Abstract
Picrorhiza kurrooa is an endangered herb known to produce the medicinally important picrosides through isoprenoid pathway. The present work showed the functionality of WRKY motifs (TGAC cis-acting elements) present in the promoters of regulatory genes 3-hydroxy-3-methylglutaryl coenzyme A reductase (Pkhmgr) and 1-deoxy-d-xylulose-5-phosphate synthase (Pkdxs) of the picrosides biosynthetic pathway by electrophoretic mobility shift assay. Also, the two WRKY genes, PkdWRKY and PksWRKY, were characterized and found to contain double and single characteristic WRKY domains, respectively along with a zinc-finger motif in each domain. Expression analysis revealed that PkdWRKY and PksWRKY exhibited a positive and negative correlation, respectively, with picrosides content under the environment of light and in different tissues. Functional evaluation in yeast showed DNA binding ability of both PksWRKY and PkdWRKY; however, only PkdWRKY exhibited transcriptional activation ability. Transient overexpression of PkdWRKY and PksWRKY in tobacco modulated the expression of selected native genes of tobacco involved in MVA and MEP pathway suggesting functionality of PkdWRKY and PksWRKY in planta. Collectively, data suggested that PkdWRKY and PksWRKY might be positive and negative regulators, respectively in the picrosides biosynthetic pathway.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02249-7) contains supplementary material, which is available to authorized users.
Keywords: Transcription factor, Gene regulation, Isoprenoid pathway, WRKY motif, DNA binding, Picroside biosynthesis
Introduction
Transcription factors (TFs) are central to complex gene-regulatory networks that regulate transcription of specific genes. Generally, TFs bind DNA elements located cis to the target genes and regulate their transcription (Calkhoven and Geert 1996). WRKY, a class of TFs originally isolated from plants is reported to be associated with several plant processes. For example, WRKY TFs play an important role in plant development (Lagace and Matton 2004; Jiang and Yu 2009) as well as in mediating response to different biotic and abiotic stresses (Park et al. 2006; Dang et al. 2014; Wang et al. 2017; Hara et al. 2000; Li et al. 2011; Dai et al. 2016). Besides, the role of WRKY TFs has also been established directly or indirectly during secondary metabolite synthesis (Phukan et al. 2016).
WRKY proteins possess a characteristic domain of 60 amino acid residues starting with the WRKYGQK sequence and a zinc finger motif at the C-terminal end. This domain binds to W box element (T)(T)TGAC(C/T) with a TGAC core. The core is crucial for binding and functionality of WRKY proteins (Eulgem et al. 2000). The classification of WRKY proteins is based on the number of WRKY domains present and the structure of zinc-finger motif. TFs with a single WRKY domain belong to group II, whereas those with two domains belong to group I and both possess zinc finger motif with same pattern (C–X4–5–C–X22–23–H–X1–H) (Berg and Shi, 1996). Another group of WRKY proteins with a distinct zinc finger motif C2–HC (C–X7–C–X23–H–X1–C) is classified into group III (Eulgem et al. 2000).
Picrorhiza kurrooa is an economically and medicinally important endangered plant species with reported hepatoprotective (Sinha et al. 2011), antioxidant (Rastogi et al. 2001), anticholestatic (Saraswat et al. 1993), cardioprotective (Nandave et al. 2013), anti-diabetic (Husain et al. 2009), immune-modulatory (Puri et al. 1992) and neuro-protective activities (Li et al. 2000, 2002). Several of the pharmacological properties of P. kurrooa are attributed to picrosides, which are iridoid glycosides. Iridoids are synthesized in plants via isoprenoid biosynthetic pathway which encompasses plastidic methylerythritol phosphate (MEP) and cytosolic mevalonate (MVA) pathways (Rohmer 1999). In our previous work, we reported a role of light in regulating accumulation of picrosides in P. kurrooa wherein Pkhmgr and Pkdxs were found to be critical in regulating picrosides biosynthesis (Kawoosa et al. 2010). In silico analysis showed the presence of multiple WRKY core sequence (TGAC) in the promoters of these regulatory genes from P. kurrooa (Kawoosa et al. 2010). However, the functionality of this core sequence (TGAC) is not yet reported.
Presence of WRKY motifs in the promoters of regulatory genes, Pkhmgr and Pkdxs suggested that WRKY TFs could play an important role in regulating picrosides biosynthesis. Further, transcriptome sequencing of P.kurrooa also revealed high enrichment of WRKY TF at 15 ºC (temperature favoring picrosides accumulation) as compared to those at 25 ºC (Gahlan et al. 2012). However, there is no knowledge neither on the functionality of these WRKY motifs, nor on the genes encoding the WRKY TFs. Accordingly, the present study reported on functionality of WRKY motif through electrophoretic mobility shift assay (EMSA). Also, the WRKY genes were cloned and functionally characterized through heterologous expression in yeast and transient overexpression in tobacco followed by expression analyses vis-à-vis picrosides accumulation in P. kurrooa.
Material and methods
Plant material
Picrorhiza kurrooa plants were collected and brought to Palampur (32° 06′ N, 76° 33′ E; 1300 m altitude, India) from Rohtang pass (32° 23′ N, 77° 15′ E; 4000 m altitude, India). Indian Biological Diversity Act 2002 permits access to biological resources to bonafide Indians for scientific research (Venkataraman 2009). At Palampur, the plants were transferred to plastic pots filled up with a mixture of sand, soil and farm yard manure in 1:2:1 ratio and kept in the experimental farm for growth and maintenance. Plants were shifted to a growth chamber set at 15 ± 2 °C (350 μmol/m2sphotosynthetic photon flux density; 16 h photoperiod) and acclimatized for two months before starting the experiment. Various analyses were performed on first leaf (youngest; top most leaf), fourth leaf (mature leaf; leaf position with respect to the first leaf), root, rhizome and inflorescence of the plants.
Electrophoretic mobility shift assay
Extraction of nuclear proteins from leaf tissue of P. kurrooa, maintained in a plant growth chamber, was performed as detailed by Kawoosa et al. (2010). Sense oligonucleotide (30 mer) having WRKY motif (with invariant TGAC core, underlined: 5′-GAATTCTTGAGAAATTTATGACTACAAACAAACA-3′) along with the corresponding antisense oligonucleotide were designed from Pkhmgr promoter of P. kurrooa (accession number, FJ228691). DNA probes were labeled with biotin using Pierce Biotin 3′ End DNA Labeling Kit (Thermo Scientific, USA) following manufacturer’s instructions. Briefly, equal number of moles of sense and antisense oligonucleotides were subjected to incubation at 95 °C for 5 min and cooled to room temperature (25 °C). EMSA was performed using the LightShift Chemiluminescent EMSA Kit (Thermo Scientific, USA). The binding reaction mixture contained 7 μmol of biotin-labeled probe, 10 μg of nuclear protein extract, 1 × binding buffer [10 mM Tris, 50 mM KCL, 1 mM DTT; pH 7.5], 2.5% glycerol, 10 mM EDTA, 50 ng/μl poly (dI–dC) and 60 mM KCL. In specific competition reaction, excess of unlabeled oligonucleotides were incubated with nuclear extract for 10 min prior to adding the probe. Resulting DNA–protein complexes were subjected to electrophoresis on a 6% acrylamide:bis-acrylamide, 0.5 X TBE gel run at 15 V/cm. The biotin labeled DNA was electro-blotted onto BrightStar™-Plus Positively Charged Nylon Membrane (Invitrogen, USA) and the membrane was cross-linked on a UV transilluminator (Thermo Scientific, USA) at 254 nm for 1 min. The signals were developed with an Azure c300 chemiluminescent detection module.
