Abstract
Aims
The use of incretin-based therapy instead of or complementary to insulin therapy is an active area of research in hospitalized patients with type 2 diabetes (T2D). We determined glycaemic efficacy and safety of linagliptin compared to basal-bolus insulin regimen in hospitalized surgical patients with T2D.
Materials and Methods
This prospective open-label multicenter study randomized T2D patients undergoing non-cardiac surgery with admission blood glucose(BG) 7.8–22.2 mmol/L treated with diet, oral agents or total insulin dose(TDD) ≤0.5 units/kg/day to linagliptin(n=128) daily or basal-bolus(n=122) with glargine once daily and rapid-acting insulin before meals. Both groups received supplemental insulin for BG>7.8 mmol/L. The primary endpoint was difference in mean daily BG between groups.
Results
Mean daily BG was inferior in linagliptin compared to basal-bolus group (9.5±2.6 vs. 8.8±2.3 mmol/L/dL, p=0.03) with mean daily BG difference of 0.6mmol/L (95% confidence interval 0.04, 1.2). In patients with randomization BG<11.1 mmol/L (63% of cohort), mean daily BG was similar in linagliptin vs basal-bolus (8.9±2.3 vs. 8.7±2.3 mmol/L, p=0.43); however, patients with BG≥11.1 mmol/L treated with linagliptin had higher BG compared to basal-bolus (10.9±2.6 vs. 9.2±2.2 mmol/L, p<0.001). Linagliptin resulted in fewer hypoglycaemic events (1.6% vs. 11%, p=0.001, 86% relative risk reduction), similar supplemental insulin (and lower number of daily insulin injections (2.0±3.3 vs 3.1±3.3, p<0.001) compared to basal-bolus.
Conclusions
In patients with T2D undergoing non-cardiac surgery presenting with mild to moderate hyperglycaemia (BG <11.1 mmol/L), daily linagliptin is a safe and effective alternative to multi-dose insulin therapy resulting in similar glucose control with lower hypoglycaemia.
Introduction
The association between hyperglycaemia and poor clinical outcomes in patients with and without diabetes is well established 1–5. Extensive data from observational and prospective randomized controlled trials in hospitalized patients have reported a strong association between hyperglycaemia and poor clinical outcomes, such as increased mortality, morbidity, length of stay (LOS), infections and overall complications 1,4,6–8. Most clinical trials in critically ill and in non-critically ill medicine and surgery patients with hyperglycaemia and diabetes have reported that improved glycaemic control reduces LOS, risk of multi-organ failure and systemic infections 9–12, as well as short- and long-term mortality 6,11, although the largest trial in critically ill patients showed increased mortality with intensive glucose control 12. Current clinical guidelines from professional societies recommend basal-bolus insulin regimens as the standard of care for hospitalized patients with type 2 diabetes (T2D) 13,14. Our group has shown that the basal-bolus insulin regimen resulted in improved glycaemic control and lower hospital complications compared to supplemental insulin alone in patients with T2D that were admitted for non-cardiac surgery to general surgical units 15. However, basal-bolus insulin regimens require multiple insulin daily injections and are associated with a significant risk of hypoglycaemia reported in up to 32% of non-critically ill patients with T2D 15–17. Thus, a simpler regimen that results in similar glycaemic efficacy to basal-bolus insulin with less risk of hypoglycaemia could improve care for non-critically ill surgical patients.
Dipeptidyl dipeptidase-4 (DPP-4) inhibitors are oral antidiabetic medications that inhibit the degradation of endogenous glucagon-like peptide-1 and can quickly lower blood glucose with a low risk of hypoglycaemia. In two recent multi-center randomized controlled studies of non-critically ill medicine and surgery patients, we showed that a DPP-4 inhibitor, sitagliptin alone or in combination with insulin glargine results in similar glycaemic control with less hypoglycaemia as compared to glargine and rapid-acting insulin with meals 18,19. However, > 80% of the patients included in these studies were admitted to the general medicine wards. Linagliptin, an orally active small-molecule inhibitor of DPP-4, licensed in the US in 2011, has been shown to improve glycaemic control in adults with T2D 20. The dose of linagliptin does not need to be modified based on renal function and therefore makes it an ideal agent to use in the hospital and in long-term care facilities.21 We hypothesized that linagliptin with correction doses of rapid-acting supplemental insulin would result in non-inferior glycaemic control as compared with a basal-bolus insulin regimen in non-cardiac surgical patients with T2D.
