The coronavirus disease 2019 (COVID-19) pandemic has caused a major disruption to our health care system. In response to the service demand surge and shortage of personal protective equipment, multiple professional societies have issued recommendations to defer elective endoscopic procedures except time-sensitive and emergency procedures.1, 2, 3 As yet, the real-life impact of this pragmatic service reduction is unknown. Here, we report the short-term impacts of the COVID-19 pandemic on the volume of gastrointestinal endoscopy and new diagnoses of gastric and colon cancers in Hong Kong and model the changes in cancer staging.
Methods
The first local COVID-19 case was reported on January 23, 2020, and through May 8, 2020, 1045 cases were reported. The total numbers of upper and lower endoscopy performed in all public hospitals in Hong Kong between October 1, 2019 and March 31, 2020 and from the same period in the preceding 3 years were retrieved from the Clinical Data Analysis and Reporting System of the Hong Kong Hospital Authority. Cancer diagnosis was verified by the Anatomic Pathology Database of the Clinical Data Analysis and Reporting System.4 , 5 The autoregressive integrated moving average (ARIMA) model was used to predict the trend of patients newly diagnosed with gastric or colorectal cancers with the Buishand U test to determine the week of turning point of the sudden changes. A linear regression model was constructed to assess the relationship between the number of cancers diagnosed and the volume of endoscopy. The potential stage shifting of these cancers with delayed diagnosis was estimated by using a state-transition Markov model (Supplementary Material). The study was approved by the institutional review board of the University of Hong Kong and the Hong Kong West Cluster of Hospital Authority, Hong Kong (reference no. UW 20-279).
Results
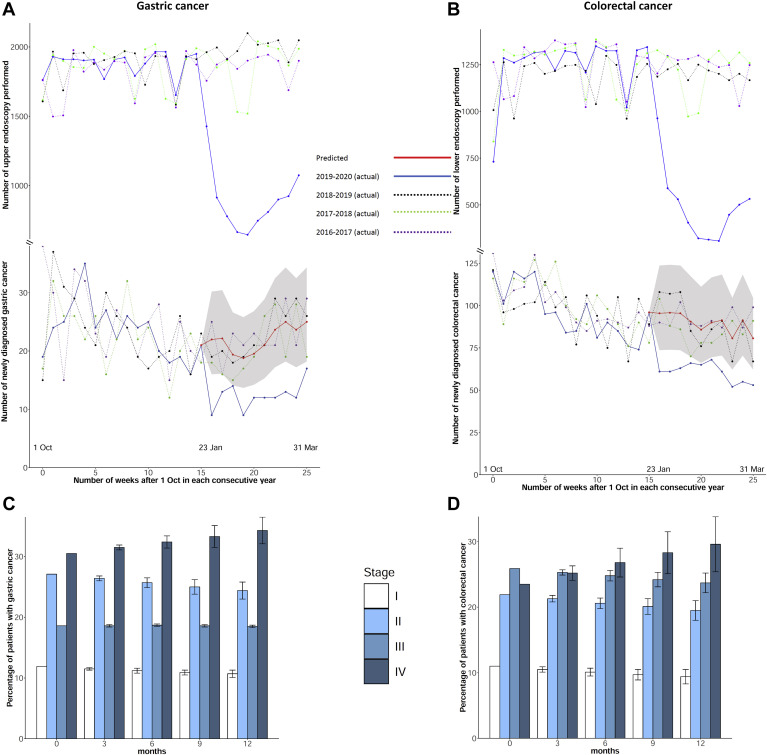
The number of procedures performed and the number of cancers diagnosed in the study period are shown in Supplementary Table 1. The Buishand U test showed a turning point in the number of upper and lower endoscopies performed (P < .001) and the number of gastric and colorectal cancers diagnosed (P < .001) during the week of January 21–27, 2020, when the first local COVID-19 case was diagnosed. From that week onward, the mean number of upper and lower endoscopies performed per week dropped by 51.0% (from 1813 to 887; P < .001) and 58.8% (from 1190 to 491; P < .001), respectively. The mean gastric cancer and colorectal cancer diagnosed per week also fell by 46.2% (from 22.9 to 12.3; P < .001) and 37.0% (from 92.1 to 58; P < .001), respectively. Notably, the positive rates (per 1000 endoscopies) for gastric cancer remained static throughout these periods (11.8 to 13.9; P = .14) (Supplementary Table 1) but increased for colorectal cancer (75.6 to 118.2; P < .001).
