Abstract
In this study, the saponin-rich fractions of five individual (two Red and three Black) sea cucumbers (Apostichopus japonicus) in South Korea were investigated for their antiproliferative effect against HL-60, B16F10, MCF-7, and Hep3B tumor cell lines. The red sea cucumber saponin-rich fraction (SSC) from Jeju Island (JRe) decreased the growth of HL-60 with an IC50 value of 23.55 ± 3.40 μg/mL, which represented the strongest anticancer activity among the extracts. Further, SSC downregulated B-cell lymphoma extra-large (Bcl-xL), while upregulating, to different degrees, Bcl-2-associated X protein (Bax), caspase-9, caspase-3, PARP cleavage, and apoptotic bodies in cancer cells. Evidence for SSC inducing apoptosis via the mitochondria-mediated pathway was found. The contents of SSCs were determined using ultra high-performance liquid chromatography coupled with a quadrupole orbitrap mass spectrometry to comparatively evaluate the regional influence. In West Sea, the total SSC content of A. japonicus was 15.5 mg/g, representing the highest content, while A. japonicus in the South Sea yielded the lowest content at 8 mg/g. The major saponin constituent in SSC was identified as Holotoxin A1, which may the anti-tumor compound in A. japonicus.
Keywords: Apostichopus japonicus, Holotoxin A1, Saponin, Anticancer activity
Introduction
Sea cucumber (Apostichopus japonicus) is one of the most economically important aquaculture species in Northeast Asian countries, including South Korea, China, and Japan, with considerable edible and medicinal values (Zhang et al. 2017). A. japonicus (also referred to as Stichopus japonicus) is divided into different types, depending on body color such as red, blue, and black (Sun et al. 2010; Kan and Kijima 2002). Commercially, color differentiation is the key factor that determines the taste and price of A. japonicus. The origin of sea cucumber is important to consumers because sea cucumber quality varies with their growth environment (Zamora et al. 2018). Red sea cucumber from Jeju Island is the most valuable type in South Korea. Worldwide, sea cucumber has been used as a functional food and tonic for thousands of years.
The bioactive compounds from sea cucumber include peptides, polysaccharides, and minerals (Nishanthan et al. 2018). These compounds possess a range of pharmacological properties, including anticancer, antibacterial, and anti-inflammatory properties (Aminin et al. 2015; Bordbar et al. 2011). Saponins are secondary metabolites found in animals, including A. japonicus (Li et al. 2016), and plants, such as ginseng (Dai et al. 2020). The saponins of sea cucumber play a role in chemical defense against predators (Kalinin et al. 2008). In addition, saponins play a significant role in regulating reproduction of sea cucumber. Saponin-rich fraction in sea cucumber (SSC), such as Holotoxin A1 and Holothurin A, possess anticancer activities, and thus have been used in functional food and tonic industries (Song et al. 2017). A previous study has shown that a sulfated saponin (Frondoside A) from sea cucumber suppresses the PAK1-dependent growth of lung and pancreatic cancer cells (Nguyen et al. 2017). In another study, saponins from tropical sea cucumber collected from five locations in the Indian Ocean were qualitatively and quantitatively investigated (Van et al. 2010), and the results indicated that total SSC content differs based on where the sea cucumber is cultivated. However, to the best of our knowledge, the anticancer properties of South Korean sea cucumber have not been reported.
Cancer prevention and its effective management have become a major concern worldwide. The screening of functional foods or drugs from marine sources is one of the promising strategies for the development of pharmaceuticals. The aim of this work was to investigate the antitumor properties of various A. japonicus from South Korea.
Material and methods
Materials and apparatus
MCF-7 human breast cancer, Hep3B human liver cancer, B16F10 Mus musculus skin melanoma, HL-60 human leukemia and Vero monkey kidney epithelial cell lines were purchased from Korean Cell Line Bank (KCLB, Seoul, Korea). Roswell Park Memorial Institute media (RPMI), Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco Inc. (Grand Island, NY, USA). Acridine orange, ethidium bromide, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (Missouri, USA). All solvents used for the experiment were of the highest grade.
Collection of sea cucumber
A total of 50 A. japonicus were collected from 4 different sites in South Korea in August of 2017, and identified by Professor You-jin Jeon of Jeju National University (Jeju, South Korea). The red type A. japonicus was harvested from the coast of Jeju Island and the East Sea, and designated JRe and ERe, respectively. The black type A. japonicus was collected from the East Sea and West Sea (also known as Yellow Sea), and designated EBa and WBa, respectively. The other black type A. japonicus was collected from the South Sea (SBa). The approximate size and weight of the A. japonicus are shown in Fig. 1a. The samples were cleaned of any attached debris with tap water and we dissected from tentacles to anus with scissors, separating the body wall and viscera. The products were lyophilized and stored at − 20 °C for further analysis. The proximate composition of all the dried A. japonicus was measured following the Association of Official Analytical Chemists methods and previous study (Shah et al. 1992; Dai et al. 2019).
