Abstract
Sarcoidosis is a multisystem granulomatous disease with nonspecific clinical manifestations that commonly affects the pulmonary system and other organs including the eyes, skin, liver, spleen, and lymph nodes. Sarcoidosis usually presents with persistent dry cough, eye and skin manifestations, weight loss, fatigue, night sweats, and erythema nodosum. Sarcoidosis is not influenced by sex or age, although it is more common in adults (< 50 years) of African-American or Scandinavians decent. Diagnosis can be difficult because of nonspecific symptoms and can only be verified following histopathological examination. Various factors, including infection, genetic predisposition, and environmental factors, are involved in the pathology of sarcoidosis. Exposures to insecticides, herbicides, bioaerosols, and agricultural employment are also associated with an increased risk for sarcoidosis. Due to its unknown etiology, early diagnosis and detection are difficult; however, the advent of advanced technologies, such as endobronchial ultrasound-guided biopsy, high-resolution computed tomography, magnetic resonance imaging, and 18F-fluorodeoxyglucose positron emission tomography has improved our ability to reliably diagnose this condition and accurately forecast its prognosis. This review discusses the causes and clinical features of sarcoidosis, and the improvements made in its prognosis, therapeutic management, and the recent discovery of potential biomarkers associated with the diagnostic assay used for sarcoidosis confirmation.
Keywords: sarcoidosis, biomarkers, diagnosis, cause, management
1. Introduction
Sarcoidosis is a systemic multisystem inflammatory disorder of unknown etiology characterized by the presence of non-caseating granulomas. The first case of sarcoidosis was reported in 1877 by Jonathan Hutchinson at the King’s College Hospital in London (United Kingdom) [1]. In 1889, Ernest Besnier described the cutaneous hallmarks of chronic sarcoidosis as lupus pernio. Later, Caesar Boeck used the term sarkoid (sarcoid) for the first time when he assumed that these lesions were similar to sarcoma, but benign. In India, the first case of sarcoid was published in the Journal of the School of Tropical Medicine, Calcutta in 1956, while in 1923 the first case of Familial sarcoidosis was recorded in two affected sisters [2].
Despite its long history, this disease remains enigmatic. Unidentified etiology and the multisystemic nature of the disease have made it more complex. Previous data suggested that at least 90% of sarcoidosis patients have manifestations in the lungs [3,4]. In addition to the lungs, the skin, liver, spleen, lymph nodes, upper respiratory tract, heart, and nervous system have all been shown to be affected by this disease and account for between 10 and 30 % [5]. Sarcoidosis occurs worldwide and has been reported in all racial and ethnic groups; no race, sex, or age is immune to it [4,6]. The clinical presentation of sarcoidosis varies depending on the specific organ involved. Sarcoidosis may present with a wide range of clinical assignations ranging from asymptomatic to fatal. The etiology of the disease is still unknown but some studies have reported that an unidentified antigen processed by activated macrophages instigates an immune response regulated by T-cells and macrophages. These activated cells discharge various mediators, including cytokines, chemokines, and reactive oxygen species that may be involved in the progression of sarcoidosis [7,8]. Many studies suggest that not only unknown antigens are responsible for this disease but also genetic susceptibility, environmental factors, and in some instances, this disease may be result from autoimmune activation [9,10].
To identify the studies included in this review, we performed an intensive search of the electronic databases, PubMed and MEDLINE, for relevant studies published between 1980 and the present using the following terms: sarcoidosis, pulmonary sarcoidosis, and extrapulmonary sarcoidosis. Bibliographies of all selected articles were reviewed, and we also included any relevant information from our personal files. More than 100 articles were extensively reviewed for the purpose of this review.
2. Epidemiology
The prevalence and incidence of sarcoidosis are not well known worldwide owing to the challenges associated with ascertaining the number of asymptomatic patients. Sarcoidosis affects individuals of all ages irrespective of race or ethnicity, with maximum incidence among people aged 20–39 years, and quite more prevalent in women, non-smokers, and in rural communities [11]. In Europe, a higher onset of the disease has been recorded in the northern part (around 60 per 100,000) than in southern European countries, including Italy (<10 per 100,000) [12,13]. In addition, the global incidence for sarcoidosis is the highest in Sweden (64/100,000) [14], 20/100 000 in the United Kingdom [15], 4.4–6.3/100,000 in Australia [16], 10/100,000 in France, 9/100,000 in Germany, 1.4/100,000 in Spain, 7/100,000 in Greece, 1.4/100,000 in Japan. Approximately 10–14/100,000 and 35.5–64/100,000 of Caucasian and in African-Americans develop sarcoidosis, respectively [17,18,19,20]. In India, the prevalence of sarcoidosis is 10–12 cases/1000 new registrations yearly, as reported by a respiratory unit in western India and 61.2/100,000 as reported by the respiratory unit of a hospital in the capital region [21,22]. Evaluation of sarcoidosis in the Indian population is still in the very early stages, and accordingly, we can assume that its prevalence is being underreported in this region. The most common comorbidities encountered in sarcoidosis patients are hyperlipidemia, obesity, thyroid disease, diabetes, osteoporosis, coronary heart disease, asthma, hypertension, chronic renal disease, and chronic obstructive pulmonary disease (COPD) [23,24]. Sarcoidosis is also often reported in patients with certain autoimmune diseases including autoimmune thyroid disease, Sjogren’s syndrome ankylosing spondylitis [25], and systemic sclerosis [26].
3. Causes
The exact cause of sarcoidosis is not known. Many researchers have hypothesized the role of genetic susceptibility, environmental factors, putative antigens, and autoimmunity in the development of this disease, but no single cause has been identified to date.
3.1. Genetic Factors
Various studies suggest that genetic factors could play a crucial role in establishing the risk and clinical development of sarcoidosis [27]. Eleven sarcoidosis risk loci (BTNL2, HLA-B, HLA-DPB1, ANXA11, IL23R, SH2B3/ATXN2, IL12B, NFKB1/MANBA, FAM177B, chromosome 11q13.1, and RAB23) have been identified to date [28]. A previous study reported that familial sarcoidosis occurred in 17% of African-Americans [29], while only 1.4% of Spanish people exhibited this same risk [30]. According to A Case-Control Etiologic Sarcoidosis Study (ACCESS) the chance of developing sarcoidosis is five-fold among siblings [31]. Monozygotic siblings with sarcoidosis had an 80-fold higher risk of developing the condition, although the estimated risk of developing sarcoidosis in dizygotic twins was only seven-fold [32].
Genome wide association studies have demonstrated that several HLA and non-HLA alleles are associated with the development of this disease [33]. HLA-DRB1*0301/ DQB1*0201 [34], transforming growth factor β (TGF-β) [35], tumor necrosis factor α (TNF-α) [36], and Toll-like receptor 4 (TLR-4) [37] are all considered significant indicators for susceptibility to sarcoidosis [38,39].
3.2. Environmental Risk Factors
Various environmental factors, including exposure to wood stoves, soil, tree pollen, inorganic particulates, insecticides, and nanoparticles, have been associated with an increased risk for developing sarcoidosis. In addition to these factors, some workers, such as those involved in hardware, gardening materials, building supplies, and metal work as well as ship servicemen in the navy, fire workers, and educators, are prone to sarcoidosis [40,41,42]. It has been suggested that silica exposure also triggers the risk of sarcoidosis [43]. The underlying hypothesis for this association is that the environment is an important risk factor for the development of sarcoidosis, which has been further strengthened by reports that US World Trade Center workers exposed to the crash debris, in particular firefighters; all experienced an increased risk for developing sarcoidosis or “sarcoid-like” disease [44].
3.3. Infection
In addition to all of the factors mentioned above, infectious agents such as mycobacteria, have been suggested to be associated with the development of sarcoidosis, because the production of granulomas is a key factor in the immune defense response against these agents. Studies have identified numerous microbial agents as a potential eliciting agents of the immune response in sarcoidosis including Leptospira species, Mycoplasma species, herpes virus, retrovirus, Chlamydia pneumoniae, Borrelia burgdorferi [45], Pneumocystis jirovecii [46], Mycobacterium (M.tb) [47], and Propionibacterium species [48]. Isolation of M.tb. DNA, from tissue specimens collected from sarcoidosis patients, with sequences specific to mycobacterial proteins, such as ESAT-6, Kat G, and SoD A, illustrate that Mycobacterium is the strongest candidate for infection-mediated sarcoidosis [49,50,51]. It has been reported that patients treated with interferon α therapy for hepatitis C infection developed sarcoidosis [52,53]. A few studies have suggested that hepatitis C infection on its own could increase the risk of developing sarcoidosis. However, it seems more likely that therapy with interferon α increases interferon-γ and interleukin-2 expression, stimulating granuloma formation and thus sarcoidosis [54,55].
3.4. Autoimmunity
Autoimmunity has not been studied as extensively but given the underlying pathological mechanism of sarcoidosis there is certainly potential for these conditions to play a contributing role in disease development. Although no disease-specific auto-antibodies have been observed, it has been shown that the major histocompatibility complex (MHC) class II molecules on antigen-presenting cells possess an autoantigen that is recognized by the T-cell receptor (TCR) of the responding T-cells in sarcoidosis patients [56,57]. Vimentin-derived peptides are the most plausible candidate for the activation of both T-cells and B-cells in the lung [58]. Autoimmunity presents a as a novel spectrum for sarcoidosis immunopathogenesis and may help elucidate sarcoid etiology [59,60,61].
Another important aspect of autoimmunity is the imbalanced gut microbiome. Gianchecchi et al. reported the associations between the presence of microbiome dysbiosis and the development of autoimmune conditions [62]. Sarcoidosis overlaps with other autoimmune diseases, including rheumatoid arthritis, autoimmune thyroid disease, Sjogren’s syndrome, and ankylosing spondylitis [63]. The role of the microbiota in these autoimmune diseases has been evaluated in previous studies and been shown to lay a significant role in their pathogenesis [64]; thus, study of the microbiome of sarcoidosis patients and its correlation with other diseases could open new avenues for investigating the underlying causes of this disease [65,66].
4. Immunopathogenesis
Many etiological agents, including infectious microbes, as well as organic and inorganic compounds, contribute to the development of sarcoidosis. These antigens are first cleared by the immune system, but this is not infallible and some undegraded antigens may remain in the cells, which can initiate an immune feedback loop. In response to this feedback loop, the antigen-presenting cells (APCs), such as dendritic cells (DCs), alveolar macrophages (AMs), and alveolar epithelial cells, produce high levels of TNF-α, and secrete interleukins-12, -15, and -18, macrophage inflammatory protein-1 (MIP-1), monocyte chemoattractant protein-1 (MCP-1), and granulocyte macrophage colony-stimulating factor (GM-CSF) [67]. These APCs also present antigens to CD4+ T-cells initiating granuloma construction, a critical feature of sarcoidosis. The growth of these granulomas establishes the primary abnormality in most cases of sarcoidosis. Sarcoid granulomas are ordered, structured masses comprised of macrophages and their derivatives, epithelioid cells, giant cells, and T-cells.
