Abstract
Kenaf seed oil was extracted with 3 different solvents, i.e. hexane, ethanol and aqueous enzymatic medium with or without ultrasonic assistance. The synergistic effects of ultrasound and extraction solvent on the content of bioactive compound in kenaf seed oil was investigated. Results show that ultrasound-assisted extraction with hexane obtained the highest yield (84.71%), while yield with aqueous enzymatic medium was the lowest (51.12%). Two endothermic peaks exhibited on the melting curve of kenaf seed oil at the temperature range − 37 to − 25 °C and − 12 to − 2 °C, respectively. Linoleic, oleic and palmitic acid are the major fatty acids, accounting for above 96% of the total fatty acids. The content of vitamin E, phosphatide, total phenols and sterol are 92.38–105.01 mg/100 g oil, 0.38–22.28 g/kg, 0.51–71.02 mg GAE/100 g and 161.79–533.12 mg/100 g, respectively. The solvent employed has significant effect (p < 0.05) on the thermal property, fatty acid composition and bioactive constituents of the extracted kenaf seed oil. The oil extracted with ethanol contained more nervonic acid and bioactive components such as β-carotene, phosphatide, total phenols and sterols. The introduction of ultrasound reduced the extraction time remarkably. The results demonstrate that extraction with ethanol combined with ultrasound is an effective method to extract kenaf seed oil, as more reasonable fatty acid composition and higher content of bioactive components can be achieved.
Keywords: Kenaf seed oil, Extraction, Ultrasound, Ethanol, Bioactive compound
Introduction
In recent years, the demand for vegetable oil for consumption and industrial purposes has been increasing because of the increase of population and economic development (Hashemi et al. 2017a). There is thereby an urgent need to explore new source of oil as well as to increase the production of existing oil crops.
Kenaf (Hibiscus cannabinus L.) is an annual herbaceous plant, which belongs to Malvaceae family (Cheng et al. 2016). It is originally native to Africa and now mainly distributes in China, India, and Thailand (Chew et al. 2017a). Kenaf seed is a by-product of kenaf processing. It has a yield of 700–1500 kg/hectare, depending on the varieties of kenaf and agro-climatic conditions (Cheng et al. 2016). The content of oil in kenaf seed ranges from 19.0 to 26.4% (Mohamed et al. 1995; Coetzee et al. 2008), which is higher than that (18–21%) in soybean (Clemente and Cahoon 2009). The fatty acid composition of kenaf seed oil is similar to that of cotton seed oil, which is rich in oleic and linoleic acid (Coetzee et al. 2008). The relatively high oil content and reasonable fatty acid composition make kenaf seed a potential source of edible oil. Furthermore, kenaf seed oil has anti-hypercholesterolemia, anti-oxidant, anti-cancer, anti-inflammatory, anti-thrombotic and anti-mutagenic properties, thus can be used not only in food but also pharmaceutical industries (Dhar et al. 2015).
The extraction of oil with organic solvent is a traditional way to produce edible oil and is still the most widely adopted one (Hashemi et al. 2016; Bhutada et al. 2016). Hexane is the most commonly used organic solvent with high extraction efficiency. However, hexane is flammable, explosive, and toxic. It has been considered as an air pollutant due to the leakage in the extraction process of oil (Tavakolpour et al. 2017; Yusoff et al. 2016).
Supercritical carbon dioxide is a non-toxic, non-explosive and environmental friendly solvent, which has been used to extract oil from kenaf seed (Chan and Ismail 2009; Mariod et al. 2011). Nevertheless, high cost and operating pressure restrict its application in industry-scale production of edible oil.
Ethanol and water are also environment- and health-friendly solvent for oil extraction (de Oliveira et al. 2013; Latif and Anwar 2011). However, the application of ethanol and water as solvent surfers from lower extraction efficiency when compared with hexane.
In order to increase the yield of oil and reduce the extraction time, several assistant technology such as enzyme, microwave and ultrasound have been adopted (Sicaire et al. 2016; Yusoff et al. 2016; Hashemi et al. 2017b). Ultrasound-assisted extraction was considered as a modern physical extraction method for vegetable oil (Dhar et al. 2015; Wang and Weller 2006). Under ultrasonic radiation Yusri et al. (2012), extracted antioxidant compounds from kenaf seed through sequential solvent extraction, while Wong et al. (2014) adopted binary solvent (ethanol and water). In these works, the phenolic content and antioxidant activity of kenaf seed extracts were fully analyzed, but the effect of ultrasound on the yield and properties of kenaf seed oil was omitted. To the best of our knowledge, limited information is available on the synergistic effect of ultrasound and extraction solvent on the bioactive compounds of kenaf seed oil. Therefore, for the first time, the kenaf seed oil was extracted by using three solvents, i.e. hexane, ethanol, and aqueous enzyme with or without ultrasonic assistance in the present work. The synergistic effects of ultrasound and extraction solvent on the yield, thermal property, fatty acid composition and content of bioactive components, i.e. vitamin E, phosphatide, carotenoid, total phenols and sterols of kenaf seed oil are evaluated.
Materials and methods
Materials
Kenaf seeds (variety Fuyou No.1) were provided by Xinyang Agricultural Research Institute (Xinyang, China). Fatty acids methyl esters (FAMEs) standards were obtained from Sigma-Aldrich Corporation (Shanghai, China). Tocopherols and tocotrienols standards were purchased from Beijing Sunky Biological Technology Co., Ltd. (Beijing, China). Alkaline protease (200 U/mg) was purchased from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China). All the reagents were of analytical grade and used without any further purification.
Extraction of kenaf seed oil
Extraction of kenaf seed oil with hexane (HE)
The extraction of kenaf seed oil with hexane was carried out following the procedure reported by Khoddami et al. (2011) with slight modification. Powdered kenaf seed was dispersed in hexane (1:8, w/v) in a beaker and then extracted for 4 h at 45 °C. After that, the mixture was centrifuged at 1860×g for 20 min at ambient temperature. The supernatant was collected and then concentrated in rotary evaporator under vacuum. The kenaf seed oil obtained was stored at 4 °C for subsequent analyses.
Extraction of kenaf seed oil with ethanol (EE)
Similar to the report by Oliveira et al. (2012), 95% aqueous ethanol was employed as extraction solvent in this study. The extraction procedure was as described in Sect. 2.2.1.