For gastric cancer, the ARIMA(0,1,1)(1,0,1)26 model with a 26-week cyclical pattern was fitted with data from previous years. The mean percent error values of the model were 2.5% and 3.5% for the training set and test set, respectively. For colorectal cancer, the mean percent error values of the ARIMA(0,0,1)(1,0,1)26 model were 2.8% and 3.3%, respectively. For the 10-week period starting from January 21, 2020, the predicted numbers of gastric cancers and colorectal cancers were higher than observed by 51.1%, (221; 95% confidence interval [CI], 136–357 vs 123; P < .01) and 32.9% (865; 95% CI, 668–1286 vs 580; P < .01), respectively (Figure 1 ). Based on the Markov model prediction, 4.6% of patients with gastric cancer and 6.4% of patients with colorectal cancer would have higher stage shifting at 6 months. In particular, the proportion of stage IV cancers increased (gastric: 30.5% to 32.4%; P < .01 and colorectal: 23.5% to 26.8%; P < .01).
Figure 1.
(A, B) Actual vs predicted numbers (by ARIMA) of endoscopy and cancers diagnosed per week: (A) gastric cancer and (B) colorectal cancer. (C, D) Prediction of changes in cancer staging with various durations of COVID-19 outbreak in Hong Kong: (C) gastric cancer and (D) colorectal cancer. The shaded areas and error bars represent 95% CIs.
The linear regression model showed a linear relationship between the number of gastric cancers and upper endoscopy volume and between the number of colorectal cancers and lower endoscopy volume, both before (gastric: 0.68; 95% CI, 0.30–1.05; colorectal: 0.77; 95% CI, 0.54–0.94) and after the turning point (gastric: 0.42; 95% CI, 0.07–0.76; colorectal: 0.34; 95% CI, 0.05–0.37). If there is a further 20% reduction in current endoscopy volume, the corresponding mean numbers of gastric cancers and colorectal cancers diagnosed per week would fall by 54.1% (10.5 vs 22.9) and 41.1% (54.0 vs 91.7), respectively, from baseline. With a 20% greater reduction in endoscopy services, the predicted proportions of patients with gastric and colorectal cancer with stage upshifting were 5.3% (vs current 4.6%) and 7.2% (vs current 6.4%), respectively, at 6 months (Supplementary Figure 1).
Supplementary Figure 1.
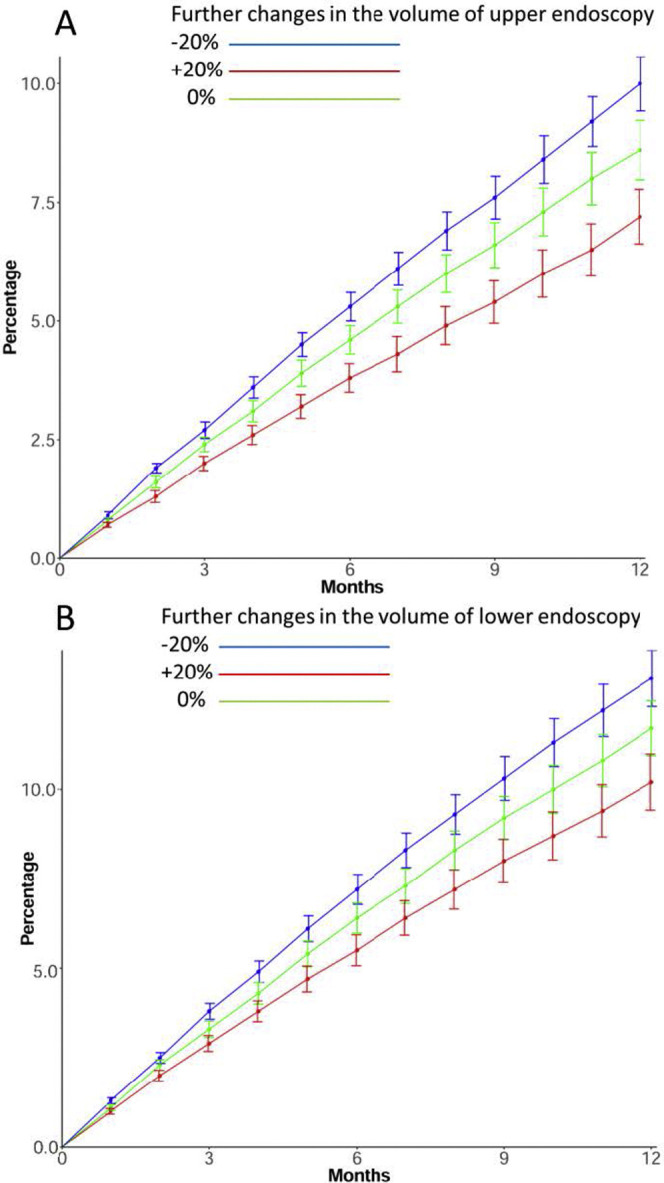
Predicted effects of various levels of endoscopy volume reduction on percentage of patients with cancer upstaging on diagnosis. (A) Gastric cancer. (B) Colorectal cancer.