Activated CD4+ T-cells can differentiate into two distinct subsets, namely, T helper 1 (Th1) and T helper 2 (Th2) cells, based on their cytokines profile. Th1 cells predominantly secrete interleukin-2 (IL-2) and interferon-gamma (IFN-γ), while IL-4 and IL-13 are the major secretions of Th2 cells. Resolution or maintenance of granuloma is determined by the proportion of Th1 and Th2 cells, respectively. Alveolar macrophages are activated in the Th2 milieu and stimulate fibroblast and collagen proliferation culminating in progressive fibrosis [68].
Incapacitation of Tregs is also a key feature of granuloma maintenance. It is presumed that infiltrating Tregs fail to reduce the exaggerated inflammatory response, thereby contributing to granuloma persistence and integrity. Tregs also release transforming growth factor β (TGF-β) that may contribute to fibrosis and granuloma organization [69].
Th17 and Th17.1 cells have only recently been linked to the pathogenesis of sarcoidosis [70]. These cells are recruited to the disease site and are involved in the construction of the granuloma. The balance between Th17 and Treg cells is thought to be disrupted in sarcoidosis [71] and is an important factor in its prognosis [72]. The regulation of antigen processing, antigen presentation to the APCs, and cytokine release are all controlled through genetic elements and may link the various causal factors of sarcoidosis together [73,74,75].
5. Clinical Features
Sarcoidosis is often diagnosed when aberrations are identified on a chest radiograph (up to 50% of patients) during a routine examination. Based on the presence of lung infiltration and/or lymphadenopathies on the radiograph, different stages of sarcoidosis have been described [3] (Box 1) Symptoms are usually negligible and nonspecific including cough, labored breathing, chest discomfort, dyspnea, and low-grade fever [76,77]. Systemic symptoms such as tiredness, weight reduction, and night sweats, are common. Hemoptysis is rare. Sarcoidosis may be acute, sub-acute, or chronic; however, in a majority of cases, it is entirely asymptomatic. Lofgren syndrome, where erythema nodosum and bilateral hilar adenopathy are both present, is one of the classic and acute presentation of sarcoidosis. Individuals suffering from sub-acute sarcoidosis have nonspecific signs comprising frailty, fever, weight reduction, arthralgia, and peripheral lymphadenopathy [9,78]. Chronic sarcoidosis is identified following serious persistent lung engagement, with a slow onset and a high degree of individual variability.
Box 1. Scadding’s staging of sarcoidosis.
| Radiographic Type | Radiographic Characteristics |
| 0 | No visible findings |
| I | Bilateral hilar lymphadenopathy |
| II | Bilateral hilar lymphadenopathy and parenchymal infiltration |
| III | Parenchymal infiltration without hilar adenopathy in regular chest radiography |
| IV | Advanced fibrosis with severe distortion of the normal lung architecture predominately in the middle and upper lobes with evidence of bronchiectasis, hilar retraction, bulla, cysts and more rarely “honeycombing” |
The multisystemic nature of sarcoidosis leads to organ specific manifestations (Table 1). Symptoms may differ from patient to patient. According to ACCESS, 95% of patients had thoracic engagement, 50% had extra thoracic symptoms, and 2% had unaccompanied extra thoracic sarcoidosis [4]. In a study that used 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18 FDG-PET/CT), the following four sarcoidosis phenotypes were identified and evaluated: thoracic nodal hilar-mediastinal, thoracic nodal hilar-mediastinal and lungs, extended thoracic and extra-thoracic only nodal phenotype including inguinal-abdominal-supraclavicular stations, and all of the above plus systemic organs and tissues such as muscles-bones-spleen and skin [79]. Most clinical studies agree that owing to the multi-organ and system granulomatous potential of sarcoidosis, a multifaceted approach is necessary to evaluate the possibility of extrapulmonary localizations of this disease.
Table 1.
List of organs involved in sarcoidosis.
| Organ Involvement | Prevalence of Organ Involvement | Manifestations | References |
|---|---|---|---|
| Lung involvement | more than 90% (With hilar and mediastinal lymph node) |
Dry cough, wheezing, dysponea, fatigue Acute: Pleural effusion, pericardial effusion, pneumothorax, and lymph-node Chronic: lung fibrosis and respiratory failure |
[80,81,82] |
| Lymph node involvement | 20% of patients | Peripheral lymphadenopathy, affected lymph nodes are moderately swollen, and are usually not painful. | [83,84,85] |
| Endocrine and exocrine involvement | Thyroid glands and parotid glands are usually affected in 20%–50% of cases | Thyroid dysfunction (5%), Parotid enlargement (5%–10%), hypothalamic-pituitary effects (for example, diabetes insipidus), | [86,87] |
| Skin involvement | 20%–30% of patients | Erythema nodosum (most common), profuse sweating, nodules, papules and plaques. | [88,89] |
| Eye involvement | more than 40% of patients | pain, photophobia, and hyperaemia, sometimes associated with the Löfgren syndrome | [90,91,92] |
| Bone involvement | 1%–13% of patients | Osteoporosis and osteopenia are common, Nodular lesions, cystic lesions involving the joints, arthritis and arthralgia | [93,94,95] |
| Upper respiratory tract | In most patients with systemic sarcoidosis | Larynx, nasopharynx and nose are affected | [96,97,98] |
| Renal involvement | 5% | Renal calculi, nephrocalcinosis, interstitial nephritis, and kidney failure | [99,100] |
| Cardiac involvement | 20%–27% of sarcoidosis | Heart failure, arrhythmias, syncope | [101,102] |
| Neurological involvement or neurosarcoidosis | less than 10% of patients | Facial palsy, Meningeal inflammation, encephalopathy, vasculopathy, seizures, hydrocephalus, and mass lesions | [103,104] |
| Liver and spleen involvement | 18% | Hepatosplenomegaly, intrahepatic cholestasis, and portal hypertension and altered liver function | [105,106,107] |
6. Screening and Diagnosis
Diagnosis of sarcoidosis always poses a challenge to clinicians. Owing to its multisystemic nature and unidentified etiology, the diagnosis of this condition can be difficult and is often delayed; however, early diagnosis is indispensable for patient management. Sarcoidosis is usually diagnosed when radiological and typical clinical data are reinforced by histological confirmation of non-necrotic granulomas. To establish any confirmed diagnosis, patients should undergo multiple clinical examinations, depending on organ involvement, as a specific diagnostic assay is still lacking (Table 2).
Table 2.
A list of conventional diagnostic tests for sarcoidosis.
| Test | Indication for Sarcoidosis | References |
|---|---|---|
| Physical examination | fever, fatigue, malaise, weight loss, and erythema nodosum | [108] |
| Routine ophthalmologic examination | orbital and eyelid granulomas | [109] |
| Peripheral blood count | Lymphopenia | [110] |
| Renal function tests | High level of calcium, urea, and creatinine | [111] |
| Urine analysis | Hypercalciurea | [112] |
| Pulmonary function Tests | Assess pulmonary involvement and disease severity | [113] |
| Tissue biopsy | For the presence of granuloma (Lungs, lymph node, skin, salivary gland, conjunctiva) |
[114] |
| Bronchial Biopsy | Detect pulmonary involvement, (Endobronchial ultrasound-guided transbronchial needle aspirate [EBUS-TBNA], Trans and endobronchial Biopsy) | [115,116] |
| Tuberculin skin test (Mantoux) | Negative in the most sarcoidosis patients | [117] |
| Chest X-ray | Bilateral hilar lymphadenopathy, Disseminated nodules in the lungs | [118,119] |
| HRCT | Differentiation of sarcoidosis from other pulmonary conditions | [120,121] |
| FDG-PET | Highly sensitive to detect cardiac and pulmonary involvement | [122] |
| Electrocardiogram (ECG) | Repolarization disturbances, Ectopic beats, Rhythm abnormalities | [123,124] |
| MRI | Detect neurological involvement, spinal cord, meninges, skull vault, and pituitary lesions. | [125,126] |
Numerous imaging techniques have also been assessed for their diagnostic utility in the identification of sarcoidosis, but their utility is mostly restricted to specific organs. Despite these limitations, high-resolution computed tomography (HRCT), magnetic resonance imaging (MRI), and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) have improved the diagnosis of sarcoidosis. These techniques are equally effective in evaluating a patient’s response to treatment.
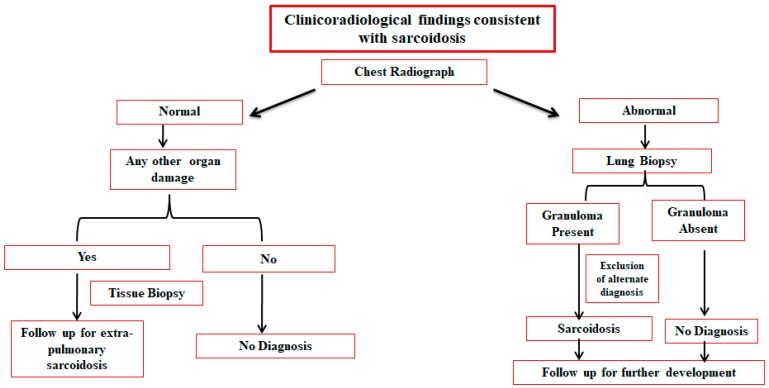
All examinations mentioned in Table 2 can be used to identify sarcoidosis in different organs; however, no assay has been established as the gold standard. One or more tests can be performed in combination to confirm the presence of sarcoidosis, but patients’ history, symptoms, clinical signs, and particularly, expert clinical discretion always complement the finding of the relevant medical examination. Stepwise evaluation of all of the available information, excluding non-specific data, should be undertaken to confirm diagnosis (Figure 1).
Figure 1.
Diagnostic management of sarcoidosis.
7. Biomarkers for Sarcoidosis
A biological marker or biomarker refers to a broad subcategory of medical signs that can be accurately, objectively, and reproducibly measured [127]. Several biomarkers have been proposed for the diagnosis of sarcoidosis and the monitoring of its progression, but none has been accepted wholly in practice [111]. The difficulty in identifying and evaluating biomarkers for sarcoidosis is linked to its dubious etiology, non-specific symptoms, and multiple disease phenotypes. Due to this lack of biomarkers, diagnosis, prognosis, treatment response, and clinical outcomes for this disease are not thoroughly predictable.
Various biomarkers, including, serological biomarkers, bronchoalveolar lavage (BAL) biomarkers, and exhaled breath biomarkers, have been proposed numerous studies, but all have been shown to have limited applicability. Serological biomarkers should be the area of most focus for researchers moving forward as these are the least invasive and most accessible [128]. Although higher serum angiotesin converting enzyme (SACE) and BAL lymphocyte ratios are widely discussed, their utility is limited as these are not specific to sarcoidosis. In Table 3, we discuss all the plausible biomarkers for the evaluation of sarcoidosis.
Table 3.
