Objective
Recent studies highlighted that many HIV-positive migrants in Europe acquired their infections post-migration. However, the timing of these infections is not always known. This study aims to estimate the timing of post-migration HIV acquisition among sub-Saharan migrants in France and to understand the correlates of post-migration infection.
Methods
Within the PARCOURS retrospective survey conducted in 2012–2013 in 74 healthcare facilities in the Paris region, life-event data were collected among a random sample of 926 patients living with HIV in HIV services and 763 patients undiagnosed with HIV in primary care centres born in sub-Saharan Africa (reference group). Based on previous analysis, we considered the first 6 years in France after migration as a settlement period. Among the persons who acquired HIV after migration, we estimated the proportion of persons infected during settlement (0–6 years after migration) and after settlement (>6 years after migration) by using an algorithm that combines life-event data and a modelisation of CD4+ T-cell count decline. We then assessed the determinants of HIV acquisition during settlement and after settlement using bivariate logistic regression models.
Results
Overall, 58% of sub-Saharan migrants who acquired HIV after migration were infected during the first 6 years in France. HIV acquisition during settlement was found to be linked to short/transactional partnerships and lack of a resident permit. 42% of migrants had contracted HIV after settlement. After settlement, HIV acquisition was associated with short/transactional but also with concurrent partnerships and not with social hardship.
Conclusion
Two profiles of HIV post-migration acquisition emerged. The majority of HIV post-migration acquisition occurs during the settlement period: comprehensive combination prevention programmes among recently arrived migrants are needed. However, long-term migrants are also at risk for HIV through multiple partnerships. Prevention programmes should address the different profiles of migrants at risk for post-migration HIV acquisition.
Keywords: life-event history survey, sub-saharan africa, france, prevention
Introduction
Migrants represent a sizeable proportion of those affected by the HIV epidemics in Western countries as they accounted for 15% of new diagnoses in Europe in 20171 and approximately 16% in the USA from 2007 to 2010.2 In France, sub-Saharan African migrants represented 39% of new HIV diagnoses in 2017.3 Virological data from the mid-2000s have already underlined an important proportion (28%) of subtype B HIV viruses among migrants from sub-Saharan Africa in France, which suggests that these persons were probably infected on the European continent, as this type of virus is rather rare in sub-Saharan Africa.4 However, only recently has a growing body of evidence highlighted the fact that HIV-positive migrants in Europe actually acquired their infection post-migration.5–9 For heterosexual transmission, it is estimated that between 33% and 58% of persons acquire their infection after migration, and estimations are as high as 63% for men having sex with men.6 7 9
Contrary to previous conceptions about sub-Saharan African migrants all being infected in their home countries, this evidence paints a totally renewed picture of the dynamics of HIV among migrants in Western countries and calls for better adjusted preventive actions based on the combined prevention paradigm among this population.
However, to better design these prevention campaigns and actions, more information is needed about the dynamics and the determinants of post-migration HIV acquisition. Nevertheless, the timing of HIV infection has not yet been assessed: is the infection concentrated during the first years after migration during the settlement period, when most migrants may face a number of social hardships, or do migrants acquire HIV infection later in their lives?
The aim of this study is twofold: first, to estimate the timing of post-migration HIV acquisition among sub-Saharan African migrants in France and then to understand the correlates of HIV post-migration acquisition during settlement or after settlement.10
Methods
PARCOURS survey
The PARCOURS study is a retrospective quantitative life-event survey that was conducted from 2012 to 2013 in healthcare facilities in the greater Paris metropolitan area among two random samples of migrants aged 18 to 59 born in sub-Saharan Africa: one receiving HIV care (HIV group, N=926) and another visiting primary care centres (reference group, N=763). Doctors asked all eligible patients to participate.
Time–location sampling was performed to obtain the study sample. Healthcare facilities, outpatient hospital HIV clinics (n=24) and general practice medical centres (n=30) were selected using a stratified random sampling procedure based on the estimated number of migrants in the caseloads of the exhaustive lists of the facilities. General practice medical centres are primary healthcare facilities of the National Federation of Health Centres (FNCS) of the Paris region, which brings together most healthcare facilities irrespective of their status (public, private or voluntary; 118 facilities) as well as the primary healthcare facilities for vulnerable populations (9 facilities). The sampling frame consisted of 127 facilities and was stratified by type of facility and by the number of African migrants in the facility’s municipality. Sampling frames were constructed for each half-day that the healthcare facilities were open. All eligible patients were included for each healthcare facility half-day visit.
