Abstract
The ideal construct for tracheal replacement remains elusive in the management of long segment airway defects. Tissue engineered tracheal grafts (TETG) have been limited by the development of graft stenosis or collapse, infection, or lack of an epithelial lining. We applied a mouse model of orthotopic airway surgery to assess the impact of three critical barriers encountered in clinical applications: the scaffold, the extent of intervention, and the impact of cell seeding and characterized their impact on graft performance. First, synthetic tracheal scaffolds electrospun from polyethylene terephthalate / polyurethane (PET/PU) were orthotopically implanted in anterior tracheal defects of C57BL/6 mice. Scaffolds demonstrated complete coverage with ciliated respiratory epithelium by 2 weeks. Epithelial migration was accompanied by macrophage infiltration which persisted at long term (>6 weeks) time points. We then assessed the impact of segmental tracheal implantation using syngeneic trachea as a surrogate for the ideal tracheal replacement. Graft recovery involved local upregulation of epithelial progenitor populations and there was no evidence of graft stenosis or necrosis. Implantation of electrospun synthetic tracheal scaffold for segmental replacement resulted in respiratory distress and required euthanasia at an early time point. There was limited epithelial coverage of the scaffold with and without seeded bone marrow-derived mononuclear cells (BM-MNCs). We conclude that synthetic scaffolds support re-epithelialization in orthotopic patch implantation, syngeneic graft integration occurs with focal repair mechanisms, however epithelialization in segmental synthetic scaffolds is limited and is not influenced by cell seeding.
Keywords: Tissue engineered trachea graft, Synthetic PET/PU scaffold, Syngeneic segmental tracheal transplantation, Bone marrow mononuclear cells, Epithelialization
Graphical Abstract

1. Introduction
The pursuit of the ideal tracheal replacement remains elusive. Airway tissue engineering holds the potential to create a living tracheal replacement capable of renewal, regeneration, and repair. Synthetic tissue engineered tracheal grafts (TETG) spare the need for decellularization and would provide an “off the shelf” solution for tracheal replacement. Recent large animal studies have supported clinical reports identifying that synthetic TETG demonstrate graft stenosis and delayed epithelialization [1]. Using a mouse model of tracheal replacement, we have noted that similar to the large animal model, orthotopic implantation of synthetic tracheal scaffolds result in delayed epithelialization and poor survival [2].
Considerable efforts have been made to improve the TETG performance, including material selection and structure optimization to enhance regeneration and confer suitable mechanical properties [3, 4]. Tissue engineered trachea fabrication has evolved with the application of novel manufacturing approaches such as three-dimensional printing and electrospinning [5]. Despite these improvements, several critical barriers remain for the successful translation of TETG. First, the scaffold should demonstrate the capability to support a functional respiratory epithelium. Delayed epithelialization results in an interruption of the mucociliary ladder, local inflammation and infection [6]. Second, a better understanding of the procedural impact of long segment tracheal replacement is necessary. In the absence of microvascular free tissue transfer, tracheal replacement results in graft devascularization, and outcomes of native trachea removal and reimplantation have led to graft infection and necrosis [7]. Third, the effect of graft seeding with autologous cells remains controversial. Early pilot studies suggested that cell seeding in TETG attenuates stenosis and accelerates epithelialization, however the outcomes remain highly variable [1, 8].
To explore these barriers, we applied an orthotopic model of mouse TETG implantation to characterize the formation of functional respiratory epithelium on a synthetic TETG patch. We then used a syngeneic mouse trachea as a surrogate for the ideal TETG to assess the role of vascular endothelial and progenitor cell populations on graft incorporation. Finally, we created a representative TETG by seeding an electrospun synthetic scaffold with autologous bone marrow-derived mononuclear cells (BM-MNCs) and assessed overall survival and persistence of seeded cells following orthotopic implantation.
2. Materials and methods
2.1. Animal care and ethics statement
The Institutional Animal Care and Use Committee of the Abigail Wexner Research Institute at Nationwide Children’s Hospital (Columbus, OH) reviewed and approved the protocol (AR15‐00090). Representatives of the animal care staff monitored the study animals during all phases of the project. All animals received humane care in accordance with standards published by the Public Health Service, National Institutes of Health (Bethesda, MD) in the Care and Use of Laboratory Animals (2011), and US Department of Agriculture (USDA) regulations outlined in the Animal Welfare Act.
2.2. Electrospun synthetic tracheal scaffold fabrication
Miniaturized tracheal scaffolds were manufactured and mechanically tested by Nanofiber Solutions, Inc. (Columbus, OH, USA) as previously described [2, 9]. Briefly, precursor solutions were prepared by dissolving 8 wt.% polyethylene terephthalate (PET) and 3 wt.% polyurethane (PU) in 1,1,1,3,3,3-hexafluoroisopropanol (HFIP), respectively. The solutions were mixed to create a 20 wt.% PET and 80 wt.% PU solution. The PET/PU solution was then electrospun on a 1 mm diameter steel rod utilizing a 20-gauge blunt tip needle, a high-voltage DC power supply set to +14 kV and a 20 cm tip-to-substrate distance. Excess length to secure the graft to the mandrel was ultimately trimmed to produce 5 mm functional scaffolds. Scaffolds were sterilized by UV illumination at 35 J/cm2. Cylindrical PET/PU scaffolds were used for segmental replacement procedures. For patch tracheoplasty procedures, representative patches (1 × 2 mm) were cut from the cylindrical scaffolds for implantation.
2.3. In-vitro analysis
2.3.1. BM-MNCs harvest and graft seeding
Bone marrow-derived mononuclear cells (BM-MNC) were harvested as previously described [2]. Six to eight-week-old female C57BL/6 mice were euthanized. Under aseptic conditions, long bones were harvested and the bone marrow was flushed from the femur and tibia with RPMI-1640 medium (Thermofisher Scientific, MA, USA) using a 25 g needle. Syngeneic BM-MNC were collected by filtration, isolation of mononuclear cells on a Ficoll 1083 gradient, and centrifugation of the bone marrow. The harvested concentrations of mouse BM-MNC (1 × 106, 10 × 106, 100 × 106 cells) were statically-seeded on 5 mm long synthetic mouse tracheal scaffolds and incubated overnight. Scaffolds were also prepared in the same manner without cell seeding as a control. Quantification of cell seeding (N = 4 / group) was obtained by DNA extraction assay (QuantiT™ PicoGreen® dsDNA Assay, Life Technologies, CA, USA). Cells attached to scaffold were lysed and dsDNA labeled with stain was detected using a fluorescence microplate reader. A standard curve of cell number as a function of fluorescence was generated using known quantity of human BM cells. The 10 × 106 dose was used for segmental tracheal replacement procedures.
2.3.2. Scaffold characterization
PET/PU Scaffolds (both seeded with BM-MNCs and unseeded controls) and native trachea specimens were lyophilized and mounted on aluminum pin mounts with carbon double-sided tape. The samples were then imaged with scanning electron microscopy (SEM, S-4800, Hitachi, Japan) after a 5 nm gold sputter coating.
2.4. Orthotopic tracheal graft implantation in a mouse model
Six to eight-week-old C57BL/6 mice were used as graft recipients for all procedures of the study. Mice were weighed and given a 0.1 mL/10g intraperitoneal (IP) dose of an anesthetic cocktail consisting of ketamine (100 mg/kg), xylazine (10 mg/kg) and ketoprofen (5 mg/kg). Upon confirming sedation, a midline incision was made from the clavicle to the hyoid bone. The strap muscles were opened to expose the thyroid cartilage, cricoid cartilage and the trachea. The individual transplant procedures are described in sections 2.4.1–2.4.4. Following graft implantation, the strap muscles and surrounding tissues were reapproximated and the midline incision was closed using sutures in a running pattern. A subcutaneous dose of buprenorphine (dose in 0.1 mL) was administered, and the animal was placed in a recovery cage on a heating pad until able to ambulate. Upon recovery, the mouse was transferred to a new cage with soft bedding, moist chow and medicated water (Ibuprofen, 30 mg/kg) for 48 hours. Animals were provided standard chow and access to automatic water after 2 days.
Animals were observed for changes in weight and evidence of respiratory distress, including labored breathing, stridor, and/or loss of 20% or more of pre-operative body weight. At planned or humane endpoint, animals were euthanized with Ketamine/Xylazine cocktail. Once euthanasia was confirmed, the trachea was exposed as previously described [2]. The entire trachea and graft were harvested and placed in 10% neutral-buffered formalin for downstream processing and analysis.
2.4.1. Patch tracheoplasty
With exposure of the anterior tracheal wall, a window was made in the anterior tracheal cartilage, creating a 1 × 2 mm defect. This defect was covered with a PET/PU patch of equivalent size. The patch was secured to the native trachea with 9–0 nylon suture. Mice were randomly assigned to experimental groups (N = 4 / group) for each of the short term (1 week, 2 weeks) and long term (>6 weeks) endpoints.
2.4.2. Syngeneic trachea transplantation
Following exposure of the trachea, the airway was dissected from the recurrent laryngeal nerves and esophagus. The trachea was transected below the third tracheal ring and 5 mm from the first incision. A syngeneic graft was then introduced to the surgical field and the distal anastomosis is first completed followed by proximal anastomosis. Experimental groups used were un-operated controls and post-procedure endpoints of 0, 3, 7, 14, 30, 60 days, 6 months, and 1 year (N = 4 / group).
2.4.3. Segmental Replacement Using TETG Seeded with BM-MNC
TETG segmental replacement was performed in the same manner as the syngeneic transplant procedure in section 2.4.2 [2]. Rather than syngeneic donor tissue, a 5 mm segment of PET/PU graft (with or without BM-MNC seeding as described in section 2.3) was sutured in place using 9–0 sterile nylon suture. Post-procedure endpoints were set at short term (7 days) and long term, and each group (considering planned endpoint and cell-seeding status) was assigned four animals. Animals exhibiting respiratory distress, stridor and more than 20% weight loss were recommended for early humane endpoint.
