Abstract
Cornelia de Lange syndrome (CdLS) is a severe genetic disorder characterised by multisystemic malformations. CdLS is due to pathogenetic variants in NIPBL, SMC1A, SMC3, RAD21 and HDAC8 genes which belong to the cohesin pathway. Cohesin plays a pivotal role in chromatid cohesion, gene expression, and DNA repair. In this review, we will discuss how perturbations in those biological processes contribute to CdLS phenotype and will emphasise the state-of-art of CdLS therapeutic approaches.
Keywords: cohesin, Cornelia de Lange syndrome, gene dysregulation, genome instability, therapeutic approaches
Introduction
The cohesin complex, composed of four core subunits, SMC1A, SMC3, RAD21 and STAG, forms a ring-shaped structure that encircles chromatin.1 It is evolutionarily conserved from prokaryotic to eukaryotic organisms. Additional factors, such as NIPBL, PDS5, WAPL, HDAC8 and ESCO, finely regulate the activity of cohesin during the cell cycle (figure 1). Cohesin loading onto chromatin is mediated from NIPBL, in association with its molecular partner MAU2.2 3 It occurs in G1 in yeast or at the end of telophase of the previous cell cycle in mammalian cells. ESCO proteins (1 and 2) allow cohesin establishment in the S phase, and PDS5 (A and B) guarantees its maintenance.4–7 WAPL contributes to cohesin dissolution and HDAC8 is necessary for cohesin recycling during the cell cycle.8 9 Though cohesin was first identified for its role in establishing sister chromatid cohesion, which is important for proper chromosome segregation, cohesin is a key regulator in gene expression10–12 and 3D genome organisation.13 14 In fact, cohesin cooperates with the sequence-specific DNA binding protein CTCF to organise the mammalian genome into structural topologically associated domains (TADs), chromatin loops and contact domains.15–19 It is thought that these features compartmentalise genes and bring together distant enhancers with promoter sequences to orchestrate gene expression.20–22 The disruption of TADs and the removal of cohesin or CTCF binding sites result in abnormal DNA domain topology, thus leading to gene expression dysregulation.14 23–25 Cohesin is also essential for genome integrity as it controls fork replication speed,26 27 promotes DNA repair by homologous recombination,28–32 safeguards telomere stability33 34 and recruits proteins involved in G2/M checkpoints.35 36 DNA damage response and gene transcription are intimately associated via cohesin. In fact, in presence of DNA double-strand breaks, cohesin represses transcription in the flanking chromatin.37 38 Because of this role, it is not surprising that cohesin’s pathogenetic variants are frequently found in cancer, including colorectal and urothelial carcinomas, Ewing sarcomas and acute myeloid leukaemia.39–45
Figure 1.
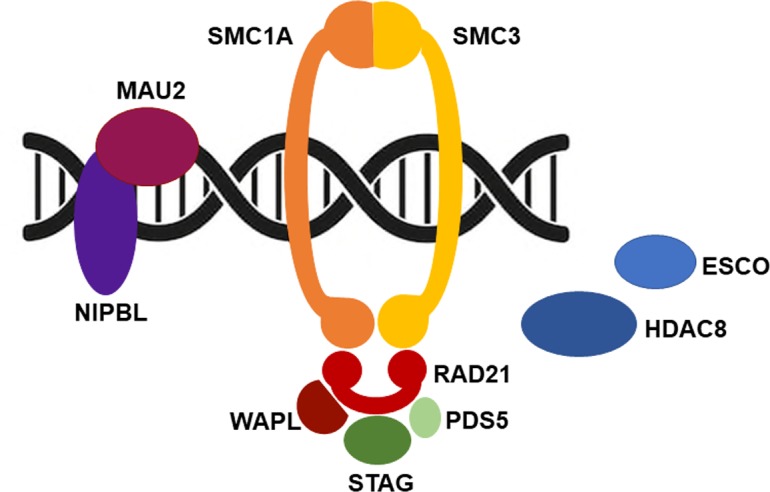
Cohesin and cell cycle. Cohesin is loaded onto chromosomes prior to DNA replication by a binary protein complex formed by NIPBL and MAU2. ESCO (1 and 2) are acetyltransferases that establish sister chromatid cohesion during DNA replication by promoting the acetylation of a pair of lysine residues within SMC3. Pds5 (A and B) has a cohesive effect on cohesin, while having the opposite impact when associated with Wapl. Finally, HDAC8, a histone deacetylase, targets SMC3 permitting cohesin recycling.
