Introduction
It is unclear whether in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, acute kidney injury (AKI) results from direct infection of the kidneys or from complications arising in the course of coronavirus disease 2019 (COVID-19). Reports of urinary abnormalities in COVID-19 patients,S1 positive staining of tubules with viral antigens and complement components in one autoptic series,1 visualization of viral particles in tubular epithelial cells and podocytes on ultrastructural examination in another,2 and isolation of SARS-CoV-2 in urineS2,S3 raised the possibility of a SARS-CoV-2 nephropathy. We describe the clinical course, renal histopathology, and molecular testing of renal tissue, serum, and urinary samples of a critically ill patient with COVID-19 who developed oligoanuric AKI. The absence of viral genetic material in tested specimens and lack of histologic evidence for cytopathic effects do not support the hypothesis of the kidneys as target organs of SARS-CoV-2 infection.
Case Presentation
A 49-year-old overweight but otherwise healthy male was admitted to the emergency room for dyspnea, cough, and fever. He had no known contacts with SARS-CoV-2–positive individuals, nor history of recent travel abroad. His peripheral capillary oxygen saturation (SpO2) was 98%, heart rate 112 bpm, and body temperature 100.4 °F. SpO2 worsened over a few hours, requiring oxygen therapy. A chest computed tomography scan showed interstitial pneumonia with ground-glass opacities. As he failed to meet strict epidemiologic criteria for COVID-19, imaging findings were deemed compatible with bacterial infection. Azithromycin and piperacillin/tazobactam were started. Real-time reverse transcriptase polymerase chain reaction (RT-PCR) for SARS-CoV-2 on nasal and oropharyngeal swabs tested positive and he was transferred to the infectious diseases unit (day 2 since hospitalization), where he received the institutional treatment protocol for COVID-19 (200 mg bid hydroxychloroquine and 400/100 mg bid lopinavir-ritonavir). SpO2 deteriorated to 84% on high-flow oxygen therapy. By day 6, he developed acute respiratory distress syndrome (ARDS) and was transferred to the general intensive care unit, where after a trial of noninvasive mechanical ventilation he was sedated, intubated, curarized, and put on lung-protective mechanical ventilation. On day 12, he was tracheostomized. The clinical course was complicated by acute heart failure with severely reduced ejecton fraction and hypotension requiring fluid resuscitation and inotropes. In the meantime (day 8), he developed oligoanuric AKI requiring overall twenty 12-hour sustained low-efficiency dialysis (SLED) sessions with citrate regional anticoagulation.3 On day 19, he was transferred to the intensive care section of the renal unit. Urinary output slowly increased without amelioration of renal function. Oropharyngeal swabs repeatedly tested positive for SARS-CoV-2 RT-PCR. A kidney biopsy was performed on day 37. The patient later became afebrile and was successfully weaned off both ventilation and tracheostomy and transferred to the inpatient clinic of the renal unit. He is currently doing well and is afebrile with 96% SpO2 in ambient air. Renal function is slowly but steadily ameliorating with no current need for dialysis (last session on day 34; time of writing: day 51).
Kidney biopsy showed extensive acute tubular injury (ATI) with marked regenerative changes and focal acute tubular necrosis (Figure 1). No nuclear atypia, syncytia, or inclusion bodies were observed. An extensive immunohistochemical stain panel for CD3, CD20, CD68, and CD163 showed minimal, focal lymphocytic tubulitis and mild diffuse mixed interstitial inflammation (Figure 2). Immunofluorescence on frozen tissue including immunostaining for IgG, IgA, IgM, kappa and lambda light chains, C3, C4d, C1q, C5b9, and fibrinogen was negative or nonspecific. Viral particles were not observed on ultrastructural examination (Figure 3).
