Abstract
Mitochondria are involved in crucial homeostatic processes in the cell: the production of adenosine triphosphate and reactive oxygen species, and the release of pro-apoptotic molecules. Thus, cell survival depends on the maintenance of proper mitochondrial function by mitochondrial quality control. The most important mitochondrial quality control mechanisms are mitochondrial unfolded protein response, mitophagy, biogenesis, and fusion-fission dynamics. This review deals with mitochondrial quality control in heart diseases, especially myocardial infarction and heart failure. Some previous studies have demonstrated that the activation of mitochondrial quality control mechanisms may be beneficial for the heart, while others have shown that it may lead to heart damage. Our aim was to describe the mechanisms by which mitochondrial quality control contributes to heart protection or damage and to provide evidence that may resolve the seemingly contradictory results from the previous studies.
Mitochondria are involved in adenosine triphosphate (ATP) generation, biosynthetic processes and redox homeostasis. However, dysfunctional mitochondria can become a source of endogenous noxious stimuli that can severely damage the cells, such as the overproduction of reactive oxygen species (ROS), cellular calcium overload, opening of mitochondrial permeability transition pore (mPTP), and release of pro-apoptotic signals (1,2). Thus, it is of vital importance to maintain mitochondrial function by (intra)mitochondrial quality control (MQC) mechanisms. MQC either repairs the damaged mitochondria by restoring or destroying impaired proteins through the activation of mitochondrial unfolded protein response (UPRmt), or removes mitochondria damaged beyond repair by mitophagy (3). Mitophagy is closely balanced with mitochondrial biogenesis to maintain total mitochondrial mass. Rapid changes in fusion and fission of mitochondria are associated with ROS generation and apoptosis, but are also interconnected with other MQC mechanisms (4).
Cardiovascular diseases, especially acute myocardial infarction (MI) and chronic heart failure (HF), account for numerous deaths and severely undermine the quality of life (5,6). A crucial etiological factor in these diseases is mitochondrial dysfunction (7). The aim of this article is to review the present data on underlying mechanisms of heart disease, especially HF and MI, mediated by improper MQC functioning. While the broad scientific community recognizes MQC as a beneficial homeostatic mechanism, numerous studies demonstrate its opposite effects on cardiac diseases. Some studies report a cardioprotective role of MQC, while others show its negative effects in major heart disease. Here, we will try to provide a plausible explanation of such discrepancies. The article addresses major components of MQC in heart disease, including UPRmt, mitophagy, mitochondrial biogenesis, and mitochondrial fusion-fission, as well as MQC in the aged heart.
Basic mechanisms of injury in myocardial infarction and heart failure
The common noxious stimuli in MI and HF are excessive ROS generation and mitochondrial calcium overload (1,2,8,9). In both diseases, ROS and mitochondrial calcium overload induce opening of the mPTP, which ultimately leads to apoptotic or necrotic cell death (1,2,10). Mitochondrial permeability transition pore opening initiates the events that lead to the release of intramitochondrial proapoptotic factors, including cytochrome c, diablo IAP-binding mitochondrial protein, HTRA serine peptidase 2 [OMI/HTRA2], apoptosis-inducing factor, and endonuclease G (11). It is believed that a less extensive mPTP opening results in apoptosis, mostly in the periphery of MI (12). More extensive mPTP opening in the center of MI leads to necrotic cell death, possibly due to severe ATP depletion and the inability to complete the energy-dependent process of apoptosis (12). A major role in the pathogenesis of heart injury is played by the substrates used for energy metabolism. For example, the use of fatty acids can enhance ROS generation and thereby damage cardiomyocytes (13).
Mitochondrial unfolded protein response
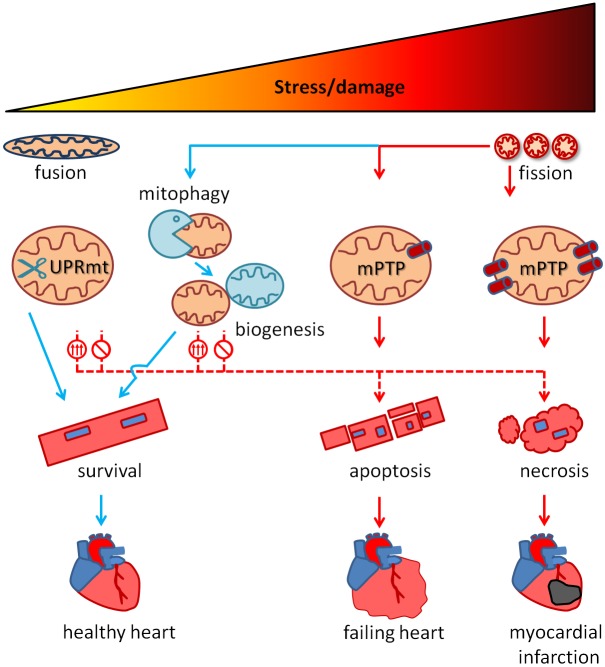
UPRmt is evolutionally a highly conserved MQC mechanism that helps maintain normal mitochondrial function under pathological conditions (Figure 1). It can be triggered by damage to mitochondrial proteins, imbalance between mitochondrial and nuclear proteome (mitonuclear imbalance), or other stressors, such as mitochondrial depolarization. UPRmt and endoplasmic reticulum UPR share some elements, especially transcription factors such as C/EBP homologous protein (CHOP), CCAAT/enhancer-binding protein β (C/EBPβ), or eukaryotic initiation factor 2α (eIF2α) (14,15). UPRmt involves a complex machinery of signaling molecules, transcription factors, proteases (OMI/HTRA2, lon peptidase 1 [LONP1], caseinolytic mitochondrial matrix peptidase proteolytic subunit [ClpP], paraplegin, YME1 like 1 ATPase [YME1L], mitochondrial-processing peptidase [MPP], and OMA1 zinc metallopeptidase [OMA1]), antioxidants (thioredoxin 2), endonuclease G, and chaperons (mitochondrial 70kDa heat shock protein, heat shock protein family D [Hsp60] member 1, heat shock protein family E [Hsp10] member 1, and DnaJ [Hsp40] homologue, subfamily A, member 3) (16-19). The peptides obtained by mitochondrial proteases are extruded from mitochondria by HAlF transporter (ATP Binding Cassette Subfamily B Member 10 in mammals), further activating transcription factors CHOP, C/EBPβ, and activating transcription factor 4 (ATF-4) via c-Jun/AP-1 (14,15).
Figure 1.