Discussion
To our knowledge, this is the first study to quantify the disruption brought by the COVID-19 pandemic on the delivery of gastrointestinal endoscopy and its impacts on cancer diagnosis and staging at a population level. Although the volume of endoscopy was reduced by more than 50% in Hong Kong, the number of patients diagnosed with gastric and colorectal cancer dropped by 49.1% and 38.1%, respectively. Based on the ARIMA prediction and Markov model, we estimated that 4.6% and 6.4% of patients with gastric and colorectal cancer would have cancer stage upshifting at 6 months.
In a recent report from a single hospital in Shanghai, China, it was noted that the endoscopy volume dropped by 6.3 times during COVID-19.6 In another North American survey, 65% of centers were performing less than 10% of their usual volume.7 However, public hospitals in Hong Kong do not routinely perform screening endoscopy, and the potential impacts on delayed cancer diagnosis could be worse than in centers that primarily perform screening procedures.
Interestingly, we noted that the impact of service reduction on upper endoscopy was more critical than on lower colonoscopy because the positive rate for cancer by lower colonoscopy actually increased after the outbreak, suggesting that triage based on lower gastrointestinal symptoms is more reliable than upper gastrointestinal symptoms in arranging early endoscopy.
In conclusion, the COVID-19 outbreak has resulted in a substantial drop in endoscopy services in Hong Kong, which is associated with a significant delay in cancer diagnosis. The delay and subsequent cancer stage upshifting will be amplified if the COVID-19 pandemic lengthens further. Gradual resumption of nonemergency endoscopy services should be considered as early as feasible and practical, balancing the risks of delayed cancer diagnosis and the personal safety of patients and staff.
Acknowledgments
CRediT Authorship Contributions
Thomas K.L. Lui, MBBS, MMedSc (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Methodology: Lead; Writing – original draft: Equal); Kathy Leung, PhD (Data curation: Equal; Formal analysis: Equal; Methodology: Equal); Chuan-Guo Guo, MMed (Data curation: Equal; Formal analysis: Supporting; Methodology: Supporting); Vivien W.M. Tsui, MBBS (Data curation: Equal; Project administration: Supporting; Validation: Supporting); Joseph T. Wu, PhD (Formal analysis: Supporting; Investigation: Supporting; Methodology: Supporting; Supervision: Supporting); Wai K. Leung, MD (Conceptualization: Lead; Methodology: Equal; Project administration: Lead; Resources: Equal; Supervision: Lead; Writing – original draft: Equal; Writing – review & editing: Lead).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.05.037.
Supplementary Methods
Time Trend Analysis of Newly Diagnosed Gastric and Colorectal Cancers During the Coronavirus 2019 Pandemic
We analyzed the numbers of upper and lower endoscopies performed and the number of new cases of gastric cancer and colorectal cancer diagnosed between October 1 and March 31 in each year on a weekly basis. The Buishand U test was used to determine the week of the turning point of sudden changes in the number of endoscopies performed and the number of cancers diagnosed (ie, week of turning point). The mean number of endoscopies performed and the mean number of cancers diagnosed per week were compared by 2-sample t test before and after the turning point. Pearson correlation was used to assess for any association between the number of cancers diagnosed and the volume of endoscopy.
ARIMA was used to predict the trend of patients diagnosed with gastric or colorectal cancers. The corrected Akaike information criterion was used to choose the ARIMA models with different parameter combinations. The weekly data were split into different training sets from October 1, 2016, to March 31, 2017; from October 1, 2017, to March 31, 2018; and from October 1, 2018 to March 31, 2019. The training data were first used to fit the model with adjustment of the holiday effect of Christmas and Lunar New Year. The test set ran from October 1, 2019, to the week of the turning point as determined by the Buishand U test for internal and external validation of the model. The mean percent error was used to test the accuracy of the forecast. Based on the numbers of new diagnoses of gastric or colorectal cancer before the week of the turning point as determined by the Pettitt test, ARMIA prediction of the numbers of patients diagnosed with gastric or colorectal cancer was made. The week-based data after excluding the Lunar New Year holiday effect were used in the ARIMA prediction. The difference between the ARIMA predictions and actual numbers of patients diagnosed with gastric cancer or colorectal cancer were used to estimate the number of patients with delayed cancer diagnosis after the turning point.
