Abstract
Changes in physicochemical properties, isoflavone composition, antioxidant activities, and microbial count of cheonggukjang during the manufacturing process were investigated. During fermentation, isoflavone glucosides are converted to isoflavone aglycones. After fermentation, the increased isoflavone aglycone content was determined. The total phenolic and total flavonoid content, as well as antioxidant activities, significantly increased in cheonggukjang at fermentation process. In proximate composition, fermented soybeans had the highest crude protein content. A gradual increase in the browning index and pH values was observed from the primary processing procedure to fermentation. The total bacterial count increased with each manufacturing step, except for the steamed step. The traditional processing methods for cheonggukjang from raw soybean induced several changes in chemical composition. In addition, the change of isoflavone glucosides to isoflavone aglycones during fermentation could enhance their bioavailability and antioxidant properties.
Keywords: Antioxidant activities, Cheonggukjang, Isoflavones composition, Physicochemical properties
Introduction
Soybean are rich in dietary isoflavones and the soy isoflavones are classified into two types: glucones and aglycones. It has been observed that 99% of the isoflavones in soybeans exist in the form of glucones, but aglycones had higher bio-availability than glucones (Kulling et al. 2001; Mariusz et al. 1999). And the bioavailability of isoflavone aglycones is higher than its glyosidic forms. The action of microorganisms during the fermentation process, which facilitate the conversion of glucones into aglycones (Coward et al. 1998). Soybean manufacturing process could alter the constitution of bioactive compounds as well. Generally, the functional compound in soybean products has been widely reported to have anti-cholesterol and cancer-preventing functions. Soybean products were obtained by kinds of pretreatment, such as soaking, boiling, roasting, streaming and fermentation. And this pretreatment could effectively enhance their flavor and palatability and improve the bioavailability of bioactivates by inactivating anti-nutritional components (Akande et al. 2010). But physicochemical processes with soaking, steaming and fermentation can affect the stability of the nutrients and bioactive in soybeans as well (Muratsugu et al. 2017). Akilligolu and Sibel (2010) was observed that the isoflavones content in soy milk was significant decrease during soaking, as well as significant change in chemical composition, physicochemical properties and bioactive compounds. Furthermore, fermentation plays a critical role in the unique flavor of cheonggukjang, as well in the enhancement of its bioactive activities. It has been reported that soybean products have many functional compounds, including those with antioxidant and antibiotic activities.
Fermented soybean products have a long history in Asian countries. such as tempeh, natto and soy sauce are richer in isoflavone aglycones than unfermented soybean products. One of these products, cheonggukjang, is manufactured by short term fermentation of soybean. It is one of the most popular traditional fermented foods in Korea. Previous studies have focused on the fermentation temperature and time to find the optimal fermentation conditions. Furthermore, the application of starter cultures for soybean products to obtain a uniform quality fermented soybean was reported (Jeon et al. 2018). Traditionally, the manufacturing of cheonggukjang includes soaking, steaming fermentation and molding.
Most previous studies focused on the effect of the quality properties of cheonggukjang. However, few studies address the effects of manufacturing steps on the physicochemical qualities and bioactivity of cheonggukjang. Thus, the aim of our study was to investigate the physicochemical properties, antioxidant activities and isoflavone composition. Monitoring changes in the quality and functional compounds of cheonggukjang during fermentation could provide a theoretical foundation to reduce the loss of nutrients during the production process and produce high-quality cheonggukjang.
Materials and methods
Materials
Raw, soaked, steamed and fermented soybeans and cheonggukjang were obtained from a local cheonggukjang factory (Jangseong, Chonnam, South Korea). All other reagents were analytical grade. Manufacture of cheonggukjang.
Steps of the preparation of cheonggukjang were as follows:
Raw soybeans were washed by tap water and soaked at room temperature for 12 h at 25 ± 1 °C.
They were steamed using an autoclave (JSAT-60, JS Research Inc., Gong-ju, S. Korea) for 45 min at 110 °C, after this the soybeans were left to stand for 1 h at 25 ± 1 °C to cool down (Park, et al. 2008).