Cohesin and Cornelia de Lange syndrome
The finding that germinal pathogenetic variants in cohesin structural and regulatory genes are associated with human diseases collectively called ‘cohesinopathies’ may be less counterintuitive. Cornelia de Lange syndrome (CdLS; OMIM 122470, 300590, 610759, 614701, 300882) is the most frequently represented among these. CdLS is a rare multiorgan development disease with a prevalence ranging from 1:10 000 to 1:30 000 live births without differences between ethnic groups.46 Its clinical presentation is characterised by facial dysmorphism (arched eyebrows with synophrys, long philtrum, thin lips, hirsute forehead), prenatal and postnatal growth retardation, cognitive impairment ranging from mild to severe, gastrointestinal malformations, congenital heart abnormalities and limb defects.47 At present, five CdLS-causative genes have been identified. Pathogenetic variants in NIPBL account for about 60% of patients with CdLS whereas about 5%–10% of CdLS probands carry pathogenetic variants in SMC1A, SMC3, RAD21 or HDAC8.9 48–52 This observation suggests that other genes responsible for CdLS have to be identified. Analysis of the mutational spectrum reveals a genotype–phenotype correlation (figure 2). NIPBL truncating, nonsense, splice site and frame shift pathogenetic variants leading to a truncated and likely non-functional NIPBL protein are associated with a severe phenotype. The clinical picture of patients with CdLS carrying SMC1A, SMC3 and RAD21 pathogenetic variants is more uniform, characterised by a mild to moderate phenotype more similar to NIPBL-mutated probands who carry missense changes. Finally, patients harbouring pathogenetic variants in HDAC8 gene show clinical traits overlapping to some extent with classic forms of CdLS characterised by typical facial dysmorphia and severe cognitive delay.53
Figure 2.
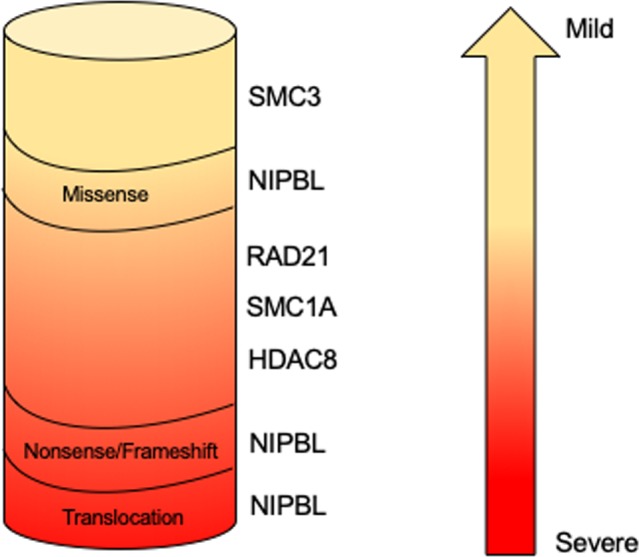
Genotype–phenotype correlation in Cornelia de Lange syndrome. NIPBL truncating pathogenetic variants result in a more severe phenotype, whereas HDAC8, NIPBL, RAD21, SMC1A and SMC3 missense mutations and in-frame deletions are associated with a milder phenotype.
The molecular diagnosis of CdLS is complicated by both the presence of somatic mosaicism and the overlap with other diseases. In fact, sequencing of DNA extracted from fibroblasts or buccal mucosa allowed the identification of mosaic variants in all five CdLS genes in patients originally reported to be mutation negative by Sanger sequencing on DNA isolated from blood.54–58 In order to provide an accurate diagnosis, these variants are of particular interest since they may escape routine molecular diagnostics and indicate that sequencing should not be restricted to DNA from blood samples. Furthermore, patients presenting CdLS or CdLS-like phenotype have been described, and interestingly they carried pathogenetic variants in chromatin-associated factors (table 1) such as BRD4 and AFF4 (responsible for CHOPS syndrome, OMIM 616368); ANKRD11 (associated with KBG syndrome, OMIM 148050); EP300 (responsible for Rubinstein-Taybi syndrome (RSTS), OMIM 613684) and KMT2A (causal gene for Wiedemann-Steiner syndrome (WDSTS), OMIM 605130); and ARID1B, ARID1A, SMARCB1, SMARCA4, SMARCE1, ARID2, SOX11 and DPF2 (associated with Coffin-Siris syndrome (CSS), OMIM 614556, 603024, 601607, 603254, 603111, 609539, 600898, 601671).59–71 Interestingly, all of these disorders display a significant clinical overlap with CdLS, each presenting with shared features that embrace facial dysmorphism, intellectual disability, growth and developmental delay and that are regularly considered in the differential diagnosis of CdLS. These findings suggest that the clinical overlap of these syndromes is mirrored by molecular interactions belonging to the same path, and chromatin dysregulation is a common step towards the pathogenesis of developmental disorders that share phenotypical features with CdLS.
Table 1.
Human diseases overlapping Cornelia de Lange syndrome (CdLS)
| Disease | Gene(s) | Role |
| CdLS-like | BRD4 | It binds to hyperacetylated genomic regions that encompass promoters and enhancers; regulates transcription elongation by paused RNA polymerase II114 |
| CHOPS | AFF4 | It is a scaffold protein comprising the core component of the super elongation complex82 |
| CSS | ARID1B, ARID1A, ARID2, DPF2, SMARCB1, SMARCA4, SMARCE1, SOX11 | They are subunits of the ATP‐dependent SWI/SNF (SWItch/sucrose non‐fermentable) chromatin remodelling complex involved in transcription115 |
| KBG | ANKRD11 | It is associated with transcription through chromatin modification116 |
| RSTS | EP300 | It functions as histone acetyltransferase that regulates transcription via chromatin remodelling117 |
| WDSTS | KMT2A | It is a histone methyltransferase that regulates chromatin-mediated transcription118 |
Biological processes dysregulated in CdLS
Sister chromatid cohesion is not affected in CdLS72 73; thus, the molecular mechanisms underlying CdLS remain elusive. Several dysregulated biological pathways have been documented in CdLS, including gene transcription, RNA biogenesis, DNA repair and oxidative stress response (figure 3).72 74–78 The cohesin complex, in association with its loader NIPBL and the insulator protein CTCF, regulates enhancer–promoter interaction and is responsible for creating the TADs.18 20 79 Genome-wide data show that cohesin binds more frequently to promoter and downstream regions and is associated with active gene in patients with CdLS.74 75 In addition, CdLS cells harbouring SMC1A pathogenetic variants or the partial decrease in NIPBL expression caused a distinctive profile of gene expression changes.74 75
Figure 3.