2.4.4. Segmental replacement using TETG seeded with GFP+ BM-MNCs
TETG segmental replacement was performed as previously mentioned in section 2.4.3. The TETG in the experiment were seeded with BM-MNC collected from transgenic mice constitutively expressing Green Fluorescent Protein (GFP) at a density of 10 × 106 cells/graft to evaluate mononuclear cell persistence within the graft. Post-procedure endpoints for graft recovery were set at 1-, 3-, and 7-day (N = 4 / group) when TETGs were explanted.
2.5. Histological analysis
Explanted tracheas from patch tracheoplasty, syngeneic tracheal transplant, and TETG segmental replacement procedures were fixed in 10% neutral buffered formalin overnight. Paraffin-embedded samples were sectioned into 4 μm thickness either axially or longitudinally. Sections were used for hematoxylin and eosin (H&E) and immunofluorescent (IF) staining procedures.
2.5.1. H&E staining and epithelial cell coverage measurement
Tissue sections were de-paraffinized with xylene, then rehydrated with a graded ethanol series to deionized H2O. Rehydrated sections were stained with Hematoxylin(Sigma–Aldrich, MO, USA) and counterstained with Eosin to visualize cellular structures. Stained sections were scanned and digitized using a bright field microscopy (Zeiss, Oberkochen, Germany) to document tracheal tissue morphology.
Hematoxylin and Eosin (H&E) staining was used to determine the extent of epithelial cell coverage on PET/PU scaffolds. The linear distance of scaffold covered by epithelial cells was measured in longitudinal sections using ImageJ software (US National Institutes of Health, MD, USA). The length of the entire graft was also measured to calculate the percent of coverage. Proximal and distal anastomoses were identified and used as the origins of measurement.
2.5.2. Preparation for Immunofluorescent (IF) staining procedures
For immunofluorescence staining, tissue sections were de-paraffinized with xylene, then rehydrated with a graded ethanol series to deionized H2O. Antigen retrieval was achieved by immersing the slides in 1X citrate buffer, microwaved on full power for 15 minutes then cooled to room temperature. Sections were blocked in blocking buffer (5% BSA, 0.1% Triton X-100 in 1X PBS) at room temperature for 30 minutes. Sections were incubated with the designated primary antibody (details below) at 4°C overnight, washed, incubated with secondary antibody (details below) at room temperature for 45 minutes, washed. Cell nuclei were identified with subsequent counterstaining with 1 μg/ml 4,6-diamidino-2-phenylindole (DAPI, Invitrogen, CA, USA). Cover slips were mounted on stained sections with Fluoromount-G mounting medium (Southern Biotech, AL, USA).
2.5.3. Acetylated tubulin (ACT) and Club cell secretory protein (CCSP) immunofluorescence and quantification
Acetylated tubulin (ACT) and Club cell secretory protein (CCSP) were stained to characterize the apical cilia and non-ciliated Club cells. The primary antibody for ACT is mouse anti-acetylated α-tubulin (1:8000 dilution, Invitrogen), and secondary antibody is goat anti-mouse IgG H&L (1:500 dilution, Alexa Fluor® 488, Invitrogen). The primary antibody used was goat anti-Club cell secretory protein (CCSP, also known as CC10 or CC16) (1:200 dilution, 07–623, graciously provided by Dr. Susan D. Reynolds’s lab). The secondary antibody used for CCSP was Alexa Fluor labeled donkey anti-goat antibody (1:200 dilution, AF-488, A-11005, Thermofisher Scientific, MA, USA). ACT coverage percentage (% width) and Club cell number per mm2 in the lumen over the patch area were analyzed by ImageJ software.
2.5.4. CD68 immunofluorescence and quantification
Immunofluorescent staining of CD68 was used to identify macrophages. Primary antibody was rabbit anti-CD68 (1:200 dilution ratio, Abcam, Cambridge, MA). Secondary antibody was goat anti-rabbit IgG H&L (Alexa Fluor® 488 Invitrogen). Images of the stained tissue were captured and analyzed using ImageJ software. The anastomosis junctions of the transplant were identified to distinguish host and transplant measurements. CD68 positive cells were counted in the subepithelial compartment of tissue. Cell density was obtained by calculating cells counted within the surveyed area.
2.5.5. Keratin5/Keratin14 Immunofluorescence and Quantification
Immunofluorescence for antigens Keratin 5 (K5, BioLegend, CA, USA) and Keratin 14 (K14, Invitrogen) were performed using a previously established method [10]. Primary antibodies specific to K5 and K14 were incubated at 4°C overnight at concentrations of 1:1000 and 1:250, respectively. Secondary antibodies were donkeyanti-rabbit Alexa Fluor 594 (1:500) and goat-anti-mouse Alexa Fluor 488 (1:500) with 1 μg/ml 4,6-diamidino-2-phenylindole (DAPI, Invitrogen, CA, USA) counterstain [10]. Photomicrographs were taken using a Zeiss Imager.M2 fluorescent microscope equipped with an Axiocam HRc black and white digital camera.
The volume densities (Vv) were determined by counting points (P) with a grid of evenly spaced points in the basement membrane between cartilages. Vv (μm3/ μm3) was calculated using the formula, Vv = Pp = PN / PT where PN is the number of points intersecting the structure of interest (defined by DAPI, red fluorescence, or green fluorescence)) and PT is the total number of intersection points in the selected space (epithelium determined by auto-fluorescence). The surface area of the basement membrane per reference volume (Sv) was determined by the formula Sv =2Io / Lr. Io is the number of intersections between the cycloid grid and a surface, the basement membrane. Lr is the total length of the test line within the reference volume, the epithelium. The thickness of a structure per unit area of basement membrane (Vs) was calculated by determining the arithmetic mean thickness: τ = Vv / Sv and is presented as μm3/ μm2.
2.5.6. CD31 immunofluorescence and quantification
Immunofluorescent staining for CD31 was used to characterize vascularization identified by endothelial cells (EC). Slides were incubated overnight with a primary antibody solution of mouse anti-CD31 (1:50, Abcam, Cambridge, United Kingdom). Antibody binding was detected with Alexa Fluor 647® goat anti-mouse IgG (1:300; Invitrogen) secondary antibody. Fluorescent images were obtained with a Zeiss inverted microscope and exposure time was determined by appropriate negative controls.
CD31 expression within the subepithelial compartment (defined as the space between the basement membrane and the luminal edge of tracheal cartilage) was measured using ImageJ software. The anastomosis junctions were identified on the distal and proximal ends of the transplanted structure (donor tissue for syngeneic procedures, and PET/PU scaffold for patch and segmental procedures), and sampling of the subepithelial compartment was performed in 200 μm lengths in either direction along the basement membrane, obtaining survey area and CD31 positive pixel measurements. Measurements were obtained out to 1 mm from the anastomosis, or until the tissue margin was reached.
2.6. Statistical analysis
Normally distributed data was compared using Welch’s t-test and non-parametric tests were used for data that were not distributed normally. Statistical tests were performed using the GraphPad Prism 8 software package (GraphPad Software Inc., CA, USA). Compared experimental groups were considered significantly different at p < 0.05. All experimental data were expressed as mean ± standard deviation (SD). CD31 and CD68 measurements were expressed as mean ± standard error of the mean (SEM).
3. Results
3.1. Orthotopic implantation of a synthetic tracheal patch results in the restoration of functional respiratory epithelium and is accompanied by macrophage infiltration
Twelve mice underwent patch implantation of synthetic tracheal scaffolds (Figure 1 A1–A3). Overall survival to planned euthanasia time point was 91.67% (11/12) and there were no signs of respiratory distress, stridor, or weight loss greater than 20%. Complete coverage with an epithelium was seen at 2 weeks and there was no graft stenosis observed (Figure 1 B1–B2). Morphologically, the neo-epithelium appeared identical to the adjacent native tracheal epithelium however the subepithelial region bearing the microvascular network and glandular structures was markedly thicker than the adjacent native trachea with cellular infiltrate (Figure 1 B2).
Figure 1.