Aqueous enzymatic extraction (AE) of kenaf seed oil
Aqueous enzymatic extraction of kenaf seed oil was carried out following the procedure reported by Latif and Anwar (2011). Powdered kenaf seed was dispersed in distilled water at 1:8 (w/v) in a flask. The mixture was boiled for 10 min and then cooled to room temperature (about 25 °C). The pH value of the mixture was adjusted to 10 using 0.5 mol/L aqueous NaOH solution prior to the addition of alkaline protease (2 wt% of the kenaf seed). The mixture was incubated at 45 °C for 4 h in a thermostat oscillator (SHZ-88, Jintan Electronics Co., Ltd., Jintan, China) with gentle agitation at 170 rpm. Subsequently, the mixture was heated to 90 °C for 10 min, followed by centrifugation at 4770×g for 20 min. The upper oil layer was collected and stored at 4 °C for subsequent analyses.
Ultrasonically assisted extraction of kenaf seed oil with different solvents
To improve the yield of kenaf seed oil, ultrasonically assisted extraction process was developed in this work. Ultrasound was introduced into the extraction medium at the stage of extraction with a Scientz-IID ultrasonic emulsifier (Ningbo Scientz Biotechnology Co., Ltd, Ningbo, China) equipped with a flat tip probe of 1.0 cm in diameter (Zhang et al. 2008). The power, frequency and duty cycle of ultrasound were 200 W, 20 kHz and 2 s on/2 s off, respectively. A DC-2006 thermostatic bath (Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China) was used to control the temperature of the sample during extraction. The extraction of kenaf seed oil assisted by ultrasound lasted for 60 min, while other conditions were the same as those described in Sects. 2.2.1–2.2.3.
The oils extracted with hexane, ethanol, and aqueous enzymatic medium at the presence of ultrasound are denoted by UHE, UEE, and UAE, respectively.
Determination of oil yield
The yield of kenaf seed oil was calculated using the following formula:
| 1 |
where total content of kenaf seed oil was 19.56%, which was determined by Soxhlet extraction procedure according to American Oil Chemists’ Society (AOCS) official method Aa 4–38 (AOCS 2001).
Thermal analysis of kenaf seed oil
The thermal analysis of kenaf seed oil was performed on a Q20 differential scanning calorimeter (DSC, TA Instruments Ltd., New Castle, USA). Prior to the analysis, DSC was calibrated for temperature and heat flow with mercury (melting point = − 38.834 °C, △H = 11.469 J/g) and indium (melting point = 156.598 °C, △H = 28.5 J/g).Five milligram of kenaf seed oil was sealed in aluminum pan. Another empty sealed aluminum pan was used as reference. Nitrogen gas (99.999%) was the purge gas at a flow rate of 40 mL/min. The sample was initially heated to 60 °C and equilibrated for 5 min. After that, the sample was cooled to − 40 °C at 5 °C/min and held isothermally for 5 min. Subsequently, the sample was heated to 60 °C again at 5 °C/min.
Fatty acid composition analysis of kenaf seed oil
Fatty acid methyl esters (FAMEs) were prepared with BF3-methanol according to ISO standard (ISO:5509, 2000). Fatty acid composition analysis of kenaf seed oil was performed using an Agilent 7890B gas chromatograph (Agilent Technologies, Wilmingston, USA) equipped with a flame ionization detector and a HP-88 chromatographic column (100 m × 0.25 mm i.d., film thickness 0.2 μm). The initial column temperature was 140 °C, held isothermally for 5 min, and then increased to 240 °C at 4 °C/min and held isothermally for another 10 min. Injector temperature was 240 °C; detector temperature was 280 °C; injection volume was 1.0 μL; split ratio was 1:50. Nitrogen gas was used as carrier gas at a flow rate of 22 mL/min. FAMEs were identified by comparing their retention time to authentic standards and quantified as relative percentage of the total peaks area.
Analysis of Vitamin E in kenaf seed oil
Vitamin E content and composition were determined according to the standard method of ISO: 9936 (ISO 2006). Half a gram of kenaf seed oil was dissolved in 10 mL hexane and then filtered using a 0.45 μm filter before HPLC analysis. The analysis of vitamin E was performed on a Waters-e2695 HPLC system (Waters Corporation, Milford, USA) equipped with a Waters 2475 fluorescence detector (Waters Corporation, Milford, USA) and an Elite NH2 column (250 mm × 4.6 mm, 5 μm particle size, Dalian Elite Analytical Co., Ltd., Dalian, China). Mobile phase was hexane/isopropanol (99:1, v/v) with a flow rate of 0.8 mL/min. Column temperature was 40 °C. Excitation and emission wavelength was 298 nm and 325 nm, respectively. The vitamin E isomers were identified by comparing their retention time with authentic standard and quantified based on the calibration curve of individual isomer standard.
Determination of β-carotene in kenaf seed oil
The β-carotene content in kenaf seed oil was determined according to the method reported by Zheng et al. (2012). Briefly, 200 mg of kenaf seed oil was diluted with 5 mL mixture of acetone-hexane (4:6, v/v). The absorbance of the solution at 453, 505, 645 and 663 nm was measured respectively using a T6 spectrophotometer (Beijing Purkinje General Instrument Co., Ltd., Beijing, China). The content of β-carotene was calculated using the following equation and expressed as mg/kg oil.
| 2 |
Determination of phosphatide in kenaf seed oil
The phosphatide content in kenaf seed oil was determined by molybdenum blue colorimetry according to AOCS official method Ca 12–55 (AOCS 1997). Phosphatide of kenaf seed oil was burned to phosphorus pentoxide, which was transferred into phosphate acid by hot hydrochloric acid. Phosphomolybdic acid sodium was generated when phosphate acid contacted with sodium molybdate and subsequently deacidized to molybdenum blue by hydrazine sulfate. Absorbance at wavelength of 650 nm was measured with a spectrophotometer (Model T6, Beijing Purkinje General Instrument Co., Ltd., Beijing, China). Phosphatide content was calculated with standard curve.
Determination of total phenols in kenaf seed oil
The total phenols content in kenaf seed oil was determined by the Folin-Ciocalteu method (Gutfinger 1981). Briefly, 2 g of kenaf seed oil dissolved in 5 mL hexane was extracted with 5 mL of aqueous methanol (60%). 0.2 mL of methanol phase was mixed with 10 mL of diluted Folin-Ciocalteu reagent. After incubation for 3 min, 1 mL of sodium carbonate solution (35%, w/v) was added to the mixture. The mixture was stored in the dark and incubated for another 2 h. The absorbance of the mixture was determined at 725 nm using spectrophotometer (Model T6, Beijing Purkinje General Instrument Co., Ltd., Beijing, China). The concentration was quantified from a calibration curve using gallic acid as reference. The results are expressed in mg gallic acid equivalents (GAE)/100 g of oil.
