Abstract
Seaweeds have been exploited as both food products and therapeutics to manage human ailments for centuries. This study investigated the metabolite profile of five seaweeds (Halimeda spp., Spyridia hypnoides (Bory de Saint-Vincent) Papenfuss, Valoniopsis pachynema (G. Martens) Børgesen, Gracilaria fergusonii J. Agardh and Amphiroa anceps (Lamarck) Decaisne using ultra-high-performance liquid chromatography coupled with electrospray ionization mass spectrometry (UHPLC-ESI-MS/MS). Furthermore, these seaweeds were assessed for antioxidant and inhibitory effects against α-amylase, α-glucosidase, acetyl-cholinesterase (AChE), butyryl-cholinesterase (BChE) and tyrosinase. Valoniopsis pachynema and A. anceps yielded the highest flavonoid (4.30 ± 0.29 mg RE/g) and phenolic content (7.83 ± 0.08 mg RE/g), respectively. Additionally, A. anceps exhibited significant antioxidant properties with all assays and significantly depressed BChE (IC50 = 6.68 ± 0.83 mg/mL) and α-amylase activities (IC50 = 5.34 ± 0.14 mg/mL). Interestingly, the five seaweeds revealed potent inhibitory effects against tyrosinase activity. In conclusion, A. anceps might be considered as a key source of phytoantioxidants and a potential candidate to develop nutritional supplements. Besides, the five tested seaweeds warrant further study and may be exploited as promising natural sources for managing hyperpigmentation.
Keywords: seaweeds, antioxidants, tyrosinase, bioactive metabolites, biological activities
1. Introduction
Seaweeds are the ‘lungs of the sea’ as well as a potential ‘wild pharmacy’. These plant like organisms produce 70–80% oxygen for the atmosphere and possess scads of metabolites with unique structures of medicinal values [1,2]. In addition to their ecological importance, seaweeds have been a source of food for humans since ancient times. Seaweeds, also referred as algae, are commonly consumed as either fresh or dried in Asian, African and European countries. The consumption of seaweeds as food products can be traced back to the fourth century in Japan. Seaweeds are rich in vitamins A, E, C, B1 and B12, carbohydrates and organic iodine. The annual human consumption of algae in a dried form is estimated to be 2,000,000 tons [3]. In addition to their nutritive value, seaweeds are known to possess a wide array of valuable pharmacological properties including use as antibiotics, anticoagulants, antiulcer, antioxidants, antimicrobials, and antifouling [4,5].
Nonetheless, there is still a dearth of scientific data on the pharmacological and chemical profiles of seaweeds. Therefore, the present study was designed to investigate the pharmacological and chemical profiles of five seaweeds originating from different families, namely Halimeda spp. (Family: Halimedaceae), Spyridia hypnoides (Bory de Saint-Vincent) Papenfuss (Family: Spyridiaceae), Valoniopsis pachynema (G. Martens) Børgesen (Family: Valoniaceae), Gracilaria fergusonii J. Agardh (Family: Gracilariaceae) and Amphiroa anceps (Lamarck) Decaisne (Family: Lithophyllaceae) collected in Tamil Nadu, India. Halimeda is a well-known green algae made up of discs containing calcium carbonate [6]. Works of literature reported that Halimeda spp. has potential apoptosis, anti-inflammatory, antioxidant, neuroprotective and hepatoprotective properties [7]. Spyridia hypnoides, belonging to the family Spyridiaceae, is a 15 cm tall plant like organism with numerous branches at short intervals. No reproductive structures were observed in this seaweed [8]. Sudharsan et al. reported that the galactans isolated from S. hypnoides exhibited anticoagulant and antioxidant properties. V. pachynema, also known as AstroTurf algae, originates from the family Valoniaceae. It is a filamentous alga, spongy and tends to cover completely the surface (dead corals, rocks) on which it grows to form a ball-like appearance [9]. A study conducted by Kumar et al. reported high level of calcium (476.67 ± 6.2%) in this seaweed species [10]. Gracilaria, originating from Gracilariaceae family is often a source of food for many people in Malaysia [11]. The aqueous extract of G. fergusonii displayed anti-inflammatory activity at a dosage of 250 µg/ml with a percentage inhibition of 63.98% [12]. On the other hand, A. anceps originating from Lithophyllaceae family, is a red macroalga usually found in sea waters at temperatures of 5 to 15 °C. This seaweed was screened for its antagonistic activity. Data collected showed that the crude extract exhibited clear inhibition zones against several pathogens: Yersinia spp., Streptococcus spp. and Vibrio spp. [13]. However, since the existing literature is insufficient, fragmented and unsystematic, we embark on this present research to try to expand the currently limited literature.
A series of enzymes were chosen based on the current challenging diseases globally such as diabetes mellitus (DM) type II, Alzheimer’s disease and skin disorders. Enzymes play a considerable role in biological reactions, contributing a diversification platform to the pharmaceutical industry. There is a wide spectrum of applicability of enzymes in the pharmaceutical industry starting from nutraceuticals, enzyme therapy, disease diagnosis, to drug synthesis [14]. Herein, enzymatic inhibitions involving α-amylase and α-glucosidase were investigated for DM type II, acetyl- (AChE) and butyryl-cholinesterase (BChE) for Alzheimer’s disease, and tyrosinase for skin disorders. Furthermore, dysfunction of the antioxidant defensive system leads to the development of chronic health conditions related to degenerative pathologies such as cardiovascular diseases, cancer, and neurodegeneration disorders [15]. Thus, to prevent health complications, the body must rely on exogenous antioxidants to effectively suppress reactive oxygen species (ROS). Since seaweeds are widely consumed, it was indeed a matter of great interest for us to investigate their antioxidant properties as well.
This work was undertaken to encompass the following objectives—(1) conduct a quantitative estimation of phytochemicals using in vitro standard chemical assays and identify the compounds using ultra-high-performance liquid chromatography coupled with an electrospray ionization mass spectrometry (UHPLC-ESI-MS/MS) technique, (2) report the antioxidant capacities in terms of radical scavenging, reducing potential, metal chelating and determine the total antioxidant capacity, (3) evaluate the enzymatic inhibitory effects against clinical enzymes associated with chronic diseases, namely diabetes mellitus (α-amylase and α-glucosidase), Alzheimer’s disease (AChE and BChE) and skin hyperpigmentation (tyrosinase) and (4) analyse the collected scientific data using multivariate analysis.
2. Results and Discussion
2.1. Antioxidant Assays
As a normal protective mechanism, the human body naturally responds to oxidative stress (caused by reactive oxygen species (ROS)) using its antioxidant defence. Nevertheless, in some cases, the enzymatic systems fail to resist to ROS, and the level of antioxidants present is insufficient to successfully ascertain healthy cellular homeostasis [16,17]. The antioxidant properties of phytochemicals are hidden behind their ability to donate electrons and/or chelate metals without them being transformed into harmful radicals [18,19]. The search for potential antioxidant activities from marine sources is still not widespread [20]. Thus, to try to fill this niche, we screened the different extracts of each seaweed for their antioxidative properties.