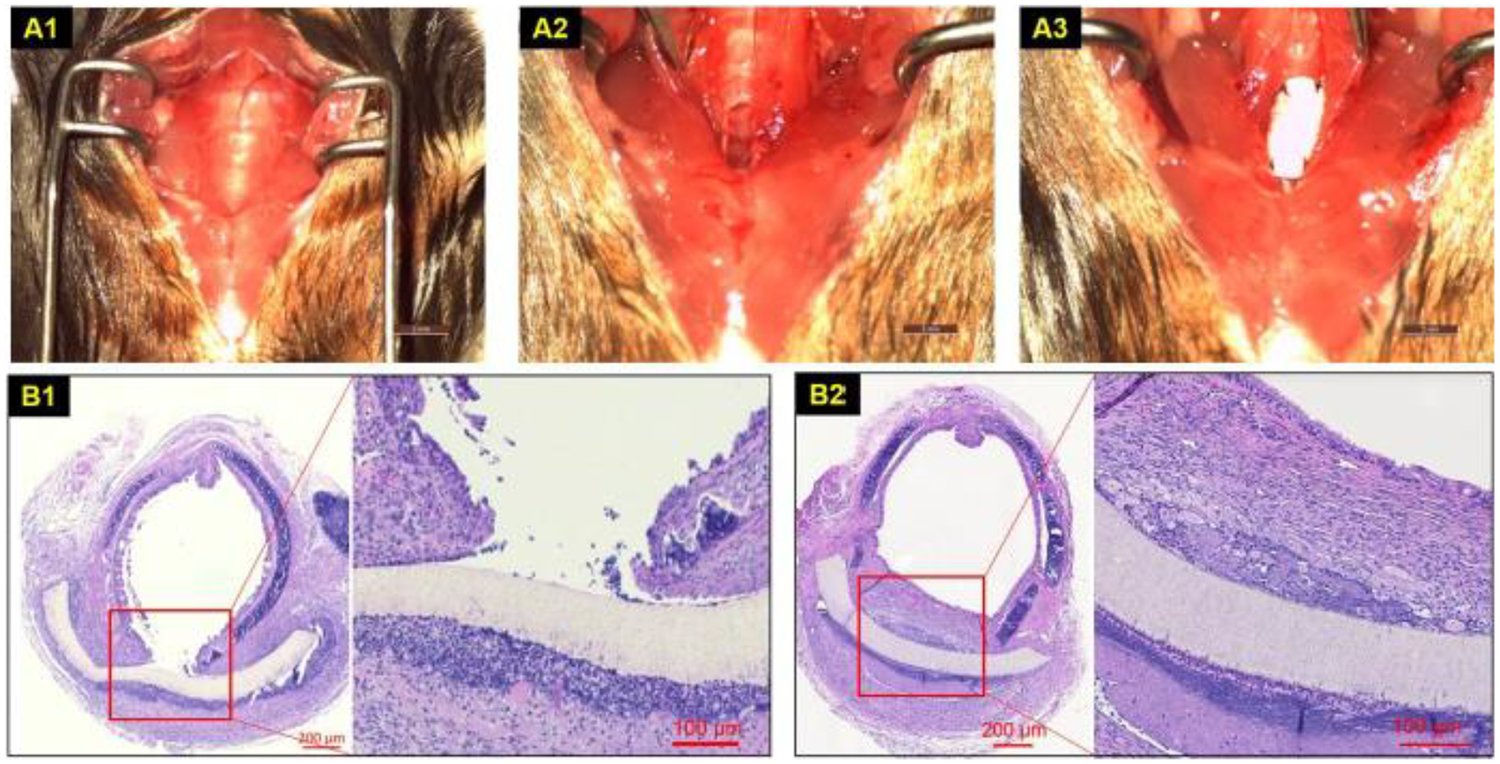
Synthetic patch implantation and outcomes. A. PET/PU patch tracheoplasty: Traches exposure (A1). 1 mm × 2 mm defect (A2), patch sutured (A3); B. Axial H&E section at patch-implanted trachea at short term (1 week) (B1) and long term (> 6 weeks) (B2).
In mice, the lumen is lined with ciliated-secretory epithelium. To assess the rate of functional tissue generation on the patch, we performed immunofluorescent staining for pseudostratified ciliated epithelium using acetylated alpha-tubulin (ACT) and club cell secretory protein (CCSP) as markers [11]. Consistent coverage with ACT+ ciliated respiratory epithelium was achieved by 2 weeks with overall coverage of 42.90% (SD ±42.08), 93.25% (SD ±26.65) and 89.71% (SD ±12.01) at 1, 2 and >6 weeks respectively (Figure 2 A1–3).
Figure 2.
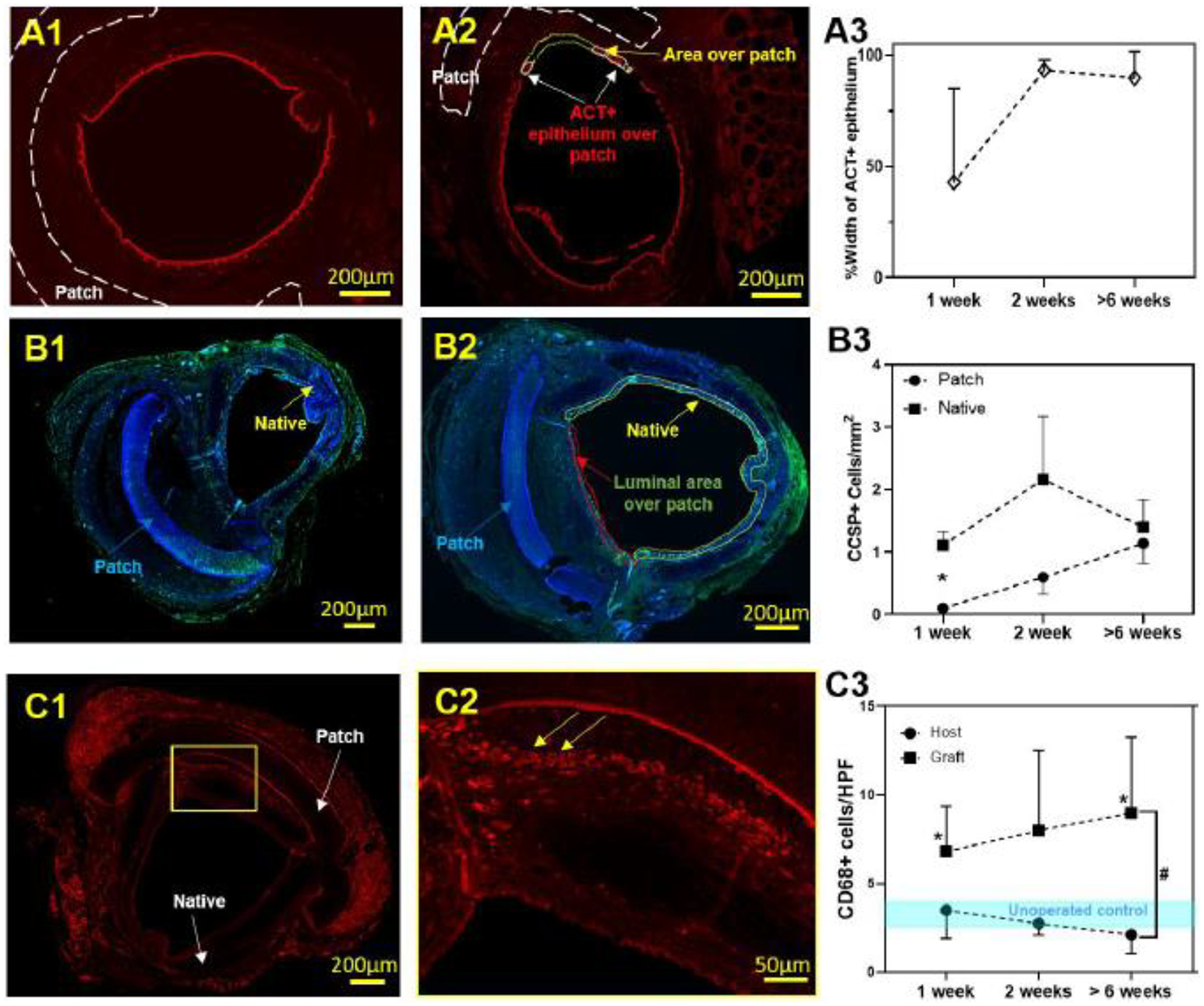
Synthetic patch implantation-host tissue interaction. A1. ACT IF, A2. % patch coverage with ciliated respiratory epithelium, A3. % width ACT+ patch coverage in time; B1. Representative CCSP immunofluorescent image, B2. CCSP+ density in time (* represent significant difference of cell density at 1 week, P < 0.05); C1. Representative CD68 immunofluorescent image, C2. CD68+ macrophage infiltration, C3. Quantified cell density in time (* represent significant difference of cell density at 1 week and over 6 weeks with unoperated control, P <0.05).; # represent significant difference of cell density over time between native and patch, P < 0.05).
We first confirmed that club cell secretory protein frequency was unchanged in the native trachea following patch implantation (Figure 2 B3, P > 0.05). Comparing club cell secretory protein (CCSP) frequency of the neoepithelium with native trachea, cell frequency in the neo-epithelium was not different by 2 weeks and more closely resembled native trachea at long term time points (Figure 2B1–3, S1).
The marked thickening of the sub-epithelial region with increased cellular infiltration was noted to be (Figure 2C) macrophage predominant (CD68+). CD68+ density (cells/mm2) in the subepithelial region of the patch persisted throughout all time points and was higher at 1 week and >6 weeks (P<0.05) than adjacent native trachea and unoperated controls (Figure 2 C1–C3).
3.2. Graft integration in a mouse model of segmental syngeneic transplantation tracheal replacement using segmental syngeneic grafts does not mirror epithelial repair seen in airway injury models
The impact of the extent of tracheal replacement was then assessed using a syngeneic tracheal transplant model. Syngeneic tracheal implantation in a mouse is a robust model, with 86.84% survival (N = 33/38) to the planned euthanasia time points of up to 1-year post-implantation. Graft recipients did not manifest with respiratory distress or stridor and histology revealed that syngeneic tracheal replacement was not complicated by stenosis. Figure S2 shows representative H&E images of syngeneic tracheal transplantation at 0 day, 6 months and 1 year.
Mapping the graft recipients’ trachea longitudinally through the host and donor regions, immunofluorescent staining was performed to identify tracheal basal cells (K5+/K14+). Tracheal basal cells (K5+) function as progenitor cells of the respiratory epithelium, upregulating K14 expression in airway epithelial injury models [12]. We identified focal upregulation of K14+ progenitor cells at the anastomosis, but there was no change in K14+ cell volume using stereological quantification, suggesting that basal cells play a minor role in graft integration (Figure 3 A, B). Stereological quantification showed higher K5 expression compared to K14 expression in the intercartilaginous areas in proximal host, distal host and donor over the period of short-and long-term time-points (P < 0.05, Figure 3B). There was no difference in progenitor cell frequency between host and donor regions.
Figure 3.