Cloning of cDNAs of PkdWRKY and PksWRKY
Total RNA was isolated from leaf tissue as described by Ghawana et al. (2011). Any contaminating DNA was removed using RNase free DNase 1 (Invitrogen, USA) following the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized as described previously by Kawoosa et al. (2010). The conserved regions of WRKY, reported from different plant species, were used for designing degenerate primers for amplification of partial gene sequences by PCR (ESM Table 1). The amplicons were cloned in pGEM-T Easy Vector system (Promega, USA) and plasmid was isolated using Gen Elute Plasmid Mini prep Kit (Sigma-Aldrich, USA). An automated DNA sequencer (ABI PRISM™ 310 and 3130 ×l Genetic Analyzer, Applied Biosystems, USA) was used for any sequencing work. Full-length cDNAs were cloned by rapid amplification of cDNA ends (RACE) using SMART™ RACE cDNA Amplification Kit (Clontech, TakaraBio, Japan) following the manufacturer’s instructions. Primer pairs and PCR conditions used for RACE (dWRKY5′ R, dWRKY 3′ F, sWRKY 5′ R and sWRKY 3′ F) are mentioned in ESM Table 1. The sequences obtained by 5′ and 3′ RACE were used to amplify full-length cDNA, which were cloned and sequenced. BLASTX, BLASTN and BLASTP, programs of National Centre for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/) were used for analysis of cDNA and deduced amino acid sequences. The secondary structure analysis of PkdWRKY and PksWRKY was done using Self-Optimized Prediction Method with Alignment (SOPMA; https://www.npsa-pbil.ibcp.fr/). Phylogenetic trees were constructed using neighbor-joining (NJ) method with MEGA5 software (Saitou and Nei 1987; Tamura et al. 2011; https://www.megasoftware.net/).
Light and dark treatment
Light and dark treatments were given by placing a set of P. kurrooa plants under continuous light and dark conditions in two separate growth chambers maintained at 15 °C. For dark treatment, lights were switched off and chamber shelves were covered with black sheets to avoid exposure to external light during sample collection. After start of the treatment (10 am was treated as 0 h), the samples were collected at 12 h interval and continued till 36 h. Long-term experiment with the plants exposed to dark was avoided since it could lead to possible starvation and limit the carbon pool.
Quantitative real-time PCR
To analyze the expression of PkdWRKY and PksWRKY, total RNA was extracted as described by Ghawana et al. (2011). RNA (1 μg) was treated with DNase I (Invitrogen, USA) and reverse transcribed using Superscript III enzyme (Invitrogen, USA) following manufacturer’s instructions. Expression primers, specific for PkdWRKY and PksWRKY, were designed using Primer Express 3.0.1 Primer Design tool (Invitrogen, USA). Three independent biological replicates were used for performing qRT PCR on Step ONE plus real time PCR system (Applied Biosystems, USA) using 2 × Brilliant II SYBR Green (Agilent Technologies, USA) QPCR Master Mix. Actin (Actin-F and Actin-R; ESM Table 1) was used as internal control. The relative expression ratio of PkdWRKY and PksWRKY with respective controls was estimated using 2−ΔΔCT method (Livak and Schmittgen 2001). Expression values were transformed (log2) to generate expression profiles. Duncan’s multiple range test (p < 0.05) was used for comparing means post hoc by using Statistica version 7.0 (Starsoft Inc. USA) software.
Estimation of picrosides
Picrosides content was estimated on an Acquity Ultra Performance Liquid Chromatography (UPLC) system (Waters, Millford, USA) as detailed by Kawoosa et al. (2010). In brief, plant tissues (100 mg) were harvested, washed, blot dried, weighed and frozen in liquid nitrogen. The samples were ground in liquid nitrogen to form a fine powder. Thereafter, 80% methanol (1 ml) was added to each sample, transferred to a micro-centrifuge tube and centrifuged for 15 min at 15,000×g. The supernatant was filtered through 0.45 micron filter (Millipore, USA) and loaded onto the chromatographic system. The mobile phase comprised of 0.05% formic acid and methanol: acetonitrile (1:1) in 70:30 ratio. Isocratic elution was performed using 5 μl of injection volume at a flow rate of 0.250 ml/min. Picrosides were monitored and quantified at 270 nm. Picroside-I and picroside-II were used as standards (ChromaDex™, USA). For each estimation, three independent biological replicates were used. Duncan's multiple range test (p < 0.05) was used for comparing means post hoc by using Statistica version 7.0 (Starsoft Inc. USA) software.
Functional evaluation of PkdWRKY and PksWRKY in Yeast One Hybrid system (Y1H)
DNA binding ability of PkdWRKY and PksWRKY was analyzed by fusing the coding sequences to the GAL4 domain of pGADT7-Rec2 vector (Clontech, TakaraBio, Japan), and Y1H assay was performed using two reporter constructs that served as bait. PkdWRKY and PksWRKY were PCR amplified using primers with SMART III, and CDS III sequences included at 5´end of forward and reverse primers, respectively to aid homologous recombination with pre-linearized pGADT7-Rec2 vector. Reporter construct was prepared by cloning oligonucleotide containing the consensus WRKY motif (TGAC) upstream of the yeast minimal promoter that regulates the expression of His3 gene in pHIS2.1 plasmid. Mutant WRKY motif (GATC) was also used to prepare a separate reporter construct and used as control. Primer sequences used in Y1H assay are mentioned in ESM Table 2. Yeast Y187 cells were co-transformed with (i) reporter construct (ii) the PCR amplified PkdWRKY/PksWRKY with SMART III (5 ' end) and CDS III (3′ end) sequences and (iii) pre-linearized pGADT7-Rec2 plasmid. The growth status of the transformed yeast cells was monitored by their ability to grow under specific nutritional deficiency, an indication of their successful transformation.
Transcriptional activation analysis of PkdWRKY and PksWRKY in yeast
Transcriptional activation ability of PksWRKY and PkdWRKY was analyzed by fusing the coding sequences in frame to GAL4 domain (DNA binding domain) of pGBKT7 vector. The constructs PkdWRKY-pGBKT7, PksWRKY–pGBKT7, negative control empty pGBKT7 plasmids, and the positive control pGBKT7-53 + pGADT7-RecT were used for transformation of AH109 yeast strain by lithium acetate-based method (Clontech, TakaraBio, Japan). The transformed cells were plated separately onto minimal medium plates without Tryptophan [SD/(–)Trp]; Tryptophan, Histidine [SD/(–)Trp/(–)His] and Tryptophan, Histidine, Adenine [SD/(–)Trp/(–)His/(–)Ade]. The α-galactosidase activity of transformed cells was measured by overlaying minimal medium plates [SD/(–)Trp] with 0.5 M potassium phosphate buffer (pH 7) containing (100 mg/ml) X-α-gal (5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside), 0.5% low melt agarose, 0.1% SDS (sodium dodecyl sulfate), 6% dimethylformamide and 0.05% of 2-mercaptoethanol. The transcriptional activation ability was analyzed according to the ability of transformed cells to grow under specific nutritional deficiency and the activation of α-galactosidase.
