Abstract
Liver transplantation is increasingly dependent on the use of extended criteria donors (ECD) to increase the organ donor pool and address rising demand. This has necessitated the adoption of innovative technologies and strategies to protect these higher-risk grafts from the deleterious effects of traditional preservation and ischaemia reperfusion injury (IRI). The advent of normothermic machine perfusion (NMP) and rapid growth in the clinical adoption of this technology has accelerated efforts to utilise NMP as a platform for therapeutic intervention to optimise donor livers. In this review we will explore the emerging preclinical data related to ameliorating the effects of IRI, protecting the microcirculation and reducing the immunogenicity of donor organs during NMP. Exploiting the window of opportunity afforded by NMP, whereby the liver can be continuously supported and functionally assessed while therapies are directly delivered during the preservation period, has clear logistical and theoretical advantages over current preservation methods. The clinical translation of many of the therapeutic agents and strategies we will describe is becoming more feasible with widespread adaptation of NMP devices and rapid advances in molecular biology and gene therapy, which have substantially improved the performance of these agents. The delivery of novel therapeutics during NMP represents one of the new frontiers in transplantation research and offers real potential for successfully tackling fundamental challenges in transplantation such as IRI.
Keywords: normothermic machine perfusion, therapeutics, liver transplantation, immunomodulation, ischaemia reperfusion injury, microcirculation, organ reconditioning, steatosis, de-fatting
1. Introduction
Liver transplantation is the only successful treatment option for many patients with end-stage liver disease. With liver disease-related mortality rising globally [1], the demand for suitable organs for transplantation is set to continue to increase. Conventionally, donor livers are preserved at 4 °C in static cold storage (SCS) prior to implantation, but if prolonged, this can be harmful, particularly if the organ is of marginal quality or deemed ‘high-risk’ owing to donor characteristics. These extended criteria donor (ECD) livers typically come from older, higher body mass index (BMI), medically co-morbid donors and donors after circulatory death (DCD). Consequently, the livers are at increased risk of primary non-function (PNF), early allograft dysfunction (EAD) and post-reperfusion syndrome (PRS), and are associated with greater complications such as non-anastomotic biliary strictures and ischaemic cholangiopathy [2,3]. The underlying mechanism or pathological process driving these complications is the increased susceptibility of ECD livers to severe ischaemia-reperfusion injury (IRI) [4,5] which is itself exacerbated by prolonged cold storage. As such, ECDs have been limited in use to situations where preservation times, in particular cold ischaemia times (CIT), are very short and no other suitable donor options exist [6,7,8].
2. Normothermic Machine Perfusion of the Liver
Alternative preservation strategies have been developed for livers, lungs, hearts and kidneys to minimise the deleterious effects of SCS and institute protective measures during preservation using machine perfusion [9,10,11,12]. In liver transplantation, these technologies have rapidly expanded with multiple devices and systems routinely being used clinically. Normothermic machine perfusion (NMP) has proven to be an exciting technology, with international multicentre randomized control trial data demonstrating its ability to reduce graft injury, prolong preservation and increase utilisation [13,14]. Further studies have reported on sequential SCS and NMP with comparable clinical outcomes to continuous NMP alone, providing robust clinical data in support of the more logistically straightforward ‘back to base model’ of liver NMP [15]. Although improved utilisation has been demonstrated and viability assessment protocols successfully established (by the Cambridge and Birmingham UK Groups) in ECD livers, NMP alone may not be sufficient to improve clinical outcomes [16,17]. As a result, multiple groups are now working on the use of NMP as a platform for delivering novel therapeutics to recondition, repair and optimise organs for transplant. In a recent landmark study by the Zürich group, Eshmuminov and colleagues were able to normothermically preserve initially porcine, then human, discarded livers for 7 days ex-situ, maintaining physiological parameters [18]. Although this study did not have a transplant recovery model and the discarded human livers were heterogenous therefore impacting the outcomes, the establishment of prolonged preservation is potentially transformative for the field, particularly in the area of therapeutic delivery during liver NMP.
The pre-clinical studies that have provided much of the evidence reviewed in this article are predominantly animal models and discarded human liver studies, which, although they provide excellent experimental models with a strong track record of clinical translation, do have several weaknesses. Animal models of ex-vivo perfusion are not exempt from the drawbacks of other animal studies, including genetic, physiological and immunological differences from human organs, limiting our ability to extrapolate results. Furthermore, transplantation animal models often require more animals, recovery of animals and carry the risk of procedure-related complications, therefore coming into conflict with the 3Rs (Replacement, Reduction, and Refinement) principles of preclinical research [19], when in some cases this can be avoided. To overcome these concerns, some groups have focused on discarded human livers, typically procured with the intent of transplantation but deemed not suitable for clinical use [20]. These organs provide valuable insights but vary dramatically in key characteristics (e.g., preservation times, malignancy, fibrosis and steatosis), many of which can directly impact the parameters being investigated. As a result, a combination of large animal transplant models and discarded human liver studies is increasingly being adopted as the optimal translational approach [18].
Transplantation is unique in that to some extent clinicians have precise timings for key stages of the process, such as donation (cold flush/cross-clamp), preservation, reperfusion and timing of donor graft exposure to the recipient immune system. This permits accurate prediction of re-infection/transfection, allorecognition and tumour seeding; thus, the modification of graft during preservation or in anticipation of these events has become logistically feasible and may dramatically alter the outcomes for transplant recipients.
In this review, we explore the role of NMP of the liver in the delivery of therapeutic agents with the ultimate aim of improving transplant outcomes.
3. Ischaemia Reperfusion Injury
Ischaemia reperfusion injury (IRI) remains a necessary component of solid organ transplantation and involves a surge in pro-inflammatory cytokines such as interleukin-1 (IL-1) & interleukin-6 (IL-6), tumour necrosis factor (TNF-α), increased reactive oxygen species (ROS), neutrophil infiltration and complement activation in response to the reperfusion of a previously ischaemic organ, ultimately resulting in apoptosis and necrosis [21,22]. Developing methods to mitigate these effects of IRI have been the subject of intense investigation in the field of transplantation for decades, as the severity of this process has been associated with higher discard rates of organs.
Gene Silencing with RNAi
Novel agents are increasingly being used to target the pathways of IRI including RNA interference (RNAi). This naturally-occurring mechanism for gene expression downregulation by specific targeting of messenger RNA (mRNA) transcripts [23] is being utilised in the context of liver transplantation to influence IRI-related pathways. Since the discovery of RNAi in 1998, it has opened up new opportunities for specific therapeutic targeting of genes involved in processes of interest and was subject the Nobel Prize in Medicine in 2006 owing to the multitude of potential applications. Pre-clinical work in liver transplantation has provided proof of concept, with Li et al. 2007 using small interfering RNA (siRNA) targeting the Fas gene and Wu et al. 2016 using short hairpin RNA (shRNA) to target interleukin-1 receptor-associated kinase-4 (IRAK 4) in rat models of liver transplantation, demonstrating improvements in markers of hepatocellular damage associated with reduced pro-inflammatory signalling via the interference of the specifically targeted pathways in both studies [24,25]. However, the successful translation of RNAi to clinical transplantation has been limited by costs, perceived risks of delivering the medication systemically and chemical structural challenges related to uptake, stability and selectivity of the therapy. Many of these hurdles are being overcome by a number of different technical advances comprehensively outlined in a recent review by Thijsesen et al., 2019 [26]. The same authors have also described the innovative lipid-based nanoparticle delivery of siRNA to genetically target livers during ex-vivo NMP in a rat model. They have been able to silence the antiapoptotic gene p53 in an in-vivo IRI model of clamping the hepatic inflow as well as demonstrate the feasibility of utilising NMP as a platform for delivering their lipid nanoparticle loaded with siRNA [27]. Interventions described in this review are predicated on normothermia; however, interesting work in this therapeutic class and others has yielded results suggesting that normothermic delivery may not have an effect on the ability to deliver these therapies, opening the possibility of incorporating these therapies into standard cold organ preservation solutions [28]. Beal and colleagues have also explored the effects of delivering an opioid agonist during NMP in a small animal model and found evidence of protection against hypoxic stress and improved mitochondrial function (Table 1).