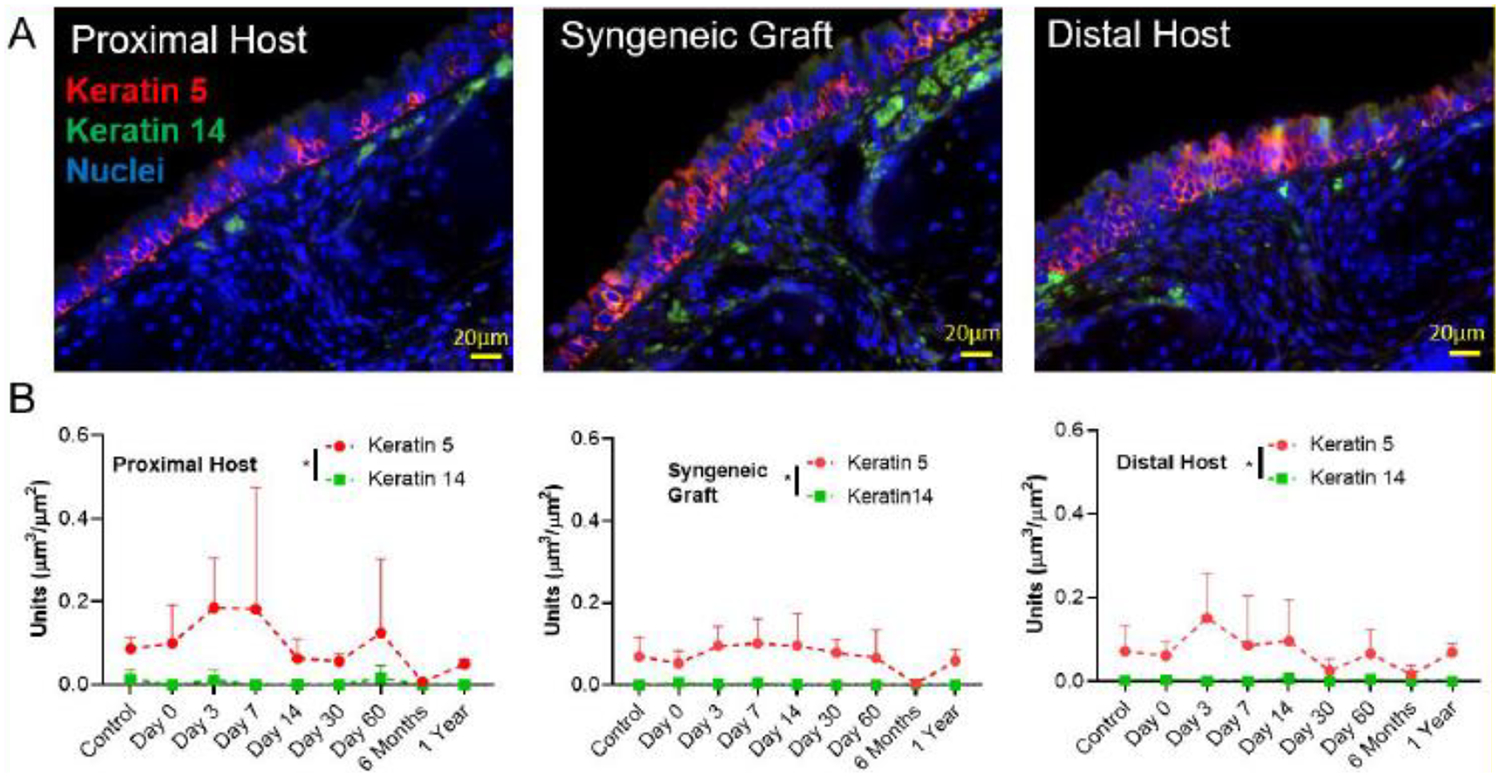
K5/K14-marked basal cells in syngeneic trachea transplantation. A. Representative immunofluorescent images of K5+/K14+ basal cell markers in Proximal Host, Syngeneic graft and Distal Host of mouse tracheas at day 3 of syngeneic tracheal transplant. Scale bar = 20 μm; B. Quantification of volume of nuclei per area of basement membrane, total K5 cell mass and total K14 cell mass for each region (proximal host, donor, and distal host). Data are presented as the mean +/− standard deviation. N = 4 for all time-points except 7 days (N = 3). * represents statistical significance (P < 0.05) between compared groups tested by two-way ANOVA.
Vascular endothelial cells (CD31+) were then quantified in longitudinal sections spanning the host and graft tissue. CD31 expression (% measured area) in the subepithelial region over each 200 μm length from the anastomosis till 1000 μm is shown in Figure S3. Quantification of the subepithelial region over the first 200 μm length from the anastomosis revealed no difference in vascular endothelial cell expression between host and graft tissue at early time points (Figure 4 A, B). Among the time points, there was an early (day 7) decrease in CD31+ area with a loss of significance by day 14 suggesting that angiogenesis takes place between the 1-and 2-week time points.
Figure 4.
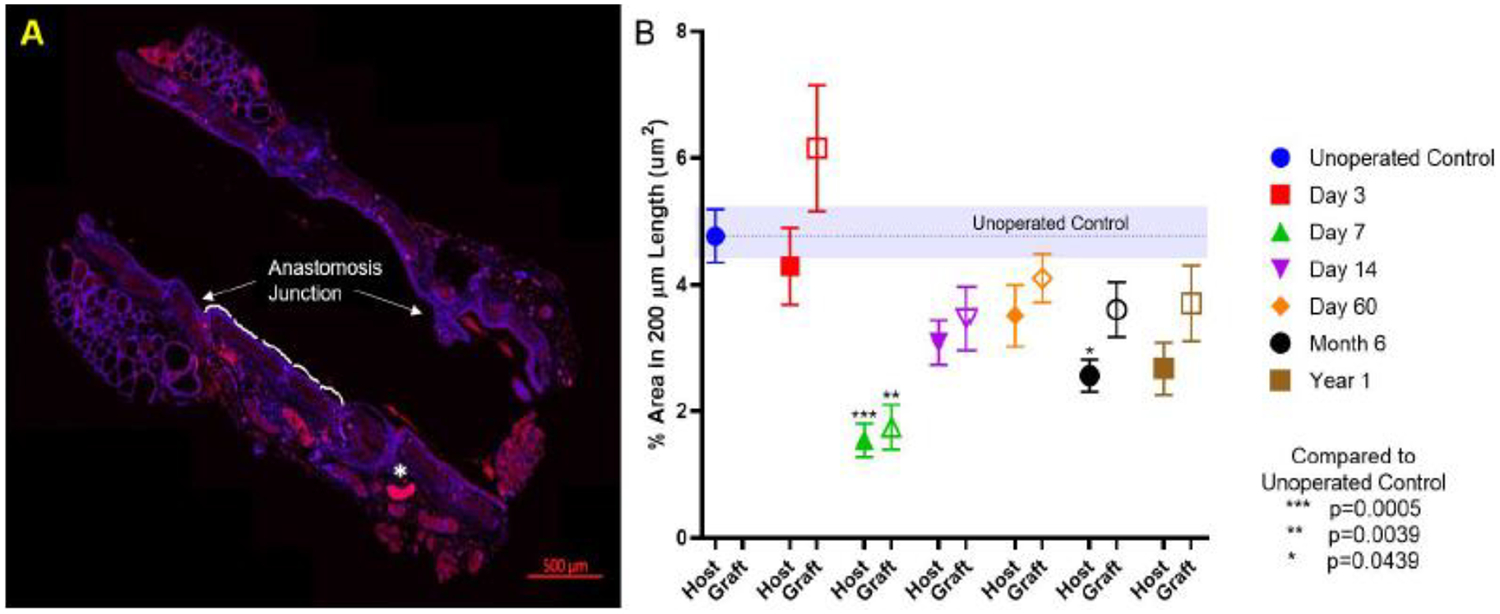
Expression of vascular markers in syngeneic transplanted-trachea for long term (1 year). A. Representative immunofluorescent image of CD31 expression in each 200 μm length along tracheal lumen from anastomosis junction (* marked out artefact); B. CD31 expression (% area in 200 μm) of host and graft with time. * represents significant difference between compared groups (P < 0.05).
3.3. Synthetic electrospun tracheal scaffolds can be seeded with BM-MNCs and demonstrate dose saturation
Static seeding of mouse tracheal scaffolds with BM-MNCs revealed that grafts exhibit dose saturation, with improved seeding efficiency with 10 × 106 cell dose (2.70 × 105 ± 1.50 × 105 cells/graft; 2.68%;) compared to 1 × 106 (1.90 × 105 ± 7.0 × 103 cells/graft; 1.91%;); an additional ten-fold increase did not yield improved cell seeding with 100 × 106 cells (1.03 × 106 ± 1.65 × 106 cells/graft; 1.03%) (Figure 5A). The synthetic PET/PU scaffold provided a porous substrate for BM-MNCs adherence and entrapment (Figure 5B, C). SEM revealed that BM-MNCs were intercalated in pores of PET/PU scaffold (Figure 5C).
Figure 5.
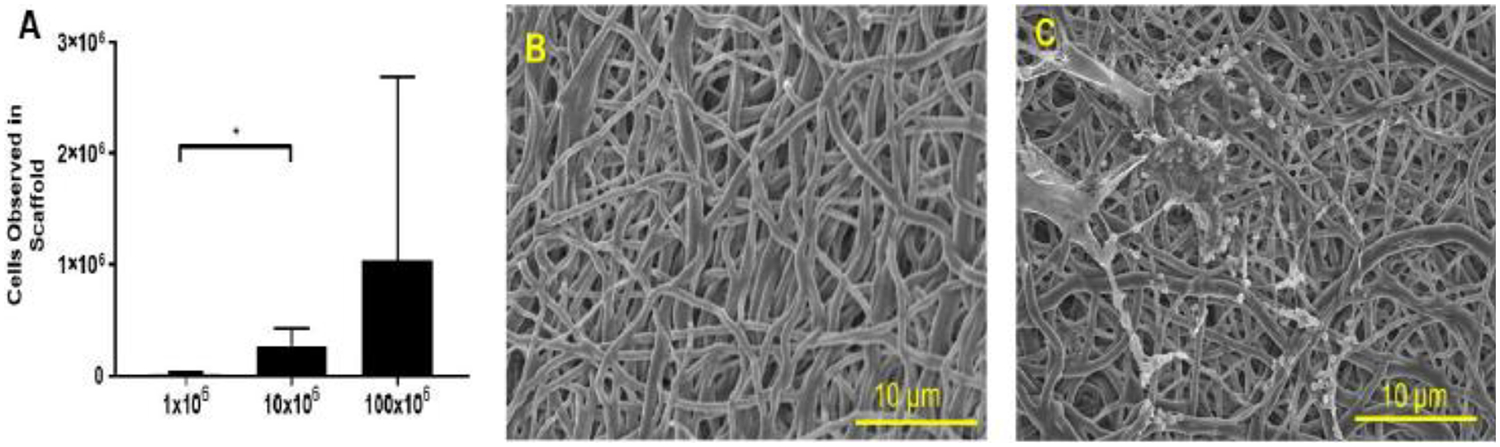
BM-MNCs seeding capacity and SEM characterization of TETG. A. Seeding BM-MNCs with 1 × 106, 10 × 106, 100 × 106 to synthetic tracheal scaffolds; B. Unseeded PET/PU scaffold morphologies; C. BM-MNCs seeded scaffold.
