Abstract
Background: We hypothesized that the change in stroke risk profile between baseline and follow-up may be a better predictor of ischemic stroke than the baseline stroke risk determination using the CHA2DS2-VASc score ((congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke/transient ischemic attack/thromboembolism (doubled), vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque), age 65–75 years, sex category (female))). Methods: We collected information for all patients treated with atrial fibrillation (AF) in French hospitals between 2010 and 2019. We studied 608,108 patients with AF who did not have risk factors of the CHA2DS2-VASc score (except for age and sex). The predictive accuracies of baseline and follow-up CHA2DS2-VASc scores, as well as the ‘Delta CHA2DS2-VASc’ (i.e., change/difference between the baseline and follow-up CHA2DS2-VASc scores) for prediction of ischemic stroke were studied. Results: The mean CHA2DS2-VASc score at baseline was 1.7, and increased to 2.4 during follow-up of 2.2 ± 2.4 years, (median (interquartile range: IQR) 1.2 (0.1–3.8) years), resulting in a mean Delta CHA2DS2-VASc score of 0.7. Among 20,082 patients suffering ischemic stroke during follow-up, 67.1% had a Delta CHA2DS2-VASc score ≥1 while they were only 40.4% in patients without ischemic stroke. The follow-up CHA2DS2-VASc score and Delta CHA2DS2-VASc score were predictors of ischemic stroke (C-index 0.670, 95% confidence interval (CI) 0.666–0.673 and 0.637, 95%CI 0.633–0.640) and they performed better than baseline CHA2DS2-VASc score (C-index 0.612, 95%CI 0.608–0.615, p < 0.0001). Conclusions: Stroke risk was non-static, and many AF patients had ≥1 new stroke risk factor(s) before ischemic stroke occurred. The follow-up CHA2DS2-VASc score and its change (i.e., ‘Delta CHA2DS2-VASc’) were better predictors of ischemic stroke than relying on the baseline CHA2DS2-VASc score.
Keywords: atrial fibrillation, ischemic stroke, risk evaluation
1. Introduction
The presence of atrial fibrillation (AF) is a recognized risk factor for ischemic stroke [1]. In these patients, the use of oral anticoagulation reduces the risk of ischemic stroke and mortality in clinical trials or observational cohorts [1].
The CHA2DS2-VASc score is calculated using the baseline risk factors for assessment of the risk of ischemic stroke risk in patients with AF [2]. However, the stroke risk in patients with AF is not static, and with time, patients get older and acquire new comorbidities. Indeed, one analysis from Taiwan reported that the change in CHA2DS2-VASc score between baseline and follow-up was a better predictor of ischemic stroke [3]. A similar observation was reported from Korea [4]. We are unaware of any similar studies in white European cohort.
We analyzed a nationwide cohort from France and we hypothesized that the change in stroke risk profile between baseline and follow-up may be a better predictor of ischemic stroke than the baseline stroke risk determination using the CHA2DS2-VASc score.
2. Methods
Our nationwide French longitudinal cohort study used the national database covering hospital care for the whole population. The data for all patients admitted with AF in France from January 2010 to December 2018 were collected from the national administrative database, the PMSI (Programme de Médicalisation des Systeèmes d’Information). This includes around 98% of the French population (67 million people) from birth (or immigration) to death (or emigration). As a result of this program implemented in 2004, medical activity is recorded in a database, computed, and rendered anonymous in the 1546 French healthcare facilities. Medical information includes the principal and secondary diagnoses which are identified with codes according to the International Classification of Diseases (ICD-10). Data for medical procedures were collected with the CCAM (classification commune des actes médicaux) which is the French medical reimbursement classification for clinical procedures. The PMSI contains individual pseudo anonymized information on each hospitalization that are linked to create a longitudinal record of hospital stays and diagnoses for each patient. Its reliability has been well evaluated [5] and this database has been used to study subjects with cardiovascular conditions, including those with AF or ischemic stroke [6,7,8]
This type of study was approved by our local institutional review board, on 1 December 2015 and was registered as a clinical audit. Ethical review was consequently not needed. The study was conducted retrospectively, patients were not involved in its conduct, and there was no impact on their care. Procedures for data collection and management have been approved by the Commission Nationale de l’Informatique et des Libertés (CNIL), the independent National ethical committee in France ensuring that information is kept confidential and anonymous (authorization number 1897139).
2.1. Study Population
We identified all patients over 18 years in the period from 1 January, 2010 to 31 December, 2018 hospitalized with a diagnosis of AF (I48 and its subsections using ICD10 codes) over the study period. The study database was constructed using the encrypted anonymized number. Patient information (including medical history and events of interest during follow-up) was described using data collected in the hospital records. Diagnoses were obtained for each hospital stay at discharge. For the present analysis, follow-up started at the date of AF diagnosis. We calculated the CHA2DS2-VASc score for every patient. In this population, there were 608,108 patients not having any risk factors of the CHA2DS2-VASc score, except for age and sex; and this was the population of interest for our study. During a follow-up of 1,322,449 person-years, 20,082 patients suffered ischemic stroke (incidence rate 1.52%/year). The flowchart of patients enrolled in the study is shown in Figure 1.
Figure 1.
Flowchart of the study population. CHA2DS2-VASc score: (congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke/transient ischemic attack/thromboembolism (doubled), vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque), age 65–75 years, sex category (female)).
