Abstract
The striatum is essential for learning which actions lead to reward and for implementing those actions. Decades of experimental and theoretical work have led to several influential theories and hypotheses about how the striatal circuit mediates these functions. However, owing to technical limitations, testing these hypotheses rigorously has been difficult. In this Review, we briefly describe some of the classic ideas of striatal function. We then review recent studies in rodents that take advantage of optical and genetic methods to test these classic ideas by recording and manipulating identified cell types within the circuit. This new body of work has provided experimental support of some longstanding ideas about the striatal circuit and has uncovered critical aspects of the classic view that are incorrect or incomplete.
Decision-making involves the selection of a motor plan based on external information (for example, sensory inputs) and internal information (such as reward history). Here, we consider the role of the striatum in sensory-based and value-based decision-making and in the learning of reward associations that underlie these behaviours.
The striatum is the primary input nucleus of the basal ganglia and is positioned within multiple parallel cortico-subcortical loops. It receives input from the cortex and thalamus and sends outputs that ultimately relay information back to the cortex via the thalamus1–3. In addition, the striatum is a site where glutamatergic input from many brain regions converges with dense innervation from midbrain dopamine (DA) neurons4. Thus, the striatum is well positioned to have a vital role in learning and decision-making.
The striatum itself is primarily composed of GABAergic projection neurons called medium spiny neurons (MSNs), which are divided into two molecularly distinct populations with largely segregated output projection pathways through the basal ganglia5–9. These two pathways oppositely modulate the output structures of the basal ganglia, which have high baseline firing rates and tonically inhibit thalamic and brainstem nuclei10–15. In addition to MSNs, there are small populations of interneurons in the striatum, including cholinergic interneurons (CINs)16, as well multiple other subclasses of GABAergic neurons, that can be distinguished on the basis of their physiological and molecular profiles17–19.
In this Review, we discuss recent work exploring how specific cell types within the striatum and its inputs are involved in learning and decision-making. We focus on five cell types: dopaminergic input neurons, the two classes of MSNs, CINs and glutamatergic input neurons (the GABAergic interneurons have been reviewed else-where18,19). For each of these components of the striatal circuit, we briefly review classic ideas about their role in learning and decision-making, which were derived primarily from anatomical, electrophysiological and pharmacological experiments. We then discuss studies from the past decade that used genetic and optical tools to more precisely monitor and manipulate these distinct cell types within the striatal circuit in rodents. In some cases, this work has confirmed classic ideas about the role of these cell types in learning and decision-making whereas in other cases the recent research has revealed that classic ideas are incomplete, opening new questions in the field that must now be resolved.
Midbrain dopamine neurons
A teaching signal.
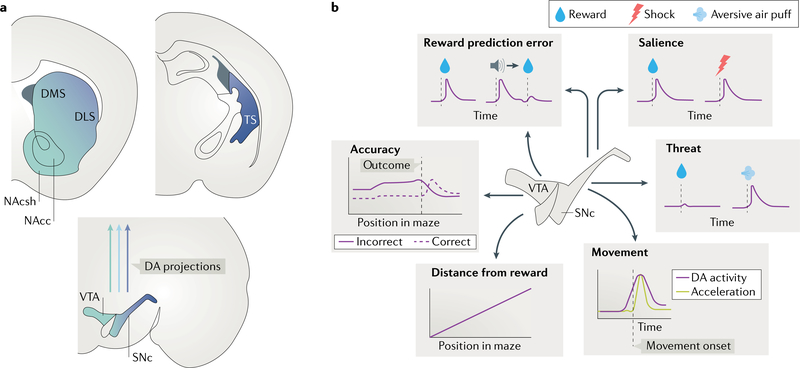
DA neurons that originate in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) provide dense, topographic innervation to the striatum20–26 (Fig. 1a). The VTA projects preferentially to the nucleus accumbens (NAc), whereas the SNc projects preferentially to the dorsomedial striatum (DMS), dorsolateral striatum (DLS) and tail of the striatum (TS; see Box 1 for an introduction to striatal subregions). Seminal experiments demonstrated that these DA neurons encode reward prediction error (RPE) — the difference between experienced and expected reward27,28. This result has been confirmed in multiple species (including mice, rats and non-human primates) and observed both in the firing patterns of putative DA neurons and in DA concentration changes in the striatum29–37.
Fig. 1 |. Heterogeneity of midbrain dopamine neurons.
a| Schematic of the organization of projections from the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) to the striatum. The VTA predominantly projects to the ventral striatum and the SNc to the dorsal striatum20–26. A distinct population of dopamine (DA) neurons in the lateral SNc project to the tail of the striatum (TS)203. b | Schematized examples of the heterogeneous activity profiles of DA neurons and their organization in the SNc and VTA. Reward prediction error signals are found throughout VTA and SNc neurons25,36,58. DA neurons increase their activity in response to both unexpected rewards (left) or cues (such as an auditory tone) that predict reward. In the medial VTA, some DA neurons signal the accuracy of the trial58. The activity of other VTA neurons is correlated with the distance of the animal from a reward58,204. Activity in some DA neurons in the lateral VTA and SNc is associated with movement58,59,63,64. Activity of some SNc DA axons in the striatum is more correlated with salience than with value, in that they respond similarly to unexpected positive events (for example, a water reward) and negative events (such as a foot shock)23. Neurons in the lateral SNc show weak responses to reward and respond more robustly to salient or threatening stimuli (such as an air puff)60,61. DLS, dorsolateral striatum; DMS, dorsomedial striatum; NAcc, nucleus accumbens core; NAcsh, nucleus accumbens shell.
Box 1 |.
Functional divisions of the striatum
The striatum is divided into functional subregions that are thought to mediate learning and expression of different types of association. In the rodent, these regions include the dorsolateral striatum (DLS; homologous to the primate putamen), dorsomedial striatum (DMS; homologous to the primate caudate) and ventral striatum (VS). These are frequently defined as the sensorimotor, associative and limbic striatum, respectively3,57,187–190.
The DLS is thought to be important for the formation of stimulus–response associations that underlie skilled movements and habitual actions3,188,190–196, whereas the DMS regulates goal-directed behaviours that rely on response–outcome associations3,57,188,190,193–195,197. Animals will stop performing goal-directed actions if the associated outcome is no longer valuable. By contrast, habitual behaviours are relatively insensitive to the value of associated outcomes. Lesions or inactivation of the DMS render learned operant behaviours habitual, whereas lesions or inactivation of the DLS prevent behaviours from becoming habitual57,191. These structures are both active during learning, but with overtraining as an action transitions from goal-directed to habitual, distinct activity patterns develop in these structures193,194.
The nucleus accumbens (NAc; the major component of the VS) is thought to be involved in outcome evaluation and motivation and in the formation of stimulus– outcome associations that are important for Pavlovian learning188,190,198,199. Furthermore, through its substantial projection to midbrain dopamine neurons200,201, the NAc regulates dopamine release throughout the striatum202.
Finally, in the primate, the tail of the striatum (TS) is increasingly recognized as a distinct subregion that is involved in processing sensory information and promoting behaviours that are reliant on sensory information177–180. Although less well studied, some evidence suggests that the TS of rodents is also specialized for sensory information88,152,158,176. The dorsal striatum can probably be further subdivided147,148, but the functional roles of these finer divisions have yet to be interrogated.
This dopaminergic RPE signal is integral to one of the critical functions ascribed to the striatum — reinforcement learning. RPE is thought to serve as a reinforcement signal that modifies glutamatergic synaptic inputs to the striatum that are active during unexpected rewards (that is, co-active with DA neurons). Thus, DA-dependent plasticity of these synapses provides a synaptic mechanism through which actions that are associated with unexpected reward are more likely to be repeated or stimuli that are associated with unexpected reward are more likely to be pursued38–42.
Classic experiments manipulating DA activity supported the idea that DA serves as a teaching signal to support reinforcement learning. For example, it has been known since the 1950s that animals will perform arbitrary actions to receive electrical stimulation of the medial forebrain bundle43,44 and that this effect can be attenuated by dopaminergic antagonists45,46.
New evidence of heterogeneity in the dopamine system.
The classic idea that DA activity supports reinforcement learning has been directly tested in recent years with optogenetic activation or inhibition of DA neurons in a wide range of learning paradigms. These studies had the cell-type specificity and temporal precision to directly test the hypothesis that DA neurons provide an RPE signal to support reinforcement learning. They have unequivocally confirmed that DA neuron activation supports Pavlovian learning25,47,48, contextual learning49,50 and operant learning50–53. Conversely, transient optogenetic inhibition of these neurons mimics a negative prediction error: inhibition of DA neurons promotes extinction of a previously conditioned response54, induces conditioned place avoidance50 and reduces the likelihood that an animal will repeat a previously selected action52,55.
Given the anatomical and functional specialization within the striatum (Box 1), this RPE signal may support different forms of learning depending on the function of the striatal target area. Consistent with this, stimulation of either VTA DA projections or SNc DA projections to the striatum is sufficient to turn a neutral cue into a conditioned stimulus, but with important differences25. Activation of the projection from the VTA to the NAc induces cue approach and leads the cue to become reinforcing on its own. By contrast, activating the projection from the SNc to the dorsal striatum induces vigorous, but undirected, movement in response to the cue and does not cause the cue to become reinforcing25. This distinction is consistent with classic ideas about striatal subregions: the NAc is thought to be important for generating stimulus–outcome associations, whereas the dorsal striatum is thought to be more important for stimulus–response associations and action–outcome associations56,57 (Box 1).
Although recent optogenetic experiments have supported the classic idea that DA activity functions as an RPE to drive reinforcement learning, and in vivo recordings from identified DA neurons have revealed RPE signals36, numerous other entirely unexpected response profiles have been recorded in vivo that cannot easily be interpreted within the RPE framework58–61 (Fig. 1b). These findings challenge the classic idea that DA projections to the striatum uniformly transmit RPE signals and instead point to anatomical specialization within the DA system and raise new questions about what functions these non-RPE signals may serve.