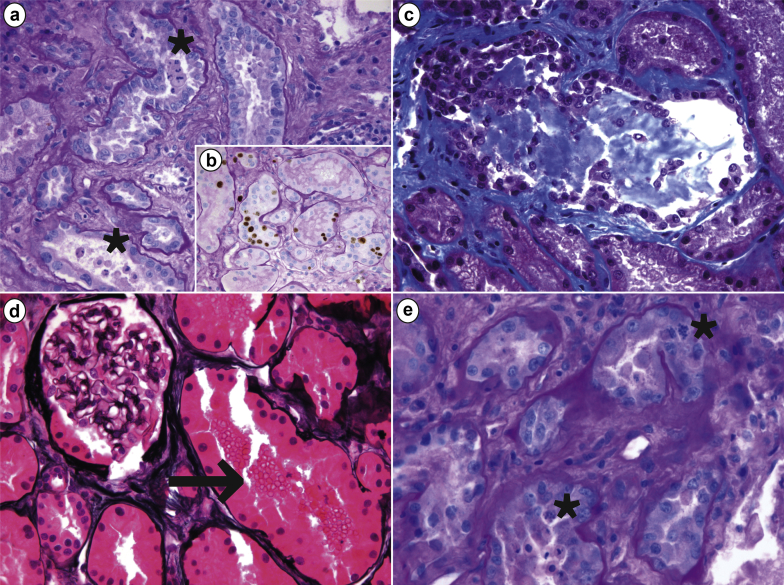
Figure 1.
Morphologic features of acute tubular injury on renal biopsy of a critically ill patient with coronavirus disease 2019 (COVID-19) and acute kidney injury. (a) Diffuse tubular injury and focal tubular necrosis with several tubules showing intraluminal epithelial sloughing (asterisks; periodic acid–Schiff [PAS], original magnification ×400); (b) epithelial cells showed an increased proliferative index, revealed by Ki67 positivity (Ki67+PAS, original magnification ×400). (c) Some tubules displayed mixed casts formation (Masson trichrome, original magnification ×400). (d) Several tubules had intraluminal red blood cells (arrow) whereas glomeruli appeared normal overall (methenamine-silver, original magnification ×400). (e) Epithelial cells showed regenerative changes with frequent mitotic figures (asterisks; PAS, original magnification ×600).
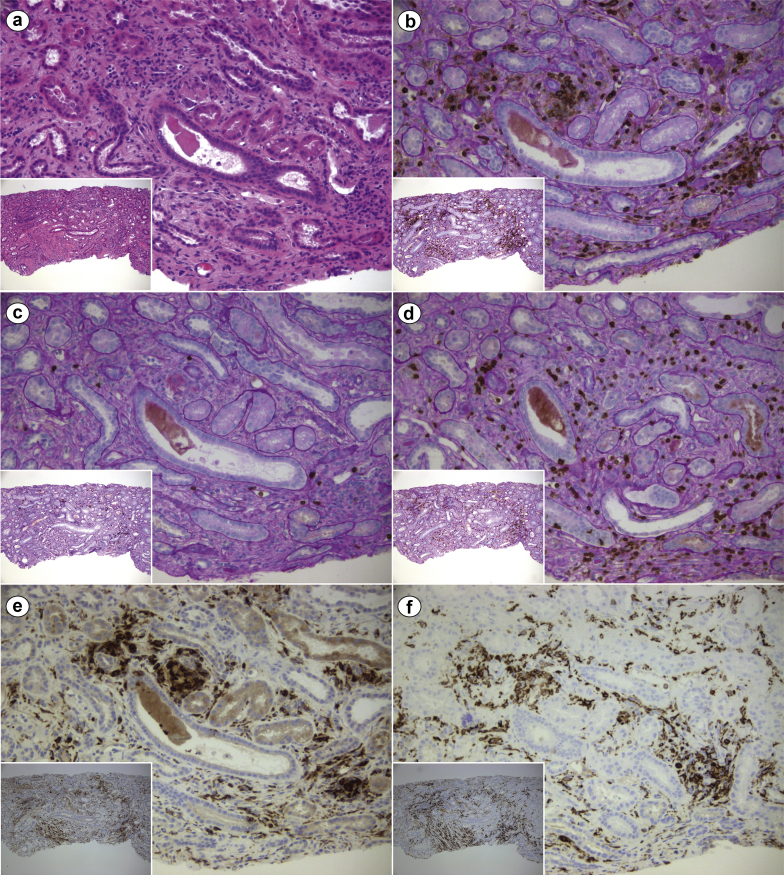
Figure 2.