2.2. Baseline, Follow-Up, Delta CHA2DS2-VASc Scores, and Its Slope
The CHA2DS2-VASc scores were determined at baseline for all patients at study entry (baseline CHA2DS2-VASc score). The CHA2DS2-VASc scores at follow-up were defined as the highest recorded CHA2DS2-VASc score of each patient during the follow-up period, before the occurrence of ischemic stroke, mortality, and/or at the last news for patients free of these events. The Delta CHA2DS2-VASc score was defined as the difference between the baseline and follow-up scores (follow-up CHA2DS2-VASc minus baseline CHA2DS2-VASc scores). Among patients with a Delta CHA2DS2-VASc score ≥ 1, the rate of change in scores was also assessed by calculating the slope. The slope of CHA2DS2-VASc score change was defined as the following: log ((Delta CHA2DS2-VASc score/follow-up duration (duration in years between the index date and the date when the highest CHA2DS2-VASc score was recorded))+1).
2.3. Statistical Analyses
Qualitative variables are described using counts and percentages and continuous quantitative variables as means ± standard deviation and also median and quartiles when necessary. Comparisons between groups were made with chi-square tests for comparing categorical variables and the Student t test or non-parametric Kruskal–Wallis test where appropriate for continuous variables. Information on outcomes during follow-up was obtained by analyzing the PMSI codes for each patient. For the outcomes analysis, the incidence rates (%/year) was estimated in the different groups. The diagnostic accuracies of baseline, follow-up, and Delta CHA2DS2-VASc scores in predicting ischemic stroke were assessed by calculating C-indexes. The areas under the receiver-operating characteristic (ROC) curves (AUCs) of these scorings were compared using the DeLong test. Net reclassification improvement (NRI) and decision-curve analysis (DCA) were used to estimate the clinical usefulness of the predictive models. In all analyses, a p value < 0.05 was considered statistically significant. All analyses were performed using Enterprise Guide ® 7.1, ©SAS Institute (SAS Campus Drive, Cary, NC, USA) and STATA® 12, (©Stata Corp, College Station, TX, USA).
2.4. Data Access
Because this study used data from human subjects, the data and everything pertaining to the data are governed by the French Health Agencies and cannot be made available to other researchers.
3. Results
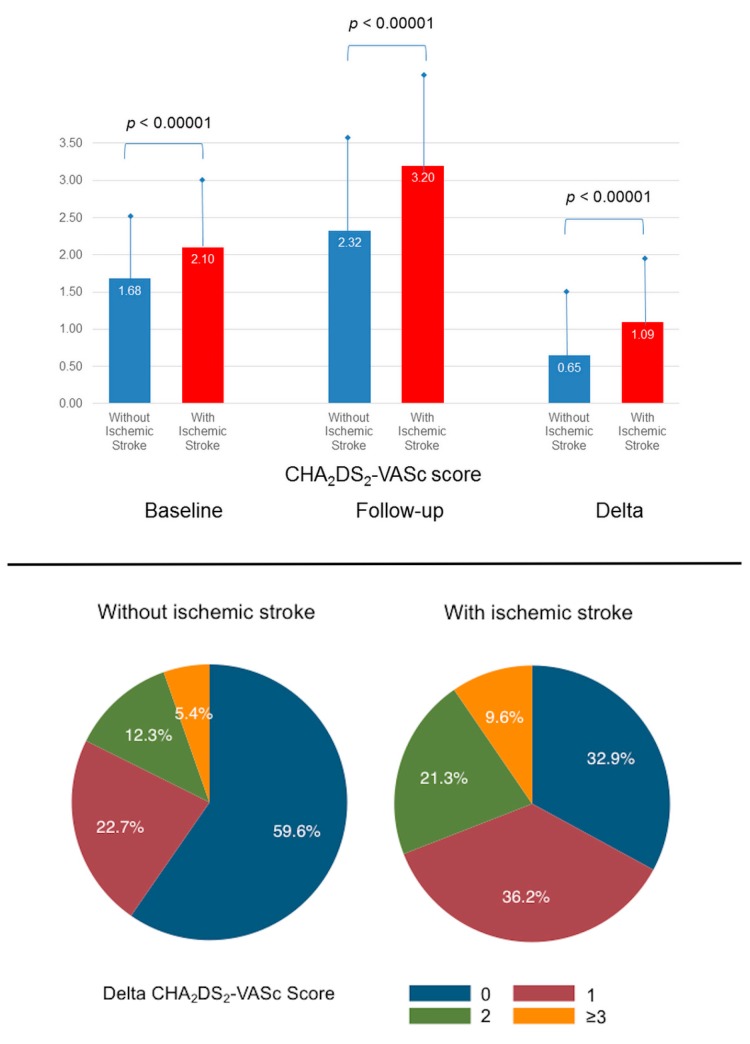
The characteristics of patients with or without ischemic stroke are in Table 1. Age increased from 72.7 to 74.8 years during a mean follow-up of 2.2 ± 2.4 years (median (interquartile range: IQR) 1.2 (0.1–3.8)). The mean of the baseline CHA2DS2-VASc score was 1.7, and it increased to 2.4 during follow-up, with a mean Delta CHA2DS2-VASc score of 0.7. The baseline, follow-up and Delta CHA2DS2-VASc scores were higher for patients suffering ischemic stroke than those with no stroke (Figure 2). The % of patients with a Delta CHA2DS2-VASc score = 0 was 58.8%, meaning that more than 40% of patients had more comorbidities or became >65 or 75 years during follow-up. Around 38% of patients had ≥1 new-onset comorbidity, the most frequent being hypertension (25.7%) and heart failure (20.7%).
Table 1.