Methods
This randomized controlled study (NCT02004366, Clinicaltrials.gov) was performed at 5 hospitals at 4 medical centers: Grady Memorial Hospital and Emory University Hospitals in Atlanta GA, Boston Medical Center in Boston MA, Rush Medical Center in Chicago IL, and University of Colorado-Anschutz Medical Center in Aurora CO between 2014–2016. The study received Institutional Review Board approval at each institution and informed consent was obtained from all subjects during the hospitalization or prior to hospital admission in some patients with elective surgery.
Subject Recruitment
Subjects with T2D who were admitted to the hospital for a non-cardiac surgical procedure and anticipated to stay as inpatient > 24 hours were included if they were between the ages of 18–80 years, had a blood glucose (BG) between 7.8–22.2 mmol/L without laboratory evidence of diabetic ketoacidosis and their outpatient diabetes regimen included diet alone, oral antidiabetic agents, or low dose insulin therapy defined as ≤ 0.5 units/kg/day of insulin. We enrolled subjects within the one to two days after hospitalization. At the University of Colorado, some subjects were consented in the preoperative clinic if hospital stay was planned for > 24 hours after the surgery. Subjects were excluded if they were admitted to or required to stay in the intensive care unit, had a history of type 1 diabetes, no known prior diagnosis of diabetes, outpatient treatment with a DPP-4 inhibitor or a glucagon-like peptide (GLP-1) receptor agonist, clinically relevant gallbladder disease, a history of pancreatitis, clinically significant hepatic or renal (GFR < 30 ml/min/1.73m2) disease, chronic steroid use. Subjects who were unable to consent, pregnant or breastfeeding were excluded. Subjects were also excluded if during the hospitalization, they received basal insulin for 48 hours prior to randomization, received insulin drip, or received correction supplemental insulin for the 72 hours prior to randomization.
Randomization Procedure and Study Protocol
Subjects who met inclusion and exclusion criteria were randomized to either oral linagliptin 5 mg daily or the basal-bolus insulin regimen. Subjects were randomized in a 1:1 manner and stratified by randomization BG (< 11.1 mmol/L or ≥ 11.1 mmol/L) to either linagliptin or basal-bolus insulin regimen. Randomization tables for each site were created by Dr. Limin Peng, the statistician for the group based at Emory University School of Public Health. Randomization was allocated by a research pharmacist at each site who was not involved in enrollment. The basal-bolus insulin regimen was administered as previously described 22. The total daily dose (TDD) of insulin was calculated based on weight and randomization BG. Subjects with randomization BG 7.8–11.1 mmol/L were started on a TDD of 0.4 U/kg/day and subjects with randomization BG 11.2–22.2 mmol/L were started on a TDD of 0.5 U/kg/day. If GFR < 45 ml/min/1.73m2, then the TDD was reduced by 50%. Half the TDD was given as glargine insulin and the other half was divided into 3 equal-fixed doses of lispro or aspart insulin for each meal. All insulin doses were titrated daily by a study physician based on BGs as previously published 22 (Appendix). Both groups were given correction supplemental insulin doses of lispro or aspart insulin for BG> 7.8 mmol/L as previously described 22 (Appendix). For subjects in the linagliptin group who were NPO, linagliptin 5 mg was continued. Supplemental insulin was given before meals in patients who were eating or every 6 hours if NPO. Capillary BGs during inpatient stay were checked with point-of-care glucose meters and monitored before each meal and bedtime or every 6 hours if patient was not eating. Additional point-of care or plasma BGs were measured if subjects had symptoms of hypoglycaemia or if the primary team deemed it necessary. Subjects continued in the study until hospital discharge or for a maximum of 10 days.
Measured Outcomes
The primary outcome of the study was to show that mean daily BG between the groups was non-inferior. Secondary outcomes were number of hypoglycaemic episodes (BG < 3.9 mmol/L and BG < 2.2 mmol/L), number of hyperglycaemic episodes (BG > 16.7 mmol/L), total daily insulin dose, length of stay, treatment failures, hospital readmission and composite of hospital complications. The composite of hospital complications included wound infections, bacteremia, respiratory failure, acute renal failure, pulmonary embolism or deep vein thrombosis, need for surgical reintervention, need for blood transfusion, skin ulcers, gastrointestinal bleed, acute myocardial infarction and stroke 15,22,23. Acute renal failure was defined as an increase of serum creatinine ≥0.5 mg/dl. Hospital mortality was defined as death occurring during the hospital admission. Subjects were considered to be in treatment failure if they had 2 consecutive BGs > 13.3 mmol/L or a mean daily BG > 13.3 mmol/L. For the subjects in treatment failure, they were treated similar to the basal-bolus group.