Considering the complexity of phytochemicals, multiple assays targeting different mechanisms of action were selected to assess antioxidant properties. For instance, radical scavenging was assessed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), reducing power by ferric reducing power antioxidant (FRAP) and cupric reducing antioxidant capacity (CUPRAC), total antioxidant capacity by phosphomolybdenum (PHPD) and metal chelating by ferrous ion chelating assay. Results are summarized in Table 1. Overall, the methanolic extract of A. anceps displayed the strongest antioxidant properties with all assays and the least potent was revealed to be methanolic extract of Halimeda spp. Existing work in literature reported that phenolic compounds are one of the most effective antioxidants [21]. Indeed, this fact was consistent with our study since A. anceps possessed the highest amount of phenolic content explaining its high antioxidant capacities. Likewise, a study suggested that A. anceps could be an effective source of antioxidants considering its high level of algal phenolic compounds [22].
Table 1.
Antioxidant properties of the tested seaweed extracts. *
| Assays | Halimeda spp. | Spyridia hypnoides | Valoniopsis pachynema | Gracilaria fergusonii | Amphiroa anceps |
|---|---|---|---|---|---|
| DPPH (mg TE/g) | 2.33 ± 0.04e | 2.55 ± 0.05d | 2.68 ± 0.03c | 3.83 ± 0.09b | 4.43 ± 0.07a |
| ABTS (mg TE/g) | 5.36 ± 0.47e | 8.91 ± 0.30d | 11.27 ± 1.07c | 13.26 ± 0.45b | 18.56 ± 0.10a |
| CUPRAC (mg TE/g) | 9.47 ± 0.17c | 10.95 ± 0.20c | 25.71 ± 0.73b | 7.31 ± 0.07d | 46.47 ± 1.60a |
| FRAP (mg TE/g) | 4.91 ± 0.05d | 6.27 ± 0.10c | 9.03 ± 0.10b | 5.24 ± 0.14d | 13.99 ± 0.30a |
| PHPD (mmol TE/g) | 0.21 ± 0.01c | 0.37 ± 0.02b | 0.30 ± 0.04bc | 0.23 ± 0.01c | 0.73 ± 0.06a |
| Chelating ability (mg EDTAE/g) |
1.62 ± 0.15d | 6.07 ± 0.56c | 9.05 ± 0.76b | NA | 16.99 ± 0.11a |
* Values are expressed as mean ± S.D. EDTAE, EDTA equivalent; PHPD, phosphomolybdenum assay; NA, not active. Different letters indicate significant differences in the extracts (p < 0.05).
Metals are entwined in our body in a very complex way. They help in the normal functioning of the body; however, an imbalance in the level of metals can lead to health complications. For instance, an excess of iron reduces hepatic extraction and metabolism of insulin leads to peripheral hyperinsulinemia causing an increase in oxidative stress which affects the normal function of insulin, hence resulting in an increase in blood glucose level [23]. Thus, iron chelation could be an effective therapeutic approach. As shown in Table 1, the ferrous ion chelating ability decreased in the following order: A. anceps > V. pachynema > S. hypnoides > Halimeda spp. > G. fergusonii, with the latter showing no activity. Radical scavenging activity estimations of the five seaweeds were evaluated using DPPH and ABTS radicals due to their simplicity, sensitivity, speed, stability of the radicals and cheap instrumentation [24]. Results from the assays showed that the methanolic extract of A. anceps was the best DPPH and ABTS scavenger (4.43 ± 0.07 and 18.56 ± 0.10 mg TE/g, respectively) while the extract of Halimeda spp. displayed the least scavenging effect with both DPPH and ABTS radicals (2.33 ± 0.04 and 5.36 ± 0.47 mg TE/g, respectively).
Furthermore, data gathered in this study showed that the crude methanolic extract of A. anceps possessed the most potent reducing power towards both Fe (III) and Cu (II) with Trolox equivalent values of 13.99 ± 0.30 and 46.47 ± 1.60 mg TE/g, respectively. However, G. fergusonii extract displayed weaker reducing potential against Cu (II) with 7.31 ± 0.07 mg TE/g, and Halimeda spp. extract was least potent on the reduction of Fe (III) with 4.91 ± 0.05 mg TE/g. The quantitative determination of total antioxidant capacity is based on the reduction of Mo (VI) to Mo (V) by the extract to form a green phosphate/Mo (V) complex under acidic condition [25]. Again, A. anceps extract with values of 0.73 ± 0.06 mmol TE/g demonstrated the optimal antioxidant capacity succeeded by S. hypnoides extract (0.37 ± 0.02 mmol TE/g), V. pachynema extract (0.30 ± 0.04 mmol TE/g), G. fergusonii extract (0.23 ± 0.01 mmol TE/g) and Halimeda spp. extract (0.21 ± 0.01 mmol TE/g) (Table 1).
2.2. Enzymatic Inhibitory Properties
The World Health Statistics 2019, an annual compilation of the World Health Organization (WHO), stated that the world is constantly under health challenges with 40 leading causes of death including ischemic heart disease, Alzheimer’s disease, lung, liver, stomach, oesophagus and prostate cancer, chronic obstructive pulmonary disease, and stroke, among others [26]. The world is unhealthy, and our existing medications are either insufficient or ineffective. Thus, our fight against these chronic pathologies should be ongoing with new strategies on how to manage these diseases. One of the strategies currently considered by many researchers is to search for potent and more effective medications from plants. Currently, the marine ecosystem is a crucial source of medicinally important metabolites with pharmaceutical importance [27]. This present work is considered as second-to-none since we have screened the methanolic crude extracts of five different seaweeds, namely Halimeda spp., S. hypnoides, V. pachynema, G. fergusonii and A. anceps with regard to five key enzymes involved in chronic health complications. Inhibition of enzymes may be considered as a therapeutic approach; for instance, acetyl- (AChE) and butyryl-cholinesterase (BChE) inhibition: Alzheimer’s disease, α-amylase and α-glucosidase inhibition: diabetes mellitus, and tyrosinase inhibition: skin disorders.
Results are summarized in Table 2. In terms of enzymatic properties, A. anceps extract significantly depressed BChE activity (IC50 = 6.68 ± 0.83 mg/mL) but weakly inhibited AChE activity (IC50 = 3.90 ± 0.83 mg/mL). Instead, the seaweed Halimeda spp. crude extract exhibited higher BChE inhibitory effect with IC50 value of 3.07 ± 0.10 mg/mL. AChE catalyses the hydrolysis of acetylcholine (ACh) into acetic acid and choline while BChE is responsible for ACh homeostasis [28]. However, an accumulation of ACh in synapses may result in muscarinic and nicotinic toxicity which subsequently cause muscle cramps, blurry vision, lacrimation, muscular weakness, and paralysis [29]. From this perspective, it is important to maintain a healthy ACh homeostasis in the body. Interestingly, it is noteworthy to highlight that BChE activity increases with the severity of dementia, thus from this background information it can be stated that searching for potent BChE inhibitors is of utmost importance, as demonstrated by the extracts of Halimeda spp. and A. anceps [30].