Table 1.
Ischaemia Reperfusion Injury.
| Study | Model | N | Preservation Details/Groups | Length of NMP | Therapeutic Agent(s) | Outcome |
|---|---|---|---|---|---|---|
| Gillooly et al. 2019 [29] | Rat | N/A | HMP ± siRNA, NMP ± SiRNA, |
4 h | Fas-short interfering RNA (siRNA) | Diffuse uptake of siRNA in both NMP and HMP groups, with increased uptake in the latter. |
| Beal et al. 2019 [30] | Rat | 6 per group | NMP, NMP + Enkephalin |
4 h | Enkephalin | Reduced hepatocyte oxidative stress and mitochondrial dysfunction via opioid receptor signalling. |
| Moore et al. 2017 [27] | Rat | N/A | NMP | 6 h | p53 siRNA-cy3 | Positive fluorescence for cy3 detected in NMP livers. |
HMP: Hypothermic Machine Perfusion; siRNA: small interfering ribonucleic acid; Enkephalin: D-Ala2, D-Leu5 (DADLE); cy3-dye.
4. Immunomodulation
Following the Consortium for Organ Preservation in Europe (COPE) international clinical trial of NMP for liver transplantation, we found that immune-mediated processes were found to be positively impacted by NMP (e.g., reduced PRS in NMP cohort) corroborating pre-clinical work previously conducted within the group [12,14,31]. Furthermore, subsequent clinical studies exploring the use of NMP following longer periods of cold preservation in both North America and Europe have shown favourable outcomes for NMP with respect to leucocytosis and inflammatory cell infiltrates histologically [15,32]. Mechanistically, this has been explored by Jassem et al. (2018), where the group found that the proportion of tissue-resident CD4posCD25highCD127negFOXP3pos regulatory T cells (Treg) seems to increase through the course of NMP, with more interferon-gamma (IFN-γ) and interleukin 17-producing T cells present and less necrosis and apoptosis in the liver parenchyma, associated with less neutrophil infiltration compared to SCS livers [33]. The immune effects of NMP observed through clinical measures such as PRS in the aforementioned clinical studies must, however, be interpreted carefully, as immunophenotyping and advance immune cell analysis of the samples is yet to be reported.
4.1. Cell Therapy during NMP
Cellular therapies (e.g., regulatory T cells, tolerogenic dendritic cells, mesenchymal stem cells) and cell-derived products (e.g., extracellular vesicles) are used in organ transplantation with immunomodulatory intent and typically delivered systemically with a well-established safety record [34,35,36]. In liver transplantation, Todo et al. 2016 published the results of a pilot that have accelerated the rate of progress in the field, where 7/10 of the patients treated with a regulatory T-cell (Treg)-based cellular therapy with tolerogenic intent were successfully weaned off all immunosuppression following living donor liver transplant (LDLT), with 16–33 months of rejection-free graft survival [37]. However, challenges remain: the delivery of these cells to the target organ remains problematic, reproducibility is a concern owing to the lack of standardisation of manufacturing processes, the paucity of good manufacturing practice (GMP) facilities and prohibitive costs are all holding back progress. Furthermore, they are largely limited to the living donor pool of recipients, which in the majority of Europe and North America remains a small proportion of overall liver transplant activity [38]. The intrinsic benefits of NMP in cellular therapy delivery are therefore intuitive; the ex-vivo circuit offers the opportunity to directly deliver cells to the liver, using lower doses (cell numbers) will reduce costs and expand therapies to deceased donor receipts. Furthermore, ECDs form a substantial and increasing portion of deceased donor organs and therefore stand to benefit most from these interventions and therapies.
4.2. Extra-Cellular Vesicles (EVs) during NMP
Mesenchymal stem cells (MSCs) are immunomodulatory cells that have received widespread attention owing to their purported capacity to alter immune responses favourably in the context of solid organ transplantation [39,40]. Their therapeutic function occurs via paracrine effects related to extracellular vesicles (MSC-EVs) released by the cells. The MSC-EVs are small vesicles released by MSCs that deliver small proteins, microRNA (miRNA), mRNA and even DNA, which exert an effect on target tissues [41]. In the lung, where the majority of the pre-clinical data exists for the use of MSC-EVs, investigators have been able to provide data supporting their efficacy in rodent IRI models. Stone and colleagues assessed the effect of MSC-EVs under both in-situ and ex-situ IRI conditions, yielding mechanistic information on the reduction of pro-inflammatory cytokines and an increase in protective cytokines [42]. This is in keeping with the findings of Lonati and colleagues, who were able to ameliorate the effects of IRI in rat lungs and demonstrate an significant alteration in gene expression related to EV treatment [43]. Overall, a limited number of studies have reported on the use of MSC-EVs during machine perfusion in the various organ systems [42,44]; however, the first group to deliver these cells or cell-derived products to livers during NMP MSC-EVs was Rigo et al. The authors used a rat liver NMP model, perfusing grafts for 4 h and delivering human liver stem-like cell (HLSC)-derived EVs, and assessed uptake within the liver and their effect on hypoxic tissue injury (the latter was induced by suboptimal oxygenation during NMP). They were able to show reduced hepatocellular damage histologically as well as reduced markers of hepatic cytolysis in the NMP perfusate while demonstrating engraftment of the delivered cell product. A limitation of this study was the lack of precision associated with “suboptimal oxygenation” during NMP, which therefore reduced the study’s validity as a model of IRI. Recently, Dondossola and colleagues have addressed this by describing a model of rat liver NMP using washed human red blood cells as an oxygen carrier to maintain optimal oxygenation during NMP and allow direct evaluation of IRI interventions during rat liver NMP in a controlled manner [45]. Although promising, the clinical application of EVs faces numerous challenges, including the standardization of isolation, qualification, characterisation and large-scale clinical-grade production of EVs, as is the case for many novel cell-based therapies.
4.3. Tolerance
Immunomodulation of grafts during NMP for transplant tolerance is an emerging area of interest, with many groups, including our own, working on this specific area. Ling Yee Chin et al. 2019 reported on a set of experiments where, during a 6 h rat liver perfusion model, they delivered a genetically engineered rat fibroblast cell suspension. They were able to engraft these cells in the donor liver while ensuring the cells were viable and the NMP remained otherwise unaffected [46].
Although progress is being made in this area, many of the studies are limited by the lack of a transplant model and the translational limitations of small animal models, and few studies are able to provide any mechanistic evidence for observed effects.