Statistical Analyses
This was a modified intention-to-treat analysis. We only analyzed data from subjects with BGs available for ≥ 24 hours after receiving study medication. We calculated glycaemic control starting from the day after randomization or day 2 of being in the study. Mean daily BG was calculated by taking an average of each subject’s daily BGs and then an average was taken for all days in the study divided by number of days in the study. We included BGs from hypoglycaemic events. We also included all BGs up to the time treatment failure was determined.
The study was powered to show the non-inferiority of mean daily BG between the linagliptin to basal-bolus insulin regimen. We set the non-inferiority margin as 1.0 mmol/L. Based on our previous studies, we have shown that a BG difference less than 1.0 mmol/L was not considered as clinically significant 24. Also based on our previous studies 15,22, we assumed the standard deviation of mean daily BG is bounded above by 2.8 mmol/L 18. With two-sample t tests or Wilcoxon tests, one sided, alpha=0.05, 121 subjects for each treatment arm would be needed to ensure 80% power to reject the hypothesis that the mean daily BG in patients treated with linagliptin is no more than 1.0 mmol/L higher than that in patients treated with basal-bolus insulin. Assuming a 10–15% attrition rate, we calculated that we would need to recruit 140 subjects in each group.
We also performed a post hoc analysis of BGs comparing the primary outcome between groups as dichotomized by randomization BG < 11.1 mmol/L and BG ≥ 11.1 mmol/L as this is the Centers for Disease Control’s threshold for risk of surgical site infection 25. All post hoc analyses were performed using two-tailed tests. Continuous variables were compared with Wilcoxon rank sum tests and discrete variables were compared using Chi-square test (or Fisher’s exact test if needed). All analyses were performed in SAS 9.2, Cary, North Carolina.
Role of the Funding Source
The study was an investigator-initiated study funded by Boehringer-Ingelheim. The funding source was not involved in the study design, data collection, interpretation, statistical analysis, manuscript preparation, or the decision to submit the manuscript for publication.
Results
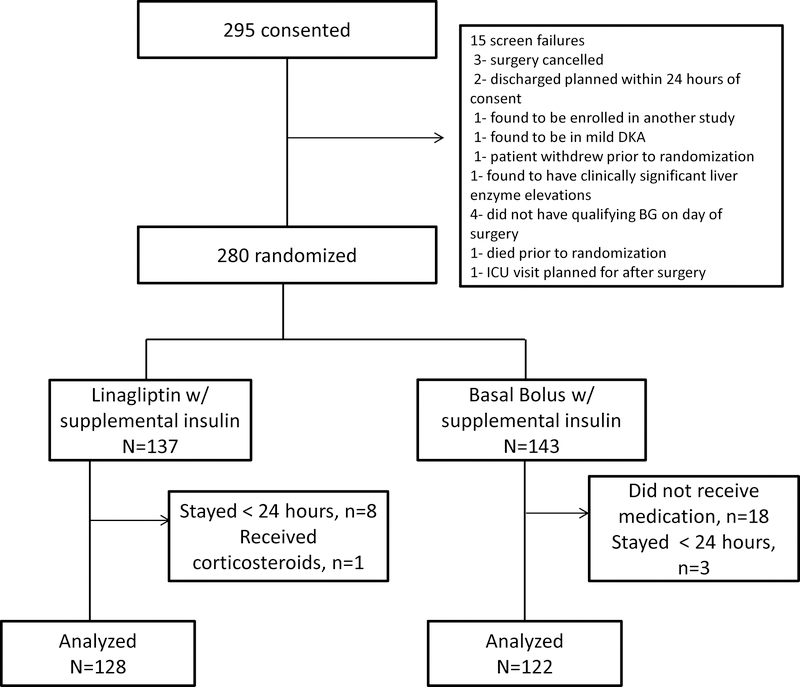
The study flow is illustrated in Figure 1. A total of 295 subjects were consented between February 2014 and October 2016. Fifteen screen failures were excluded and 280 subjects were randomized. Of these, 137 were randomized to the linagliptin group and 143 to the basal-bolus group. Nine subjects in the linagliptin and 21 subjects in the basal-bolus group were excluded from the analyses for reasons outlined in Figure 1. In the basal-bolus group, 18 subjects did not receive a basal insulin dose as the treating physician’s discretion. There were no differences in age, BMI, body weight or duration of diabetes between subjects who were excluded from the analysis compared to those who were included in the analysis. Final analysis included 128 subjects in the linagliptin group and 122 subjects in the basal-bolus group. Demographics for subjects in both groups are shown in Table 1. There we no differences in the number of male or female subjects, age, duration of diabetes, or length of stay. However, subjects in the linagliptin group had significantly higher BMI and body weight than the basal-bolus group (BMI: p=0.018, weight: p=0.039). There were no differences in preadmission antidiabetic therapy. Most subjects were admitted for elective surgery in both groups without differences between the groups.