Fig. 1.
Collection and extraction of sea cucumbers Apostichopus japonicus. a Harvesting regions, approximate sizes, and weights of A. japonicus. b Preparation of saponin-rich fraction from A. japonicus (SSC)
Preparation of SSCs
The extraction of SSCs followed a previously reported protocol (Yang et al. 2015), shown in Fig. 1b. The A. japonicus body wall was ground into powder. Forty grams of the powder was extracted two times in 70% ethanol in a water bath at 90 °C. The mixed solutions were dried and divided into water and hexane layers, then water and chloroform layers, further partitioned between water and ethyl acetate. The water layer was continuously separated with n-butanol, and the n-butanol fraction was evaporated under vacuum.
Semi-quantitative determination of SSCs
The orcinol reaction was used to determine the differences in saponin content, following the method of Kabat and Mayer (Kabat and Mayer 1967). The mixture contained the following ingredients: SSC extracts, 60% sulfuric acid solution and 1.6% orcinol solution at the ratio of 1:7.5:1 (v/v). After incubating for 15 min at 80 °C, the mixed solution was cooled in ice water to stop the reaction, and absorbance was measured at the UV wavelength of 540 nm. A standard curve was made using a series of solutions of increasing concentration, by diluting with a d-xylose stock solution (10 mg/mL).
Ultra-high-performance liquid chromatography equipped with a quadrupole orbitrap mass spectrometer (UHPLC/Q-Orbitrap MS) analysis
Each sample was separated by liquid chromatography (Dionex U3000 UHPLC, CA, USA) using a Syncronis C18 column (2.1 mm × 100 mm, 1.7 μm) at 35 °C, with mobile phase A (0.1% formic acid in water, v/v) and B (acetonitrile). The gradient was as follows: 0–10 min, 25–30% B; 10–20 min, 30–50% B; 20–30 min, 50–70% B; 30–40 min, 70–100% B. The post-run time was 5 min. The flow rate was set at 0.2 mL/min, and 5 μL of filtered sample (0.22 μm) was injected for analysis.
The MS conditions (Thermo Fisher Scientific Q-Orbitrap mass spectrometer, CA, USA) were optimized as follows: mass resolution of 70,000 FWHM; microscans of 1; CID of 0.0 eV; AGC target level of 1.0 E6; IT (injection time) of 200 ms; Full Ms screening mode. Scan range of m/z 200–2500. All the mass spectrometry data were acquired in the negative-ion mode.
Cell culture
B16F10, MCF-7 and Hep3B cells were maintained in DMEM, Vero and HL-60 cells were cultured in RPMI; both medium contained 10% FBS and 1% penicillin–streptomycin mixture. All cells were cultured at 37 °C with 5% CO2 humidified atmosphere. The cells were seeded at the density of 1 × 105 cells/mL in 96-well culture plates. The cells were treated with each sample at various concentrations 24 h after seeding. The cell survival rate was evaluated by MTT assay 48 h after seeding.
Apoptosis analysis by flow cytometry
Apoptotic cells were analyzed by a flow cytometer system following Annexin V/7-AAD staining (Sigma-Aldrich). JRe and WBa were applied with increasing concentrations (12.5, 25, 50 μg/mL) to HL-60 cells for 24 h. Cells were collected and suspended in binding buffer, then stained with 7-AAD and Annexin V. Data were analyzed using Cell Quest Pro software (BD Biosciences, San Jose, CA, USA).
Apoptotic and necrotic body formation
The acridine orange-ethidium bromide staining protocol identifies early, late apoptotic and necrosis cells (Bagheri et al. 2018). HL-60 cells were seeded at the concentration of 1 × 105 cells/mL in 24-well culture plates and treated with different amounts of JRe and WBa. After 24 h, 10 μL of 100 μg/mL acridine orange-ethidium bromide was added into each well. Cells were stained for 10 min. The double stain was washed twice using PBS and images were captured by a fluorescence microscope (CoolSNAP-Pro color digital camera Meyer Instruments, Inc., Houston, TX, USA).