Notably, specialization of DA neuron activity seems to be related to where they are located or where in the striatum they project. For example, DA neuron terminals in the dorsal striatum have relatively weak reward responses but respond robustly during locomotion59 or contralateral movements55,62. Similarly, individual DA neurons in the SNc increase their activity during movement initiation, and this increase in activity correlates with the vigour of those movements63 (Fig. 1b). Consistent with these neural correlates, optogenetic stimulation of SNc cell bodies or their terminals in the DMS increases movement59,63,64 whereas their inactivation decreases movement initiation and reduces the vigour of movements that do occur63.
Another aspect of DA activity that is seemingly inconsistent with RPE is the positive response that had been observed in some putative DA neurons, particularly in the SNc, to aversive events23,60,65–67. Calcium imaging of identified DA neurons has also suggested that these responses to aversion are projection-specific, similar to the movement-related increases in activity. In particular, DLS-projecting SNc DA neurons respond with increased activity to a foot shock23, whereas TS-projecting SNc DA neurons respond with increased activity to an air puff60 (Fig. 1b). Optogenetic activation of TS-projecting DA neurons reinforces avoidance behaviour60, suggesting that even populations of DA neurons that do not encode RPE support specific forms of reinforcement learning.
Given the increased appreciation that there are DA signals that cannot easily be considered RPE-related, an important question is how RPE and non-RPE signals are organized across individual DA neurons. In the VTA of mice navigating a virtual maze in a decision-making task, DA neurons show surprisingly heterogeneous activity, with most individual neurons representing one or two specific behavioural variables, such as reward history, trial accuracy, kinematics and/or spatial position (Fig. 1b). DA neurons within the VTA with similar activity profiles are more likely to be spatially localized58. Although RPE does not offer a clear explanation for the heterogeneous and specialized selectivity for these variables observed during this task, many of the same neurons that encode specific behavioural variables also encode RPE58.
In contrast to the overlap of RPE and non-RPE responses observed within the same neurons in the VTA, DA neurons in the SNc that are activated by reward seem to be largely distinct from those activated around the time of movement initiation59,63. This observation suggests that RPE signals are less ubiquitous in the SNc than in the VTA.
Direct and indirect output pathways
Promoting and suppressing actions.
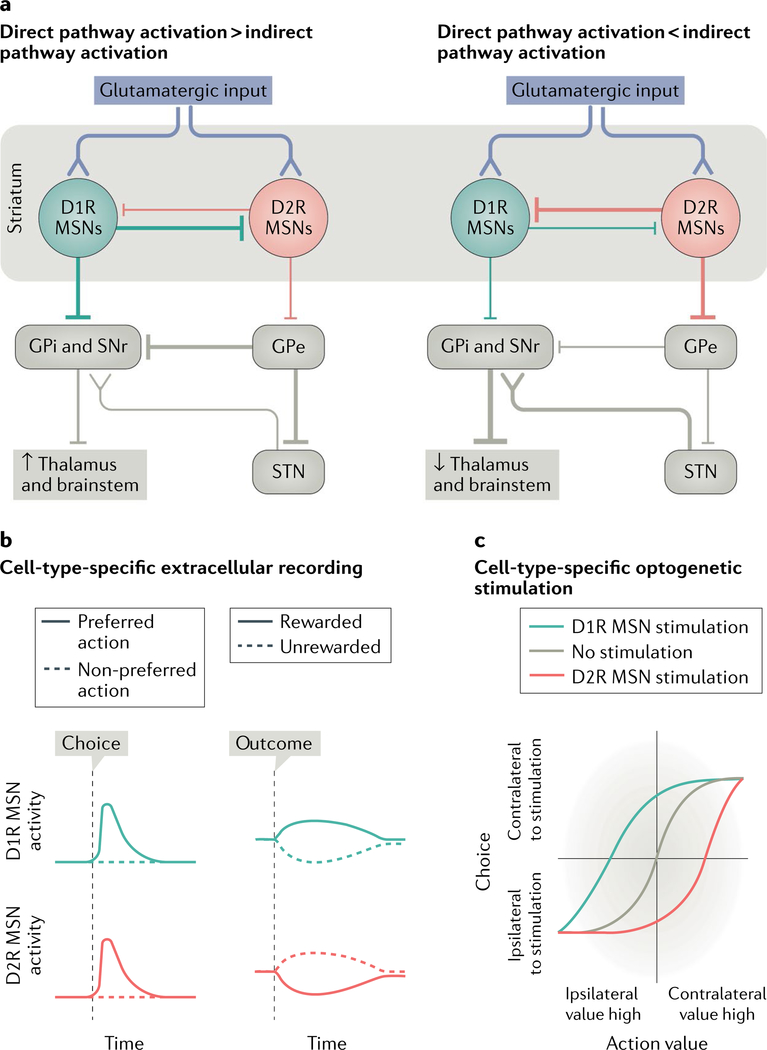
In the dorsal striatum, MSNs of the direct pathway express the D1 dopamine receptor (D1R) and inhibit the main out-put nuclei of the basal ganglia — the internal globus pallidus (GPi) and the substantia nigra pars reticulata (SNr). By contrast, indirect pathway MSNs express the D2 dopamine receptor (D2R) and indirectly increase basal ganglia output7,8,68 (Fig. 2a). The classic view of these two pathways’ functions is that they differentially regulate behaviour by oppositely modulating the firing rate of basal ganglia output nuclei2,10,12,13,15. For example, direct pathway activation would lead to disinhibition of brainstem motor structures, as well as of thalamic nuclei that target motor cortex, to promote movement. The indirect pathway drives further activation of basal ganglia output nuclei and thus promotes suppression of their targets, inhibiting movements2,10,12,13,69–72. This proposal is often referred to as the ‘go/no-go’ model.
Fig. 2 |. Direct and indirect pathway regulation of behaviour.
a | Simplified schematic of the direct and indirect pathways through the basal ganglia. The ‘go/no-go’ model of direct pathway and indirect pathway function proposes that when the D1 dopamine receptor (D1R) medium spiny neurons (MSNs) that make up the direct pathway are activated (left), they inhibit the primary output nuclei of the basal ganglia: the internal globus pallidus (GPi) and the substantia nigra pars reticulata (SNr). The GPi and SNr tonically inhibit brainstem and thalamic nuclei, which become disinhibited via D1R MSN activation. When the dopamine D2 receptor (D2R) MSNs of the indirect pathway are activated (right), they inhibit the external globus pallidus (GPe), which sends inhibitory projections to the GPi, SNr and subthalamic nucleus (STN). Thus, the GPi, SNr and STN are disinhibited. The STN sends excitatory input to the GPi and SNr, further activating it and inhibiting their brainstem and thalamic targets2,10,12,13,69,72,205. b | Schematized data from cell-type-specific extracellular recordings90. D1R and D2R neurons have similar activation patterns and selectivity during chosen actions but oppositely encode outcome. c | In a decision-making task, optogenetic stimulation of D1R or D2R MSNs induces opposite biases that depend on the difference in action value (the estimated value of the outcome resulting from an action) between the two options. Part b is adapted with permission from reF.90, Elsevier. Part c is adapted with from reF.102, Springer Nature Limited.
In its simplest form, the go/no-go model poses a straightforward hypothesis about what information is represented by each pathway: D1R neurons would be active during actions (because they promote them) and D2R neurons would be inactive during actions (because they suppress them). A related proposal suggests that D1R MSNs encode selected behaviours whereas D2R MSNs encode unselected behaviours13,69.
The go/no-go model may extend to learning and decision-making, with the two pathways exerting opposing controls on those processes. For example, striatal regions such as the DMS or VS, which receive inputs from the prefrontal cortex, could oppositely influence value-based decision-making, and subregions such as the TS, which receives inputs from sensory cortex, could oppositely influence perceptual decisions. However, to date, most studies have primarily examined the go/no-go model in the setting of spontaneous movements in which the learning and decision-making variables that control those movements are not explicitly controlled. For completeness and because the study of spontaneous movement may provide insight into how these anatomical pathways control movements in the setting of decision-making, we review below both studies of spontaneous movements and those on decision-making.
Opposing effects despite similar activity patterns.
Although electrophysiological recordings from striatal MSNs have revealed neural correlates of sensory stimuli, movement and value73–76, these studies could not differentiate between D1R and D2R MSNs, making it difficult to test predictions of the go/no-go model. The development of transgenic mouse lines to target D1R and D2R MSNs77,78 has enabled the identification and manipulation of these two populations independently in order to assess hypotheses about their endogenous activity.
Whereas the classic go/no-go model would predict opposite activity patterns in the two pathways during movement, surprisingly, recordings from the two types of MSN in the dorsal striatum have instead revealed very similar activity patterns. For example, both pathways are more active during movement than immobility79–85, are similarly active during trained79,86–91 and spontaneous movements80–84, encode the velocity of the animal80–83 and are preferentially active during contralateral movements79,91,92. These data suggest that the direct and indirect pathways simultaneously coordinate movement; indeed, there is considerable communication between the two pathways7,9,17,70,93. Thus, the simple go/no-go model of the direct versus indirect pathway function is probably incomplete.
Although these data contradict the simplest interpretation of the go/no-go model, they could allow for the possible interpretation that the direct pathway promotes the selected action whereas the indirect pathway suppresses alternative actions13,69,79. This interpretation gives rise to the interesting and testable prediction that direct pathway neurons are more selective for actions than indirect pathway neurons as there are far more unselected actions than selected actions at any point in time. Testing this model will require examination of behaviours in which neural correlates of more than two actions can be analysed, which has not been the case in the majority of studies of spontaneous movement and, to our knowledge, has not been achieved during decision-making tasks at all. However, recent investigations of spontaneous behaviour in which machine-learning algorithms classified movements into multiple discrete components suggest that this version of the model is probably incomplete as well81,82. These detailed analyses reveal that both populations concurrently encode spontaneous behaviours81,82, with a similar degree of specificity across the two pathways81,83. This similarity in specificity suggests that ensembles of D2R MSNs are unlikely to suppress all unselected actions in a particular context. Thus, so far, the data do not clearly support the extension of the go/no-go model in which the indirect pathway suppresses a wider range of actions than the direct pathway. Therefore, new ideas are needed to interpret the function of these two pathways.