Table 2.
Enzyme inhibitory effects (IC50 = mg/mL) of the tested seaweed extracts. *
| Assays | AChE | BChE | Tyrosinase | Alpha-Amylase | Alpha-Glucosidase |
|---|---|---|---|---|---|
| Halimeda spp. | 3.07 ± 0.10a | 7.82 ± 0.67a | 3.70 ± 0.06b | 8.19 ± 0.23a | 3.20 ± 0.31b |
| Spyridia hypnoides | 3.18 ± 0.05a | 7.96 ± 1.01a | 3.73 ± 0.04b | 7.31 ± 0.36b | 4.11 ± 0.40a |
| Valoniopsis pachynema | 3.25 ± 0.06a | 8.75 ± 1.31a | 3.68 ± 0.03b | 7.02 ± 0.28b | 2.57 ± 0.02c |
| Gracilaria fergusonii | 3.27 ± 0.10a | 7.43 ± 1.00a | 3.70 ± 0.05b | 8.27 ± 0.17a | 4.90 ± 0.33a |
| Amphiroa anceps | 3.90 ± 0.83a | 6.68 ± 0.83a | 4.49 ± 0.15a | 5.34 ± 0.14c | 5.64 ± 1.19a |
| Galantamine | 0.003 ± 0.0001b | 0.004 ± 0.0001b | NT | NT | NT |
| Kojic acid | NT | NT | 0.09 ± 0.01c | NT | NT |
| Acarbose | NT | NT | NT | 0.50 ± 0.01d | 0.75 ± 0.02d |
* Values are expressed as mean ± S.D. NT: not tested. Different letters indicate significant differences in the extracts (p < 0.05).
Diabetes is on the rise; since 1980, the number of patients with diabetes has increased four-fold to 422 million people irrespective of their age and gender [31]. Thus, there is still an urgent need to develop more efficient medications with lesser side effects. Results collected herein reported that the crude extract of A. anceps showed similar inhibitory effects against α-amylase (IC50 = 5.34 ± 0.14 mg/mL) and α-glucosidase (IC50 = 5.64 ± 1.19 mg/mL). On the other hand, V. pachynema extract demonstrated a higher inhibition against α-glucosidase (IC50 = 2.57 ± 0.02 mg/mL) in contrast to α-amylase (IC50 = 7.02 ± 0.28 mg/mL). Interestingly, pronounced inhibition of α-glucosidase is usually preferred over moderate α-amylase inhibition to develop antihyperglycemic agents with lesser gastrointestinal discomfort due to undigested carbohydrates [32].
The inhibitory effects of Halimeda spp., S. hypnoides, V. pachynema, G. fergusonii and A. anceps extracts on tyrosinase enzyme were also evaluated. Tyrosinase is a copper-containing enzyme responsible for the process of converting l-tyrosine and 3,4-dihydroxyphenylalanine (l-DOPA) to melanin through the formation of dopaquinone. It is reported that excessive production of dopaquinone in the brain causes neurodegeneration and cell mortality. These disorders are the primary cause and the hallmark of Parkinson’s and Huntington’s disease [33]. Inhibition of tyrosinase may be considered as a therapeutic approach for managing skin disorders and more complicated diseases. Our present study showed that the crude methanolic extracts of the five seaweeds displayed relatively the same tyrosinase activity. For instance, the extract of V. pachynema exhibited IC50 value of 3.68 ± 0.03 mg/mL, Halimeda spp.: 3.70 ± 0.06 mg/mL, S. hypnoides: 3.73 ± 0.04 mg/mL, G. fergusonii 3.7. ± 0.05 mg/mL and A. anceps: 4.49 ± 0.15 mg/mL.
2.3. Bioactive Composition
The ongoing quest to discover novel natural bioactive compounds remains of utmost importance for a world which is currently facing multiple health challenges. It is highly acknowledged that plants, culinary herbs or even spices are incorporated with a myriad of phytochemicals possessing medicinal values. Phytoconstituents are known to minimize the risk of chronic and inflammatory conditions [34]. Interestingly, a recent review compiled by Gnanavel et al. reported that more than 15,000 bioactive metabolites have been identified from marine sources and seaweeds are among them [35]. Among the different classes of phytochemicals, polyphenols or phenolic compounds and flavonoids are the two most widely studied classes of bioactive compounds [36]. Thus, in our present study, we screened the investigated seaweed extracts Halimeda spp., S. hypnoides, V. pachynema, G. fergusonii and A. anceps for phenolic and flavonoid contents.
Among the tested seaweeds, A. anceps possessed the highest phenolic content (7.83 ± 0.08 mg GAE/g) while Halimeda spp. yielded the lowest amount (2.24 ± 0.06 mg GAE/g). In terms of flavonoid content, V. pachynema yielded the highest amount, succeeded by A. anceps, S. hypnoides, Halimeda spp. and G. fergusonii (4.30 ± 0.29, 2.47 ± 0.22, 2.45 ± 0.16, 1.74 ± 0.08 and 0.42 ± 0.04 mg RE/g, in order of magnitude).
To have an overview of the chemical diversity possessed by each seaweed, an extensive profiling technique, ultra-high-performance liquid chromatography coupled with electrospray ionization mass spectrometry (UHPLC-ESI-MS/MS), was used. The results are summarized in Table 3. Results showed that A. anceps possessed a greater diversity of compounds (23) in contrast to V. pachynema (19), G. fergusonii (11), S. hypnoides (10) and Halimeda spp. (9). However, three compounds remain unidentified in A. anceps and two in Halimeda spp.
Table 3.
Chemical composition of five seaweeds.