Mitochondrial quality control (MQC) in heart disease. Everyday moderate stress/damage to mitochondria is repaired by MQC mechanisms (blue arrow), which prevent the occurrence of dysfunctional mitochondria that may exacerbate stress/damage. Less extensive damage is repaired by mitochondrial unfolded protein response (UPRmt), which properly folds misfolded proteins (chaperons) or cleaves them (proteases). Mitochondria that are beyond repair undergo mitophagy, which is tightly associated with mitochondrial biogenesis, serving to maintain a pool of healthy mitochondria. Mitochondrial fusion is beneficial as it reduces reactive oxygen species (ROS) generation. Mitochondrial fission promotes healthy phenotype when allowing mitophagy. Extreme stress beyond compensation by MQC systems induces mitochondrial permeability transition pore (mPTP) opening and cardiomyocytes death (solid red arrows). Less pronounced mPTP opening allows cell death by apoptosis, which is dominant in heart failure. The most extensive cell stress induces widespread mPTP opening in cardiomyocytes, which leads to necrotic cell death, a predominant mechanism in acute ischemia-reperfusion, ie, myocardial infarction. Extremely high cell stresses are linked to mitochondrial fission, which exacerbates ROS generation. Inadequate MQC system activation induces cell death even during exposure to less extensive cell stress (red dotted arrows). This includes excessive activation of either UPRmt, mitophagy, or mitochondrial fission, which cause further dysfunction of mitochondria, or insufficient activation of MQC mechanisms that do not repair/eliminate dysfunctional mitochondria.
One of the important mechanisms that may regulate UPRmt consists of mutually-dependent activity of two antagonistic mitochondrial proteases, YME1L and OMA1. Human ATP-dependent YME1L is an orthologue of the YME1 subunit of the yeast i-AAA complex, and OMA1 is a zinc metallopeptidase. Depending on the conditions within the cell, the two proteases can cleave and inactivate each other (20). YME1L up-regulation will result in OMA1 inactivation, and vice versa. Both YME1L and OMA1 can be up-regulated by stress. Constitutively active YME1L is further activated by mitochondrial depolarization with preserved ATP levels, while OMA1, which is quiescent in non-stressed cells, is activated by mitochondrial depolarization with ATP loss (20). In addition, YME1L may also be inactivated by high oxidative stress, which promotes cell death (21). Although the majority of studies indicate a negative effect of Oma1 on mitochondrial function and cellular viability, Bohovych (22) showed that OMA1 deletion impeded the stability of respiratory chain supercomplexes and mitochondrial bioenergetics in mouse embryonic fibroblasts. In the active state, YME1L triggers UPRmt by cleaving mitochondrial proteins and creating mitonuclear protein imbalance (23). The loss of activity of YME1L impairs normal mitochondrial fusion-fission dynamic, which is associated with an increase in ROS generation and increase in sensitivity to oxidative stress. This is caused by the up-regulation of OMA1 and OMA1-induced cleavage/inactivation of pro-fusion OPA1 (24). The loss of YME1L decreases cell proliferation, diminishes resistance to apoptosis, and increases protein carbonylation (16).
The beneficial effects of UPRmt are reflected in the preservation of ATP production, attenuation of excess mitochondrial ROS emission, and prevention of release/activation of mitochondrial pro-apoptotic factors (25). UPRmt-inducing transcription factor ATFS-1 promotes the assembly of oxidative phosphorylation components during mitochondrial stress, which can preserve ATP production (26). At the same time, UPRmt proteases, such as YME1L (16) or ClpP (17), can cleave dysfunctional respiratory chain components. Thus, UPRmt promotes respiratory chain recovery and helps maintaining oxidative phosphorylation in stressed mitochondria via ATFS-1 actions. However, ClpP hyperactivation by ONC201 (imipiridone) treatment diminishes oxidative phosphorylation and induces cell death in cancer cells (17).
Mitochondrial unfolded protein response in heart disease
UPRmt is protective in chronic (27) and acute cardiac injury (18). However and seemingly contradictory, studies have also found that blocking several UPRmt elements can reduce the signs of HF of a different etiology.
One of key breakthroughs delineating the role of mitochondria in HF was a recent publication by Wai et al, who showed that cardiac-specific YME1L deletion caused HF and premature death of mice (28). An additional OMA1 deletion restored normal mitochondrial morphology and rescued the mice from HF and premature death. However, OMA1 deletion was also shown to cause developmental heart defects (22), indicating the importance of fine tuning of UPRmt effector proteases for cardiac viability. The activity of another component of UPRmt, LONP1, reduced by oxidative stress leads to the accumulation of dysfunctional respiratory chain subunits and left ventricle contractile dysfunction (29). On the other hand, LONP1 up-regulation protects cardiomyocytes from ischemia-reperfusion (I/R) injury (18). Moreover, the down-regulation of endonuclease G contributes to ROS overproduction, reduces mitochondrial DNA replication, and induces cardiac hypertrophy in rodents (19).
Conversely, there is evidence that UPRmt can be associated with harmful events in the heart. Physical exercise reduces CCAAT/enhancer-binding protein β expression in mice, and its reduction is associated with neonatal cardiomyocyte proliferation and improved resistance to pressure-induced hypertrophy (14). Furthermore, excessive eIF2α activation via double-stranded RNA-dependent protein kinase (PKR) promotes cardiomyocyte apoptosis and HF (30). Parvostatin blocks UPRmt activator c-Jun, which improves left ventricular function and slows the progression of HF in mice (31). Moreover, elevated LONP1 activity is a mediator of hypoxia-induced cardiomyocyte apoptosis and its down-regulation attenuates ROS generation and protects the cells (32), while ClpP deletion increases the expression of respiratory chain subunits and reduces cardiomyopathy (33). In the aged rat heart, the elevated activity of OMI/HTRA2 protease promotes mitochondrial depolarization and apoptosis (34), and its overexpression causes apoptosis and cardiac dysfunction in transgenic mice (35). Gain-of-function mutation of paraplegin (SPG7) increases mitochondrial ROS generation and coronary artery disease risk (36). Lin et al (37) linked high HSP60 expression to proinflammatory state and cardiomyocyte damage in human HF. Elevated expression/activity of several UPRmt elements was demonstrated in humans and animals with HF, including LON and ClpP (38), CHOP (15), eIF2α (15), ATF-4 (39), and c-Jun N-terminal kinases (40).
A potential explanation for such seemingly contradictory findings is that UPRmt is cardioprotective when moderately active, while its excessive activity may be cardiotoxic. A moderate activation of UPRmt may be beneficial for removing/repairing damaged mitochondrial proteins and thereby maintaining normal mitochondrial and cardiac function. An excessive UPRmt activation could result in a massive cleavage of mitochondrial proteins, exacerbating mitochondrial dysfunction and promoting heart damage. It is difficult to discriminate cardiotoxic from cardioprotective actions of UPRmt in the absence of reliable pharmacological inhibitors/activators and using only gene overexpression/knockouts models, which are not well suited for testing fine dose-responses.