After, the cooled soybeans were moved to the fermentation room for 72 h at 46 ± 1 °C.
Then, they were moved to the mixing room and 4% salt was added, workers continued to mold and package until cheonggukjang was obtained.
The samples were raw soybeans and cheonggukjang product. Samples were also collected from each manufacturing step, including soaking, steaming and fermentation.
Analytical methods
Proximate analysis
Moisture content was determined by drying the samples at 105 °C to a constant weight (AOAC 2005). Crude protein content was calculated by converting the nitrogen content as determined by the Kjeldahl method (AOAC 2005). Crude fat content was determined by using the method described by Bligh and Dyer (1959). The crude ash content was determined by using the method of Ludorff and Meyer (1973).
pH
Four grams of samples with 10 volumes of distilled water (w/v) were homogenized (T25-Sl, Janke and Kunkel GmbH and Co KG, Breisgau, Germany) and centrifuged using a centrifuge (UNION32R plus, Hanil Scientific Co., Lrd., Incheon, Korea) at 4000 rpm for 15 min at 4 °C. The pH of supernatant was measured using a pH meter (EF-7732, Istek, Seoul, S. Korea) (Ko et al. 2014).
Color
The color values were determined using a colorimeter (CR-400, MINOLTA, Osaka, Japan). The results were expressed as color values, L* (lightness), a* (redness), and b* (yellowness). The measurement of color value was repeated in triplicate.
High-performance liquid chromatography (HPLC) of isoflavones
Isoflavone composition was analyzed quantitatively by HPLC (LC-20Avp Shimadzu Co., JAPAN). A sample (20 µL) of 80% methanol extract was injected onto a Water Spherisorb column (25 cm × 4.6 mm, 5 µm) and the column temperature was set to 40 °C. Isoflavones were detected by monitoring the elution at 260 nm using a UV–VIS detector. Identification of the isoflavones was carried out by comparing their retention times to those of standards. The mobile phase was composed of 0.1% acetic acid (solution A) and 100% methanol (solution B). The gradient conditions were as follows: 0–7 min, 34% B; 15 min, 38% B; 35 min, 65% B; 36 min, 34% B. Solvent flow rate was maintained at 1.0 mL/min.
Total phenolic content (TPC)
The TPC of the samples was determined using the Folin–Ciocalteu reagent based on the method by Lee et al. (2012) with minor modifications. Briefly, a 500 µL sample (1/10 with distilled water) mixed 250 µL 2 N Folin-Ciocalteu reagent. After mixing and standing at room temperature for 5 min, 500 µL of 7.5% Na2CO3 solution was added, and the mixture was allowed to stand at room temperature in the dark for 30 min. The absorbance of the solution was measured at 765 nm. The quantification was prepared using the linear regression equation of the gallic acid standard curve.
Total flavone content (TFC)
TFC was measured by the aluminum chloride-based colorimetric assay (Hajimahmoodi et al. 2008). A sample of 500 µL (1/10 with distilled water) was mixed with 70 µL of 5% NaNO2. After mixing and standing at room temperature for 5 min, 150 µL of 10% AlCl3 solution was added and left to stand room temperature for 5 min. Then, 500 µL of 1 M NaOH was added with 1.30 ml water. The absorbance of the solution was measured at 415 nm.
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activities
The DPPH radical scavenging activities of the samples were determined according to the method by Brand-Williams et al. (1995). Briefly, the sample solution (1/10 with distilled water) were allowed to react with 2580 µL of the DPPH solution (DPPH solution were made by dissolving 24 mg DPPH with 100 ml methanol and then storing at − 20 °C) for 30 min in the dark at 24 ± 1 °C. Then, the absorbance was measured at 517 nm using a UV spectrophotometer (Optizrn 2120UV, Daejeon, Korea). As a control, distilled water was used in place of the sample solution. The standard curve was linear between 0 and 800 µM Trolox. Result were expressed in µM TE/g DW (dry weight).