Western blot analysis
HL-60 cells were seeded at the concentration of 2 × 105 cells/mL in single culture plates, incubated for 24 h, treated and cells were collected after 24 h. Cells were homogenized using lysis buffer and centrifuged to remove the pellet, and proteins in the supernatants were measured using a BCA protein kit (Thermo Fisher, MA, USA). Polyacrylamide gels (12%) were loaded with 30 μg of proteins from each sample treated with lysis buffer. Protein bands were separated electrophoretically then transferred onto nitrocellulose membranes. The membranes were blocked and incubated with primary antibodies followed by secondary antibodies. The blots were treated by a chemiluminescent substrate (Cyanagen Srl, Bologna, Italy) and the fluorescence was imaged using a FUSION SOLO Vilber Lourmat system (FUSION, Paris, France) (Fernando et al. 2018). Band intensities were measured using the Image J program (developed at the US National Institutes of Health and available at https://rsb.info.nih.gov/nih-image).
Statistical analysis
All the results are presented as mean ± standard error (SE) with three independent experiments (n = 3). Statistical analyses were carried out using IBM SPSS Statistics 2.0 software (IBM Corp., Armonk, NY, USA). Student’s t-test (*p < 0.05 and **p < 0.01) was used to analysis the means of parameters of significant differences.
Results and discussion
Proximate compositions of A. japonicus
The proximate compositions of all dried A. japonicus are shown in Table 1. Moisture content ranged from 3.87 to 7.26%, ash content from 33.51 to 40.41%, protein content from 39.63 to 48.39%, carbohydrate content from 3.64 to 12.72%, and lipid content from 4.07 to 7.2%. Among five A. japonicus, JRe showed highest moisture (7.26%), protein (48.39%), and lipid contents (7.2%). ERe showed the highest ash content (40.41%), and SBa showed the highest carbohydrate content (12.72%).
Table 1.
The proximate composition of dried A. japonicus
| Carbohydrate (%) | Protein (%) | Lipid (%) | Ash (%) | Moisture (%) | |
|---|---|---|---|---|---|
| JRe | 3.64 ± 0.01 | 48.39 ± 2.8 | 7.2 ± 0.05 | 33.51 ± 1.8 | 7.26 ± 0.05 |
| ERe | 10.58 ± 0.02 | 41.07 ± 5.5 | 4.07 ± 0.01 | 40.41 ± 1.9 | 3.87 ± 0.02 |
| EBa | 7.59 ± 0.01 | 42.96 ± 3.6 | 4.78 ± 0.05 | 37.86 ± 1.8 | 6.81 ± 0.09 |
| WBa | 10.18 ± 0.02 | 42.44 ± 4.9 | 5.05 ± 0.04 | 37.11 ± 1.4 | 5.22 ± 0.07 |
| SBa | 12.72 ± 0.03 | 39.63 ± 2.6 | 6.66 ± 0.02 | 34.42 ± 1.5 | 6.57 ± 0.04 |
Semi-quantitative determination and UHPLC/Q-Orbitrap MS analysis of SSCs
A semi-quantitative method was used to measure the total saponin content of the different sea cucumber types. This complementary method detected saponins based on their structures, while also measuring SSC content. The spectrophotometric measurements were converted to milligram equivalents of saponins, based on the standard curve and wet weight.
Saponin content was quantified through measurement of yield and by orcinol reaction. The results obtained from different production areas are shown in Table 2. In the West Sea, the total SSC content of WBa was 15.5 mg/g, which was the highest amount measured in all collected areas, whereas the total SSC content of SBa was 8 mg/g. Table 2 lists the total SSCs from the two red sea cucumber-producing areas in South Korea, from the south to the north region. These places (JRe and ERe) had a total SSC content of 11.9 and 11.5 mg/g, respectively. Black sea cucumber from the east to the west locations, EBa and WBa, showed a total SSC content of 13.2 and 15.5 mg/g, respectively. Black A. japonicus was collected from the South Sea (SBa), and showed the lowest total SSC content of 8 mg/g. The two quantitative methods (yield and orcinol reaction) gave slightly different orders of total SSC content (Table 2). Hence, we consider that SSC content differed according to the sea cucumber-growing area. Moreover, SSC content differed according to the color of sea cucumber. The representative total ion current (TIC) chromatograms of LC–MS for JRe and WBa are shown in Fig. 2a, b.
Table 2.
Saponin contents of sea cucumbers measured by the orcinol reaction and yield
| JRe | ERe | EBa | WBa | SBa | |
|---|---|---|---|---|---|
| Yield (mg/g) | 11.9 ± 0.1 | 11.5 ± 0.2 | 13.2 ± 0.2 | 15.5 ± 0.2 | 8.0 ± 0.1 |
| Orcinol reaction (mg glycoside/g) | 0.12 ± 0.09 | 0.13 ± 0.08 | 0.17 ± 0.07 | 0.19 ± 0.09 | 0.05 ± 0.03 |
Fig. 2.