List of potential biomarkers of sarcoidosis.
| Biomarkers | Indication for Sarcoidosis | Diagnostic Value | Prognostic Value | Disease Severity Assessment | References |
|---|---|---|---|---|---|
| Serological Biomarkers | |||||
| SACE |
|
+ | − | ++ | [129,130,131] |
| Chitotriosidase |
|
− | − | ++ | [132,133,134] |
| Lysozyme |
|
− | − | + | [135,136] |
| Neopterin |
|
− | − | + | [137,138,139] |
| Hypercalcemia |
|
− | − | + | [140,141,142] |
| Soluble IL2 receptor |
|
− | + | ++ | [143,144,145] |
| SAA |
|
+ | − | + | [146,147,148] |
| Chemokines |
|
− | + | + | [149,150,151] |
| KL 6 |
|
− | + | + | [152] |
| IFN-gamma |
|
− | − | − | [153,154] |
| TGF-β |
|
− | + | + | [155,156] |
| TNF-α |
|
− | − | − | [157,158] |
| Biomarkers in BAL | |||||
| CD4/CD8 ratio in BAL |
|
+ | − | + | [159,160] |
| Percentage of White Blood cells in BAL |
|
- | − | + | [161,162] |
| Exhaled Breath Biomarkers | |||||
| 8-isoprostane |
|
+ | − | − | [163,164] |
| Carbon monoxide |
|
− | − | − | [165] |
| Nitric oxide |
|
− | − | − | [166,167] |
SACE: Serum angiotensin converting enzyme; SAA: Serum Amyloid A; IL2: Interleukin 2; CCL18: Chemokine ligand 18; CXCL9: C-X-C Motif; Chemokine Ligand 9; CXCL10: C-X-C Motif Chemokine Ligand 10; TNF-α: Tumor Necrosis Factor-α; IL-18: Interleukin 18; IL-10: Interleukin 10; KL 6: Kerbs von Lungren 6 antigen; TGF-β: Transforming Growth Factor-β.
The application of biomarkers in the diagnosis and prognosis of sarcoidosis is still in its infancy with relatively few biomarkers appearing to have any real clinical application. However, the advent of “omics” type approaches (consisting of genomics, proteomics, transcriptomics, metabolomics, microbiomics, and metallomics) and the increasing number of studies applying these techniques suggest that we may soon have more valid candidates to choose from. Thus, while biomarkers are not currently a viable alternative for diagnostic applications, they may soon become effective [168].
In the previous decade, transcriptomics have identified novel gene expression profiles underlying the pathogenesis of sarcoidosis [169]. All these transcriptomic datasets validate the major role of IFN-γ-driven STAT1 signaling and type I IFN signaling in sarcoidosis [170]. Micro-RNAs have also been shown to have some potential as biomarkers for the diagnosis of sarcoidosis. miRNA-29A, hsa-miR-4306, and hsa-miR-6729-5p have been shown to be associated with sarcoidosis, acting as non-invasive biomarkers [171,172]. Metabolic changes play a crucial role in the progression of inflammation. 1H nuclear magnetic resonance (NMR)-based metabolomic analysis identified metabolites and metabolic pathways that can discriminate sarcoidosis patients from healthy ones. Acetoacetate, 3-hydroxybutyrate, carnitine, cystine, and trimethylamine N-oxide levels are significantly increased in sarcoidosis, with dysregulation of ketone bodies and citric cycle metabolism also being identified as hallmarks of this disease [173,174].
8. Treatment
In sarcoidosis, a decision on the appropriate intervention precedes the decision of whether or not to treat the patient. Not every patient needs to be treated. The decision to treat a sarcoidosis patient is predicated according to the development of specific symptoms and disease progression evidenced by worsening functional status and imaging abnormalities [175,176]. Patients can be followed-up over long periods because spontaneous resolution may occur during this time frame. Development of dangerous clinical conditions and a significant impairment in the quality of life are two major indications for clinicians to start interventional treatment [177]. Therapeutic strategies should include mental and emotional well-being, in addition to physical well-being. If treatment is to be initiated, oral corticosteroids are the first line of treatment. Corticosteroids have proved reliable in providing symptomatic relief and reversing organ dysfunction, but the risks of using corticosteroids is always a matter of concern [178].
Treatment is often initiated with 0.5–0.75 mg of prednisolone per kg (body weight) daily for 4 weeks and tapered by 10 mg every 4 weeks, depending on the disease response [179]. It is sometimes advised that the dose of 0.5-0.75 mg/kg of prednisolone is too high and doses of 20 mg of prednisone can be used as an alternative. When pulmonary function has improved, therapy can be terminated, which is usually within 6-12 months. For many patients who have mild clinical manifestations, such as skin lesions, anterior uveitis, or cough, corticosteroid treatment should be instigated. For those necessitating systemic treatment, most will recover in a reasonably short time frame but there is a small group of patients who develop chronic disorders that do not recuperate after 2–5 years. These chronic patients frequently need long-term treatment, which can necessitate the use of corticosteroids or additional therapies for more than 5 years.
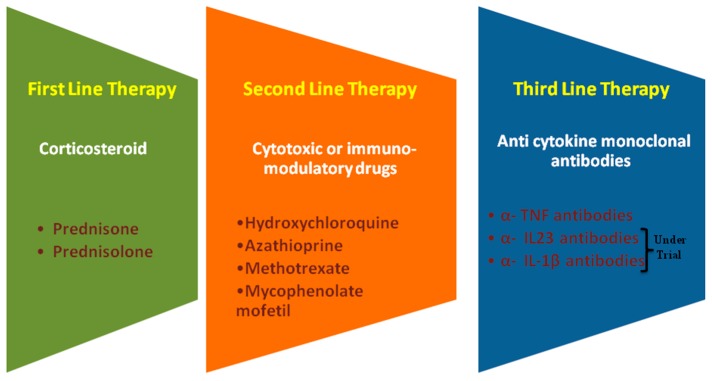
For patients with intolerable adverse responses to steroids, corticosteroid-sparing regimens can also be administered. These are considered second line treatments and rely on therapeutics such as azathioprine [180], methotrexate [181], mycophenolate mofetil [182,183], cyclosporine [184], cyclophosphamide [185], leflunomide [186], and hydroxychloroquine [187] for symptomatic relief, but all of these drugs have been shown to be less effective than the steroid interventions (Figure 2).
Figure 2.
Therapeutic options for first, second, and third-line treatment of sarcoidosis.
Mechanism-based therapeutic treatment is the most advanced and targeted approach for the treatment of sarcoidosis. Different cytokines play a pivotal role in the immunopathogenesis of sarcoidosis. Anti-cytokine monoclonal antibodies are a specific way to modulate cytokine networks, thus influencing disease progression [188]. These cytokine-directed treatments are manifested as third line therapies. TNF-α is known to play a significant role in the formation of the granulomas associated with sarcoidosis [189]. The use of anti-TNF antibodies such as infliximab [190,191] or adalimumab [192] has shown some therapeutic benefits, although these gains have been relatively low. Recent studies describing the involvement of Th17 cells and their related cytokines in the pathogenesis of sarcoidosis, have suggested that IL-23 and IL-1β, inducers of Th17 differentiation, are useful targets for therapeutic interventions. Treatment with ustekinumab and canakinumab was recently evaluated with mixed results. Ustekinumab did not show any efficacy in pulmonary sarcoidosis and the results for canakinumab are still awaited [193] (NCT2888080). In addition, there are still relatively few guidelines for the clinical intervention of sarcoidosis [194,195,196,197] but surveillance for 3–12 months is typically endorsed to determine the overall course of the disease [198].
Personalized medicine is a novel medical doctrine focused on tailoring therapeutic management of various diseases [199]. The goal of precision medicine is to address disease prevention, diagnosis, and treatment while considering individual patient variability. Integration of different omics data presents comprehensive overviews of pathological molecular pathways that can be targeted for the development and application of precision medicine [200]. Multi-omics integrative analysis generates vast amounts of big data from sarcoidosis samples including genomic, transcriptomics, proteomic, and phenomic studies, all of which have been used to describe novel candidate regions and genes, altered in sarcoidosis [201]. These new data analysis methods are bridging the gap between conventional therapies and advanced care and are bound to open new therapeutic paradigms for this complex disease.
9. Conclusions
Despite extensive research over the past several decades, the etiological agents of sarcoidosis remain unknown. Numerous potential etiological agents have been identified and the most recent hypothesis suggests that host-microbe interaction and genetic factors play an important role in the pathogenesis of this disease when they interact with various environmental factors, which results in the clinical presentation of this disease. To cure this disease, timely diagnosis is important; therefore, there is a critical need for clinicians to develop potent diagnostic tools for the identification and prognosis of sarcoidosis. Recently, new diagnostic strategies for sarcoidosis, including HRCT, FDG-PET scanning, TBNA, and EBUS technologies, have reinforced its prognosis. More focus should be concentrated on the development of non-invasive biomarkers. Big data analysis with the integration of ‘-omics’ data might elucidate the etiology and pathogenesis of sarcoidosis. Corticosteroids play an important role in the treatment of sarcoidosis, but they evoke many side effects if used for a long period. Second line and targeted treatments could be promising alternatives for the treatment of sarcoidosis in the near future. Precision medicine is the new hope in this field and should be monitored closely for progress toward targeted interventions. For better disease management, multifaceted approaches remain the best practice to ensure competent and effective patient care.
Author Contributions
Writing—Original Draft Preparation, R.J., D.Y. and N.P.; Writing—Review and Editing, R.J. and R.G.; Supervision, J.-O.J.; Project Administration, D.Y. and J.-O.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2019R1G1A1008566) and 13th Five-Year National Major Science and Technology Project on Discovery of New Drugs: Construction of Technology Platform for Clinical Evaluation on Anti-HIV Drugs (2017ZX09304027).