Ferric reducing antioxidant power (FRAP)
The FRAP assay of the samples was used based on a published method (Benzia and Strain 1996). The freshly FRAP reagent was prepared by mixing acetate buffer (100 mL, 300 mM, pH 3.6), TPTZ (2,4,6-tripyridyl-s-trizazine) solution (10 mL, 1 mM TPTZ in 40 mM HCl), and FeCl3·H2O (10 mL, 20 mM) solution in a ratio of 10:1:1 at 36 ± 1 °C before using. To perform the assay, the sample solution was allowed to react with 2580 µL of the FRAP solution for 30 min in the dark at 24 ± 1 °C. The absorbance was measured at 593 nm against a blank using a UV spectrophotometer (Optizrn 2120UV, Daejeon, Korea). The standard curve was linear between 30 and 1000 µM Trolox. Result were expressed in µM TE/g DW (dry weight).
Statistical analysis
The results are presented as the mean ± standard deviation. Each was performed in three replicates. Data were analyzed statistically by ANOVA and Duncan’s multiple range tests using SPSS 23.0 for windows software (SPSS Inc., Chicago, Illinois, USA). The level of significance p < 0.05 was considered.
Results and discussion
Proximate compositions
The proximate composition of cheonggukjang during manufacturing is shown in Table 1. No significant changes in moisture, protein and ash content between soaked and steamed soybeans were observed. High values of protein and fat content were observed in the fermented step compared to other steps. This result might be due to loss of moisture content and enhancement of soluble protein content, which was caused by the action of a protease enzyme produced by mold during fermentation. The crude ash content increased in the final product of cheonggukjang, which was 14.13 mg/100 g. Similar results have been reported during the manufacturing of doenjang (soy paste) (Son et al. 2017). This result could be due to adding salt at the final step of cheonggukjang manufacturing. Each step has an effect on the proximate composition of cheonggukjang. In particular, the fermentation step has a positive effect on protein and fat content.
Table 1.
Changes in proximate composition of cheonggukjang during manufacturing (mg/100 g)
| Moisture | Protein | Ash | Fat | |
|---|---|---|---|---|
| Raw soybean | 24.12 ± 0.75d | 18.83 ± 0.07c | 7.94 ± 0.73b | 20.70 ± 0.63b |
| Soaked | 57.68 ± 0.27a | 15.67 ± 0.74d | 3.39 ± 0.32cd | 18.91 ± 0.30bc |
| Steamed | 56.81 ± 0.33a | 13.74 ± 0.77d | 3.86 ± 0.25c | 15.37 ± 0.49d |
| Fermented | 45.88 ± 0.30d | 23.90 ± 0.75a | 2.19 ± 0.11d | 23.90 ± 0.43a |
| Product | 43.13 ± 0.32c | 20.90 ± 0.85b | 14.13 ± 0.45a | 18.36 ± 0.90c |
Values are expressed as mean ± SD (n = 3). Means in the same column with different letters are significantly different by Duncan’s multiple range test (p < 0.05)
pH, L* values and browning index
Table 2 shows the pH, L* values and browning index. There was no distinctive change in the pH values of raw, soaked and steamed soybeans, which were 6.560, 6.573, 6.560, respectively. After fermentation, the pH value increased to 6.90. This result might be related to amino acid production by the action of a microorganism (Cho et al. 2011). A significant decrease of L* values from 71.64 in raw soybeans to 40.22 in the final products and increase in browning index, was observed during manufacturing. These changes are due to the Maillard reaction in steaming and the enzymatic browning reaction of microorganisms during fermentation. The manufacturing process of cheonggukjang has a great influence on the color, which is dark during processing.
Table 2.