Western blot analysis was also performed to confirm the expression of PkdWRKY and PksWRKY by yeast cells. Yeast protein extracts were prepared following method described by Printen et al. (1994). For western blotting, extracted proteins were loaded on 10% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) gel and transferred to preactivated polyvinylidene difluoride (PVDF) membrane (Cat No. 10600023 GE healthcare life science) using semi dry blotter (Bio-Rad, USA). The membrane was blocked overnight with Tris buffer saline (TBS) + 0.1% TWEEN 20 + 5% BSA and was incubated with c-Myc specific primary antibody (Clontech, TakaraBio, Japan). For each primary antibody horseradish peroxidase conjugated anti-rabbit secondary antibody was used at 1:10,000 dilution. The signals were developed using an Azure c300 chemiluminescent detection module.
Transient overexpression of PksWRKY and PkdWRKY in tobacco
A potato virus X (PVX) based vector, pGR106 (Lacomme et al. 2008), was utlized for Agrobacterium- mediated transient overexpession of PksWRKY and PkdWRKY in tobacco. The full-length coding sequences of PksWRKY and PkdWRKY were amplified such that restriction sites NotI and SalI were incorporated at 5′and 3′ end of the amplified sequences, respectively. Primer sequences and PCR cycling profiles used in the preparation of expression construct are mentioned in ESM Table 3. Upon restriction digestion with NotI and SalI, PksWRKY and PkdWRKY were independently cloned in pGR106. The positive recombinant plasmids were mobilized in Agrobacterium strain GV3101 using freeze thaw method (Weigel and Glazebrook 2006). Agrobacterium from positive clones were cultured in LB liquid medium supplemented with 50 µg/ml kanamycin at 28 °C for 12 h. Cells were harvested by centrifugation and resuspended in 1 ml of agromix (10 mM MgCl2; 150 µM acetosyringone; and 10 mM MES, pH 5.6). The optical density of resuspension was adjusted to A600 of 0.5 with Agromix and used for inflteration of leaves of 6 week-old tabocco plants. After 14-days post infiltration, infiltrated leaves were harvested and used for isolation of genomic DNA and total RNA using methods as decribed previously (Allen et al. 2006; Ghawana et al. 2011). Presence and expression of transgenes were confirmed by PCR assays with genomic DNA and cDNA, respectively. Quantitative RT-PCR (qRT PCR) was performed to compare the expression levels of selected native genes of tobacco involved in MVA and MEP pathways. Actin serves as endogenous control for qRT PCR analysis. Primer sequences and PCR cycling profiles used for different analyses are given in ESM Table 3.
Results and discussion
TFs are central to gene regulation. Any alteration in the expression of TFs, in contrast to structural genes, is likely to have broader impact on plant performance including an effect on metabolic flux (Gaudinier et al. 2015). Further, regulatory regions of genes having similar expression patterns have been reported to contain common motifs in their promoters so that a single TF could regulate their expression (Klok et al. 2002).
Our previous work identified the presence of W-box [(T)TGAC(C/T); termed as WRKY motifs) in several positions in the promoters of Pkhmgr and Pkdxs (Kawoosa et al. 2010). Also, these genes exhibited light mediated regulation. Since, W-box is the binding site for WRKY group of TFs (Eulgem et al. 2000), it was reasonable to assume a role of PkWRKY in the regulation of Pkhmgr and Pkdxs, which are associated with picrosides biosynthetic pathway. Moreover, several members of WRKY family of TFs have been reported to play a regulatory role in the synthesis of secondary metabolites (Ma et al. 2009; Zhang et al. 2012; Wang et al. 2013; Li et al. 2014). Therefore, the present was undertaken to functionally evaluate the WRKY motif and the TFs involved thereof.
EMSA suggests binding of proteins to WRKY motif
To detect the binding of putative regulatory proteins to the WRKY motif, EMSA was performed with TGAC as a core sequence. Two shifted nucleo-protein bands were seen for the motif (Fig. 1, lane 2). In order to verify if these shifts were ascribed to the same protein, excess of unlabeled WRKY oligonucleotide (specific competitor) was added to the reaction mixture (Fig. 1, lane 3). On addition of excess specific competitor, upper nucleo-protein band was not detectable suggesting it to be a specific interaction. A slight reduction in intensity of lower nucleo-protein band suggested it to be a non-specific interaction. Several studies have suggested sequence specific binding of WRKY TFs to W-box in the promoter of genes via gel shift assays (Rushton et al. 1996; Zheng et al. 2007; Fan et al. 2017). Therefore, EMSA suggested functionality of WRKY domains and indicated the probable role of WRKY TFs in regulating gene expression. Accordingly, WRKYs were cloned from P. kurrooa for further analysis.
Fig. 1.