Mitophagy
Mitochondrial damage with the loss of polarity on the outer mitochondrial membrane (OMM) is a signal for the removal of mitochondria by mitochondrial autophagy (mitophagy). The complex processes involved in mitophagy are in more detail described elsewhere (3). Mitochondrial fragmentation is essential for mitophagy, whereby fission enables the sequestration of damaged mitochondrial parts into a mitochondrion, which is eliminated by mitophagy. Crucial molecular regulators of mitophagy are Parkin (E3 ubiquitin ligase) and PTEN-induced putative kinase protein 1 (PINK1) (41,42). Under normal conditions, when mitophagy is not occurring, Parkin is self-ubiquitinated and inactivated and PINK1 is degraded (3,24). PINK1 is degraded by MPP and presenilin-associated rhomboid-like (PARL) after it is imported into the mitochondrial matrix via the translocase of the outer membrane 40 and translocase of the inner membrane 23 complexes (24). The loss of mitochondrial membrane potential prevents the import of PINK1, which then accumulates in the TOM complex. PINK1 autophosphorylates and activates itself and phosphorylates and activates Parkin. Parkin ubiquitinates mitochondrial OMM proteins and thereby targets them for phagosomal digestion. In the process of mitophagy, Parkin also interacts with PRKR-like endoplasmic reticulum kinase, ATF-4, MFN2, mitochondrial rho GTPase 1, voltage-dependent anion-selective channel 1, and hexokinase-1 (3,39,43). The mitochondrion is engulfed by the autophagosome, which eventually fuses with lysosome for digestion. The autophagosome formation depends on the proteins optineurin, nuclear domain 10 protein 52, sequestosome 1 (p62), microtubule-associated proteins 1A/1B light chain 3B (LC3), and TANK-binding kinase 1 (3,42). The phagophore membrane elongation is conveyed by proteins from the autophagy-related genes (ATG) family. An important factor in autophagosome formation is unc-51 like autophagy activating kinase 1 complex. It phosphorylates a large number of mitophagy proteins, such as Beclin-1, p62 and FUN14 domain containing 1 (FUNDC1) (5). The autophagosome maturation and fusion with lysosome is mediated by Ras-associated protein 7, TBC1 domain family member 15 (TBC1D15), TBC1D17, homotypic fusion and protein sorting complex, pleckstrin homology and RUN domain containing M1, syntaxin-17, and vesicle-associated membrane protein 8 (3). Parkin-independent mitophagy occurs via BCL2 interacting protein (BNIP3), NIP3-like protein X (NIX), FUNDC1, BCL2 like 13, GABA(A) receptor-associated protein (GABARAP) and GABARAPL1 (5).
Mitophagy in heart disease
Mitophagy is crucial for heart function and development. The interruption of PINK1/MFN2/Parkin pathway in mice causes lethal cardiomyopathy by 7-8 weeks, while the surviving mice exhibit abnormal energy metabolism in the heart (43).
Similarly to UPRmt, there is a fine line between protective and deleterious effects of mitophagy in cardiomyocytes. BNIP3 is transcriptionally up-regulated in the heart by hypoxia, whereas myocardial NIX up-regulation appears to be a specific transcriptional response to pathological hypertrophy (44). Forced NIX expression in cardiomyocytes leads to progressive apoptotic cardiomyopathy and premature death (45). The ablation of BNIP3 reduces apoptosis in ischemic cardiomyocytes (46). A very similar effect in heart remodeling is observed in Nix knockout mice, which were protected from ventricular dilation, wall thinning, and systolic dysfunction following heart pressure overload (45). Conversely, Nix and Bnip3 double knockouts develop cardiomyopathy with reduced ejection function of the left ventricle. This indicates that both NIX and BNIP3 may play a detrimental and beneficial role in the heart, depending on the (patho)physiological context and perhaps the extent of their activity.
Hypoxia activates FUNDC1, which interacts with LC3, thereby, activating mitophagy in platelets, diminishing their activity, which ameliorates cardiac reperfusion injury (47). The reduced levels of FUNDC1 (5) and PINK1 (41) are found in human samples of HF. Conversely, other studies show an elevated expression of mitophagy markers, including Parkin and LC3-mediated formation of autophagosome (42) and BNIP3 (48), in human and animal samples of HF of different etiologies. Cardiac-specific Fundc1 knockout mice exhibit impaired cardiac function, the accumulation of elongated and dysfunctional mitochondria and a greater degree of MI-induced HF (5). Similarly, PINK1-deficient mice are more susceptible to pressure overload-induced HF (41) and I/R heart injury (49). Moreover, the stimulation of PINK1/Parkin-mediated mitophagy by AMP-activated protein kinase α2 (AMPKα2) overexpression protects from pressure-induced HF (50). Parkin overexpression protects the heart from aging-induced dysfunction and cell senescence (51).
Mitochondrial function is unaffected in Parkin deficiency, although such mitochondria seem smaller, with a disorganized network (52). However, Parkin-deficient mice are more sensitive to I/R injury, which can be reduced by Parkin overexpression in isolated cardiac myocytes (52). Moreover, ATG5 depletion induces HF (53), while ATG7 induction may ameliorate desmin-related cardiomyopathy (54). Impaired mitochondrial fusion-fission balance (55) and AMPKα2 expression (50,56) may lead to cardiomyopathy and/or cardiomyocyte necrosis in part by disrupting mitophagy. Pharmacological and non-pharmacological mitophagy inducers, such as rapamycin, mainly reduce cardiac I/R injury or pathological cardiac remodeling and HF (57).
Overall, similarly to UPRmt, studies have showed positive and negative effects of mitophagy in heart disease, which could be related to the extent of mitophagy activation.
Mitochondrial biogenesis
Mitochondrial biogenesis is described in detail in the review by Ploumi et al (58). In brief, mitochondrial biogenesis is driven to a lesser extent by mitochondrial DNA (mtDNA) and to a greater extent by nuclear DNA that harbors essential regulatory processes. The key factors of mtDNA replication include mitochondrial DNA polymerase γ (POLG) and twinkle (resembles helicase) (59,60). POLG, mitochondrial transcription factor A (TFAM), and adenine nucleotide translocase (ANT) are important for mtDNA maintenance and repair (61-63). Mitochondrial DNA transcription elongation factor defines whether replication or transcription takes place. The transcription initiation complex consists of TFAM, mitochondrial DNA-directed RNA polymerase, and mitochondrial dimethyladenosine transferase 2 enzyme (58). These transcription factors and coactivators regulate other processes, such as respiratory chain assembly or fatty acid oxidation, and their impairment can affect cardiac function in multiple ways.
Nuclear respiratory factors 1 and 2 (NRF1 and NRF2) are transcription factors that regulate the expression of numerous genes involved in mitochondrial assembly (64,65). NRF2 is negatively regulated by cytoplasmic protein KEAP1, which marks it for degradation by ubiquitination (58). The expression of genes involved in mitochondrial metabolism is controlled by estrogen related receptors (ERR)α and ERRγ, whereby ERRα activates peroxisome proliferator activated receptor (PPAR)α and up-regulates NRF1 (66-68). The central role in the regulation of mitochondrial biogenesis belongs to PGC-1α. It is a transcription coactivator of PPARα, PPARβ, PPARγ, ERRα, ERRβ, ERRγ, NRF1 and NRF2 (69). A similar role is played by PGC-1β (69).
Mitochondrial biogenesis in heart disease
An impaired mitochondrial biogenesis leads to HF and increases the sensitivity to MI. A factor that interferes with mitochondrial biogenesis is ANT deficiency, which destabilizes mtDNA, increases ROS production, and causes HF (63). Cardiac-specific Tfam knockout mice display reduced ATP generation, increased apoptosis, atrioventricular conduction block, and dilated cardiomyopathy (70-72). Conversely, TFAM overexpression protects the heart from MI-induced HF (62). Similarly, the overexpression of TFAM or twinkle improves cardiac function in a pressure or volume overload and reduces ROS overproduction (59,60). Poor mtDNA replication and accumulation of mutations, induced by inactive POLG, leads to dilated cardiomyopathy and interstitial fibrosis (61).