Changes in pH, L* values and browning index during manufacturing of cheonggukjang
| pH | L* | Browning index | |
|---|---|---|---|
| Raw soybean | 6.566 ± 0.003c | 71.64 ± 0.63a | 29.64 ± 0.59d |
| Soaked | 6.573 ± 0.008c | 64.53 ± 0.22b | 35.30 ± 0.41c |
| Steamed | 6.560 ± 0.010c | 55.07 ± 0.63c | 40.54 ± 1.76b |
| Fermented | 6.900 ± 0.000b | 42.12 ± 0.03d | 52.61 ± 0.68a |
| Product | 7.180 ± 0.000a | 40.22 ± 0.07e | 53.08 ± 1.09a |
Values are expressed as mean ± SD (n = 3). Means in the same column with different letters are significantly different by Duncan’s multiple range test (p < 0.05)
Total bacterial count
Changes in the total bacterial count of cheonggukjang during manufacturing are shown in Table 3. The bacterial count was 3.20 log CFU/g of raw soybeans. There was a slight difference in the count between the raw and soaked soybeans. The total bacterial count decreased from 3.20 to 1.51 log CFU/g after steam treatment. In addition, the total bacterial count reached 7.71 log CFU/g after fermentation, and there was no significant change in total bacterial count between the fermented and cheonggukjang product. This result might be due to the metabolic processes of microorganisms, which play an important role in the physicochemical changes during the manufacturing of cheonggukjang (Kim et al. 2011), and will affect the flavor and quality of cheonggukjang. In this study, cheonggukjang was manufactured by fermenting steamed soybeans in a natural environment where steamed soy was exposed to airborne microorganisms. Therefore, it is important to control the microorganisms which can assess the fermentation process.
Table 3.
Changes in total bacterial count during manufacturing of cheonggukjang (unit: log CFU/g)
| Total bacterial count | |
|---|---|
| Raw soybean | 3.20 ± 0.00d |
| Soaked | 4.67 ± 0.00c |
| Steamed | 1.51 ± 0.34e |
| Fermented | 7.71 ± 0.24b |
| Product | 8.56 ± 0.01a |
Values are expressed as mean ± SD (n = 3). Means in the same column with different letters are significantly different by Duncan’s multiple range test (p < 0.05)
Total phenolic and flavonoid content and antioxidant activities
The total phenolic and flavonoid content of raw, soaked, steamed and fermented soybeans, and cheonggukjang, are presented in Table 4. No significant change in TPC between soaked and steamed soybeans was observed, while there was a marked increase from 1.52 to 1.98 mg GAE/g after fermentation. The soaked and steamed soybeans showed lower TPC than raw soybeans, which might to attributed to water-soluble phenolic leaching and broken phenolic structure under high temperature during steaming (Nicoli et al. 1999). The TPC increased during fermentation. Hydroxycinnamic acid, a phenolic compound of beans, deceased during soaking, which might be related to the enzymes and metabolic activities of microorganisms. The TFC showed a similar trend to TPC during the manufacturing of cheonggukjang. This significant loss could be attributed to water-soluble phenolic leaching to water (Xu and Chang 2008), so there was the lowest flavonoid content in soaked soybeans. TFC could have also decreased due to extraction by condensed water when steamed. The significant increase in the fermentation step is supported by the fact that the bacteria in the fermentation step contribute to the production of phenols and flavonoids. In addition, soybeans might produce functional substances that are beneficial to health after fermentation. So, fermentation plays an important role in the processing of cheonggukjang.