| No. | Name | Formula | Rt | [M + H]+ | [M − H]− | Fragment 1 | Fragment 2 | Fragment 3 | Fragment 4 | Fragment 5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Halimeda spp. | ||||||||||
| 1 | Acetylcholine | C7H15NO2 | 1.20 | 146.11810 | 87.0446 | 60.0816 | ||||
| 2 | Unidentified hydroxycarboxylic acid | C10H18O4 | 13.50 | 201.11268 | 183.1017 | 57.1221 | 139.1116 | |||
| 3 | Hydroxyethylimino-phenylpropanol derivative | C11H15NO2 | 17.08 | 194.11810 | 176.1072 | 152.1071 | 134.0967 | 117.0702 | 91.0547 | |
| 4 | 3-Methyladipic acid | C7H12O4 | 17.32 | 159.06574 | 141.0544 | 115.0750 | 97.0642 | |||
| 5 | Loliolide or isololiolide | C11H16O3 | 18.19 | 197.11777 | 179.1068 | 161.0962 | 135.1171 | 133.1014 | 107.0859 | |
| 6 | Loliolide or isololiolide | C11H16O3 | 19.47 | 197.11777 | 179.1069 | 161.0963 | 135.1170 | 133.1014 | 107.0860 | |
| 7 | Bromocarboxylic acid | C7H5BrO3 | 19.73 | 214.93438 | 170.9440 | 78.9173 | ||||
| 8 | Caulerpin | C24H18N2O4 | 34.52 | 399.13449 | 385.1174 | 367.1080 | 340.1209 | 308.0944 | 280.0995 | |
| 9 | Unidentified alkaloid | C24H18N2O4 | 37.16 | 399.13449 | 363.1574 | |||||
| Spyridia hypnoides | ||||||||||
| 1 | Pantothenic acid | C9H17NO5 | 5.16 | 220.11850 | 202.1076 | 184.0971 | 174.1123 | 116.0346 | 90.0555 | |
| 2 | 3-(4-Hydroxyphenyl) lactic acid | C9H10O4 | 8.93 | 181.05009 | 163.0392 | 135.0441 | 119.0491 | 72.9917 | ||
| 3 | Kynurenic acid isomer | C10H7NO3 | 14.34 | 190.05042 | 162.0550 | 144.0445 | 116.0498 | |||
| 4 | 3-Phenyllactic acid | C9H10O3 | 16.84 | 165.05517 | 147.0438 | 119.0489 | 72.9915 | |||
| 5 | 3-Methyladipic acid | C7H12O4 | 17.33 | 159.06574 | 141.0546 | 115.0750 | 97.0645 | |||
| 6 | Loliolide or isololiolide | C11H16O3 | 18.18 | 197.11777 | 179.1069 | 161.0962 | 135.1171 | 133.1014 | 107.0859 | |
| 7 | Riboflavin | C17H20N4O6 | 18.60 | 377.14611 | 359.1344 | 243.0879 | 200.0819 | 172.0869 | 99.0444 | |
| 8 | Loliolide or isololiolide | C11H16O3 | 19.46 | 197.11777 | 179.1069 | 161.0962 | 135.1171 | 133.1014 | 107.0860 | |
| 9 | N-(2-Phenylethyl) acetamide | C10H13NO | 20.00 | 164.10754 | 122.0967 | 105.0703 | 90.9482 | 79.0548 | ||
| 10 | Lumichrome | C12H10N4O2 | 23.88 | 243.08821 | 216.0768 | 200.0825 | 198.0665 | 172.0871 | ||
| Valoniopsis pachynema | ||||||||||
| 1 | Betaine | C5H11NO2 | 1.22 | 118.08681 | 59.0737 | 58.0659 | ||||
| 2 | Ectoine | C6H10N2O2 | 1.22 | 143.08206 | 101.0715 | 97.0766 | 73.0768 | 68.0502 | 56.0502 | |
| 3 | Acetylcholine | C7H15NO2 | 1.23 | 146.11810 | 87.0446 | 60.0816 | ||||
| 4 | Pantothenic acid | C9H17NO5 | 5.17 | 220.11850 | 202.1078 | 184.0972 | 174.1123 | 116.0347 | 90.0555 | |
| 5 | 4-Hydroxybenzoic acid | C7H6O3 | 8.99 | 137.02387 | 93.0330 | 65.0382 | ||||
| 6 | Kynurenic acid | C10H7NO3 | 13.02 | 190.05042 | 162.0551 | 144.0445 | 116.0495 | 89.0386 | ||
| 7 | Kynurenic acid isomer | C10H7NO3 | 14.33 | 190.05042 | 162.0549 | 144.0446 | 116.0496 | |||
| 8 | Methyladipic acid isomer | C7H12O4 | 15.04 | 159.06574 | 141.0544 | 115.0750 | 97.0644 | |||
| 9 | 3-Phenyllactic acid | C9H10O3 | 16.82 | 165.05517 | 147.0440 | 119.0487 | 72.9914 | |||
| 10 | Loliolide or isololiolide | C11H16O3 | 18.19 | 197.11777 | 179.1070 | 161.0963 | 135.1171 | 133.1015 | 107.0860 | |
| 11 | Azelaamic acid (9-Amino-9-oxononanoic acid) | C9H17NO3 | 18.64 | 186.11302 | 125.0958 | 123.0803 | 97.0645 | |||
| 12 | Loliolide or isololiolide | C11H16O3 | 19.46 | 197.11777 | 179.1069 | 161.0962 | 135.1171 | 133.1015 | 107.0860 | |
| 13 | Chicoric acid (2,3-Di-O-caffeoyltartaric acid) | C22H18O12 | 19.53 | 473.07201 | 311.0414 | 293.0303 | 219.0298 | 179.0340 | 149.0080 | |
| 14 | N-(2-Phenylethyl) acetamide | C10H13NO | 19.99 | 164.10754 | 122.0967 | 105.0704 | 90.9483 | 79.0549 | ||
| 15 | Hydroxycapric acid | C10H20O3 | 33.27 | 187.13342 | 141.1270 | 59.0123 | ||||
| 16 | Caulerpin | C24H18N2O4 | 34.51 | 399.13449 | 385.1177 | 367.1078 | 340.1208 | 308.0943 | 280.0998 | |
| 17 | Hydroxyundecanoic acid isomer 1 | C11H22O3 | 35.38 | 201.14907 | 59.0123 | |||||
| 18 | Hydroxyundecanoic acid isomer 2 | C11H22O3 | 35.87 | 201.14907 | 59.0123 | |||||
| 19 | Hydroxydodecanoic acid | C12H24O3 | 38.04 | 215.16472 | 169.1581 | 59.0123 | ||||
| Gracilaria fergusonii | ||||||||||
| 1 | Gigartinine | C7H15N5O3 | 1.20 | 218.12532 | 133.0973 | 116.0709 | 115.0869 | 86.0354 | 70.0657 | |
| 2 | Phenethylamine | C8H11N | 3.67 | 122.09698 | 105.0703 | 103.0548 | 79.0548 | |||
| 3 | Methyladipic acid isomer | C7H12O4 | 15.03 | 159.06574 | 141.0547 | 115.0750 | 97.0645 | |||
| 4 | 3-Phenyllactic acid | C9H10O3 | 16.82 | 165.05517 | 147.0440 | 119.0488 | 72.9915 | |||
| 5 | Loliolide or isololiolide | C11H16O3 | 18.17 | 197.11777 | 179.1069 | 161.0961 | 135.1171 | 133.1014 | 107.0859 | |
| 6 | Loliolide or isololiolide | C11H16O3 | 19.46 | 197.11777 | 179.1069 | 161.0962 | 135.1170 | 133.1014 | 107.0860 | |
| 7 | Chicoric acid (2,3-Di-O-caffeoyltartaric acid) | C22H18O12 | 19.53 | 473.07201 | 311.0414 | 293.0303 | 219.0298 | 179.0340 | 149.0080 | |
| 8 | N-(2-Phenylethyl) acetamide | C10H13NO | 19.98 | 164.10754 | 122.0967 | 105.0703 | 90.9482 | 79.0549 | ||
| 9 | Lumichrome | C12H10N4O2 | 23.88 | 243.08821 | 216.0772 | 200.0819 | 198.0671 | 172.0869 | ||
| 10 | Dihydrololiolide or dihydroisololiolide | C11H18O3 | 30.06 | 199.13342 | 181.1225 | 163.1118 | 153.1275 | 135.1170 | 107.0859 | |
| 11 | Hydroxydodecanoic acid | C12H24O3 | 38.04 | 215.1647 | 169.1595 | 59.0123 | ||||
| Amphiroa anceps | ||||||||||