5. Microcirculation and Endothelial Protection
DCD liver transplantation is increasingly common, as with optimised protocols and novel technologies being employed, outcomes have dramatically improved [3,7,8]. These approaches have focused on ways of protecting DCD grafts from the effects of IRI on the microcirculation of the liver, where sinusoidal endothelial cells (SEC) are particularly vulnerable to IRI. During the warm ischaemic phase of DCD procurements, hypo-oxygenation results in rapid depletion of adenosine triphosphate (ATP), mitochondrial stress, activation of Kupffer cells and endothelial dysfunction as SECs undergo necrosis and apoptosis resulting in microcirculatory disturbance [4,5]. NMP has been an effective strategy in mitigating some of these effects, with extensive pre-clinical [47,48] and clinical work [17,49] demonstrating its benefits. Goldarecena et al. have sought to investigate approaches that may demonstrate additional benefit beyond NMP through the delivery of a combination of anti-inflammatory agents and other strategies employable during machine perfusion. They used a porcine liver transplant model, comparing NMP alone with a sub-normothermic machine perfusion (33 °C) intervention group within which the anti-inflammatory medications were delivered (Table 2) [50]. Although the integration of multiple combinations of interventions made it difficult to determine which agents were the most effective and in which combination, it proved some evidence that these agents could ameliorate the effects of IRI and therefore have a role in therapeutic interventions during NMP.
Table 2.
Microcirculation protection. HBD: Heart-beating donor; UNHBD: Uncontrolled non-heart-beating donor; SNMP: sub-normothermic machine perfusion.
| Study | Model | N | Perfusion Details/Groups | Length of NMP | Therapeutic Agent(s) | Outcome |
|---|---|---|---|---|---|---|
| Hara et al. 2013 [51] | Rat (Reperfusion) |
5 per group | HBD (SCS), UNHBD + NMP, UNHBD + NMP +PGE1 |
30 min | Prostaglandin E1 (PGE1) | Improved mitochondrial function and reduced inflammatory cytokines in NMP + PGE1 group |
| Goldaracena et al. 2016 [50] | Porcine (Transplant) |
5 per group | NMP, SNMP + anti-inflammatory agents, HBD (SCS) |
4 h | Anti-inflammatory additives: BQ123, prostaglandin E1, Acetylcystine, prostacycline, gas composition 95% O2 and 5% CO2 | Significantly lower markers of hepatocellular damage in NMP groups, Improved endothelial (microcirulatory) function |
6. Microbial Transmission
The process of transplantation carries a risk of microbial transmission from donor to recipient and re-infection of graft following reperfusion [52]. NMP may provide an opportunity to minimise this. The use of antibiotics during NMP of the liver is well established and has been a part of our group’s clinical protocol [12]; however, this not true of all organs, for example, the kidney [11], where no antimicrobial therapies given during perfusion and infective complications have been reported [53].
Hepatitis C Virus (HCV) has previously been the leading indication in North America for liver transplantation until very recently, having been surpassed by alcohol-related liver disease (ARLD) and non-alcoholic fatty liver disease (NAFLD) due to the advent of improved treatments for HCV. It remains an important condition globally for the development of end-stage liver disease and the consequent requirement for liver transplantation [54]. HCV is associated with high re-infection of the graft following reperfusion and the development of cirrhosis due to aggressive recurrence of HCV, and even in the era of direct-acting antiviral agents (DAAs), there is no consensus on how best to avoid it [55]. The Toronto group have investigated the use of Miravirsen in the induction of resistance to re-infection of livers for transplant during NMP. Miravirsen or antisense miRNA is a locked nucleic acid antisense oligonucleotide which inactivated miRNA-122, a critical factor in the hepatic replication of HCV. In this proof of concept study, Miravirsen was delivered to porcine livers during NMP vs. SCS, and they were able to demonstrate increased functional miRNA-122 sequestration, higher target gene suppression and enhanced absorption [56]. Although this was a key study in the development of NMP as a platform for therapeutic intervention, there were some limitations, including the lack of a porcine HCV model, resulting in an inability to directly assess the efficacy of the intervention and limiting the generalisability of the findings.
7. Hepatic Steatosis and Implications on Liver Transplantation
In efforts to increase the donor pool, more “marginal” donor livers are being transplanted [57]. These include livers with substantial intra-cellular fat deposition (steatosis). Steatosis results from altered metabolism of fatty acids within hepatocytes and is characterised by intracytoplasmic accumulation of triacylglycerol (TG) in the form of lipid droplets (LDs) [58]. Large intracystoplasmic LDs cause peripheral displacement of the cell nucleus resulting in macrovesicular steatosis (MaS). Livers with evidence of MaS are much more susceptible to ischaemia reperfusion injury (IRI) during conventional cold storage of the organ, termed static cold storage (SCS). The consequent organ injury is attributed to impaired microcirculation, reduced mitochondrial function and excessive inflammatory response, and is associated with subsequent poor post-transplant outcomes [59].
A recent systematic review concluded that livers with moderate to severe steatosis (more than 30% MaS) are associated with primary non-function and increased graft loss of 71% (RR 1.71, p = 0.007), and such high-risk organs are frequently declined for transplantation [60]. As a result, 1000 steatotic livers are retrieved but discarded each year in the USA [61]; in the UK, 39% of liver discards are due to steatosis [62].
The incidence of hepatic steatosis, which is commonly associated with obesity, is increasing and currently affects 33% of the UK population [63,64]. Increasing obesity in the population is reflected in deceased organ donors; those with a BMI > 30 kg/m2 have increased from 16% to 26% in the last decade [64]. Steatosis in donor livers is inevitably increasing, and methods to render these organs suitable for transplantation are urgently needed. Recently, the benefits of NMP have been described (Table 3) [14,17,65,66].
Table 3.
Normothermic machine perfusion (NMP) in liver transplantation.
| Key Benefits: |
|---|
| (i) Allows recovery from acute injury (hypoxia) sustained prior to or during retrieval [65]; |
| (ii) Permits objective assessment of organ function prior to transplantation: a number of studies have shown that this enables identification of organs in the ‘high-risk’ category that can safely be transplanted [14,17,66]; |
| (iii) Enables extended preservation times (up to 24 h) [14] |
| (iv) Provides the opportunity for therapeutic intervention to a functioning organ before it is transplanted. |
7.1. NMP, Hepatic Steatosis and Pre-Clinical Animal Studies
Steatotic livers constitute the largest individual cohort of organs which might benefit from active intervention during NMP [67,68,69,70,71]. Pre-clinical models demonstrate that ex-situ liver function can be enhanced by NMP, and hepatic triglyceride content can be reduced by enhancing intracellular lipid metabolism and potentiating the reversal of steatosis [67,68].
Jamieson et al. [67] investigated the effect of NMP alone on steatotic porcine livers during 48 h perfusions. Hepatic steatosis was induced by pre-treatment with streptozotocin together with a high fat diet to facilitate hyperglycaemia and a ketotic state prior to organ procurement for the NMP experiments. This study demonstrated that steatotic porcine livers were able to maintain perfusate base excess, factor V and bile production during NMP. In addition, the steatotic livers demonstrated comparable haemodynamics and markers of liver injury to lean controls. Importantly, these livers had higher perfusate glucose and urea levels. Overall, there was a reduction in MaS from 28% to 15% with reduction in lipid droplet size at the end of preservation without any defatting adjunct therapy.