Baseline characteristics of AF patients.
| Total | No Ischemic Stroke | Ischemic Stroke | p | |
|---|---|---|---|---|
| (n = 608108) | (n = 588026) | (n = 20082) | ||
| Characteristics at baseline | ||||
| Age, years | 72.7 ± 14.6 | 72.5 ± 14.6 | 78.3 ± 10.8 | <0.0001 |
| Age 65–74 yrs | 135994 (22.4) | 132380 (22.5) | 3614 (18.0) | <0.0001 |
| Age ≥75 yrs | 313332 (51.5) | 299213 (50.9) | 14119 (70.3) | <0.0001 |
| Gender (male) | 341299 (56.1) | 331608 (56.4) | 9691 (48.3) | <0.0001 |
| CHA2DS2-VASc score | 1.7 ± 1.1 | 1.7 ± 1.1 | 2.1 ± 0.9 | <0.0001 |
| CHA2DS2-VASc score = 0 | 110373 (18.2) | 108728 (18.5) | 1645 (8.2) | <0.0001 |
| CHA2DS2-VASc score = 1 | 131674 (21.7) | 128849 (21.9) | 2825 (14.1) | <0.0001 |
| CHA2DS2-VASc score = 2 | 197673 (32.5) | 190410 (32.4) | 7263 (36.2) | <0.0001 |
| CHA2DS2-VASc score = 3 | 168388 (27.7) | 160039 (27.2) | 8349 (41.6) | <0.0001 |
| Coronary artery disease | 52807 (8.7) | 50744 (8.6) | 2063 (10.3) | <0.0001 |
| Previous pacemaker or Defibrillator | 8317 (1.4) | 8022 (1.4) | 295 (1.5) | 0.21 |
| Smoker | 26202 (4.3) | 25687 (4.4) | 515 (2.6) | <0.0001 |
| Dyslipidemia | 38136 (6.3) | 36967 (6.3) | 1169 (5.8) | 0.01 |
| Obesity | 33410 (5.5) | 32700 (5.6) | 710 (3.5) | <0.0001 |
| Alcohol related diagnoses | 24514 (4.0) | 23803 (4.0) | 711 (3.5) | 0.0003 |
| Abnormal renal function | 10898 (1.8) | 10555 (1.8) | 343 (1.7) | 0.36 |
| Lung disease | 60242 (9.9) | 58721 (10.0) | 1521 (7.6) | <0.0001 |
| Liver disease | 13894 (2.3) | 13572 (2.3) | 322 (1.6) | <0.0001 |
| Thyroid diseases | 39551 (6.5) | 38244 (6.5) | 1307 (6.5) | 0.98 |
| Inflammatory disease | 22307 (3.7) | 21587 (3.7) | 720 (3.6) | 0.52 |
| Anemia | 54504 (9.0) | 52925 (9.0) | 1579 (7.9) | <0.0001 |
| Previous cancer | 93633 (15.4) | 91466 (15.6) | 2167 (10.8) | <0.0001 |
| Characteristics after the follow-up | ||||
| Age, years | 74.8 ± 14.6 | 74.6 ± 14.7 | 80.8 ± 10.8 | <0.0001 |
| Age 65–74 yrs | 128281 (21.1) | 125254 (21.3) | 3027 (15.1) | <0.0001 |
| Age ≥75 yrs | 343328 (56.5) | 328135 (55.8) | 15193 (75.7) | <0.0001 |
| CHA2DS2-VASc score | 2.4 ± 1.5 | 2.3 ± 1.5 | 3.2 ± 1.3 | <0.0001 |
| CHA2DS2-VASc score = 0 | 76845 (12.6) | 76186 (13.0) | 659 (3.3) | <0.0001 |
| CHA2DS2-VASc score = 1 | 100752 (16.6) | 99369 (16.9) | 1383 (6.9) | <0.0001 |
| CHA2DS2-VASc score = 2 | 144335 (23.7) | 140771 (23.9) | 3564 (17.7) | <0.0001 |
| CHA2DS2-VASc score = 3 | 160270 (26.4) | 154124 (26.2) | 6146 (30.6) | <0.0001 |
| CHA2DS2-VASc score = 4 | 78774 (13.0) | 73630 (12.5) | 5144 (25.6) | <0.0001 |
| CHA2DS2-VASc score = 5 | 37770 (6.2) | 35291 (6.0) | 2479 (12.3) | <0.0001 |
| CHA2DS2-VASc score = 6 | 8584 (1.4) | 7941 (1.4) | 643 (3.2) | <0.0001 |
| CHA2DS2-VASc score = 7 | 778 (0.1) | 714 (0.1) | 64 (0.3) | <0.0001 |
| New-onset comorbidities | ||||
| Hypertension | 156081 (25.7) | 146131 (24.9) | 9950 (49.5) | <0.0001 |
| Heart failure | 125684 (20.7) | 119795 (20.4) | 5889 (29.3) | <0.0001 |
| Diabetes mellitus | 27105 (4.5) | 25531 (4.3) | 1574 (7.8) | <0.0001 |
| Vascular disease | 40512 (6.7) | 37503 (6.4) | 3009 (15.0) | <0.0001 |
| Any new-onset comorbidity | 227993 (37.5) | 214952 (36.6) | 13041 (64.9) | <0.0001 |
| Delta CHA2DS2-VASc score | 0.7 ± 0.9 | 0.6 ± 0.9 | 1.1 ± 1.0 | <0.0001 |
| Delta CHA2DS2-VASc score = 0 | 357349 (58.8) | 350738 (59.6) | 6611 (32.9) | <0.0001 |
| Delta CHA2DS2-VASc score = 1 | 140768 (23.1) | 133503 (22.7) | 7265 (36.2) | <0.0001 |
| Delta CHA2DS2-VASc score = 2 | 76373 (12.6) | 72090 (12.3) | 4283 (21.3) | <0.0001 |
| Delta CHA2DS2-VASc score = 3 | 27067 (4.5) | 25498 (4.3) | 1569 (7.8) | <0.0001 |
| Delta CHA2DS2-VASc score = 4 | 5809 (1.0) | 5484 (0.9) | 325 (1.6) | <0.0001 |
| Delta CHA2DS2-VASc score = 5 | 742 (0.1) | 713 (0.1) | 29 (0.1) | 0.36 |
Values are mean ± SD or n (%). AF = atrial fibrillation; CHA2DS2-VASc = congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, age 65–74 years, sex category (female).