Sterol composition analysis of kenaf seed oil
Sterol composition of kenaf seed oil was determined according to the method of ISO: 12228 (ISO, 1999). Briefly, one gram of kenaf seed oil and 1 mL of internal standard (α-cholestanol) was saponified by ethanolic potassium hydroxide solution. Unsaponifiable matter was extracted with hexane and the solvent was removed under a vacuum. Subsequently, the dried hexane extract was dissolved in diethyl ether. Sterols were separated by thin layer chromatography. The separated sterols were converted to the trimethylsilyl ether derivatives using Bis (trimethylsilyl) trifluoroacetamide/trimethylchlorosilane (99:1). The transferred sterols were analyzed on a gas chromatograph (GC-7890B, Agilent Technologies, Wilmingston, USA) equipped with a flame ionization detector and a HP-5 column (30 m × 320 μm, particle size 0.25 μm). Nitrogen gas was carrier gas at a flow rate of 1.0 mL/min. Injection volume was 1.0 μL, spilt ratio was 1:20. Injector and detector temperature were 240 °C and 360 °C, respectively. The sterols were identified by relative retention time. The results were expressed as percentage of peak area of all sterols.
Statistical analysis
All experiments were repeated for at least 3 times. The results were expressed as mean ± standard deviation (SD). The analysis of variance (ANOVA) of the data were performed using SPSS 24.0 statistical software (IBM Corp, Armonk, NY, USA) as well as Duncan’s test. Differences were considered statistically significant with a p-value less than 0.05.
Results and discussion
Kenaf seed oil yield
Extraction solvent has a significant effect (p < 0.05) on the yield of kenaf seed oil. As expected, hexane obtained the highest yield of kenaf seed oil (~ 81%), followed by ethanol (~ 65%) and aqueous enzyme (~ 51%) in the absence of ultrasound. This might be attributed to the different polarities of extraction solvents. Triacylglycerol is a nonpolar substance which is less dissolvable in polar solvent. The polarities of the employed solvents follow the order hexane < ethanol < water. Thus extraction with hexane demonstrate the highest efficiency.
The application of ultrasound increased the yield of kenaf seed oil remarkably. The oil yield extracted with hexane, ethanol and water under ultrasound radiation for 60 min was 84.7%, 67.3% and 55.8%, respectively. The yield is equal to or even higher than that obtained at 4 h without ultrasound. It implies that ultrasonically assisted extraction process reduces the extraction time required evidently. The positive effect might be attributed to the damage of cell structure by ultrasound. The application of ultrasound during extraction process could disrupt the cell wall and accelerate the release of oil from matrix because of the cavitation effects (Zhang et al. 2008; Sicaire et al. 2016).
Even though the yield of kenaf seed oil extracted with ethanol is relatively low, ethanol is still a promising green solvent for the extraction of kenaf seed oil considering the cost, environmental and healthy benefits. In addition, the yield of oil could be improved further by optimizing the extraction conditions. Oliveira et al. (2012) reported that 99.9% of the oil available in fresh rice bran could be extracted using ethanol under optimized extraction conditions.
Thermal properties of kenaf seed oil
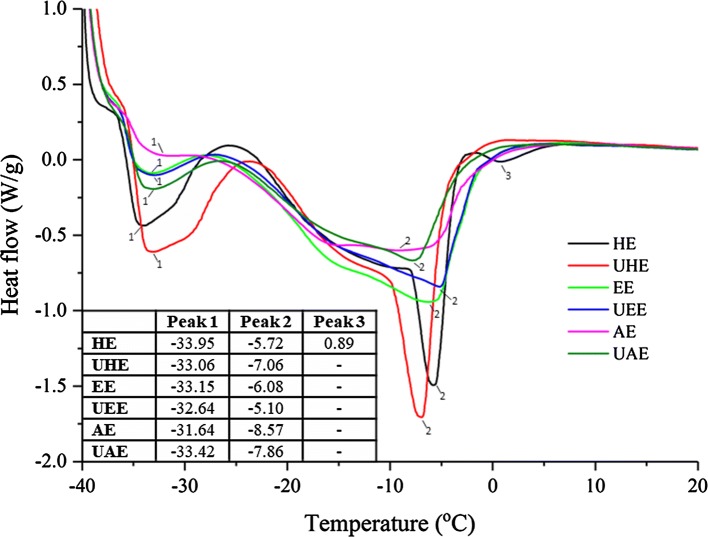
Figure 1 shows the DSC curves of kenaf seed oil. All the curves show two peaks: the peak around − 32 °C is attributed to the melting of unsaturated triacylglycerol while the peak around − 6 °C is attributed to the melting of saturated triacylglycerol (Zhang et al. 2014). This observation is slightly different from the report by Nyam et al. (2009a), in which the melting points appeared at − 19.32 °C and − 4.23 °C, respectively. The inconsistence might be due to the different genotypes, planting environments and processing conditions of kenaf seeds. Yanty et al. (2011) and Zhang et al. ( 2011) observed that the avocado oil and flaxseed oil obtained from different varieties showed different thermal properties
Fig. 1.
Melting profile of kenaf seed oil extracted with different methods
Figure 1 also indicates that extraction solvent affects the thermal properties of kenaf seed oil significantly. The hexane-extracted oil shows the sharpest melting peaks and the largest enthalpy on the DSC curves, followed by ethanol-extracted oil and enzyme-extracted oil. The variation is partly due to the difference in their fatty acid and triacylglycerol molecular compositions (which will be discussed later), since the thermal behavior of lipid are the result of overlapping effect from composition and polymorphism of the triacylglycerols (Samyn et al. 2012; Samaram et al. 2014)
The kenaf seed oils extracted with or without ultrasonic assistance are slightly different in the melting profile, suggesting that there are some differences in components between the oils extracted with and without ultrasound.
Fatty acid composition of kenaf seed oil
As listed in Table 1, 10 types of fatty acid were identified in kenaf seed oil. Linoleic is the most predominant fatty acid, followed by oleic, palmitic, stearic and palmitoleic, which account for more than 96% of total fatty acids. Minor fatty acids such as nervonic acid (C24:1), lignoceric acid (C24:0), arachidic acid (C20:0), linolenic acid (C18:3) and myristic acid (C14:0) were also found in kenaf seed oil. The contents of these fatty acids are close to those reported by Mohamed et al. (1995). However, Coetzee et al. (2008) found that the relative contents of fatty acids varied greatly among different kenaf varieties, oleic acid was the predominant fatty acid in some varieties.