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- 1.Hutchinson J. Anomalous disease of the skin of the fingers: Case of livid papillary psoriasis. Illus. Clin. Surg. 1877;1:42–43. [Google Scholar]
- 2.Gupta S.K. Sarcoidosis: A journey through 50 years. Indian J. Chest Dis. Allied Sci. 2002;44:247–254. [PubMed] [Google Scholar]
- 3.Hunninghake G. Statement on sarcoidosis. Am. J. Respir. Crit. Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 4.Baughman R.P., Teirstein A.S., Judson M.A., Rossman M.D., Yeager H., Jr., Bresnitz E.A., DePalo L., Hunninghake G., Iannuzzi M.C., Johns C.J., et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am. J. Respir. Crit. Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 5.Li C.-W., Tao R.-J., Zou D.-F., Li M.-H., Xu X., Cao W.-J. Pulmonary sarcoidosis with and without extrapulmonary involvement: A cross-sectional and observational study in china. BMJ Open. 2018;8:e018865. doi: 10.1136/bmjopen-2017-018865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siltzbach L.E., James D.G., Neville E., Turiaf J., Battesti J.P., Sharma O.P., Hosoda Y., Mikami R., Odaka M. Course and prognosis of sarcoidosis around the world. Am. J. Med. 1974;57:847–852. doi: 10.1016/0002-9343(74)90160-0. [DOI] [PubMed] [Google Scholar]
- 7.Miyara M., Amoura Z., Parizot C., Badoual C., Dorgham K., Trad S., Kambouchner M., Valeyre D., Chapelon-Abric C., Debré P., et al. The immune paradox of sarcoidosis and regulatory t cells. J. Exp. Med. 2006;203:359–370. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zissel G. Cellular activation in the immune response of sarcoidosis. Semin. Respir. Crit. Care Med. 2014;35:307–315. doi: 10.1055/s-0034-1376861. [DOI] [PubMed] [Google Scholar]
- 9.Iannuzzi M.C., Rybicki B.A., Teirstein A.S. Sarcoidosis. N. Engl. J. Med. 2007;357:2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 10.Prior C., Knight R.A., Herold M., Ott G., Spiteri M.A. Pulmonary sarcoidosis: Patterns of cytokine release in vitro. Eur. Respir. J. 1996;9:47–53. doi: 10.1183/09031936.96.09010047. [DOI] [PubMed] [Google Scholar]
- 11.Peros-Golubicic T., Ljubic S. Cigarette smoking and sarcoidosis. Acta Med. Croat. 1995;49:187–193. [PubMed] [Google Scholar]
- 12.Siltzbach L.E. Current thoughts on the epidemiology and etiology of sarcoidosis. Am. J. Med. 1965;39:361–368. doi: 10.1016/0002-9343(65)90205-6. [DOI] [PubMed] [Google Scholar]
- 13.Arkema E.V., Cozier Y.C. Epidemiology of sarcoidosis: Current findings and future directions. Ther. Adv. Chronic Dis. 2018;9:227–240. doi: 10.1177/2040622318790197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arkema E.V., Grunewald J., Kullberg S., Eklund A., Askling J. Sarcoidosis incidence and prevalence: A nationwide register-based assessment in sweden. Eur. Respir. J. 2016;48:1690–1699. doi: 10.1183/13993003.00477-2016. [DOI] [PubMed] [Google Scholar]
- 15.Bresnitz E.A., Strom B.L. Epidemiology of sarcoidosis. Epidemiol. Rev. 1983;5:124–156. doi: 10.1093/oxfordjournals.epirev.a036255. [DOI] [PubMed] [Google Scholar]
- 16.Gillman A., Steinfort C. Sarcoidosis in australia. Intern. Med. J. 2007;37:356–359. doi: 10.1111/j.1445-5994.2007.01365.x. [DOI] [PubMed] [Google Scholar]
- 17.Hosoda Y., Yamaguchi M., Hiraga Y. Global epidemiology of sarcoidosis. What story do prevalence and incidence tell us? Clin. Chest Med. 1997;18:681–694. doi: 10.1016/S0272-5231(05)70412-3. [DOI] [PubMed] [Google Scholar]
- 18.James D.G. Epidemiology of sarcoidosis. Sarcoidosis. 1992;9:79–87. [PubMed] [Google Scholar]
- 19.Reich J., Johnson R. Incidence of clinically identified sarcoidosis in a northwest United States population. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG. 1996;13:173–177. [PubMed] [Google Scholar]
- 20.Karakatsani A., Papakosta D., Rapti A., Antoniou K.M., Dimadi M., Markopoulou A., Latsi P., Polychronopoulos V., Birba G., Ch L., et al. Epidemiology of interstitial lung diseases in greece. Respir. Med. 2009;103:1122–1129. doi: 10.1016/j.rmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Sharma S., Mohan A. Sarcoidosis in India: Not so rare. J. Indian Acad. Clin. Med. 2004;5:12–21. [Google Scholar]
- 22.Gupta S., Gupta S. Sarcoidosis in India: A review of 125 biopsy-proven cases from eastern India. Sarcoidosis. 1990;7:43–49. [PubMed] [Google Scholar]
- 23.Martusewicz-Boros M.M., Boros P.W., Wiatr E., Roszkowski-Śliż K. What comorbidities accompany sarcoidosis? A large cohort (n = 1779) patients analysis. Sarcoidosis Vasc. Diffus. Lung Dis. 2015;32:115–120. [PubMed] [Google Scholar]
- 24.Nowinski A., Puscinska E., Goljan-Geremek A., Bednarek M., Kaminski D., Gorecka D. Comorbidities associated with sarcoidosis—Results from long-term observational study. Eur. Respir. J. 2014;44:461. [Google Scholar]
- 25.Wu C.H., Chung P.I., Wu C.Y., Chen Y.T., Chiu Y.W., Chang Y.T., Liu H.N. Comorbid autoimmune diseases in patients with sarcoidosis: A nationwide case-control study in taiwan. J. Dermatol. 2017;44:423–430. doi: 10.1111/1346-8138.13654. [DOI] [PubMed] [Google Scholar]
- 26.Carmi O., Berla M., Edelstein E., Levy Y. Coexisting systemic sclerosis-polymyositis and sarcoidosis: Case report and review of the literature. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 2018;24:238–240. doi: 10.1097/RHU.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 27.Grunewald J., Spagnolo P., Wahlstrom J., Eklund A. Immunogenetics of disease-causing inflammation in sarcoidosis. Clin. Rev. Allergy Immunol. 2015;49:19–35. doi: 10.1007/s12016-015-8477-8. [DOI] [PubMed] [Google Scholar]
- 28.Fischer A., Ellinghaus D., Nutsua M., Hofmann S., Montgomery C.G., Iannuzzi M.C., Rybicki B.A., Petrek M., Mrazek F., Pabst S., et al. Identification of immune-relevant factors conferring sarcoidosis genetic risk. Am. J. Respir. Crit. Care Med. 2015;192:727–736. doi: 10.1164/rccm.201503-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rybicki B.A., Iannuzzi M.C. Epidemiology of sarcoidosis: Recent advances and future prospects. Semin. Respir. Crit. Care Med. 2007;28:22–35. doi: 10.1055/s-2007-970331. [DOI] [PubMed] [Google Scholar]
- 30.Fabrellas E.F. Epidemiología de la sarcoidosis. Arch. Bronconeumol. 2007;43:92–100. doi: 10.1016/S1579-2129(07)60030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rybicki B.A., Iannuzzi M.C., Frederick M.M., Thompson B.W., Rossman M.D., Bresnitz E.A., Terrin M.L., Moller D.R., Barnard J., Baughman R.P., et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (access) Am. J. Respir. Crit. Care Med. 2001;164:2085–2091. doi: 10.1164/ajrccm.164.11.2106001. [DOI] [PubMed] [Google Scholar]
- 32.Sverrild A., Backer V., Kyvik K.O., Kaprio J., Milman N., Svendsen C.B., Thomsen S.F. Heredity in sarcoidosis: A registry-based twin study. Thorax. 2008;63:894–896. doi: 10.1136/thx.2007.094060. [DOI] [PubMed] [Google Scholar]
- 33.Schurmann M., REICHEL P., Muller-Myhsok B., Schlaak M., Muller-Quernheim J., Schwinger E. Results from a genome-wide search for predisposing genes in sarcoidosis. Am. J. Respir. Crit. Care Med. 2001;164:840–846. doi: 10.1164/ajrccm.164.5.2007056. [DOI] [PubMed] [Google Scholar]
- 34.Ishihara M., Ohno S., Ishida T., Ando H., Naruse T., Nose Y., Inoko H. Molecular genetic studies of hla class ii alleles in sarcoidosis. Tissue Antigens. 1994;43:238–241. doi: 10.1111/j.1399-0039.1994.tb02331.x. [DOI] [PubMed] [Google Scholar]
- 35.Pabst S., Fränken T., Schönau J., Stier S., Nickenig G., Meyer R., Skowasch D., Grohé C. Transforming growth factor-β gene polymorphisms in different phenotypes of sarcoidosis. Eur. Respir. J. 2011;38:169–175. doi: 10.1183/09031936.00120410. [DOI] [PubMed] [Google Scholar]
- 36.Sharma S., Ghosh B., Sharma S. Association of tnf polymorphisms with sarcoidosis, its prognosis and tumour necrosis factor (tnf)-α levels in Asian Indians. Clin. Exp. Immunol. 2008;151:251–259. doi: 10.1111/j.1365-2249.2007.03564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pabst S., Baumgarten G., Stremmel A., Lennarz M., Knüfermann P., Gillissen A., Vetter H., Grohe C. Toll-like receptor (tlr) 4 polymorphisms are associated with a chronic course of sarcoidosis. Clin. Exp. Immunol. 2006;143:420–426. doi: 10.1111/j.1365-2249.2006.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grunewald J. Role of genetics in susceptibility and outcome of sarcoidosis. Semin. Respir. Crit. Care Med. 2010;31:380–389. doi: 10.1055/s-0030-1262206. [DOI] [PubMed] [Google Scholar]
- 39.Iannuzzi M.C. Genetics of sarcoidosis. Semin. Respir. Crit. Care Med. 2007;28:15–21. doi: 10.1055/s-2007-970330. [DOI] [PubMed] [Google Scholar]
- 40.Kucera G.P., Rybicki B.A., Kirkey K.L., Coon S.W., Major M.L., Maliarik M.J., Iannuzzi M.C. Occupational risk factors for sarcoidosis in african-american siblings. Chest. 2003;123:1527–1535. doi: 10.1378/chest.123.5.1527. [DOI] [PubMed] [Google Scholar]
- 41.Newman L.S., Rose C.S., Bresnitz E.A., Rossman M.D., Barnard J., Frederick M., Terrin M.L., Weinberger S.E., Moller D.R., McLennan G., et al. A case control etiologic study of sarcoidosis: Environmental and occupational risk factors. Am. J. Respir. Crit. Care Med. 2004;170:1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 42.Newman K.L., Newman L.S. Occupational causes of sarcoidosis. Curr. Opin. Allergy Clin. Immunol. 2012;12:145–150. doi: 10.1097/ACI.0b013e3283515173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vihlborg P., Bryngelsson L., Andersson L., Graff P. Risk of sarcoidosis and seropositive rheumatoid arthritis from occupational silica exposure in swedish iron foundries: A retrospective cohort study. BMJ Open. 2017;7:e016839. doi: 10.1136/bmjopen-2017-016839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izbicki G., Chavko R., Banauch G.I., Weiden M.D., Berger K.I., Aldrich T.K., Hall C., Kelly K.J., Prezant D.J. World trade center “sarcoid-like” granulomatous pulmonary disease in new york city fire department rescue workers. Chest. 2007;131:1414–1423. doi: 10.1378/chest.06-2114. [DOI] [PubMed] [Google Scholar]
