1016/j.lwt.2014.03.019. [DOI] [Google Scholar]
- Altunkaya A, Gokmen V. Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Lactuca sativa) Food Chem. 2008;107(3):1173–1179. doi: 10.1016/j.foodchem.2007.09.046. [DOI] [Google Scholar]
- An J, Zhang M, Lu Q. Changes in some quality indexes in fresh-cut green asparagus pretreated with aqueous ozone and subsequent modified atmosphere packaging. J Food Eng. 2007;78:340–344. doi: 10.1016/j.jfoodeng.2005.10.001. [DOI] [Google Scholar]
- Anwar F, Bhanger MI. Analytical characterization of Moringa oleifera seed oil grown in temperate regions of Pakistan. J Agric Food Chem. 2003;51:6558–6563. doi: 10.1021/jf0209894. [DOI] [PubMed] [Google Scholar]
- Anwar F, Ashraf M, Bhanger MI. Interprovenance variation in the composition of Moringa oleifera oilseeds from Pakistan. J Am Oil Chem Soc. 2005;82:45–51. doi: 10.1007/s11746-005-1041-1. [DOI] [Google Scholar]
- Aquino-Bolanos EN, Mercado-Silva E. Effects of polyphenol oxidase and peroxidase activity, phenolics and lignin content on the browning of cut jicama. Postharvest Biol Technol. 2004;33(3):275–283. doi: 10.1016/j.postharvbio.2004.03.009. [DOI] [Google Scholar]
- Azevedo VM, Dias MV, de Siqueira Elias HH, Fukushima KL, Silva EK, Carneiro JDDS, Borges SV. Effect of whey protein isolate films incorporated with montmorillonite and citric acid on the preservation of fresh-cut apples. Food Res Int. 2018;107:306–313. doi: 10.1016/j.foodres.2018.02.050. [DOI] [PubMed] [Google Scholar]
- Cantos E, Tudela JA, Gil MI, Espin JC. Phenolic compounds and related enzymes are not rate-limiting in browning development of fresh-cut potatoes. J Agric Food Chem. 2002;50:3015–3023. doi: 10.1021/jf0116350. [DOI] [PubMed] [Google Scholar]
- Chen HZ, Zhang M, Bhandari B, Guo Z. Evaluation of the freshness of fresh-cut green bell pepper (Capsicum annuum var. grossum) using electronic nose. LWT-Food Sci Technol. 2018;87:77–84. doi: 10.1016/j.lwt.2017.08.052. [DOI] [Google Scholar]
- Chung H-S, Moon KD. Browning characteristics of fresh-cut ‘Tsugaru’ apples as affected by pre-slicing storage atmospheres. Food Chem. 2009;114:1433–1437. doi: 10.1016/j.foodchem.2008.11.027. [DOI] [Google Scholar]
- Degl’Innocenti E, Pardossi A, Tognoni F, Guidi L. Physiological basis of sensitivity to enzymatic browning in ‘lettuce’,‘escarole’and ‘rocket salad’when stored as fresh-cut products. Food Chem. 2007;104:209–215. doi: 10.1016/j.foodchem.2006.11.026. [DOI] [Google Scholar]
- Deng Y, Wu Y, Li YF. Effects of high O2 levels on post-harvest quality and shelf life of table grapes during long-term storage. Eur Food Res Technol. 2005;221:392–397. doi: 10.1007/s00217-005-1186-4. [DOI] [Google Scholar]
- Ghasi S, Nwobodo E, Ofili JO. Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam in high-fat diet fed Wistar rats. J Ethnopharmacol. 2000;69:21–25. doi: 10.1016/S0378-8741(99)00106-3. [DOI] [PubMed] [Google Scholar]
- Gorny JR, Hess-Pierce B, Cifuentes RA, Kader AA. Quality changes in fresh-cut pear slices as affected by controlled atmospheres and chemical preservatives. Postharvest Biol Technol. 2002;24:271–278. doi: 10.1016/S0925-5214(01)00139-9. [DOI] [Google Scholar]
- He Q, Luo Y. Enzymatic browning and its control in fresh-cut produce. Stewart Postharvest Review. 2007;3(6):1–7. doi: 10.2212/spr.2007.6.16. [DOI] [Google Scholar]