Table 1.
Fatty acid composition of kenaf seed oil extracted with different methods (%)
| Fatty acids | HE | UHE | EE | UEE | AE | UAE |
|---|---|---|---|---|---|---|
| C14:0 | 0.15 ± 0.00a | 0.15 ± 0.00a | 0.15 ± 0.00a | 0.16 ± 0.01a | 0.15 ± 0.00a | 0.15 ± 0.00a |
| C16:0 | 22.43 ± 0.10 cd | 22.63 ± 0.11d | 23.53 ± 0.15e | 22.08 ± 0.30c | 21.35 ± 0.23b | 18.82 ± 0.12a |
| C16:1 | 1.03 ± 0.00ab | 1.03 ± 0.01ab | 1.02 ± 0.01a | 1.05 ± 0.01bc | 1.04 ± 0.01abc | 1.06 ± 0.01d |
| C18:0 | 2.52 ± 0.02c | 2.50 ± 0.03c | 2.42 ± 0.01b | 2.41 ± 0.03b | 2.44 ± 0.02b | 2.13 ± 0.02a |
| C18:1 | 28.55 ± 0.13b | 28.77 ± 0.11bc | 27.87 ± 0.15a | 28.49 ± 0.26b | 29.17 ± 0.13c | 30.11 ± 0.40d |
| C18:2 | 43.07 ± 0.05c | 42.87 ± 0.06c | 41.98 ± 0.05a | 42.46 ± 0.08b | 43.9 ± 0.11d | 44.90 ± 0.25e |
| C18:3 | 0.45 ± 0.01a | 0.42 ± 0.01a | 0.45 ± 0.02a | 0.50 ± 0.00b | 0.42 ± 0.01a | 0.49 ± 0.01b |
| C20:0 | 0.55 ± 0.01c | 0.46 ± 0.02b | 0.46 ± 0.01b | 0.39 ± 0.00a | 0.38 ± 0.01a | 0.38 ± 0.00a |
| C24:0 | 0.65 ± 0.01b | 0.57 ± 0.02a | 0.98 ± 0.01d | 1.15 ± 0.03e | 0.55 ± 0.00a | 0.89 ± 0.02c |
| C24:1 | 0.62 ± 0.00a | 0.62 ± 0.00a | 1.17 ± 0.03c | 1.33 ± 0.04d | 0.62 ± 0.03a | 1.09 ± 0.03b |
| ∑MUFA | 30.2 ± 0.13a | 30.41 ± 0.11ab | 30.05 ± 0.13a | 30.86 ± 0.31b | 30.83 ± 0.12b | 32.26 ± 0.39c |
| ∑PUFA | 43.52 ± 0.06c | 43.29 ± 0.07c | 42.42 ± 0.03a | 42.96 ± 0.08b | 44.32 ± 0.01d | 45.39 ± 0.27e |
| ∑UFA | 73.72 ± 0.06b | 73.7 ± 0.04b | 72.47 ± 0.16a | 73.82 ± 0.23b | 75.15 ± 0.22c | 77.65 ± 0.12d |
| ∑SFA | 26.29 ± 0.06c | 26.3 ± 0.04c | 27.53 ± 0.16d | 26.19 ± 0.23c | 24.86 ± 0.22b | 22.36 ± 0.12a |
| UFA/SFA | 2.81 ± 0.01b | 2.81 ± 0.01b | 2.64 ± 0.02a | 2.82 ± 0.03b | 3.03 ± 0.04c | 3.48 ± 0.02d |
Values of each line with different labels were significantly different (p < 0.05)
MUFA monounsaturated fatty acid, PUFA polyunsaturated fatty acid, UFA unsaturated fatty acid, SFA saturated fatty acid
The fatty acid compositions of kenaf seed oils extracted with different solvents are statistically different (p < 0.05) from each other. The oil extracted with ethanol contains more C24:0, C24:1 but less C18:2, while the one extracted with aqueous enzymatic medium has more C18:1, C18:2 and less C16:0. Lee et al. (2015) extracted passion fruit oil with three different solvents including ethanol, hexane and acetone. They found that the ethanol-extracted oil was lower in linoleic acid, while higher in oleic, palmitic and stearic acids than hexane-extracted oil. The results can be interpreted partially by the difference in solubility of vegetable oil in different solvents. Besides, polar lipids, for instance phospholipid which esterified with fatty acid and phosphoric acid also have influence on the fatty acid profile of extracted oil, since the phospholipid are more soluble in polar solvents than in nonpolar solvent like hexane (Lee et al. 2015).
It is found that the effect of ultrasound on fatty acid composition depends on the type of solvent employed. No significant difference (p > 0.05) is found in the fatty acid composition between the oils extracted using hexane with or without ultrasound. On the contrary, a significant difference (p < 0.05) is observed when ethanol or aqueous enzymatic medium is used as extraction solvent. Hashemi et al. (2016) reported the extraction of Pistacia khinjuk hull oil using hexane. No significant difference in the fatty acid composition was observed under conventional and ultrasonic conditions.
It is worth noting that nervonic acid is extremely important for human body and polyunsaturated fatty acids (PUFA) are effective in preventing hypertension, atherosclerosis and coronary disease, etc. Higher contents of nervonic acid and PUFA were found in ethanol-extracted kenaf seed oil.
Vitamin E composition of kenaf seed oil
Vitamin E is a very important antioxidant in vegetable oils. It refers to a group of compounds that include 4 tocopherol (TP) isomers (α-, β-, γ- and δ-) and 4 tocotrienol (TT) isomers (α-, β-, γ- and δ-). As shown in Table 2, 7 kinds of isomer were found in kenaf seed oils except α-TT. The content of vitamin E in kenaf seed oil was 92.38–105.01 mg/ 100 g. The result is consistent with the report by Mariod et al. (2011), in which the highest vitamine E content was 88.20 mg/ 100 g in kenaf seed oil extracted using supercritical carbon dioxide.
Table 2.