Figure 1:
Study Flow for the Randomized Controlled Study Comparing Linagliptin and Basal Bolus Insulin Regimen in Patients with Type 2 Diabetes undergoing non-cardiac surgery.
Table 1:
Baseline Characteristics
| Linagliptin (n=128) | Basal Bolus (n=122) | p-value | |
|---|---|---|---|
| Age, years | 58 ± 11 | 58 ± 12 | 0.99 |
| Female, n (%) | 66 (52) | 58 (48) | 0.52 |
| BMI, kg/m2 | 35.4 ± 8.5 | 32.6 ± 6.9 | 0.02 |
| Weight, kg | 101.7 ± 24.8 | 94.6 ± 22.3 | 0.04 |
| Race, n (%) | 0.67 | ||
| White | 44 (35) | 46 (38) | |
| Black | 59 (46) | 58 (48) | |
| Other | 25 (19) | 18 (15) | |
| Duration of Diabetes, years | 8 ± 7 | 9 ± 8 | 0.47 |
| Length of stay, days (median, IQR) | 4 (3, 5) | 3 (3, 6) | 0.76 |
| Admission BG, mg/dl | 9.7 ± 4.2 | 9.9 ± 3.8 | 0.39 |
| Admission HbA1c, mmol/mol | 60 ± 20.8 | 64 ± 21.9 | 0.04 |
| Randomization BG, mmol/L | 10.7 ± 2.2 | 11.0 ± 2.3 | 0.26 |
| Admission diabetes therapy, n (%) | 0.41 | ||
| No medications | 15 (12) | 9 (7.4) | |
| OAD only | 89 (70) | 81 (66) | |
| Insulin only | 11 (8.6) | 14 (11) | |
| OAD + Insulin | 13 (10) | 18 (15) | |
| Type of Surgery, n (%) | |||
| Orthopedic | 51 (40) | 50 (41) | 0.85 |
| Abdominal | 14 (11) | 7 (5.7) | 0.17 |
| Urologic | 11 (8.6) | 10 (8.2) | 1.00 |
| Amputation | 6 (4.7) | 12 (9.8) | 0.14 |
| Gynecologic | 10 (7.8) | 9 (7.4) | 1.00 |
| I&D | 9 (7) | 9 (7.4) | 1.00 |
| Head & Neck | 1 (0.8) | 5 (4.1) | 0.11 |
| Other | 26 (20.3) | 20 (16.4) | 0.45 |
All data are expressed as mean ± SD unless otherwise specified. IQR: interquartile range, OAD: oral antidiabetic agent, I&D: Incision and drainage. BG: blood glucose
Subjects in the linagliptin group had lower admission HbA1c (60.0± 20.8 vs 64.0±21.9 mmol/mol vs, p=0.04) compared to the basal-bolus group (Table 2). There were no differences in admission BG, randomization BG or preoperative BG between groups.
Table 2:
Primary and Secondary Outcomes
| Linagliptin (n=128) | Basal Bolus (n=122) | p-value | |
|---|---|---|---|
| Mean Daily BG, mmol/L, Days 2–10 | |||
| All patients | 9.5 ± 2.6 | 8.8 ± 2.3 | 0.03 |
| Randomization BG < 11.1 mmol/L | 8.9 ± 2.3 | 8.7 ± 2.3 | 0.43 |
| Randomization BG ≥ 11.1 mmol/L | 10.9 ± 2.6 | 9.2 ± 2.2 | <0.001 |
| Meal Time BG, mmol/L | |||
| Pre-Breakfast | 9.3 ± 2.5 | 8.9 ± 2.4 | 0.15 |
| Pre-Lunch | 10.0 ± 2.5 | 9.7± 2.7 | 0.15 |
| Pre-Dinner | 9.5 ± 2.6 | 9.5 ± 2.3 | 0.82 |
| Bedtime | 9.8 ± 2.5 | 9.4 ± 2.4 | 0.18 |
| % of BG Readings within range | |||