- 45.Newman L. Aetiologies of sarcoidosis. Eur. Respir. Monogr. 2005;32:23–48. [Google Scholar]
- 46.Vidal S., De la Horra C., Martin J., Montes-Cano M., Rodríguez E., Respaldiza N., Rodriguez F., Varela J., Medrano F., Calderón E. Pneumocystis jirovecii colonisation in patients with interstitial lung disease. Clin. Microbiol. Infect. 2006;12:231–235. doi: 10.1111/j.1469-0691.2005.01337.x. [DOI] [PubMed] [Google Scholar]
- 47.Drake W.P., Newman L.S. Mycobacterial antigens may be important in sarcoidosis pathogenesis. Curr. Opin. Pulm. Med. 2006;12:359–363. doi: 10.1097/01.mcp.0000239554.01068.94. [DOI] [PubMed] [Google Scholar]
- 48.Ishige I., Eishi Y., Takemura T., Kobayashi I., Nakata K., Tanaka I., Nagaoka S., Iwai K., Watanabe K., Takizawa T. Propionibacterium acnes is the most common bacterium commensal in peripheral lung tissue and mediastinal lymph nodes from subjects without sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2005;22:33–42. [PubMed] [Google Scholar]
- 49.Allen S.S., Evans W., Carlisle J., Hajizadeh R., Nadaf M., Shepherd B.E., Pride D.T., Johnson J.E., Drake W.P. Superoxide dismutase a antigens derived from molecular analysis of sarcoidosis granulomas elicit systemic th-1 immune responses. Respir. Res. 2008;9:36. doi: 10.1186/1465-9921-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song Z., Marzilli L., Greenlee B.M., Chen E.S., Silver R.F., Askin F.B., Teirstein A.S., Zhang Y., Cotter R.J., Moller D.R. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J. Exp. Med. 2005;201:755–767. doi: 10.1084/jem.20040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drake W.P., Dhason M.S., Nadaf M., Shepherd B.E., Vadivelu S., Hajizadeh R., Newman L.S., Kalams S.A. Cellular recognition of mycobacterium tuberculosis esat-6 and katg peptides in systemic sarcoidosis. Infect. Immun. 2007;75:527–530. doi: 10.1128/IAI.00732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirano A., Kataoka M., Nakata Y., Takeda K., Kamao T., Hiramatsu J., Kimura G., Tanimoto Y., Kanehiro A., Tanimoto M. Sarcoidosis occurring after interferon-alpha therapy for chronic hepatitis c: Report of two cases. Respirology. 2005;10:529–534. doi: 10.1111/j.1440-1843.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- 53.Trien R., Cooper C.J., Paez D., Colon E., Ajmal S., Salameh H. Interferon-alpha-induced sarcoidosis in a patient being treated for hepatitis c. Am. J. Case Rep. 2014;15:235–238. doi: 10.12659/AJCR.890180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brjalin V., Salupere R., Tefanova V., Prikk K., Lapidus N., Jõeste E. Sarcoidosis and chronic hepatitis c: A case report. World J. Gastroenterol. 2012;18:5816–5820. doi: 10.3748/wjg.v18.i40.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramos-Casals M., Mana J., Nardi N., Brito-Zeron P., Xaubet A., Sanchez-Tapias J.M., Cervera R., Font J. Sarcoidosis in patients with chronic hepatitis c virus infection: Analysis of 68 cases. Medicine. 2005;84:69–80. doi: 10.1097/01.md.0000157577.69729.e6. [DOI] [PubMed] [Google Scholar]
- 56.Grunewald J., Kaiser Y., Ostadkarampour M., Rivera N.V., Vezzi F., Lötstedt B., Olsen R.-A., Sylwan L., Lundin S., Käller M. T-cell receptor—Hla-drb1 associations suggest specific antigens in pulmonary sarcoidosis. Eur. Respir. J. 2016;47:898–909. doi: 10.1183/13993003.01209-2015. [DOI] [PubMed] [Google Scholar]
- 57.Wahlström J., Dengjel J., Winqvist O., Targoff I., Persson B., Duyar H., Rammensee H.-G., Eklund A., Weissert R., Grunewald J. Autoimmune t cell responses to antigenic peptides presented by bronchoalveolar lavage cell hla-dr molecules in sarcoidosis. Clin. Immunol. 2009;133:353–363. doi: 10.1016/j.clim.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Zissel G., Müller-Quernheim J. Specific antigen(s) in sarcoidosis: A link to autoimmunity? Eur. Respir. Soc. 2016;47:707–709. doi: 10.1183/13993003.01791-2015. [DOI] [PubMed] [Google Scholar]
- 59.Kaiser Y., Eklund A., Grunewald J. Moving target: Shifting the focus to pulmonary sarcoidosis as an autoimmune spectrum disorder. Eur. Respir. J. 2019;54:1802153. doi: 10.1183/13993003.021532018. [DOI] [PubMed] [Google Scholar]
- 60.Starshinova A.A., Malkova A.M., Basantsova N.Y., Zinchenko Y.S., Kudryavtsev I.V., Ershov G.A., Soprun L.A., Mayevskaya V.A., Churilov L.P., Yablonskiy P.K. Sarcoidosis as an autoimmune disease. Front. Immunol. 2019;10:2933. doi: 10.3389/fimmu.2019.02933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haggmark A., Hamsten C., Wiklundh E., Lindskog C., Mattsson C., Andersson E., Lundberg I.E., Gronlund H., Schwenk J.M., Eklund A., et al. Proteomic profiling reveals autoimmune targets in sarcoidosis. Am. J. Respir. Crit. Care Med. 2015;191:574–583. doi: 10.1164/rccm.201407-1341OC. [DOI] [PubMed] [Google Scholar]
- 62.Gianchecchi E., Fierabracci A. Recent advances on microbiota involvement in the pathogenesis of autoimmunity. Int. J. Mol. Sci. 2019;20:283. doi: 10.3390/ijms20020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korsten P., Tampe B., Konig M.F., Nikiphorou E. Sarcoidosis and autoimmune diseases: Differences, similarities and overlaps. Curr. Opin. Pulm. Med. 2018;24:504–512. doi: 10.1097/MCP.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 64.Chu F., Shi M., Lang Y., Shen D., Jin T., Zhu J., Cui L. Gut microbiota in multiple sclerosis and experimental autoimmune encephalomyelitis: Current applications and future perspectives. Mediat. Inflamm. 2018 doi: 10.1155/2018/8168717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Becker A., Vella G., Galata V., Rentz K., Beisswenger C., Herr C., Walter J., Tierling S., Slevogt H., Keller A., et al. The composition of the pulmonary microbiota in sarcoidosis—An observational study. Respir. Res. 2019;20:46. doi: 10.1186/s12931-019-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inaoka P.T., Shono M., Kamada M., Espinoza J.L. Host-microbe interactions in the pathogenesis and clinical course of sarcoidosis. J. Biomed. Sci. 2019;26:45. doi: 10.1186/s12929-019-0537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agostini C., Adami F., Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr. Opin. Rheumatol. 2000;12:71–76. doi: 10.1097/00002281-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 68.Wynn T.A., Barron L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Branton M.H., Kopp J.B. Tgf-beta and fibrosis. Microbes. Infect. 1999;1:1349–1365. doi: 10.1016/S1286-4579(99)00250-6. [DOI] [PubMed] [Google Scholar]
- 70.Miedema J.R., Kaiser Y., Broos C.E., Wijsenbeek M.S., Grunewald J., Kool M. Th17-lineage cells in pulmonary sarcoidosis and löfgren’s syndrome: Friend or foe? J. Autoimmun. 2018;87:82–96. doi: 10.1016/j.jaut.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 71.Huang H., Lu Z., Jiang C., Liu J., Wang Y., Xu Z. Imbalance between th17 and regulatory t-cells in sarcoidosis. Int. J. Mol. Sci. 2013;14:21463–21473. doi: 10.3390/ijms141121463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y., Qiu L., Wang Y., Aimurola H., Zhao Y., Li S., Xu Z. The circulating treg/th17 cell ratio is correlated with relapse and treatment response in pulmonary sarcoidosis patients after corticosteroid withdrawal. PLoS ONE. 2016;11:e0148207. doi: 10.1371/journal.pone.0148207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelly A., Trowsdale J. Genetics of antigen processing and presentation. Immunogenetics. 2019;71:161–170. doi: 10.1007/s00251-018-1082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee J.A., Sinkovits R.S., Mock D., Rab E.L., Cai J., Yang P., Saunders B., Hsueh R.C., Choi S., Subramaniam S., et al. Components of the antigen processing and presentation pathway revealed by gene expression microarray analysis following b cell antigen receptor (bcr) stimulation. BMC Bioinformatics. 2006;7:237. doi: 10.1186/1471-2105-7-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bennett D., Bargagli E., Refini R.M., Rottoli P. New concepts in the pathogenesis of sarcoidosis. Expert Rev. Respir. Med. 2019;13:981–991. doi: 10.1080/17476348.2019.1655401. [DOI] [PubMed] [Google Scholar]
- 76.Pierce T.B., Margolis M., Razzuk M.A. Sarcoidosis: Still a mystery? Bayl. Univ. Med Cent. Proc. 2001;14:8–12. doi: 10.1080/08998280.2001.11927724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu X., Carmona E.M., Yi E.S., Pellikka P.A., Ryu J. Causes of death in patients with chronic sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2016;33:275–280. [PubMed] [Google Scholar]
- 78.Wirnsberger R.M., de Vries J., Wouters E.F., Drent M. Clinical presentation of sarcoidosis in the netherlands an epidemiological study. Neth. J. Med. 1998;53:53–60. doi: 10.1016/S0300-2977(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 79.Papiris S.A., Georgakopoulos A., Papaioannou A.I., Pianou N., Kallergi M., Kelekis N.L., Gialafos H., Manali E.D., Chatziioannou S. Emerging phenotypes of sarcoidosis based on 18f-fdg pet/ct: A hierarchical cluster analysis. Expert Rev. Respir. Med. 2020;14:229–238. doi: 10.1080/17476348.2020.1684902. [DOI] [PubMed] [Google Scholar]
- 80.Tavana S., Alizadeh M., Mohajerani S., Hashemian S. Pulmonary and extra-pulmonary manifestations of sarcoidosis. Niger. Med. J. 2015;56:258–262. doi: 10.4103/0300-1652.169702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nunes H., Uzunhan Y., Gille T., Lamberto C., Valeyre D., Brillet P.Y. Imaging of sarcoidosis of the airways and lung parenchyma and correlation with lung function. Eur. Respir. J. 2012;40:750–765. doi: 10.1183/09031936.00025212. [DOI] [PubMed] [Google Scholar]
- 82.Spagnolo P., Rossi G., Trisolini R., Sverzellati N., Baughman R.P., Wells A.U. Pulmonary sarcoidosis. Lancet Respir. Med. 2018;6:389–402. doi: 10.1016/S2213-2600(18)30064-X. [DOI] [PubMed] [Google Scholar]