Nagarth et al. [68] developed an experimental oxygenated normothermic model investigating the effect of a “defatting cocktail” on steatotic livers procured from Zucker rats over 3 h perfusions. The “defatting cocktail” consisted of a combination of pharmacological agents, including the peroxisome proliferator-activated receptor delta (PPARδ) ligand GW501516, the peroxisome proliferator-activated receptor alpha (PPARα) ligand GW7647, the cyclic adenosine monophosphate (cAMP) activator forskolin, the pregnane X receptor ligand hypericin, the insulin-mimetic adipokine visfatin and the constitutive androstane receptor ligand scorparone (see Table 4). In combination with NMP, there was a reduction in hepatic triglyceride content of 65% and intracellular lipid content by 50% with increased hepatic lipid metabolism. However, a 30% reduction in hepatic triglyceride was seen in the control perfusate with no defatting agents.
Table 4.
Defatting agents.
| Defatting Agent | Function |
|---|---|
| PPARδ ligand GW501516 | Increase fatty acid β-oxidation |
| Peroxisome proliferator-activated receptor (PPAR) α ligand GW7647 | Increase mitochondrial fatty acid oxidation |
| Cyclic adenosine monophosphate (cAMP) activator forskolin | A glucagon mimetic cAMP activator, increases lipolysis and fatty acid oxidation |
| Pregnane X receptor ligand hypericin | Increase β-oxidation (very long chain fatty acids) |
| Visfatin | An insulin-memetic adipokine, role not fully understood |
| Scorparone | An androstane receptor ligand, upregulates PPAR |
Defatting agents and mechanism of action in amelioration of hepatic steatosis.
Raigani et al. [72] demonstrated similar results using an addition of l-carnitine (to increase fatty acid β-oxidation) to the “defatting cocktail”. The overall reduction of MaS was 41.5% to 8.5% following deffating interventions. In addition, there was an increase in perfusate ketone content (a marker of fatty acid β-oxidation), bile bicarbonate content and lactate clearance in steatotic livers that received the interventions.
These pre-clinical animal studies have provided an insight into the potential of NMP as a platform to provide active intervention to treat donor hepatic steatosis. They provide evidence that both hepatic triglyceride content and MaS can be reduced during ex-situ perseveration. However, these studies include a small sample size of homogenous livers in which steatosis has been induced as part of the experimental design. Therefore, there is a risk of translational bias if results are applied to clinical practice in a heterogenous cohort of steatotic human livers procured for transplantation. Overall, the benefits of defatting agents in animal studies appear conflicting; NMP alone has been shown to reduce hepatic triglyceride content with improved liver function comparable with lean counterparts. The effect of defatting agents during NMP would be better investigated in a discarded steatotic human liver model (Table 5).
Table 5.
Summary of defatting interventions and experimental design.
| Ref. | Defatting Interventions | Model | Total Ex-Situ Perfusion Time (h) | Percentage (%) Reduction in Macrosteatosis (MaS) | Main Outcomes |
|---|---|---|---|---|---|
| Jamieson et al. 2011 [67] | NMP alone | Porcine | 48 | 13 | Reduction in hepatic triglyceride content of 31% and markers of hepatocyte injury comparable to lean counterparts |
| Nagarth et al. 2009 [68] | GW501516, GW7647, forskolin, hypericin, visfatin and scorparone | Zucker rats | 3 | 50 | Reduction in hepatic triglyceride content of 65% Increased hepatic lipid metabolism |
| Raigani et al. 2019 [72] | GW501516, GW7647, forskolin, hypericin, visfatin, scorparone and L-carnitine | Zucker rats | 6 | 33 | Hepatic triglyceride content not reported Increased perfusate ketone content, bile bicarbonate content and lactate clearance |
| Boteon et al. 2019 [69] | GW501516, GW7647, forskolin, hypericin, visfatin, scorparone and L-carnitine | Discarded human livers | 6 12 |
40 50 |
Reduction in hepatic triglyceride level of 38% at 6 h and 30% at 12 h Increased hepatic lipid metabolism, improved metabolic liver function, reduced vascular resistance and reduced markers of hepatocyte injury Reduced immune cell activation and release of inflammatory cytokines |
Outcomes following defatting interventions, including percentage reduction in steatosis, metabolic and synthetic liver function.
7.2. NMP, Hepatic Steatosis and Discarded Human Livers
Although animal models have demonstrated the benefit of NMP in hepatic lipid metabolism, a recent study involving NMP alone of human steatotic livers subjected to 24 h perfusions did not demonstrate a reduction in macrosteatosis [73]. However, Ceresa et al. have recently explored the effect of NMP on steatotic human livers and observed clear differences in fat metabolism during preservation compared to lean livers, with greater mobilisation of TG and mitochondrial fatty acid β-oxidation. However, the degree of MaS did not change significantly during NMP when compared to SCS [71]. In a recent trial, previously declined livers were perfused, and those meeting pre-defined functional criteria were transplanted: 22 of 31 perfused organs were transplanted, all with immediate function. Notably, of the livers that did not meet viability criteria, a large proportion had evidence of moderate to severe steatosis and were therefore not transplanted [74]. These data suggest that steatotic human livers require active intervention beyond that of simply replacing SCS with NMP.
Boteon et al. [69] have demonstrated the potential of a defatting strategy in organs retrieved for clinical transplantation but discarded due to advanced steatosis using a “defatting cocktail” described in pre-clinical animal studies (Table 4) with the addition of l-carnitine during NMP. Using groups of five steatotic human livers, pharmacological defatting was associated with improved metabolic function, reduced vascular resistance, lower levels of liver injury and increased bile production. There was evidence of reduction in markers of oxidative injury, immune cell activation, release of inflammatory cytokines and tissue triglycerides. There was a reduction of MaS by 40% at 6 h of perfusion (Table 5).
Interestingly, the authors demonstrated that five livers that received defatting therapies were able to achieve viability criteria for transplantation compared to two in the control group (p = 0.04). However, not all treated livers that meet the viability criteria achieved MaS of <30% [69]. This would suggest that evidence MaS of <30% following intervention on NMP may not be a necessary criterion to proceed with transplantation. In fact, the results would suggest that organ recovery during NMP could be a separate process to defatting. Thereby, activation of cytoprotective and vasoprotective pathways might be what is required to render such organs suitable for transplantation [75].
Although Boteon et al. [69] demonstrated metrics that would have rendered the organs transplantable, further evaluation of the safety profile of the proposed “defatting cocktail” is required prior to approval for human use and translation of findings. Currently, many of the agents included in the “defatting cocktail” lack important safety data (although there is some cytotoxicity reported in vitro) [76]. Hypericin is a component of St John’s Wort that is involved in up-regulation of the cytochrome P450 3A4 enzyme [77]. This enzyme is involved in the metabolism of medications including cyclosporine and tacrolimus. In addition, the peroxisome proliferator-activated receptor agonists GW501516 and GW7647 have not been tested in human trials and concern has been raised regarding carcinogenesis in preliminary animal studies [78].
8. Conclusions and Future Perspectives
The recent studies of ex-situ defatting in discarded human livers have demonstrated a “proof of concept”, and if these were translated into clinical practice, it would significantly increase the number of safely transplantable high-risk donor organs. Overall, further research would provide a platform for therapeutic ex-situ organ optimisation with novel agents to increase organ utilisation, reduce waiting list deaths and address implications of the global obesity crisis in the context of liver transplantation.