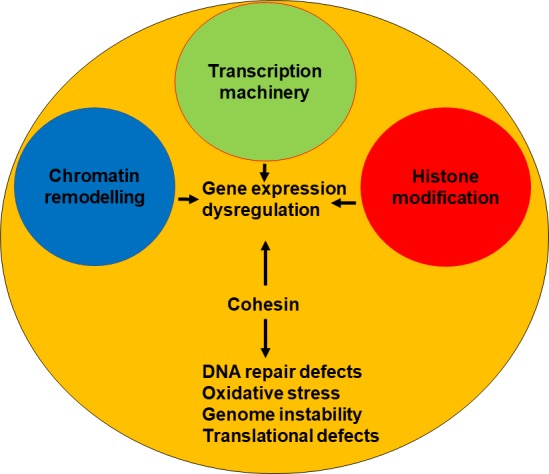
Most of CdLS are caused by mutations in cohesin and cohesin-regulatory genes. These mutations cause alterations in many fundamental biological processes such as transcription, DNA repair and translation, leading to gene expression dysregulation, high levels of oxidative stress and genome instability. A few cases are instead due to mutations in genes coding transcription machinery members or involved in chromatin remodelling and histone modification, which in turn cause gene changes.
For this reason, CdLS is now grouped in a growing broader class of neurodevelopmental diseases, called disorders of transcriptional regulation (DTRs).80 In addition to CdLS, DTRs include genetic diseases resulting from mutations in gene coding proteins for the transcriptional machinery, histone modification and chromatin remodelling. For example, CSS is characterised by facial dysmorphism, developmental delay, cognitive impairment, absence of terminal phalanges and fifth fingernail hypoplasia.81 CSS is a disorder of chromatin remodelling. In fact, it is caused by mutations in genes belonging to the SWI/SNF chromatin remodeller complex.68 69 CHOPS syndrome is characterised by round faces and arched eyebrows, intellectual disability, heart defects and short stature.59 CHOPS patients carry a mutation in AFF4 gene that encodes a component of the super elongation complex.59 82 Therefore, CHOPS syndrome may be considered a disorder of transcriptional elongation. KBG syndrome is characterised by facial dysmorphism, macrodontia of the upper central incisor, skeletal defects and cognitive impairment.83 KBG syndrome is caused by mutations in ANKRD11 gene which regulates histone acetylation,84 thus it is a disorder of histone modifications. Gene transcription regulation is a fundamental biological process involving many aspects of cell life such as the interaction between enhancer and promoter by chromatin loop, histone modification and the formation of transcription apparatus. The observation that the number of diseases belonging to DTRs is increasing over the years indicates that perturbations in transcription regulation cause multiple developmental syndromes. In addition to gaining insight into the pathogenetic basis of human disorders, the study of DTRs also contributes to dissecting the molecular mechanism of gene transcription regulation.
The findings that CdLS cells show gene expression dysregulation and no defects in sister chromatid cohesion reveal a dosage-sensitive functional hierarchy of cohesin. Mitotic functions appear to be the least dose sensitive, while gene regulation is more dose sensitive. This view is further supported by the findings that the depletion of cohesin or Nipped-B (homologue of human NIPBL) in Drosophila cells and Nipbl in mice affected gene expression but did not cause cohesion or chromosome segregation defects.85 86
The notion that gene transcription is dysregulated in CdLS is further supported by studies in animal models.86–88 Up to 1000 genes have been found dysregulated, but interestingly their fold changes were modest74 75 86 87 suggesting that phenotypic consequences arise from multiple collective perturbations in gene expression. The mechanism leading to gene dysregulation in CdLS is still poorly understood. However, pieces of data may help us to set up the puzzle. In fact, though cohesin is preferentially associated with transcription site start, the number of cohesin sites on differentially expressed genes is significantly reduced in CdLS, whereas the reduction is moderate for the non-differentially expressed genes.74 In addition, mutant cohesin displays an increased affinity for chromatin making the cohesin–DNA binding more stable and impairs PolII recruitment at the promoter regions of dysregulated genes.72 75 It is reasonable to deduce that cohesin mutations cause chromatin modification, leading to global transcription disturbance in CdLS.
Cohesin binds to the ribosomal DNA, plays a role in nucleolus organisation and facilitates protein translation.89 90 It has been shown that rRNA production and protein synthesis are decreased in a zebrafish model of CdLS.91 This finding suggests that cohesin has the potential to affect the functions of the nucleolus, and some of the transcriptional changes observed in CdLS may occur as a result of translational defects.