Vitamin E composition of kenaf seed oil extracted with different methods (mg/100 g)
| Isomers | HE | UHE | EE | UEE | AE | UAE |
|---|---|---|---|---|---|---|
| α-TP | 23.36 ± 2.14a | 25.54 ± 1.79a | 21.99 ± 2.16a | 24.45 ± 1.65a | 31.97 ± 1.05b | 34.03 ± 1.75b |
| β-TP | 8.84 ± 0.13ab | 8.53 ± 0.18a | 8.99 ± 0.09b | 8.71 ± 0.13ab | 8.94 ± 0.18ab | 8.81 ± 0.25ab |
| γ-TP | 25.7 ± 1.06a | 25.02 ± 0.58a | 29.92 ± 1.19b | 29.08 ± 1.49b | 28.12 ± 0.66ab | 28.16 ± 1.85ab |
| (β + γ)-TT | 20.58 ± 0.96a | 19.70 ± 0.81a | 20.85 ± 1.15a | 20.21 ± 0.66a | 20.61 ± 0.81a | 20.07 ± 1.03a |
| δ-TP | 4.39 ± 0.04ab | 4.21 ± 0.11a | 4.52 ± 0.16b | 4.39 ± 0.11ab | 4.41 ± 0.11ab | 4.41 ± 0.13ab |
| δ-TT | 9.85 ± 0.18a | 9.40 ± 0.22a | 9.94 ± 0.17a | 9.60 ± 0.21a | 9.81 ± 0.19a | 9.55 ± 0.33a |
| Total | 92.72 ± 4.42a | 92.38 ± 3.34a | 96.20 ± 4.26ab | 96.43 ± 0.95ab | 103.84 ± 2.39c | 105.01 ± 3.92c |
Values of each line with different labels were significantly different (p < 0.05)
The content and profile of vitamin E in kenaf seed oil depend on the solvents employed. The oils extracted with hexane and ethanol have similar composition and content of vitamin E except a higher γ-TP in ethanol-extracted oil. The most prevalent vitamin E isomer in these oils was γ-TP, followed by α-TP. The results are in good agreement with that of Chew et al. (2017c) and Nyam et al. (2009a). However, the most abundant vitamin E isomer was α-TP in the oil extracted with aqueous enzymatic medium. This might be partly related to the recovery method of kenaf seed oil. Evaporation was replaced by the centrifugal separation in aqueous enzymatic extraction process, in which more nonpolar α-TP was partitioned into oil phase.
Furthermore, ultrasound has no significant effect (p > 0.05) on the composition and content of vitamin E in kenaf seed oil regardless of the extraction solvent used.
Sterols composition of kenaf seed oil
Sterols are important bioactive substance in vegetable oils, which are beneficial to human health in lowing blood cholesterol, antioxidant, anti-inflammation, and anti-tumor (Racette et al. 2010). Table 3 shows that 7 types of sterol are identified in kenaf seed oil. The major sterols are β-sitosterol (70.88–72.54%) and campesterol (12.01–13.71%), which account for 83.04–86.25% of the total sterols. The values are consistent with previous study by Mohamed et al. (1995), in which the average content of β-sitosterol and campesterol for 9 kenaf genotypes were 72.3% and 9.9%, respectively. The type of extraction solvent has significant effect (p < 0.05) on the total content of sterols. Ethanol-extracted oils have higher sterol content which may be attributed to the fact that sterols have weaker polarity due to their 3-OH group (Tir et al. 2012). The finding that ethanol tends to extract more sterols was also reported by several studies (Bhatnagar and Krishna 2013; Nyam et al. 2009b). Bhatnagar and Krishna (2013) found that ethanol-extracted oil contained almost fivefold total sterols compared to hexane-extracted oil (6309.3 ppm vs. 1351.4 ppm).
Table 3.
Sterol composition and content of kenaf seed oil extracted with different methods
| Sterol | HE | UHE | EE | UEE | AE | UAE |
|---|---|---|---|---|---|---|
| Cholesterol (%) | 2.76 ± 0.10ab | 2.64 ± 0.13a | 3.12 ± 0.03ab | 3.88 ± 1.08b | 3.19 ± 0.10ab | 2.30 ± 0.12a |
| Campesterol (%) | 12.24 ± 0.91ab | 12.01 ± 0.38a | 12.73 ± 0.10ab | 12.27 ± 0.90ab | 12.82 ± 0.36ab | 13.71 ± 0.15b |
| Stigmasterol (%) | 3.92 ± 0.35ab | 4.43 ± 0.26b | 3.7 ± 0.60ab | 3.94 ± 0.26ab | 3.81 ± 0.33ab | 3.32 ± 0.02a |
| Δ7-Campesterol (%) | 4.83 ± 0.03b | 4.57 ± 0.53b | 3.69 ± 0.06ab | 4.444 ± 0.88b | 3.61 ± 0.77ab | 2.56 ± 0.25a |
| β-sitosterol (%) | 70.93 ± 1.03a | 71.03 ± 1.44a | 71.09 ± 0.91a | 70.88 ± 0.53a | 71.83 ± 0.41a | 72.54 ± 0.8a |
| Sitostanol (%) | 4.67 ± 0.41a | 4.64 ± 0.09a | 5.05 ± 0.34a | 4.00 ± 0.85a | 4.06 ± 0.57a | 4.87 ± 0.51a |
| 5,24-Stigmastadienol (%) | 0.67 ± 0.01a | 0.70 ± 0.02a | 0.65 ± 0.09a | 0.61 ± 0.06a | 0.71 ± 0.07a | 0.72 ± 0.02a |
| Total sterols (mg/100 oil) | 161.79 ± 8.94a | 327.31 ± 12.23c | 471.06 ± 5.2d | 533.12 ± 10.49e | 259.04 ± 9.03b | 259.75 ± 11.21b |
Values of each line with different labels were significantly different (p < 0.05)
The data in Table 3 show that ultrasound has an insignificant influence on the sterol composition of extracted kenaf seed oil, while it has a significant influence (p < 0.05) on the total content of sterols.
β-carotene content in kenaf seed oil
Table 4 shows that the content of β-carotene is significantly different (p < 0.05) in the oils extracted with different solvents. In the absence of ultrasound, the highest content of β-carotene is obtained in ethanol-extracted oil (7.75 mg/kg oil), followed by aqueous enzymatic extracted oil (3.50 mg/kg oil) and hexane-extracted oil (2.73 mg/kg oil). This is consistent with the report by Bhatnagar and Krishna (2013), in which the β-carotene content in niger seed oil extracted by ethanol was about 6 times of the one extracted by hexane (181.01 vs. 26.85 mg/kg oil).
Table 4.