Immunohistochemical characterization of the interstitial inflammatory infiltrate on renal biopsy of a critically ill patient with coronavirus disease 2019 (COVID-19) and acute kidney injury. (a) Mild diffuse interstitial inflammatory infiltrate in the setting of interstitial edema and acute tubular injury with tubuloepithelial flattening and simplification (hematoxylin and eosin, original magnification ×200 and ×100 [inset]). Immunohistochemistry studies showed (b) diffuse leukocyte common antigen (LCA) (CD45)–positive infiltrate (LCA + periodic acid–Schiff [PAS], original magnification ×200 and ×100 [inset]), with (c) a scarce B-cell component (CD20 + PAS, original magnification ×200 and ×100 [inset]), but relevant presence of (d) T cells (CD3 + PAS, original magnification ×200 and ×100 [inset]) and CD68–CD163–positive macrophages (e [CD68] and f [CD163], original magnification ×200 and ×100 [insets]). LCA, as the name implies, is a pan-leukocyte marker; CD3 and C20 are T cell– and B cell–specific, respectively; CD68 and CD163 are both macrophage markers. Overall, the inflammatory infiltrate was mixed and nonspecific, as commonly observed in a variety of conditions, including the typical inflammation associated with tubular atrophy/interstitial fibrosis. CD, cluster of differentiation.
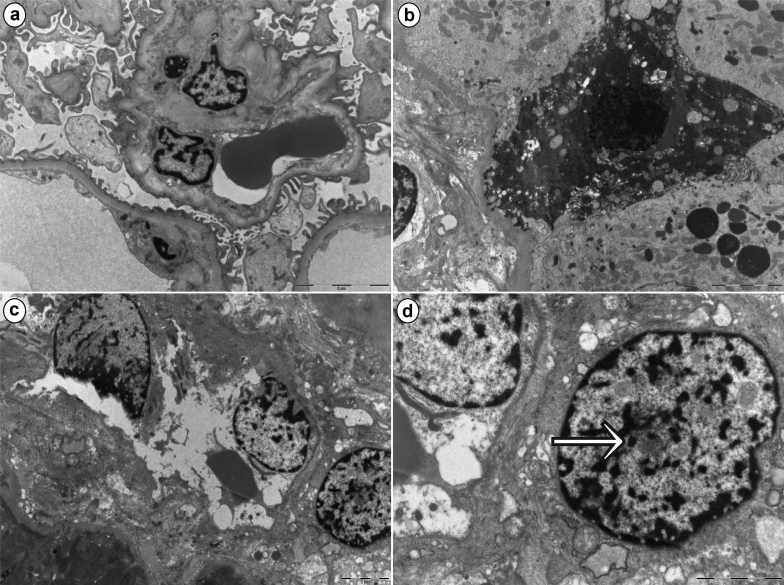
Figure 3.
Ultrastructural findings on renal biopsy of a critically ill patient with coronavirus disease 2019 (COVID-19) and acute kidney injury. Low magnification of a glomerulus showing overall well-preserved architecture, with patent capillaries and irregular flattening of podocyte foot processes. (a) Effacement of the latter was minimal and confined to a few areas (bar = 5 μm). (b) A degenerating epithelial cell with a pyknotic nucleus is consistent with acute tubular injury (bar = 5 μm). (c) A blood vessel, likely a peritubular capillary, shows degenerating endothelial cells (bar = 2 μm). Higher magnification of the same image as in (c), highlighting (d) the presence of a “nuclear body” (arrow) in a fibroblast adjacent to the peritubular capillary (bar = 2 μm). Nuclear bodies are thought to be the expression of “cellular hyperactivity” due to several causes, including physiological and disease states.
Real-time RT-PCR (per CDC guidelines, https://www.cdc.gov/coronavirus/2019-ncov/lab/) on frozen renal tissue, urine, and serum collected the same day of the biopsy was negative for SARS-CoV-2 RNA.