Another possibility is that whereas D1R and D2R MSNs are both active during the same action, the relative activation of the two pathways determines whether that action is selected or avoided94–96. A potentially related idea is that the striatum is involved in the learning or decision-making processes that underlie motor outputs but is not directly involved in generating the motor output. In this framework, D1R and D2R MSNs may have opposing representations of the decision variables underlying a motor output despite having similar activation during movement82,97. This possibility can be best tested when internal variables related to decision-making (for example, the value of an action or the sensory evidence that drives a decision98) are parametrically controlled during decision-making, but not during spontaneous behaviour, when movements are monitored without knowledge of the decision-making process.
In support of these ideas, recent evidence suggests that value may oppositely modulate activity in the two pathways, despite both pathways showing similar activity during movement90,91,99. For example, in value-based decision-making tasks, many D1R MSNs increase their activity during reward presentation90 whereas D2R MSNs are more active during unrewarded outcomes90,100 (Fig. 2b). Opposite outcome-dependent responses are also observed in a Pavlovian conditioning task; here, D1R and D2R MSN responses to reward-predicting cues are positively or negatively correlated with reward value, respectively99. The fact that the activity of D1R and D2R MSNs seems to be differently modulated by value suggests that these neurons could oppositely encode the internal variables that underlie behavioural decisions rather than the actions themselves.
Specific optogenetic manipulations of D1R or D2R MSNs further support the idea that D1R MSNs and D2R MSNs oppositely modulate decision-making rather than influencing motor output (Fig. 2c). Animals trained in a probabilistic reversal learning task tend to repeat previously selected actions if they lead to reward but switch if they do not90,101,102. Transient stimulation of D1R MSNs or D2R MSNs just before mice execute their selected action induces a contralateral or ipsilateral bias, respectively. This is not simply a motor effect because the bias is dependent on the difference in estimated value of the two available choices, such that the bias was greater when the estimated value of the two choices was more similar102 (Fig. 2c). In addition, stimulation of D1R MSNs during outcome presentation decreases switches following reward whereas stimulation of D2R MSNs increases switching after unrewarded trials, suggesting that outcome-period activity in these pathways regulates animals’ outcome-dependent decision strategy90.
Activation of the direct and indirect pathways also seems to oppositely modulate learning. Activation of D1R MSNs in the dorsal striatum reinforces the behaviour or spatial location paired with stimulation103 and can reinforce specific features of trained movements, such as velocity104. D2R MSN activation has the opposite effects: decreasing performance of stimulation-paired behaviour, inducing aversion for a spatial location and decreasing selection of a particular movement velocity103,104. Similarly, stimulation of D1R MSNs in the NAc increases cocaine conditioned place preference (CPP) whereas stimulating D2R MSNs decreases cocaine CPP105.
Thus, considerable support from optogenetic activation suggests that D1R and D2R neurons exert antagonistic control over learning and decision-making. However, there are also recent studies that suggest surprising functions for the indirect pathway that seem to be entirely distinct from ‘no-go’. For example, in a sensory go/no-go task, activation of D1R MSNs or D2R MSNs causes a bias towards go responses, with no change in the perception of sensory information106. The increase in go responses with D1R MSN activation is consistent with classic models of direct pathway function; however, according to the classic model, D2R MSN stimulation would be expected to reduce rather than increase go responses. Similarly, optogenetic inhibition of D1R MSNs slows action initiation, consistent with the classic model, whereas D2R MSN inhibition does not speed action initiation but instead increases the probability that the mouse disengages from the task89,107.
In addition, although in the DMS direct and indirect pathway activity oppositely modulates reinforcement103, this does not seem to be the case in the DLS or NAc. D2R MSN activation in the DLS does not decrease pressing a stimulation-paired lever but instead increases pressing of both the paired and an unpaired lever108. In the NAc, activation of D1R or D2R MSNs promotes self-stimulation, although only D1R stimulation increases time spent in a simulated spatial location109. Moreover, in a task designed to test motivation, stimulating either D1R or D2R MSNs in the NAc increases motivation, and inhibition of D2R MSNs decreases motivation, causing animals to give up earlier than controls110 (but see reFs111,112). Taken together, these studies suggest that, under some conditions, the indirect pathway can have entirely unexpected roles that seem to be very different from the classically proposed role of antagonizing direct pathway function. When and why the indirect pathway serves these unexpected roles remain to be understood.
Cholinergic interneurons
Salience signalling.
Despite comprising only 1–2% of the total neurons within the striatum, CINs provide a major source of acetylcholine to the structure113 (Fig. 3a). As acetylcholine has been implicated in attention and learning in other brain regions114, and levels of cholinergic markers are particularly high in the striatum115–117, there has been great interest in understanding the function of striatal CINs. However, their sparse and distributed nature has made this goal particularly challenging.
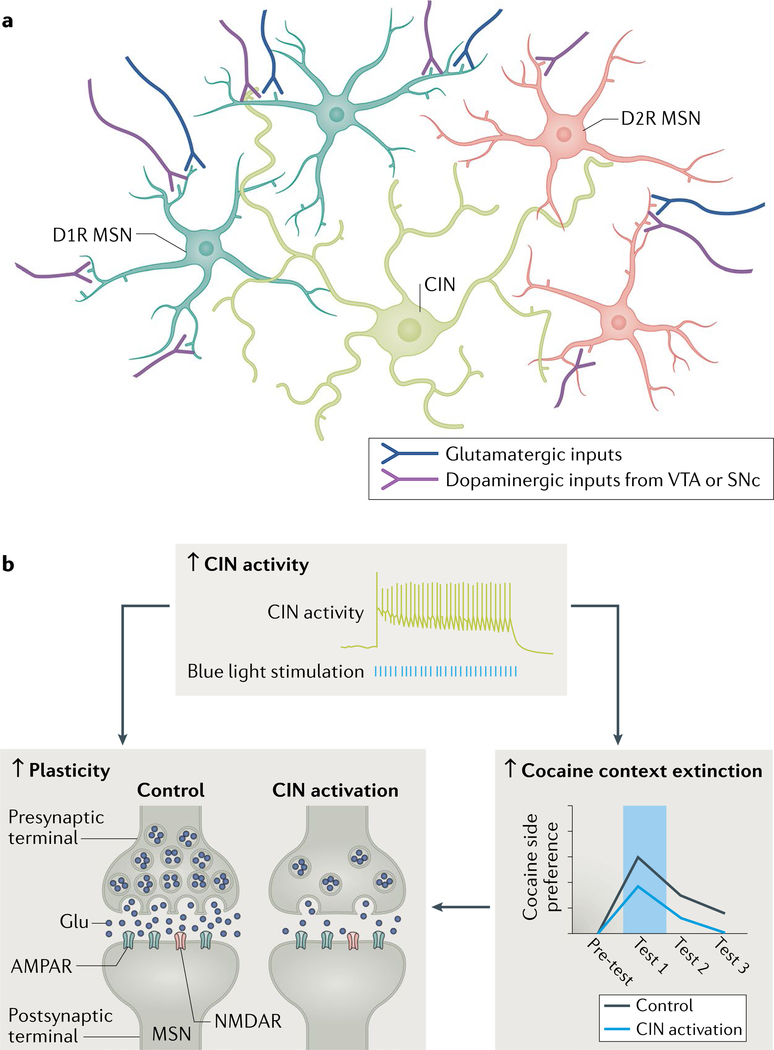
Fig. 3 |. Cholinergic interneurons modulate synaptic plasticity and cocaine context extinction learning.
a | Simplified schematic of the striatal circuit, highlighting the cholinergic interneurons (CINs) as well as D1 (D1R) and D2 dopamine receptor (D2R) medium spiny neurons (MSNs). All cell types receive glutamatergic and dopaminergic inputs from external structures. b | A recent study128 manipulated CINs during the extinction of a cocaine-context association. Increased CIN activity was associated with increased presynaptic plasticity (corresponding to reduced glutamate release) and increased extinction learning. In addition, extended extinction training in control animals was associated with a similar change in synaptic plasticity, suggesting that CINs accelerate the plasticity associated with extinction learning. SNc, substantia nigra pars compacta; VTA, ventral tegmental area. Part b is adapted with permission from reF.128, Elsevier.
Classic ideas about the role of CINs in learning and decision-making come from extracellular recordings of tonically active neurons (TANs), which are thought to be CINs on the basis of in vitro characterization or in vivo intracellular or juxtacellular recordings followed by histological identification113,118–120. TANs transiently respond to motivationally relevant stimuli with brief pauses that are often flanked by bursts of increased activity65,113,121–126. Interestingly, TANs tend to exhibit these pause–burst responses to both appetitive and aversive stimuli. This tendency is in stark contrast to DA neurons, which tend to respond positively to reward and negatively to aversive stimuli (signalling RPE). Thus, CINs are thought to represent the ‘salience’ or ‘motivational significance’ of stimuli, which potentially has a role in modulating the rate of learning rather than providing a reinforcement signal that can directly drive learning.
Modulating plasticity in medium spiny neurons.
The notion that putative CINs (TANs) represent salience or motivational significance leads to an interesting hypothesis — these neurons may modulate the ‘gain’ of learning and plasticity without being sufficient to drive these processes on their own, because although they respond at moments when learning should occur, they do not provide information about the direction of learning, as responses are similar for appetitive and aversive stimuli.
Recent optogenetic experiments have provided support for the idea that CINs modulate the gain on learning. For example, increasing CIN activity in the NAc hastens the extinction of a cocaine CPP; conversely, decreasing CIN activity can slow the extinction (or acquisition) of these associations127,128. Enhanced extinction learning is accompanied by a decrease in the synaptic strength of glutamatergic inputs to MSNs128 (Fig. 3b). However, this change in synaptic strength does not occur when CINs are activated outside of the learning context. Similarly, activation of CINs is not sufficient on its own (for example, in real-time CPP or intra-cranial self-stimulation tasks) to support learning128. Together, these findings suggest that CINs regulate the gain on learning when it occurs but do not drive reinforcement learning themselves.