Electrophoretic mobility shift assay displaying binding of nuclear proteins to WRKY motif. Lane 1, biotin labeled WRKY oligonucleotide without nuclear protein; lane 2, biotin labeled WRKY oligonucleotide with nuclear protein; lane 3, biotin labeled WRKY oligonucleotide with nuclear protein and unlabeled WRKY oligonucleotide (specific competitor). Solid arrow highlights upper (specific) nucleo-protein complex
Cloning of PkdWRKY and PksWRKY
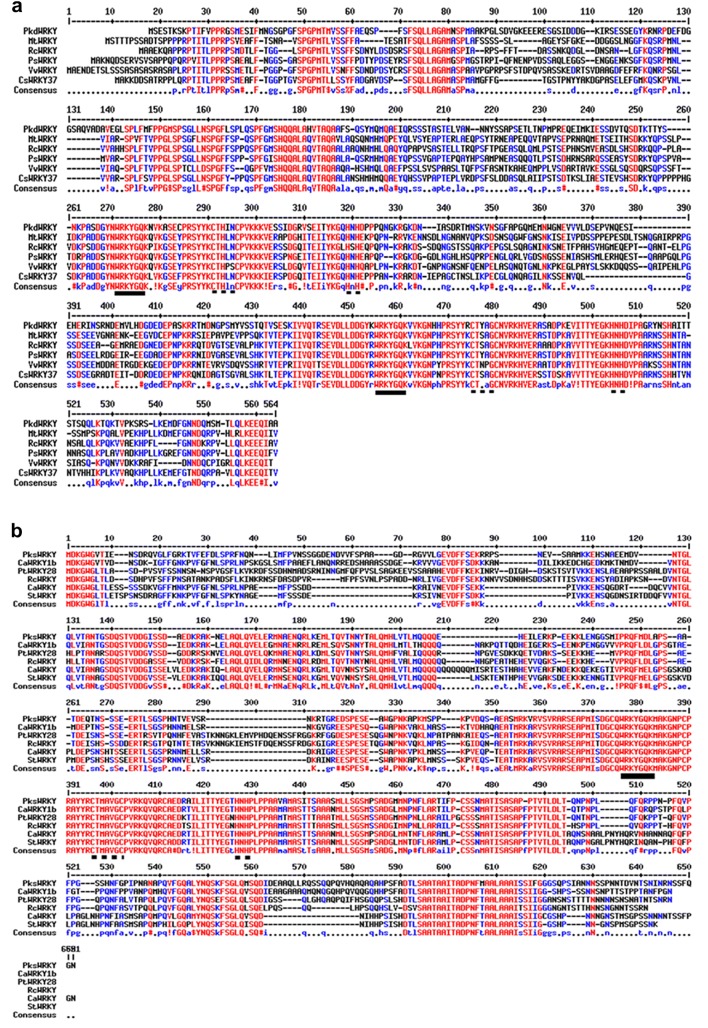
Partial sequences of two WRKYs, one with single and other with double WRKYGQK motif were amplified by degenerate primers and confirmed by BLASTX analysis. Specific primers designed from the cloned sequences were used to clone their respective 5′ and 3′ ends using RACE that amplified 1985 bp of PkdWRKY (accession no. DQ347962; double WRKY motif) and 1825 bp of PksWRKY (Accession No. EU561005; single WRKY motif). The cDNAs comprised of open reading frames (ORF) of 1545 and 1671 bp; 194 and 54 bp of 5′ untranslated region and 243 and 100 bp of 3′ untranslated regions for PkdWRKY and PksWRKY, respectively. The deduced proteins, referred to as PkdWRKY and PksWRKY, had isoelectric points/molecular masses of 6.15/56.89 kDa and 5.62/60.37 kDa, respectively. Deduced protein of PkdWRKY exhibited 52, 50, 50, 47 and 54% sequence identity with WRKY protein from Medicago truncatula (AES77535.1), Ricinus communis (EEF32386.1), Prunus salicina (AEQ28760.1), Vitis vinifera (AAT46067.1) and Cucumis sativus (ADU52521.1), respectively (Fig. 2a). Deduced protein of PksWRKY exhibited 62, 70, 63, 60 and 57% sequence identity with WRKY protein from Coffea arabica (ABC86709.1), Capsicum annuum (ACT80136.1), Solanum tuberosum (ABN69038.1), Populus tomentosa (ACV92030.1) and Ricinus communis (EEF43831.1), respectively (Fig. 2b). Most of the conservation was exhibited at WRKY domains and zinc finger like motifs while rest of the sequence showed minimum similarity with corresponding homologous proteins. An alignment of the putative WRKY protein with related sequences indicated a highly conserved WRKY domain consisting of 60 amino acids at position Glu293–Val352 in PksWRKY (ESM Fig. 1b) and two WRKY domains at positions Glu238–Arg298 and Glu408–Asn468 in PkdWRKY (ESM Fig. 1a). In addition, these genes possessed similar zinc finger motifs (C–X4–C–X22–23–H–X1–H) along the C- terminus of each WRKY domain. Conserved domain analysis confirmed that PkdWRKY and PksWRKY consisted of two and one WRKY domains, respectively (ESM Fig. 2). Therefore, PkdWRKY and PksWRKY belonged to group I and II of the WRKY transcription factor family, respectively (Wu et al. 2005). SOPMA analysis showed that PkdWRKY and PksWRKY consisted of 20.78% and 34.35% alpha helices; 52.23% and 43.17% random coils; 17.67% and 16.19% extended strands; 9.32% and 6.29% β-turns (ESM Fig. 3). The evolutionary relationship of PksWRKY and PkdWRKY inferred from phylogenetic tree indicated closeness of these TFs with that of Cucumis sativus and Capsicum annuum in comparison to other plant species (ESM Fig. 4).
Fig. 2.
a Alignment of amino acid sequence of PkdWRKY with different plant species. Dots indicate gaps introduced to optimize alignment. Two WRKY domains are indicated as solid lines and the histidine and cysteine residues essential for zinc-finger motif are indicated with broken lines. The GenBank accession numbers of different WRKYs used for alignment were Picrorhiza kurrooa (PkdWRKY), ACI90293.2; Medicago truncatula (MtWRKY), AES77535.1; Ricinus communis (RcWRKY), EEF32386.1; Prunus salicina, (PsWRKY) AEQ28760.1; Vitis vinifera (VvWRKY), AAT46067.1 and Cucumis sativus, (CsWRKY37), ADU52521.1. b Alignment of amino acid sequence of PksWRKY with different plant species. Dots indicate gaps introduced to optimize alignment. WRKY domain is indicated as solid line and the histidine and cysteine, essential for zinc-finger motif are indicated with broken lines. The GenBank accession numbers of various WRKY sequences used for alignment were Picrorhiza kurrooa (PksWRKY), ACI90292.1; Coffea Arabica (CaWRKY), ABC86708.1; Capsicum annuum (CaWRKY1b), ACT80136.1; Solanum tuberosum (StWRKY), ABN69038.1; Populus tomentosa (PtWRKY28), ACV92030.1 and Ricinus communis (RcWRKY), EEF43831.1
Expression analysis of PkdWRKY and PksWRKY and picrosides estimation
WRKY comprises of a large family of TFs that regulate diverse biological processes in different tissues and varied developmental stages of plants (Rushton et al. 2010). For example, expression analysis of fifteen WRKY in wheat leaves of different developmental age revealed their role in senescence (Wu et al. 2008). An analysis of expression pattern of WRKY gene family in different tissues of Phyllostachys edulis seedling suggested their regulatory role in flower development and shoot growth (Long et al. 2017). In the present study, expression analysis of PkdWRKY and PksWRKY in different tissues of P. kurrooa showed highest expression of PkdWRKY in rhizome followed by in inflorescence, first leaf and root tissues. On the contrary to this, expression of PksWRKY was highest in the root tissue followed by in the rhizome. A downregulation of PksWRKY gene was observed in the first leaf and in the inflorescence tissues (Fig. 3a). Picrosides content (PI + PII) was highest in the rhizome tissue followed by in inflorescence, root and first leaf tissues (Fig. 3b). Data suggested expression of PkdWRKY and PksWRKY exhibited positive and negative correlation, respectively, with picrosides content in different tissues.
Fig. 3.