NRF1- or NRF2-deficient mice exhibit low levels of mtDNA, left ventricular dysfunction, and die before birth (64,65). Conversely, NRF2 induction by preconditioning or pharmacological treatment protects against MI (73). ERRα-deficient mice exhibit cardiac dysfunction only after pressure overload (66), while ERRγ deficient mice exhibit lethal HF (67). The concomitant deletion of ERRα and ERRγ leads to prenatal death (74). While mice with prenatal knockout of either Pgc-1α or Pgc-1β experience mild cardiac dysfunction only after the exposure to noxious stimuli (75,76), prenatal knockouts of both proteins cause HF and prenatal death (69), indicating an overlap in their function. On the other hand, their deletion in adulthood is not associated with significant heart dysfunction (77). The same study also demonstrated that PGC-1α and PGC-1β deficiency decreases the expression of pro-fusion factors MFN2 and OPA1, and pro-fission factor fission 1 (FIS1), leading to abnormal mitochondrial morphology. This further shows multiple interplays among different MQC mechanisms. PGC-1α-induced biogenesis may act beneficially by reducing mitochondrial calcium uptake (78), which is a powerful stressor and inducer of mPTP opening (1,79). Mitochondrial permeability transition pore opening is induced by ROS and mitochondrial calcium overload and precedes cardiomyocyte death (80,81). However, in dilated (and not ischemic) cardiomyopathy, mitochondrial biogenesis is enhanced (increased expression of PGC-1α, POLG, POLG2, and TFAM) (82). This possibly reflects a compensatory response to mitochondrial dysfunction arising from mtDNA mutations that were the most prevalent in dilated cardiomyopathy (82). Conversely, in samples of HF with reduced ejection fraction, PGC-1α levels are reduced together with complex IV activity (48), which may suggest that poor biogenesis contributes to cardiac dysfunction.
Altogether, impaired mitochondrial biogenesis predisposes to HF and increases the extent of MI, while enhanced expression of biogenesis genes may correspond to compensatory response to mitochondrial dysfunction.
Mitochondrial fusion-fission balance
Mitochondria constantly undergo fusion and fission. Mitochondrial dynamics is associated with the metabolic state of the cell, presence of various stressors, and other important processes, such as cell proliferation and differentiation. Mitochondrial fusion-fission is reviewed in detail by Ježek et al (4). Fusion-fission dynamics work tightly with other MQC mechanisms, UPRmt, mitophagy, and biogenesis.
The key protein responsible for mitochondrial fission is GTP-ase dynamin-related protein 1 (Drp1) (83). Other pro-fission factors that interact with Drp1 and promote mitochondrial fission include FIS1 and mitochondrial dynamics proteins mitochondrial dynamics protein of 49 KDa (MID49) and MID51 (4,84). Once activated, Drp1 translocates from the cytoplasm onto mitochondria causing fission, which also requires the action of dynamin-2 (85). The mitochondrial fusion depends on three GTP-ases, MFN1, MFN2, and OPA1 (86,87). While MFN1 and MFN2 mediate OMM fusion, OPA1 mediates inner mitochondrial membrane fusion. In addition to regulating the morphology of mitochondrial cristae, OPA1 also prevents apoptosis in physiological conditions, while pro-fission factors, such as Drp1, promote apoptosis (4,86,88). OMA1 and YME1L proteases are key regulators of OPA1 activity, whereby OMA1 cleaves and inactivates it (88).
Mitochondrial fusion-fission balance in heart disease
Extensive fission (fragmentation) of mitochondria has been associated with cardiac I/R injury (84,89), which is caused by enhanced FIS1 and Drp1 expression in mitochondria and/or reduced MFN1 and OPA1 expression (90). OPA1 deficiency reduces mtDNA copy number, decreases mitochondrial and heart function, and leads to cardiomyopathy (91). Conversely, the induction of mitochondrial fusion by OPA1 overexpression protects cardiac (92) and other cell types (93) from various types of stressors. Zaja et al (83) showed that I/R injury induced mitochondrial fission, cardiomyocyte death and Drp1 activation (phosphorylation of Ser616 and dephosphorylation of Ser637), while it did not significantly change the expression of MFN1, MFN2 and OPA1. Drp1 activation results in cardiomyocyte death, with ROS and calcineurin acting as upstream modulators of Drp1 activity (83). Pharmacological Drp1 inhibition with mdivi-1 or calcineurin inhibition with FK506 reduces mitochondrial fission and cell death (83). Ong et al (84) have demonstrated that both the transfection of HL-1 cells with the Mfn1, Mfn2, or a dominant-negative mutant form of Drp1 (Drp1K38A), or pharmacological Drp1 inhibition, promote mitochondrial elongation and reduce mPTP opening and cell death after simulated I/R injury. They also showed that human FIS1 overexpression reduced mitochondrial elongation, increased cell death, but without an effect on mPTP opening. Disatnik et al (89) used selective Drp1 inhibitor (P110) in ex vivo and in vivo rat heart model of MI to assess the role of Drp1/FIS1 interaction in reperfusion injury. They found that increased mitochondrial fragmentation during reperfusion facilitated long-term cardiac dysfunction in rats, whereas acute inhibition of mitochondrial fission early at the onset of reperfusion resulted in long-term benefits. Conversely, FUNDC1 ablation promoted mitochondrial elongation via FIS1 down-regulation, which caused mitochondrial dysfunction and HF in mice (5). Moreover, FUNDC1 and FIS1 are down-regulated and mitochondria are elongated in HF samples (5). This suggests that the absence of mitochondrial fission and predominance of mitochondrial elongation (fusion) could be detrimental to the heart. However, this is inconsistent with the majority of other studies, which show an increase in mitochondrial fission in different types of HF and cardiomyocyte injury (48,82,86). The expression of pro-fusion and pro-fission proteins varies among studies and types of HF. Ahuja et al (82) and Chen et al (86) found OPA1 decrease in ischemic heart and MFN2 increase in dilated cardiomyopathy. Conversely, these two studies found an opposite change in MFN2 in ischemic cardiomyopathy. In the first study it was decreased and in the second it was increased. In dilated cardiomyopathy, Ahuja et al found increased OPA1 and Chen et al found no change in OPA1. The latter study also found an increase in MFN1 and Drp1 and no change in FIS1 in both types of cardiomyopathy (86). Increased Drp1 expression was also found in the samples of HF with reduced ejection fraction (48). HF of different etiology in animal models is associated with increased mitochondrial fragmentation, decrease in pro-fusion factors, and increase in pro-fission factors: OPA1, MFN1, and Drp1 (94); OPA1, MFN1, MFN2, Drp1, and FIS1 (87); MFN2 and Drp1 (95) and MFN2 (Drp1 was reduced) (42). In the majority of these studies, mitochondrial fragmentation was associated with elevated ROS generation, diminished ATP production, and cardiac dysfunction/damage, while the shift toward mitochondrial fusion exhibited beneficial effects. Along these lines, Drp1 inhibition by mdivi-1 ameliorates the signs of HF arising from pressure overload (96). However, Drp1 is important for cardiac function. Its mutation causes HF due to impaired mitophagy, mitochondrial depolarization, poor calcium handling, and diminished ATP synthesis (97).