Figure 2.
CHA2DS2-VASc scores of patients with AF with or without ischemic stroke: baseline, follow-up, and Delta CHA2DS2-VASc scores (top panel) and repartition of Delta CHA2DS2-VASc in these patients (lower panel). CHA2DS2-VASc score: (congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke/transient ischemic attack/thromboembolism (doubled), vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque), age 65–75 years, sex category (female)).
Among 20,082 patients who suffered ischemic stroke, 67.1% had a Delta CHA2DS2-VASc score ≥1 compared while this was the case for only 40.4% in patients with no ischemic stroke (Figure 2), and 13,041 (64.9%) patients were identified as having ≥1 new-onset comorbidity (hypertension in 9950 (49.5%), heart failure in 5889 (29.3%), diabetes mellitus in 1574 (7.8%), and vascular diseases in 3009 (15.0%)) (Table 1). In patients who experienced ischemic stroke, the number of new-onset comorbidities is shown in Supplemental Figure S1. This shows that 53.9% of patients developed 1 new comorbidity, whilst 2, 3, and 4 new comorbidities were identified in 31.8%, 11.6%, and 2.6% of patients.
3.1. Baseline, Follow-Up and Delta CHA2DS2-VASc Scores, and the Risk of Ischemic Stroke
The characteristics of patients by the Delta CHA2DS2-VASc score are in Table 2. The yearly risk of ischemic stroke increased continuously from 0.67% for those patients with a baseline CHA2DS2-VASc score = 0 to 2.52% for those with a baseline CHA2DS2-VASc score = 3, and from 0.60% for those with a follow-up CHA2DS2-VASc score = 0 to 1.98% for patients with a follow-up CHA2DS2-VASc score = 4 (Table 3). Considering Delta CHA2DS2-VASc score, the highest risk was seen for patients with a Delta CHA2DS2-VASc score = 1.
Table 2.
Characteristics of patients with AF stratified by delta CHA2DS2-VASc score.
| Delta CHA2DS2-VASc score = 0 |
Delta CHA2DS2-VASc score = 1 |
Delta CHA2DS2-VASc score = 2 |
Delta CHA2DS2-VASc score ≥3 |
|
|---|---|---|---|---|
| (n = 357349) | (n = 140768) | (n = 76373) | (n = 33618) | |
| Characteristics at baseline | ||||
| Age, years | 70.7 ± 16.1 | 75.1 ± 12.2 | 76.7 ± 10.8 | 74.6 ± 9.6 |
| Age 65–74 yrs | 75426 (21.1) | 31574 (22.4) | 17578 (23.0) | 11416 (34.0) |
| Age ≥75 yrs | 171231 (47.9) | 79587 (56.5) | 46575 (61.0) | 15939 (47.4) |
| Gender (male) | 200713 (56.2) | 77384 (55.0) | 41750 (54.7) | 21452 (63.8) |
| CHA2DS2-VASc score | 1.6 ± 1.1 | 1.8 ± 1.0 | 1.9 ± 1.0 | 1.6 ± 1.0 |
| CHA2DS2-VASc score = 0 | 76841 (21.5) | 20344 (14.5) | 8444 (11.1) | 4607 (13.7) |
| CHA2DS2-VASc score = 1 | 79937 (22.4) | 28338 (20.1) | 14311 (18.7) | 9081 (27.0) |
| CHA2DS2-VASc score = 2 | 107111 (30.0) | 49157 (34.9) | 28689 (37.6) | 12829 (38.2) |
| CHA2DS2-VASc score = 3 | 93460 (26.2) | 42929 (30.5) | 24929 (32.6) | 7101 (21.1) |
| Coronary artery disease | 18290 (5.1) | 17106 (12.2) | 10545 (13.8) | 6866 (20.4) |
| Previous pacemaker or Defibrillator | 4331 (1.2) | 2237 (1.6) | 1244 (1.6) | 505 (1.5) |
| Smoker | 17859 (5.0) | 5074 (3.6) | 2231 (2.9) | 1038 (3.1) |
| Dyslipidemia | 21218 (5.9) | 9811 (7.0) | 4929 (6.5) | 2178 (6.5) |
| Obesity | 19348 (5.4) | 7493 (5.3) | 4323 (5.7) | 2246 (6.7) |
| Alcohol related diagnoses | 15704 (4.4) | 5050 (3.6) | 2492 (3.3) | 1268 (3.8) |
| Abnormal renal function | 5846 (1.6) | 2733 (1.9) | 1585 (2.1) | 734 (2.2) |
| Lung disease | 35331 (9.9) | 13682 (9.7) | 7692 (10.1) | 3537 (10.5) |
| Liver disease | 8938 (2.5) | 2956 (2.1) | 1407 (1.8) | 593 (1.8) |
| Thyroid diseases | 23446 (6.6) | 9591 (6.8) | 4866 (6.4) | 1648 (4.9) |
| Inflammatory disease | 13299 (3.7) | 5104 (3.6) | 2773 (3.6) | 1131 (3.4) |