Expression of two WRKYs (a) and the picrosides content (b) in P. kurrooa in different tissues. a Relative expression of PksWRKY and PkdWRKY wherein L1, L4, Rh, Rt and Inf represent first leaf, fourth leaf, rhizome, root and inflorescence, respectively. Relative expression ratios in different tissues have been calculated with reference to L4 i.e. fourth leaf and the relative expression values were log2 transformed. Actin was used as endogenous control to normalize the data. Each value in the bar diagram represents mean of three independent biological replicates and error bar represents standard error. Different letters above the bar show significant difference at p < 0.05 according to Duncan’s multiple range test. b Picrosides content in different tissues of P. kurrooa wherein L1, L4, Rh, Rt and Inf represent first leaf, fourth leaf, rhizome, root and inflorescence, respectively. PI and PII represent picroside I and II, respectively. Each value in the bar diagram represents mean of three independent biological replicates and the error bar represents standard error. Different letters above the bar show significant difference at p < 0.05 according to Duncan’s multiple range test
Although WRKYs have been characterised in different plant species, but there are no reports on their possible involvement in relation to light mediated gene regulation. Our earlier work reported on light and dark conditions in modulating picrosides accumulation and gene expression in P. kurrooa (Kawoosa et al. 2010). Therefore, light and dark mediated expression of PkdWRKY and PksWRKY was analyzed at various time points in P. kurrooa placed under continuous light/dark regimes (Fig. 4a). Analysis was carried out in fourth leaf (mature leaf, to avoid development mediated change in gene expression) of P. kurrooa plants kept at 15 °C (near to native day time temperature of the niche and also picrosides content were higher at this temperature, as compared to 25 °C; Kawoosa et al. 2010). Interestingly, the expression of PksWRKY and PkdWRKY exhibited opposite trends under light and dark regimes. PkdWRKY transcript constantly accumulated at higher level under light and PksWRKY was found to be up-regulated under dark conditions. Also, the total picrosides content (PI + PII) followed an increasing trend in light as compared to that under the dark condition (Fig. 4b). Thus, a positive correlation between expression of PkdWRKY and picrosides content was observed under the condition of continuous light. Data suggested that PkdWRKY and PksWRKY could be positive and negative regulators of picrosides biosynthesis, respectively. Therefore, genes were functionally evaluated by yeast hybrid assays and transient overexpression in tobacco.
Fig. 4.

Effect of light (L) and dark (D) periods on expression of two WRKYs in P. kurrooa (a) and picrosides content (b). Different time of exposure (in h) to continuous light (L) and darkness (D) is indicated below the x-axis of (a) and (b). a The relative expression ratio at different time of exposure has been calculated with reference to 0 h sample and the values were log2 transformed. Actin was used as endogenous control to normalize the data. Each value in the bar diagram represents mean of three independent biological replicates and error bar represents standard error. Different letters above the bar show significant difference at p < 0.05 according to Duncan’s multiple range test. b Picrosides content under L and D periods; PI and PII represent picroside I and II, respectively. Each value in the bar diagram represents mean of three independent biological replicates and error bar represents standard error. Different letters above the bar show significant difference at p < 0.05 according to Duncan’s multiple range test
Functional evaluation in YIH system revealed DNA binding ability
PkdWRKY and PksWRKY were functionally evaluated for their interaction with WRKY motifs through Y1H system. Interaction of TFs with the WRKY motif was studied by co-transforming yeast Y187 cells with a reporter construct and the amplified PksWRKY/PkdWRKY and pre-linearized pGADT7-Rec2 plasmid. The growth on specific nutrition conditions of the transformed yeast cells was analyzed. The transformed yeast cells carrying PkdWRKY-pGADT7-Rec2, PksWRKY-pGADT7-Rec2 and pGAD-Rec2-53-p53HIS2 (a positive control) grew well on SD medium lacking histidine, leucine and tryptophan [SD/(–)His/(–)Leu/(–)Trp] as well as on [SD/(–)His/(–)Leu/(–)Trp] supplemented with 100 mM of 3-amino-1,2,4- triazole (3-AT) to control leaky expression of His3 in absence of an activating WRKY protein (ESM Figs. 5 and 6). Transformants with the vector plasmid pGAD-Rec2-53/pHIS2 served as a negative control and were unable to grow on [SD/(–)His/(–)Leu/(–)Trp]. PkdWRKY-pGADT7-Rec2 and PksWRKY-pGADT7-Rec2 were also transformed into mutated W box reporter yeasts, and the transformants showed no growth on [SD/(–)His/(–)Leu/(–)Trp], which indicated that PksWRKY and PkdWRKY could specifically bind to WRKY motif in vivo.
Transcriptional activation ability in yeast
To analyze the abilities of PkdWRKY and PksWRKY for activating transcription, a GAL4-responsive reporter system was used to perform transcriptional activation assay. The transformants containing pGBKT7 vectors (empty) and pGBKT7-53-pGADT7-T (DBD–P53 + T-antigen) were used as negative and positive controls, respectively. The transformed yeast cells containing pGBKT7-PkdWRKY and pGBKT7-53-pGADT7-T could grow on SD medium lacking adenine, histidine and tryptophan [SD/(–)ADE/(–)HIS/(–)TRP] and exhibited α-galactosidase activity (ESM Fig. 7 and Fig. 5), whereas cells containing pGBKT7-PksWRKY, pGBKT7 (negative control) could not grow in SD/(-)ADE/(-)HIS/(-)TRP and displayed no α-galactosidase activity in overlay assay. The results suggested that only PkdWRKY exhibited transcriptional activation ability. Therefore, an inverse relationship between expression of PksWRKY and picrosides content, as well as lack of transcriptional activation ability of PksWRKY suggested its negative association with picrosides biosynthesis. Western blot analysis confirmed expression of PkdWRKY and PKsWRKY by yeast cells (Fig. 6).
Fig. 5.
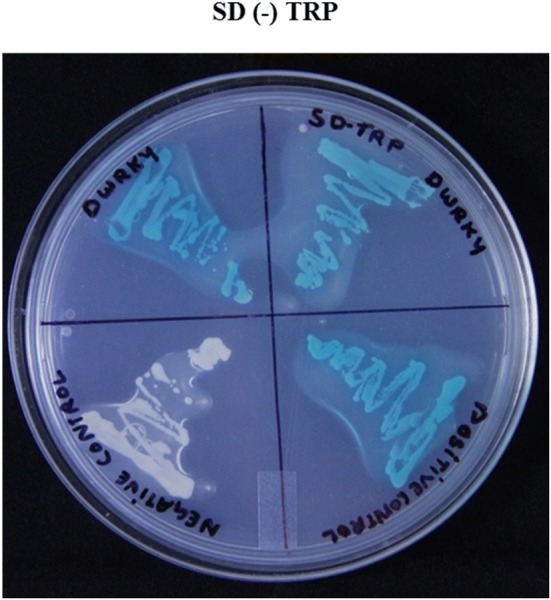
X-α- gal overlay assay showing transcriptional activation by PkdWRKY
Fig. 6.