Representative total ion current chromatograms (TICs) of SSCs obtained from a JRe and b WBa, and the fragmentation pattern of the [M-H]− ion of Holotoxin A1 (m/z 1391.6) c in UHPLC/Q-Orbitrap MS
The sugar chain of saponins are composed mainly of d-xylose (Xyl), d-quinovose (Qui), 3-O-methyl-d-glucose (MeGlc), 3-O-methyl-d-xylose, and d-glucose (Glc). The nomenclature for saponin fragments follows the conventions of Domon and Costello (Domon and Costello 1988). The [M-H]− ion (m/z 1391.6) and adduct ion [M-H-CO2]− ion (m/z 1347.6) were determined, and the mass spectrum is shown in Fig. 2c. In Holotoxin A1, the Y3α ion at m/z 1171.6, Y2α ion at m/z 1039.5, and Y1α ion at m/z 893.5 were generated by the loss of MeGlc (176 Da), Xyl (132 Da), and Qui residues (146 Da), respectively. The Y2β ion at m/z 1153.6 was produced by the loss of H2O (18 Da) and MeGlc (176 Da). The Y1β ion at m/z 1009.5 was produced by the loss of CO2 (44 Da), MeGlc (176 Da), and Glc (162 Da). The ion at m/z 701.4 was produced by [M-H-H2O-MeGlc-Xyl-MeGlc-Glc]−. The Y0 ion at m/z 423.5 represents the aglycon moiety produced by losses of all sugar residues at the C3 position. Thus, with ion m/z 1347, 1171, 1153, 1039, 1009, 893, 701, and 423 peaks, we considered the mass spectrum in Fig. 2c to be that of Holotoxin A1. Table 3 summarizes the results of SSC analysis, showing that the identified compounds matched well with previously reported properties (Yang et al. 2015).
Table 3.
The molecular ion and MS/MS product ions in five saponin-rich fractions
| No | Identity | Molecular formula | JRe | ERe | EBa | WBa | SBa | [M-H]− (m/z) mass accuracy < 10−5 | MS/MS fragment ion (m/z) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cladoloside B | C59H92O26 | − | − | + | + | − | 1215.5805 | 1171.8, 995.6, 863.6, 701.5, 555.4, 423.4 |
| 2 | Holotoxin A1 | C66H104O31 | + | + | + | + | + | 1391.6488 | 1347.6, 1171.6, 1153.6, 1039.5, 1009.5, 893.5, 701.4, 423.4 |
| 3 | Echinoside A | C54H87O26SNa | + | + | + | + | + | 1205.5031 | 1183.5, 1085.2, 909.3, 747.4 |
| 4 | Holothurin A | C54H85O27SNa | + | + | + | + | + | 1219.4823 | 1201.8, 1093.6, 1039.8, 893.6, 917.6, 785.5, 423.4 |
| 5 | Holothurin A1 | C54H87O27SNa | + | + | − | + | − | 1221.4980 | 1203.8, 1095.6, 1041.7, 895.6, 733.6, 787.5, 425.3 |
“−” not detected, “+” detected
Cytotoxicity and anticancer effects of SSCs
Figure 3 shows the effect of five species of SSCs on the cell death rate of Vero (kidney epithelial), HL-60 (leukemia), B16F10 (mouse skin), MCF-7 (breast cancer), and Hep3B (liver cancer) cells. All SSCs reduced the survival rates of HL-60, B16F10, Hep3B, and MCF-7 cells in a concentration-dependent manner. Vero cells, representing a normal cell line, showed higher survival rate after treatment with SSC at each concentration than that of the four malignant cell lines. Among SSCs, JRe had the strongest antiproliferative effects with IC50 values of 23.55 ± 3.40 and 46.54 ± 2.75 μg/mL for HL-60 and B16F10 cells, respectively. In addition, the survival rates of Vero cells were above 85% following treatment with SSC at all concentrations. Among five A. japonicus, WBa had the highest SSC content. Hence, JRe and WBa were selected for further investigation.
Fig. 3.
Percentage of viable cells as a measure of cell proliferation after treatment with different concentrations of five SSCs. a Vero cells, b HL-60, c B16F10, d Hep3B and e MCF-7 cells. Experiments were performed in triplicate (n = 3) and the data are expressed as mean ± SE. *p < 0.05, **p < 0.001
In Fig. 4a, HL-60 cells were treated with JRe, resulting in the induction of apoptotic body formation. Likewise, WBa induced cell apoptosis, showing that SSCs induced apoptosis in multiple cancer cells types in a dose-dependent manner.