The data were weighted according to each individual’s probability of inclusion in the survey (ie, considering the probability of inclusion in the sample for each healthcare facility, the number of half-days of weekly consultations in each facility included and the individual study participation per half-day of the included consultations).
Thirty trained interviewers administered a face-to-face standardised life-event history questionnaire to each participant. Information collected included conditions of migration and life in France, relational and sexual history, and healthcare pathways, including HIV testing. Each dimension of interest was documented year by year from birth until the time of data collection. Clinical and laboratory information was collected from medical records.
All information was collected anonymously. Participants received €15 vouchers. The complete survey protocol is registered on ClinicalTrials.gov (NCT02566148) and is available online (http://ceped.org/parcours/protocol-en.pdf).
Timing of settlement in the study population
We have previously shown that it takes a median of 6 years for PARCOURS participants to have access to three basic elements of settlement: a personal dwelling, resident permit of at least 1 year, and paid job11 with no difference according to sex or study group.12 Here, we consider the first 6 years after migration to be the settlement period and 7 years and more after migration to be the post-settlement period based on a previous study using PARCOURS data.11
Groups of interest
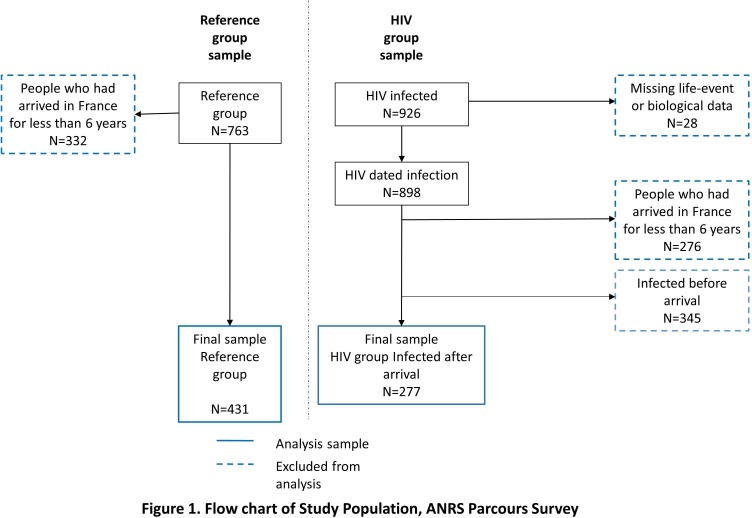
We excluded 608 persons (332 in the reference group and 276 in the HIV group) who had arrived in France for less than 6 years to ensure a minimum time of observation of 6 years since we aimed to measure whether HIV infection had taken place before or after settlement. Additionally, in the HIV group, we excluded 28 people for whom there were missing data to date HIV infection (life-event data or biological data).7
Variables of interest
Information on the year of HIV diagnosis was documented using both the participants’ reports and medical records. Patient-reported years of arrival in France, negative HIV tests (if ever) and year of first intercourse were available in the life-event questionnaire. CD4+ T-cell counts at the time of (1) HIV diagnosis, (2) initiation of antiretroviral therapy (ART) and (3) survey were extracted from medical records. We defined the first measurement of CD4+ T-cell count as CD4+ T-cell count at HIV diagnosis or, in case of missing information at diagnosis (n=30), as first CD4+ T-cell count available before initiation of ART.
For each year between migration to France and year of data collection, sexual partnerships were documented and then classified as follows: partnerships that lasted at least 1 year (‘stable’), partnerships that lasted less than 1 year (grouping short relationships and reports of transactional sex, both at risk for HIV and STIs13 14), or two or more partnerships within 1 year (‘concurrent’, based on participants’ declaration of having two or more relationships overlapping during 1 year). Information on administrative and housing situations was also collected year by year.