a Coomassie stained PAGE gel loaded with yeast protein extracts carrying PksWRKY-pGBKT7 and PkdWRKY-pGBKT7 constructs, respectively. b Western blot showing expression of PksWRKY and PkdWRKY by yeast cells using c-Myc specific primary antibody
Transient overexpression of PksWRKY and PkdWRKY modulated the expression of selected native genes involved in MVA and MEP pathway in tobacco
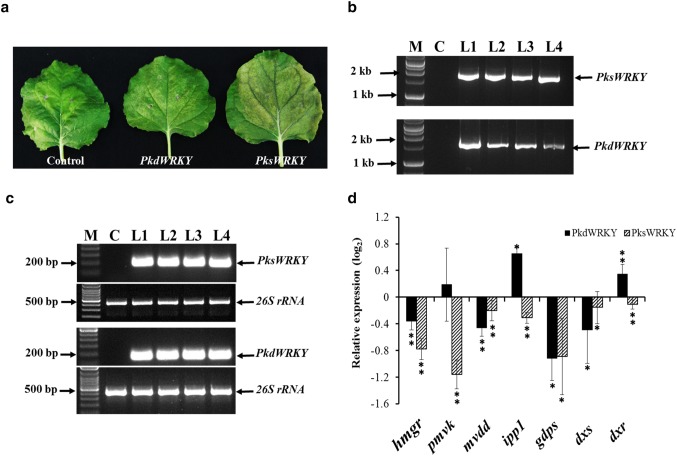
Full length nucleotide sequences of PksWRKY and PkdWRKY were independently cloned in pGR106 vector for PVX-based transient overexpression in tobacco. After 14-days post infiltration, leaves showed evident symptoms of infections as characterized by the development of yellow necrotic lesions (Fig. 7a). Leaves infiltrated with empty pGR106 vector served as a control for different analyses. PCR analysis with genomic DNA confirmed the presence of transgenes, while reverse transcription (RT)-PCR showed the expression of transgenes in the leaves of infiltrated plants. No amplification was observed in leaves from control plants, which established the specificity of the primers used for PCR assay (Fig. 7b, c). Transient overexpression of PksWRKY and PkdWRKY in tobacco resulted in significant change in expression levels of genes involved in MVA pathway i.e. hydroxy 3 methylglutaryl coenzyme A reductase (hmgr), mevalonate 5-disphosphate decarboxylase (mvdd), isopentenyl pyrophosphate isomerase (ipp1), geranyl diphosphate synthase (gdps) as compared to the control leaves (Fig. 7d). Expression levels of another gene from MVA pathway, phosphomevalonate kinase (pmvk) was found to be significantly different only in PksWRKY overexpressing leaves. Also, two genes from MEP pathway, 1-deoxy-d-xylulose-5-phosphate synthase (dxs) and 1-deoxy-d-xylulose-5-phosphate reductoisomerase (dxr) showed significant change in expression in PksWRKY and PkdWRKY overexpressing leaves as compared to the control leaves. These results further suggested the functionality of PksWRKY and PkdWRKY in planta.
Fig. 7.
a Representative photograph showing symptoms of infection in the leaves of tobacco after 14-days of agro-infiltration. b PCR analysis with genomic DNA isolated from tobacco leaves infiltrated with empty pGR 106 vector (C) and pGR106 harboring PksWRKY and PkdWRKY (L1 to L4). M represents 1 kb DNA ladder. c Semi-quantitative RT-PCR for evaluation of transgene expression in tobacco leaves infiltrated with empty pGR 106 vector (C) and pGR106 harboring PksWRKY and PkdWRKY (L1–L4). 26 s rRNA served as endogenous control for RT-PCR analysis. M represents 100 bp DNA ladder. d Quantitative real time (RT-qPCR) for relative expression of selected native genes of tobacco involved in MVA and MEP pathway in leaves overexpressing PksWRKY and PkdWRKY. Values indicate expression relative to the control leaves. hmgr hydroxy 3 methylglutaryl coenzyme A reductase, pmvk Phosphomevalonate kinase, mvdd mevalonate 5-disphosphate decarboxylase, ipp1 isopentenyl pyrophosphate isomerase, gdps geranyl diphosphate synthase, dxs 1-deoxy-d-xylulose-5-phosphate synthase, dxr 1-deoxy-d-xylulose-5-phosphate reductoisomerase. Each data point indicates mean of three independent biological replicates. Error bars denotes SE. * and ** Shows significant differences from the control at p ≤ 0.05 and p ≤ 0.01, respectively, according to Duncan's multiple range test
Owing to the ability of TFs to regulate multiple genes of the same metabolic pathway, TFs serve an efficient tool for plant metabolic engineering. Understanding the role of TFs in secondary metabolism pathway can aid in increasing the yield of valuable metabolites. WRKY TFs are known to regulate genes of terpenoid biosynthesis in many plant species (Schluttenhofer and Yuan, 2015). A WRKY TF, GaWRKY1 has been reported to regulate biosynthesis of sesquiterpene in cotton (Xu et al. 2004). Overexpression of TcWRKY1 from Taxus chinensis resulted in enhanced expression of a regulatory gene of taxol biosynthesis pathway (Li et al. 2013). In yet another study, a two-fold increase in the yield of artemisinin was obtained by overexpression of AaWRKY in Artemisia annua (Han et al. 2014). OsWRKY45 has been reported to regulate the synthesis of momilactone, oryzalexin and phytocassane in rice (Akagi et al. 2014). Furthermore, Kawauchi et al. (2016) suggested a role of HaWRKY1 in regulating the expression of hahpo1 gene of solavetivone biosynthesis in Hyoscyamus albus. These studies suggested that WRKY TFs could be used as potential candidates for engineering terpenoid production in plants.
The present study showed that accumulation of picrosides and PkdWRKY transcript exhibited a positive correlation with picrosides content under continous light conditions and in different tissues. Moreover, PkdWRKY showed both DNA binding and transcriptional activation ability in yeast. It is likely that PkdWRKY might act as transcriptional activator of regulatory genes of pathway and participate in the regulation of picrosides biosynthesis. Coordinated transcriptional control of biosynthetic genes by specific TFs has evolved as a major mechanism governing secondary metabolite production in plants (Vom Endt et al. 2002). Therefore, over-expression of this TF might be promising to modulate picrosides biosynthetic pathway.
Data also suggested that PksWRKY might function as a negative regulator under the stated conditions. Previous reports suggested a role of WRKY TFs in transcriptional repression (Wang et al. 2013). There are reports where WRKY acted as a transcriptional repressor in abscisic acid (ABA) (Shang et al. 2010), gibberellic acid (GA) (Zhang et al. 2004), and both ABA as well as GA (Zhang et al. 2009) signaling simultaneously. Thus, PksWRKY could also be used as a potential candidate gene in metabolic engineering of P. kurrooa.
Conclusion
The present work showed the functionality of WRKY motif through electrophoretic mobility shift assay (EMSA). Two WRKY genes, PkdWRKY and PksWRKY were cloned and characterized from P. kurrooa. Tissue specific and light mediated gene expression studies suggested a positive and a negative association of PkdWRKY and PksWRKY, respectively with picrosides content. Yeast one hybrid analysis revealed DNA binding ability of both PkdWRKY and PksWRKY, whereas only PkdWRKY showed transcriptional activation ability. Transient overexpression in tobacco further suggested functionality of PkdWRKY and PksWRKY in planta. Collectively, experimental findings suggested the involvement of WRKY TFs (PkdWRKY and PksWRKY) in picrosides biosynthesis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
TS is thankful to Indian Council of Medical Research (ICMR), for junior/ senior research fellowship. The authors acknowledge financial support from the Council of Scientific and Industrial Research (CSIR), India for funding projects entitled “Genomics of medicinal plants and agronomically important traits (PlaGen), BSC0107” and “Plant diversity: studying adaptation biology and understanding/exploiting medicinally important plants for useful bioactives, SIMPLE-BSC0109”. The manuscript represents CSIR-IHBT communication no. 4232.