CINs may be particularly essential in regulating the rate of learning in contexts that require flexible updating of previously learned associations129–133. Cell-type-specific lesions of CINs in the dorsal striatum or VS do not affect initial learning of action–outcome associations but do impair performance when task contingencies change130,132. CIN ablation increases perseveration with an old strategy when relevant task features change130 and impairs the ability of the animal to discriminate different action–outcome contingencies in a devaluation test132 (but see reF.131).
How do CINs modulate MSNs to support learning? CINs inhibit MSNs through various mechanisms127,134–136; thus, pauses in CIN activity may disinhibit MSNs, increasing their responsiveness to behaviourally relevant information. In addition, CINs trigger DA release from the striatal terminals of midbrain DA neurons, which may directly enhance plasticity137,138. Moreover, as mentioned above, CINs can regulate plasticity of glutamatergic input–MSN synapses128. How each of these mechanisms affects learning and decision-making, and what other mechanisms may be important, are key open areas of research in the field.
Glutamatergic inputs
A crucial component of the striatal circuit is the glutamatergic input that converges in the striatum from the cortex as well as from subcortical structures such as the thalamus, amygdala and hippocampus. Cortical and thalamic neurons project topographically to the striatum, such that different striatal subregions receive distinct combinations of cortical and thalamic inputs139–146 (Fig. 4a). In fact, unsupervised clustering of the anatomical distribution of glutamatergic inputs has been used to recover boundaries between traditional striatal subregions (for example, the DMS, DLS and NAc) and to discover new subregions of the striatum (mostly within the DMS)147,148.
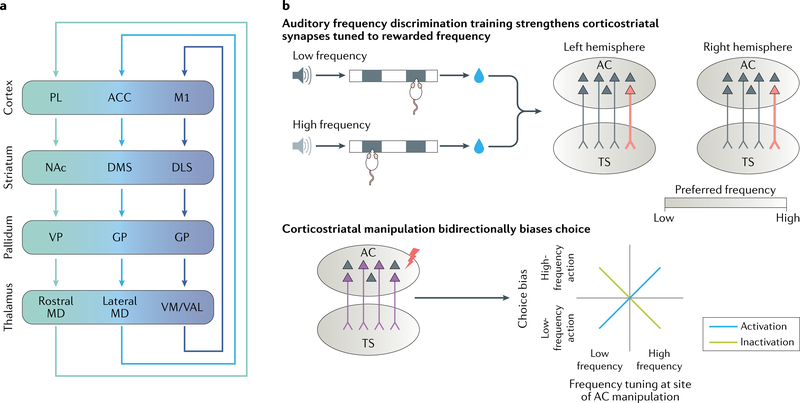
Fig. 4 |. Glutamatergic inputs to the striatum.
a | Schematic of three example cortico–basal ganglia–thalamo–cortical loops with corticostriatal projections from the prelimbic cortex (PL), anterior cingulate cortex (ACC) and primary motor cortex (M1). Inputs to the striatum are topographically organized, and this organization persists throughout the basal ganglia140,147,148. b | Glutamatergic inputs provide functional specialization to striatal subregions. For example, the projection from the auditory cortex (AC) to the tail of the striatum (TS) is tonotopically organized, and frequency tuning of TS neurons corresponds to that of their AC inputs158. When rats learn to perform a right or left nose poke in response to a low-frequency or high-frequency auditory stimulus to obtain reward (upper left), corticostriatal synapses tuned to the rewarded frequency are selectively potentiated (indicated in red, upper right). Thus, if mice learn to perform a right nose poke following a low-frequency stimulus, inputs from the left (that is, contralateral) AC to the left striatum that are tuned to low frequencies will be strengthened (upper right)158. In the same task, optogenetic manipulation of neurons projecting to the TS from the AC bidirectionally biases choice (lower)152. Activation of these neurons biases choice towards, whereas inhibition biases choice away from, the preferred frequency of the manipulated AC neurons. DLS, dorsolateral striatum; DMS, dorsomedial striatum; GP, globus pallidus; MD, medial dorsal thalamus; NAc, nucleus accumbens; VAL, ventral anterior-lateral complex; VM, ventromedial nucleus; VP, ventral pallidum. Part b is adapted from reF.152, Springer Nature Limited.
Providing functional specialization to striatal sub regions.
A classic idea regarding the striatal circuit is that the neural activity of each glutamatergic input is specific to its target region and determines the function of that region. To test this model, numerous important studies have begun to examine the functional specialization of glutamatergic inputs to the striatum in learning and decision-making tasks by specifically targeting neurons on the basis of their projections149–176.
Several of these studies have supported the idea that glutamatergic inputs provide functional specialization to striatal subregions. For example, glutamatergic inputs to the TS are thought to be specialized for processing sensory information and supporting sensory-guided decisions177–180. Projections from the auditory cortex to the TS are tonotopically organized158, and neurons recorded in the TS have similar auditory responses as the auditory cortex neurons innervating them152 (Fig. 4b). In a two-choice auditory discrimination task, specifically stimulating auditory cortical neurons projecting to the striatum biases choice towards the action associated with the preferred frequency of the simulated neurons, whereas inhibition induces the opposite effect152. In addition, after rats learn this auditory discrimination task, synapses from corticostriatal neurons that encode the rewarded auditory stimuli are selectively potentiated158.
Further evidence of input specialization comes from examining the role of inputs from the mPFC to the NAc in learning (TABle 1). These neurons are involved in learning associations between a conspecific and a spatial location165 but are not required for the acquisition of Pavlovian conditioning (although they are involved in expression of conditioned behaviour)164. Furthermore, mPFC–NAc projections are not involved in learning the association of a particular action or cue with a reward (although they are involved in switching between these tasks)171. Thus, the projection from the mPFC to the NAc seems specialized to support only some types of learning.
Table 1 |.
Optogenetic tests of glutamatergic inputs to the NAc in learning and decision-making
| Projection | Target | Behaviours | Resulta | Refs |
|---|---|---|---|---|
| mPFC | NAc | ICSS and CPPb | Activation of terminals reinforces the behaviour that triggers stimulation | 150 |
| Activation of terminals has no effect on the behaviour that triggers stimulation | 149,164 | |||
| Activation of mPFC–NAc neurons reduces time spent in the stimulated spatial location | 168 | |||
| mPFC (PL) | NAc | Pavlovian conditioning | Activation of mPFC–NAc neurons increases, and inactivation decreases, expression of conditioned reward-seeking behaviour | 164 |
| mPFC (PL) | NAc core | Social CPP | Activation of mPFC–NAc neurons increases, and inactivation decreases, learning of association between a social target and spatial location | 165 |
| mPFC (PL) | NAc core | Task switching | Activation of PL terminals decreases and inhibition of PL terminals increases perseverative errors | 171 |
| BLA | NAc | ICSS and CPP | Stimulation of BLA–NAc projection increases performance of operant behaviour that triggers stimulation | 149,150,157 |
| Stimulation of BLA–NAc projection increases time spent in stimulation-paired spatial location | 150 | |||
| BLA | NAc | Pavlovian conditioning | Inhibition of BLA–NAc terminals decreases conditioned reward-seeking | 149 |
| vHipp | NAc shell | ICSS and real-time CPP | Activation of vHipp–NAc terminals reinforces operant behaviour and increases time spent in stimulated spatial location | 150 |
| vHipp | NAc shell | Social memory | Inhibition of vHipp–NAc terminals impairs social discrimination | 161 |
| vHipp | NAc | CPP | Optogenetically induced LTP of vHipp–NAc synapses increases time spent in stimulated spatial location; inhibition of vHipp–NAc terminals impairs association of a social target with a spatial location | 173 |
| dCA1 | NAc | CPP | Inhibition of dCA1–NAc terminals impairs retrieval of sucrose CPP | 175 |
| PVT | NAc shell | CPA | Activation of PVT–NAc terminals decreases time spent in stimulated spatial location | 160 |
| ILT | NAc | Social stress | Inhibition of ILT–NAc terminals decreases social avoidance following chronic social defeat stress | 159 |
| VTA (glutamatergic neurons) | NAc | ICSS | Stimulation of VTA–NAc glutamatergic terminals reinforces operant behaviour | 162 |
BLA, basolateral amygdala; CPA, conditioned place aversion; CPP, conditioned place preference; dCA1, dorsal area CA1 of the hippocampus; ICSS, intracranial self-stimulation; ILT, intralaminar thalamus; LTP, long-term potentiation; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; PL, prelimbic cortex; PVT, paraventricular thalamus; vHipp, ventral hippocampus; VTA, ventral tegmental area.
All experiments used optogenetics to activate or inhibit neurons or terminals.
Results differ across experiments.
In addition to whether a specific input is specialized for a specific behavioural function, a related question is whether multiple inputs to the same target region have different or redundant functions. In fact, several inputs to the NAc seem to be specialized for reward learning, which is thought to be a major function of that subregion (TABle 1). For example, several inputs to the NAc seem to be reinforcing: mice will learn to perform an action that triggers optogenetic stimulation of the projection to the NAc from the basolateral amygdala (BLA)149,150,157 or the ventral hippocampus150. Consistent with these observations, inactivation of the projection from the BLA to the NAc reduces conditioned licking in response to a reward-predicting cue149. In contrast, inhibiting this projection does not affect the acquisition of fear learning157. Whereas multiple inputs to the NAc support reward learning, several inputs from the thalamus may have opposing, aversive effects. Stimulation of the projection from the paraventricular thalamus (PVT) to the NAc is aversive, and weakening this projection through optogenetically induced long-term depression attenuates the expression of aversive symptoms of opiate withdrawal160. Moreover, chronic social defeat strengthens the projection from the intralaminar thalamus to the NAc, and optogenetic inhibition of this projection reduces the resultant social avoidance, whereas optogenetic activation reduces social interaction159.
Comparing inputs to the DMS from different regions of the prefrontal cortex also reveals evidence of functional differences across projections. In a T-maze, optogenetic manipulation of inputs from the prelimbic cortex (PL) affects decision-making only when the choice that maximizes reward is distinct from the choice that minimizes an aversive stimulus (in this case, bright light)155. By contrast, manipulation of the projection from the anterior cingulate cortex (ACC) affects multiple types of cost–benefit comparison155.