3.4. BM-MNC seeding does not improve overall segmental TETG recipient survival
Segmental TETG recipients demonstrate poor survival regardless of graft seeding. Comparing these cohorts with syngeneic segmental tracheal replacement, both scaffold groups demonstrated worse survival (Figure 6A, P < 0.05). There was no difference in survival between the seeded and unseeded scaffold groups (Figure 6A). Microscopic examination of the graft anastomosis demonstrated graft patency, suggesting that mortality is not entirely due to airway obstruction. When assessing the extent of epithelialization of seeded vs. unseeded scaffolds at 1 week, there was no difference observed in the percentage of epithelial coverage between groups (Figure 7).
Figure 6.
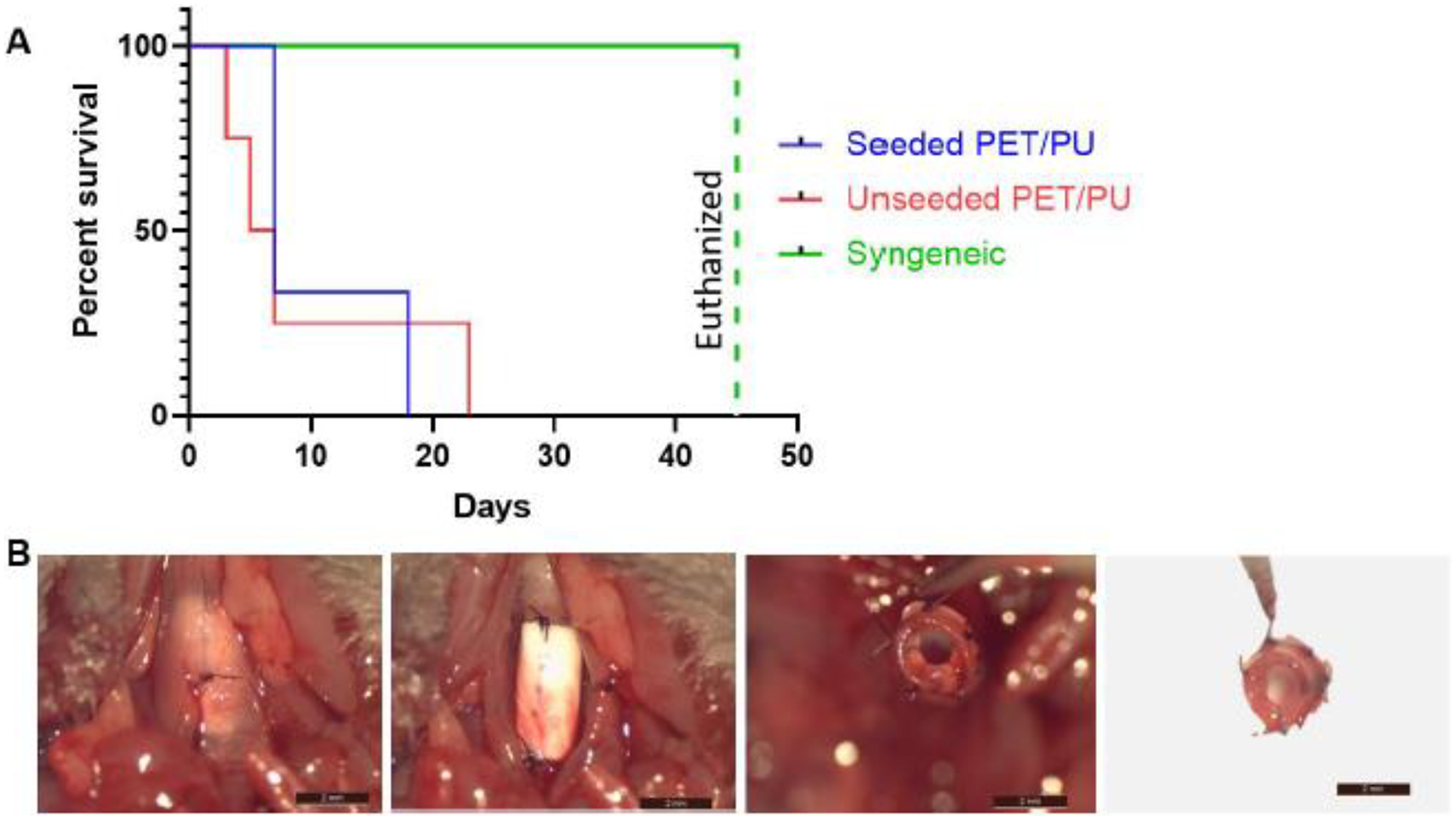
Post-operative outcomes of segmental TETG implantation. A. Survival curve (Kaplan Meier Survival Analysis); B. Representative process of grafts explantation showing no stenosis formation in the graft lumen, 4 days post-operatively. (Scale bar = 2 mm).
Figure 7.
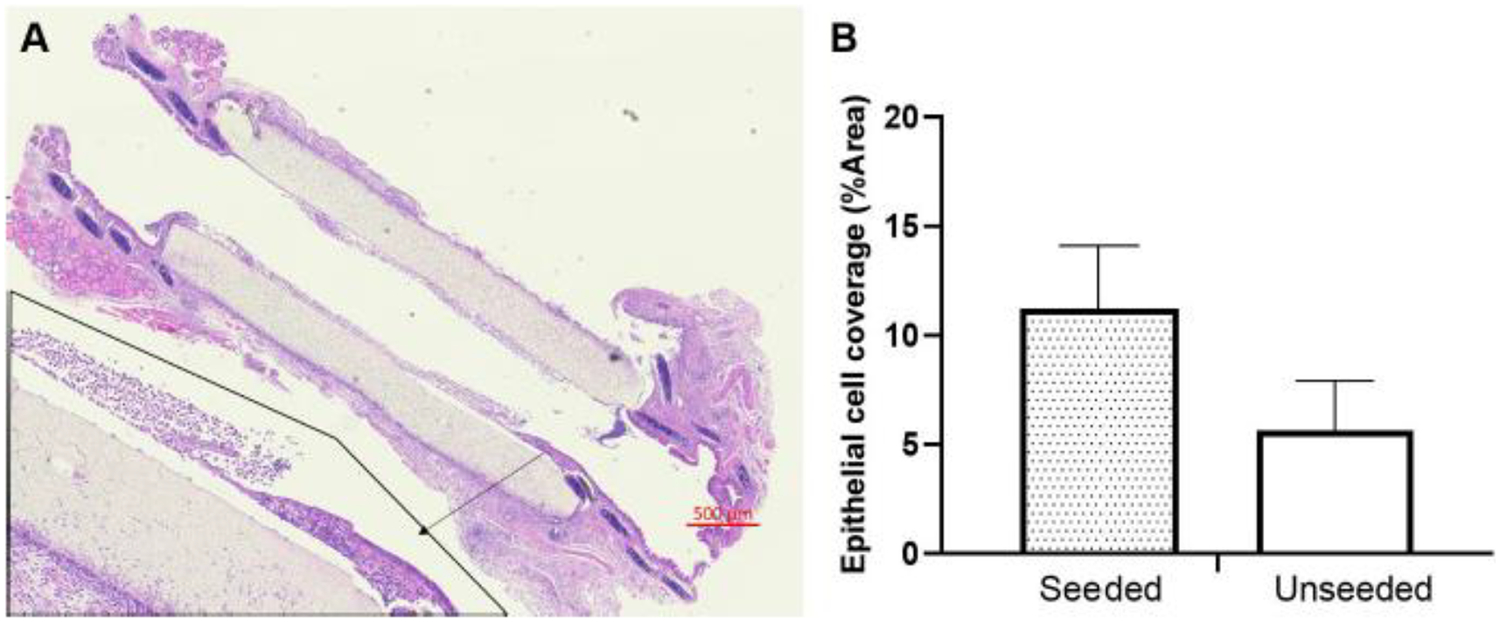
Epithelial cells coverage on scaffold at 7th day after segmental TETG implantation. A. Longitudinal H&E section; B. Quantified epithelial cell coverage percentage on graft in both seeded and unseeded groups.
The expression of K5+/K14+ basal cells (orange/yellow, white arrow pointed) near the anastomosis of syngeneic, seeded and unseeded PET/PU graft was abluminal (Figure 8). In Figure 8 C, epithelium exhibited squamous metaplasia at the anastomosis of unseeded PET/PU graft. The basal cells were observed with expression of K5+/K14−(red) in the syngeneic and seeded PET/PU groups. CD31 staining marked out the vasculature in host tissue and there was no obvious vasculature formed on graft (Figure 9 A1, A2). Stereological data confirmed the observation. Both seeded and unseeded PET/PU grafts showed significantly lower CD31+ cells compared with un-operated control (P < 0.05, Figure 9). This result is similar with the syngeneic implantation result (Figure 4). There is significantly less host tissue-CD31 expression in seeded scaffolds (P < 0.05, Figure 9).