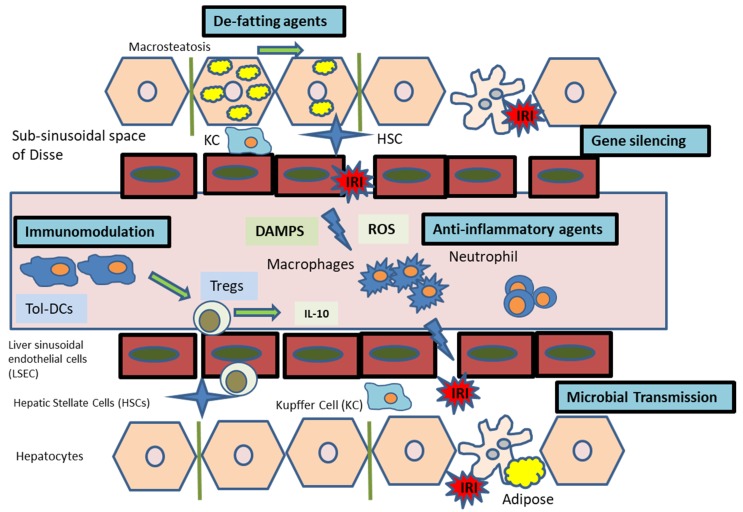
We have reviewed the rapidly evolving field of therapeutic delivery during normothermic machine perfusion for liver transplantation (Figure 1). Technological advances in perfusion devices coupled with advances in molecular medicine and development of novel therapeutics have propelled this to the forefront of transplant research. To date, the research has focused on pre-clinical perfusion models, and we expect to see many of these therapies progress to phase I/II studies as acceptance increases.
Figure 1.
Overview of interventions during NMP for IRI.
Abbreviations
| °C | Degrees Celsius |
| % | Percent |
| 3R | Reduction, Refinement and Replacement |
| ARLD | Alcohol-related liver disease |
| ATP | Adenosine triphosphate |
| BMI | Body mass index |
| BQ-123 | D-tryptamine-D-aspartic acid-L-proline-D-valine-L-leucine |
| cAMP | Cyclic adenosine monophosphate |
| CD4 | Cluster of differentiation 4 |
| CD25 | Cluster of differentiation 25 |
| CD127 | Cluster of differentiation 127 |
| CIT | Cold ischaemia time |
| CO2 | Carbon dioxide |
| COPE | Consortium for Organ Preservation in Europe |
| Cy3 | Cyanine Cy3 |
| DAA | Direct-acting antivirals |
| DCD | Donors after circulatory death |
| DNA | Deoxyribonucleic acid |
| EAD | Early allograft dysfunction |
| ECD | Extended criteria donor |
| FOXP3 | Forkhead box P3 |
| GMP | Good manufacturing practice |
| GW7647 | 2-(4-(2-(1-Cyclohexanebutyl)-3-cyclohexylureido)ethyl)-phenyl-thio)-2-methyl-propionic acid |
| GW501516 | 2-[2-Methyl-4-[[[4-methyl-2-[4-(trifluoromethyl)phenyl]-5-thiazolyl]methyl]thio]phenoxy]-acetic acid |
| h | Hour/hours |
| HBD | Heart-beating donor |
| HCV | Hepatitis C virus |
| HLSC | Human liver stem-like cells |
| HMP | Hypothermic machine perfusion |
| IFN-γ | Interferon gamma |
| IL-1 | Interleukin 1 |
| IL-6 | Interleukin 6 |
| IL-17 | Interleukin 17 |
| IRAK-4 | Interleukin-1 receptor-associated kinase 4 |
| IRI | Ischaemia reperfusion injury |
| kg/m2 | Kilogram per square meter |
| LD | Lipid droplet |
| LDLT | Living donor liver transplant |
| MaS | Macrovesicular steatosis |
| mRNA | Messenger RNA |
| miRNA | MicroRNA |
| MSC | Mesenchymal stem cells |
| MSC-EV | Extracellular vesicles of mesenchymal stem cells |
| NAFLD | Non-alcoholic fatty liver disease |
| NMP | Normothermic machine perfusion |
| O2 | Oxygen |
| PGE1 | Prostaglandin E1 |
| PNF | Primary non-function |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| PPARδ | Peroxisome proliferator-activated receptor delta |
| PRS | Post reperfusion syndrome |
| RNA | Ribonucleic acid |
| RNAi | RNA interference |
| ROS | Reactive oxygen species |
| SCS | Static cold storage |
| SEC | Sinusoidal endothelial cell |
| shRNA | Short hairpin RNA |
| siRNA | Small interfering RNA |
| SNMP | Sub-normothermic machine perfusion |
| TG | Triacylglycerol |
| TNF-α | Tumour necrosis factor alpha |
| Treg | Regulatory T cell |
| UNHBD | Uncontrolled non-heart-beating donor |
Author Contributions
Writing—Original Draft Preparation, F.D. and S.H.A.; Writing—Review and Editing, G.E. and D.N.; Supervision, D.N.; Project Administration, F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J. Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Foley D.P., Fernandez L.A., Leverson G., Anderson M., Mezrich J., Sollinger H.W., D’Alessandro A. Biliary complications after liver transplantation from donation after cardiac death donors: An analysis of risk factors and long-term outcomes from a single center. Ann. Surg. 2011;253:817–825. doi: 10.1097/SLA.0b013e3182104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayant K., Reccia I., Virdis F., Shapiro A.M.J. Systematic Review and Meta-Analysis on the Impact of Thrombolytic Therapy in Liver Transplantation Following Donation after Circulatory Death. J Clin. Med. 2018;7:425. doi: 10.3390/jcm7110425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dar W.A., Sullivan E., Bynon J.S., Eltzschig H., Ju C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int. 2019;39:788–801. doi: 10.1111/liv.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhai Y., Petrowsky H., Hong J.C., Busuttil R.W., Kupiec-Weglinski J.W. Ischaemia-reperfusion injury in liver transplantation-from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wind J., Faut M., van Smaalen T.C., van Heurn E.L.W. Variability in protocols on donation after circulatory death in Europe. Crit. Care. 2013;17:R217. doi: 10.1186/cc13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callaghan C.J., Charman S.C., Muiesan P., Powell J.J., Gimson A.E., van der Meulen J.H.P. Outcomes of transplantation of livers from donation after circulatory death donors in the UK: A cohort study. BMJ Open. 2013;3:e003287. doi: 10.1136/bmjopen-2013-003287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlegel A., Kalisvaart M., Scalera I., Laing R.W., Mergental H., Mirza D.F., Perera T., Isaac J., Dutkowski P., Muiesan P. The UK DCD Risk Score: A new proposal to define futility in donation-after-circulatory-death liver transplantation. J. Hepatol. 2018;68:456–464. doi: 10.1016/j.jhep.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Cypel M., Yeung J.C., Machuca T., Chen M., Singer L.G., Yasufuku K., de Perrot M., Pierre A., Waddel T.K., Keshavjee S. Experience with the first 50 Ex Vivo lung perfusions in clinical transplantation. J. Thorac. Cardiovasc. Surg. 2012;144:1200–1206. doi: 10.1016/j.jtcvs.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Rojas S.V., Ius F., Schibilsky D., Kaufeld T., Sommer W., Benk C., Goecke T., Siemeni T., Poyanmehr R., Rümke S., et al. Cardiac Transplantation in Higher Risk Patients: Is Ex Vivo Heart Perfusion a Safe Preservation Technique? A Two Center Experience. J. Heart Lung Transplant. 2019;38:S43. doi: 10.1016/j.healun.2019.01.091. [DOI] [Google Scholar]
- 11.Hosgood S.A. Renal transplantation after Ex Vivo normothermic perfusion: The first clinical study. Am. J. Transplant. 2013;13:1246–1252. doi: 10.1111/ajt.12179. [DOI] [PubMed] [Google Scholar]
- 12.Ravikumar R., Jassem W., Mergental H., Heaton N., Mirza D., Perera M.T.P.R., Quaglia A., Holroyd D., Vogel T., Coussios C.C., et al. Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am. J. Transplant. 2016;16:1779–1787. doi: 10.1111/ajt.13708. [DOI] [PubMed] [Google Scholar]
- 13.Goodman L.F., Yasmine E.S., Maija Y., Benno C., Ozgediz D., Adoyi Ameh E., Bickler S., Poenaru D., Oldham K., Farmer D. The Global Initiative for Children’s Surgery: Optimal Resources for Improving Care. Eur. J. Pediatr. Surg. 2018;28:58–59. doi: 10.1055/s-0037-1604399. [DOI] [PubMed] [Google Scholar]