Statistical analysis
First, based on previous work, we could already identify the persons infected before arrival.7 Then, to estimate the probability that an individual was infected before or after 6 years in France, we used a combined method that mixed life-event and CD4+ T-cell data that has been fully described elsewhere.15
Based on life-event data, we considered that among persons who had acquired HIV in France, HIV infection had been acquired during the first 6 years in France if HIV diagnosis occurred during the first 6 years in France. In addition, we assumed that HIV infection had probably been acquired after 6 years in France if at least one of the following life-event criteria was fulfilled: (1) HIV diagnosis at least 11 years after 6 years in France (ie, 17 years after arrival),16 (2) at least one negative HIV test after the first 6 years in France and (3) sexual debut after 6 years in France. If none of these criteria were fulfilled, we estimated the duration from HIV acquisition to measurement of first CD4+ T-cell count using statistical modelling of the decline in CD4+ T-cell count based on a previously described method.17 We modelled the decline in CD4+ T cells using a cohort of West African seroconverters: the ANRS 1220 PRIMO-CI cohort of HIV-1 seroconverters in Abidjan, Côte d’Ivoire.18 The decline in CD4+ T-cell count (square root-transformed) over time was estimated using a linear mixed model with random intercept and slope, adjusted for individual CD4+ T-cell count at first CD4+ T-cell count measure duration from estimated date of HIV seroconversion to first CD4+ T-cell count measure and age at HIV seroconversion. The proportion of individuals who acquired HIV infection after 6 years in France and the 95% CIs were estimated according to a conservative scenario if more than 95% of the simulated durations fell after 6 years in France.
To identify the factors associated with HIV post-migration acquisition during settlement or after settlement, we used univariate logistic regression to model the probability of being in the group who acquired HIV during the settlement period (0–6 years) and being in the group who acquired HIV after settlement (>6 years) compared with the reference group.
Finally, although life-event data are more reliable at the individual level, we conducted a sensitivity analysis using only the CD4+ T-cell count modelisation to assign patients to HIV acquisition during settlement or after. Analyses were conducted using Stata V.14.0.19
Results
A total of 1053 persons were included in the study (622 in the HIV group, 431 in the reference group) (figure 1), who had all lived in France for at least 6 years. A full description of the study population is available in online supplementary material 1. Apart from slight differences in period and age at arrival; the main difference observed was that women infected post-settlement came more frequently to France for family reasons.
Figure 1.
Flow chart of study population, ANRS PARCOURS survey.
sextrans-2019-054080supp001.pdf (213.8KB, pdf)
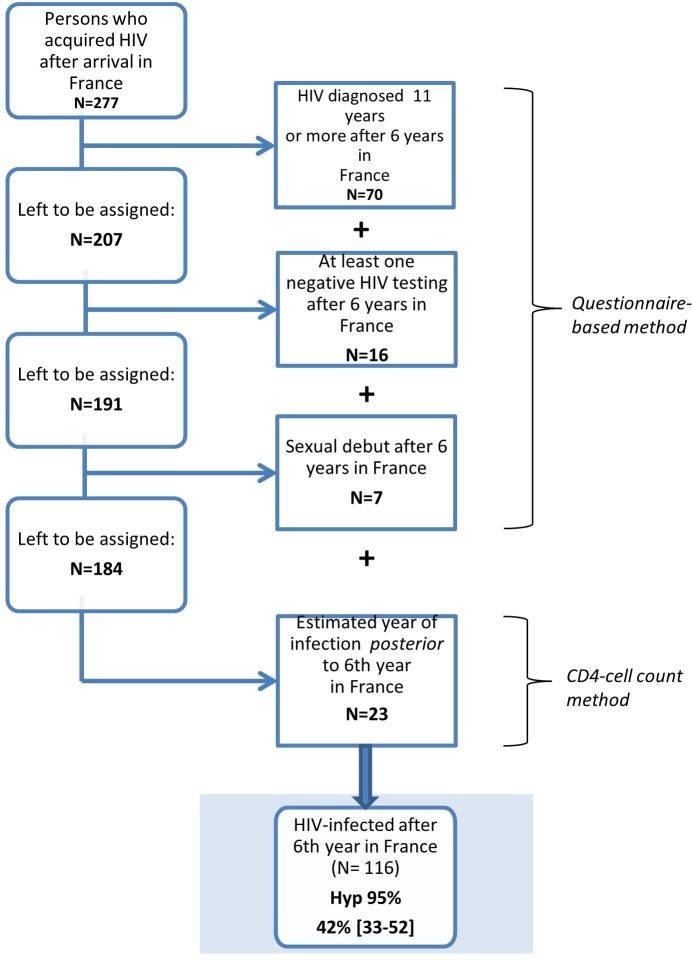
In the HIV group, the proportion of persons infected during settlement (0–6 years after migration) was 58% (55% among men and 61% among women). Conversely, 42% were infected after the settlement period (>6 years after migration) (45% of men and 39% of women) (figure 2).