Discussion
During the 2003 Hong Kong outbreak of SARS, AKI occurred in 8 patients out of a series of 536 (6.7%).4 AKI was more frequently reported in Middle East respiratory syndrome (MERS), although with a wide variability, reaching a prevalence of 58% in one small series of critically ill patients.5,S4 Early reports of COVID-19 showed a low incidence of AKI (0.5%–15%S2,S5–S7). SARS-CoV and MERS-CoV differ in their point of entry into cells, the former entering via angiotensin-converting enzyme-2 and the latter via dipeptidyl peptidase-4, both extensively expressed by human kidney epithelial cells.S8,S9 Despite extensive expression, in vitro studies showed that while both viruses could enter and replicate in immortalized human kidney epithelial cells, only MERS-CoV determined cytopathic effects (cell detachment and death), therefore implicating a possible causal relationship between kidney infection and AKI in MERS.6 The first live COVID-19 patient who underwent a kidney biopsy was a non-critically ill African American female with prior comorbidities (including chronic kidney disease) who developed AKI that eventually required dialysis. She never developed ARDS. As kidney biopsy showed collapsing focal segmental glomerulosclerosis, a high-interferon state induced by viral infection in the setting of apolipoprotein-1 high-risk status was hypothesized, and no evidence for virus infection was established based on in situ RNA hybridization and ultrastructural examination.7
“Viral nephropathy” is a somewhat misleading term, encompassing (i) nephropathies resulting from systemic (e.g., capillary leak due to cytokine storm, as in Dengue-associated AKIS10,S11) or local (e.g., in situ antigen deposition, as in hepatitis B virus–associated membranous nephropathy) consequences of the infection without actual infection of renal cells, and (ii) nephropathies due to infection of the kidneys (i.e., viral tropism of the kidney), as with direct cytopathic effects on tubular epithelial cells in BK virus nephropathy and on endothelial cells in Hantavirus nephropathy.S12 AKI during acute viral illness can derive from a plethora of causes. In SARS-related AKI, there was no evidence of viral tropism of the kidney despite detection of viral RNA fragments by RT-PCR in urinary samplesS13 and distal tubular cells.S14 Thus, kidney injury was ascribed to hemodynamic changes secondary to cytokine storm and ensuing capillary leak syndrome, rather than to direct target organ infection.4 An in vitro study, however, showed that productive infection of tubular and mesangial cells was attained by SARS-CoV, albeit with no cytopathic effect.8 In the current dramatic SARS-CoV-2 outbreak, indirect evidence from autopsy series raised the possibility of viral kidney tropism leading to AKI, although the low incidence of the latter argues against it.1,2
In their series of 6 COVID-19 autopsies, Diao et al.1 reported ATI, and in contrast with our biopsy, tubulointerstitial nephritis with a predominant macrophagic infiltrate and deposition of the terminal complement cascade component C5b9 along tubules. Immunohistochemistry for SARS-CoV-2 antigen nucleocapsid protein was positive in epithelial cells, which showed syncytia, a morphologic correlate of viral cytopathy, leading the authors to argue that altogether these findings proved viral tropism of the kidney. In a further autopsy series of 26 cases by Su et al.,2 similar morphologic and immunohistochemical findings were reported, with the addition of ultrastructural evidence of viral particles in both podocytes and tubular epithelial cells, with features similar to those of MERS-CoV and SARS-CoV.2 Based on these findings, the authors openly state that SARS-CoV-2 infection of renal parenchyma occurs, the virus being a direct effector of ATI. These claims, however, are based on fragile grounds.
Syncytia by light microscopy (described by Diao et al.1 but not Su et al.2) and viral particles by ultrastructural examination can be difficult to unequivocally identify, whereas the inherent limits of such techniques can be partly overcome with the aid, for example, of in situ hybridization. Immunohistochemistry for viral antigens as compared to genetic material demonstration is intrinsically limited by low specificity; this also applies to SARS-CoV-2 nucleocapsid protein immunohistochemistry, as reported by Larsen et al.7 More importantly, neither observation provides reproducible proof of viral tropism with productive infection, which would require the concurrent demonstration of renal cell infection (i.e., viral entry) together with viral replication and/or cytopathy.S15 In fact, even the presence of viral antigens or genetic material in organs might not necessarily translate into tissue damage, as with cytomegalovirus infection.9
Finally, ATI is notoriously hard, if not impossible, to differentiate from autolysis, one of the most common findings in autopsy kidneys, and although Su et al.2 reported no cases showed autolysis in their series, it is unclear how they excluded it, as no reliable histologic criteria exist to tell them apart; they do, however, state that all autopsies were performed with a postmortem interval of less than 6 hours, which might be short enough to prevent significant autolysis.S16,S17 Regardless, the attribution of ATI to viral infection based solely on the observation of sparse viral particles is questionable, considering the multitude of complications capable of inducing ATI that a critically ill patient with ARDS commonly experiences in the intensive care unit.