| 1 | 3-(4-Hydroxyphenyl) lactic acid | C9H10O4 | 9.01 | 181.05009 | 163.0386 | 135.0441 | 119.0487 | 72.9916 | ||
| 2 | Methyladipic acid isomer | C7H12O4 | 15.07 | 159.06574 | 141.0546 | 115.0750 | 97.0644 | |||
| 3 | N-Acetylisoleucine | C8H15NO3 | 15.92 | 172.09737 | 130.0860 | 128.1068 | ||||
| 4 | N-Acetylleucine | C8H15NO3 | 16.75 | 172.09737 | 130.0860 | 128.1068 | ||||
| 5 | 3-Phenyllactic acid | C9H10O3 | 16.85 | 165.05517 | 147.0439 | 119.0489 | 72.9915 | |||
| 6 | Indoleacetic acid | C10H9NO2 | 17.26 | 174.05551 | 130.0649 | 128.0491 | ||||
| 7 | 3-Methyladipic acid | C7H12O4 | 17.36 | 159.06574 | 141.0547 | 115.0750 | 97.0644 | |||
| 8 | 4-Coumaric acid | C9H8O3 | 17.72 | 163.03952 | 119.0488 | 93.0331 | ||||
| 9 | Loliolide or isololiolide | C11H16O3 | 18.20 | 197.11777 | 179.1068 | 161.0961 | 135.1170 | 133.1015 | 107.0859 | |
| 10 | Riboflavin | C17H20N4O6 | 18.58 | 377.14611 | 359.1341 | 243.0876 | 200.0822 | 172.0866 | 99.0445 | |
| 11 | Indole carboxaldehyde | C9H7NO | 18.91 | 146.06059 | 118.0654 | 117.0577 | 91.0547 | |||
| 12 | Loliolide or isololiolide | C11H16O3 | 19.47 | 197.11777 | 179.1068 | 161.0961 | 135.1170 | 133.1014 | 107.0859 | |
| 13 | Chicoric acid (2,3-Di-O-caffeoyltartaric acid) | C22H18O12 | 19.53 | 473.07201 | 311.0414 | 293.0303 | 219.0298 | 179.0340 | 149.0080 | |
| 14 | N-(2-Phenylethyl) acetamide | C10H13NO | 20.00 | 164.10754 | 122.0967 | 105.0703 | 90.9482 | 79.0549 | ||
| 15 | Caffeoyl phenylethanoid glycoside isomer 1 | C29H36O15 | 21.97 | 623.19760 | 161.0232 | 133.0280 | ||||
| 16 | Caffeoyl phenylethanoid glycoside isomer 2 | C29H36O15 | 23.22 | 623.19760 | 161.0232 | 133.0282 | ||||
| 17 | Lumichrome | C12H10N4O2 | 23.85 | 243.08821 | 216.0768 | 200.0823 | 198.0660 | 172.0870 | ||
| 18 | Unidentified terpene 1 | C11H16O2 | 26.59 | 181.12285 | 163.1119 | 145.1014 | 135.1171 | 121.1014 | 107.0859 | |
| 19 | Unidentified terpene 2 | C11H18O3 | 30.06 | 199.13340 | 181.1224 | 163.1117 | 145.1013 | 135.1171 | 111.0443 | |
| 20 | Unidentified terpene 3 | C20H30O4 | 32.47 | 335.22223 | 317.2114 | 299.2006 | 281.1903 | 273.1854 | 255.1740 | |
| 21 1 | Eicosapentaenoic acid | C20H30O2 | 44.57 | 301.21676 | 257.2273 | 203.1801 | 135.1166 | |||
| 22 | Pheophytin A | C55H74N4O5 | 62.78 | 871.57375 | 593.2763 | 533.2549 | 460.2259 | |||
| 23 | Pheophytin A isomer | C55H74N4O5 | 64.99 | 871.57375 | 593.2764 | 533.2552 | 459.2172 | |||
1 Confirmed by standard.
In the nontargeted analysis, we tried to identify all components present in the samples using UHPLC-ESI-MS/MS. Using HPLC-single stage Orbitrap MS, a broad spectrum of chemical classes could be separated and detected within 70 minutes. High resolution (35000) and high mass accuracy (<5 ppm) enabled identification of most compounds. Structural identification and characterization were carried out on the comparisons of their chromatographic and ESI-MS/MS data (retention time, exact mass and fragmentation pathway) with the corresponding standards and data reported in the previous literature. Loliolide, phenolic acids, C9-C20 carboxylic acids, amino acids and their derivatives, terpenes were identified in the extracts, but their number was quite different.
2.3.1. Halimeda spp.
Three carboxylic acids were detected in negative ion mode. Compounds with a retention time of 13.50 had [M − H]− ion at m/z 201.11268 (C10H18O4) suggesting one unsaturated bond in the aliphatic chain and they yielded a characteristic fragment ion at m/z 183.1017 corresponding to neutral loss of one water molecule (Table 3). 3-Methyladipic acid with a retention time of 17.32 is a well-known and characterized compound. Carboxylic acid with a retention time of 19.73 exhibited a molecular ion [M − H]− at m/z 214.93438 (Figure S1 in the Supplementary Information). The isotopic pattern indicated the presence of one bromo atom in the compound and a fragment ion [M – H − 44]− at m/z 170.9440 corresponding to loss of CO2. Two monoterpenoid lactones: loliolide and isololiolide were detected in positive ion mode [M + H]+ at m/z 197.11777. Their structures were identified by comparison of their mass spectra with previously reported values. Ions at m/z 179.1069 and m/z 111.92 confirmed the loss of H2O [M + H − H2O]+, and also the loss of the ring adjacent to the lactone [M + H − H2O − C5H8]+. In addition to caulerpin, we have detected another compound at m/z 399.13449, but the fragmentation of this unidentified alkaloid was different from caulerpin.
2.3.2. Spyridia hypnoides
In this species, we have identified several well-known and characterized compounds, for example, pantothenic acid, 3-phenyllactic acid, riboflavin or lumichrome, loliolide and isololiolide were also present in this species (Table 3).
2.3.3. Valoniopsis pachynema
We also detected loliolide and isololiolide in this species. In addition to some known compounds, we detected two carboxylic acids with retention times 35.38 and 35.87 (Table 3). These compounds had the same [M − H]− molecular ions at m/z 201.14907 (C11H22O3) (Figures S2 and S3 in the Supplementary Information). In the MS2 spectrums we did not find water losses suggesting the presence of omega hydroxyl group or ether bond. We tentatively identified these two compounds as ω-hydroxyundecanoic acid isomers. In the case of compound 19 in Table 3 the situation was the same. We did not find water loss and the main fragment was m/z 59.0123 (CH3-COO−), so we tentatively identified this carboxylic acid as a ω-hydroxydodecanoic acid isomer.