CdLS cells are characterised by genome instability, as evidenced by the presence of chromosomal rearrangements and aberrations such as translocations, deletions, gaps and breaks.48 49 72 92 In addition, cells are sensitive to genotoxic drugs such as aphidicolin and mytomicin C, and X-ray exposure,72 93 suggesting that CdLS cells have a reduced ability to tolerate DNA damage, likely as a result of reduced DNA repair capability. These data raise the possibility that mutant cohesin may contribute to DNA damage sensitivity by altering the dynamic association of cohesin with DNA, which in turn impairs the recruitment of proteins involved in DNA repair. Oxidative stress could provide a further contribution to the genome instability detected in CdLS. In fact, it is well known that high levels of oxidative stress promote genome instability, apoptosis and cell growth arrest.94–96 Of note, experimental evidence shows the downregulation of proteins involved in the response to oxidative stress and an increase in global oxidative stress in CdLS cell lines.78
Therapeutic approaches in CdLS
The discoveries made in recent years regarding both the identification of CdLS-causative genes and the cellular and molecular characterisation of CdLS cells (figure 3) are the basis for attempting a therapeutic approach in CdLS.
As reported above, CdLS is associated with defects in ribosome biogenesis and translation.91 It has been shown that treatment with l-leucine improves rRNA production, protein synthesis and cell survival and partially rescues developmental defects (embryo length, cartilage formation, and head and eye size) in a zebrafish model of CdLS.91 l-Leucine treatment results in TOR (target of rapamycin) pathway activation through a mechanism that involves the activity of leucyl tRNA synthase and of GTP activating proteins.97 98 The TOR pathway controls cell proliferation, protein translation and ribosome biogenesis.99–101 Treatment with lithium chloride (LiCl) partially rescues neural development in nipblb knockdown zebrafish embryos and cell death in fibroblasts of patients with CdLS.102 Interestingly, LiCl promotes the activation of both Wnt-b-catenin and TOR signalling pathways.103
CdLS is characterised by several phenotypic markers, including growth delay, short stature and delayed puberty.104 Recently, a girl carrying a de novo splicing mutation in NIPBL gene was treated at 4.3 years of age with recombinant human growth hormone (r-hGH). The r-hGH treatment led to a height gain of 1.6 SD score over 8 years105 suggesting that hormonal therapy may be effective for patients with CdLS with short stature.
The relationship between oxidative stress and genome instability in neurodegeneration and senescence is well established.106–108 Oxidative stress is induced by the accumulation of reactive oxygen species. Interestingly, an intriguing link between oxidative stress and TOR pathway has been described, in particular related to age-dependent cognitive decline, pathogenesis of Alzheimer disease and Down syndrome.109–111 Treatment with antioxidant drugs reduces both the level of oxidative stress and genome instability, leading to the extension of in vitro lifespan of CdLS cell lines. In addition, treatment ameliorates the phenotypic feature of zebrafish modelling of CdLS.92 Antioxidant drugs protect the DNA from damage and prevent cell death, as shown in cerebellar cells using N-acetyl-cysteine.112 113 Altogether, these data indicate that antioxidant therapy could provide a means of improving specific phenotypic features of CdLS.
Conclusion
There is increasing evidence that CdLS is caused by a combination of factors, such as gene expression dysregulation, accumulation of cellular damage and cellular phenotype ageing, which collectively contribute to the CdLS phenotype. CdLS therapy is taking its first steps. Chemical or hormonal treatment represents a valuable attempt to identify potential therapeutic targets for future treatment of patients with CdLS, and hopefully clinical trials are now becoming closer.
Acknowledgments
We acknowledge the financial support of Fondazione Pisa to AM.
Footnotes
Contributors: PS and MMP wrote the paper. AM conceived the structure and content, wrote and revised the manuscript.
Funding: This study was funded by Fondazione Pisa.
Disclaimer: The funder had no involvement.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Nasmyth K, Haering CH. Cohesin: its roles and mechanisms. Annu Rev Genet 2009;43:525–58. 10.1146/annurev-genet-102108-134233 [DOI] [PubMed] [Google Scholar]
- 2. Ciosk R, Shirayama M, Shevchenko A, Tanaka T, Toth A, Shevchenko A, Nasmyth K. Cohesin's binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Mol Cell 2000;5:243–54. 10.1016/S1097-2765(00)80420-7 [DOI] [PubMed] [Google Scholar]