The majority of studies demonstrate beneficial effects of mitochondrial fusion and detrimental effects of fission in acute cardiac I/R injury, suggesting that mitochondrial fusion increases the resistance to cell death possibly by attenuating ROS generation and preserving ATP production. In chronic HF experiments, some of the results reported are not congruent with those by other investigators. Inconsistencies in results among studies testing human HF samples may arise from different reasons, such as methodological issues, biological variability of individuals/populations, and different etiologies of HF. Thus, a fusion-fission imbalance can lead to or accelerate HF, reflecting the importance of both states for long term mitochondrial and cardiac function.
Mitochondrial quality control in the aged heart
Aging is associated with a decline in cardiac function and increased incidence of MI and HF. Aging impairs mitochondrial function in part by dysregulation of MQC (98). Limited perturbations of mitochondrial function can induce UPRmt, a process that is associated with lifespan extension (99). Conversely, there are studies that demonstrate that triggering of UPRmt may reduce lifespan (99). UPRmt protease LON is down-regulated in aged cells (100). However, there is a lack of studies investigating UPRmt in the aged heart. The overexpression of ATG5 protein may extend lifespan (101), and its inhibition could lead to age-related cardiomyopathy in mice (102), suggesting that impairment of autophagy/mitophagy could contribute to degenerative changes in the aged heart. Parallel ablation of MFN1, MFN2, and Drp1, which impaired mitochondrial dynamics, causes “mitochondrial senescence” and cardiac changes that resemble aging-related cardiomyopathy (103). In agreement to this study, Zhao et al found a reduced expression of p62, LC3-IIm PGC-1α and MFN2 in the aged (25-month-old) rat heart, indicating reduced autophagy/mitophagy, mitochondrial biogenesis and fusion (104). Using 24-month-old mice, hearts Whitehead et al also found a reduced expression of MQC genes: PGC-1α, PGC-1β, TFAM, ERRα, MFN1, MFN2 and OPA1 but observed no change in FIS1 and Drp1 (105). Conversely, another study with older rats (36-months-old) showed elevated cardiac Drp1 and OPA1 expression (106).
Most of the existing studies suggest that the MQC is reduced in the aged heart and that its down-regulation shortens lifespan and leads to phenotypes found in the aged heart. However, there are data showing that some MQC components may be up-regulated in the aged heart. It is possible that, as in heart disease, MQC could exert beneficial or maybe even detrimental effects in the aged heart. More studies are required to resolve such seemingly conflicting data.
Conclusions
MQC consists of several interconnected mechanisms that serve to maintain proper mitochondrial function. Numerous studies demonstrate that failure in some of MQC mechanisms may induce HF or exacerbate MI, and that stimulation of MQC attenuates cardiac injury/dysfunction. On the contrary, a large number of studies also show that MQC elements are elevated in HF and that MQC down-regulation has protective effects on the heart. It is possible that the effects of MQC on mitochondrial and cardiac functions are nonlinear or biphasic (107). In other words, a moderate activity of MQC may improve overall mitochondrial function and be beneficial for the heart as a compensatory response. However, hyperactive MQC could lead to excessive perturbation of mitochondrial homeostasis (eg, excessive mitochondrial removal or protein cleavage), leading to cardiac injury or exacerbating the existing pathological processes.
Acknowledgments
Funding This work is in part supported by the Croatian Science Foundation grant IP-2019-04-1449 to F.S. and European Social Fund grant HR.3.2.01-0182 to A.S. and University of Zagreb research fund to F.S.
Ethical approval Not required.
Declaration of authorship TS, MiM, MaM, LK, AŠ, DM, JB, BS, MSS, FrS, AK, FiS conceived and designed the study; TS, MiM, MaM, LK, FiS acquired the data; TS, MiM, MaM, LK, FiS analyzed and interpreted the data; TS, MiM, MaM, LK, AŠ, DM, JB, BS, MSS, FrS, AK, FiS drafted the manuscript; TS, MiM, MaM, LK, AŠ, DM, JB, BS, MSS, FrS, AK, FiS critically revised the manuscript for important intellectual content; TS, MiM, MaM, LK, AŠ, DM, JB, BS, MSS, FrS, AK, FiS gave approval of the version to be submitted; TS, MiM, MaM, LK, AŠ, DM, JB, BS, MSS, FrS, AK, FiS agree to be accountable for all aspects of the work.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
REFERENCES
- 1.Sedlic F, Sepac A, Pravdic D, Camara AK, Bienengraeber M, Brzezinska AK, et al. Mitochondrial depolarization underlies delay in permeability transition by preconditioning with isoflurane: roles of ROS and Ca2+. Am J Physiol Cell Physiol. 2010;299:C506–15. doi: 10.1152/ajpcell.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Q, Sedlic F, Pravdic D, Bosnjak ZJ, Kwok WM. Biphasic effect of nitric oxide on the cardiac voltage-dependent anion channel. FEBS Lett. 2011;585:328–34. doi: 10.1016/j.febslet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamacher-Brady A, Brady NR. Mitophagy programs: mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mol Life Sci. 2016;73:775–95. doi: 10.1007/s00018-015-2087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jezek J, Cooper KF, Strich R. Reactive oxygen species and mitochondrial dynamics: the yin and yang of mitochondrial dysfunction and cancer progression. Antioxidants. 2018;7 doi: 10.3390/antiox7010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu S, Lu Q, Wang Q, Ding Y, Ma Z, Mao X, et al. Binding of FUN14 domain containing 1 with inositol 1,4,5-trisphosphate receptor in mitochondria-associated endoplasmic reticulum membranes maintains mitochondrial dynamics and function in hearts in vivo. Circulation. 2017;136:2248–66. doi: 10.1161/CIRCULATIONAHA.117.030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Qiao S, Hong L, Sun J, Che T, An J, et al. NOS cofactor tetrahydrobiopterin contributes to anesthetic preconditioning induced myocardial protection in the isolated ex vivo rat heart. Int J Mol Med. 2020;45:615–22. doi: 10.3892/ijmm.2019.4445. [DOI] [PubMed] [Google Scholar]