Figure 2.
Assignment of HIV acquisition post-settlement (>6 years after migration) among sub-Saharan African immigrants living with HIV in the Paris metropolitan area and who acquired HIV after arrival in France, February 2012–May 2013 (n=277).
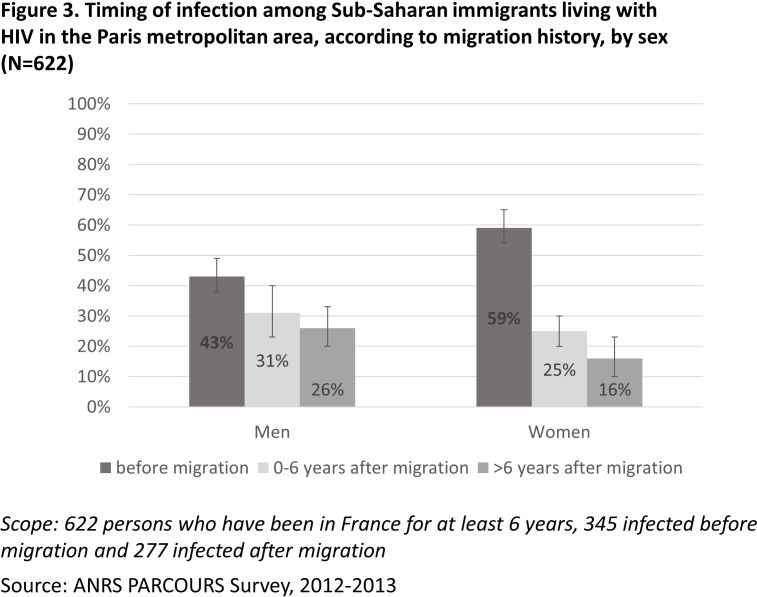
Combined with previous work,7 this analysis allows us to have a global picture of the HIV acquisition timing for the whole HIV group in the PARCOURS study (figure 3). Among sub-Saharan men living with HIV in France, 43% (95% CI 38 to 49) acquired HIV before arrival, 31% (95% CI 23 to 40) acquired it in the settlement period (0–6 years after migration) and 26% (95% CI 20 to 33) acquired it after settlement (>6 years after migration). Among women, the proportion of infection before arrival was higher than among men (59% (95% CI 54 to 65), p<0.01); 25% (95% CI 20 to 30) acquired HIV during the settlement period and 16% (95% CI 10 to 23) acquired it after settlement.
Figure 3.
Timing of infection among sub-Saharan immigrants living with HIV in the Paris metropolitan area, according to migration history, by sex (N=622).
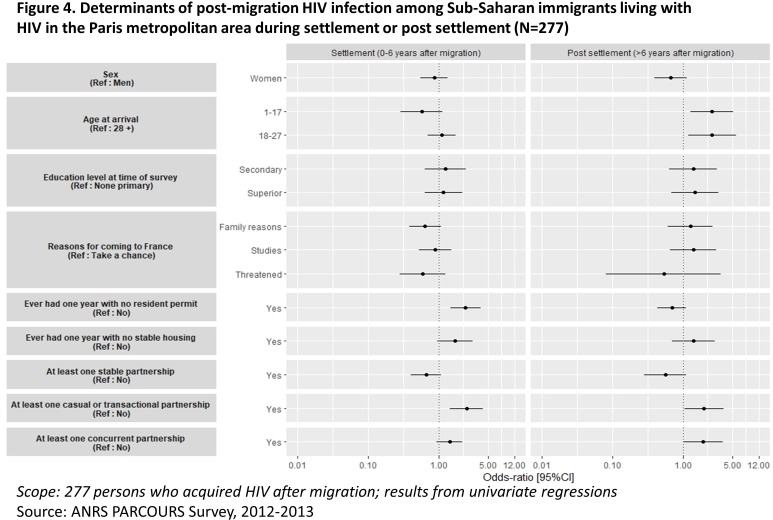
Compared with the reference non-infected group, the persons infected during settlement (0–6 years after migration) more often experienced years without resident permits (OR 2.40 (1.47 to 3.92)) and more short/transactional partnerships (OR 2.47 (1.45 to 4.21)) than the reference group (figure 4).