Together, these studies suggest that projections to the striatum show some functional differentiation. However, more work is needed to determine how much redundancy exists across glutamatergic inputs.
Summary and future outlook
The recent application of techniques for cell-type-specific monitoring and manipulation of distinct neuronal populations in the striatum has allowed rigorous testing of several classic ideas of striatal function. As summarized in TABle 2, many of these studies support classic models, whereas others provide unexpected insights that challenge and contradict certain prevailing ideas. Thus, new models are needed to better understand striatal contributions to learning and decision-making.
Table 2 |.
Genetic and optical tests of models of striatal circuits in learning and decision-making
| Classic view | Method | Result | Supports classic view? | Refs |
|---|---|---|---|---|
| DA functions as a teaching signal | Optogenetic manipulation | DA activation promotes Pavlovian conditioning and inhibition promotes extinction of Pavlovian overexpectation | Yes | 25,47,54,206 |
| DA manipulation bidirectionally modulates time spent in a laser-paired location | Yes | 49,50 | ||
| DA manipulation bidirectionally modulates model-based associations | Yes | 48 | ||
| DA manipulation bidirectionally modulates performance of Yes stimulation-associated operant behaviours | Yes | 50–52,55 | ||
| DA neurons encode RPE | Recording from DA neurons or their striatal axons (using Ca2+ imaging or optotagging) | Identified DA neurons encode RPE and/or reward | Yes | 36,58,59,63 |
| Identified DA neurons encode non-RPE information | No | 23,55,58–61,63 | ||
| Optogenetic manipulation | Manipulation of SNc DA cell bodies or terminals bidirectionally modulates movement | No | 59,63,64 | |
| Activation of DA neurons projecting to the TS reinforces avoidance behaviour | No | 60 | ||
| D1R and D2R neurons oppositely modulate behaviour | Optogenetic manipulation | Activation of D1R MSNs increases whereas activation of D2R MSNs decreases spontaneous movement | Yes | 72,183,184 |
| In value-based decision-making, activation of D1R and D2R Yes MSNs oppositely biases choice | Yes | 90,102 | ||
| Activation of D1R MSNs promotes performance of a stimulation-paired behaviour and activation of D2R MSNs decreases performance of a stimulation-paired behaviour (in the DMS and NAc) | Yes | 103–105 | ||
| Activation of D1R and D2R MSNs in the DLS promotes pressing of stimulation-paired lever, but activation of D2R MSNs in the DLS also increases pressing of an unpaired lever | No | 108 | ||
| Activation of D1R and D2R MSNs promotes performance of a stimulation-paired behaviour, but only D1R activation increases time spent in a stimulation-paired location (in the NAc) |
No | 109 | ||
| Activation of D1R and D2R MSNs promotes go responses in No a sensory go/no-go task | No | 106 | ||
| Activation of D1R and D2R MSNs increases motivation | No | 110 | ||
| D1R MSN inhibition slows action initiation but D2R MSN inhibition decreases task engagement | No | 89,107 | ||
| D1R and D2R MSNs oppositely encode behavioural variables | Recording from identified neurons (Ca2+ imaging or optotagging) | D1R and D2R neurons are concurrently active during spontaneous and trained movement | No | 79–84,87,89–91 |
| D1R and D2R neurons oppositely encode value | Yes | 90,91,99 | ||
| CIN pause–burst activity signals salience and modulates learning | Optogenetic manipulation | Modulation of CINs bidirectionally modulates the rate of cocaine CPP extinction | Yes | 127,128 |
| Cell-type-specific ablation | CIN ablation impairs flexible updating of learned associations | Yes | 130–132 |
CIN, cholinergic interneuron; CPP, conditioned place preference; D1R, D1 dopamine receptor; D2R, D2 dopamine receptor; DA, dopamine; DLS, dorsolateral striatum; DMS, dorsomedial striatum; MSN, medium spiny neuron; NAc, nucleus accumbens; RPE, reward prediction error; SNc, substantia nigra pars compacta; TS, tail of the striatum.
For example, DA neurons terminating in the striatum can show heterogeneous signals during complex decision-making58. This suggests that the model positing that these neurons provide only RPE signals to the striatum is incomplete. It is possibile that heterogeneous signals in DA neurons actually represent specialized types of prediction error to support specific types of learning. For example, DA inputs to the TS have been suggested to signal errors in threat prediction60. However, at this point, whether heterogenous DA signals can be viewed as specialized types of prediction error to support specific aspects of learning is not clear. Indeed, a recent study examined whether activation of DA projections to DMS during a contralateral choice in a value-based decision-making task correlates more with contralateral movement or with a specialized RPE with respect to contralateral movements, and concluded that the signal is more related to movement62. Thus, some DA signals may not reflect a prediction error at all.
Even if all DA signals may not reflect RPE, all DA neurons presumably modulate plasticity and excitability in the striatum by releasing DA. Thus, inasmuch as DA activity correlates with movement, striatal plasticity and excitability would be modulated by movement59,63 rather than (or in addition to) reward. Such movement-generated plasticity may modulate the continuity and vigour of ongoing movements. Similarly, inasmuch as DA activity correlates with an internal state, such as behavioural accuracy during decision-making58, DA release and subsequent DA-mediated plasticity may maintain the continuity of the ongoing internal state. To interrogate the function of specialized non-RPE DA signals, new studies are needed that specifically target functional subpopulations of DA neurons during learning and decision-making paradigms that elicit these signals.
Recordings of indirect and direct pathway MSNs also provide exciting new challenges to classic models. The major challenge arises from the fact that D1R and D2R MSNs seem to be co-active during trained and spontaneous movements79–82,84,90,91. Consistent with the classic model, however, the activity of D1R and D2R neurons is oppositely modulated by value in reinforcement learning and decision-making paradigms90,91,99, potentially through differential effects of a DA signal on the synaptic plasticity at (or excitability of) D1R and D2R MSNs40,42,181,182. In this framework, inputs that are active around the time of reward will be potentiated if they target D1R MSNs or weakened if they target D2R MSNs. Thus, opposing activity patterns in D1R and D2R neurons may be most evident in the case of learning and decision-making paradigms, when DA is released at specific time points to differentially modulate the two pathways. The specific learning paradigms that are relevant to each striatal subregion might differ; therefore, opposing activity in the two pathways may be behaviour-specific. This idea would be best tested by inhibiting endogenous activity in each subpopulation during learning and decision-making tasks rather than through artificial activation. To date, most optogenetic interrogations of MSN function have relied on excitatory opsins72,90,102–105,183,184, which strongly and synchronously activate many neurons in an artificial pattern and thus provide little insight into whether the endogenous activity in the two populations is opposing. Thus, despite many foundational experiments, the classic go/no-go model remains to be fully tested.
Indeed, despite extensive progress in recent years, several hypotheses from classic models of striatal function have yet to be fully tested. For example, CINs are thought to signal salient events through their pause–burst firing and are presumed to support learning. However, these activity patterns have not yet been directly replicated during learning and decision-making, and whether pauses in CIN activity are indeed crucial to their modulation of learning is unknown (although see ref.185 for indirect manipulation of CINs).
In addition, more work is needed to relate neural activity in glutamatergic inputs to classic ideas that their plasticity underlies learning and decision-making. For example, a basic test of the idea that corticostriatal plasticity is a neural substrate of reward-based learning is whether glutamatergic projections that are involved in learning new behavioural associations are also required for their expression186. Whether or not that is the case is not yet clear. Furthermore, whether specific glutamatergic inputs are specialized to support or modulate different elements of task execution, such as motivation or action selection, is also unclear. Addressing these questions will require systematic comparisons of multiple glutamatergic inputs at different time points, both within a trial and across learning, within a consistent behavioural framework.
In summary, studies that have recorded and manipulated identified neuronal populations have addressed many longstanding hypotheses regarding the role of striatal circuits in learning and decision-making. Some components of these classic models have been upheld by this new evidence, although important challenges to classic ideas have also emerged. Future experiments must be designed to address these challenges and the important ideas that remain untested.
Acknowledgements
The authors thank L. Pinto for comments on this manuscript and W. Fleming for providing a figure schematic. This work was funded by New York Stem Cell Foundation (NYSCF), Pew, McKnight, NARSAD (US National Alliance for Research on Schizophrenia and Depression) and Sloan Foundation grants to I.B.W.; US National Institutes of Health (NIH) grants U19 NS104648-01, DP2 DA035149-01 and 5R01MH106689-02 (to I.B.W.) and F32 MH112320-02 (to J.C.); and Army Research Office grant W911NF-17-1-0554. I.B.W. is an NYSCF-Robertson Investigator.
Glossary
- Basal ganglia
An evolutionarily conserved group of interconnected subcortical nuclei that are involved in motor, cognitive and limbic processes
- Reinforcement learning
learning process in which performance of a behaviour is modified by positive or negative feedback
- Medial forebrain bundle
A white-matter tract that contains dopaminergic axons travelling from the ventral tegmental area and substantia nigra pars compacta to the striatum
- Stimulus–outcome associations
Associations between sensory stimuli and the outcomes they predict, which induce conditioned behaviours, although experience of the outcome is independent of that behaviour
- Stimulus–response associations
Associations that result in the performance of actions in response to sensory stimuli, regardless of the value of the outcomes of the actions
- Action–outcome associations
Associations between actions (or responses) and the outcomes of those actions, the performance of which depends on the value of the outcomes
- Probabilistic reversal learning task
A behavioural task in which participants learn associations between actions and reward probabilities that are then reversed, requiring updating of learned associations
- Conditioned place preference: (CPP)
An assay for measuring context-reward associations that evaluates how much time animals spend in a spatial location associated with a particular stimulus
- Devaluation test
A measurement of performance of an action with a learned outcome that becomes devalued (for example, with satiety) to assess whether a behaviour is more goal-directed or habitual
- Cost–benefit comparison
A comparison between actions that are associated with both a benefit (such as reward) and a cost (such as punishment)
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Neuroscience thanks D. Sulzer and the other, anonymous reviewers for their contribution to the peer review of this work.