Fig. 4.
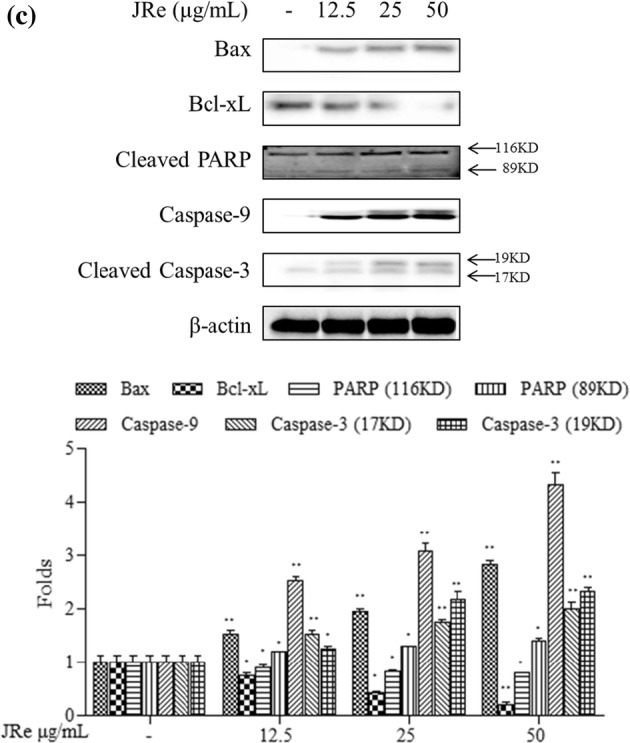
Effects of JRe and WBa on the induction of apoptosis in HL-60 cells. a Flow cytometry analysis of JRe and WBa on the nuclear morphology in HL-60 cancer cell. JRe and WBa were treated with 12.5, 25, 50 μg/mL concentrations to HL-60 cells for 24 h. Cells were stained with 7-AAD and Annexin V. b Fluorescent microscopic evaluation of the effects of JRe and WBa on the nuclear morphology in HL-60 cancer cell. SSC treatment was performed using 12.5, 25 and 50 μg/mL sample concentrations. Observations were made 24 h after treatment by the acridine orange/ethidium bromide double-staining method. c Western blot analyses of JRe induced HL-60 cells for measuring the expression of Bax, Bcl-xL, cleaved PARP, caspase-9, and cleaved caspase-3. Experiments were performed in triplicate (n = 3) and the data are expressed as mean ± SE. *p < 0.05, **p < 0.001. Results are reproducible based on three independent determinations
Apoptosis morphology of HL-60 cell line due to JRe and WBa
Viable cells had a green homogeneous nucleus, whereas early apoptotic cells were characterized by fragmented green patches. Late apoptotic cells were characterized by both green and orange or orange particulate matter, and necrotic cells were characterized by damaged cytoplasmic membranes, appearing homogenously red (Baskić et al. 2006). Figure 4b shows the effects of JRe and WBa on HL-60 cells, which were characterized by increasing apoptotic body formation in a dose-dependent manner. Small amounts (12.5 μg/mL) of both JRe and WBa induced early apoptotic body formation in cells at 24 h after treatment, with a small number of cells in the late apoptotic and necrotic stages. At the medium and high concentrations, an increasing number of late apoptotic bodies were observed, as well as a small number of necrotic cells.
Apoptosis pathway regulated by JRe
Western blot analysis showed that increasing concentrations of JRe up to 50 μg/mL induced the expression of apoptotic and anti-apoptotic factors, as shown by a twofold increase in Bcl-2-associated X protein (Bax) expression and a threefold decrease in B-cell lymphoma extra-large (Bcl-xL) expression, compared to that in the untreated group. In Fig. 4c, cleavage of the PARP protein was also observed, with the expression of 116 kDa PARP decreasing as the expression of cleaved 89 kDa fragment increased in a dose-dependent manner. Moreover, JRe increased the expression of caspase-9 and caspase-3 in a concentration-dependent manner.
Currently, the most frequently harvested species of sea cucumber in East Asia is A. japonicus, which has been evaluated for its secondary metabolites, which possess various bioactivities (Chen 2003). Previous studies reported that the anticancer property of SSCs is mediated by cytotoxic effects involving the molecular structures of saponins (Kim and Himaya 2012). For instance, the antitumor effect of an n-butanol fraction purified from red A. japonicus on HL-60, HT-29, and HepG2 cancer cells was evaluated (Park et al. 2011). LC–MS method is an effective tool to identify saponins (Dai et al. 2020). This study aimed to obtain and purify SSCs from different types of A. japonicus from South Korea and to evaluate their anticancer properties and characterize their structural features using LC–MS analysis.