Figure 4.
Determinants of post-migration HIV infection among sub-Saharan migrants living with HIV in the Paris metropolitan area during settlement or post-settlement (N=277).
Migrants who had acquired HIV after settlement (>6 years after arrival) were younger at migration, had more short/transactional (OR 1.98 (1.05 to 3.75)) and concurrent (OR 1.91 (1.00 to 3.63)) partnerships than the reference group. There was no other sociodemographic difference between people who acquired HIV after settlement and the reference group.
The results of the sensitivity analysis confirmed the robustness of the method. By exclusively using CD4+ T-cell count modelisation, we found a 90% concordance with our results using both life-event and CD4+ T-cell count modelisation.
Discussion
To our knowledge, our results provide the first estimation of the timing of post-migration HIV acquisition after arrival in a European country. Although the majority of these infections occurred during settlement (0–6 years after migration), 42% of them occurred after settlement (>6 years after migration). Although we could not perform multivariate analysis of the determinants of this HIV post-migration acquisition for lack of statistical power, we were still able to clearly identify two profiles of infection. Consistent with previous analyses, HIV acquisition during settlement affects the migrants during periods when they are still undocumented.20 Indeed, it is well known that undocumented migrants encounter numerous obstacles in their daily life, as well as anxiety linked to their legal status.21 In such conditions, prevention of sexual risk is not necessarily a priority. Additionally, being undocumented was found to be associated with transactional relationships and sexual violence, which are high risk for HIV.20 22 The first profile should be put in perspective with previous findings on social hardship as a distant factor for HIV acquisition.
Furthermore, a second profile emerges of migrants, mostly men, who arrive at a later age and who have long been settled in France. They are more at risk of HIV acquisition after settlement through short/transactional and concurrent partnerships. These HIV acquisitions post-settlement are not explained by specific characteristics that could differentiate this infected population from the reference group. However, several elements can explain this HIV post-settlement infection. The fact that migrants mostly engage in intra-African sexual networks and are more exposed to HIV could be one of the drivers for this post-settlement HIV acquisition.23 In another study conducted on PARCOURS data, it was shown that only a small proportion of unions started after migration were with non-African partners: 19% of unions among women and 30% among men.24 Furthermore, we found that the level of systematic use of condoms with occasional partners in the last year (83%, 60% and 29% for acquisition during the settlement group, post-settlement acquisition group and reference group, respectively) was too low to prevent new STIs in a context where the prevalence of HIV is high.25 Thus, sub-Saharan African migrants are engaged mostly in intra-African networks that are more exposed to HIV, and the use of condoms is not sufficiently systematic to prevent new infections. It is interesting to note that among persons infected after settlement, more arrived in France at a very young age. It is probable that these persons who were not yet sexually active when they arrived became infected with HIV after a longer time in France. However, the other side of the coin is that children who arrived in France, who grew up in the same educational and healthcare context as natives, are still at risk of being infected with HIV years after their arrival. This suggests a certain lack of prevention among the youth in particular.
These results hold several implications in terms of prevention. First, newly arrived migrants are a target group for HIV prevention. To reduce new infections among them, the focus should be on accelerating settlement, hence reducing the period when people lack resident permit or stable housing, together with free and easy access to combined prevention tools.26 27 Second, the settled migrants (>6 years in France) also acquire HIV; this means that prevention programmes should not be limited to newly arrived migrants but should also be directed to long-settled migrants who remain exposed to HIV through unprotected sex. Previous studies have shown how low the perception of their exposure to HIV and STIs can be28 29; thus, to enhance the use of prevention tools in sub-Saharan African migrants living in France, specifically tailored programmes favouring repeated testing should be designed and tested.
Although based on information given by the interviewees, histories of migratory path, HIV testing and sexual debut can be considered reliable since the life-event method greatly supports the recall process and the order of life events as shown previously.30 31 Moreover, this mode of collection decreases the risk of desirability bias because the interviewer can compare the answers given for different periods and fields of life. If inconsistencies are detected, they can check the reliability of the answer with the patient and correct it.