| Anemia | 34058 (9.5) | 12223 (8.7) | 6011 (7.9) | 2212 (6.6) |
| Previous cancer | 61422 (17.2) | 20163 (14.3) | 8798 (11.5) | 3250 (9.7) |
| Characteristics after the follow-up | ||||
| Follow-up duration (years) | 1.2 ± 1.9 | 3.0 ± 2.4 | 3.8 ± 2.4 | 4.8 ± 2.3 |
| Age, years | 71.9 ± 15.9 | 78.1 ± 11.9 | 80.5 ± 10.4 | 79.3 ± 9.2 |
| Age 65–74 yrs | 75426 (21.1) | 31250 (22.2) | 14225 (18.6) | 7380 (22.0) |
| Age ≥75 yrs | 171231 (47.9) | 91132 (64.7) | 56420 (73.9) | 24545 (73.0) |
| CHA2DS2-VASc score | 1.6 ± 1.1 | 2.8 ± 1.0 | 3.9±1.0 | 4.9 ± 1.0 |
| CHA2DS2-VASc score = 0 | 76845 (21.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| CHA2DS2-VASc score = 1 | 79940 (22.4) | 20812 (14.8) | 0 (0.0) | 0 (0.0) |
| CHA2DS2-VASc score = 2 | 107108 (30.0) | 28419 (20.2) | 8808 (11.5) | 0 (0.0) |
| CHA2DS2-VASc score = 3 | 93456 (26.2) | 48898 (34.7) | 14432 (18.9) | 3484 (10.4) |
| CHA2DS2-VASc score = 4 | 0 (0.0) | 42639 (30.3) | 28480 (37.3) | 7655 (22.8) |
| CHA2DS2-VASc score = 5 | 0 (0.0) | 0 (0.0) | 24653 (32.3) | 13117 (39.0) |
| CHA2DS2-VASc score = 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8584 (25.5) |
| CHA2DS2-VASc score = 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 778 (2.3) |
| Hypertension | 0 (0.0) | 61486 (43.7) | 62627 (82.0) | 31968 (95.1) |
| Heart failure | 0 (0.0) | 45143 (32.1) | 51028 (66.8) | 29513 (87.8) |
| Diabetes mellitus | 0 (0.0) | 4590 (3.3) | 9115 (11.9) | 13400 (39.9) |
| Vascular disease | 0 (0.0) | 6783 (4.8) | 13639 (17.9) | 20090 (59.8) |
| Any new-onset comorbidity | 0 (0.0) | 118002 (83.8) | 76373 (100.0) | 33618 (100.0) |
Values are mean ± SD or n (%). Abbreviation as in Table 1. IQR = interquartile range.
Table 3.
Risk of ischemic stroke in AF patients with different baseline follow-up and delta CHA2DS2-VASc score (mean (SD) 2.2 (2.4), median (IQR) 1.2 (0.1–3.8) years).
| Number of Patients | Number of Incident Ischemic Stroke | Incidence of Ischemic Stroke (%/year) | Hazard. Ratio (95%CI) | p | |
|---|---|---|---|---|---|
| Baseline CHA2DS2-VASc score | |||||
| 0 (reference group) | 111,269 | 1687 | 0.67 | 1.00 | |
| 1 | 131,937 | 2852 | 0.91 | 1.35 (1.27–1.43) | <0.0001 |
| 2 | 197,176 | 7238 | 1.69 | 2.54 (2.41–2.68) | <0.0001 |
| 3 | 167,726 | 8305 | 2.52 | 3.80 (3.61–4.01) | <0.0001 |
| Follow-up CHA2DS2-VASc score | |||||
| 0 (reference group) | 76,845 | 659 | 0.60 | 1.00 | |
| 1 | 100,752 | 1383 | 0.77 | 1.30 (1.18–1.42) | <0.0001 |
| 2 | 144,335 | 3564 | 1.34 | 2.25 (2.07–2.44) | <0.0001 |
| 3 | 160,270 | 6146 | 1.91 | 3.20 (2.95–3.47) | <0.0001 |
| 4 | 78,774 | 5144 | 1.98 | 3.38 (3.12–3.67) | <0.0001 |
| ≥5 | 47,132 | 3186 | 1.70 | 2.90 (2.66–3.15) | <0.0001 |
| Delta CHA2DS2-VASc score | |||||
| 0 (reference group) | 357,349 | 6611 | 1.50 | 1.00 | |
| 1 | 140,768 | 7265 | 1.69 | 1.16 (1.12–1.20) | <0.0001 |
| 2 | 76,373 | 4283 | 1.46 | 0.99 (0.96–1.03) | 0.75 |
| 3 | 27,067 | 1569 | 1.27 | 0.85 (0.81–0.90) | <0.0001 |
| ≥4 | 6551 | 354 | 0.99 | 0.65 (0.58–0.72) | <0.0001 |
Values are n (incidence rate, %/year); CI = confidence interval. AF = atrial fibrillation; SD = standard deviation; IQR = interquartile range.
When considering the baseline, follow-up and delta CHA2DS2-VASc scores, using their respective score = 0 as the reference, the hazard ratios for the strata of the increasing scores are in Table 3. Increasing baseline and follow-up CHA2DS2-VASc scores were associated with a higher risk of ischemic stroke. This was also the case for subjects with delta CHA2DS2-VASc score of 1 (compared to 0 as the reference), but this association was not seen when Delta CHA2DS2-VASc score got higher.