Altogether, the histologic features we observed were consistent with ATI of any etiology, and particularly ischemic ATI/acute tubular necrosis,S18 which fits well with prolonged hypotension. Tubulitis and nonspecific interstitial inflammation were also observed, but the former was minimal, whereas the latter was confined to areas of initial tubular atrophy, which is hardly consistent with a diagnosis of acute interstitial nephritis.
By failing to show evidence of the virus on serum, urine, and renal tissue using the most sensitive method available (real-time RT-PCR), despite repeatedly positive nasopharyngeal swabs, our case stands as evidence against the hypothesis of a SARS-CoV-2 nephropathy. However, we are reporting a single case, and formal proof can only derive from functional studies in a rigorous experimental setting, such as those conducted for MERS-CoV.6 Our case hopefully shed some light on kidney involvement (or, rather, lack thereof) by SARS-CoV-2, while showing how pathomorphology alone is insufficient to prove or disprove viral tropism, even when supported by sophisticated techniques such as PCR and in situ hybridization.
Larger studies on kidney biopsies of patients with COVID-19 are needed to definitively rule out the existence of a SARS-CoV-2 nephropathy, provided they are coupled with scientifically sound experimental evidence of direct infection of renal cells by the virus (Table 1). During this pandemic, we grew accustomed to unorthodox means of diffusion of scientific knowledge, including social media, articles not yet peer-reviewed, recommendations of empiric therapy based on very thin evidence (often changing overnight), and lots of buzz on presumed pathophysiology. We argue that our case is a reminder of the necessity, even during global emergencies, to support scientific statements with reproducible evidence, and to rigorously distinguish between observation, association, and causal relationship. Indeed, failure to do so harbors the risk of translating questionable theories of disease pathogenesis into therapeutic trials with potential harm to patients.
Table 1.
Key teaching points
|
|
|
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Disclosure
All the authors declared no competing interests.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Diao B., Wang C., Wang R. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. 2020 doi: 10.1038/s41467-021-22781-1. 2020.03.04.20031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiaccadori E., Regolisti G., Cademartiri C. Efficacy and safety of a citrate-based protocol for sustained low-efficiency dialysis in AKI using standard dialysis equipment. Clin J Am Soc Nephrol. 2013;8:1670–1678. doi: 10.2215/CJN.00510113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu K.H., Tsang W.K., Tang C.S. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackay I.M., Arden K.E. MERS coronavirus: diagnostics, epidemiology and transmission. Virol J. 2015;12:222. doi: 10.1186/s12985-015-0439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckerle I., Muller M.A., Kallies S. In-vitro renal epithelial cell infection reveals a viral kidney tropism as a potential mechanism for acute renal failure during Middle East Respiratory Syndrome (MERS) Coronavirus infection. Virol J. 2013;10:359. doi: 10.1186/1743-422X-10-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen C.P., Bourne T.D., Wilson J.D. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep. 2020;5:935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pacciarini F., Ghezzi S., Canducci F. Persistent replication of severe acute respiratory syndrome coronavirus in human tubular kidney cells selects for adaptive mutations in the membrane protein. J Virol. 2008;82:5137–5144. doi: 10.1128/JVI.00096-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabrielli L., Bonasoni M.P., Lazzarotto T. Histological findings in foetuses congenitally infected by cytomegalovirus. J Clin Virol. 2009;(suppl 4):S16–S21. doi: 10.1016/j.jcv.2009.09.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.