Table 4.
Changes in antioxidant capacity, total flavone composition and total phenol composition during manufacturing of cheonggukjang
| DPPH Radical scavenging activity (μM TE/g DW) |
FRAP (μM TE/g DW) | TP (mg GAE/g DW) | TF (mg QE/g DW) | |
|---|---|---|---|---|
| Raw soybean | 151.28 ± 3.10a | 65.33 ± 2.03a | 2.41 ± 0.23a | 42.30 ± 0.32a |
| Soaked | 97.06 ± 0.98d | 17.63 ± 3.53c | 1.52 ± 0.02c | 31.06 ± 0.47d |
| Steamed | 99.88 ± 0.81cd | 38.00 ± 1.57b | 1.49 ± 0.08c | 23.66 ± 1.03c |
| Fermented | 104.24 ± 0.94c | 63.49 ± 0.12a | 1.97 ± 0.03b | 31.40 ± 1.60c |
| Product | 118.17 ± 1.49b | 67.01 ± 0.59a | 2.01 ± 0.00b | 37.07 ± 1.94b |
Values are expressed as mean ± SD (n = 3). Means in the same column with different letters are significantly different by Duncan’s multiple range test (p < 0.05)
Antioxidant activities
The antioxidant activities of the raw, soaked, steamed and fermented soybeans, and cheonggukjang, were investigated based on DPPH radical scavenging activity and FRAP values. As shown in Table 4, the DPPH radical scavenging activity and FRAP values were 54.22 µM TE/g and 47.70 µM TE/g, respectively, which was a significant decrease compared to raw soybeans. This might be caused by the loss of water-soluble phenolic compounds during the soaking process. In addition, the steamed step showed a negligible effect on DPPH radical scavenging activity, while FRAP values were significantly affected. During fermentation, the DPPH radical scavenging activity went from 99.80 to 104.24 µM TE/g and FRAP values dramatically increased from 38.00 to 63.49 µM TE/g. The increased FRAP values might be related to bioactive peptides which are produced by the degradation of soybean proteins during fermentation. Fan et al. (2009) and Torino et al. (2013) reported that amino acids in solid-state food, such as glutamic acid, glutamine, lysine and arginine, could contribute to the superior Fe2+-chelating ability of antioxidants, as well as the high radical scavenging potential of fermentation. Hur et al. (2014) reported that increasing the number of low-molecular-weight peptides by enzymatic hydrolysis may influence antioxidative activity during fermentation. In Xu et al. (2015), it was observed a high positive relationship between DPPH radical scavenging activity and antioxidant activities in 28 types of fermented soybeans. Considering the above results, the soaking and fermentation steps would be two major control points for nutrient changes, so could be targeted to obtain high-quality cheonggukjang.
Isoflavone composition