Other bioactive compounds in kenaf seed oil extracted with different methods
| HE | UHE | EE | UEE | AE | UAE | |
|---|---|---|---|---|---|---|
| β-carotene | 2.73 ± 0.04b | 2.67 ± 0.03b | 7.75 ± 0.11e | 6.81 ± 0.1d | 3.50 ± 0.08c | 1.87 ± 0.04a |
| Phosphatide | 4.58 ± 0.31b | 18.79 ± 1.32d | 9.23 ± 0.58c | 22.28 ± 1.46e | 0.38 ± 0.07a | 1.54 ± 0.20a |
| Total phenols | 17.18 ± 0.62b | 23.5 ± 0.28c | 66.78 ± 1.7d | 71.02 ± 1.83e | 3.21 ± 0.34a | 0.51 ± 0.72a |
Values of each line with different labels were significantly different (p < 0.05)
The effect of ultrasound on the β-carotene content in kenaf seed oil depends on the type of extraction solvent used. As shown in Table 4, application of ultrasound caused a significant (p < 0.05) decrease in β-carotene content for the oils extracted with ethanol and aqueous enzyme, while non-significant change was found in the oils obtained with hexane. The lower β-carotene content may be ascribed to the degradation of β-carotene, since vapor pressures of ethanol and water are lower than that of hexane. In general, the lower the vapor pressure of extraction solvent, the more the cavitation bubble. The cavitation bubble implode with higher strength when the vapor pressure is lower, which further cause a higher local temperature and pressure (Rodrigues et al. 2017). Previous studies indicated that the degradation of carotenoids followed a first-order kinetic model and was sensitive to thermal treatment and light illumination. The β-carotene in crude palm oil completely eliminated after the oil was oxidized at 120 °C for 8 h (Damanik and Murkovic 2018).
Phosphatide content
Table 4 shows that the content of phosphatide in kenaf seed oil. In the absence of ultrasound, kenaf seed oil extracted with ethanol had the highest phosphatide content (9.23 g/kg oil), followed by the one extracted with hexane (4.58 g/kg oil) and aqueous enzymatic medium (0.38 g/kg oil). Higher phosphatide content was observed in the ultrasonically extracted kenaf seed oils. This might be resulted from the fact that ultrasound ruptured the cell membrane and released more phosphatide into solvents, as the phosphatide exist mainly in the cell membrane. A higher phosphatide content in ethanol-extracted oil could be partially explained by the fact that phosphatide contains a hydrophilic polar phosphate, which render it having a high affinity towards polar solvent (Perrier et al. 2017). While a lower phosphatide content in kenaf seed oil extracted with aqueous enzymatic medium could be attributed to the precipitation of hydratable phosphatide in water (Mehanni et al. 2017), which was removed subsequently from oil by centrifugation.
Total phenols content
Table. 4 shows that the content of total phenols in the kenaf seed oil ranges from 71.02 to 0.51 mg GAE/100 g oil. The highest content of total phenols is found in the oil extracted with ethanol combined with ultrasound (71.02 mg GAE/100 g), followed by ethanol extraction without ultrasound (66.78 GAE/100 g). This could be explained by the ability of ethanol to extract both hydrophilic and lipophilic polyphenols (Lee et al. 2015). In general, the content of total phenols in enzyme-extracted oil is higher than hexane-extracted oil as reported by Latif and Anwar (2011) and Li et al. (2017). It was explained by enzymatic hydrolysis which reduce the complexation of phenolic compounds with polysaccharides, proteins and pectins, and consequently enhanced bioactive components partitioning into the oil phase (Ranalli et al. 2005). In contrast, the content of total phenols in the enzyme-extracted oils is the lowest in this work. This is due to the face that more phenolic compounds was partitioned into aqueous phase and then washed away. It is further supported by the study of Yusri et al. (2012) and Ryu et al (2017). They reported that most of phenolic compounds in kenaf seed oil are highly polar in nature, and more extractable by polar solvents. Moreover, Nyam et al. (2009a) reported that the main phenolic acids in kenaf seed are vanillic, caffeic, gallic, p-hydroxybenzoic, ferulic, p-coumaric and protocatechuic acid.
The higher content of total phenols in kenaf seed oil extracted under ultrasonic radiation suggests that ultrasound could rupture cell structure of kenaf seeds, facilitating the release of bioactive compound such as polyphenol from the matrix.
Interactive analysis
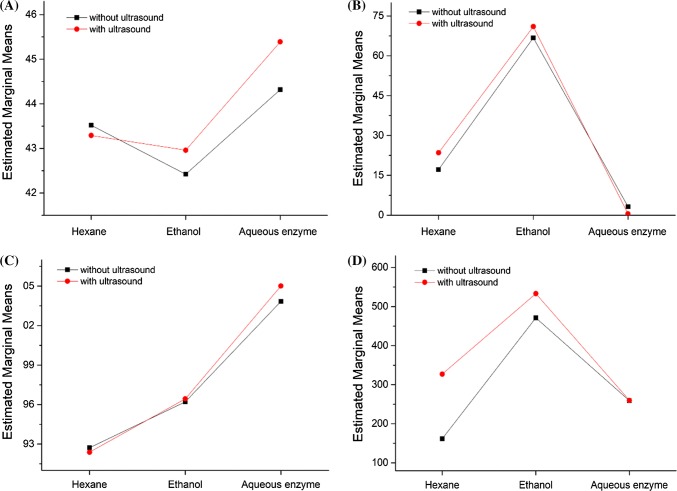
Figure 2 shows that the introduction of ultrasound during extraction process results in a change in the indexes such as PUFA percentage and content of some bioactive compounds, i.e. vitamin E, sterols and total phenols of the obtained oils. Moreover, the change depends on the type of extraction solvent. Therefore, there is a synergistic effect between extraction solvent and ultrasound on the components of kenaf seed oil. As shown in Fig. 2, the introduction of ultrasound decreases the content of PUFA and vitamin E in hexane-extracted oil, as well as total phenols in enzyme-extracted oil. However, when ethanol is used as extraction solvent, PUFA percentage, content of vitamin E, sterol and total phenols in kenaf seed oil increase under ultrasonic radiation
Fig. 2.
Interactive effect of extraction solvent and ultrasound on the kenaf seed oil. a Percentage of polyunsaturated fatty acid in the oil, b total phenols content, c vitamin E content and d sterol content
Conclusion
Kenaf seed oil was extracted with 3 different solvents, i.e. hexane, ethanol and aqueous enzymatic medium with or without ultrasonic assistance. The oil obtained was characterized comprehensively to explore the synergistic effect between extraction solvent and ultrasound. The results show that physiochemical properties varied dramatically among the oils extracted with hexane, ethanol and water, respectively. More nervonic acid, sterols, β-carotene, phosphatide and total phenols were obtained when ethanol was adopted, suggesting that ethanol is efficient in the extraction of bioactive compounds in kenaf seed oil. Ultrasound increased the yield of kenaf oil and reduced the extraction time required remarkably. Therefore, ultrasonic-assisted ethanol extraction is considered as a promising approach for the extraction of kenaf seed oil.