- 3. Haering CH, Farcas A-M, Arumugam P, Metson J, Nasmyth K. The cohesin ring concatenates sister DNA molecules. Nature 2008;454:297–301. 10.1038/nature07098 [DOI] [PubMed] [Google Scholar]
- 4. Rolef Ben-Shahar T, Heeger S, Lehane C, East P, Flynn H, Skehel M, Uhlmann F. Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science 2008;321:563–6. 10.1126/science.1157774 [DOI] [PubMed] [Google Scholar]
- 5. Unal E, Heidinger-Pauli JM, Kim W, Guacci V, Onn I, Gygi SP, Koshland DE. A molecular determinant for the establishment of sister chromatid cohesion. Science 2008;321:566–9. 10.1126/science.1157880 [DOI] [PubMed] [Google Scholar]
- 6. Panizza S, Tanaka T, Hochwagen A, Eisenhaber F, Nasmyth K. Pds5 cooperates with cohesin in maintaining sister chromatid cohesion. Curr Biol 2000;10:1557–64. 10.1016/S0960-9822(00)00854-X [DOI] [PubMed] [Google Scholar]
- 7. Losada A, Yokochi T, Hirano T. Functional contribution of Pds5 to cohesin-mediated cohesion in human cells and Xenopus egg extracts. J Cell Sci 2005;118:2133–41. 10.1242/jcs.02355 [DOI] [PubMed] [Google Scholar]
- 8. Tedeschi A, Wutz G, Huet S, Jaritz M, Wuensche A, Schirghuber E, Davidson IF, Tang W, Cisneros DA, Bhaskara V, Nishiyama T, Vaziri A, Wutz A, Ellenberg J, Peters J-M. Wapl is an essential regulator of chromatin structure and chromosome segregation. Nature 2013;501:564–8. 10.1038/nature12471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deardorff MA, Bando M, Nakato R, Watrin E, Itoh T, Minamino M, Saitoh K, Komata M, Katou Y, Clark D, Cole KE, De Baere E, Decroos C, Di Donato N, Ernst S, Francey LJ, Gyftodimou Y, Hirashima K, Hullings M, Ishikawa Y, Jaulin C, Kaur M, Kiyono T, Lombardi PM, Magnaghi-Jaulin L, Mortier GR, Nozaki N, Petersen MB, Seimiya H, Siu VM, Suzuki Y, Takagaki K, Wilde JJ, Willems PJ, Prigent C, Gillessen-Kaesbach G, Christianson DW, Kaiser FJ, Jackson LG, Hirota T, Krantz ID, Shirahige K. Hdac8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature 2012;489:313–7. 10.1038/nature11316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang J, Li K, Cai W, Liu X, Zhang Y, Orkin SH, Xu J, Yuan G-C. Dissecting super-enhancer hierarchy based on chromatin interactions. Nat Commun 2018;9:943 10.1038/s41467-018-03279-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dowen JM, Bilodeau S, Orlando DA, Hübner MR, Abraham BJ, Spector DL, Young RA. Multiple structural maintenance of chromosome complexes at transcriptional regulatory elements. Stem Cell Reports 2013;1:371–8. 10.1016/j.stemcr.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cuartero S, Weiss FD, Dharmalingam G, Guo Y, Ing-Simmons E, Masella S, Robles-Rebollo I, Xiao X, Wang Y-F, Barozzi I, Djeghloul D, Amano MT, Niskanen H, Petretto E, Dowell RD, Tachibana K, Kaikkonen MU, Nasmyth KA, Lenhard B, Natoli G, Fisher AG, Merkenschlager M. Control of inducible gene expression links cohesin to hematopoietic progenitor self-renewal and differentiation. Nat Immunol 2018;19:932–41. 10.1038/s41590-018-0184-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rao SSP, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES, Aiden EL. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 2014;159:1665–80. 10.1016/j.cell.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schwarzer W, Abdennur N, Goloborodko A, Pekowska A, Fudenberg G, Loe-Mie Y, Fonseca NA, Huber W, Haering CH, Mirny L, Spitz F. Two independent modes of chromatin organization revealed by cohesin removal. Nature 2017;551:51–6. 10.1038/nature24281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wendt KS, Yoshida K, Itoh T, Bando M, Koch B, Schirghuber E, Tsutsumi S, Nagae G, Ishihara K, Mishiro T, Yahata K, Imamoto F, Aburatani H, Nakao M, Imamoto N, Maeshima K, Shirahige K, Peters J-M. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 2008;451:796–801. 10.1038/nature06634 [DOI] [PubMed] [Google Scholar]
- 16. Rubio ED, Reiss DJ, Welcsh PL, Disteche CM, Filippova GN, Baliga NS, Aebersold R, Ranish JA, Krumm A. Ctcf physically links cohesin to chromatin. Proc Natl Acad Sci U S A 2008;105:8309–14. 10.1073/pnas.0801273105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parelho V, Hadjur S, Spivakov M, Leleu M, Sauer S, Gregson HC, Jarmuz A, Canzonetta C, Webster Z, Nesterova T, Cobb BS, Yokomori K, Dillon N, Aragon L, Fisher AG, Merkenschlager M. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell 2008;132:422–33. 10.1016/j.cell.2008.01.011 [DOI] [PubMed] [Google Scholar]