Figure 8.
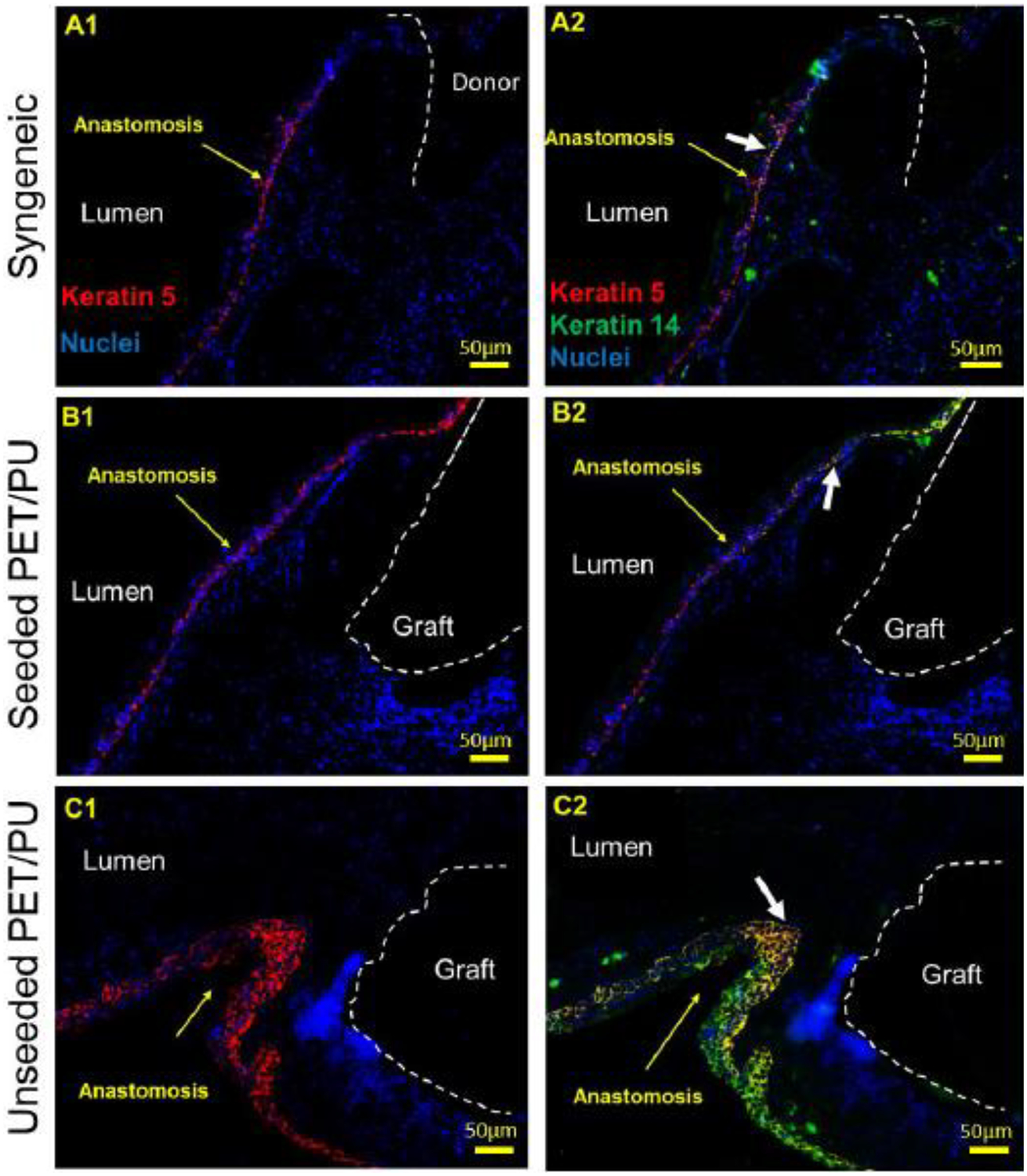
Representative anastomosis IF images of K5/K14 basal cell markers at 7th day of syngeneic and segmental TETG (seeded and unseeded) implantation. The white dotted lines separate the donor trachea (A1 and A2) and the TETG (B1 to C2) from the host trachea and the lumen. The white arrows point out K5+/K14+basal cells. The yellow arrows point out anastomosis. Scale bar = 50 μm.
Figure 9.
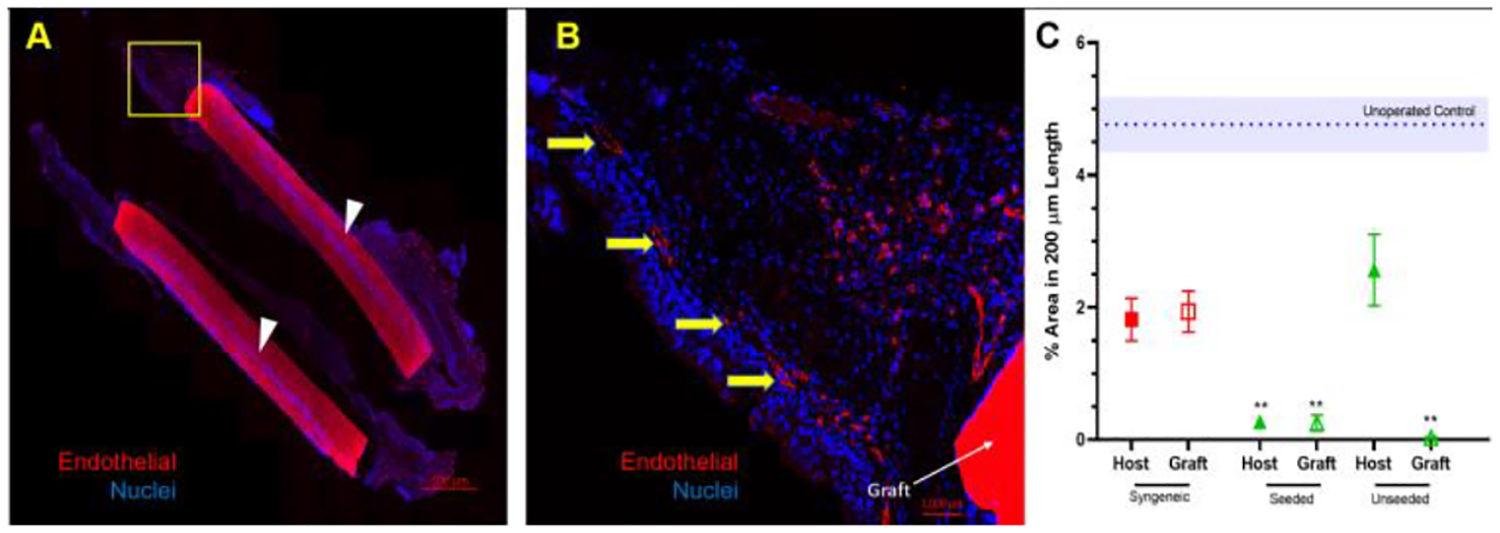
Expression of vascular marker, CD31, in segmental TETG implanted-trachea: A. Representative immunofluorescent images of CD31 staining of longitudinal section; B. Representative high power field image of CD31 stained endothelial cells; C. CD31 quantification (% area in 200 μm length) in 7 days (* represent significant difference between unoperated control and seeded host, graft and unseeded graft, P < 0.05). The yellow arrows point out vascular marker, CD31. The white arrows indicate the autofluorescent graft.
Scaffolds seeded with GFP+ BM-MNCs and implanted in wild-type mice demonstrate that seeded cells are rapidly cleared from the scaffold from 329.00 ± 29.82 cells on post-operative day 1 to 11.25 ± 5.32 cells on post-operative day 3 (Figure 10). The result (Figure 10) showed that the number of seeded BM-MNCs decreased from day 1 to day 3 (P = 0.0024) and day 7 (P = 0.0028).
Figure 10.