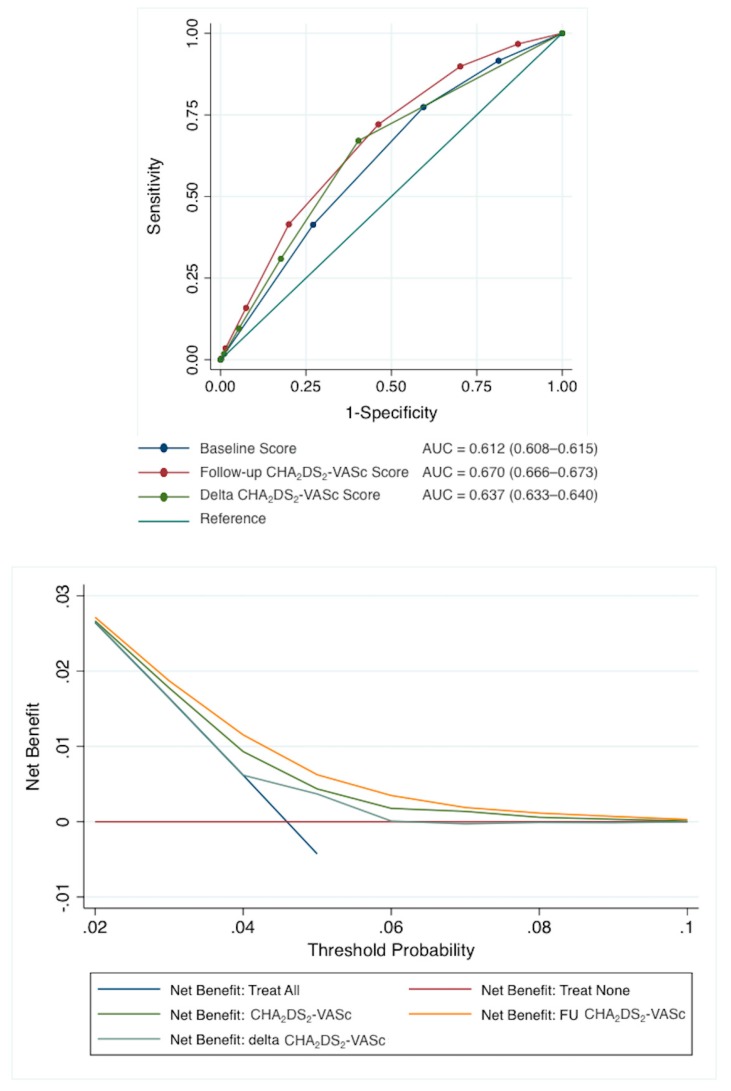
Figure 3 presents the AUC for the baseline, follow-up, and Delta CHA2DS2-VASc scores for prediction of ischemic stroke. The AUC was lower for the baseline CHA2DS2-VASc score (0.612, 95%CI 0.608–0.615) compared to that of follow-up (0.670, 95%CI 0.666–0.673) or Delta (0.637, 95%CI 633–640) scores (p < 0.0001 for DeLong test).
Figure 3.
Upper panel: AUCs for the baseline follow-up and Delta CHA2DS2-VASc scores in predicting ischemic stroke. Lower panel: Decision curve analysis: net number of true positives gained with different models with different thresholds probabilities, compared to no model. AUC = area under curve
When we used NRI, we found a significant difference in the follow-up score compared to the baseline score, NRI being 14.4% (95% CI: 13.5 to 15.4%; p < 0.0001). Considering the Delta CHA2DS2-VASc score versus baseline score, the NRI was 14.4% (95% CI: 13.5 to 15.4%; p < 0.0001). Regarding the follow-up score vs the Delta score, NRI was 7.5% (95% CI: 6.5% to 8.4%; p < 0.0001). Using DCA, assuming that a high risk identified by one of the tests would result in different treatment, FU CHA2DS2-VASc score was superior to the “treat all” strategy and the “treat none” strategy. The FU CHA2DS2-VASc score was likewise superior to the CHA2DS2-VASc score and Delta CHA2DS2-VASc score for any threshold probability (Figure 3).
3.2. Slope of the Change in CHA2DS2-VASc Score
The slope was higher for patients with ischemic stroke than in those with no ischemic stroke (0.9 vs. 0.7; p < 0.0001), and this was also seen with different Delta CHA2DS2-VASc score (Table 4). The OR for suffering ischemic stroke was 1.29 (95% CI: 1.27 to 1.31; p < 0.0001) per 1 unit of the slope for patients with a Delta CHA2DS2-VASc score ≥1. This increased from 1.22 (95% CI: 1.19 to 1.24) for patients with a Delta CHA2DS2-VASc score = 1 to 1.64 (95% CI: 1.37 to 1.96) for patients with a Delta CHA2DS2-VASc score ≥4. The AUCs of the slope for prediction of ischemic stroke were marginally higher for patients with higher Delta CHA2DS2-VASc scores (2 and ≥3) than for those with lower Delta CHA2DS2-VASc score = 1 (Supplemental Figure S2).
Table 4.
Slope of the change of c CHA2DS2-VASc score and the risk of ischemic stroke in each group of delta CHA2DS2-VASc score.
| No Ischemic Stroke | Ischemic Stroke | p value | Odds Ratio (95% CI) of Ischemic Stroke Per Unit Slope Change | p value | |
|---|---|---|---|---|---|
| (n = 237288) | (n = 13471) | ||||
| Delta CHA2DS2-VASc Score | |||||
| All (≥1) | 0.7 ± 0.8 | 0.9 ± 1.2 | <0.0001 | 1.29 (1.27–1.31) | <0.0001 |
| 1 | 0.7 ± 0.9 | 0.9 ± 1.3 | <0.0001 | 1.22 (1.19–1.24) | <0.0001 |
| 2 | 0.7 ± 0.6 | 0.9 ± 1.2 | <0.0001 | 1.48 (1.44–1.53) | <0.0001 |
| 3 | 0.7 ± 0.5 | 0.9 ± 0.9 | <0.0001 | 1.54 (1.44–1.64) | <0.0001 |
| ≥ 4 | 0.6 ± 0.4 | 0.8 ± 0.8 | <0.0001 | 1.64 (1.37–1.96) | <0.0001 |
4. Discussion
In this study from a white European nationwide AF cohort, our main findings are: (1) Stroke risk (assessed by CHA2DS2-VASc score) was non-static, and many patients developed ≥1 new stroke risk factor(s) before presenting with ischemic stroke; and (2) the follow-up CHA2DS2-VASc score and its evolution (i.e.,‘Delta CHA2DS2-VASc’, reflecting the modification in stroke risk profile between baseline and follow-up) were better predictors of ischemic stroke than relying on the baseline CHA2DS2-VASc score.