| % BG 3.9–7.8 mmol/L | 30 ± 28 | 34 ± 24 | 0.10 |
| % BG 3.9–10 mmol/L | 61 ± 34 | 64 ± 27 | 0.80 |
| BG > 16.7 mmol/L, n (%) | 22 (18) | 18 (14) | 0.38 |
| Treatment failures, n (%) | 19 (15) | 10 (8.2) | 0.12 |
| Insulin doses | |||
| Total insulin (units/day) | 29.3 ± 15.8 | <0.001 | |
| Total insulin dose (units/kg/day) | 0.31 ± 0.15 | <0.001 | |
| Total basal (units/day) | 15.7 ± 8.1 | <0.001 | |
| Total prandial (units/day) | 9.8 ± 7.2 | <0.001 | |
| Total supplemental insulin (units/day) | 5.6 ± 6.6 | 3.8 ± 5.5 | 0.23 |
| Number of injections, Day 2–10 | 2 ± 3 | 3 ± 3 | <0.001 |
| Subjects with Hypoglycaemia, n (%) | |||
| BG < 3.9 mmol/L | 2 (1.6) | 14 (11) | 0.001 |
| BG < 3.1 mmol/L | 1 (0.8) | 4 (3.3) | 0.20 |
| BG < 2.2 mmol/L | 1 (0.8) | 0 (0) | >0.99 |
| Subjects with Hospital Complications, n (%) | |||
| Composite complications | 14 (11) | 11 (9) | 0.61 |
| Acute renal failure | 6 (4.7) | 4 (3.3) | 0.75 |
| Surgical reintervention | 6 (4.7) | 4 (3.3) | 0.75 |
| Wound infection | 1 (0.8) | 0 (0) | >0.99 |
| Other infection | 3 (2.3) | 1 (0.8) | 0.62 |
| Need for blood transfusion | 3 (2.3) | 2 (1.6) | >0.99 |
All data are expressed as means ± SD. BG: blood glucose
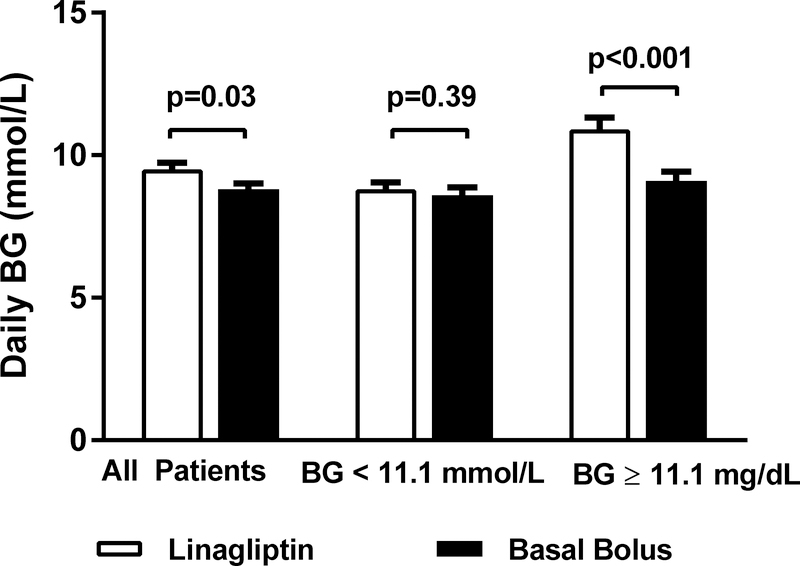
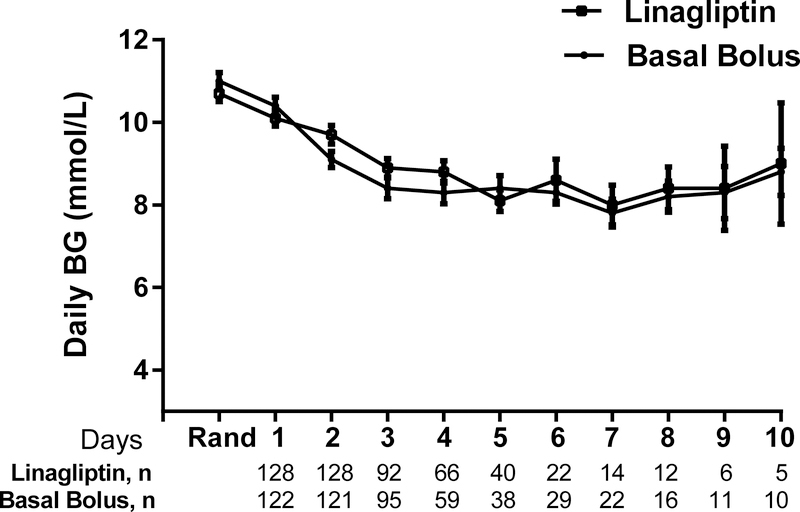
For glycaemic control, data was calculated starting the day after randomization (day 2) and included patients who failed treatment. The mean daily BG, the primary outcome, for the entire cohort was inferior in the linagliptin group (9.5±2.3 mmol/L) compared to the basal-bolus group (8.8 ± 2.3 mmol/L) (p=0.03) with a mean difference of 0.6 mmol/L (95% confidence interval: 0.04, 1.2 mmol/L, Figure 2). The mean daily BG during most study days did not significantly differ between the groups except for day 3 of the study (Figure 3). There were also no differences in mealtime BGs between the groups (Table 2). There were no differences between groups in the percentage of subjects in the BG range 3.9–7.8 mmol/L and 3.9–10.0 mmol/L. The proportion of subjects with BGs in the hyperglycaemia range > 16.7 mg/dl did not differ between the groups. The number of subjects who failed treatment were not statistically different between the groups although the linagliptin group had higher failure rates compared to the basal-bolus group. The median time to failure was 2 (range 2–10) days in the linagliptin group and was 2.5 (range 2–4) days in the basal-bolus group.