- 83.Ozgul M., Cetinkaya E., Kirkil G., Ozgul G., Abul Y., Acat M., Onaran H., Urer H., Tutar N., Dincer H. Lymph node characteristics of sarcoidosis with endobronchial ultrasound. Endosc. Ultrasound. 2014;3:232–237. doi: 10.4103/2303-9027.144541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koo H.J., Kim M.Y., Shin S.Y., Shin S., Kim S.-S., Lee S.W., Choi C.-M. Evaluation of mediastinal lymph nodes in sarcoidosis, sarcoid reaction, and malignant lymph nodes using ct and fdg-pet/ct. Medicine. 2015;94:e1095. doi: 10.1097/MD.0000000000001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robinson L.A., Smith P., Sengupta D.J., Prentice J.L., Sandin R.L. Molecular analysis of sarcoidosis lymph nodes for microorganisms: A case-control study with clinical correlates. BMJ Open. 2013;3:e004065. doi: 10.1136/bmjopen-2013-004065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Porter N., Beynon H.L., Randeva H.S. Endocrine and reproductive manifestations of sarcoidosis. QJM. 2003;96:553–561. doi: 10.1093/qjmed/hcg103. [DOI] [PubMed] [Google Scholar]
- 87.Bell N.H. Endocrine complications of sarcoidosis. Endocrinol. Metab. Clin. N. Am. 1991;20:645–654. doi: 10.1016/S0889-8529(18)30262-7. [DOI] [PubMed] [Google Scholar]
- 88.Yanardag H., Pamuk O.N., Karayel T. Cutaneous involvement in sarcoidosis: Analysis of the features in 170 patients. Respir. Med. 2003;97:978–982. doi: 10.1016/S0954-6111(03)00127-6. [DOI] [PubMed] [Google Scholar]
- 89.Yanardag H., Tetikkurt C., Bilir M., Demirci S., Iscimen A. Diagnosis of cutaneous sarcoidosis; clinical and the prognostic significance of skin lesions. Multidiscip. Respir. Med. 2013;8:26. doi: 10.1186/2049-6958-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pasadhika S., Rosenbaum J.T. Ocular sarcoidosis. Clin. Chest Med. 2015;36:669–683. doi: 10.1016/j.ccm.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raevis J.J., Antonova N., Agemy S. Ocular involvement in sarcoidosis. J. Rheumatol. 2018;45:580. doi: 10.3899/jrheum.171058. [DOI] [PubMed] [Google Scholar]
- 92.Kansal V., Dollin M. Ocular involvement in sarcoidosis. CMAJ. 2017;189:E609. doi: 10.1503/cmaj.160569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nessrine A., Zahra A.F., Taoufik H. Musculoskeletal involvement in sarcoidosis. J. Brasileiro Pneumol. 2014;40:175–182. doi: 10.1590/S1806-37132014000200012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou Y., Lower E.E., Li H., Farhey Y., Baughman R.P. Clinical characteristics of patients with bone sarcoidosis. Semin. Arthritis Rheum. 2017;47:143–148. doi: 10.1016/j.semarthrit.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 95.Conte G., Zugni F., Colleoni M., Renne G., Bellomi M., Petralia G. Sarcoidosis with bone involvement mimicking metastatic disease at (18)f-fdg pet/ct: Problem solving by diffusion whole-body mri. Ecancermedicalscience. 2015;9:537. doi: 10.3332/ecancer.2015.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rottoli P., Bargagli E., Chidichimo C., Nuti D., Cintorino M., Ginanneschi C., Caruso G. Sarcoidosis with upper respiratory tract involvement. Respir. Med. 2006;100:253–257. doi: 10.1016/j.rmed.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 97.Soares M.T., Sousa C., Garanito L., Freire F. Extensive upper respiratory tract sarcoidosis. BMJ Case Rep. 2016 doi: 10.1136/bcr-2015-213325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilson R., Lund V., Sweatman M., Mackay I., Mitchell D. Upper respiratory tract involvement in sarcoidosis and its management. Eur. Respir. J. 1988;1:269–272. [PubMed] [Google Scholar]
- 99.Hilderson I., Van Laecke S., Wauters A., Donck J. Treatment of renal sarcoidosis: Is there a guideline? Overview of the different treatment options. Nephrol. Dial. Transplant. 2013;29:1841–1847. doi: 10.1093/ndt/gft442. [DOI] [PubMed] [Google Scholar]
- 100.Kala D., Naik N., Agarwal A. Our experience in the management of vaginal agenesis: Its psychosocial impact and role of contrast magnetic resonance imaging scan with vaginal mold in the interpretation of high transverse vaginal septum. Saudi J. Kidney Dis. Transpl. 2008;19:67–71. doi: 10.4103/jhrs.JHRS_82_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Birnie D.H., Kandolin R., Nery P.B., Kupari M. Cardiac manifestations of sarcoidosis: Diagnosis and management. Eur. Heart J. 2017;38:2663–2670. doi: 10.1093/eurheartj/ehw328. [DOI] [PubMed] [Google Scholar]
- 102.Lynch J.P., III, Hwang J., Bradfield J., Fishbein M., Shivkumar K., Tung R. Cardiac involvement in sarcoidosis: Evolving concepts in diagnosis and treatment. Semin. Respir. Crit. Care Med. 2014;35:372–390. doi: 10.1055/s-0034-1376889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ibitoye R.T., Wilkins A., Scolding N.J. Neurosarcoidosis: A clinical approach to diagnosis and management. J. Neurol. 2017;264:1023–1028. doi: 10.1007/s00415-016-8336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lacomis D. Neurosarcoidosis. Curr. Neuropharmacol. 2011;9:429–436. doi: 10.2174/157015911796557975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Warshauer D.M., Molina P.L., Hamman S.M., Koehler R.E., Paulson E.K., Bechtold R.E., Perlmutter M.L., Hiken J.N., Francis I.R., Cooper C.J., et al. Nodular sarcoidosis of the liver and spleen: Analysis of 32 cases. Radiology. 1995;195:757–762. doi: 10.1148/radiology.195.3.7754007. [DOI] [PubMed] [Google Scholar]
- 106.Patel I., Ismajli M., Steuer A. Sarcoidosis presenting as massive splenic infarction. Case Rep. Rheumatol. 2012 doi: 10.1155/2012/834758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raber E.L., Haba J., Beck P. Splenic sarcoidosis: A case report and review of the imaging findings of multiple incidental splenic lesions as the initial presentation of sarcoidosis. Can. J. Gastroenterol. 2011;25:477–478. doi: 10.1155/2011/748920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Valeyre D., Prasse A., Nunes H., Uzunhan Y., Brillet P.-Y., Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383:1155–1167. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 109.Prasse A. The diagnosis, differential diagnosis, and treatment of sarcoidosis. Deutsches Ärzteblatt Int. 2016;113:565–574. doi: 10.3238/arztebl.2016.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sweiss N.J., Salloum R., Ghandi S., Alegre M.-L., Sawaqed R., Badaracco M., Pursell K., Pitrak D., Baughman R.P., Moller D.R. Significant cd4, cd8, and cd19 lymphopenia in peripheral blood of sarcoidosis patients correlates with severe disease manifestations. PLoS ONE. 2010;5:e9088. doi: 10.1371/annotation/a75007e1-492a-4bcb-80a8-28b4d432c099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chopra A., Kalkanis A., Judson M.A. Biomarkers in sarcoidosis. Expert Rev. Clin. Immunol. 2016;12:1191–1208. doi: 10.1080/1744666X.2016.1196135. [DOI] [PubMed] [Google Scholar]
- 112.Kikuchi H., Mori T., Rai T., Uchida S. Acute kidney injury caused by sarcoid granulomatous interstitial nephritis without extrarenal manifestations. CEN Case Rep. 2015;4:212–217. doi: 10.1007/s13730-015-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tanizawa K., Handa T., Nagai S., Niimi A., Oguma T., Kubo T., Ito Y., Aihara K., Ikezoe K., Matsumoto H., et al. Comprehensive evaluation of airway involvement in pulmonary sarcoidosis. ERJ Open Res. 2017;3 doi: 10.1183/23120541.00105-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mitchell D.N., Scadding J.G., Heard B.E., Hinson K.F. Sarcoidosis: Histopathological definition and clinical diagnosis. J. Clin. Pathol. 1977;30:395–408. doi: 10.1136/jcp.30.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Akten H.S., Kilic H., Celik B., Erbas G., Isikdogan Z., Turktas H., Kokturk N. Diagnostic yield of transbronchial biopsy in comparison to high resolution computerized tomography in sarcoidosis cases. Asian Pac. J. Cancer Prev. 2018;19:1029. doi: 10.22034/APJCP.2018.19.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Navasakulpong A., Auger M., Gonzalez A.V. Yield of ebus-tbna for the diagnosis of sarcoidosis: Impact of operator and cytopathologist experience. BMJ Open Respir. Res. 2016;3:e000144. doi: 10.1136/bmjresp-2016-000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smith-Rohrberg D., Sharma S.K. Tuberculin skin test among pulmonary sarcoidosis patients with and without tuberculosis: Its utility for the screening of the two conditions in tuberculosis-endemic regions. Sarcoidosis Vasc. Diffuse Lung Dis. 2006;23:130–134. [PubMed] [Google Scholar]
- 118.Mambretti J. Chest x-ray stages of sarcoidosis. J. Insur. Med. 2004;36:91–92. [PubMed] [Google Scholar]
- 119.Avital M., Hadas-Halpern I., Deeb M., Izbicki G. Radiological findings in sarcoidosis. Isr. Med. Assoc. J. 2008;10:572–574. [PubMed] [Google Scholar]
- 120.Lynch J.P., III Computed tomographic scanning in sarcoidosis. Semin. Respir. Crit. Care Med. 2003;24:393–418. doi: 10.1055/s-2003-42375. [DOI] [PubMed] [Google Scholar]
- 121.Keijsers R.G.M., van den Heuvel D.A.F., Grutters J.C. Imaging the inflammatory activity of sarcoidosis. Eur. Respir. J. 2013;41:743–751. doi: 10.1183/09031936.00088612. [DOI] [PubMed] [Google Scholar]
- 122.Piotrowski W.J. Radiological examinations in the diagnosis and monitoring of pulmonary sarcoidosis. Polski Merkuriusz Lekarski Organ Polskiego Towarzystwa Lekarskiego. 2018;44:118–123. [PubMed] [Google Scholar]
- 123.Thunell M., Bjerle P., Stjernberg N. Ecg abnormalities in patients with sarcoidosis. Acta Med. Scand. 1983;213:115–118. doi: 10.1111/j.0954-6820.1983.tb03701.x. [DOI] [PubMed] [Google Scholar]
- 124.Błaut-Jurkowska J., Kaźnica-Wiatr M., Żygadło A., Tomkiewicz-Pająk L., Podolec P., Olszowska M. Electrocardiographic abnormalities in patients with pulmonary sarcoidosis (Rcd Code: Iii) J. Rare Cardiovasc. Dis. 2017;3:81–85. doi: 10.20418/jrcd.vol3no3.266. [DOI] [Google Scholar]