HPLC quantitative detection was used to observe total isoflavone changes during the manufacturing process. Table 5 shows that isoflavone glycoside content is represented by a combination of the daidzin, genistin and glycitin content of soybean. In soaked soybean, the glycoside content decreased from 253.17 to 109.06 µg/g compared to the raw soybean, due to the loss of water-soluble isoflavones. No significant differences in isoflavone composition were found between soaked and steamed soybean, except for daidzin content. In the fermentation step a significant increase in the isoflavone content was observed, especially in aglycone content, which increased from 16.74 µg/g when soaked to 31.44 µg/g at fermentation. These results are in agreement with those reported in Chang et al. (2007), that fungi can decompose isoflavones during fermentation and produce daidzein and genistein. Wang et al. (2007) and Chien et al. (2006) suggested that soybean is rich in the physiologically active isoflavone aglycones daidzein and genistein. This study suggests that the health function of soybean can be enhanced through the conversion of isoflavone glycosides into isoflavone aglycones during fermentation.
Table 5.
Changes in isoflavones glycosides and aglycones of cheonggukjang
| Glycosides | Aglycones | |||||
|---|---|---|---|---|---|---|
| Daidzin | Genisin | Glycitin | Daidzein | Genistein | Glycitein | |
| Raw soybean | 80.34 ± 0.72b | 82.05 ± 0.80a | 90.77 ± 0.91 | 4.53 ± 0.10b | 9.98 ± 0.12b | 3.70 ± 0.09c |
| Soaked | 30.10 ± 0.122d | 38.65 ± 0.05b | 89.93 ± 0.18 | 3.98 ± 0.09c | 1.03 ± 0.04e | 3.28 ± 0.19d |
| Steamed | 99.89 ± 0.63a | 36.96 ± 0.37c | 89.81 ± 0.02 | 3.04 ± 0.00d | 1.86 ± 0.01d | 2.84 ± 0.00e |
| Fermented | 57.46 ± 1.04c | 28.25 ± 0.47d | 89.82 ± 0.00 | 13.77 ± 0.34a | 8.19 ± 0.15c | 9.47 ± 0.00b |
| Product | 55.71 ± 0.41c | 24.28 ± 0.31e | 87.95 ± 1.08 | 13.31 ± 0.32a | 11.25 ± 0.11a | 12.86 ± 0.30a |
Values are expressed as mean ± SD (n = 3). Means in the same column with different letters are significantly different by Duncan’s multiple range test (p < 0.05)
Correlation analysis
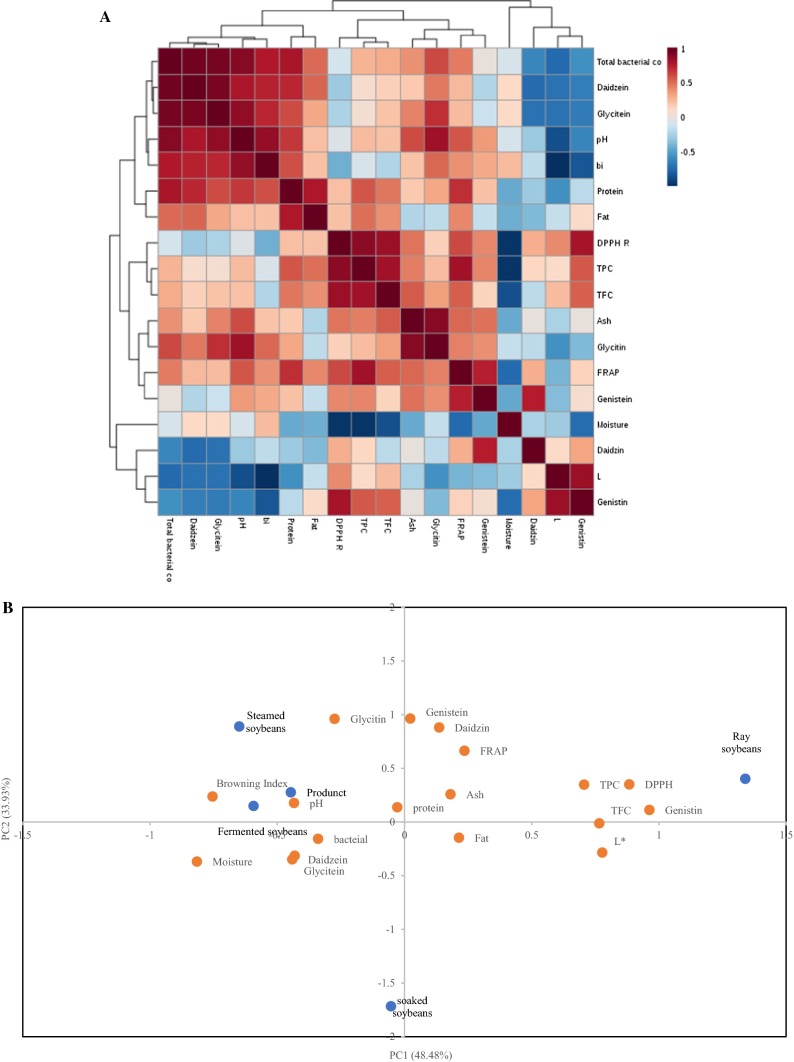
Figure 1a shows the correlations among all quality attributes of raw, soaked, steamed and fermented soybeans, and cheonggukjang. The correlation between DPPH radical scavenging activity and TPC in cheonggukjang is very high. There is also a good correlation between FRAP value and total phenol content in cheonggukjang. There is also a high correlation between DPPH free radical scavenging activity, FRAP value and TFC in cheonggukjang. Therefore, fermentation can enhance antioxidant activity, increase the total phenol and flavonoid content, and the correlation between them is relatively large. According to the correlation analysis, a significant positive correlation between total bacteria content, daidzein and genistein was observed. This result might be due to bacteria breaking down isoflavones to produce daidzein and genistein. In addition, browning index and pH values showed a positive correlation. This explains increases in browning index during fermentation.
Fig. 1.