We identified five saponins (Cladoloside B, Holotoxin A1, Echinoside A, Holothurin A, and Holothurin A1) from sea cucumber using LC–MS. Their properties agree well with those of previously reported saponins (Wang et al. 2012). Recent reports specifically highlighted the antiproliferative effect of SSCs. Fan et al. reported that the SC-2 fraction of A. japonicus induces apoptosis and DNA fragmentation in HeLa cells (Fan et al. 2009). Sea cucumber (Holothuria leucospilota) saponin was reported to exert an antiapoptotic activity by increasing the expression of Bax while decreasing Bcl-2 expression (Soltani et al. 2015). Moreover, the anticancer property of holothurian saponin on breast cancer cells has been reported (Kovalchuk et al. 2006). In previous years, there have been a series of studies screening the anticancer properties of saponins. Holotoxin A1, in particular, exerts an anticancer effect against K562 and HL-60 cancer cells (Yun et al. 2018). However, the present study is the first report of the antitumor activities of saponin-rich fractions of different types of A. japonicus obtained from different regions in South Korea.
One of the main SSCs, Holotoxin A1, was found in A. japonicus collected from various areas in the northern part of the Yellow Sea (also named West Sea) (Wang et al. 2012). Holotoxin A1 content in A. japonicus from the Yellow sea was higher than that in sea cucumber grown in the other areas; moreover, the production pattern from south to north was low to high (Yang et al. 2015). Holotoxin A1 may be the key anticancer compound in black and red sea cucumber. Our data showed that the content of biological compounds varied among the different types of A. japonicus even though they all belong to the same species. The regions and environments in which they were grown were the main determinants of the composition and amount of saponins in A. japonicus, making distinctions among the sea cucumber types relatively easy. In the wild, red A. japonicus inhabits offshore sandy beds, whereas black and blue A. japonicus are more likely to be found in the sandy-muddy bottom of inshore areas (Nishimura 1995). There are many possible external factors, such as ambient temperature, light, and salinity that may have influenced the metabolism of sea cucumber. Therefore, the effect of sea cucumber growth environment on their metabolism could be an interesting topic for further studies.
Saponins from black and red sea cucumber were extracted and used to treat cancer cell lines, and the results showed that red sea cucumber had significantly higher antiproliferative effects against HL-60 and B16F10 cells than black sea cucumber. The results of this study elucidated the significant role of SSCs in apoptotic body formation in cancer cells. During apoptosis, the chromatin condenses, the cell shrinks, the membrane blebs, and finally the cell disassembles into vesicles enclosed by membranes (Thornberry and Lazebnik 1998). Our results showed that JRe and WBa regulated apoptosis in HL-60 cells in a dose-dependent manner, proving the hypothesis that the antiproliferative activity of JRe and WBa against HL-60 cells was mediated by regulating apoptosis, which is a process that operates through a series of signaling cascades. The apoptotic factors belonging to the Bcl-2 protein family and caspases stay dormant under inactive conditions (Barry et al. 1990). The members of the Bcl-2 protein family, including proapoptotic (Bax) and antiapoptotic (Bcl-xL) proteins, are the main apoptotic regulators (Mooney et al. 2002). In cellular mitochondria, when the voltage-dependent anion channels are disturbed by Bax, cytochrome c is released, leading to the continuous stimulation of caspases (Weng et al. 2005). The upregulation of Bax, coupled with the downregulation of Bcl-xL, increases the ratio of Bax/Bcl-xL, which is considered to be the hallmark of apoptosis (Salakou et al. 2007). Western blotting analysis showed an increase in Bax/Bcl-xL ratio, indicating apoptosis, and this effect occurred in a dose-dependent manner. Cytochrome c and initiator caspases contribute to activate the proenzyme form of caspases. These initiator caspases include caspase-3 and caspase-9, which play a significant role in the proteolytic cleavage of the PARP protein (Jänicke et al. 1998). The increased Bax/Bcl-xL ratio caused cytochrome c to be released, and increased the expression of caspase-3 and -9. The observation of PARP protein cleavage products agrees well with our analysis. The hypothesis was proven in the SSC bioactivity study, in which the anticancer properties of SSC via the mitochondrial-mediated apoptosis pathway were demonstrated in four cancer cell lines.