- 125.Komada T., Suzuki K., Ishiguchi H., Kawai H., Okumura T., Hirashiki A., Naganawa S. Magnetic resonance imaging of cardiac sarcoidosis: An evaluation of the cardiac segments and layers that exhibit late gadolinium enhancement. Nagoya J. Med. Sci. 2016;78:437–446. doi: 10.18999/nagjms.78.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Craig D.A., Colletti P.M., Ratto D., Gordonson J.S., Raval J.K., Sharma O.P. Mri findings in pulmonary sarcoidosis. Magn. Reson. Imaging. 1988;6:567–573. doi: 10.1016/0730-725X(88)90131-2. [DOI] [PubMed] [Google Scholar]
- 127.Strimbu K., Tavel J.A. What are biomarkers? Curr. Opin. HIV AIDS. 2010;5:463. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ramos-Casals M., Retamozo S., Siso-Almirall A., Perez-Alvarez R., Pallares L., Brito-Zeron P. Clinically-useful serum biomarkers for diagnosis and prognosis of sarcoidosis. Expert Rev. Clin. Immunol. 2019;15:391–405. doi: 10.1080/1744666X.2019.1568240. [DOI] [PubMed] [Google Scholar]
- 129.Bunting P.S., Szalai J.P., Katic M. Diagnostic aspects of angiotensin converting enzyme in pulmonary sarcoidosis. Clin. Biochem. 1987;20:213–219. doi: 10.1016/S0009-9120(87)80123-6. [DOI] [PubMed] [Google Scholar]
- 130.Ungprasert P., Carmona E.M., Crowson C.S., Matteson E.L. Diagnostic utility of angiotensin-converting enzyme in sarcoidosis: A population-based study. Lung. 2016;194:91–95. doi: 10.1007/s00408-015-9826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kahkouee S., Samadi K., Alai A., Abedini A., Rezaiian L. Serum ace level in sarcoidosis patients with typical and atypical hrct manifestation. Pol. J. Radiol. 2016;81:458–461. doi: 10.12659/PJR.897708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bargagli E., Bennett D., Maggiorelli C., Di Sipio P., Margollicci M., Bianchi N., Rottoli P. Human chitotriosidase: A sensitive biomarker of sarcoidosis. J. Clin. Immunol. 2013;33:264–270. doi: 10.1007/s10875-012-9754-4. [DOI] [PubMed] [Google Scholar]
- 133.Popević S., Šumarac Z., Jovanović D., Babić D., Stjepanović M., Jovičić S., Šobić-Šaranović D., Filipović S., Gvozdenović B., Omčikus M., et al. Verifying sarcoidosis activity: Chitotriosidase versus ace in sarcoidosis—A case-control study. J. Med Biochem. 2016;35:390–400. doi: 10.1515/jomb-2016-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bennett D., Cameli P., Lanzarone N., Carobene L., Bianchi N., Fui A., Rizzi L., Bergantini L., Cillis G., d’Alessandro M., et al. Chitotriosidase: A biomarker of activity and severity in patients with sarcoidosis. Respir. Res. 2020;21:6. doi: 10.1186/s12931-019-1263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sahin O., Ziaei A., Karaismailoglu E., Taheri N. The serum angiotensin converting enzyme and lysozyme levels in patients with ocular involvement of autoimmune and infectious diseases. BMC Ophthalmol. 2016;16:19. doi: 10.1186/s12886-016-0194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tomita H., Sato S., Matsuda R., Sugiura Y., Kawaguchi H., Niimi T., Yoshida S., Morishita M. Serum lysozyme levels and clinical features of sarcoidosis. Lung. 1999;177:161–167. doi: 10.1007/PL00007637. [DOI] [PubMed] [Google Scholar]
- 137.Prior C., Frank A., Fuchs D., Hausen A., Judmaier G., Reibnegger G., Werner E.R., Wachter H. Urinary neopterin excretion in pulmonary sarcoidosis: Correlation to clinical course of the disease. Clin. Chim. Acta. 1988;177:211–220. doi: 10.1016/0009-8981(88)90065-4. [DOI] [PubMed] [Google Scholar]
- 138.Eklund A., Blaschke E. Elevated serum neopterin levels in sarcoidosis. Lung. 1986;164:325–332. doi: 10.1007/BF02713657. [DOI] [PubMed] [Google Scholar]
- 139.Lacronique J., Auzeby A., Valeyre D., Traore B.M., Barbosa M.L., Soler P., Choudat D., Battesti J.P., Touitou Y., Marsac J. Urinary neopterin in pulmonary sarcoidosis. Relationship to clinical and biologic assessment of the disease. Am. Rev. Respir. Dis. 1989;139:1474–1478. doi: 10.1164/ajrccm/139.6.1474. [DOI] [PubMed] [Google Scholar]
- 140.Sharma O.P. Hypercalcemia in granulomatous disorders: A clinical review. Curr. Opin. Pulm. Med. 2000;6:442–447. doi: 10.1097/00063198-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 141.Ibrik O., Samon R., Roda A., Roca R., Gonzalez J.C., Viladoms J., Vilaseca J., Serrano M. Sarcoidosis: Diagnosis from the renal function and hypercalcaemia study. Nefrologia. 2011;31:371–372. doi: 10.3265/Nefrologia.pre2011.Mar.10832. [DOI] [PubMed] [Google Scholar]
- 142.Demetriou E.T., Pietras S.M., Holick M.F. Hypercalcemia and soft tissue calcification owing to sarcoidosis: The sunlight-cola connection. J. Bone Miner. Res. 2010;25:1695–1699. doi: 10.1002/jbmr.51. [DOI] [PubMed] [Google Scholar]
- 143.Grutters J.C., Fellrath J.M., Mulder L., Janssen R., van den Bosch J.M., van Velzen-Blad H. Serum soluble interleukin-2 receptor measurement in patients with sarcoidosis: A clinical evaluation. Chest. 2003;124:186–195. doi: 10.1378/chest.124.1.186. [DOI] [PubMed] [Google Scholar]
- 144.Thi Hong Nguyen C., Kambe N., Kishimoto I., Ueda-Hayakawa I., Okamoto H. Serum soluble interleukin-2 receptor level is more sensitive than angiotensin-converting enzyme or lysozyme for diagnosis of sarcoidosis and may be a marker of multiple organ involvement. J. Dermatol. 2017;44:789–797. doi: 10.1111/1346-8138.13792. [DOI] [PubMed] [Google Scholar]
- 145.Ina Y., Takada K., Sato T., Yamamoto M., Noda M., Morishita M. Soluble interleukin 2 receptors in patients with sarcoidosis. Possible origin. Chest. 1992;102:1128–1133. doi: 10.1378/chest.102.4.1128. [DOI] [PubMed] [Google Scholar]
- 146.Bargagli E., Olivieri C., Landi C., Magi B., Bennett D., Bianchi N., Perrone A., Fossi A., Rottoli P. Serum amyloid a as a potential biomarker of sarcoidosis. Eur. Respir. J. 2011;38:3807. [Google Scholar]
- 147.Chen E.S., Song Z., Willett M.H., Heine S., Yung R.C., Liu M.C., Groshong S.D., Zhang Y., Tuder R.M., Moller D.R. Serum amyloid a regulates granulomatous inflammation in sarcoidosis through toll-like receptor-2. Am. J. Respir. Crit. Care Med. 2010;181:360–373. doi: 10.1164/rccm.200905-0696OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huho A., Foulke L., Jennings T., Koutroumpakis E., Dalvi S., Chaudhry H., Chopra A., Modi A., Rane N., Prezant D.J., et al. The role of serum amyloid a staining of granulomatous tissues for the diagnosis of sarcoidosis. Respir. Med. 2017;126:1–8. doi: 10.1016/j.rmed.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 149.Boot R.G., Hollak C.E., Verhoek M., Alberts C., Jonkers R.E., Aerts J.M. Plasma chitotriosidase and ccl18 as surrogate markers for granulomatous macrophages in sarcoidosis. Clin. Chim. Acta. 2010;411:31–36. doi: 10.1016/j.cca.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 150.Cai M., Bonella F., He X., Sixt S.U., Sarria R., Guzman J., Costabel U. Ccl18 in serum, bal fluid and alveolar macrophage culture supernatant in interstitial lung diseases. Respir. Med. 2013;107:1444–1452. doi: 10.1016/j.rmed.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 151.Su R., Nguyen M.-L.T., Agarwal M.R., Kirby C., Nguyen C.P., Ramstein J., Darnell E.P., Gomez A.D., Ho M., Woodruff P.G. Interferon-inducible chemokines reflect severity and progression in sarcoidosis. Respir. Res. 2013;14:121. doi: 10.1186/1465-9921-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Miyoshi S., Hamada H., Kadowaki T., Hamaguchi N., Ito R., Irifune K., Higaki J. Comparative evaluation of serum markers in pulmonary sarcoidosis. Chest. 2010;137:1391–1397. doi: 10.1378/chest.09-1975. [DOI] [PubMed] [Google Scholar]
- 153.Ramstein J., Broos C.E., Simpson L.J., Ansel K.M., Sun S.A., Ho M.E., Woodruff P.G., Bhakta N.R., Christian L., Nguyen C.P., et al. Ifn-gamma-producing t-helper 17.1 cells are increased in sarcoidosis and are more prevalent than t-helper type 1 cells. Am. J. Respir. Crit. Care Med. 2016;193:1281–1291. doi: 10.1164/rccm.201507-1499OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Prior C., Haslam P.L. Increased levels of serum interferon-gamma in pulmonary sarcoidosis and relationship with response to corticosteroid therapy. Am. Rev. Respir. Dis. 1991;143:53–60. doi: 10.1164/ajrccm/143.1.53. [DOI] [PubMed] [Google Scholar]
- 155.Salez F., Gosset P., Copin M.C., Lamblin Degros C., Tonnel A.B., Wallaert B. Transforming growth factor-beta1 in sarcoidosis. Eur. Respir. J. 1998;12:913–919. doi: 10.1183/09031936.98.12040913. [DOI] [PubMed] [Google Scholar]
- 156.Piotrowski W.J., Kiszalkiewicz J., Pastuszak-Lewandoska D., Antczak A., Gorski P., Migdalska-Sek M., Gorski W., Czarnecka K., Nawrot E., Domanska D., et al. Tgf-beta and smads mrna expression in pulmonary sarcoidosis. Adv. Exp. Med. Biol. 2015;852:59–69. doi: 10.1007/5584_2014_106. [DOI] [PubMed] [Google Scholar]
- 157.Drent M. Sarcoidosis: Is there a role for anti-tnf-α? Rev. Port. Pneumol. 2007;13:S51–S57. doi: 10.1016/S0873-2159(15)30404-9. [DOI] [Google Scholar]
- 158.Amber K.T., Bloom R., Mrowietz U., Hertl M. Tnf-alpha: A treatment target or cause of sarcoidosis? J. Eur. Acad. Dermatol. Venereol. 2015;29:2104–2111. doi: 10.1111/jdv.13246. [DOI] [PubMed] [Google Scholar]
- 159.James W.E. Leaving history behind: Cd4/cd8 ratio as a diagnostic tool in sarcoidosis. EBioMedicine. 2016;8:20. doi: 10.1016/j.ebiom.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shen Y., Pang C., Wu Y., Li D., Wan C., Liao Z., Yang T., Chen L., Wen F. Diagnostic performance of bronchoalveolar lavage fluid cd4/cd8 ratio for sarcoidosis: A meta-analysis. EBioMedicine. 2016;8:302–308. doi: 10.1016/j.ebiom.2016.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Winterbauer R.H., Lammert J., Selland M., Wu R., Corley D., Springmeyer S.C. Bronchoalveolar lavage cell populations in the diagnosis of sarcoidosis. Chest. 1993;104:352–361. doi: 10.1378/chest.104.2.352. [DOI] [PubMed] [Google Scholar]