- 7.Yang M, Xu Y, Heisner JS, Sun J, Stowe DF, Kwok WM, et al. Peroxynitrite nitrates adenine nucleotide translocase and voltage-dependent anion channel 1 and alters their interactions and association with hexokinase II in mitochondria. Mitochondrion. 2019;46:380–92. doi: 10.1016/j.mito.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moris D, Spartalis M, Spartalis E, Karachaliou GS, Karaolanis GI, Tsourouflis G, et al. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann Transl Med. 2017;5:326. doi: 10.21037/atm.2017.06.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A. 2015;112:11389–94. doi: 10.1073/pnas.1513047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol. 2012;32:1552–62. doi: 10.1161/ATVBAHA.111.224915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–70. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 12.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovasc Res. 2004;61:372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 13.Cortassa S, Sollott SJ, Aon MA. Mitochondrial respiration and ROS emission during beta-oxidation in the heart: An experimental-computational study. PLOS Comput Biol. 2017;13:e1005588. doi: 10.1371/journal.pcbi.1005588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, Panakova D, et al. C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–83. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Y, Lu Q, Hu Z, Yu Y, Chen Q, Wang QK. A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure. Nat Commun. 2017;8:133. doi: 10.1038/s41467-017-00171-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stiburek L, Cesnekova J, Kostkova O, Fornuskova D, Vinsova K, Wenchich L, et al. YME1L controls the accumulation of respiratory chain subunits and is required for apoptotic resistance, cristae morphogenesis, and cell proliferation. Mol Biol Cell. 2012;23:1010–23. doi: 10.1091/mbc.e11-08-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishizawa J, Zarabi SF, Davis RE, Halgas O, Nii T, Jitkova Y, et al. Mitochondrial ClpP-mediated proteolysis induces selective cancer cell lethality. Cancer Cell. 2019;35:721–37.e9. doi: 10.1016/j.ccell.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkatesh S, Li M, Saito T, Tong M, Rashed E, Mareedu S, et al. Mitochondrial LonP1 protects cardiomyocytes from ischemia/reperfusion injury in vivo. J Mol Cell Cardiol. 2019;128:38–50. doi: 10.1016/j.yjmcc.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Blasco N, Camara Y, Nunez E, Bea A, Bares G, Forne C, et al. Cardiomyocyte hypertrophy induced by Endonuclease G deficiency requires reactive oxygen radicals accumulation and is inhibitable by the micropeptide humanin. Redox Biol. 2018;16:146–56. doi: 10.1016/j.redox.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rainbolt TK, Lebeau J, Puchades C, Wiseman RL. Reciprocal degradation of YME1L and OMA1 adapts mitochondrial proteolytic activity during stress. Cell Reports. 2016;14:2041–9. doi: 10.1016/j.celrep.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rainbolt TK, Saunders JM, Wiseman RL. YME1L degradation reduces mitochondrial proteolytic capacity during oxidative stress. EMBO Rep. 2015;16:97–106. doi: 10.15252/embr.201438976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohovych I, Fernandez MR, Rahn JJ, Stackley KD, Bestman JE, Anandhan A, et al. Metalloprotease OMA1 fine-tunes mitochondrial bioenergetic function and respiratory supercomplex stability. Sci Rep. 2015;5:13989. doi: 10.1038/srep13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen MB, Jasper H. Mitochondrial proteostasis in the control of aging and longevity. Cell Metab. 2014;20:214–25. doi: 10.1016/j.cmet.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wai T, Saita S, Nolte H, Muller S, Konig T, Richter-Dennerlein R, et al. The membrane scaffold SLP2 anchors a proteolytic hub in mitochondria containing PARL and the i-AAA protease YME1L. EMBO Rep. 2016;17:1844–56. doi: 10.15252/embr.201642698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497:451–7. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nargund AM, Fiorese CJ, Pellegrino MW, Deng P, Haynes CM. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt). Mol Cell. 2015;58:123–33. doi: 10.1016/j.molcel.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smyrnias I, Gray SP, Okonko DO, Sawyer G, Zoccarato A, Catibog N, et al. Cardioprotective effect of the mitochondrial unfolded protein response during chronic pressure overload. J Am Coll Cardiol. 2019;73:1795–806. doi: 10.1016/j.jacc.2018.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wai T, Garcia-Prieto J, Baker MJ, Merkwirth C, Benit P, Rustin P, et al. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science. 2015;350:aad0116. doi: 10.1126/science.aad0116. [DOI] [PubMed] [Google Scholar]
- 29.Hoshino A, Okawa Y, Ariyoshi M, Kaimoto S, Uchihashi M, Fukai K, et al. Oxidative post-translational modifications develop LONP1 dysfunction in pressure overload heart failure. Circ Heart Fail. 2014;7:500–9. doi: 10.1161/CIRCHEARTFAILURE.113.001062. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Xu X, Fassett J, Kwak D, Liu X, Hu X, et al. Double-stranded RNA-dependent protein kinase deficiency protects the heart from systolic overload-induced congestive heart failure. Circulation. 2014;129:1397–406. doi: 10.1161/CIRCULATIONAHA.113.002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao S, Zeng Z, Wang X, Bin J, Xu D, Liao Y. Pravastatin slows the progression of heart failure by inhibiting the c-Jun N-terminal kinase-mediated intrinsic apoptotic signaling pathway. Mol Med Rep. 2013;8:1163–8. doi: 10.3892/mmr.2013.1622. [DOI] [PubMed] [Google Scholar]
- 32.Kuo CY, Chiu YC, Lee AY, Hwang TL. Mitochondrial Lon protease controls ROS-dependent apoptosis in cardiomyocyte under hypoxia. Mitochondrion. 2015;23:7–16. doi: 10.1016/j.mito.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Seiferling D, Szczepanowska K, Becker C, Senft K, Hermans S, Maiti P, et al. Loss of CLPP alleviates mitochondrial cardiomyopathy without affecting the mammalian UPRmt. EMBO Rep. 2016;17:953–64. doi: 10.15252/embr.201642077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Lei J, Wang K, Ma L, Liu D, Du Y, et al. Mitochondrial Omi/HtrA2 promotes caspase activation through cleavage of HAX-1 in aging heart. Rejuvenation Res. 2017;20:183–92. doi: 10.1089/rej.2016.1861. [DOI] [PubMed] [Google Scholar]
- 35.Wang K, Yuan Y, Liu X, Lau WB, Zuo L, Wang X, et al. Cardiac specific overexpression of mitochondrial Omi/HtrA2 induces myocardial apoptosis and cardiac dysfunction. Sci Rep. 2016;6:37927. doi: 10.1038/srep37927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Almontashiri NA, Chen HH, Mailloux RJ, Tatsuta T, Teng AC, Mahmoud AB, et al. SPG7 variant escapes phosphorylation-regulated processing by AFG3L2, elevates mitochondrial ROS, and is associated with multiple clinical phenotypes. Cell Reports. 2014;7:834–47. doi: 10.1016/j.celrep.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 37.Lin L, Kim SC, Wang Y, Gupta S, Davis B, Simon SI, et al. HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2007;293:H2238–47. doi: 10.1152/ajpheart.00740.2007. [DOI] [PubMed] [Google Scholar]
- 38.Guillon B, Bulteau AL, Wattenhofer-Donze M, Schmucker S, Friguet B, Puccio H, et al. Frataxin deficiency causes upregulation of mitochondrial Lon and ClpP proteases and severe loss of mitochondrial Fe-S proteins. FEBS J. 2009;276:1036–47. doi: 10.1111/j.1742-4658.2008.06847.x. [DOI] [PubMed] [Google Scholar]
- 39.Freundt JK, Frommeyer G, Wotzel F, Huge A, Hoffmeier A, Martens S, et al. The transcription factor ATF4 promotes expression of cell stress genes and cardiomyocyte death in a cellular model of atrial fibrillation. BioMed Res Int. 2018;2018:3694362. doi: 10.1155/2018/3694362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook SA, Sugden PH, Clerk A. Activation of c-Jun N-terminal kinases and p38-mitogen-activated protein kinases in human heart failure secondary to ischaemic heart disease. J Mol Cell Cardiol. 1999;31:1429–34. doi: 10.1006/jmcc.1999.0979. [DOI] [PubMed] [Google Scholar]