- 14.Nasralla D., Coussios C.C., Mergental H., Akhtar M.Z., Butler A.J., Ceresa C.D.L., Chiocchia V., Dutton S.J., Garcia-Valdecasas J.C., Heaton N., et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50–56. doi: 10.1038/s41586-018-0047-9. [DOI] [PubMed] [Google Scholar]
- 15.Ceresa C.D.L., Nasralla D., Watson C.J.E., Butler A.J., Coussios C.C., Crick K., Hodson L., Imber C., Jassem W., Knight S.R., et al. Transient Cold Storage Prior to Normothermic Liver Perfusion May Facilitate Adoption of a Novel Technology. Liver Transplant. 2019;25:1503–1513. doi: 10.1002/lt.25584. [DOI] [PubMed] [Google Scholar]
- 16.Watson C.J.E., Jochmans I. From “Gut Feeling” to Objectivity: Machine Preservation of the Liver as a Tool to Assess Organ Viability. Curr. Transplant. Rep. 2018;5:72–81. doi: 10.1007/s40472-018-0178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laing R.W., Mergental H., Yap C., Kirkham A., Whilku M., Darren B., Curbishley S., Boteon Y.L., Neil D.A., Hübscher S.G., et al. Viability testing and transplantation of marginal livers (VITTAL) using normothermic machine perfusion: Study protocol for an open-label, nonrandomised, prospective, singlearm trial. BMJ Open. 2017;7:e017733. doi: 10.1136/bmjopen-2017-017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eshmuminov D., Becker D., Bautista Borrego L., Hefti M., Schuler M.J., Hagedorn C., Muller X., Mueller M., Onder C., Graf R., et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat. Biotechnol. 2020;38:189–198. doi: 10.1038/s41587-019-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rusche B. The 3Rs and animal welfare—Conflict or the way forward? Altex. 2003;20:63–76. [PubMed] [Google Scholar]
- 20.Vogel T., Brockmann J.G., Quaglia A., Morovat A., Jassem W., Heaton N.D., Coussios C.C., Friend P.J. The 24-hour normothermic machine perfusion of discarded human liver grafts. Liver Transplant. 2017;23:207–220. doi: 10.1002/lt.24672. [DOI] [PubMed] [Google Scholar]
- 21.Chouchani E.T., Pell V.R., Gaude E., Aksentijević D., Sundier S.Y., Robb E.L., Logan A., Nadtochiy S.M., Ord E.N.J., Smith A.C., et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martins R.M., Soeiro Teodoro J., Furtado E., Rolo A.P., Marques Palmeira C., Tralhão J.G. Recent insights into mitochondrial targeting strategies in liver transplantation. Int. J. Med. Sci. 2018;15:248–256. doi: 10.7150/ijms.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carthew R.W., Sontheimer E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y., Liu Y., Li M., Liu Z., Gong J. IRAK-4-shRNA Prevents Ischemia/Reperfusion Injury Via Different Perfusion Periods Through the Portal Vein After Liver Transplantation in Rat. Transplant Proc. 2016;48:2803–2808. doi: 10.1016/j.transproceed.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 25.Li X., Zhang J.F., Lu M.Q., Yang Y., Xu C., Li H., Wang G.S., Cai C.J., Chen G.H. Alleviation of ischemia-reperfusion injury in rat liver transplantation by induction of small interference RNA targeting Fas. Langenbeck’s Arch. Surg. 2007;392:345–351. doi: 10.1007/s00423-006-0142-5. [DOI] [PubMed] [Google Scholar]
- 26.Thijssen M.F., Brüggenwirth I.M.A., Gillooly A., Khvorova A., Kowalik T.F., Martins P.N. Gene Silencing With siRNA (RNA Interference): A New Therapeutic Option During Ex Vivo Machine Liver Perfusion Preservation. Liver Transplant. 2019;25:140–151. doi: 10.1002/lt.25383. [DOI] [PubMed] [Google Scholar]
- 27.Moore C., Thijssen M., Wang X., Mandrekar P., Xiaofei E., Abdi R., Porte R., Bozorgzadeh A., Leuvenink H., Kowalik T., et al. Gene Silencing with p53 si-RNA Downregulates Inflammatory Markers in the Liver: Potential Utilization during Normothermic Machine Preservation. Am. J. Transplant. 2017;18:503. [Google Scholar]
- 28.Buchwald J.E., Xu J., Bozorgzadeh A., Martins P.N. Therapeutics administered during Ex Vivo liver machine perfusion: An overview. World J. Transplant. 2020;10:1–14. doi: 10.5500/wjt.v10.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillooly A.R., Perry J., Martins P.N. First Report of siRNA Uptake (for RNA Interference) during Ex Vivo Hypothermic and Normothermic Liver Machine Perfusion. Transplantation. 2019;103:e56–e57. doi: 10.1097/TP.0000000000002515. [DOI] [PubMed] [Google Scholar]
- 30.Beal E.W., Kim J.L., Reader B.F., Akateh C., Maynard K., Washburn W.K., Zweier J.L., Whitson B.A., Black S.M. [D-Ala 2, D-Leu 5] Enkephalin Improves Liver Preservation During Normothermic Ex Vivo Perfusion. J. Surg. Res. 2019;241:323–335. doi: 10.1016/j.jss.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Vogel T., Brockmann J.G., Pigott D., Neil D.A.H., Muthusamy A.S.R., Coussios C.C., et al. Successful transplantation of porcine liver grafts following 48-hour normothermic preservation. PLoS ONE. 2017;12:e0188494. doi: 10.1371/journal.pone.0188494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bral M., Dajani K., Leon Izquierdo D., Bigam D., Kneteman N., Ceresa C.D.L., Friend P.J., Shapiro A.M.J. A Back-to-Base Experience of Human Normothermic Ex Situ Liver Perfusion: Does the Chill Kill? Liver Transplant. 2019;25:848–858. doi: 10.1002/lt.25464. [DOI] [PubMed] [Google Scholar]
- 33.Jassem W., Xystrakis E., Ghnewa Y.G., Yuksel M., Pop O., Martinez-Llordella M., Jabri Y., Huang X., Lozano J.J., Quaglia A., et al. Normothermic machine perfusion (NMP) inhibits proinflammatory responses in the liver and promotes regeneration. Hepatology. 2018;70:682–695. doi: 10.1002/hep.30475. [DOI] [PubMed] [Google Scholar]
- 34.Safinia N., Grageda N., Scottà C., Thirkell S., Fry L.J., Vaikunthanathan T., Lechler R.I., Lombardi G. Cell Therapy in Organ Transplantation: Our Experience on the Clinical Translation of Regulatory T Cells. Front. Immunol. 2018;9:354. doi: 10.3389/fimmu.2018.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beriou G., Moreau A., Cuturi M.C. Tolerogenic dendritic cells: Applications for solid organ transplantation. Curr. Opin. Organ. Transplant. 2012;17:42–47. doi: 10.1097/MOT.0b013e32834ee662. [DOI] [PubMed] [Google Scholar]
- 36.Detry O., Vandermeulen M., Delbouille M.H., Somja J., Bletard N., Briquet A., et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: A phase I-II, open-label, clinical study. J. Hepatol. 2017;67:47–55. doi: 10.1016/j.jhep.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Todo S., Yamashita K., Goto R., Zaitsu M., Nagatsu A., Oura T., Watanabe M., Ayoura T., Suzuki T., Shimamura T., et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64:632–643. doi: 10.1002/hep.28459. [DOI] [PubMed] [Google Scholar]
- 38.Global Observatory on Donation and Transplantation (GODT) 2017 Global Report. [(accessed on 25 February 2020)]; Available online: http://www.transplant-observatory.org/download/2017-activity-data-report/