Prediction of Cancer Stage Shifting at Diagnosis
The baseline stage distributions of colorectal cancer in Hong Kong were as follows: stage I, 11.0%; stage II, 21.9%; stage III, 25.9%; stage IV, 23.5%; and unstaged, 17.7%.1 The corresponding stage distributions of gastric cancer were as follows: stage I, 11.8%; stage II, 27.1%; stage III, 18.6%; stage IV, 30.5%; and unstaged, 16.3%.
Based on currently available data for gastric cancer, the annual transition rate from stage I to II was 0.20, from stage II to III was 0.30, and from stage III to IV was 0.40.2 For colorectal cancer, the annual transition rate from stage I to II was 0.58, from stage II to III was 0.66, and from stage III to IV was 0.83.3 The potential stage shifting of these cancers with delayed diagnosis was estimated by using a state-transition Markov model. Linear regression models were formulated for the association between upper endoscopy volume and number of newly diagnosed gastric cancers and between lower endoscopy volume and number of newly diagnosed colorectal cancers.
All statistical analyses were performed with R statistical software, version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables are expressed as median and interquartile range. The Mann-Whitney U test was used to compare continuous variables between 2 groups. The chi-squared test or Fisher exact test, where appropriate, was applied for comparing categorical variables.
Supplementary Table 1.
Number of Endoscopic Procedures and Diagnoses in Hong Kong During the Study Period (October 1–March 31, 2016–2020)
| Endoscopic procedures and diagnoses | Oct 2016–Mar 2017 |
Oct 2017–Mar 2018 |
Oct 2018–Mar 2019 |
Oct 2019–Mar 2020 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before week of turning point (Jan 21–27) |
After week of turning point (Jan 21–27) |
|||||||||
| Whole period | Average per week | Whole period | Average per week | Whole period | Average per week | Whole period | Average per week | Whole period | Average per week | |
| Upper endoscopy, n | 46,129 | 1774.2 | 47,588 | 1830.3 | 48,302 | 1857.8 | 28,932 | 1808.3 | 8878 | 887.8 |
| Lower endoscopy, n | 31,142 | 1197.8 | 31,835 | 1224.4 | 29,522 | 1135.5 | 19,433 | 1214.6 | 4909 | 490.9 |
| New gastric cancer, n | 629 | 24.2 | 562 | 21.6 | 587 | 22.6 | 374 | 23.4 | 123 | 12.3 |
| New colorectal cancer, n | 2484 | 95.5 | 2388 | 91.8 | 2314 | 89.0 | 1469 | 91.8 | 580 | 58.0 |
| Positive rate for gastric cancer per 1000 upper endoscopies | 13.6 | — | 11.8 | — | 12.2 | — | 12.9 | — | 13.9 | — |
| Positive rate for colorectal cancer per 1000 lower endoscopies | 79.8 | — | 75.0 | — | 78.4 | — | 75.6 | — | 118.2 | — |
References
- 1.Sultan S., et al. Gastroenterology. 2020;159:739–758. doi: 10.1053/j.gastro.2020.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gralnek I.M., et al. Endoscopy. 2020;52(6):483–490. doi: 10.1055/a-1155-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu P.W.Y., et al. Gut. 2020;69(6):991–996. doi: 10.1136/gutjnl-2020-321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung K.S., et al. Gut. 2018;67:28–35. doi: 10.1136/gutjnl-2017-314605. [DOI] [PubMed] [Google Scholar]
- 5.Leung W.K., et al. Gastroenterology. 2018;155:67–75. doi: 10.1053/j.gastro.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Zhu L., et al. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:327–331. doi: 10.3760/cma.j.issn.1671-0274.2020-0316-00147. [DOI] [PubMed] [Google Scholar]
- 7.Forbes N., et al. Gastroenterology. 2020;159:772–774. doi: 10.1053/j.gastro.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplementary References
- 1.Hospital Authority. Hong Kong Cancer Registry. Available at: https://www3.ha.org.hk/cancereg/. Accessed Aug 19, 2020.
- 2.Oh S.Y., et al. Ann Surg Oncol. 2019;26:2905–2911. doi: 10.1245/s10434-019-07455-z. [DOI] [PubMed] [Google Scholar]
- 3.Li Z.F., et al. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38:253–260. doi: 10.3760/cma.j.issn.0254-6450.2017.02.024. [DOI] [PubMed] [Google Scholar]