- 41.Billia F, Hauck L, Konecny F, Rao V, Shen J, Mak TW. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci U S A. 2011;108:9572–7. doi: 10.1073/pnas.1106291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thai PN, Seidlmayer LK, Miller C, Ferrero M, Dorn Ii GW, Schaefer S, et al. Mitochondrial quality control in aging and heart failure: influence of ketone bodies and mitofusin-stabilizing peptides. Front Physiol. 2019;10:382. doi: 10.3389/fphys.2019.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Dorn GW., II PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–5. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yussman MG, Toyokawa T, Odley A, Lynch RA, Wu G, Colbert MC, et al. Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat Med. 2002;8:725–30. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- 45.Dorn GW., II Mitochondrial pruning by Nix and BNip3: an essential function for cardiac-expressed death factors. J Cardiovasc Transl Res. 2010;3:374–83. doi: 10.1007/s12265-010-9174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, et al. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest. 2007;117:2825–33. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W, Siraj S, Zhang R, Chen Q. Mitophagy receptor FUNDC1 regulates mitochondrial homeostasis and protects the heart from I/R injury. Autophagy. 2017;13:1080–1. doi: 10.1080/15548627.2017.1300224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaanine AH, Joyce LD, Stulak JM, Maltais S, Joyce DL, Dearani JA, et al. Mitochondrial morphology, dynamics, and function in human pressure overload or ischemic heart disease with preserved or reduced ejection fraction. Circ Heart Fail. 2019;12:e005131. doi: 10.1161/CIRCHEARTFAILURE.118.005131. [DOI] [PubMed] [Google Scholar]
- 49.Siddall HK, Yellon DM, Ong SB, Mukherjee UA, Burke N, Hall AR, et al. Loss of PINK1 increases the heart’s vulnerability to ischemia-reperfusion injury. PLoS One. 2013;8:e62400. doi: 10.1371/journal.pone.0062400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang B, Nie J, Wu L, Hu Y, Wen Z, Dong L, et al. AMPKalpha2 protects against the development of heart failure by enhancing mitophagy via PINK1 phosphorylation. Circ Res. 2018;122:712–29. doi: 10.1161/CIRCRESAHA.117.312317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoshino A, Mita Y, Okawa Y, Ariyoshi M, Iwai-Kanai E, Ueyama T, et al. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat Commun. 2013;4:2308. doi: 10.1038/ncomms3308. [DOI] [PubMed] [Google Scholar]
- 52.Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK, et al. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem. 2013;288:915–26. doi: 10.1074/jbc.M112.411363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–24. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 54.Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM, et al. Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest. 2013;123:5284–97. doi: 10.1172/JCI70877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW., II Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2015;21:273–86. doi: 10.1016/j.cmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim M, Shen M, Ngoy S, Karamanlidis G, Liao R, Tian R. AMPK isoform expression in the normal and failing hearts. J Mol Cell Cardiol. 2012;52:1066–73. doi: 10.1016/j.yjmcc.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu X, He L, Chen F, He X, Cai Y, Zhang G, et al. Impaired autophagy contributes to adverse cardiac remodeling in acute myocardial infarction. PLoS One. 2014;9:e112891. doi: 10.1371/journal.pone.0112891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ploumi C, Daskalaki I, Tavernarakis N. Mitochondrial biogenesis and clearance: a balancing act. FEBS J. 2017;284:183–95. doi: 10.1111/febs.13820. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka A, Ide T, Fujino T, Onitsuka K, Ikeda M, Takehara T, et al. The overexpression of Twinkle helicase ameliorates the progression of cardiac fibrosis and heart failure in pressure overload model in mice. PLoS One. 2013;8:e67642. doi: 10.1371/journal.pone.0067642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ikeda M, Ide T, Fujino T, Arai S, Saku K, Kakino T, et al. Overexpression of TFAM or twinkle increases mtDNA copy number and facilitates cardioprotection associated with limited mitochondrial oxidative stress. PLoS One. 2015;10:e0119687. doi: 10.1371/journal.pone.0119687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang D, Mott JL, Farrar P, Ryerse JS, Chang SW, Stevens M, et al. Mitochondrial DNA mutations activate the mitochondrial apoptotic pathway and cause dilated cardiomyopathy. Cardiovasc Res. 2003;57:147–57. doi: 10.1016/S0008-6363(02)00695-8. [DOI] [PubMed] [Google Scholar]
- 62.Ikeuchi M, Matsusaka H, Kang D, Matsushima S, Ide T, Kubota T, et al. Overexpression of mitochondrial transcription factor a ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation. 2005;112:683–90. doi: 10.1161/CIRCULATIONAHA.104.524835. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, Chen XJ. Adenine nucleotide translocase, mitochondrial stress, and degenerative cell death. Oxid Med Cell Longev. 2013;2013:146860. doi: 10.1155/2013/146860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huo L, Scarpulla RC. Mitochondrial DNA instability and peri-implantation lethality associated with targeted disruption of nuclear respiratory factor 1 in mice. Mol Cell Biol. 2001;21:644–54. doi: 10.1128/MCB.21.2.644-654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ristevski S, O’Leary DA, Thornell AP, Owen MJ, Kola I, Hertzog PJ. The ETS transcription factor GABPalpha is essential for early embryogenesis. Mol Cell Biol. 2004;24:5844–9. doi: 10.1128/MCB.24.13.5844-5849.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, et al. The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, et al. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 2007;6:13–24. doi: 10.1016/j.cmet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, et al. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci U S A. 2004;101:6570–5. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, et al. Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart. Genes Dev. 2008;22:1948–61. doi: 10.1101/gad.1661708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Silva JP, Gustafsson CM, Rustin P, Larsson NG. Increased in vivo apoptosis in cells lacking mitochondrial DNA gene expression. Proc Natl Acad Sci U S A. 2001;98:4038–43. doi: 10.1073/pnas.061038798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hansson A, Hance N, Dufour E, Rantanen A, Hultenby K, Clayton DA, et al. A switch in metabolism precedes increased mitochondrial biogenesis in respiratory chain-deficient mouse hearts. Proc Natl Acad Sci U S A. 2004;101:3136–41. doi: 10.1073/pnas.0308710100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J, Wilhelmsson H, Graff C, Li H, Oldfors A, Rustin P, et al. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat Genet. 1999;21:133–7. doi: 10.1038/5089. [DOI] [PubMed] [Google Scholar]
- 73.Cheng L, Jin Z, Zhao R, Ren K, Deng C, Yu S. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: role of Nrf2/ARE pathway. Int J Clin Exp Med. 2015;8:10420–8. [PMC free article] [PubMed] [Google Scholar]