References
- 1.Alexander GE, DeLong MR & Strick PL Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci 9, 357–381 (1986). [DOI] [PubMed] [Google Scholar]
- 2.Alexander GE & Crutcher MD Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266–271 (1990). [DOI] [PubMed] [Google Scholar]
- 3.Redgrave P et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat. Rev. Neurosci 11, 760–772 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerfen CR & Bolam JP in Handbook of Basal Ganglia Structure and Function 2nd edn Vol. 24 (eds Steiner H & Tseng KY) 3–32 (Elsevier, 2016). [Google Scholar]
- 5.Loopuijt LD & van der Kooy D Organization of the striatum: collateralization of its efferent axons. Brain Res. 348, 86–99 (1985). [DOI] [PubMed] [Google Scholar]
- 6.Gerfen CR & Scott Young W Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 460, 161–167 (1988). [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi Y, Wilson CJ & Emson PC Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J. Neurosci. 10, 3421–3438 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerfen CR et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432 (1990). [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Richard S & Parent A The organization of the striatal output system: a single-cell juxtacellular labeling study in the rat. Neurosci. Res. 38, 49–62 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Albin RL, Young AB & Penney JB The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 (1989). [DOI] [PubMed] [Google Scholar]
- 11.Chevalier G & Deniau JM Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 13, 277–280 (1990). [DOI] [PubMed] [Google Scholar]
- 12.DeLong MR Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285 (1990). [DOI] [PubMed] [Google Scholar]
- 13.Mink JW The basal ganglia: focused selection and inhibition of competing motor programs. Prog. Neurobiol. 50, 381–425 (1996). [DOI] [PubMed] [Google Scholar]
- 14.Lanciego JL, Luquin N & Obeso JA Functional neuroanatomy of the basal ganglia. Cold Spring Harb. Perspect. Med 2, a009621 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson AB & Kreitzer AC Reassessing models of basal ganglia function and dysfunction. Annu. Rev. Neurosci 37, 117–135 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolam JP, Wainer BH & Smith AD Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience 12, 711–718 (1984). [DOI] [PubMed] [Google Scholar]
- 17.Burke DA, Rotstein HG & Alvarez VA Striatal local circuitry: a new framework for lateral inhibition. Neuron 96, 267–284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tepper JM & Koós T in Handbook of Basal Ganglia Structure and Function 2nd edn Vol. 24 (eds Steiner H & Tseng KY) 157–178 (Elsevier, 2016). [Google Scholar]
- 19.Berke JD Functional properties of striatal fast-spiking interneurons. Front. Syst. Neurosci. 5, 45 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckstead RM, Domesick VB & Nauta WJ Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 175, 191–217 (1979). [DOI] [PubMed] [Google Scholar]
- 21.Swanson LW The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 9, 321–353 (1982). [DOI] [PubMed] [Google Scholar]
- 22.Lammel S et al. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57, 760–773 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Lerner TN et al. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beier KT et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162, 622–634 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders BT, Richard JM, Margolis EB & Janak PH Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat. Neurosci. 21, 1072–1083 (2018). This study shows how VTA DA activation increases the value of an associated conditioned stimulus whereas SNc DA activation increases conditioned responding to the conditioned stimulus without increasing its value. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poulin J-F et al. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci. 21, 1260–1271 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montague PR, Dayan P & Sejnowski TJ A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J. Neurosci. 16, 1936–1947 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultz W, Dayan P & Montague PR A neural substrate of prediction and reward. Science 275, 1593–1599 (1997). This seminal paper connects DA activity with reinforcement learning models. [DOI] [PubMed] [Google Scholar]
- 29.Mirenowicz J & Schultz W Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature 379, 449–451 (1996). [DOI] [PubMed] [Google Scholar]
- 30.Hollerman JR & Schultz W Dopamine neurons report an error in the temporal prediction of reward during learning. Nat. Neurosci. 1, 304–309 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Schultz W Predictive reward signal of dopamine neurons. J. Neurophysiol. 80, 1–27 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Fiorillo CD, Tobler PN & Schultz W Discrete coding of reward probability and uncertainty by dopamine neurons. Science 299, 1898–1902 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Roesch MR, Calu DJ & Schoenbaum G Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nat. Neurosci. 10, 1615–1624 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Day JJ, Roitman MF, Wightman RM & Carelli RM Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat. Neurosci. 10, 1020–1028 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Bromberg-Martin ES, Matsumoto M & Hikosaka O Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen JY, Haesler S, Vong L, Lowell BB & Uchida N Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482, 85–88 (2012). This study employs phototagging to confirm that VTA DA neurons represent RPE whereas VTA GABA neurons represent expected reward. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eshel N et al. Arithmetic and local circuitry underlying dopamine prediction errors. Nature 525, 243–246 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds JNJ, Hyland BI & Wickens JR A cellular mechanism of reward-related learning. Nature 413, 67–70 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Reynolds JNJ & Wickens JR Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 15, 507–521 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Shen W, Flajolet M, Greengard P & Surmeier DJ Dichotomous dopaminergic control of striatal synaptic plasticity. Science 321, 848–851 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerfen CR & Surmeier DJ Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441–466 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bamford NS, Wightman RM & Sulzer D Dopamine’s effects on corticostriatal synapses during reward-based behaviors. Neuron 97, 494–510 (2018). This recent review discusses mechanisms by which DA affects corticostriatal synapses and MSN activity during reward-seeking behaviours. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olds J Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science 127, 315–324 (1958). [DOI] [PubMed] [Google Scholar]
- 44.Corbett D & Wise RA Intracranial self-stimulation in relation to the ascending dopaminergic systems of the midbrain: a moveable electrode mapping study. Brain Res. 185, 1–15 (1980). [DOI] [PubMed] [Google Scholar]
- 45.Fouriezos G & Wise RA Pimozide-induced extinction of intracranial self-stimulation: response patterns rule out motor or performance deficits. Brain Res. 103, 377–380 (1976). [DOI] [PubMed] [Google Scholar]
- 46.Wise RA Forebrain substrates of reward and motivation. J. Comp. Neurol. 493, 115–121 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinberg EE et al. A causal link between prediction errors, dopamine neurons and learning. Nat. Neurosci. 16, 966–973 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharpe MJ et al. Dopamine transients are sufficient and necessary for acquisition of model-based associations. Nat. Neurosci. 20, 735–742 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai H-C et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ilango A et al. Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J. Neurosci. 34, 817–822 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Witten IB et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 72, 721–733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamid AA et al. Mesolimbic dopamine signals the value of work. Nat. Neurosci. 19, 117–126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adamantidis AR et al. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J. Neurosci. 31, 10829–10835 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang CY et al. Brief optogenetic inhibition of dopamine neurons mimics endogenous negative reward prediction errors. Nat. Neurosci. 19, 111–116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parker NF et al. Reward and choice encoding in terminals of midbrain dopamine neurons depends on striatal target. Nat. Neurosci. 19, 845–854 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Doherty J et al. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304, 452–454 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Balleine BW, Delgado MR & Hikosaka O The role of the dorsal striatum in reward and decision-making. J. Neurosci. 27, 8161–8165 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engelhard B et al. Specialized coding of sensory, motor and cognitive variables in VTA dopamine neurons. Nature 10.1038/s41586-019-1261-9 (2019). Cellular resolution imaging of VTA DA neurons reveals widespread reward representations multiplexed with specialized representations of task variables. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howe MW & Dombeck DA Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535, 505–510 (2016). Axonal imaging of DA terminals in the dorsal striatum reveals that distinct axons signal locomotion and reward. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Menegas W, Akiti K, Amo R, Uchida N & Watabe-Uchida M Dopamine neurons projecting to the posterior striatum reinforce avoidance of threatening stimuli. Nat. Neurosci. 275, 1593 (2018). This paper shows that DA in the TS supports learning to avoid threatening stimuli whereas DA in the NAc supports learning to pursue rewarding stimuli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menegas W, Babayan BM, Uchida N & Watabe-Uchida M Opposite initialization to novel cues in dopamine signaling in ventral and posterior striatum in mice. eLife 6, e21886 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee RS, Mattar MG, Parker NF, Witten IB & Daw ND Reward prediction error does not explain movement selectivity in DMS-projecting dopamine neurons. eLife 8, e42992 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.da Silva JA, Tecuapetla F, Paixão V & Costa RM Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 554, 244–248 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Barter JW et al. Beyond reward prediction errors: the role of dopamine in movement kinematics. Front. Integr. Neurosci. 9, 39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joshua M, Adler A, Mitelman R, Vaadia E & Bergman H Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J. Neurosci. 28, 11673–11684 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsumoto M & Hikosaka O Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459, 837–841 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brischoux F, Chakraborty S, Brierley DI & Ungless MA Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl Acad. Sci. USA 106, 4894–4899 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gangarossa G et al. Spatial distribution of D1R-and D2R-expressing medium-sized spiny neurons differs along the rostro-caudal axis of the mouse dorsal striatum. Front. Neural Circuits 7, 124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hikosaka O, Takikawa Y & Kawagoe R Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol. Rev. 80, 953–978 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Calabresi P, Picconi B, Tozzi A, Ghiglieri V & Di Filippo M Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat. Neurosci. 17, 1022–1030 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Oldenburg IA & Sabatini BL Antagonistic but not symmetric regulation of primary motor cortex by basal ganglia direct and indirect pathways. Neuron 86, 1174–1181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roseberry TK et al. Cell-type-specific control of brainstem locomotor circuits by basal ganglia. Cell 164, 526–537 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lauwereyns J, Watanabe K, Coe B & Hikosaka O A neural correlate of response bias in monkey caudate nucleus. Nature 418, 413–417 (2002). [DOI] [PubMed] [Google Scholar]