- 18. Zuin J, Dixon JR, van der Reijden MIJA, Ye Z, Kolovos P, Brouwer RWW, van de Corput MPC, van de Werken HJG, Knoch TA, van IJcken WFJ, Grosveld FG, Ren B, Wendt KS. Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells. Proc Natl Acad Sci U S A 2014;111:996–1001. 10.1073/pnas.1317788111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, Taatjes DJ, Dekker J, Young RA. Mediator and cohesin connect gene expression and chromatin architecture. Nature 2010;467:430–5. 10.1038/nature09380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rao SSP, Huang S-C, Glenn St Hilaire B, Engreitz JM, Perez EM, Kieffer-Kwon K-R, Sanborn AL, Johnstone SE, Bascom GD, Bochkov ID, Huang X, Shamim MS, Shin J, Turner D, Ye Z, Omer AD, Robinson JT, Schlick T, Bernstein BE, Casellas R, Lander ES, Aiden EL. Cohesin loss eliminates all loop domains. Cell 2017;171:e24:305–20. 10.1016/j.cell.2017.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vian L, Pękowska A, Rao SSP, Kieffer-Kwon K-R, Jung S, Baranello L, Huang S-C, El Khattabi L, Dose M, Pruett N, Sanborn AL, Canela A, Maman Y, Oksanen A, Resch W, Li X, Lee B, Kovalchuk AL, Tang Z, Nelson S, Di Pierro M, Cheng RR, Machol I, St Hilaire BG, Durand NC, Shamim MS, Stamenova EK, Onuchic JN, Ruan Y, Nussenzweig A, Levens D, Aiden EL, Casellas R. The energetics and physiological impact of cohesin extrusion. Cell 2018;175:292–4. 10.1016/j.cell.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haarhuis JHI, van der Weide RH, Blomen VA, Yáñez-Cuna JO, Amendola M, van Ruiten MS, Krijger PHL, Teunissen H, Medema RH, van Steensel B, Brummelkamp TR, de Wit E, Rowland BD. The cohesin release factor WAPL restricts chromatin loop extension. Cell 2017;169:e14:693–707. 10.1016/j.cell.2017.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hnisz D, Weintraub AS, Day DS, Valton A-L, Bak RO, Li CH, Goldmann J, Lajoie BR, Fan ZP, Sigova AA, Reddy J, Borges-Rivera D, Lee TI, Jaenisch R, Porteus MH, Dekker J, Young RA. Activation of proto-oncogenes by disruption of chromosome neighborhoods. Science 2016;351:1454–8. 10.1126/science.aad9024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seitan VC, Hao B, Tachibana-Konwalski K, Lavagnolli T, Mira-Bontenbal H, Brown KE, Teng G, Carroll T, Terry A, Horan K, Marks H, Adams DJ, Schatz DG, Aragon L, Fisher AG, Krangel MS, Nasmyth K, Merkenschlager M. A role for cohesin in T-cell-receptor rearrangement and thymocyte differentiation. Nature 2011;476:467–71. 10.1038/nature10312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Narendra V, Rocha PP, An D, Raviram R, Skok JA, Mazzoni EO, Reinberg D. CTCF establishes discrete functional chromatin domains at the Hox clusters during differentiation. Science 2015;347:1017–21. 10.1126/science.1262088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cucco F, Palumbo E, Camerini S, D'Alessio B, Quarantotti V, Casella ML, Rizzo IM, Cukrov D, Delia D, Russo A, Crescenzi M, Musio A. Separase prevents genomic instability by controlling replication fork speed. Nucleic Acids Res 2018;46:267–78. 10.1093/nar/gkx1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Terret M-E, Sherwood R, Rahman S, Qin J, Jallepalli PV. Cohesin acetylation speeds the replication fork. Nature 2009;462:231–4. 10.1038/nature08550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Potts PR, Porteus MH, Yu H. Human Smc5/6 complex promotes sister chromatid homologous recombination by recruiting the SMC1/3 cohesin complex to double-strand breaks. Embo J 2006;25:3377–88. 10.1038/sj.emboj.7601218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gelot C, Guirouilh-Barbat J, Le Guen T, Dardillac E, Chailleux C, Canitrot Y, Lopez BS. The cohesin complex prevents the end joining of distant DNA double-strand ends. Mol Cell 2016;61:15–26. 10.1016/j.molcel.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 30. Schär P, Fäsi M, Jessberger R. Smc1 coordinates DNA double-strand break repair pathways. Nucleic Acids Res 2004;32:3921–9. 10.1093/nar/gkh716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jessberger R, Podust V, Hübscher U, Berg P. A mammalian protein complex that repairs double-strand breaks and deletions by recombination. J Biol Chem 1993;268:15070–9. [PubMed] [Google Scholar]