Correlation analysis (a) and principle composition analysis (b) of all quality attributes of cheonggukjang during manufacturing. bi browning index
Principle composition analysis (PCA)
PCA was performed to evaluate the influences of cheonggukjang processing on physicochemical properties, antioxidant activities and isoflavone composition. In Fig. 1b, the loading plot shows that PC1 explained 48.48% of the total variation, whereas PC2 explained only 33.93% of the total variability. From the PCA plot it was observed that the TPC, TFC and DPPH radical scavenging activity was close, which indicated high correlation among them. The result agreed with the correlation analysis (Table 5). A similar result, that phenolic and flavonoid compounds have a great effect on DPPH radical scavenging activity, has been reported by other researchers. Furthermore, in the raw soybean samples, TFC, TPC and DPPH radical scavenging activity was observed in the positive side of PC1. This result could be explained by the raw soybean sample with the highest TPC, TFC and DPPH radical scavenging activity (Table 4). On the country, steamed and fermented soybeans, and cheonggukjang were negatively related to PC1, which might be due to the relative loss of phenol and flavone compounds during the manufacturing of cheonggukjang. In addition, we found that daidzein, glycitein, genistein and total bacteria was close in the fermented soybeans and Cheonggukjang. This result might be due to fungi affecting isoflavone compounds during fermentation and inducing the conversion of isoflavone glucones to isoflavone aglycones (Table 5).
Summary and conclusion
The manufacturing process had a significant influence on the physicochemical properties, isoflavone compounds and antioxidant activities of cheonggukjang. During soaking, significant decreases in daidzin, TFC, TPC, and antioxidant activities were observed, which might be due to the loss of water-soluble compounds. Furthermore, the transformation of isoflavone glycosides to isoflavone aglycones, as well as the increase of TPC, TFC and antioxidant activities during fermentation significantly improved bioavailability and the functional properties of cheonggukjang. Thus, monitoring the changes in the physicochemical properties of cheonggukjang during manufacturing could provide important information for controlling and improving quality-properties of cheonggukjang.
Complice with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akande KE, Doma U, Agu HO, Adamu HM. Major antinutrients found in plant protein sources: theireffect on nutrition. Pak J Nutr. 2010;9(8):827–832. doi: 10.3923/pjn.2010.827.832. [DOI] [Google Scholar]
- Akilligolu HG, Sibel K. Changes in total phenols, total flavonoids and antioxidant activities of common beans and pinto beans after soaking, cooking and in vireo digestion process. Food Sci Biotechnol. 2010;19:633–639. doi: 10.1007/s10068-010-0089-8. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 18. Washington: Association of Official Analytical Chemists; 2005. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chang TS, Ding HY, Sorgan S-KT, Wu CY. Metabolism of the soy isoflavones daidzein and genistein by fungi used in the preparation of various fermented soybean foods. Biosci Biotechnol Biochem. 2007;71:1330–1333. doi: 10.1271/bbb.60573. [DOI] [PubMed] [Google Scholar]
- Chien HL, Huang HY, Chou CC. Transformation of isoflavone phytoestrogens during the fermentation of soymilk with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006;23:772–778. doi: 10.1016/j.fm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Cho KM, Lee JH, Han DY, Ahn BY, Kim H, Seo WT. Changes of phytochemical constituents (isoflavones, flavonols, and phenolic acids) during Cheonggukjang soybeans fermentation using potential probiotics Bacillus subtilis CS90. J Food Compos Anal. 2011;24:402–410. doi: 10.1016/j.jfca.2010.12.015. [DOI] [Google Scholar]
- Coward L, Smith M, Kirk M, Barnes S. Chemical modification of isoflavones in soy foods during cooking and processing. Am J Clin Nutr. 1998;68:1486–1491. doi: 10.1093/ajcn/68.6.1486S. [DOI] [PubMed] [Google Scholar]