- 74.Samejima K, Ueda Y, Doya K & Kimura M Representation of action-specific reward values in the striatum. Science 310, 1337–1340 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Lau B & Glimcher PW Value representations in the primate striatum during matching behavior. Neuron 58, 451–463 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ding L & Gold JI Caudate encodes multiple computations for perceptual decisions. J. Neurosci. 30, 15747–15759 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gong S et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci. 27, 9817–9823 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gerfen CR, Paletzki R & Heintz N GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 80, 1368–1383 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cui G et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238–242 (2013). This study is the first to show that D1R and D2R MSNs are co-activated during movement and inactive during immobility, contrary to some theories of striatal function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barbera G et al. Spatially compact neural clusters in the dorsal striatum encode locomotion relevant information. Neuron 92, 202–213 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klaus A et al. The spatiotemporal organization of the striatum encodes action space. Neuron 96, 949 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Markowitz JE et al. The striatum organizes 3D behavior via moment-to-moment action selection. Cell 174, 44–58 (2018). This study uses machine learning algorithms to characterize spontaneous behaviour into discrete, subsecond components and describes D1R and D2R MSN responses to the identity and sequence of these behavioural components. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parker JG et al. Diametric neural ensemble dynamics in parkinsonian and dyskinetic states. Nature 557, 177–182 (2018). This study thoroughly probes the effects of DA depletion and subsequent administration of dopaminergic agonists and antagonists on the activity of D1R-expressing and D2R-expressing MSNs in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meng C et al. Spectrally resolved fiber photometry for multi-component analysis of brain circuits. Neuron 98, 707–717 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.London TD et al. Coordinated ramping of dorsal striatal pathways preceding food approach and consumption. J. Neurosci. 38, 3547–3558 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Isomura Y et al. Reward-modulated motor information in identified striatum neurons. J. Neurosci. 33, 10209–10220 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jin X, Tecuapetla F & Costa RM Basal ganglia subcircuits distinctively encode the parsing and concatenation of action sequences. Nat. Neurosci. 17, 423–430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sippy T, Lapray D, Crochet S & Petersen CCH Cell-type-specific sensorimotor processing in striatal projection neurons during goal-directed behavior. Neuron 88, 298–305 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Geddes CE, Li H & Jin X Optogenetic editing reveals the hierarchical organization of learned action sequences. Cell 174, 32–43 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nonomura S et al. Monitoring and updating of action selection for goal-directed behavior through the striatal direct and indirect pathways. Neuron 99, 1302–1314 (2018). [DOI] [PubMed] [Google Scholar]
- 91.Donahue CH, Liu M & Kreitzer A Distinct value encoding in striatal direct and indirect pathways during adaptive learning. Preprint at bioRxiv 10.1101/277855 (2018). [DOI] [Google Scholar]
- 92.Tecuapetla F, Matias S, Dugue GP, Mainen ZF & Costa RM Balanced activity in basal ganglia projection pathways is critical for contraversive movements. Nat. Commun. 5, 4315 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cazorla M et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron 81, 153–164 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Collins AGE & Frank MJ Opponent actor learning (OpAL): modeling interactive effects of striatal dopamine on reinforcement learning and choice incentive. Psychol. Rev. 121, 337–366 (2014). [DOI] [PubMed] [Google Scholar]
- 95.Bariselli S, Fobbs WC, Creed MC & Kravitz AV A competitive model for striatal action selection. Brain Res. 1713, 70–79 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frank MJ, Seeberger LC & O’reilly RC By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science 306, 1940–1943 (2004). [DOI] [PubMed] [Google Scholar]
- 97.Peak J, Hart G & Balleine BW From learning to action: the integration of dorsal striatal input and output pathways in instrumental conditioning. Eur. J. Neurosci. 49, 658–671 (2019). [DOI] [PubMed] [Google Scholar]
- 98.Yartsev MM, Hanks TD, Yoon AM & Brody CD Causal contribution and dynamical encoding in the striatum during evidence accumulation. eLife 7, e34929 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shin JH, Kim D & Jung MW Differential coding of reward and movement information in the dorsomedial striatal direct and indirect pathways. Nat. Commun. 9, 404 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zalocusky KA et al. Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature 531, 642–646 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lau B & Glimcher PW Dynamic response-by-response models of matching behavior in rhesus monkeys. J. Exp. Anal. Behav. 84, 555–579 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tai L-H, Lee AM, Benavidez N, Bonci A & Wilbrecht L Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat. Neurosci. 15, 1281–1289 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kravitz AV, Tye LD & Kreitzer AC Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat. Neurosci. 15, 816–818 (2012). This paper shows how D1R and D2R MSN activity in DMS is sufficient to positively and negatively reinforce intracranial self-stimulation, respectively, but this learning does not depend on DA transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yttri EA & Dudman JT Opponent and bidirectional control of movement velocity in the basal ganglia. Nature 533, 402–406 (2016). This study shows that D1R and D2R MSN activity in DMS is sufficient to positively and negatively reinforce movement velocity, respectively, and this learning depends on DA transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lobo MK et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330, 385–390 (2010). D1R and D2R MSN activity in NAc enhances or suppresses, respectively, the establishment of a cocaine CPP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang L, Rangarajan KV, Gerfen CR & Krauzlis RJ Activation of striatal neurons causes a perceptual decision bias during visual change detection in mice. Neuron 98, 669 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tecuapetla F, Jin X, Lima SQ & Costa RM Complementary contributions of striatal projection pathways to action initiation and execution. Cell 166, 703–715 (2016). [DOI] [PubMed] [Google Scholar]
- 108.Vicente AM, Galvão-Ferreira P, Tecuapetla F & Costa RM Direct and indirect dorsolateral striatum pathways reinforce different action strategies. Curr. Biol. 26, R267–269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cole SL, Robinson MJF & Berridge KC Optogenetic self-stimulation in the nucleus accumbens: D1 reward versus D2 ambivalence. PLOS ONE 13, e0207694 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soares-Cunha C et al. Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat. Commun. 7, 11829 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carvalho Poyraz F et al. Decreasing striatopallidal pathway function enhances motivation by energizing the initiation of goal-directed action. J. Neurosci. 36, 5988–6001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gallo EF et al. Accumbens dopamine D2 receptors increase motivation by decreasing inhibitory transmission to the ventral pallidum. Nat. Commun. 9, 1086 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Apicella P The role of the intrinsic cholinergic system of the striatum: what have we learned from TAN recordings in behaving animals? Neuroscience 360, 81–94 (2017). [DOI] [PubMed] [Google Scholar]
- 114.Hasselmo ME The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 16, 710–715 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Macintosh FC The distribution of acetylcholine in the peripheral and the central nervous system. J. Physiol. 99, 436–442 (1941). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hebb CO & Silver A Gradient of cholinesterase activity and of choline acetylase activity in nerve fibres: gradient of choline acetylase activity. Nature 189, 123–125 (1961). [DOI] [PubMed] [Google Scholar]
- 117.Lim SAO, Kang UJ & McGehee DS Striatal cholinergic interneuron regulation and circuit effects. Front. Synaptic Neurosci. 6, 22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wilson CJ, Chang HT & Kitai ST Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J. Neurosci. 10, 508–519 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Inokawa H, Yamada H, Matsumoto N, Muranishi M & Kimura M Juxtacellular labeling of tonically active neurons and phasically active neurons in the rat striatum. Neuroscience 168, 395–404 (2010). [DOI] [PubMed] [Google Scholar]
- 120.Schulz JM, Oswald MJ & Reynolds JNJ Visual-induced excitation leads to firing pauses in striatal cholinergic interneurons. J. Neurosci. 31, 11133–11143 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kimura M, Rajkowski J & Evarts E Tonically discharging putamen neurons exhibit set-dependent responses. Proc. Natl Acad. Sci. USA 81, 4998–5001 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aosaki T et al. Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. J. Neurosci. 14, 3969–3984 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Graybiel AM, Aosaki T, Flaherty AW & Kimura M The basal ganglia and adaptive motor control. Science 265, 1826–1831 (1994). [DOI] [PubMed] [Google Scholar]
- 124.Aosaki T, Graybiel AM & Kimura M Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science 265, 412–415 (1994). [DOI] [PubMed] [Google Scholar]
- 125.Ravel S, Legallet E & Apicella P Tonically active neurons in the monkey striatum do not preferentially respond to appetitive stimuli. Exp. Brain Res. 128, 531–534 (1999). [DOI] [PubMed] [Google Scholar]
- 126.Goldberg JA & Reynolds JNJ Spontaneous firing and evoked pauses in the tonically active cholinergic interneurons of the striatum. Neuroscience 198, 27–43 (2011). [DOI] [PubMed] [Google Scholar]
- 127.Witten IB et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330, 1677–1681 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee J, Finkelstein J, Choi JY & Witten IB Linking cholinergic interneurons, synaptic plasticity, and behavior during the extinction of a cocaine-context association. Neuron 90, 1071–1085 (2016). This study shows that CINs regulate glutamatergic synaptic plasticity in the NAc during cocaine context extinction in a manner that can explain the associated behavioural changes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bradfield LA, Bertran-Gonzalez J, Chieng B & Balleine BW The thalamostriatal pathway and cholinergic control of goal-directed action: interlacing new with existing learning in the striatum. Neuron 79, 153–166 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aoki S, Liu AW, Zucca A, Zucca S & Wickens JR Role of striatal cholinergic interneurons in set-shifting in the rat. J. Neurosci. 35, 9424–9431 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Okada K et al. Enhanced flexibility of place discrimination learning by targeting striatal cholinergic interneurons. Nat. Commun. 5, 3778 (2014). [DOI] [PubMed] [Google Scholar]
- 132.Matamales M et al. Aging-related dysfunction of striatal cholinergic interneurons produces conflict in action selection. Neuron 90, 362–373 (2016). [DOI] [PubMed] [Google Scholar]
- 133.Collins AL et al. Nucleus accumbens cholinergic interneurons oppose cue-motivated behavior. Biol. Psychiatry 10.1016/j.biopsych.2019.02.014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.English DF et al. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat. Neurosci. 15, 123–130 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nelson AB et al. Striatal cholinergic interneurons drive GABA release from dopamine terminals. Neuron 82, 63–70 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tritsch NX, Oh W-J, Gu C & Sabatini BL Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis. eLife 3, e01936 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cachope R et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2, 33–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Threlfell S et al. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75, 58–64 (2012). [DOI] [PubMed] [Google Scholar]