Acknowledgements
The work was supported financially by National Key Research and Development Plan of China (2016YFD0400205-3), Fundamental Research Funds for the Central Universities (20720190038), National Natural Science Foundation of China (31101367) and China Agriculture Research System (CARS14-1-29).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhen-shan Zhang, Email: zzsan010@126.com.
Liming Che, Email: lmc@xmu.edu.cn.
References
- AOCS, (1997, 2001). Ca 12–55, Aa 4–38. In: Firestone, D. (Ed.), Official Methods and Recommended Practices of the American Oil Chemists’ Society. AOCS Press, Champaign III USA.
- Bhatnagar, A.S., Krishna, A.G.G., (2013). Effect of extraction solvent on oil and bioactives composition of commercial Indian niger (Guizotia abyssinica (L.f.) Cass.) seed. Journal of the American Oil Chemists Society 90(8), 1203–1212.
- Bhutada PR, Jadhav AJ, Pinjari DV, Nemade PR, Jain RD. Solvent assisted extraction of oil from Moringa oleifera Lam. seeds. Ind Crops Prod. 2016;82:74–80. [Google Scholar]
- Chan, K.W., Ismail, M., (2009). Supercritical carbon dioxide fluid extraction of Hibiscus cannabinus L. seed oil: A potential solvent-free and high antioxidative edible oil. Food Chemistry 114(3), 970–975.
- Cheng WY, Akanda JMH, Nyam KL. Kenaf seed oil: A potential new source of edible oil. Trends Food Sci Technol. 2016;52:57–65. [Google Scholar]
- Chew SC, Tan CP, Nyam KL. Optimization of bleaching parameters in refining process of kenaf seed oil with a central composite design model. J Food Sci. 2017;82(7):1622–1630. doi: 10.1111/1750-3841.13758. [DOI] [PubMed] [Google Scholar]
- Chew SC, Tan CP, Nyam KL. Optimization of degumming parameters in chemical refining process to reduce phosphorus contents in kenaf seed oil. Sep Purif Technol. 2017;188:379–385. [Google Scholar]
- Chew SC, Tan CP, Nyam KL. Application of response surface methodology for optimizing the deodorization parameters in chemical refining of kenaf seed oil. Sep Purif Technol. 2017;184:144–151. [Google Scholar]
- Clemente TE, Cahoon EB. Soybean oil: Genetic approaches for modification of functionality and total content. Plant Physiol. 2009;151(3):1030–1040. doi: 10.1104/pp.109.146282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee, R., Labuschagne, M.T., Hugo, A., (2008). Fatty acid and oil variation in seed from kenaf (Hibiscus cannabinus L.). Industrial Crops and Products 27(1), 104–109.
- Damanik M, Murkovic M. The stability of palm oils during heating in a rancimat. Eur Food Res Technol. 2018;244(7):1293–1299. [Google Scholar]
- de Oliveira RC, Davantel de Barros ST, Gimenes ML. The extraction of passion fruit oil with green solvents. J Food Eng. 2013;117(4):458–463. [Google Scholar]
- Dhar P, Kar CS, Ojha D, Pandey SK, Mitra J. Chemistry, phytotechnology, pharmacology and nutraceutical functions of kenaf (Hibiscus cannabinus L.) and roselle (Hibiscus sabdariffa L.) seed oil: An overview. Ind Crops Prod. 2015;77:323–332. [Google Scholar]
- Gutfinger T. Polyphenols in olive oils. J Am Oil Chem Soc. 1981;58(11):966–968. [Google Scholar]
- Hashemi SMB, Mousavi Khaneghah A, Akbarirad H. Effects of ultrasound treatment and zenyan essential oil on lipid oxidation of blended vegetable oil. International Food Research Journal. 2015;22(5):1918–1923. [Google Scholar]
- Hashemi SMB, Khaneghah AM, Akbarirad H. The effects of amplitudes ultrasound-assisted solvent extraction and pretreatment time on the yield and quality of Pistacia khinjuk hull oil. J Oleo Sci. 2016;65(9):733–738. doi: 10.5650/jos.ess15252. [DOI] [PubMed] [Google Scholar]
- Hashemi SMB, Amarowicz R, Khaneghah AM, Vardehsara MS, Hosseini M, Yousefabad SHA. Kangar (Gundelia tehranica) seed oil: Quality measurement and frying performance. Journal of Food and Nutrition Research. 2017;56(1):86–95. [Google Scholar]
- Hashemi SMB, Mousavi Khaneghah A, Koubaa M, Lopez-Cervantes J, Yousefabad SHA, Hosseini SF, Karimi M, Motazedian A, Asadifard S. Novel edible oil sources: Microwave heating and chemical properties. Food Res Int. 2017;92:147–153. doi: 10.1016/j.foodres.2016.11.033. [DOI] [PubMed] [Google Scholar]
- ISO, (2000, 2006, 1999). ISO:5509, ISO:9936, ISO:12228. International Organization for Standardization, Geneva, Switzerland
- Khoddami, A., Ghazali, H.M., Yassoralipour, A., Ramakrishnan, Y., Ganjloo, A., (2011). Physicochemical characteristics of nigella seed (Nigella sativa L.) oil as affected by different extraction methods. Journal of the American Oil Chemists Society 88(4), 533–540.
- Latif S, Anwar F. Aqueous enzymatic sesame oil and protein extraction. Food Chem. 2011;125(2):679–684. [Google Scholar]
- Lee SY, Fu SY, Chong GH. Ultrasound-assisted extraction kinetics, fatty acid profile, total phenolic content and antioxidant activity of green solvents' extracted passion fruit oil. Int J Food Sci Technol. 2015;50(8):1831–1838. [Google Scholar]
- Li H, Zhang Z, He D, Xia Y, Liu Q, Li X. Ultrasound-assisted aqueous enzymatic extraction of oil from perilla seeds and determination of its physicochemical properties, fatty acid composition and antioxidant activity. Food Science and Technology. 2017;37:71–77. [Google Scholar]
- Mariod AA, Matthäus B, Ismail M. Comparison of supercritical fluid and hexane extraction methods in extracting kenaf (Hibiscus cannabinus) seed oil lipids. J Am Oil Chem Soc. 2011;88(7):931–935. [Google Scholar]
- Mehanni, A.E.S., El-Reffaei, W.H.M., Melo, A., Casal, S., Ferreira, I.M.P.L.V.O., (2017). Enzymatic extraction of oil from Balanites Aegyptiaca (Desert Date) kernel and comparison with solvent extracted oil. Journal of Food Biochemistry 41(2), e12270, 1–6.