To our knowledge, this is the first report assessing the dynamic nature of stroke risk in a non-Asian cohort, using a large dataset from the large France nationwide administrative hospital-discharge database. The present study from France is the first from a non-Asian cohort, and was substantially larger than either the Taiwan and South Korea studies. In contrast to prior studies, we found that the follow-up CHA2DS2-VASc score had the best value for predicting ischemic stroke, as shown by AUCs and NRI.
In the study from Taiwan, Chao et al [3] reported that among AF patients with ischemic stroke, 89% had a Delta CHA2DS2-VASc score ≥1 whereas this was the case for only 55% in patients without ischemic stroke, and 64% patients had ≥1 new-onset comorbidity, the most common being hypertension. The Delta CHA2DS2-VASc score was a significant predictor of ischemic stroke performing better than baseline or follow-up CHA2DS2-VASc scores.
By contrast to the follow-up CHA2DS2-VASc score, an increasing Delta CHA2DS2-VASc score above 1 (2, 3, and ≥4) in our study was not significantly associated with a higher risk of ischemic stroke. This may seem counterintuitive and different to Chao et al [3], but our patients with highest Delta CHA2DS2-VASc in our study had longer follow-up, lower prevalence of those age above 75 and lower baseline CHA2DS2-VASc score. This may partly explain why the univariate analysis by strata of the Delta CHA2DS2-VASc score did not find increasing risk when Delta CHA2DS2-VASc score increased above 2. Of note, the predictive value of the slope of CHA2DS2-VASc score change in the different strata confirms that the increasing number of risk factors per year provides consistent evidence on the increasing risk of stroke in our population.
In a similar analysis in Korea, studying more than 160,000 AF patients with no anticoagulation, Yoon et al [4] reported that during follow-up of 10 years, the rate of ischemic stroke was higher when patients had incident risk factors, and were re-classified into categories with higher CHA2DS2-VASc score. Thus, the two Asian study and our results reaffirm why assessment of stroke risk is needed at each contact with the patient, as addition of risk factors and increasing CHA2DS2-VASc score results over time in a greater stroke risks. Of note, the dynamic nature of risk is not only evident for stroke risk, but also for bleeding risk. Chao et al also showed that the best predictive value for major bleeding was the follow-up and change HAS-BLED score [9,10].
Assessment of clinical usefulness (using Decision Curve Analysis) re-emphasizes the value of follow-up stroke risk assessment. When assessing the slope of change in CHA2DS2-VASc scores, the predictive value was better for patients when they had higher Delta CHA2DS2-VASc scores (2 and ≥3) compared to those who had a lower Delta CHA2DS2-VASc score of 1. Indeed, the “take home” message should be that clinicians should not rely on baseline ’one off’ stroke risk assessment, but regular re-assessment, for the follow-up or change in stroke risk profile, which is a better predictor compared to the baseline assessment.
Limitations
One limitation of this study was related to its observational and retrospective nature. This analysis is limited by its registry design, and errors for classification or coding may be present. Identifying AF and comorbidities is challenging. However, ICD-10 is considered reliable for identifying AF, stroke and stroke risk factors [11,12,13]. The PMSI was previously verified and used for epidemiologic purpose in AF patients or those with ischemic stroke [7]. We were not able to differentiate paroxysmal AF from persistent and permanent AF, whereas patients with permanent AF may have a higher risk of stroke. Events included were only in-hospital, and we had no outpatient data. Results apply to inpatients with AF and may not apply to all AF patients. Data were based on the diagnostic codes registered for reimbursement purposes and were not externally checked with a potential information bias. However, our study aimed to provide a simple clinical approach and a global picture at a national level not limited to tertiary referral centers. Drug therapies, particularly oral anticoagulation use, were not included in the analysis, which may impact on event rates at follow-up. The recent EORP-AF registry indicates that the rate of anticoagulation therapy based on CHA2DS2VASc score (≥2) in Europe is generally above 85% [14] and this should apply to our nationwide analysis. Another limitation is the lack of available information concerning time in therapeutic range for international normalized ratios for patients taking vitamin K antagonist. However, the study size of the population including every hospitalization in France with a negligible risk of follow-up loss may compensate for some of these limitations.
5. Conclusions
Stroke risk (CHA2DS2-VASc score) was dynamic, and many AF patients had ≥1 new stroke risk factor(s) before they suffered ischemic stroke. The follow-up CHA2DS2-VASc score and its change (i.e., Delta CHA2DS2-VASc, picturing the evolution in stroke risk profile between baseline and follow-up) were better predictors of ischemic stroke than relying on the baseline CHA2DS2-VASc score. This emphasizes that stroke risk in AF is an evolving process when age increases and when new comorbidities appear, and regular re-assessment of risk is needed.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/4/1234/s1, Supplemental Figure S1. Number of new-onset comorbidities for patients who experienced ischemic stroke. Supplemental Figure S2. AUCs for the slopes in predicting ischemic stroke in different Delta CHA2DS2-VASc scores.