2.3.4. Gracilaria fergusonii
In this species, we have identified several compounds that we have already found in the previous algae. Besides these compounds, we have identified a terpene with molecular ion at m/z 199.13342 in the positive ionisation mode (Table 3). The fragmentation of this molecule was very similar to loliolide and isololiolide, but the compound and most of its fragments had two hydrogen atoms more related to loliolide. Based on the exact molecular mass and the similarity of the fragmentation to loliolide, we tentatively identified this compound as dihydrololiolide or dihydroisololiolide.
2.3.5. Amphiroa anceps
In addition to some known and characterized compounds, we have detected three terpenes with molecular ions m/z 181.12285 (C11H16O2), 199.13340 (C11H18O3) and 335.22223 (C20H30O4), respectively, in the positive ionisation mode (Figure S4 in the Supplementary Information). Compound 18 yielded characteristic fragment ions at m/z 163.1119 and 145.1014 corresponding to neutral loss of two water molecules. Compounds 19 and 20 yielded characteristic fragment ions at m/z 181.1224, 163.1117, 145.1013 and 317.2114, 299.2008, 281.1904, respectively, matching a neutral loss of three water molecules (Table 3).
2.4. Multivariate Analysis
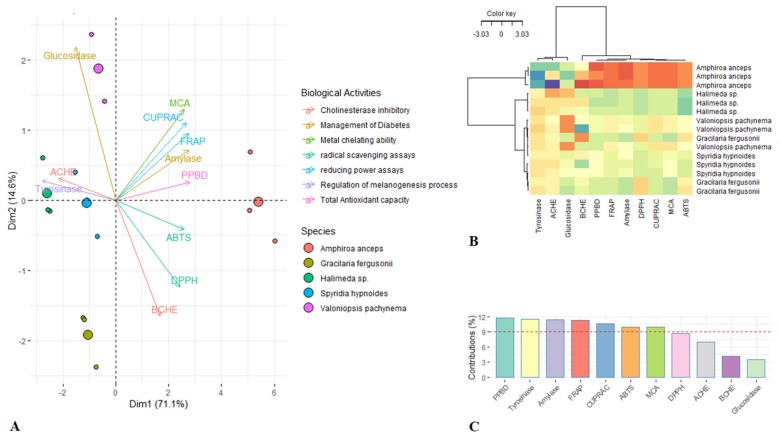
The unsupervised multivariate analysis, principal component analysis (PCA) and Hierarchical cluster analysis (HCA) were used to comprehensively screen trends or resemblance between samples, i.e., whether they clustered according to the evaluated biological activities and to identify the key biological activities that contribute to the explanation of most the variance in the dataset. PCA aims to reduce the dimensionality of the data through summarizing as much information as possible. As shown in the PCA score plot, A. anceps was effectively discriminated from the other species (V. pachynema, G. fergusonii, Halimena spp., and S. hypnoides) (Figure 1A). In more detail, this segregation was done along the first component that was defined as the linear combination of seven biological activities (PPBD, tyrosinase, ABTS, CUPRAC, FRAP, metal chelating ability (MCA), α-amylase) (Figure 1C). Alongside this, the clustered image map (CIM) displayed a good classification of the samples into two distinct groups (Figure 1B). CIM was based on the hierarchical clustering simultaneously operating on the use of “Euclidean” distance and “Ward” linkage method. The HCA result was consistent with the principal component analysis, indicating the seven biological activities mentioned above effectively characterize the differences between A. anceps and the other species. Additionally, biological activities recorded for A. anceps were most potent among all the studied seaweeds, suggesting A. anceps as the most bioactive species.
Figure 1.
Multivariate analysis outcomes. (A) Score plot of multilevel the principle component analysis (PCA) model on the first two principal components. (B) Clustered image map based on the use of “Euclidean” distance and “Ward” linkage method. (C) Biological activities discriminating the species as gained by the evaluation of the relation between the 11 studied biological activities and the first component of the PCA.
3. Materials and Methods
3.1. Materials and Extraction
The five seaweeds, namely Halimeda spp., Spyridia hypnoides (Bory de Saint-Vincent) Papenfuss, Valoniopsis pachynema (G. Martens) Børgesen, Gracilaria fergusonii J. Agardh and Amphiroa anceps (Lamarck) Decaisne were collected from Mandapam coast, Gulf of Mannar, Tamil Nadu, India during March 2018. The collected seaweeds were identified by Dr. R. Arumugam, Department of Botany, A. V. C. College, Mannampanthal, Tamil Nadu, India. The collected sample was brought to the laboratory and washed thoroughly with tap water to remove all the extraneous materials and shade dried. The extracts were prepared as described in a previous study [37].
3.2. Determination of Antioxidant and Enzyme Inhibitory Effects
The radical scavenging (1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS)), reducing power (cupric ion reducing activity (CUPRAC), ferric reducing antioxidant power (FRAP)), total antioxidant capacity (phosphomolybdenum (PHPD)) and metal chelating power using ferrous ions were conducted to evaluate antioxidant properties. Acetylcholinesterase (AChE), butyrylcholinesterase (BChE), α-amylase, α-glucosidase and tyrosinase were used to evaluate enzymatic inhibitory properties.
Antioxidant abilities were evaluated as standard equivalents (trolox (TE, for ABTS, DPPH, FRAP, CUPRA and PHPD) and EDTA (EDTAE, for metal chelating)). Enzyme inhibitory assays results were expressed as IC50 values. Galantamine (GALAE, for cholinesterase), kojic acid (KAE, for tyrosinase), acarbose (ACAE, for amylase and glucosidase) were used as standard inhibitors in the enzyme assays. The detailed experimental procedures were given in the Supplementary Information as mentioned by our previous papers [38,39]. The detailed experimental procedures were given in the Supplementary File.
3.3. Profiling of Bioactive Metabolites Using Ultra Performance High-Pressure Liquid Chromatography (UHPLC)
Dionex Ultimate 3000RS HPLC instrument was used to analyse the phytochemical composition of the extracts. Before RP-HPLC analysis, the extracts were filtered through 0.22 μm PTFE filter membrane (Labex Ltd., Hungary). The filtered samples were injected onto a Thermo Accucore C18 (100 mm × 2, i.d., 2.6 μm) column thermostated at 25 °C (±1 °C). The solvents used were water (A) and methanol (B). Both were acidified with 0.1% formic acid. The flow rate was maintained at 0.2 mL min−1. The elution gradient was isocratic 5% B (0–3 min), a linear gradient increasing from 5% B to 100% (3–43 min), 100% B (43–61 min), a linear gradient decreasing from 100% B to 5% (61–62 min) and 5% B (62–70 min). The column was coupled with a Thermo Q Exactive Orbitrap mass spectrometer (Thermo Scientific, USA) equipped with electrospray ionization source. Spectra were recorded in positive- and negative-ion mode, respectively, between m/z 100 and 1500.