- 39.Girdlestone J. Mesenchymal stromal cells with enhanced therapeutic properties. Immunotherapy. 2016;8:1405–1416. doi: 10.2217/imt-2016-0098. [DOI] [PubMed] [Google Scholar]
- 40.Obermajer N., Popp F.C., Johnson C.L., Benseler V., Dahlke M.H. Rationale and prospects of mesenchymal stem cell therapy for liver transplantation. Curr. Opin. Organ. Transplant. 2014;19:60–64. doi: 10.1097/MOT.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 41.El Andaloussi S., Mäger I., Breakefield X.O., Wood M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug. Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 42.Stone M.L., Zhao Y., Smith J.R., Weiss M.L., Kron I.L., Laubach V.E., Sharma A.K. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir. Res. 2017;18:212. doi: 10.1186/s12931-017-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lonati C., Bassani G.A., Brambilla D., Leonardi P., Carlin A., Maggioni M., Zanella A., Dondossola D., Fonsato V., Grange C., et al. Mesenchymal stem cell–derived extracellular vesicles improve the molecular phenotype of isolated rat lungs during ischemia/reperfusion injury. J. Heart Lung Transplant. 2019;38:1306–1316. doi: 10.1016/j.healun.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 44.Varchetta S., Oliviero B., Francesca Donato M., Agnelli F., Rigamonti C., Paudice E., Arosio E., Berra M., Rossi G., Tinelli C., et al. Prospective study of natural killer cell phenotype in recurrent hepatitis C virus infection following liver transplantation. J. Hepatol. 2009;50:314–322. doi: 10.1016/j.jhep.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Dondossola D., Santini A., Lonati C., Zanella A., Merighi R., Vivona L., Battistin M., Galli A., Biancolilli O., Maggioni M., et al. Human Red Blood Cells as Oxygen Carriers to Improve Ex-Situ Liver Perfusion in a Rat Model. J. Clin. Med. 2019;8:1918. doi: 10.3390/jcm8111918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chin L.Y., Carroll C., Raigani S., Detelich D.M., Tessier S.N., Wojtkiewicz G.R., Schmidt S.P., Weissleder R., Yeh H., Uygun K., et al. Ex Vivo perfusion-based engraftment of genetically engineered cell sensors into transplantable organs. PLoS ONE. 2019;14:e0225222. doi: 10.1371/journal.pone.0225222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reddy S.P., Bhattacharjya S., Maniakin N., Greenwood J., Guerreiro D., Hughes D., Imber C.J., Piggot D.W., Susan F., Richard T., et al. Preservation of porcine non-heart-beating donor livers by sequential cold storage and warm perfusion. Transplantation. 2004;77:1328–1332. doi: 10.1097/01.TP.0000119206.63326.56. [DOI] [PubMed] [Google Scholar]
- 48.Xu H., Berendsen T., Kim K., Soto-Gutiérrez A., Bertheium F., Yarmush M.L., Hertl M. Excorporeal normothermic machine perfusion resuscitates pig DCD livers with extended warm ischemia. J. Surg. Res. 2012;173:e83–e88. doi: 10.1016/j.jss.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlegel A., De Rougemont O., Graf R., Clavien P.A., Dutkowski P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 2013;58:278–286. doi: 10.1016/j.jhep.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Goldaracena N., Echeverri J., Spetzler V.N., Kaths J.M., Barbas A.S., Louis K.S., Adeyi O.A., Grant D.R., Selzner N., Selzner R. Anti-inflammatory signaling during Ex Vivo liver perfusion improves the preservation of pig liver grafts before transplantation. Liver Transplant. 2016;22:1573–1583. doi: 10.1002/lt.24603. [DOI] [PubMed] [Google Scholar]
- 51.Hara Y., Akamatsu Y., Maida K., Kashiwadate T., Kobayashi Y., Ohuchi N., et al. A new liver graft preparation method for uncontrolled non-heart-beating donors, combining short oxygenated warm perfusion and prostaglandin E1. J. Surg. Res. 2013;184:1134–1142. doi: 10.1016/j.jss.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 52.Lin M., Mah A., Wright A.J. Infectious complications of liver transplantation. AME Med. J. 2018;3:5. doi: 10.21037/amj.2017.12.10. [DOI] [Google Scholar]
- 53.Phillips B.L., Chandak P., Uwechue R., Van Nispen Tot Pannerden C., Hemsley C., Callaghan C.J. Microbial contamination during kidney Ex Vivo normothermic perfusion. Transplantation. 2018;102:E186–E188. doi: 10.1097/TP.0000000000002076. [DOI] [PubMed] [Google Scholar]
- 54.Spearman C.W., Dusheiko G.M., Hellard M., Sonderup M. Hepatitis C. Lancet. 2019;394:1451–1466. doi: 10.1016/S0140-6736(19)32320-7. [DOI] [PubMed] [Google Scholar]
- 55.Herzer K., Gerken G. Hepatitis C virus reinfection after liver transplantation. New chances and new challenges in the era of direct-acting antiviral agents. World J. Hepatol. 2015;7:532–538. doi: 10.4254/wjh.v7.i3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldaracena N., Spetzler V.N., Echeverri J., Kaths J.M., Cherepanov V., Persson R., Hodges M.R., Janssen H.L.A., Selzner N., Grant D.R., et al. Inducing Hepatitis C Virus Resistance After Pig Liver Transplantation—A Proof of Concept of Liver Graft Modification Using Warm Ex Vivo Perfusion. Am. J. Transplant. 2017;17:970–978. doi: 10.1111/ajt.14100. [DOI] [PubMed] [Google Scholar]
- 57.Durand F., Renz J., Alkofer B., Burra P., Clavien P., Porte R., Freeman R.B., Belghiti J. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transplant. 2008;14:1694–1707. doi: 10.1002/lt.21668. [DOI] [PubMed] [Google Scholar]
- 58.Parekh S., Anania F. Abnormal Lipid and Glucose Metabolism in Obesity: Implications for Nonalcoholic Fatty Liver Disease. Gastroenterology. 2007;132:2191–2207. doi: 10.1053/j.gastro.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 59.Chu M., Dare A., Phillips A., Bartlett A. Donor Hepatic Steatosis and Outcome After Liver Transplantation: A Systematic Review. J. Gastrointest. Surg. 2015;19:1713–1724. doi: 10.1007/s11605-015-2832-1. [DOI] [PubMed] [Google Scholar]
- 60.Spitzer A.L., Lao O.B., Dick A.A.S., Bakthavatsalam R., Halldorson J.B., Yeh M.M., Upton M.P., Reyes J.D., Perkins J.D. The biopsied donor liver: Incorporating macrosteatosis into high-risk donor assessment. Liver Transplant. 2010;16:874–884. doi: 10.1002/lt.22085. [DOI] [PubMed] [Google Scholar]
- 61.Nativ N.I., Maguire T.J., Yarmush G., Brasaemle D.L., Henry S.D., Guarrera J., Berthiaume F., Yarmush M.L. Liver Defatting: An Alternative Approach to Enable Steatotic Liver Transplantation. Am. J. Transplant. 2012;12:3176–3183. doi: 10.1111/j.1600-6143.2012.04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.NHS Blood and Transfusion (NHSBT) Annual Report on Liver Transplantation: Report for 2014/2015/2016. [(accessed on 22 November 2019)]; Available online: http://www.odt.nhs.uk/pdf/organ_specific_report_liver_2014.pdf.