- Mohamed A, Bhardwaj H, Hamama A, Webber C. Chemical composition of kenaf (Hibiscus cannabinus L.) seed oil. Ind Crops Prod. 1995;4:157–165. [Google Scholar]
- Nyam KL, Tan CP, Lai OM, Long K, Man YBC. Physicochemical properties and bioactive compounds of selected seed oils. LWT-Food Science and Technology. 2009;42(8):1396–1403. [Google Scholar]
- Nyam KL, Tan CP, Che Man YB, Lai OM, Long K. Physicochemical properties of Kalahari melon seed oil following extractions using solvent and aqueous enzymatic methods. Int J Food Sci Technol. 2009;44(4):694–701. [Google Scholar]
- Oliveira R, Oliveira V, Aracava KK, Rodrigues CEDC. Effects of the extraction conditions on the yield and composition of rice bran oil extracted with ethanol-A response surface approach. Food Bioprod Process. 2012;90(C1):22–31. [Google Scholar]
- Perrier A, Delsart C, Boussetta N, Grimi N, Citeau M, Vorobiev E. Effect of ultrasound and green solvents addition on the oil extraction efficiency from rapeseed flakes. Ultrason Sonochem. 2017;39:58–65. doi: 10.1016/j.ultsonch.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Racette SB, Lin X, Lefevre M, Spearie CA, Most MM, Ma L, Ostlund RE., Jr Dose effects of dietary phytosterols on cholesterol metabolism: a controlled feeding study. Am J Clin Nutr. 2010;91(1):32–38. doi: 10.3945/ajcn.2009.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranalli A, Lucera AM, Contento S, Sotiriou E. Effects of processing techniques on the natural colourings and the other functional constituents in virgin olive oil. Food Res Int. 2005;38(9):873–878. [Google Scholar]
- Rodrigues, G.d.M., Ferreira de Mello, B.T., dos Santos Garcia, V.A., da Silva, C., (2017). Ultrasound-assisted extraction of oil from macauba pulp using alcoholic solvents. Journal of Food Process Engineering 40(5), e12530, 1–8.
- Ryu, J., Kwon, S.-J., Ahn, J.-W., Jo, Y.D., Kim, S.H., Jeong, S.W., Lee, M.K., Kim, J.-B., Kang, S.-Y., (2017). Phytochemicals and antioxidant activity in the kenaf plant (Hibiscus cannabinus L.). Journal of Plant Biotechnology 44(2), 191–202.
- Samaram S, Mirhosseini H, Tan CP, Ghazali HM. Ultrasound-assisted extraction and solvent extraction of papaya seed oil: Crystallization and thermal behavior, saturation degree, color and oxidative stability. Ind Crops Prod. 2014;52:702–708. [Google Scholar]
- Samyn P, Schoukens G, Vonck L, Stanssens D, Van den Abbeele H. Quality of Brazilian vegetable oils evaluated by (modulated) differential scanning calorimetry. J Therm Anal Calorim. 2012;110(3):1353–1365. [Google Scholar]
- Sicaire A-G, Vian MA, Fine F, Carre P, Tostain S, Chemat F. Ultrasound induced green solvent extraction of oil from oleaginous seeds. Ultrason Sonochem. 2016;31:319–329. doi: 10.1016/j.ultsonch.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Tavakolpour, Y., Moosavi-Nasab, M., Niakousari, M., Haghighi-Manesh, S., Hashemi, S.M.B., Khaneghah, A.M., (2017). Comparison of four extraction methods for essential oil from Thymus daenensis subsp. Lancifolius and chemical analysis of extracted essential oil. Journal of Food Processing and Preservation 41(4): e13046.
- Tir R, Dutta PC, Badjah-Hadj-Ahmed AY. Effect of the extraction solvent polarity on the sesame seeds oil composition. Eur J Lipid Sci Technol. 2012;114(12):1427–1438. [Google Scholar]
- Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol. 2006;17(6):300–312. [Google Scholar]
- Wong YH, Lau HW, Tan CP, Long K, Nyam KL. Binary solvent extraction system and extraction time effects on phenolic antioxidants from kenaf seeds (Hibiscus cannabinus L.) extracted by a pulsed ultrasonic-assisted extraction. The Scientific World Journal 2014. Article. 2014;789346:1–7. doi: 10.1155/2014/789346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanty NAM, Marikkar JMN, Long K. Effect of varietal differences on composition and thermal characteristics of avocado oil. J Am Oil Chem Soc. 2011;88(12):1997–2003. [Google Scholar]
- Yusoff MM, Gordon MH, Ezeh O, Niranjan K. Aqueous enzymatic extraction of Moringa oleifera oil. Food Chem. 2016;211:400–408. doi: 10.1016/j.foodchem.2016.05.050. [DOI] [PubMed] [Google Scholar]
- Yusri, N.M., Chan, K.W., Iqbal, S., Ismail, M., (2012). Phenolic content and antioxidant activity of Hibiscus cannabinus L. seed extracts after sequential solvent extraction. Molecules 17(11), 12612–12621. [DOI] [PMC free article] [PubMed]
- Zhang ZS, Wang L-J, Li D, Jiao SS, Chen XD, Mao Z-H. Ultrasound-assisted extraction of oil from flaxseed. Sep Purif Technol. 2008;62(1):192–198. [Google Scholar]
- Zhang Z-S, Wang L-J, Li D, Li S-J, Özkan N. Characteristics of flaxseed oil from two different flax plants. Int J Food Prop. 2011;14(6):1286–1296. [Google Scholar]
- Zhang ZS, Li D, Zhang LX, Liu YL, Wang XD. Heating effect on the DSC melting curve of flaxseed oil. J Therm Anal Calorim. 2014;115(3):2129–2135. [Google Scholar]
- Zheng, L., Huang, X., Wang, L., Chen, Z., (2012). Physicochemical properties, chemical composition and antioxidant activity of Dalbergia odorifera T. Chen seed oil. Journal of the American Oil Chemists Society 89(5), 883–890