Conclusion
Variations in the anticancer effects of different types of A. japonicus may be due to the differences in their biological compositions. Growth environment could affect the biological composition of these sea cucumbers. Moreover, the present study also showed that JRe acts as the one of the most promising anticancer agents among different sea cucumbers. The specific growth environment of JRe needs to be explored in depth using meteorological equipment, and the further anticancer mechanism JRe also needs to be evaluated for further clinical applications.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2019R1A6A1A03033553) and a research grants from the Korea Institute of Ocean Science and Technology (PE99822).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Soo-Jin Heo, Email: sjheo@kiost.ac.kr.
You-Jin Jeon, Email: youjin2014@gmail.com.
References
- Aminin DL, Menchinskaya ES, Pisliagin EA, Silchenko AS, Avilov SA, Kalinin VI. Anticancer activity of sea cucumber triterpene glycosides. Mar Drugs. 2015;13(3):1202–1223. doi: 10.3390/md13031202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri E, Hajiaghaalipour F, Nyamathulla S, Salehen N. Ethanolic extract of Brucea javanica inhibit proliferation of HCT-116 colon cancer cells via caspase activation. RSC Adv. 2018;8(2):681–689. doi: 10.1039/c7ra09618f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MA, Behnke CA, Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990;40(10):2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- Baskić D, Popović S, Ristić P, Arsenijević NN. Analysis of cycloheximide-induced apoptosis in human leukocytes: fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol Int. 2006;30(11):924–932. doi: 10.1016/j.cellbi.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Bordbar S, Anwar F, Saari N. High-value components and bioactives from sea cucumbers for functional foods—a review. Mar Drugs. 2011;9(10):1761–1805. doi: 10.3390/md9101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Overview of sea cucumber farming and sea ranching practices in China. SPC beche-de-mer Inf Buln. 2003;18:18–23. [Google Scholar]
- Dai Y-L, Qiao M-D, Yu P, Zheng F, Yue H, Liu S-Y. Comparing eight types of ginsenosides in ginseng of different plant ages and regions using RRLC-Q-TOF MS/MS. J Ginseng Res. 2020;44(2):205–214. doi: 10.1016/j.jgr.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y-L, Jiang Y-F, Lee HG, Jeon Y-J, Kang M-C. Characterization and screening of anti-tumor activity of fucoidan from acid-processed hijiki (Hizikia fusiforme) Int J Biol Macromol. 2019;139:170–180. doi: 10.1016/j.ijbiomac.2019.07.119. [DOI] [PubMed] [Google Scholar]
- Domon B, Costello CE. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj J. 1988;5(4):397–409. [Google Scholar]
- Fan T, Yuan W, Cong R, Yang X, Wang W, Jing Z. Studies on the purification of water-soluble holothurian glycosides from Apostichopus japonicus and their tumor suppressing activity. Acta Pharm Sin. 2009;44(1):25–31. [PubMed] [Google Scholar]
- Fernando IS, Lee WW, Jayawardena TU, Kang M-C, Ann Y-S, Ko C-I, et al. 3β-Hydroxy-Δ5-steroidal congeners from a column fraction of Dendronephthya puetteri attenuate LPS-induced inflammatory responses in RAW 264.7 macrophages and zebrafish embryo model. RSC Adv. 2018;8(33):18626–18634. doi: 10.1039/c8ra01967c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273(16):9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- Kabat E, Mayer M (1967) Immunological and immunochemical methodology. In: Experimental immunochemistry, chapter1, pp 22–97
- Kalinin V, Aminin D, Avilov S, Silchenko A, Stonik V. Studies in natural product chemistry (bioactive natural products) The Netherlands: Elsevier Science; 2008. [Google Scholar]
- Kan M, Kijima A. Quantitative and qualitative evaluation on the color variation of the Japanese sea cucumber Stichopus japonicus. Aquac Sci. 2002;50(1):63–69. [Google Scholar]
- Kim S-K, Himaya S. Triterpene glycosides from sea cucumbers and their biological activities. Adv Food Nutr Res. 2012;65:297–319. doi: 10.1016/B978-0-12-416003-3.00020-2. [DOI] [PubMed] [Google Scholar]