- 63.Dyson J., Anstee Q., McPherson S. Non-alcoholic fatty liver disease: A practical approach to diagnosis and staging. Front. Gastroenterol. 2013;5:211–218. doi: 10.1136/flgastro-2013-100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.NHS Digital Statistics on Obesity, Physical Activity and Diet: England 2015. [(accessed on 4 November 2019)]; Available online: https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet/statistics-on-obesity-physical-activity-and-diet-england-2015.
- 65.Brockmann J., Reddy S., Coussios C., Pigott D., Guirriero D., Hughes D., Morovat A., Roy D., Winter L., Friend P.J. Normothermic Perfusion. Ann. Surg. 2009;250:1–6. doi: 10.1097/SLA.0b013e3181a63c10. [DOI] [PubMed] [Google Scholar]
- 66.Watson C., Kosmoliaptsis V., Pley C., Randle L., Fear C., Crick K., et al. Observations on the Ex Situ perfusion of livers for transplantation. Am. J. Transplant. 2018;18:2005–2020. doi: 10.1111/ajt.14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jamieson R.W., Zilvetti M., Roy D., Hughes D., Morovat A., Coussios C.C., Friend P.J. Hepatic Steatosis and Normothermic Perfusion—Preliminary Experiments in a Porcine Model. Transplantation. 2011;92:289–295. doi: 10.1097/TP.0b013e318223d817. [DOI] [PubMed] [Google Scholar]
- 68.Nagrath D., Xu H., Tanimura Y., Zuo R., Berthiaume F., Avila M., Yarmush R., Yarmush M.L. Metabolic preconditioning of donor organs: Defatting fatty livers by normothermic perfusion Ex Vivo. Metab. Eng. 2009;11:274–283. doi: 10.1016/j.ymben.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boteon Y.L., Attard J., Boteon A.P.C.S., Wallace L., Reynolds G., Hubscher S., Mirza D.F., Mergenthal H., Bhogal R.H., Afford S.C. Manipulation of Lipid Metabolism During Normothermic Machine Perfusion: Effect of Defatting Therapies on Donor Liver Functional Recovery. Liver Transplant. 2019;25:1007–1022. doi: 10.1002/lt.25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banan B., Watson R., Xu M., Lin Y., Chapman W. Development of a normothermic extracorporeal liver perfusion system toward improving viability and function of human extended criteria donor livers. Liver Transplant. 2016;22:979–993. doi: 10.1002/lt.24451. [DOI] [PubMed] [Google Scholar]
- 71.Ceresa C., Nasralla D., Neil D., Mergental H., Jassem W., Butler A., Imber C., Barrett A., Clark A., Coussios C., et al. A Histological and Biochemical Assessment of Steatotic Livers Undergoing Normothermic Machine Perfusion; Proceedings of the 18th Congress of the European Society for Organ Transplantation; Barcelona, Spain. 24–27 September 2017; Padova, Italy: European Society for Organ Transplantation; 2017. [Google Scholar]
- 72.Raigani S., Carrol C., Cronin S., Pendexter C., Rosales I., Yarmush M., Uygun K.Y.H. Defatting Steatotic Rat Livers during Ex Situ Normothermic Perfusion Improves Lactate Clearance and Bile Quality—ATC Abstracts; Proceedings of the 2019 American Transplant Congress; Boston, MA, USA. 1–5 June 2019; Mt. Laurel, NJ, USA: American Society of Transplantation; 2019. pp. 14–17. [Google Scholar]
- 73.Liu Q., Nassar A., Buccini L., Iuppa G., Soliman B., Pezzati D., Hassan A., Blum M., Baldwin W., Bennett A., et al. Lipid metabolism and functional assessment of discarded human livers with steatosis undergoing 24 hours of normothermic machine perfusion. Liver Transplant. 2018;24:233–245. doi: 10.1002/lt.24972. [DOI] [PubMed] [Google Scholar]
- 74.Laing R.W., Boteon Y.L., Kirkham A., Perera T., Attard J., Barton D., Curbishley S., Neil D.A., Hubscher S.G., Musien P., et al. Transplantation of Discarded Livers Following Viability Testing With Normothermic Machine Perfusion: The VITTAL (VIability Testing and Transplantation of mArginal Livers) Trial Outcomes. Transplantation. 2019;103:3–4. [Google Scholar]
- 75.Raigani S., Markmann J.F., Yeh H. Rehabilitation of Discarded Steatotic Livers Using Ex Situ Normothermic Machine Perfusion: A Future Source of Livers for Transplantation. Liver Transplant. 2019;25:991–992. doi: 10.1002/lt.25490. [DOI] [PubMed] [Google Scholar]
- 76.Boteon Y., Wallace L., Boteon A., Mirza D., Mergental H., Bhogal R., Afford S. An effective protocol for pharmacological defatting of primary human hepatocytes which is non-toxic to cholangiocytes or intrahepatic endothelial cells. PLoS ONE. 2018;13:e0201419. doi: 10.1371/journal.pone.0201419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moore L., Goodwin B., Jones S., Wisely G., Serabjit-Singh C., Willson T., Collins J.L., Kliewer S.A., St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc. Natl. Acad. Sci. USA. 2000;97:7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pollock C., Rodriguez O., Martin P., Albanese C., Li X., Kopelovich L., Glazer R.I. Induction of Metastatic Gastric Cancer by Peroxisome Proliferator-Activated Receptorδ Activation. PPAR Res. 2010;2010:1–12. doi: 10.1155/2010/571783. [DOI] [PMC free article] [PubMed] [Google Scholar]