- 74.Wang T, McDonald C, Petrenko NB, Leblanc M, Wang T, Giguere V, et al. Estrogen-related receptor alpha (ERRalpha) and ERRgamma are essential coordinators of cardiac metabolism and function. Mol Cell Biol. 2015;35:1281–98. doi: 10.1128/MCB.01156-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, et al. Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol. 2006;4:e369. doi: 10.1371/journal.pbio.0040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin OJ, Lai L, Soundarapandian MM, Leone TC, Zorzano A, Keller MP, et al. A role for peroxisome proliferator-activated receptor gamma coactivator-1 in the control of mitochondrial dynamics during postnatal cardiac growth. Circ Res. 2014;114:626–36. doi: 10.1161/CIRCRESAHA.114.302562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bianchi K, Vandecasteele G, Carli C, Romagnoli A, Szabadkai G, Rizzuto R. Regulation of Ca2+ signalling and Ca2+-mediated cell death by the transcriptional coactivator PGC-1alpha. Cell Death Differ. 2006;13:586–96. doi: 10.1038/sj.cdd.4401784. [DOI] [PubMed] [Google Scholar]
- 79.Tampo A, Hogan CS, Sedlic F, Bosnjak ZJ, Kwok WM. Accelerated inactivation of cardiac L-type calcium channels triggered by anaesthetic-induced preconditioning. Br J Pharmacol. 2009;156:432–43. doi: 10.1111/j.1476-5381.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sedlic F, Muravyeva MY, Sepac A, Sedlic M, Williams AM, Yang M, et al. Targeted modification of mitochondrial ros production converts high glucose-induced cytotoxicity to cytoprotection: effects on anesthetic preconditioning. J Cell Physiol. 2017;232:216–24. doi: 10.1002/jcp.25413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muravyeva M, Baotic I, Bienengraeber M, Lazar J, Bosnjak ZJ, Sedlic F, et al. Cardioprotection during diabetes: the role of mitochondrial DNA. Anesthesiology. 2014;120:870–9. doi: 10.1097/ALN.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahuja P, Wanagat J, Wang Z, Wang Y, Liem DA, Ping P, et al. Divergent mitochondrial biogenesis responses in human cardiomyopathy. Circulation. 2013;127:1957–67. doi: 10.1161/CIRCULATIONAHA.112.001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zaja I, Bai X, Liu Y, Kikuchi C, Dosenovic S, Yan Y, et al. Cdk1, PKCdelta and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death. Biochem Biophys Res Commun. 2014;453:710–21. doi: 10.1016/j.bbrc.2014.09.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–22. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 85.Lee JE, Westrate LM, Wu H, Page C, Voeltz GK. Multiple dynamin family members collaborate to drive mitochondrial division. Nature. 2016;540:139–43. doi: 10.1038/nature20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84:91–9. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang D, Ma J. Mitochondrial dynamics in rat heart induced by 5-fluorouracil. Med Sci Monit. 2018;24:6666–72. doi: 10.12659/MSM.910537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, et al. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol. 2014;204:919–29. doi: 10.1083/jcb.201308006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, et al. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2:e000461. doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stotland A, Gottlieb RA. Mitochondrial quality control: Easy come, easy go. Biochim Biophys Acta. 2015;1853(10 Pt B):2802–11. doi: 10.1016/j.bbamcr.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen L, Liu T, Tran A, Lu X, Tomilov AA, Davies V, et al. OPA1 mutation and late-onset cardiomyopathy: mitochondrial dysfunction and mtDNA instability. J Am Heart Assoc. 2012;1:e003012. doi: 10.1161/JAHA.112.003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Makino A, Suarez J, Gawlowski T, Han W, Wang H, Scott BT, et al. Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1296–302. doi: 10.1152/ajpregu.00437.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alaimo A, Gorojod RM, Beauquis J, Munoz MJ, Saravia F, Kotler ML. Deregulation of mitochondria-shaping proteins Opa-1 and Drp-1 in manganese-induced apoptosis. PLoS One. 2014;9:e91848. doi: 10.1371/journal.pone.0091848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Naruse G, Kanamori H, Yoshida A, Minatoguchi S, Kawaguchi T, Iwasa M, et al. The intestine responds to heart failure by enhanced mitochondrial fusion through glucagon-like peptide-1 signaling. Cardiovasc Res. 2019 doi: 10.1093/cvr/cvz002. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Zhang L, Zhang Y, Fan X, Yang W, Yu B, et al. YiQiFuMai powder injection attenuates coronary artery ligation-induced heart failure through improving mitochondrial function via regulating ROS generation and CaMKII signaling pathways. Front Pharmacol. 2019;10:381. doi: 10.3389/fphar.2019.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N, Tyagi SC. Mitochondrial division/mitophagy inhibitor (Mdivi) ameliorates pressure overload induced heart failure. PLoS One. 2012;7:e32388. doi: 10.1371/journal.pone.0032388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cahill TJ, Leo V, Kelly M, Stockenhuber A, Kennedy NW, Bao L, et al. Resistance of dynamin-related protein 1 oligomers to disassembly impairs mitophagy, resulting in myocardial inflammation and heart failure. J Biol Chem. 2015;290:25907–19. doi: 10.1074/jbc.M115.665695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tocchi A, Quarles EK, Basisty N, Gitari L, Rabinovitch PS. Mitochondrial dysfunction in cardiac aging. Biochim Biophys Acta. 2015;1847:1424–33. doi: 10.1016/j.bbabio.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bennett CF, Kaeberlein M. The mitochondrial unfolded protein response and increased longevity: cause, consequence, or correlation? Exp Gerontol. 2014;56:142–6. doi: 10.1016/j.exger.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ngo JK, Pomatto LC, Davies KJ. Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol. 2013;1:258–64. doi: 10.1016/j.redox.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pyo JO, Yoo SM, Ahn HH, Nah J, Hong SH, Kam TI, et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun. 2013;4:2300. doi: 10.1038/ncomms3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–6. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 103.Song M, Franco A, Fleischer JA, Zhang L, Dorn GW., II Abrogating mitochondrial dynamics in mouse hearts accelerates mitochondrial senescence. Cell Metab. 2017;26:872–83.e5. doi: 10.1016/j.cmet.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao L, Zou X, Feng Z, Luo C, Liu J, Li H, et al. Evidence for association of mitochondrial metabolism alteration with lipid accumulation in aging rats. Exp Gerontol. 2014;56:3–12. doi: 10.1016/j.exger.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 105.Whitehead N, Gill JF, Brink M, Handschin C. Moderate modulation of cardiac PGC-1alpha expression partially affects age-associated transcriptional remodeling of the heart. Front Physiol. 2018;9:242. doi: 10.3389/fphys.2018.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ljubicic V, Menzies KJ, Hood DA. Mitochondrial dysfunction is associated with a pro-apoptotic cellular environment in senescent cardiac muscle. Mech Ageing Dev. 2010;131:79–88. doi: 10.1016/j.mad.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 107.Sedlic F, Kovac Z. Non-linear actions of physiological agents: Finite disarrangements elicit fitness benefits. Redox Biol. 2017;13:235–43. doi: 10.1016/j.redox.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]