- Fan JF, Zhang YY, Chang XJ, Masayoshi S, Li ZG. Changes in the radical scavenging activity of bacterial-type Douchi, a traditional fermented soybean product, during the primary fermentation process. Biosci Biotechnol Biochem. 2009;73:2749–2753. doi: 10.1271/bbb.90361. [DOI] [PubMed] [Google Scholar]
- Hajimahmoodi M, Hanifeh M, Oveisi MR, Sadeghi N, Jannat B. Determination of total antioxidant capacity of green teas by the ferric reducing/antioxidant power assay. Iran J Environ Health. 2008;5:167–172. [Google Scholar]
- Hur SJ, Lee SY, Kim YC, Choi I, Kim GB. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014;160:346–356. doi: 10.1016/j.foodchem.2014.03.112. [DOI] [PubMed] [Google Scholar]
- Jeon HL, Yang SJ, Son SH, Kim WS, Lee NK, Park HD. Evaluation of probiotic bacillus subtilis P229 isolated from Cheonggukjang and its application in soybean fermentation. Food Sci Technol. 2018;97:94–99. [Google Scholar]
- Kim JY, Choi JN, Kang DJ, Son GH, Kim YS, Choi HK, Kwon DY, Lee CH. Correlation between antioxidative activities and metabolite changes during Cheonggukjang fermentation. Biosci Biotechnol Biochem. 2011;75:732–739. doi: 10.1271/bbb.100858. [DOI] [PubMed] [Google Scholar]
- Ko HM, Choi SJ, Lee WS, Choi UK. Quality characteristics of cheonggukjang made with the smoked soybeans. Korean J Food Nutr. 2014;27(02):274–279. doi: 10.9799/ksfan.2014.27.2.274. [DOI] [Google Scholar]
- Kulling SE, Honig DM, Metzler M. Oxidative metabolism of the soy isoflavones daidzein and genistein in humans in vitro and in vivo. J Agric Food Chem. 2001;49(6):3024–3033. doi: 10.1021/jf0012695. [DOI] [PubMed] [Google Scholar]
- Lee BR, Muneer S, Jung WJ, Avice JC, Ourry A, Kim TH. Mycorrhizal colonization alleviates drought-induced oxidative damage and lignification in the leaves of drought-stressed perennial ryegrass (Lolium perenne) Physiol Plant. 2012;145(470):440–449. doi: 10.1111/j.1399-3054.2012.01586.x. [DOI] [PubMed] [Google Scholar]
- Ludorff W, Meyer V (1973) Fische und fischerzeugnisse, vol. 6. Paul parey
- Mariusz KP, Jun U, Yukihiko L. Daidzein and genistein but not their Glucoside are absorbed from the rat stomach. FEBSI Lett. 1999;447:287–291. doi: 10.1016/S0014-5793(99)00307-5. [DOI] [PubMed] [Google Scholar]
- Muratsugu M, Washino K, Onchi Y, Yamaguchi A, Kumaki A, Shimooka I, Nakanishi M. Changes in biotin levels during production of natto, Japanese fermented soybean. Int J Food Allied Sci. 2017;3(1):1–9. doi: 10.21620/ijfaas.201711-9. [DOI] [Google Scholar]
- Nicoli MC, Anese M, Parpinel M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci Technol. 1999;10(3):94–100. doi: 10.1016/S0924-2244(99)00023-0. [DOI] [Google Scholar]
- Park NY, Seong JH, Choi MS, Moon KD, Kwon JH, Jeong YJ. Comparison of functional properties of cheonggukjang by using red ginseng. J Korean Soc Food Sci Nutr. 2008;37(03):261–266. doi: 10.3746/jkfn.2008.37.3.261. [DOI] [Google Scholar]
- Son YJ, Kang SH, Ko JN, Lee YK, Hwang IK, Kang HJ. Changes in physicochemical characteristics and nutritional values of soybean, meju, and doenjang by varying sowing periods. J Food Sci Technol. 2017;49:56–62. [Google Scholar]
- Torino MI, Limón RI, Martínez-Villaluenga C, Mäkinen S, Pihlanto A, Vidal-Valverde C, Frias J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2013;136(2):1030–1037. doi: 10.1016/j.foodchem.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Yin LJ, Li D, Zou L, Massayoushi S, Eizo T, Li LT. Influences of processing and NaCl supplementation on isoflavone contents and composition during Douchi manufacturing. Food Chem. 2007;101:1247–1253. doi: 10.1016/j.foodchem.2006.03.029. [DOI] [Google Scholar]
- Xu BJ, Chang SKC. Total phenolics, phenolic acids isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. J Agric Food Chem. 2008;56:7165–7175. doi: 10.1021/jf8012234. [DOI] [PubMed] [Google Scholar]
- Xu L, Du B, Xu BJ. A systematic, comparative study on the beneficial health components and antioxidant activities of commercially fermented soy products marketed in China. Food Chem. 2015;174:202–213. doi: 10.1016/j.foodchem.2014.11.014. [DOI] [PubMed] [Google Scholar]