- Kovalchuk S, Kozhemyako V, Atopkina L, Silchenko A, Avilov S, Kalinin V, et al. Estrogenic activity of triterpene glycosides in yeast two-hybrid assay. J Steroid Biochem Mol Biol. 2006;101(4):226–231. doi: 10.1016/j.jsbmb.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Li S, Wang Y, Jiang T, Wang H, Yang S, Lv Z. Absorption and transport of sea cucumber saponins from Apostichopus japonicus. Mar Drugs. 2016;14(6):114. doi: 10.3390/md14060114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney L, Al-Sakkaf K, Brown B, Dobson P. Apoptotic mechanisms in T47D and MCF-7 human breast cancer cells. Br J Cancer. 2002;87(8):909–917. doi: 10.1038/sj.bjc.6600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BCQ, Yoshimura K, Kumazawa S, Tawata S, Maruta H. Frondoside A from sea cucumber and nymphaeols from Okinawa propolis: natural anti-cancer agents that selectively inhibit PAK1 in vitro. Drug Discov Therap. 2017;11(2):110–114. doi: 10.5582/ddt.2017.01011. [DOI] [PubMed] [Google Scholar]
- Nishanthan G, Kumara P, de Croos M, Prasada D, Dissanayake D. Effects of processing on proximate and fatty acid compositions of six commercial sea cucumber species of Sri Lanka. J Food Sci Technol. 2018;55(5):1933–1941. doi: 10.1007/s13197-018-3111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S. Guide to seashore animals of Japan with color pictures and keys. Osaka: Hoikusha; 1995. [Google Scholar]
- Park S-Y, Lim HK, Lee S, Cho SK, Park S, Cho M. Biological effects of various solvent fractions derived from Jeju Island red sea cucumber (Stichopus japonicus) J Korean Soc Appl Biol Chem. 2011;54(5):718–724. [Google Scholar]
- Salakou S, Kardamakis D, Tsamandas AC, Zolota V, Apostolakis E, Tzelepi V, et al. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In Vivo. 2007;21(1):123–132. [PubMed] [Google Scholar]
- Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, et al. Analytical methods validation: bioavailability, bioequivalence, and pharmacokinetic studies. J Pharm Sci. 1992;81(3):309–312. doi: 10.1007/BF03189968. [DOI] [PubMed] [Google Scholar]
- Soltani M, Parivar K, Baharara J, Kerachian MA, Asili J. Putative mechanism for apoptosis-inducing properties of crude saponin isolated from sea cucumber (Holothuria leucospilota) as an antioxidant compound. Iran J Basic Med Sci. 2015;18(2):180. [PMC free article] [PubMed] [Google Scholar]
- Song S, Zhang L, Cao J, Xiang G, Cong P, Dong P, et al. Characterization of metabolic pathways and absorption of sea cucumber Saponins, Holothurin A and Echinoside A, in vitro and in vivo. J Food Sci. 2017;82(8):1961–1967. doi: 10.1111/1750-3841.13759. [DOI] [PubMed] [Google Scholar]
- Sun X-J, Li Q, Kong L-F. Comparative mitochondrial genomics within sea cucumber (Apostichopus japonicus): Provide new insights into relationships among color variants. Aquaculture. 2010;309(1):280–285. [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281(5381):1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Van S, Gerbaux P, Flammang P. Qualitative and quantitative saponin contents in five sea cucumbers from the Indian Ocean. Mar Drugs. 2010;8(1):173–189. doi: 10.3390/md8010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhang H, Yuan W, Gong W, Tang H, Liu B, et al. Antifungal nortriterpene and triterpene glycosides from the sea cucumber Apostichopus japonicus Selenka. Food Chem. 2012;132(1):295–300. doi: 10.1016/j.foodchem.2011.10.080. [DOI] [PubMed] [Google Scholar]
- Weng C, Li Y, Xu D, Shi Y, Tang H. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. J Biol Chem. 2005;280(11):10491–10500. doi: 10.1074/jbc.M412819200. [DOI] [PubMed] [Google Scholar]
- Yang J, Wang Y, Zhang R, Jiang T, Lv Z. Determination of the triterpene glycosides in sea cucumbers by liquid chromatography with evaporative light scattering and mass spectrometry detection. J Sep Sci. 2015;38(7):1117–1122. doi: 10.1002/jssc.201401253. [DOI] [PubMed] [Google Scholar]
- Yun S-H, Sim E-H, Han S-H, Han J-Y, Kim S-H, Silchenko AS, et al. Holotoxin A1 induces apoptosis by activating acid sphingomyelinase and neutral sphingomyelinase in K562 and human primary leukemia cells. Mar drugs. 2018;16(4):123. doi: 10.3390/md16040123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora L-N, Yuan X, Carton A-G, Slater M-J. Role of deposit-feeding sea cucumbers in integrated multitrophic aquaculture: progress, problems, potential and future challenges. Rev Aquac. 2018;10(1):57–74. [Google Scholar]
- Zhang K, Hou H, Bu L, Li B, Xue C, Peng Z, et al. Effects of heat treatment on the gel properties of the body wall of sea cucumber (Apostichopus japonicus) J Food Sci Technol. 2017;54(3):707–717. doi: 10.1007/s13197-017-2509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]