The main limitation of our study is its lack of statistical power due to sample size, which prevented us from examining short and transactional relationships separately, although these are not the same in terms of subjective experience and capacity to negotiate the use of prevention tools. Additionally, it prevented us from conducting separate factor analysis between men and women although gender norms can play an important role in sexuality. More research is then needed to better understand the mechanisms underlying HIV infection once one has arrived in Europe.
Finally, our data were collected in 2012–2013 before the arrival of asylum seekers from sub-Saharan Africa from 2015 onwards. However, if anything, we think this recent policy crisis makes our results even more relevant: many recently arrived migrants live in harsh conditions in different European countries, including France, as they flee from conflicts and wars in their own countries. Unfortunately, it is likely that these populations will be exposed to HIV risk during their settlement years if nothing is done to improve their living conditions.
Conclusion
Among sub-Saharan migrants who acquired HIV after migration, 58% acquired HIV during the settlement period (0–6 years after migration) and 42% acquired it after settlement (>6 years after migration). Prevention programmes should ensure that all sub-Saharan African migrants are provided with free and facilitated access to repeated HIV testing, linkage to care and treatment if they are HIV-positive and PrEP for HIV-negative persons if relevant. In particular, vulnerable populations who are more at risk for HIV should benefit from combination prevention programmes in the years that follow their arrival. The path is still ahead as migrants have been almost completely absent from clinical trials proposing PrEP32 until now in France.
The newly arrived migrants are the most exposed to HIV in times of social hardship, and action should be taken to improve their sexual and global health, bearing in mind the importance of accelerating migrants’ settlement: a call for a shift in migration European and national policy. Second, it must not be forgotten that long-settled sub-Saharan migrants are also at risk of HIV: specifically tailored prevention programmes should be directed at them so they can have access to appropriate diversified prevention tools.
Key messages.
The majority of HIV post-migration acquisition among sub-Saharan migrants in France occurs during the first 6 years after migration.
Two profiles of HIV post-migration acquisition exist: one concerns newly arrived migrants, the other long-settled migrants.
HIV acquisitions during the first 6 years are associated to lack of resident permit.
HIV acquisitions after more than 6 years in France are related to multiple partnerships.
Specific prevention programmes should ensure access to free combined prevention tools among all sub-Saharan migrants.
Acknowledgments
The authors would like to thank all the persons who participated in the study, the RAAC-Sida, COMEDE, FORIM and SOS hepatitis associations for their support in preparing and conducting the survey, G Vivier, E Lelièvre (INED) and A Gervais (AP-HP) for their support in preparing the questionnaire, H Panjo for statistical support, A Guillaume for communication tools, the ClinSearch and Ipsos societies for data collection, and staff at all participating centres.
Footnotes
Handling editor: Professor Anna Maria Geretti
Collaborators: The PARCOURS Study Group included A Desgrées du Loû, F Lert, R Dray Spira, N Bajos, N Lydié (scientific coordinators), J Pannetier, A Ravalihasy, A Gosselin, E Rodary, D Pourette, J Situ, P Revault, P Sogni, J Gelly, Y Le Strat and N Razafindratsima.
Contributors: AG, FL, JP and ADdL conceived the objective and analysis plan of this paper. AG and AR conducted the statistical analysis. AG first drafted the paper and it was critically revised by AR, FL, JP and ADdL.
Funding: The ANRS Parcours Study was supported by the French National Agency for research on AIDS and Viral hepatitis (ANRS) and the Directorate General of Health (DGS, French Ministry of Health).
Disclaimer: The sponsor of the study had no role in study design, data collection, data analysis, data interpretation or writing of the paper.
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: The Advisory Committee on Data Collection in Health Research (CCTIRS) and the French Data Protection Authority (CNIL) (DR-2011-484).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request.