The TraceFinder 3.1 (Thermo Scientific, USA) software was used for nontargeted screening. Most of the compounds were identified based on literature data and/or our previously published works. All cases, retention time, exact molecular mass, isotopic pattern, essential fragments with a given (5 ppm) mass tolerance were used for the identification of the compounds. Peaks that were detected in blank runs and those were also detected in the samples were rejected. Compounds which were confirmed by standards are marked in Table 2.
3.4. Statistical Analysis
All the data were given as mean ± SD and the statistical procedures were performed using R software v. 3.5.1. One-way ANOVA followed by Tukey’s multiple range was conducted to measure differences (p < 0.05) between the tested samples. Principal component analysis (PCA) and hierarchical cluster analysis (HCA) were performed to evaluate the differences of the tested seaweeds in terms of biological activities.
4. Conclusions
The present study highlights for the first time the phytochemical profile, antioxidant capacities and enzyme inhibitory properties of five seaweeds. The seaweeds showed low to moderate antioxidant and enzymatic activities, with A. anceps showing the highest antioxidant properties, attributed to its high level of phenolics compounds. Hence, A. anceps could be considered as an effective source of natural antioxidants which deserves further consideration. This observation was further supported via multivariate analysis. Halimeda spp. was the least potent seaweed in terms of antioxidant and enzymatic properties but with potent anti-tyrosinase activity which needs further attention. The presence of loliolide compound in all of the five seaweeds warrants further investigations particularly in terms of cytotoxicity analysis and bioavailability. This study has established baseline data on these seaweeds which could be further explored for potential sustainable development of novel bioproducts.
Acknowledgments
This paper was supported by KU Brain Pool Program of the Konkuk University, Seoul, South Korea.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/18/4/198/s1, Figure S1: Extracted Ion Chromatogram (XIC) of compound 7 in Halimeda spp. at m/z 214.93438 (A) MS2 spectrum of compound 7 in Halimeda spp (B). Compound details available in Table 2. Figure S2: Extracted Ion Chromatogram (XIC) of compounds 10 and 12 in Valoniopsis pachynema at m/z 197.11777 (A). MS2 spectrum of compound 12 in Valoniopsis pachynema (B). Compounds details available in Table 2. Figure S3: Extracted Ion Chromatogram (XIC) of compounds 17 and 18 in Valoniopsis pachynema at m/z 201.14907 (A). MS2 spectrum of compound 18 in Valoniopsis pachynema (B). Compounds details available in Table 2. Figure S4: Extracted Ion Chromatogram (XIC) of compounds 20 in Amphiroa anceps at m/z 335.22223 (A). MS2 spectrum of compound 20 in Amphiroa anceps (B). Compounds details available in Table 2. The detailed experimental procedures for antioxidant and enzyme inhibitory bioassays were given in supplementary file.
Author Contributions
Conceptualization, M.F.M., N.B.S. and K.R.R.R.; data curation, K.R.R.R.; formal analysis, M.F.M., G.Z., Z.C., J.J. and K.R.R.R.; investigation, D.H.K.; methodology, G.Z., Z.C., J.J., A.D. and K.I.S.; project administration, K.R.R.R.; software, Z.C., J.J., A.D. and K.I.S.; validation, M.F.M., N.B.S., G.Z., Z.C., J.J., A.D., K.I.S. and K.R.R.R.; writing—original draft, M.F.M., N.B.S., A.D. and K.R.R.R.; writing—review and editing, M.F.M., N.B.S., G.Z., Z.C., J.J., A.D., K.I.S., K.P., D.H.K. and K.R.R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Davis G.D.J., Vasanthi A.H.R. Seaweed metabolite database (SWMD): A database of natural compounds from marine algae. Bioinformation. 2011;5:361. doi: 10.6026/97320630005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perun B. Blane’s Perun: The Sea. [(accessed on 11 September 2019)];2019 Available online: https://www.thesea.org/does-seaweed-produce-oxygen/
- 3.Fleurence J. Chapter 5—Seaweeds as Food. In: Fleurence J., Levine I., editors. Seaweed in Health and Disease Prevention. Academic Press; San Diego, CA, USA: 2016. pp. 149–167. [Google Scholar]
- 4.De Almeida C.L.F., Falcão H.d.S., Lima G.R.d.M., Montenegro C.d.A., Lira N.S., de Athayde-Filho P.F., Rodrigues L.C., de Souza M.d.F.V., Barbosa-Filho J.M., Batista L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011;12:4550–4573. doi: 10.3390/ijms12074550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shelar P., Kumar V., Gauri S., Harkulkar G., Kavitha M., Kumar G., Vidya G., Reddy S. Medicinal value of seaweeds and its applications—A review. Cont. J. Pharmacol. Toxicol. Res. 2012;5:1–22. [Google Scholar]
- 6.Adey W.H., Loveland K. Dynamic Aquaria: Building Living Ecosystems. Elsevier; London, UK: 2011. [Google Scholar]
- 7.Silva A., Novoa A., Gutierrez D., Filho J. Seaweeds from Halimeda Genus as Sources of Natural Antioxidants. J. Anal. Pharm. Res. 2017:5. doi: 10.15406/japlr.2017.05.00158. [DOI] [Google Scholar]
- 8.Anderson R., Stegenga H., Bolton J. Seaweeds of the South African South Coast. World Wide Web Electronic Publication, University of Cape Town. [(accessed on 20 September 2019)];2016 Available online: http://southafrseaweeds.uct.ac.za.