Abbreviations
- MVA
Mevalonate
- MEP
Methylerythritol phosphate
- EMSA
Electrophoretic mobility shift assay
- NP
Nucleo-protein
- TF
Transcription factor
Author contributions
TS performed EMSA, gene expression analysis, yeast hybrid assays and wrote the manuscript. TK performed cloning and in silico analysis and wrote the first draft of manuscript. PG performed picroside estimation. DS performed agro-infilteration in tobacco and isolated RNA and DNA from infilterated leaves. AK prepared transient overexpression constructs and performed PCR and qRT-PCR analysis. VH supervised yeast hybrid experiments and finalised the manuscript. SK conceived the study, designed the experiments, coordinated the study and finalised the manuscript. All authors have read and approved the manuscript.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Tanvi Sharma and Tabasum Kawoosa contributed equally to this work.
Contributor Information
Tanvi Sharma, Email: Tanvisharma15@gmail.com.
Tabasum Kawoosa, Email: Tabasumihbt@gmail.com.
Parul Gahlan, Email: parulgahlan@gmail.com.
Damini Sharma, Email: damini.samnol.21.ds@gmail.com.
Anish Kaachra, Email: aneebioinf@gmail.com.
Vipin Hallan, Email: rnaivi@gmail.com.
Sanjay Kumar, Email: sanjayplp1@gmail.com, Email: sanjaykumar@ihbt.res.in.
References
- Akagi A, Fukushima S, Okada K, Jiang CJ, Yoshida R, Nakayama A, et al. WRKY45 dependent priming of diterpenoid phytoalexin biosynthesis in rice and the role of cytokinin in triggering the reaction. Plant Mol Biol. 2014;86:171–183. doi: 10.1007/s11103-014-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GC, Flores-Vergara MA, Krasynanski S, Kumar S, Thompson WF. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat Protoc. 2006;1(5):2320–2325. doi: 10.1038/nprot.2006.384. [DOI] [PubMed] [Google Scholar]
- Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271:1081–1085. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- Calkhoven CF, Geert AB. Multiple steps in the regulation of transcription factor level and activity. Biochem J. 1996;317:329–342. doi: 10.1042/bj3170329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Wang Y, Zhang WH. OsWRKY74, a WRKY transcription factor, modulates tolerance to phosphate starvation in rice. J Exp Bot. 2016;67:947–960. doi: 10.1093/jxb/erv515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang FF, Wang YN, She JJ, Lei YF, Liu ZQ, Eulgem T, et al. Overexpression of CaWRKY27, a subgroup IIe WRKY transcription factor of Capsicum annuum, positively regulates tobacco resistance to Ralstonia solanacearum infection. Physiol Plant. 2014;150:397–411. doi: 10.1111/ppl.12093. [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/s1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- Fan ZQ, Tan XL, Shan W, Kuang JF, Lu WJ, Chen JY. BrWRKY65, a WRKY transcription factor, is involved in regulating three leaf senescence-associated genes in Chinese flowering cabbage. Int J Mol Sci. 2017;18:1228. doi: 10.3390/ijms18061228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahlan P, Singh HR, Shankar R, Sharma N, Kumari A, Chawla V, et al. De novo sequencing and characterization of Picrorhiza kurrooa transcriptome at two temperatures showed major transcriptome adjustments. BMC Genom. 2012;13:126. doi: 10.1186/1471-2164-13-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudinier A, Tang M, Kliebenstein DJ. Transcriptional networks governing plant metabolism. Curr Plant Biol. 2015;3–4:56–64. [Google Scholar]
- Ghawana S, Paul A, Kumar H, Kumar A, Singh H, Bhardwaj PK, et al. An RNA isolation system for plant tissues rich in secondary metabolites. BMC Res Notes. 2011;4:85. doi: 10.1186/1756-0500-4-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Wang H, Lundgren A, Brodelius PE. Effects of overexpression of AaWRKY1 on artemisinin biosynthesis in transgenic Artemisia annua plants. Phytochemistry. 2014;102:89–96. doi: 10.1016/j.phytochem.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Hara K, Yagi M, Kusano T, Sano H. Rapid systemic accumulation of transcripts encoding a tobacco WRKY transcription factor upon wounding. Mol Gen Genet. 2000;263:30–37. doi: 10.1007/pl00008673. [DOI] [PubMed] [Google Scholar]
- Husain GM, Singh PN, Kumar V. Antidiabetic activity of standardized extract of Picrorhiza kurroa in rat model of NIDDM. Drug Discov Ther. 2009;3:88–92. [PubMed] [Google Scholar]
- Jiang W, Yu D. Arabidopsis WRKY2 transcription factor mediates seed germination and post-germination arrest of development by abscisic acid. BMC Plant Biol. 2009;9:96. doi: 10.1186/1471-2229-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi M, Arima TH, Kuroyanagi M. Molecular cloning and transcriptional analysis of WRKY and solavetivone biosynthetic genes in the hairy roots of Hyoscyamus albus. Plant Gene. 2016;5:78–86. [Google Scholar]
- Kawoosa T, Singh H, Kumar A, Sharma SK, Devi K, Dutt S, et al. Light and temperature regulated terpene biosynthesis: hepatoprotective monoterpene picroside accumulation in Picrorhiza kurrooa. Funct Integr Genom. 2010;10:393–404. doi: 10.1007/s10142-009-0152-9. [DOI] [PubMed] [Google Scholar]
- Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, et al. Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell. 2002;14:2481–2494. doi: 10.1105/tpc.004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacomme C, Chapman S. Use of potato virus X (PVX)–based vectors for gene expression and virus induced gene silencing (VIGS) Curr Protoc Microbiol. 2008;8(1):16I–I21. doi: 10.1002/9780471729259.mc16i01s8. [DOI] [PubMed] [Google Scholar]
- Lagace M, Matton DP. Characterization of a WRKY transcription factor expressed in late torpedo-stage embryos of Solanum chacoense. Planta. 2004;219:185–189. doi: 10.1007/s00425-004-1253-2. [DOI] [PubMed] [Google Scholar]
- Li HL, Guo D, Yang ZP, Tang X, Peng SQ. Genome-wide identification and characterization of WRKY gene family in Hevea brasiliensis. Genomics. 2014;104:14–23. doi: 10.1016/j.ygeno.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Li P, Matsunaga K, Yamakuni T, Ohizumi Y. Potentiation of nerve growth factor-action by picrosides I and II, natural iridoids, in PC12D cells. Eur J Pharmacol. 2000;406:203–208. doi: 10.1016/s0014-2999(00)00662-2. [DOI] [PubMed] [Google Scholar]
- Li P, Matsunaga K, Yamakuni T, Ohizumi Y. Picrosides I and II, selective enhancers of the mitogen-activated protein kinase-dependent signaling pathway in the action of neuritogenic substances on PC12D cells. Life Sci. 2002;71:1821–1835. doi: 10.1016/s0024-3205(02)01949-5. [DOI] [PubMed] [Google Scholar]