Contributor Information
On behalf of the PARCOURS Study Group:
Desgrées du Loû, F Lert, R Dray Spira, N Bajos, N Lydié, J Pannetier, A Ravalihasy, A Gosselin, E Rodary, D Pourette, J Situ, P Revault, P Sogni, J Gelly, Y Le Strat, and N Razafindratsima
References
- 1. European Centre for Disease Prevention and Control, WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2018–2017 data. Copenhagen: WHO Regional Office for Europe, 2018. Available: https://www.ecdc.europa.eu/sites/portal/files/documents/hiv-aids-surveillance-europe-2018.pdf
- 2. Prosser AT, Tang T, Hall HI. HIV in persons born outside the United States, 2007–2010. JAMA 2012;308:601–7. 10.1001/jama.2012.9046 [DOI] [PubMed] [Google Scholar]
- 3. Santé Publique France Point épidémiologique—Infection par le VIH et IST bactériennes, 2017. Available: http://invs.santepubliquefrance.fr/Dossiers-thematiques/Maladies-infectieuses/VIH-sida-IST/Infection-a-VIH-et-sida/Actualites/Infection-par-le-VIH-et-les-IST-bacteriennes.-Point-epidemiologique-du-28-novembre-2017 [Accessed 28 Nov 2017].
- 4. Lucas E, Cazein F, Brunet S, et al. HIV types, groups and subtypes diagnosed in France since 2003: data from eight years of surveillance. Bull Epidémiol Hebd 2012:46–7. [Google Scholar]
- 5. Burns FM, Arthur G, Johnson AM, et al. United Kingdom acquisition of HIV infection in African residents in London: more than previously thought. AIDS 2009;23:262–6. 10.1097/QAD.0b013e32831c546b [DOI] [PubMed] [Google Scholar]
- 6. Rice BD, Elford J, Yin Z, et al. A new method to assign country of HIV infection among heterosexuals born abroad and diagnosed with HIV. AIDS 2012;26:1961–6. 10.1097/QAD.0b013e3283578b80 [DOI] [PubMed] [Google Scholar]
- 7. Desgrées-du-Loû A, Pannetier J, Ravalihasy A, et al. Sub-Saharan African migrants living with HIV acquired after migration, France, ANRS PARCOURS study, 2012 to 2013. Euro Surveill 2015;20 10.2807/1560-7917.ES.2015.20.46.30065 [DOI] [PubMed] [Google Scholar]
- 8. Fakoya I, Álvarez-del Arco D, Woode-Owusu M, et al. A systematic review of post-migration acquisition of HIV among migrants from countries with generalised HIV epidemics living in Europe: implications for effectively managing HIV prevention programmes and policy. BMC Public Health 2015;15:561 10.1186/s12889-015-1852-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alvarez-del Arco D, Fakoya I, Thomadakis C, et al. High levels of postmigration HIV acquisition within nine European countries. AIDS 2017;31:1979–88. 10.1097/QAD.0000000000001571 [DOI] [PubMed] [Google Scholar]
- 10. Gosselin A, Ravalihasy A, Pannetier J, et al. When and who? timing of Post-Migration HIV acquisition among sub-Saharan immigrants in France. AIDS 2018, Amsterdam, Netherlands, 2018. [Google Scholar]
- 11. Gosselin A, Desgrées du Loû A, Lelièvre E, et al. Understanding settlement pathways of African immigrants in France through a capability approach: do Pre-migratory characteristics matter? Eur J Popul 2018;34:849–71. 10.1007/s10680-017-9463-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gosselin A, Desgrées du Loû A, Lelièvre E, et al. Understanding sub-Saharan migrant settlement in France through a capability approach: evidence from a life-event history survey. Working Paper du Ceped, n°33, 2016. Available: http://www.ceped.org/wp
- 13. Padian NS, McCoy SI, Karim SSA, et al. HIV prevention transformed: the new prevention research agenda. Lancet 2011;378:269–78. 10.1016/S0140-6736(11)60877-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wamoyi J, Stobeanau K, Bobrova N, et al. Transactional sex and risk for HIV infection in sub-Saharan Africa: a systematic review and meta-analysis. J Int AIDS Soc 2016;19:20992 10.7448/IAS.19.1.20992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Desgrées-du-Loû A, Pannetier J, Ravalihasy A, et al. Sub-Saharan African migrants living with HIV acquired after migration, France, ANRS PARCOURS study, 2012 to 2013. Eurosurveillance 2015;20:31–8. 10.2807/1560-7917.ES.2015.20.46.30065 [DOI] [PubMed] [Google Scholar]