- 9.Ahmed S., Hasan M., Ali M., Azhar I. Antiemetic activity of Iyengaria stellata and Valoniopsis pachynema in chicks. Int. J. Phycol. Phycochem. 2012;8:127–132. [Google Scholar]
- 10.N Kumar R., Patel K., Viyol S., Bhoi R. Nutrient Composition and Calorific Value of Some Seaweeds from Bet Dwarka, West Coast of Gujarat, India. Our Nat. 2010:7. doi: 10.3126/on.v7i1.2565. [DOI] [Google Scholar]
- 11.Othman M.N.A., Hassan R., Harith M.N., Sah A.S.R.M. Morphological Characteristics and Habitats of Red Seaweed Gracilaria spp. (Gracilariaceae, Rhodophyta) in Santubong and Asajaya, Sarawak, Malaysia. Trop. Life Sci. Res. 2018;29:87–101. doi: 10.21315/tlsr2018.29.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalini K., Johnson M., Adaikalaraj G., Vidyarani G., Ramakrishnan P. Anti-Inflammatory Activity of Aqueous Extracts of Gracilaria. Int. J. Curr. Pharm. Res. 2017:9. doi: 10.22159/ijcpr.2017v9i5.22130. [DOI] [Google Scholar]
- 13.Lubobi S., Matunda C., Kumar V., Omboki B. Isolation of Bioactive Secondary Metabolites from Seaweeds Amphiroa anceps against Chicken Meat Associated Pathogens. J. Antimicrob. 2016;2:2. [Google Scholar]
- 14.Vimal A., Kumar A. Chapter 35—Transforming the Healthcare System Through Therapeutic Enzymes. In: Kuddus M., editor. Enzymes in Food Biotechnology. Academic Press; London, UK: 2019. pp. 603–625. [Google Scholar]
- 15.San Miguel-Chávez R. Phenolic antioxidant capacity: A review of the state of the art. Phenolic Compd. Biol. Act. 2017 doi: 10.5772/66897. [DOI] [Google Scholar]
- 16.Mozahheb N., Arefian E., Amoozegar M.A. Designing a whole cell bioreporter to show antioxidant activities of agents that work by promotion of the KEAP1-NRF2 signaling pathway. Sci. Rep. 2019;9:3248. doi: 10.1038/s41598-019-39011-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos-Sánchez N.F., Salas-Coronado R., Villanueva-Cañongo C., Hernández-Carlos B. Antioxidants. IntechOpen; London, UK: 2019. Antioxidant Compounds and Their Antioxidant Mechanism. [Google Scholar]
- 18.Ahmad R. Introductory Chapter: Basics of Free Radicals and Antioxidants, Free Radicals, Antioxidants and Diseases, IntechOpen. [(accessed on 8 September 2019)]; doi: 10.5772/intechopen.76689. Available online: https://www.intechopen.com/books/free-radicals-antioxidants-and-diseases/introductory-chapter-basics-of-free-radicals-and-antioxidants. [DOI]
- 19.Muddathir A.M., Yamauchi K., Batubara I., Mohieldin E.A.M., Mitsunaga T. Anti-tyrosinase, total phenolic content and antioxidant activity of selected Sudanese medicinal plants. S. Afr. J. Bot. 2017;109:9–15. doi: 10.1016/j.sajb.2016.12.013. [DOI] [Google Scholar]
- 20.De S., Devasagayam T.P.A., Menon V. Antioxidant properties of a novel marine analogue of dendrodoine. BARC News Lett. 2006;273:511–512. [Google Scholar]
- 21.Nagai T., Yukimoto T. Preparation and functional properties of beverages made from sea algae. Food Chem. 2003;81:327–332. doi: 10.1016/S0308-8146(02)00426-0. [DOI] [Google Scholar]
- 22.Marimuthu Antonisamy J., Sankara Raj E.D. UV–VIS and HPLC studies on Amphiroa anceps (Lamarck) Decaisne. Arab. J. Chem. 2016;9:S907–S913. doi: 10.1016/j.arabjc.2011.09.005. [DOI] [Google Scholar]
- 23.Choi J.S., Koh I.-U., Lee H.J., Kim W.H., Song J. Effects of excess dietary iron and fat on glucose and lipid metabolism. J. Nutr. Biochem. 2013;24:1634–1644. doi: 10.1016/j.jnutbio.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Olszowy M., Dawidowicz A.L. Is it possible to use the DPPH and ABTS methods for reliable estimation of antioxidant power of colored compounds? Chem. Pap. 2018;72:393–400. doi: 10.1007/s11696-017-0288-3. [DOI] [Google Scholar]
- 25.Prieto P., Pineda M., Aguilar M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 26.WHO . World Health Statistics Overview 2019: Monitoring Health for the SDGs, Sustainable Development Goals. World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 27.Jeewon R., Luckhun A.B., Bhoyroo V., Sadeer N.B., Mahomoodally M.F., Rampadarath S., Puchooa D., Sarma V.V., Durairajan S.S.K., Hyde K.D. Pharmaceutical Potential of Marine Fungal Endophytes. In: Jha S., editor. Endophytes and Secondary Metabolites. Springer International Publishing; Cham, Switzerland: 2019. pp. 1–23. [Google Scholar]
- 28.Reale M., Costantini E., Di Nicola M., D’Angelo C., Franchi S., D’Aurora M., Di Bari M., Orlando V., Galizia S., Ruggieri S., et al. Butyrylcholinesterase and Acetylcholinesterase polymorphisms in Multiple Sclerosis patients: Implication in peripheral inflammation. Sci. Rep. 2018;8:1319. doi: 10.1038/s41598-018-19701-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adeyinka A., Kondamudi N. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2019. [(accessed on 9 September 2019)]. Cholinergic Crisis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482433/ [Google Scholar]
- 30.Lane R.M., Potkin S.G., Enz A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int. J. Neuropsychopharmacol. 2006;9:101–124. doi: 10.1017/S1461145705005833. [DOI] [PubMed] [Google Scholar]
- 31.WHO. [(accessed on 9 September 2019)];2019 Available online: https://www.who.int/news-room/feature-stories/detail/treating-diabetes-takes-more-than-insulin-senegal-mobile-phone-project-promoting-public-health.
- 32.Akata I., Zengin G., Picot C.M.N., Mahomoodally M.F. Enzyme inhibitory and antioxidant properties of six mushroom species from the Agaricaceae family. S. Afr. J. Bot. 2019;120:95–99. doi: 10.1016/j.sajb.2018.01.008. [DOI] [Google Scholar]
- 33.Barros M.R., Menezes T.M., da Silva L.P., Pires D.S., Princival J.L., Seabra G., Neves J.L. Furan inhibitory activity against tyrosinase and impact on B16F10 cell toxicity. Int. J. Biol. Macromol. 2019;136:1034–1041. doi: 10.1016/j.ijbiomac.2019.06.120. [DOI] [PubMed] [Google Scholar]
- 34.Cseke L.J., Kirakosyan A., Kaufman P.B., Warber S., Duke J.A., Brielmann H.L. Natural Products from Plants. CRC Press; Boca Raton, FL, USA: 2016. [Google Scholar]
- 35.Gnanavel V., Roopan S.M., Rajeshkumar S. Aquaculture: An overview of chemical ecology of seaweeds (food species) in natural products. Aquaculture. 2019;507:1–6. doi: 10.1016/j.aquaculture.2019.04.004. [DOI] [Google Scholar]
- 36.Watson R.R. Polyphenols in Plants: Isolation, Purification and Extract Preparation. Academic Press; London, UK: 2018. [Google Scholar]
- 37.Rengasamy K.R.R., Sadeer N.B., Zengin G., Mahomoodally M.F., Cziáky Z., Jekő J., Diuzheva A., Abdallah H.H., Kim D.H. Biopharmaceutical potential, chemical profile and in silico study of the seagrass–Syringodium isoetifolium (Asch.) Dandy. S. Afr. J. Bot. 2019;127:167–175. doi: 10.1016/j.sajb.2019.08.043. [DOI] [Google Scholar]
- 38.Zengin G., Aktumsek A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement.Altern. Med. 2014;11:481–488. doi: 10.4314/ajtcam.v11i2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zengin G. A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: New sources of natural inhibitors for public health problems. Ind. Crops Prod. 2016;83:39–43. doi: 10.1016/j.indcrop.2015.12.033. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.