Figure 2:
Mean Daily BG Levels During Study Period in the Linagliptin and the Basal Bolus Insulin Regimens. Linagliptin (open bars) treatment was inferior to basal-bolus insulin (closed bars) treatment. Mean daily BGs were higher in the linagliptin group compared to basal-bolus group, p=0.03. After stratifying by randomization BG, there was no difference in mean daily glucose levels between the linagliptin and basal groups with randomization BG < 11.1 mmol/L. In patients with randomization BG ≥ 11.1 mmol/L, linagliptin group had higher mean daily glucose levels compared to basal bolus group, p<0.001. Data are expressed as mean ± SEM. Abbreviations: BG, Blood Glucose
Figure 3:
Mean BG Levels During Days on Study between Linagliptin and Basal Bolus Insulin Regimens. There were no differences in glucose levels on most days during the study between the linagliptin (open squares) and the basal bolus insulin regimen (closed circles) except on Day 3 of the study (p=0.03). Data are expressed as mean ± SEM. Abbreviations: BG, Blood Glucose
We performed post hoc analyses between the linagliptin and basal-bolus groups stratified by randomization BG < 11.1 mmol/L (that accounted for 63% of cohort) or BG ≥ 11.1 mmol/L. In the subjects with a randomization BG < 11.1 mmol/L, there were no differences in baseline characteristics between the linagliptin and basal-bolus groups except for weight (Appendix Table 1). Subjects in the linagliptin group weighed more than those in the basal bolus group. There was no difference in mean daily BG between linagliptin (8.9 ± 2.3 mmol/L) compared to the basal-bolus group (8.7 ± 2.3 mmol/L), p=0.43 (Figure 2). In subjects with randomization BG ≥ 11.1 mmol/L, there were no differences in baseline characteristics between the groups (Appendix Table 2). The mean daily BG was significantly higher in the linagliptin compared to basal-bolus group (10.9 ± 2.6 vs. 9.2 ± 2.2 mmol/L, p<0.001).
Rates of hypoglycaemia < 3.9 mmol/L mg/dl were significantly lower in the linagliptin group compared to the basal-bolus group (1.6% vs. 11%, p=0.001), a relative risk reduction of 86%, Table 2. There were no differences between groups for BG < 3.1 mmol/L or severe hypoglycaemia < 2.2 mmol/L. In post hoc analyses, in the subjects with a randomization BG < 11.1 mmol/L, fewer subjects in the linagliptin group experienced hypoglycaemia (BG < 3.9 mmol/L) compared to the basal-bolus group (1.1% vs 11%, p=0.01). There were no differences between groups in the number of subjects who experienced BG < 3.9 mmol/L and BG < 2.2 mmol/L (data not shown). In the subjects with a randomization BG ≥ 11.1 mmol/L, there were no differences between groups in the number of subjects who experienced BG < 3.9 mmol/L, BG < 3.1 mmol/L and BG < 2.2 mmol/L (data not shown).
Both groups received supplemental insulin before meals or every 6 hours if NPO for correction of hyperglycaemia. The amount of supplemental insulin did not differ between the groups, linagliptin 5.6±6.6 U/day and basal-bolus: vs 3.8±5.5 U/day, p=0.23. There were no differences between groups in LOS or in the number of subjects with postsurgical composite of complications including wound infections, acute kidney injury, readmission and blood transfusion (Table 2). In addition, one subject in the linagliptin group developed nausea and another developed urinary retention. Two subjects in the basal-bolus group developed nausea and vomiting.