- 32. Jessberger R, Berg P. Repair of deletions and double-strand gaps by homologous recombination in a mammalian in vitro system. Mol Cell Biol 1991;11:445–57. 10.1128/MCB.11.1.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cipressa F, Morciano P, Bosso G, Mannini L, Galati A, Raffa GD, Cacchione S, Musio A, Cenci G. A role for separase in telomere protection. Nat Commun 2016;7:10405 10.1038/ncomms10405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Daniloski Z, Smith S. Loss of tumor suppressor STAG2 promotes telomere recombination and extends the replicative lifespan of normal human cells. Cancer Res 2017;77:5530–42. 10.1158/0008-5472.CAN-17-1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watrin E, Peters J-M. The cohesin complex is required for the DNA damage-induced G2/M checkpoint in mammalian cells. Embo J 2009;28:2625–35. 10.1038/emboj.2009.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Musio A, Montagna C, Mariani T, Tilenni M, Focarelli ML, Brait L, Indino E, Benedetti PA, Chessa L, Albertini A, Ried T, Vezzoni P. Smc1 involvement in fragile site expression. Hum Mol Genet 2005;14:525–33. 10.1093/hmg/ddi049 [DOI] [PubMed] [Google Scholar]
- 37. Meisenberg C, Pinder SI, Hopkins SR, Wooller SK, Benstead-Hume G, Pearl FMG, Jeggo PA, Downs JA. Repression of transcription at DNA breaks requires cohesin throughout interphase and prevents genome instability. Mol Cell 2019;73:212–23. 10.1016/j.molcel.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brownlee PM, Chambers AL, Cloney R, Bianchi A, Downs JA. BAF180 promotes cohesion and prevents genome instability and aneuploidy. Cell Rep 2014;6:973–81. 10.1016/j.celrep.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kon A, Shih L-Y, Minamino M, Sanada M, Shiraishi Y, Nagata Y, Yoshida K, Okuno Y, Bando M, Nakato R, Ishikawa S, Sato-Otsubo A, Nagae G, Nishimoto A, Haferlach C, Nowak D, Sato Y, Alpermann T, Nagasaki M, Shimamura T, Tanaka H, Chiba K, Yamamoto R, Yamaguchi T, Otsu M, Obara N, Sakata-Yanagimoto M, Nakamaki T, Ishiyama K, Nolte F, Hofmann W-K, Miyawaki S, Chiba S, Mori H, Nakauchi H, Koeffler HP, Aburatani H, Haferlach T, Shirahige K, Miyano S, Ogawa S. Recurrent mutations in multiple components of the cohesin complex in myeloid neoplasms. Nat Genet 2013;45:1232–7. 10.1038/ng.2731 [DOI] [PubMed] [Google Scholar]
- 40. Barber TD, McManus K, Yuen KWY, Reis M, Parmigiani G, Shen D, Barrett I, Nouhi Y, Spencer F, Markowitz S, Velculescu VE, Kinzler KW, Vogelstein B, Lengauer C, Hieter P. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci U S A 2008;105:3443–8. 10.1073/pnas.0712384105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cucco F, Servadio A, Gatti V, Bianchi P, Mannini L, Prodosmo A, De Vitis E, Basso G, Friuli A, Laghi L, Soddu S, Fontanini G, Musio A. Mutant cohesin drives chromosomal instability in early colorectal adenomas. Hum Mol Genet 2014;23:6773–8. 10.1093/hmg/ddu394 [DOI] [PubMed] [Google Scholar]
- 42. Solomon DA, Kim J-S, Bondaruk J, Shariat SF, Wang Z-F, Elkahloun AG, Ozawa T, Gerard J, Zhuang D, Zhang S, Navai N, Siefker-Radtke A, Phillips JJ, Robinson BD, Rubin MA, Volkmer B, Hautmann R, Küfer R, Hogendoorn PCW, Netto G, Theodorescu D, James CD, Czerniak B, Miettinen M, Waldman T. Frequent truncating mutations of STAG2 in bladder cancer. Nat Genet 2013;45:1428–30. 10.1038/ng.2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Solomon DA, Kim T, Diaz-Martinez LA, Fair J, Elkahloun AG, Harris BT, Toretsky JA, Rosenberg SA, Shukla N, Ladanyi M, Samuels Y, James CD, Yu H, Kim J-S, Waldman T. Mutational inactivation of STAG2 causes aneuploidy in human cancer. Science 2011;333:1039–43. 10.1126/science.1203619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, Meyerson M, Gabriel SB, Lander ES, Getz G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 2014;505:495–501. 10.1038/nature12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sarogni P, Palumbo O, Servadio A, Astigiano S, D'Alessio B, Gatti V, Cukrov D, Baldari S, Pallotta MM, Aretini P, Dell'Orletta F, Soddu S, Carella M, Toietta G, Barbieri O, Fontanini G, Musio A. Overexpression of the cohesin-core subunit SMC1A contributes to colorectal cancer development. J Exp Clin Cancer Res 2019;38 10.1186/s13046-019-1116-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ramos FJ, Puisac B, Baquero-Montoya C, Gil-Rodríguez MC, Bueno I, Deardorff MA, Hennekam RC, Kaiser FJ, Krantz ID, Musio A, Selicorni A, FitzPatrick DR, Pié J. Clinical utility gene card for: Cornelia de Lange syndrome. Eur J Hum Genet 2015;23:1431 10.1038/ejhg.2014.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kline AD, Moss JF, Selicorni A, Bisgaard A-M, Deardorff MA, Gillett PM, Ishman SL, Kerr LM, Levin AV, Mulder PA, Ramos FJ, Wierzba J, Ajmone PF, Axtell D, Blagowidow N, Cereda A, Costantino A, Cormier-Daire V, FitzPatrick D, Grados M, Groves L, Guthrie W, Huisman S, Kaiser FJ, Koekkoek G, Levis M, Mariani M, McCleery JP, Menke LA, Metrena A, O'Connor J, Oliver C, Pie J, Piening S, Potter CJ, Quaglio AL, Redeker E, Richman D, Rigamonti C, Shi A, Tümer Z, Van Balkom IDC, Hennekam RC. Diagnosis and management of Cornelia de Lange syndrome: first international consensus statement. Nat Rev Genet 2018;19:649–66. 10.1038/s41576-018-0031-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tonkin ET, Wang T-J, Lisgo S, Bamshad MJ, Strachan T. Nipbl, encoding a homolog of fungal Scc2-type sister chromatid cohesion proteins and fly Nipped-B, is mutated in Cornelia de Lange syndrome. Nat Genet 2004;36:636–41. 10.1038/ng1363 [DOI] [PubMed] [Google Scholar]