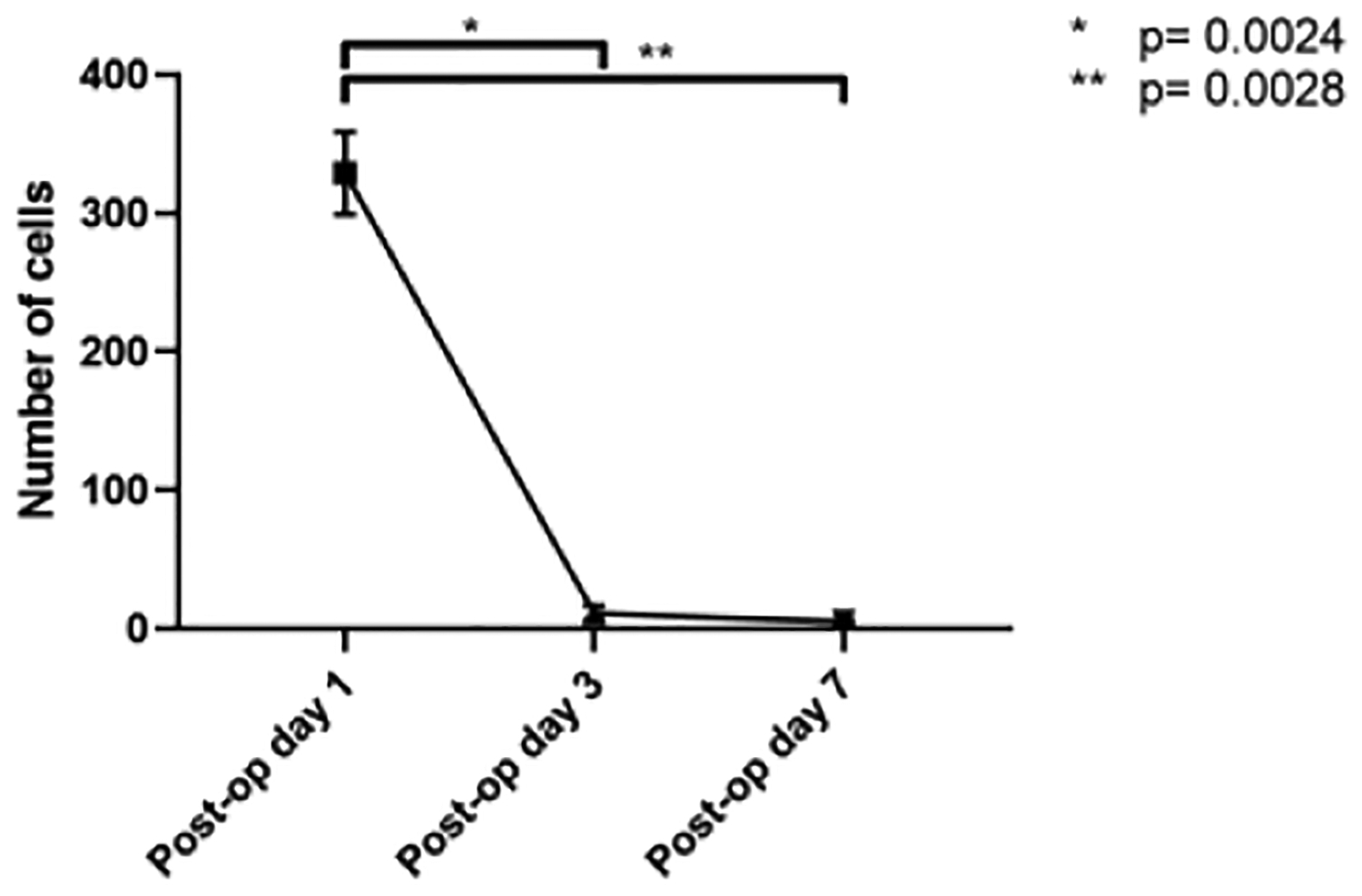
Amount of GFP+ BM-MNCs in scaffold after segmental TETG implantation at day 1, day 3, and day 7. * & ** represents significant difference of cells number between post-op day 1 and day 3, day 7, P < 0.05).
4. Discussion
The success of synthetic TETG has been limited due to chronic inflammation, graft stenosis, and delayed epithelialization [13]. To assess several critical barriers of translation, we deconstructed segmental tissue engineered tracheal replacement by studying the polymer composition of the scaffold, graft integration during segmental replacement, and the impact of cell seeding(S4).
We first demonstrate that orthotopic implantation of a synthetic tracheal patch can support a functional respiratory epithelium. Tracheal scaffolds fabricated with PET/PU have been shown to possess biomimetic mechanical properties, BM-MNC biocompatibility, and support neo-epithelium formation for tracheal reconstruction applications [8, 9]. While patch tracheoplasty has been applied to numerous large animal models of TETG, we first demonstrate that it is feasible in a mouse model [14, 15]. We found that by two weeks, the PET/PU patch is populated by a neo-epithelium that is morphologically normal and contains both ciliated (ACT+) and secretory (CCSP+) cells at frequencies that are comparable to native trachea. This finding is in contrast with protracted epithelialization as seen in PET/PU (non-resorbable), PGA (resorbable) and polypropylene-collagen grafts [2, 16]. Notably, despite the absence of luminal stenosis, the subepithelial space of the patch is markedly thickened and exhibits macrophage infiltration. The role of infiltrating macrophages in TETG re-epithelialization is unclear; in other applications, macrophages can play a role in either graft integration or foreign body reaction [17].
We then studied the mechanisms of graft integration in segmental syngeneic tracheal transplantation. End to end anastomosis of host and donor trachea was tolerated well by the cohort. Despite microscopic regions of graft overlap or telescoping, grafts remained patent without histologic evidence of stenosis or fibrosis. Syngeneic graft recipients did not exhibit signs of airway obstruction. Syngeneic tracheal transplant in the mouse model has been previously applied in tracheal allografts and decellularized tracheas [18, 19]. Large animal models of segmental trachea re-implantation results in a devascularized graft that is susceptible to ischemia, necrosis, stenosis, and infection [7, 20]. Using syngeneic trachea, we demonstrate that a living construct is a viable solution for tracheal replacement and is not limited by the morbidity of the procedure itself.
Finally, in contrast to patch tracheoplasty and syngeneic tracheal transplant models, we found that segmental tracheal replacement with synthetic TETG results in poor survival [2, 21]. There was no difference in survival and graft epithelialization between seeded and unseeded scaffolds. We hypothesize that this mortality is in part related to the large segment of non-functional trachea with interruption of the mucociliary ladder. In contrast to complete epithelial coverage seen patch tracheoplasty model, the extent of segmental TETG epithelialization was minimal. Although the PET / PU scaffold is fabricated to structurally mimic the native tracheal extracellular matrix, this suggests that additional scaffold modifications to the synthetic matrisome are needed to enhance epithelialization at the segmental scale.
With poor epithelialization of our segmental TETG, we assessed the tracheal basal cells, the progenitor cell population of the respiratory epithelium that mediate epithelial repair [22]. In steady state, most basal cells in mouse tracheobronchial epithelium are K5+/K14− with only 20% that are K5+/K14+ [10, 23]. Experimental models of airway injury and repair result in an upregulation of K5+/K14+ basal cells [24]. With our model of the “ideal” graft (syngeneic implant), graft integration occurs by way of focal upregulation of K5+/K14+ basal cells without any regional upregulation in progenitor cell activity seen in epithelial injury and repair models. In TETG implantation, basal cell expression was similar in seeded and unseeded scaffold group. However, despite similar focal upregulation of K5+/K14+ basal cells graft epithelialization remained limited (Figure 8).
Our findings support that graft survival in syngeneic tracheal implantation relies on a functional microvascular network within the TETG. With syngeneic tracheal implantation, endothelial expression of the microvascular network of the host and graft remains similar in both short term and long-term time points, supporting previous work demonstrating that restoration of perfusion to the graft is dependent upon establishing continuity with host vessels, rather than neovascularization [25]. Despite similar vascular endothelial cell expression in host tissues seen in the syngeneic replacement model, there were no vascular endothelial cells associated with the scaffold, indicating the lack of microvascular network within or atop the scaffold. We hypothesize that neotissue formation can form on a patch in spite of a functional microvascular network due to the scale of defect, and that poor survival outcomes of segmental TETG implantation are in part related to a lack of functional microvascular network. A functional TETG microvascular network TETG would permit the efficient delivery of nutrients and oxygen following implantation and improve epithelial survival, anastomotic regeneration and maintenance of tracheal architecture [26–28].
Cell seeding of synthetic scaffolds have demonstrated benefit in numerous regenerative medicine applications. Bone marrow-derived mononuclear cells (BM-MNCs) induce neotissue formation and improve tissue repair following vascular damage and lung injuries, reducing inflammation [29–33]. More specifically, a tissue engineered vascular graft that was seeded with BM-MNCs displayed well-organized vascular neo-tissue formation via attenuation of platelet activation [34]. Furthermore, biocompatibility between a polyethylene terephthalate (PET) tracheal scaffold and autologous bone marrow mononuclear cells was demonstrated leading to re-epithelialization and vascularization in humans [35].
Comparing seeded and unseeded tracheal scaffolds, we found no difference in overall survival, basal cell expression, vascularization and graft epithelialization. This represents either a lack of effect or lack of persistence of seeded BM-MNC; we demonstrated that seeded BM-MNC are rapidly cleared from the TETG once implanted. Inadequate scaffold porosity can lead to poor cell attachment, migration and tissue in-growth [36], which could contribute to the poor persistence of BM-MNCs on our scaffolds. Future work to assess the paracrine effect of seeded cells and improve cell persistence will help elucidate the true impact of graft seeding.
Conclusion
Segmental tracheal replacement with synthetic TETG is limited due to poor epithelialization and lack of functional microvascular network, among several other factors. Future work should be devoted to scaffold modulations that would promote neovascularization and accelerate epithelialization.
Supplementary Material
Statement of Significance.
The life-threatening nature of long-segment tracheal defects has led to clinical use of tissue engineered tracheal grafts in the last decade for cases of compassionate use. However, the ideal tracheal reconstruction using tissue-engineered tracheal grafts (TETG) has not been clarified. We addressed the core challenges in tissue engineered tracheal replacement (re-epithelialization and graft patency) by defining the role of cell seeding with autologous bone marrow-derived mononuclear cells, the mechanism of respiratory epithelialization and proliferation, and the role of the inflammatory immune response in regeneration. This research will facilitate comprehensive understanding of cellular regeneration and neotissue formation on TETG, which will permit targeted therapies for accelerating re-epithelialization and attenuating stenosis in tissue engineered airway replacement.
Acknowledgments
We would like to express our gratitude to the animal care and veterinary staff and the Morphology core at the Abigail Wexner Research Institute at Nationwide Children’s Hospital.
This study was supported by NIH NHLBIK08HL 138460 (Tendy Chiang is the recipient).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of conflict of interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Jed Johnson is a co-founder and chief technology officer of Nanofiber Solutions, Inc. Christopher Breuer receives research support from Cook Medical (Bloomington, Indiana, USA) and Gunze Ltd (Kyoto, Japan). The remaining authors have no disclosures.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version.