Discussion
This is the first randomized controlled study to evaluate the efficacy and safety of an oral antidiabetic agent in non-cardiac surgical patients with T2D. Our study showed that treatment with linagliptin, an oral DPP4-inhibitor was inferior and resulted in higher mean daily BGs compared to the basal-bolus group. However, two-thirds of patients in our study had a BG < 11.1 mmol/L prior to randomization. In these patients, treatment with linagliptin resulted in clinically similar glycaemic control as the basal-bolus insulin regimen. There were no differences in length of hospital stay or in the rate of perioperative complications between groups. However, linagliptin resulted in fewer patients with hypoglycaemia compared to basal-bolus insulin.
Professional guidelines recommend the use of the basal-bolus insulin regimen for management of hyperglycaemia in hospitalized patients with T2D 13,26. Three studies have shown that treatment with a DPP-4 inhibitor alone or in combination with basal insulin resulted in similar glycaemic efficacy as basal-bolus insulin regimen in mainly general medical patients with T2D 18,19,27. Two of these studies were limited to small numbers of patients in each study group 19,27, and subjects in the study by Pasquel et al. were mostly non-surgical and were randomized to receive a DPP4 inhibitor with basal insulin or the basal bolus insulin regimen 18. This current study shows that linagliptin monotherapy with supplemental insulin was inferior to basal-bolus therapy by 0.6 mmol/l in patients undergoing noncardiac surgery. In patients who had a randomization BG < 11.1 mmol/L, which accounted for 63% of cohort, linagliptin provided similar glycaemic control to basal-bolus insulin treatment. Therefore, this simplified regimen with a single oral agent is efficacious in patients with mild to moderate hyperglycaemia
In addition to showing efficacy, linagliptin caused less hypoglycaemia than the basal-bolus insulin regimen. One episode of hypoglycaemia was prevented for every 11 patients treated with linagliptin compared to basal-bolus insulin. The percentage of subjects with hypoglycaemia in the basal-bolus group in the current study was lower than in our previous studies where the rate of hypoglycaemic ranged from 16–23% 15,22,23. In our current study, despite receiving similar amounts of supplemental insulin, only 2 subjects in the linagliptin experienced a BG < 3.9 mmol/L and only one subject experienced a BG < 2.2 mmol/L. Since linagliptin does not need dose titration, it offers an easier alternative than the basal-bolus insulin regimen for glycaemic control in most non-cardiac surgical patients with T2D. Further, reluctance with insulin titration due to fear of hypoglycaemia has been documented in physicians 28; therefore this simplified regimen using linagliptin with supplemental insulin in patients presenting with BG < 11.1 mmol/L can be helpful in achieving glycaemic control.
In patients with BG ≥ 11.1 mmol/L, insulin was superior to linagliptin and should be the preferred treatment of choice. Several reasons could explain this finding. In our cohort of patients, patients presenting with a BG ≥ 11.1 mmol/L had longer duration of diabetes and higher admission HbA1c levels compared to patients that presented with BG < 11.1 mmol/L. Despite the higher HbA1c, many were not on insulin as outpatient. The patients with the presentation BG ≥ 11.1 mmol/l were likely poorly controlled with more advanced diabetes. Due to the duration of diabetes, these patients likely needed to be on insulin therapy as outpatient and therefore need to be on insulin therapy as inpatient.
There are several limitations to our study. In the cohort of patients analyzed, patients in the basal-bolus group had a lower BMI than patients in the linagliptin group. It is possible that if BMI was similar in both groups, glycaemic control for linagliptin and basal-bolus groups would be similar. The results of this study are not applicable to all patients undergoing surgery. Most of the subjects in this study were admitted for elective surgery and likely have lower acuity of surgical disease as indicated by the lower number of perioperative complications. We also excluded patients who were receiving more than 0.5 units/kg/day of insulin, patients who were already receiving a DPP-4 inhibitor or a GLP-1 receptor agonist, and patients undergoing gastrointestinal surgery and patients with an eGFR < 30 ml/min/1.73m2.
Overall, treatment with oral linagliptin combined with supplemental insulin offers an effective alternative to the basal-bolus insulin therapy in most surgical patients with T2D that are undergoing non cardiac surgery, especially in patients who present with a BG < 11.1 mmol/L. Even though we did not power this study to assess differences in complications, the low percentage of subjects with hypoglycaemia suggests that linagliptin represents an alternative to the basal-bolus insulin regimen in surgical patients with mild-to-moderate hyperglycaemia.
Supplementary Material
Acknowledgements
Linagliptin Inpatient Research Group: These collaborators contributed to the recruitment and care of study subjects and collected the data. University of Colorado, Division of Endocrinology: Hoda Bakhtiari, Cecilia Low Wang, MD and the Glucose Management Team; Rush University, Division of Endocrinology: Jocelyn Jones; Boston University, Division of Endocrinology: Katherine Modzelewski, Elizabeth Ensminger; Emory University, Division of Endocrinology, Metabolism and Lipids: J. Sonya Haw, Maya Fayfman, Clementina Ramos, Patricia Gomez.