- 162.Drent M., Mansour K., Linssen C. Bronchoalveolar lavage in sarcoidosis. Semin. Respir. Crit. Care Med. 2007;28:486–495. doi: 10.1055/s-2007-991521. [DOI] [PubMed] [Google Scholar]
- 163.Psathakis K., Papatheodorou G., Plataki M., Panagou P., Loukides S., Siafakas N.M., Bouros D. 8-isoprostane, a marker of oxidative stress, is increased in the expired breath condensate of patients with pulmonary sarcoidosis. Chest. 2004;125:1005–1011. doi: 10.1378/chest.125.3.1005. [DOI] [PubMed] [Google Scholar]
- 164.Piotrowski W.J., Antczak A., Marczak J., Nawrocka A., Kurmanowska Z., Górski P. Eicosanoids in exhaled breath condensate and bal fluid of patients with sarcoidosis. Chest. 2007;132:589–596. doi: 10.1378/chest.07-0215. [DOI] [PubMed] [Google Scholar]
- 165.Ciarleglio G., Refini R., Pieroni M. Exhaled carbon monoxide in sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2008;25:46–50. [PubMed] [Google Scholar]
- 166.Moodley Y., Chetty R., Lalloo U. Nitric oxide levels in exhaled air and inducible nitric oxide synthase immunolocalization in pulmonary sarcoidosis. Eur. Respir. J. 1999;14:822–827. doi: 10.1034/j.1399-3003.1999.14d17.x. [DOI] [PubMed] [Google Scholar]
- 167.Wilsher M.L., Fergusson W., Milne D., Wells A.U. Exhaled nitric oxide in sarcoidosis. Thorax. 2005;60:967–970. doi: 10.1136/thx.2004.033852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Carleo A., Bennett D., Rottoli P. Biomarkers in sarcoidosis: The contribution of system biology. Curr. Opin. Pulm. Med. 2016;22:509–514. doi: 10.1097/MCP.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 169.Su R., Li M.M., Bhakta N.R., Solberg O.D., Darnell E.P., Ramstein J., Garudadri S., Ho M., Woodruff P.G., Koth L.L. Longitudinal analysis of sarcoidosis blood transcriptomic signatures and disease outcomes. Eur. Respir. J. 2014;44:985–993. doi: 10.1183/09031936.00039714. [DOI] [PubMed] [Google Scholar]
- 170.Schupp J.C., Vukmirovic M., Kaminski N., Prasse A. Transcriptome profiles in sarcoidosis and their potential role in disease prediction. Curr. Opin. Pulm. Med. 2017;23:487–492. doi: 10.1097/MCP.0000000000000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ascoli C., Huang Y., Schott C., Turturice B.A., Metwally A., Perkins D.L., Finn P.W. A circulating microrna signature serves as a diagnostic and prognostic indicator in sarcoidosis. Am. J. Respir. Cell Mol. Biol. 2018;58:40–54. doi: 10.1165/rcmb.2017-0207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kiszałkiewicz J., Piotrowski W.J., Pastuszak-Lewandoska D., Górski P., Antczak A., Górski W., Domańska-Senderowska D., Migdalska-Sęk M., Czarnecka K.H., Nawrot E. Altered mirna expression in pulmonary sarcoidosis. BMC Med. Genet. 2016;17:2. doi: 10.1186/s12881-016-0266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Geamanu A., Gupta S.V., Bauerfeld C., Samavati L. Metabolomics connects aberrant bioenergetic, transmethylation, and gut microbiota in sarcoidosis. Metab. Off. J. Metab. Soc. 2016;12:35. doi: 10.1007/s11306-015-0932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Banoei M.M., Iupe I., Bazaz R.D., Campos M., Vogel H.J., Winston B.W., Mirsaeidi M. Metabolomic and metallomic profile differences between veterans and civilians with pulmonary sarcoidosis. Sci. Rep. 2019;9:19584. doi: 10.1038/s41598-019-56174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Judson M.A., Baughman R.P., Costabel U., Mack M., Barnathan E.S. The potential additional benefit of infliximab in patients with chronic pulmonary sarcoidosis already receiving corticosteroids: A retrospective analysis from a randomized clinical trial. Respir. Med. 2014;108:189–194. doi: 10.1016/j.rmed.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 176.Pande A., Culver D.A. Knowing when to use steroids, immunosuppressants or biologics for the treatment of sarcoidosis. Expert Rev. Respir. Med. 2020;14:285–298. doi: 10.1080/17476348.2020.1707672. [DOI] [PubMed] [Google Scholar]
- 177.James W.E., Judson M.A. Therapeutic strategies for pulmonary sarcoidosis. Expert Rev. Respir. Med. 2020;14:391–403. doi: 10.1080/17476348.2020.1721284. [DOI] [PubMed] [Google Scholar]
- 178.Gibson G., Prescott R., Muers M., Middleton W., Mitchell D., Connolly C., Harrison B. British thoracic society sarcoidosis study: Effects of long term corticosteroid treatment. Thorax. 1996;51:238–247. doi: 10.1136/thx.51.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Grunewald J., Grutters J.C., Arkema E.V., Saketkoo L.A., Moller D.R., Müller-Quernheim J. Sarcoidosis. Nat. Rev. Dis. Primers. 2019;5:45. doi: 10.1038/s41572-019-0096-x. [DOI] [PubMed] [Google Scholar]
- 180.Muller-Quernheim J., Kienast K., Held M., Pfeifer S., Costabel U. Treatment of chronic sarcoidosis with an azathioprine/prednisolone regimen. Eur. Respir. J. 1999;14:1117–1122. doi: 10.1183/09031936.99.14511179. [DOI] [PubMed] [Google Scholar]
- 181.Baughman R., Winget D., Lower E. Methotrexate is steroid sparing in acute sarcoidosis: Results of a double blind, randomized trial. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG. 2000;17:60–66. [PubMed] [Google Scholar]
- 182.Brill A.-K., Ott S.R., Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: A retrospective study. Respiration. 2013;86:376–383. doi: 10.1159/000345596. [DOI] [PubMed] [Google Scholar]
- 183.Papiris S., Stagaki E., Papadaki G., Kolilekas L., Korbila I., Apollonatou V., Kallieri M., Gialafos H., Chatziioannou S., Papaioannou A.I., et al. Mycophenolate mofetil as an alternative treatment in sarcoidosis. Pulm. Pharmacol. Ther. 2019;58:101840. doi: 10.1016/j.pupt.2019.101840. [DOI] [PubMed] [Google Scholar]
- 184.Wyser C.P., van Schalkwyk E.M., Alheit B., Bardin P.G., Joubert J.R. Treatment of progressive pulmonary sarcoidosis with cyclosporin a: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 1997;156:1371–1376. doi: 10.1164/ajrccm.156.5.9506031. [DOI] [PubMed] [Google Scholar]
- 185.Korsten P., Mirsaeidi M., Sweiss N.J. Nonsteroidal therapy of sarcoidosis. Curr. Opin. Pulm. Med. 2013;19:516. doi: 10.1097/MCP.0b013e3283642ad0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Baughman R., Lower E. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG. 2004;21:43–48. doi: 10.1007/s11083-004-5178-y. [DOI] [PubMed] [Google Scholar]
- 187.Sharma O.P. Effectiveness of chloroquine and hydroxychloroquine in treating selected patients with sarcoidosis with neurological involvement. Arch. Neurol. 1998;55:1248–1254. doi: 10.1001/archneur.55.9.1248. [DOI] [PubMed] [Google Scholar]
- 188.Saketkoo L.A., Baughman R.P. Biologic therapies in the treatment of sarcoidosis. Expert Rev. Clin. Immunol. 2016;12:817–825. doi: 10.1080/1744666X.2016.1175301. [DOI] [PubMed] [Google Scholar]
- 189.Muller-Quernheim J. Sarcoidosis: Immunopathogenetic concepts and their clinical application. Eur. Respir. J. 1998;12:716–738. doi: 10.1183/09031936.98.12030716. [DOI] [PubMed] [Google Scholar]
- 190.Hostettler K.E., Studler U., Tamm M., Brutsche M.H. Long-term treatment with infliximab in patients with sarcoidosis. Respiration. 2012;83:218–224. doi: 10.1159/000328738. [DOI] [PubMed] [Google Scholar]
- 191.Baughman R.P., Drent M., Kavuru M., Judson M.A., Costabel U., du Bois R., Albera C., Brutsche M., Davis G., Donohue J.F. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am. J. Respir. Crit. Care Med. 2006;174:795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 192.Loza M.J., Brodmerkel C., Du Bois R.M., Judson M.A., Costabel U., Drent M., Kavuru M., Flavin S., Lo K.H., Barnathan E.S. Inflammatory profile and response to anti-tumor necrosis factor therapy in patients with chronic pulmonary sarcoidosis. Clin. Vaccine Immunol. 2011;18:931–939. doi: 10.1128/CVI.00337-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Judson M.A., Baughman R.P., Costabel U., Drent M., Gibson K.F., Raghu G., Shigemitsu H., Barney J.B., Culver D.A., Hamzeh N.Y. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur. Respir. J. 2014;44:1296–1307. doi: 10.1183/09031936.00000914. [DOI] [PubMed] [Google Scholar]
- 194.Carmona E.M., Kalra S., Ryu J.H. Pulmonary sarcoidosis: Diagnosis and treatment. Mayo Clin. Proc. 2016;91:946–954. doi: 10.1016/j.mayocp.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 195.Sauer W.H., Stern B.J., Baughman R.P., Culver D.A., Royal W. High-risk sarcoidosis. Current concepts and research imperatives. Ann. Am. Thorac. Soc. 2017;14:S437–S444. doi: 10.1513/AnnalsATS.201707-566OT. [DOI] [PubMed] [Google Scholar]
- 196.Ahmadzai H., Huang S., Steinfort C., Markos J., Allen R.K., Wakefield D., Wilsher M., Thomas P.S. Sarcoidosis: A state of the art review from the thoracic society of australia and new zealand. Med. J. Aust. 2018;208:499–504. doi: 10.5694/mja17.00610. [DOI] [PubMed] [Google Scholar]
- 197.Baughman R.P., Grutters J.C. New treatment strategies for pulmonary sarcoidosis: Antimetabolites, biological drugs, and other treatment approaches. Lancet Respir. Med. 2015;3:813–822. doi: 10.1016/S2213-2600(15)00199-X. [DOI] [PubMed] [Google Scholar]
- 198.Dastoori M., Fedele S., Leao J.C., Porter S.R. Sarcoidosis—A clinically orientated review. J. Oral Pathol. Med. 2013;42:281–289. doi: 10.1111/j.1600-0714.2012.01198.x. [DOI] [PubMed] [Google Scholar]
- 199.Landi C., Carleo A., Cillis G., Rottoli P. Sarcoidosis: Proteomics and new perspectives for improving personalized medicine. Expert Rev. Proteom. 2018;15:829–835. doi: 10.1080/14789450.2018.1528148. [DOI] [PubMed] [Google Scholar]
- 200.Gligorijevic V., Malod-Dognin N., Przulj N. Integrative methods for analyzing big data in precision medicine. Proteomics. 2016;16:741–758. doi: 10.1002/pmic.201500396. [DOI] [PubMed] [Google Scholar]
- 201.Hočevar K., Maver A., Kunej T., Peterlin B. Sarcoidosis related novel candidate genes identified by multi-omics integrative analyses. Omics J. Integr. Biol. 2018;22:322–331. doi: 10.1089/omi.2018.0027. [DOI] [PubMed] [Google Scholar]