- Jang JH, Moon KD. Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chem. 2011;124:444–449. doi: 10.1016/j.foodchem.2010.06.052. [DOI] [Google Scholar]
- Kuwar U, Sharma S, Tadapaneni VRR. A loe vera gel and honey-based edible coatings combined with chemical dip as a safe means for quality maintenance and shelf life extension of fresh-cut papaya. J Food Qual. 2015;38(5):347–358. doi: 10.1111/jfq.12150. [DOI] [Google Scholar]
- Lamikanra O, Watson MA. Effects of ascorbic acid on peroxidase and polyphenoloxidase activities in fresh-cut cantaloupe melon. J Food Sci. 2001;66(9):1283–1286. doi: 10.1111/j.1365-2621.2001.tb15202.x. [DOI] [Google Scholar]
- Li-Qin Z, Jie Z, Shu-Hua Z, Lai-Hui G. Inhibition of browning on the surface of peach slices by short-term exposure to nitric oxide and ascorbic acid. Food Chem. 2009;114(1):174–179. doi: 10.1016/j.foodchem.2008.09.036. [DOI] [Google Scholar]
- Luo Z, Wang Y, Jiang L, Xu X. Effect of nano-CaCO3-LDPE packaging on quality and browning of fresh-cut yam. LWT-Food Sci Technol. 2015;60:1155–1161. doi: 10.1016/j.lwt.2014.09.021. [DOI] [Google Scholar]
- Lurie S, Fallik E, Handros A, Shapira R. The possible involvement of peroxidase in resistance to Botrytis cinerea in heat treated tomato fruit. Physiol Mol Plant Pathol. 1997;50:141–149. doi: 10.1006/pmpp.1996.0074. [DOI] [Google Scholar]
- Meng X, Zhang M, Adhikari B. Extending shelf-life of fresh-cut green peppers using pressurized argon treatment. Postharvest Biol Technol. 2012;71:13–20. doi: 10.1016/j.postharvbio.2012.04.006. [DOI] [Google Scholar]
- Oms-Oliu G, Rojas-Graü MA, González LA, Varela P, Soliva-Fortuny R, Hernando MIH, Martín-Belloso O. Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: a review. Postharvest Biol Technol. 2010;57:139–148. doi: 10.1016/j.postharvbio.2010.04.001. [DOI] [Google Scholar]
- Qadri OS, Yousuf B, Srivastava AK. Fresh-cut fruits and vegetables: critical factors influencing microbiology and novel approaches to prevent microbial risks—a review. Cogent Food Agric. 2015;1(1):1121606. [Google Scholar]
- Raymond LV, Zhang M, Karangwa E, Chesereka MJ. Mixed noble gas effect on cut green peppers. Int Agrophys. 2013;27(1):75–79. doi: 10.2478/v10247-012-0070-2. [DOI] [Google Scholar]
- Rico D, Martin-Diana AB, Barat JM, Barry-Ryan C. Extending and measuring the quality of fresh-cut fruit and vegetables: a review. Trends Food Sci Technol. 2007;18:373–386. doi: 10.1016/j.tifs.2007.03.011. [DOI] [Google Scholar]
- Sothornvit R, Kiatchanapaibul P. Quality and shelf-life of washed fresh-cut asparagus in modified atmosphere packaging. LWT-Food Sci Technol. 2009;42(9):1484–1490. doi: 10.1016/j.lwt.2009.05.012. [DOI] [Google Scholar]
- Ventura-Aguilar RI, Colinas-León MT, Bautista-Baños S. Combination of sodium erythorbate and citric acid with MAP, extended storage life of sliced oyster mushrooms. LWT-Food Sci Technol. 2017;79:437–444. doi: 10.1016/j.lwt.2017.01.053. [DOI] [Google Scholar]
- Vina SZ, Chaves AR. Texture changes in fresh cut celery during refrigerated storage. J Sci Food Agric. 2003;83(13):1308–1314. doi: 10.1002/jsfa.1540. [DOI] [Google Scholar]
- Yousuf B, Qadri OS, Srivastava AK. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: a review. LWT-Food Sci Technol. 2018;89:198–209. doi: 10.1016/j.lwt.2017.10.051. [DOI] [Google Scholar]