- 139.Selemon LD & Goldman-Rakic PS Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J. Neurosci. 5, 776–794 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Groenewegen HJ, Berendse HW, Wolters JG & Lohman AH The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog. Brain Res. 85, 95–116 (1990). [DOI] [PubMed] [Google Scholar]
- 141.Flaherty AW & Graybiel AM Corticostriatal transformations in the primate somatosensory system. Projections from physiologically mapped body-part representations. J. Neurophysiol. 66, 1249–1263 (1991). [DOI] [PubMed] [Google Scholar]
- 142.Berendse HW, Galis-de Graaf Y & Groenewegen HJ Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 316, 314–347 (1992). [DOI] [PubMed] [Google Scholar]
- 143.Pan WX, Mao T & Dudman JT Inputs to the dorsal striatum of the mouse reflect the parallel circuit architecture of the forebrain. Front. Neuroanat. 4, 147 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wall NR, De La Parra M, Callaway EM & Kreitzer AC Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron 79, 347–360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guo Q et al. Whole-brain mapping of inputs to projection neurons and cholinergic interneurons in the dorsal striatum. PLOS ONE 10, e0123381 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ & Haber SN Circuit-based corticostriatal homologies between rat and primate. Biol. Psychiatry 80, 509–521 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hunnicutt BJ et al. A comprehensive excitatory input map of the striatum reveals novel functional organization. eLife 5, e19103 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hintiryan H et al. The mouse cortico-striatal projectome. Nat. Neurosci. 19, 1100–1114 (2016). This paper and that of Hunnicutt et al. (2016) provide detailed cortical and thalamic input maps to the striatum and use clustering methods on the anatomical distribution of these inputs to identify striatal subdomains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stuber GD et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Britt JP et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76, 790–803 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Koralek AC, Jin X, Long JD 2nd, Costa RM & Carmena JM Corticostriatal plasticity is necessary for learning intentional neuroprosthetic skills. Nature 483, 331–335 (2012). This study shows that corticostriatal plasticity is required for learning neuroprosthetic control of motor cortex neurons, irrespective of movement, and that the activity of striatal neurons is modulated by this type of goal-directed learning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Znamenskiy P & Zador AM Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature 497, 482–485 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.MacAskill AF, Cassel JM & Carter AG Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens. Nat. Neurosci. 17, 1198–1207 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pascoli V et al. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509, 459–464 (2014). [DOI] [PubMed] [Google Scholar]
- 155.Friedman A et al. A corticostriatal path targeting striosomes controls decision-making under conflict. Cell 161, 1320–1333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rothwell PE et al. Input- and output-specific regulation of serial order performance by corticostriatal circuits. Neuron 88, 345–356 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Namburi P et al. A circuit mechanism for differentiating positive and negative associations. Nature 520, 675–678 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xiong Q, Znamenskiy P & Zador AM Selective corticostriatal plasticity during acquisition of an auditory discrimination task. Nature 521, 348–351 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Christoffel DJ et al. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat. Neurosci. 18, 962–964 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhu Y, Wienecke CFR, Nachtrab G & Chen X A thalamic input to the nucleus accumbens mediates opiate dependence. Nature 530, 219–222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Okuyama T, Kitamura T, Roy DS, Itohara S & Tonegawa S Ventral CA1 neurons store social memory. Science 353, 1536–1541 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yoo JH et al. Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat. Commun. 7, 13697 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Beyeler A et al. Divergent routing of positive and negative information from the amygdala during memory retrieval. Neuron 90, 348–361 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Otis JM et al. Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature 543, 103–107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Murugan M et al. Combined social and spatial coding in a descending projection from the prefrontal cortex. Cell 171, 1663–1677 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kupferschmidt DA, Juczewski K, Cui G, Johnson KA & Lovinger DM Parallel, but dissociable, processing in discrete corticostriatal inputs encodes skill learning. Neuron 96, 476–489 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Friedman A et al. Chronic stress alters striosome-circuit dynamics, leading to aberrant decision-making. Cell 171, 1191–1205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim CK et al. Molecular and circuit-dynamical identification of top-down neural mechanisms for restraint of reward seeking. Cell 170, 1013–1027 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Amadei EA et al. Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature 546, 297–301 (2017). This paper demonstrates that pair bonding in prairie voles modulates the projection from the mPFC to the NAc and that stimulation of this projection increases preference for a social target. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sweis BM, Larson EB, Redish AD & Thomas MJ Altering gain of the infralimbic-to-accumbens shell circuit alters economically dissociable decision-making algorithms. Proc. Natl Acad. Sci. USA 115, E6347–E6355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cui Q, Li Q, Geng H, Chen L & Ip NY Dopamine receptors mediate strategy abandoning via modulation of a specific prelimbic cortex–nucleus accumbens pathway in mice. Proc. Natl Acad. Sci. USA 115, E4890–E4899 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Díaz-Hernández E et al. The thalamostriatal projections contribute to the initiation and execution of a sequence of movements. Neuron 100, 739–752 (2018). [DOI] [PubMed] [Google Scholar]
- 173.LeGates TA et al. Reward behaviour is regulated by the strength of hippocampus-nucleus accumbens synapses. Nature 564, 258–262 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hart G, Bradfield LA, Fok SY, Chieng B & Balleine BW The bilateral prefronto-striatal pathway is necessary for learning new goal-directed actions. Curr. Biol. 28, 2218–2229 (2018). [DOI] [PubMed] [Google Scholar]
- 175.Trouche S et al. A hippocampus-accumbens tripartite neuronal motif guides appetitive memory in space. Cell 176, 1393–1406 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen L, Wang X, Ge S & Xiong Q Medial geniculate body and primary auditory cortex differentially contribute to striatal sound representations. Nat. Commun. 10, 418 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yamamoto S, Monosov IE, Yasuda M & Hikosaka O What and where information in the caudate tail guides saccades to visual objects. J. Neurosci. 32, 11005–11016 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yamamoto S, Kim HF & Hikosaka O Reward value-contingent changes of visual responses in the primate caudate tail associated with a visuomotor skill. J. Neurosci. 33, 11227–11238 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim HF & Hikosaka O Distinct basal ganglia circuits controlling behaviors guided by flexible and stable values. Neuron 79, 1001–1010 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim HF, Amita H & Hikosaka O Indirect pathway of caudal basal ganglia for rejection of valueless visual objects. Neuron 94, 920–930 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Surmeier DJ, Ding J, Day M, Wang Z & Shen W D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 30, 228–235 (2007). [DOI] [PubMed] [Google Scholar]
- 182.Pawlak V & Kerr JND Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J. Neurosci. 28, 2435–2446 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kravitz AV et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bartholomew RA et al. Striatonigral control of movement velocity in mice. Eur. J. Neurosci. 43, 1097–1110 (2016). [DOI] [PubMed] [Google Scholar]
- 185.Brown MTC et al. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature 492, 452–456 (2012). [DOI] [PubMed] [Google Scholar]
- 186.Kawai R et al. Motor cortex is required for learning but not for executing a motor skill. Neuron 86, 800–812 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Graybiel AM Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 31, 359–387 (2008). [DOI] [PubMed] [Google Scholar]
- 188.Liljeholm M & O’Doherty JP Contributions of the striatum to learning, motivation, and performance: an associative account. Trends Cogn. Sci. 16, 467–475 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gruber AJ & McDonald RJ Context, emotion, and the strategic pursuit of goals: interactions among multiple brain systems controlling motivated behavior. Front. Behav. Neurosci. 6, 50 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Balleine BW & O’Doherty JP Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35, 48–69 (2010). This paper reviews human and rodent studies investigating striatal involvement in goal-directed and habitual behaviour. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yin HH & Knowlton BJ The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 7, 464–476 (2006). [DOI] [PubMed] [Google Scholar]
- 192.Barnes TD, Kubota Y, Hu D, Jin DZ & Graybiel AM Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature 437, 1158–1161 (2005). [DOI] [PubMed] [Google Scholar]
- 193.Yin HH et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat. Neurosci. 12, 333–341 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Thorn CA, Atallah H, Howe M & Graybiel AM Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron 66, 781–795 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dolan RJ & Dayan P Goals and habits in the brain. Neuron 80, 312–325 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.O’Hare JK et al. Pathway-specific striatal substrates for habitual behavior. Neuron 89, 472–479 (2016). This paper shows correlations between performance of habitual behaviour and the strengthening of cortically evoked activity in D1R and D2R MSNs in the DLS as well as changes in the relative timing of activation of the two pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yin HH, Ostlund SB, Knowlton BJ & Balleine BW The role of the dorsomedial striatum in instrumental conditioning. Eur. J. Neurosci. 22, 513–523 (2005). [DOI] [PubMed] [Google Scholar]
- 198.Setlow B, Schoenbaum G & Gallagher M Neural encoding in ventral striatum during olfactory discrimination learning. Neuron 38, 625–636 (2003). [DOI] [PubMed] [Google Scholar]
- 199.Roitman MF, Wheeler RA & Carelli RM Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron 45, 587–597 (2005). [DOI] [PubMed] [Google Scholar]
- 200.Groenewegen HJ, Wright CI, Beijer AV & Voorn P Convergence and segregation of ventral striatal inputs and outputs. Ann. NY Acad. Sci. 877, 49–63 (1999). [DOI] [PubMed] [Google Scholar]
- 201.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A & Uchida N Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012). [DOI] [PubMed] [Google Scholar]
- 202.Mannella F, Gurney K & Baldassarre G The nucleus accumbens as a nexus between values and goals in goal-directed behavior: a review and a new hypothesis. Front. Behav. Neurosci. 7, 135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Menegas W et al. Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. eLife 4, e10032 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Howe MW, Tierney PL, Sandberg SG, Phillips PEM & Graybiel AM Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature 500, 575–579 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Freeze BS, Kravitz AV, Hammack N, Berke JD & Kreitzer AC Control of basal ganglia output by direct and indirect pathway projection neurons. J. Neurosci. 33, 18531–18539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kim KM et al. Optogenetic mimicry of the transient activation of dopamine neurons by natural reward is sufficient for operant reinforcement. PLOS ONE 7, e33612 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]