Funding:
This was an investigator-initiated study funded by Boehringer Ingelheim and Atlanta and Colorado CTSA grants, Public Health Service Grant UL1 RR025008 and UL1 TR001082 from the Clinical and Translational Science Award program, and 1P30DK111024-01 from the National Institutes of Health and National Center for Research Resources. PV was funded in part by K12HD085850.
Conflicts of Interest Disclosures:
G.E.U. has received unrestricted research support for inpatient studies (to Emory University) from Merck, Novo Nordisk, AstraZeneca, Boehringer Ingelheim, and Sanofi and has received advisory/consulting fees from Sanofi and Merck. P.V. has received consulting fees from Boehringer Ingelheim and Merck. F.J.P. has received consulting fees from Merck, Sanofi, and Boehringer Ingelheim. N.R. has received research support from Novo Nordisk, AstraZeneca, Boehringer Ingelheim, and Intarcia. The remaining authors have no others conflicts of interest.
Footnotes
Trial Registration: NCT02004366, https://clinicaltrials.gov
Bibliography
- 1.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–982. [DOI] [PubMed] [Google Scholar]
- 2.Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. Jama. 2003;290(15):2041–2047. [DOI] [PubMed] [Google Scholar]
- 3.Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31(2):359–366. [DOI] [PubMed] [Google Scholar]
- 4.Pomposelli JJ, Baxter JK 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22(2):77–81. [DOI] [PubMed] [Google Scholar]
- 5.Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26(1):57–65. [DOI] [PubMed] [Google Scholar]
- 6.Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553–597. [DOI] [PubMed] [Google Scholar]
- 7.McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28(4):810–815. [DOI] [PubMed] [Google Scholar]
- 8.Baker EH, Janaway CH, Philips BJ, et al. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2006;61(4):284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–1021. [DOI] [PubMed] [Google Scholar]
- 10.van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. [DOI] [PubMed] [Google Scholar]
- 11.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. [DOI] [PubMed] [Google Scholar]
- 12.Investigators N-SS, Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. [DOI] [PubMed] [Google Scholar]
- 13.Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes A. 14. Diabetes Care in the Hospital. Diabetes Care. 2017;40(Suppl 1):S120–S127.27979901 [Google Scholar]
- 15.Umpierrez GE, Smiley D, Jacobs S, et al. RAndomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes Undergoing General Surgery (RABBIT Surgery). Diabetes Care. 2011;34(2):256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umpierrez GE, Hor T, Smiley D, et al. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(2):564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120(7):563–567. [DOI] [PubMed] [Google Scholar]
- 18.Pasquel FJ, Gianchandani R, Rubin DJ, et al. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): a multicentre, prospective, open-label, non-inferiority randomised trial. The lancet Diabetes & endocrinology. 2017;5(2):125–133. [DOI] [PubMed] [Google Scholar]
- 19.Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes Care. 2013;36(11):3430–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graefe-Mody U, Retlich S, Friedrich C. Clinical pharmacokinetics and pharmacodynamics of linagliptin. Clinical pharmacokinetics. 2012;51(7):411–427. [DOI] [PubMed] [Google Scholar]
- 21.Umpierrez GE, Cardona S, Chachkhiani D, et al. A Randomized Controlled Study Comparing a DPP4 Inhibitor (Linagliptin) and Basal Insulin (Glargine) in Patients With Type 2 Diabetes in Long-term Care and Skilled Nursing Facilities: Linagliptin-LTC Trial. Journal of the American Medical Directors Association. 2018;19(5):399–404 e393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181–2186. [DOI] [PubMed] [Google Scholar]
- 23.Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a Basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vellanki P, Bean R, Oyedokun FA, et al. Randomized controlled trial of insulin supplementation for correction of bedtime hyperglycemia in hospitalized patients with type 2 diabetes. Diabetes Care. 2015;38(4):568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berrios-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017;152(8):784–791. [DOI] [PubMed] [Google Scholar]
- 26.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg R, Schuman B, Hurwitz S, Metzger C, Bhandari S. Safety and efficacy of saxagliptin for glycemic control in non-critically ill hospitalized patients. BMJ open diabetes research & care. 2017;5(1):e000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross SA, Tildesley HD, Ashkenas J. Barriers to effective insulin treatment: the persistence of poor glycemic control in type 2 diabetes. Curr Med Res Opin. 2011;27 Suppl 3:13–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.