- 16. Supervie V, Ndawinz JDA, Lodi S, et al. The undiagnosed HIV epidemic in France and its implications for HIV screening strategies. AIDS 2014;28:1797–804. 10.1097/QAD.0000000000000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ndawinz JDA, Costagliola D, Supervie V. New method for estimating HIV incidence and time from infection to diagnosis using HIV surveillance data: results for France. AIDS 2011;25:1905–13. 10.1097/QAD.0b013e32834af619 [DOI] [PubMed] [Google Scholar]
- 18. Ndawinz JDA, Anglaret X, Delaporte E, et al. New indicators for delay in initiation of antiretroviral treatment: estimates for Cameroon. Bull World Health Organ 2015;93:521–8. 10.2471/BLT.14.147892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. StataCorp Stata statistical software: release 14. College Station, TX: StataCorp LP, 2015. [Google Scholar]
- 20. Desgrées du Loû A, Pannetier J, Ravalihasy A, et al. Is hardship during migration a determinant of HIV infection? Results from the ANRS PARCOURS study of sub-Saharan African migrants in France. AIDS 2016;30:645–56. 10.1097/QAD.0000000000000957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larchanché S. Intangible obstacles: health implications of stigmatization, structural violence, and fear among undocumented immigrants in France. Soc Sci Med 2012;74:858–63. 10.1016/j.socscimed.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 22. Pannetier J, Ravalihasy A, Lydié N, et al. Prevalence and circumstances of forced sex and post-migration HIV acquisition in sub-Saharan African migrant women in France: an analysis of the ANRS-PARCOURS retrospective population-based study. Lancet Public Health 2018;3 10.1016/S2468-2667(17)30211-6 [DOI] [PubMed] [Google Scholar]
- 23. Marsicano E, Lydié N, Bajos N. Migrants from over there or racial minority here? Sexual networks and prevention practices among sub-Saharan African migrants in France. Cult Health Sex 2013;15:819–35. 10.1080/13691058.2013.785024 [DOI] [PubMed] [Google Scholar]
- 24. Guen ML, du LAD, Bajos N, et al. Migration et évolutions des situations conjugales : entre diversification des partenaires et persistance des asymétries de genre [Internet]. La Découverte, 2017. Available: https://www.cairn.info/parcours-de-vie-et-sante-des-africains-immigres-9782707196453-page-92.htm
- 25. Larsen C, Limousi F, Rahib D, et al. Infections VIH et VHB parmi les Afro-Caribéens d’Île-de-France : des prévalences élevées et des dépistages insuffisants. Bull Épidémiol Hebd 2017:29–30. [Google Scholar]
- 26. Loos J, Nöstlinger C, Reyniers T, et al. PrEP for African migrants in Europe? A research agenda. Lancet HIV 2016;3:e505–7. 10.1016/S2352-3018(16)30173-4 [DOI] [PubMed] [Google Scholar]
- 27. Carillon S. Médicamenter ou accueillir: L’approche de la prep pour les migrants en france est-elle pertinente. AFRAVIH, Bordeaux, France, 2018. [Google Scholar]
- 28. Elam G, Fenton KA, Johnson AM, et al. Exploring ethnicity and sexual health. A qualitative study of the sexual attitudes and lifestyles of five ethnic minority communities in Camden and Islington, 1999. Available: http://discovery.ucl.ac.uk/188927/
- 29. Prost A, Elford J, Imrie J, et al. Social, behavioural, and intervention research among people of sub-Saharan African origin living with HIV in the UK and Europe: literature review and recommendations for intervention. AIDS Behav 2008;12:170–94. 10.1007/s10461-007-9237-4 [DOI] [PubMed] [Google Scholar]
- 30. Courgeau D. Analyse des données biographiques erronées. Population 1991;46:89–104. 10.2307/1533611 [DOI] [Google Scholar]
- 31. Le Cœur S, Im-em W, Koetsawang S, et al. Living with HIV in Thailand: assessing vulnerability through a life-event history approach. Population 2005;60:551–68. [Google Scholar]
- 32. Molina J, Ghosn J, Béniguel L. Incidence of HIV-infection in the ANRS Prevenir study in the Paris region with daily or on demand PreP with TDF/FTC. AIDS, In Amsterdam, Netherlands, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
sextrans-2019-054080supp001.pdf (213.8KB, pdf)