- Li S, Fu Q, Chen L, Huang W, Yu D. Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermotolerance. Planta. 2011;233:1237–1252. doi: 10.1007/s00425-011-1375-2. [DOI] [PubMed] [Google Scholar]
- Li S, Zhang P, Zhang M, Fu C, Yu L. Functional analysis of a WRKY transcription factor involved in transcriptional activation of the DBAT gene in Taxus chinensis. Plant Biol. 2013;15:19–26. doi: 10.1111/j.1438-8677.2012.00611.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Long L, Shaohua M, Zhanchao C, Yuanwen C, Ying Z, Ying M, et al. Characterization and expression analysis of the WRKY gene family in moso bamboo. Sci Rep. 2017;7:6675. doi: 10.1038/s41598-017-06701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Pu G, Lei C, Ma L, Wang H, Guo Y, et al. Isolation and characterization of AaWRKY1, an Artemisia annua transcription factor that regulates the amorpha-4,11-diene synthase gene, a key gene of artemisinin biosynthesis. Plant Cell Physiol. 2009;50:2146–2161. doi: 10.1093/pcp/pcp149. [DOI] [PubMed] [Google Scholar]
- Nandave M, Ojha SK, Kumari S, Nag TC, Mehra R, Narang R, et al. Cardioprotective effect of root extract of Picrorhiza kurroa (Royle Ex Benth) against isoproterenol-induced cardiotoxicity in rats. Indian J Exp Biol. 2013;51:694–701. [PubMed] [Google Scholar]
- Park CJ, Shin YC, Lee BJ, Kim KJ, Kim JK, Paek KH. A hot pepper gene encoding WRKY transcription factor is induced during hypersensitive response to tobacco mosaic virus and Xanthomonas campestris. Planta. 2006;223:168–179. doi: 10.1007/s00425-005-0067-1. [DOI] [PubMed] [Google Scholar]
- Phukan UJ, Jeena GS, Shukla RK. WRKY transcription factors: molecular regulation and stress responses in plants. Front Plant Sci. 2016;7:760. doi: 10.3389/fpls.2016.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Printen JA, Sprague GFJ. Protein-protein interactions in the yeast pheromone response pathway: Ste5p interacts with all members of the MAP kinase cascade. Genetics. 1994;138:609–619. doi: 10.1093/genetics/138.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri A, Saxena RP, Sumati GPY, Kulshreshtha DK, Saxena KC, Dhawan BN. Immunostimulant activity of Picroliv, the iridoid glycoside fraction of Picrorhiza kurrooa, and its protective action against Leishmania donovani infection in hamsters. Planta Med. 1992;58:528–532. [PubMed] [Google Scholar]
- Rastogi R, Srivastava AK, Rastogi K. Long term effect of aflatoxin B1 on lipid peroxidation in rat liver and kidney: effect of picroliv and silymarin. Phytotherapy Res. 2001;15:307–310. doi: 10.1002/ptr.722. [DOI] [PubMed] [Google Scholar]
- Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep. 1999;16:565–574. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Somssich IE, Ringler P, Shen QJ. WRKY transcription factors. Trends Plant Sci. 2010;15:247–258. doi: 10.1016/j.tplants.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Torres JT, Parniske M, Wernert P, Hahlbrock K, Somssich IE. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J. 1996;15:5690–5700. [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Saraswat B, Visen PK, Patnaik GK, Dhawan BN. Anticholestatic effect of picroliv, active hepatoprotective principle of Picrorhiza kurrooa, against carbon tetrachloride induced cholestasis. Indian J Exp Biol. 1993;31(4):316–318. [PubMed] [Google Scholar]
- Schluttenhofer C, Yuan L. Regulation of specialized metabolism by WRKY transcription factors. Plant Physiol. 2015;167:295–306. doi: 10.1104/pp.114.251769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Yan L, Liu Z, Cao Z, Mei C, Xin Q, et al. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell. 2010;22:1909–1935. doi: 10.1105/tpc.110.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Bhat J, Joshi M, Sinkar V, Ghaskadbi S. Hepatoprotective activity of Picrorhiza kurroa Royle Ex. Benth extract against alcohol cytotoxicity in mouse liver slice culture. Int J Green Pharmacy. 2011;5(3):244–253. [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman K. India’s Biodiversity Act 2002 and its role in conservation. Trop Ecol. 2009;50(1):23–30. [Google Scholar]
- Vom Endt D, Kijne JW, Memelink J. Transcription factors controlling plant secondary metabolism: what regulates the regulators? Phytochemistry. 2002;61:107–114. doi: 10.1016/s0031-9422(02)00185-1. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Tao F, An F, Zou YP, Tian W, Chen XM, et al. Wheat transcription factor TaWRKY70 is positively involved in high-temperature seedling plant resistance to Puccinia striiformis f. sp. tritici. Mol Plant Pathol. 2017;18:649–661. doi: 10.1111/mpp.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo D, Li HL, Peng SQ. Characterization of HbWRKY1, a WRKY transcription factor from Hevea brasiliensis that negatively regulates HbSRPP. Plant Physiol Biochem. 2013;71:283–289. doi: 10.1016/j.plaphy.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J. Transformation of agrobacterium using the freeze-thaw method. CSH protocols. 2006;7:1031–1036. doi: 10.1101/pdb.prot4666. [DOI] [PubMed] [Google Scholar]
- Wu HL, Ni ZF, Yao YY, Guo GG, Sun QX. Cloning and expression profiles of 15 genes encoding WRKY transcription factor in wheat (Triticum aestivem L.) Prog Nat Sci. 2008;18:697–705. [Google Scholar]
- Wu KL, Guo ZJ, Wang HH, Li J. The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Res. 2005;12:9–26. doi: 10.1093/dnares/12.1.9. [DOI] [PubMed] [Google Scholar]
- Xu YH, Wang JW, Wang S, Wang JY, Chen XY. Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-delta-cadinene synthase-A. Plant Physiol. 2004;135:507–515. doi: 10.1104/pp.104.038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhu J, Ni Y, Cai Y, Zhang Z. Expression profiling of HbWRKY1, an ethephon-induced WRKY gene in latex from Hevea brasiliensis in responding to wounding and drought. Trees (Berl) 2012;26:587–595. [Google Scholar]
- Zhang ZL, Shin M, Zou X, Huang J, Ho TH, Shen Q. A negative regulator encoded by a rice WRKY gene represses both abscisic acid and gibberellins signaling in aleurone cells. Plant Mol Biol. 2009;70:139–151. doi: 10.1007/s11103-009-9463-4. [DOI] [PubMed] [Google Scholar]
- Zhang ZL, Xie Z, Zou XL, Casaretto J, Ho THD, Shen QXJ. A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells. Plant Physiol. 2004;134:1500–1513. doi: 10.1104/pp.103.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Mosher SL, Fan B, Klessig DF, Chen Z. Functional analysis of Arabidopsis WRKY25 transcription factor in plant defense against Pseudomonas syringae. BMC Plant Biol. 2007;7:2. doi: 10.1186/1471-2229-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.