Author Contributions
Conceptualization, L.F., T.-F.C. and G.Y.H.L.; Data curation, L.F., A.B. (Alexandre Bodin), A.B. (Arnaud Bisson) and J.H.; Formal analysis, L.F. and A.B. (Arnaud Bisson); Methodology, A.B. (Alexandre Bodin), A.B. (Arnaud Bisson), P.S. and G.Y.H.L.; Software, A.B. (Alexandre Bodin) and J.H.; Supervision, T.-F.C. and G.Y.H.L.; Validation, P.S. and D.B.; Writing—original draft, L.F.; Writing—review and editing, N.C., T.-F.C. and G.Y.H.L. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
LF: speaker or consultant for Boehringer Ingelheim, Bayer, BMS/Pfizer, Medtronic, and Novartis. NC: Consultant or speaker for Boston Scientific and Medtronic. DB: Speaker for BMS/Pfizer and Medtronic. GYHL: Consultant for BMS/Pfizer, Bayer/Janssen, Boehringer Ingelheim, Medtronic, Novartis, Verseon, and Daiichi-Sankyo. Speaker for BMS/Pfizer, Bayer, Boehringer Ingelheim, Medtronic, and Daiichi-Sankyo. No fees are directly received personally. Other authors—none.
References
- 1.Lip G.Y.H., Freedman B., De Caterina R., Potpara T.S. Stroke prevention in atrial fibrillation: Past, present and future. Comparing the guidelines and practical decision-making. Thromb. Haemost. 2017;117:1230–1239. doi: 10.1160/TH16-11-0876. [DOI] [PubMed] [Google Scholar]
- 2.Lip G.Y.H., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J.G.M. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 3.Chao T.F., Lip G.Y.H., Liu C.J., Lin Y.J., Chang S.L., Lo L.W., Hu Y.F., Tuan T.C., Liao J.N., Chung F.P., et al. Relationship of aging and incident comorbidities to stroke risk in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2018;71:122–132. doi: 10.1016/j.jacc.2017.10.085. [DOI] [PubMed] [Google Scholar]
- 4.Yoon M., Yang P.S., Jang E., Yu H.T., Kim T.H., Uhm J.S., Kim J.Y., Pak H.N., Lee M.H., Lip G.Y.H., et al. Dynamic changes of cha2ds2-vasc score and the risk of ischaemic stroke in asian patients with atrial fibrillation: A nationwide cohort study. Thromb. Haemost. 2018;118:1296–1304. doi: 10.1055/s-0038-1651482. [DOI] [PubMed] [Google Scholar]
- 5.Chantry A.A., Deneux-Tharaux C., Cans C., Ego A., Quantin C., Bouvier-Colle M.-H., Group G.S. Hospital discharge data can be used for monitoring procedures and intensive care related to severe maternal morbidity. J. Clin. Epidemiol. 2011;64:1014–1022. doi: 10.1016/j.jclinepi.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Lorgis L., Cottenet J., Molins G., Benzenine E., Zeller M., Aube H., Touzery C., Hamblin J., Gudjoncik A., Cottin Y., et al. Outcomes after acute myocardial infarction in hiv-infected patients: Analysis of data from a french nationwide hospital medical information database. Circulation. 2013;127:1767–1774. doi: 10.1161/CIRCULATIONAHA.113.001874. [DOI] [PubMed] [Google Scholar]
- 7.Fauchier L., Clementy N., Pelade C., Collignon C., Nicolle E., Lip G.Y. Patients with ischemic stroke and incident atrial fibrillation: A nationwide cohort study. Stroke. 2015;46:2432–2437. doi: 10.1161/STROKEAHA.115.010270. [DOI] [PubMed] [Google Scholar]
- 8.Fauchier L., Chaize G., Gaudin A.F., Vainchtock A., Rushton-Smith S.K., Cotte F.E. Predictive ability of has-bled, hemorr2hages, and atria bleeding risk scores in patients with atrial fibrillation. A french nationwide cross-sectional study. Int. J. Cardiol. 2016;217:85–91. doi: 10.1016/j.ijcard.2016.04.173. [DOI] [PubMed] [Google Scholar]
- 9.Pisters R., Lane D.A., Nieuwlaat R., de Vos C.B., Crijns H.J.G.M., Lip G.Y.H. A novel user-friendly score (has-bled) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The euro heart survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 10.Chao T.F., Lip G.Y.H., Lin Y.J., Chang S.L., Lo L.W., Hu Y.F., Tuan T.C., Liao J.N., Chung F.P., Chen T.J., et al. Incident risk factors and major bleeding in patients with atrial fibrillation treated with oral anticoagulants: A comparison of baseline, follow-up and delta has-bled scores with an approach focused on modifiable bleeding risk factors. Thromb. Haemost. 2018;118:768–777. doi: 10.1055/s-0038-1636534. [DOI] [PubMed] [Google Scholar]
- 11.Kokotailo R.A., Hill M.D. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 12.Norberg J., Backstrom S., Jansson J.H., Johansson L. Estimating the prevalence of atrial fibrillation in a general population using validated electronic health data. Clin. Epidemiol. 2013;5:475–481. doi: 10.2147/CLEP.S53420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter J., Mondor L., Kapral M.K., Fang J., Hall R.E. How reliable are administrative data for capturing stroke patients and their care. Cerebrovasc. Dis. Extra. 2016;6:96–106. doi: 10.1159/000449288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boriani G., Proietti M., Laroche C., Fauchier L., Marin F., Nabauer M., Potpara T., Dan G.A., Kalarus Z., Diemberger I., et al. Contemporary stroke prevention strategies in 11 096 european patients with atrial fibrillation: A report from the eurobservational research programme on atrial fibrillation (eorp-af) long-term general registry. Europace. 2018;20:747–757. doi: 10.1093/europace/eux301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.