References
- [1].Pepper V, Best CA, Buckley K, Schwartz C, Onwuka E, King N, White A, Dharmadhikari S, Reynolds SD, Johnson J, Grischkan J, Breuer CK, Chiang T, Factors Influencing Poor Outcomes in Synthetic Tissue-Engineered Tracheal Replacement, Otolaryngol Head Neck Surg (2019) 194599819844754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dharmadhikari S, Best C, King N, Henderson M, Johnson J, Breuer C, Chiang T, Mouse model of tracheal replacement with electrospun nanofiber scaffolds, Ann Otol Rhinol Laryngol 128((5)) (2019) 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Park JH, Hong JM, Ju YM, Jung JW, Kang H-W, Lee SJ, Yoo JJ, Kim SW, Kim SH, Cho D-W, A novel tissue-engineered trachea with a mechanical behavior similar to native trachea, Biomaterials 62 (2015) 106–115. [DOI] [PubMed] [Google Scholar]
- [4].Xu Y, Li D, Yin Z, He A, Lin M, Jiang G, Song X, Hu X, Liu Y, Wang J, Tissue-engineered trachea regeneration using decellularized trachea matrix treated with laser micropore technique, Acta biomaterialia 58 (2017) 113–121. [DOI] [PubMed] [Google Scholar]
- [5].Kang Y, Wang C, Qiao Y, Gu J, Zhang H, Peijs T, Kong J, Zhang G, Shi X, Tissue-engineered trachea consisting of electrospun patterned sc-PLA/GO-g-IL fibrous membranes with antibacterial property and 3D-printed skeletons with elasticity, Biomacromolecules 20(4) (2019) 1765–1776. [DOI] [PubMed] [Google Scholar]
- [6].Soleas JP, Paz A, Marcus P, McGuigan A, Waddell TK, Engineering airway epithelium, BioMed Research International 2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bertolotti AM, Alvarez FA, Defranchi S, Alvarez M, Laguens RP, Favaloro RR, Successful circumferential free tracheal transplantation in a large animal model, J Invest Surg 25(4) (2012) 227–34. [DOI] [PubMed] [Google Scholar]
- [8].Clark ES, Best C, Onwuka E, Sugiura T, Mahler N, Bolon B, Niehaus A, James I, Hibino N, Shinoka T, Effect of cell seeding on neotissue formation in a tissue engineered trachea, Journal of pediatric surgery 51(1) (2016) 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Best CA, Pepper VK, Ohst D, Bodnyk K, Heuer E, Onwuka EA, King N, Strouse R, Grischkan J, Breuer CK, Designing a tissue-engineered tracheal scaffold for preclinical evaluation, International journal of pediatric otorhinolaryngology 104 (2018) 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cole BB, Smith RW, Jenkins KM, Graham BB, Reynolds PR, Reynolds SD, Tracheal basal cells: a facultative progenitor cell pool, The American journal of pathology 177(1) (2010) 362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reynolds SD, Malkinson AM, Clara cell: progenitor for the bronchiolar epithelium, Int J Biochem Cell Biol 42(1) (2010) 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ghosh M, Ahmad S, Jian A, Li B, Smith RW, Helm KM, Seibold MA, Groshong SD, White CW, Reynolds SD, Human tracheobronchial basal cells. Normal versus remodeling/repairing phenotypes in vivo and in vitro, Am J Respir Cell Mol Biol 49(6) (2013) 1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chiang T, Pepper V, Best C, Onwuka E, Breuer CK, Clinical Translation of Tissue Engineered Trachea Grafts, Ann Otol Rhinol Laryngol 125(11) (2016) 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gilbert TW, Gilbert S, Madden M, Reynolds SD, Badylak SF, Morphologic assessment of extracellular matrix scaffolds for patch tracheoplasty in a canine model, The Annals of thoracic surgery 86(3) (2008) 967–974. [DOI] [PubMed] [Google Scholar]
- [15].Jung SY, Lee SJ, Kim HY, Park HS, Wang Z, Kim HJ, Yoo JJ, Chung SM, Kim HS, 3D printed polyurethane prosthesis for partial tracheal reconstruction: a pilot animal study, Biofabrication 8(4) (2016) 045015. [DOI] [PubMed] [Google Scholar]
- [16].Nomoto M, Nomoto Y, Tada Y, Tani A, Otsuki K, Suzuki R, Nakamura T, Omori K, Bioengineered trachea using autologous chondrocytes for regeneration of tracheal cartilage in a rabbit model, The Laryngoscope 123(9) (2013) 2195–2201. [DOI] [PubMed] [Google Scholar]
- [17].Klopfleisch R, Macrophage reaction against biomaterials in the mouse model - Phenotypes, functions and markers, Acta Biomater (2016). [DOI] [PubMed] [Google Scholar]
- [18].Genden EM, Boros P, Liu J, Bromberg JS, Mayer L, Orthotopic tracheal transplantation in the murine model, Transplantation 73(9) (2002) 1420–5. [DOI] [PubMed] [Google Scholar]
- [19].Kutten JC, McGovern D, Hobson CM, Luffy SA, Nieponice A, Tobita K, Francis RJ, Reynolds SD, Isenberg JS, Gilbert TW, Decellularized tracheal extracellular matrix supports epithelial migration, differentiation, and function, Tissue Engineering Part A 21(1–2) (2014) 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maughan EF, Butler CR, Crowley C, Teoh GZ, den Hondt M, Hamilton NJ, Hynds RE, Lange P, Ansari T, Urbani L, Janes SM, de Coppi P, Birchall MA, Elliott MJ, A comparison of tracheal scaffold strategies for pediatric transplantation in a rabbit model, Laryngoscope 127(12) (2017) E449–E457. [DOI] [PubMed] [Google Scholar]
- [21].Wiet MG, Dharmadhikari S, White A, Reynolds SD, Johnson J, Breuer CK, Chiang T, Seeding and Implantation of a Biosynthetic Tissue-engineered Tracheal Graft in a Mouse Model, JoVE (146) (2019) e59173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Musah S, Chen J, Hoyle GW, Repair of tracheal epithelium by basal cells after chlorine-induced injury, Respiratory research 13(1) (2012) 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rock JR, Randell SH, Hogan BL, Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling, Disease models & mechanisms 3(9–10) (2010) 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Que J, The initial establishment and epithelial morphogenesis of the esophagus: a new model of tracheal–esophageal separation and transition of simple columnar into stratified squamous epithelium in the developing esophagus, Wiley Interdisciplinary Reviews: Developmental Biology 4(4) (2015) 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Babu AN, Murakawa T, Thurman JM, Miller EJ, Henson PM, Zamora MR, Voelkel NF, Nicolls MR, Microvascular destruction identifies murine allografts that cannot be rescued from airway fibrosis, J Clin Invest 117(12) (2007) 3774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Heim C, Motsch B, Jalilova S, Bernhardt WM, Ramsperger-Gleixner M, Burzlaff N, Weyand M, Eckardt K-U, Ensminger SM, Reduction of obliterative bronchiolitis (OB) by prolyl-hydroxylase-inhibitors activating hypoxia-inducible transcription factors in an experimental mouse model, Transplant immunology 39 (2016) 66–73. [DOI] [PubMed] [Google Scholar]
- [27].Jiang X, Khan MA, Tian W, Beilke J, Natarajan R, Kosek J, Yoder MC, Semenza GL, Nicolls MR, Adenovirus-mediated HIF-1α gene transfer promotes repair of mouse airway allograft microvasculature and attenuates chronic rejection, The Journal of clinical investigation 121(6) (2011) 2336–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jiang X, Malkovskiy AV, Tian W, Sung YK, Sun W, Hsu JL, Manickam S, Wagh D, Joubert L-M, Semenza GL, Promotion of airway anastomotic microvascular regeneration and alleviation of airway ischemia by deferoxamine nanoparticles, Biomaterials 35(2) (2014) 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tse H-F, Kwong Y-L, Chan JK, Lo G, Ho C-L, Lau C-P, Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation, The Lancet 361(9351) (2003) 47–49. [DOI] [PubMed] [Google Scholar]
- [30].Araújo IM, Abreu SC, Maron-Gutierrez T, Cruz F, Fujisaki L, Carreira H, Ornellas F, Ornellas D, Vieira-de-Abreu A, Castro-Faria-Neto HC, Bone marrow-derived mononuclear cell therapy in experimental pulmonary and extrapulmonary acute lung injury, Critical care medicine 38(8) (2010) 1733–1741. [DOI] [PubMed] [Google Scholar]
- [31].Prota LFM, Lassance RM, Maron-Gutierrez T, Castiglione RC, Garcia CSB, Santana MCE, Souza-Menezes J, Abreu SC, Samoto V, Santiago MF, Bone marrow mononuclear cell therapy led to alveolar-capillary membrane repair, improving lung mechanics in endotoxin-induced acute lung injury, Cell transplantation 19(8) (2010) 965–971. [DOI] [PubMed] [Google Scholar]
- [32].Krause DS, Bone marrow–derived cells and stem cells in lung repair, Proceedings of the American Thoracic Society 5(3) (2008) 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Areu SC, Antunes AM, Maron-Gutierre T, FCruz F, Carmo LG, Ornellas DS, Junior HC, AbSaber AM, Parra ER, Capelozzi VL, Effects of bone marrow-derived mononuclear cells on airway and lung parenchyma remodeling in a murine model of chronic allergic inflammation, Respiratory physiology & neurobiology 175(1) (2011) 153–163. [DOI] [PubMed] [Google Scholar]
- [34].Fukunishi T, Best CA, Ong CS, Groehl T, Reinhardt J, Yi T, Miyachi H, Zhang H, Shinoka T, Breuer CK, Role of bone marrow mononuclear cell seeding for nanofiber vascular grafts, Tissue Engineering Part A 24(1–2) (2018) 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gilevich I, Polyakov I, Porkhanov V, Chekhonin V, Morphological analysis of biocompatibility of autologous bone marrow mononuclear cells with synthetic polyethylene terephthalate scaffold, Bulletin of experimental biology and medicine 163(3) (2017) 400–404. [DOI] [PubMed] [Google Scholar]
- [36].Murphy CM, Haugh MG, O’brien FJ, The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering, Biomaterials 31(3) (2010) 461–466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