- 49. Krantz ID, McCallum J, DeScipio C, Kaur M, Gillis LA, Yaeger D, Jukofsky L, Wasserman N, Bottani A, Morris CA, Nowaczyk MJM, Toriello H, Bamshad MJ, Carey JC, Rappaport E, Kawauchi S, Lander AD, Calof AL, Li H-H, Devoto M, Jackson LG. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat Genet 2004;36:631–5. 10.1038/ng1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Musio A, Selicorni A, Focarelli ML, Gervasini C, Milani D, Russo S, Vezzoni P, Larizza L. X-Linked Cornelia de Lange syndrome owing to SMC1L1 mutations. Nat Genet 2006;38:528–30. 10.1038/ng1779 [DOI] [PubMed] [Google Scholar]
- 51. Deardorff MA, Kaur M, Yaeger D, Rampuria A, Korolev S, Pie J, Gil-Rodríguez C, Arnedo M, Loeys B, Kline AD, Wilson M, Lillquist K, Siu V, Ramos FJ, Musio A, Jackson LS, Dorsett D, Krantz ID. Mutations in cohesin complex members SMC3 and SMC1A cause a mild variant of Cornelia de Lange syndrome with predominant mental retardation. Am J Hum Genet 2007;80:485–94. 10.1086/511888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deardorff MA, Wilde JJ, Albrecht M, Dickinson E, Tennstedt S, Braunholz D, Mönnich M, Yan Y, Xu W, Gil-Rodríguez MC, Clark D, Hakonarson H, Halbach S, Michelis LD, Rampuria A, Rossier E, Spranger S, Van Maldergem L, Lynch SA, Gillessen-Kaesbach G, Lüdecke H-J, Ramsay RG, McKay MJ, Krantz ID, Xu H, Horsfield JA, Kaiser FJ. Rad21 mutations cause a human cohesinopathy. Am J Hum Genet 2012;90:1014–27. 10.1016/j.ajhg.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mannini L, Cucco F, Quarantotti V, Krantz ID, Musio A. Mutation spectrum and genotype–phenotype correlation in Cornelia de Lange syndrome. Hum Mutat 2013;34:1589–96. 10.1002/humu.22430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huisman SA, Redeker EJW, Maas SM, Mannens MM, Hennekam RCM. High rate of mosaicism in individuals with Cornelia de Lange syndrome. J Med Genet 2013;50:339–44. 10.1136/jmedgenet-2012-101477 [DOI] [PubMed] [Google Scholar]
- 55. Pozojevic J, Parenti I, Graul-Neumann L, Ruiz Gil S, Watrin E, Wendt KS, Werner R, Strom TM, Gillessen-Kaesbach G, Kaiser FJ. Novel mosaic variants in two patients with Cornelia de Lange syndrome. Eur J Med Genet 2018;61:680–4. 10.1016/j.ejmg.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 56. Slavin TP, Lazebnik N, Clark DM, Vengoechea J, Cohen L, Kaur M, Konczal L, Crowe CA, Corteville JE, Nowaczyk MJ, Byrne JL, Jackson LG, Krantz ID. Germline mosaicism in Cornelia de Lange syndrome. Am J Med Genet A 2012;158A:1481–5. 10.1002/ajmg.a.35381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ansari M, Poke G, Ferry Q, Williamson K, Aldridge R, Meynert AM, Bengani H, Chan CY, Kayserili H, Avci S, Hennekam RCM, Lampe AK, Redeker E, Homfray T, Ross A, Falkenberg Smeland M, Mansour S, Parker MJ, Cook JA, Splitt M, Fisher RB, Fryer A, Magee AC, Wilkie A, Barnicoat A, Brady AF, Cooper NS, Mercer C, Deshpande C, Bennett CP, Pilz DT, Ruddy D, Cilliers D, Johnson DS, Josifova D, Rosser E, Thompson EM, Wakeling E, Kinning E, Stewart F, Flinter F, Girisha KM, Cox H, Firth HV, Kingston H, Wee JS, Hurst JA, Clayton-Smith J, Tolmie J, Vogt J, Tatton-Brown K, Chandler K, Prescott K, Wilson L, Behnam M, McEntagart M, Davidson R, Lynch S-A, Sisodiya S, Mehta SG, McKee SA, Mohammed S, Holden S, Park S-M, Holder SE, Harrison V, McConnell V, Lam WK, Green AJ, Donnai D, Bitner-Glindzicz M, Donnelly DE, Nellåker C, Taylor MS, FitzPatrick DR. Genetic heterogeneity in Cornelia de Lange syndrome (CdLS) and CdLS-like phenotypes with observed and predicted levels of mosaicism. J Med Genet 2014;51:659–68. 10.1136/jmedgenet-2014-102573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baquero-Montoya C, Gil-Rodríguez MC, Braunholz D, Teresa-Rodrigo ME, Obieglo C, Gener B, Schwarzmayr T, Strom TM, Gómez-Puertas P, Puisac B, Gillessen-Kaesbach G, Musio A, Ramos FJ, Kaiser FJ, Pié J. Somatic mosaicism in a Cornelia de Lange syndrome patient with NIPBL mutation identified by different next generation sequencing approaches. Clin Genet 2014;86:595–7. 10.1111/cge.12333 [DOI] [PubMed] [Google Scholar]
- 59. Izumi K, Nakato R, Zhang Z, Edmondson AC, Noon S, Dulik MC, Rajagopalan R, Venditti CP, Gripp K, Samanich J, Zackai EH, Deardorff MA, Clark D, Allen JL, Dorsett D, Misulovin Z, Komata M, Bando M, Kaur M, Katou Y, Shirahige K, Krantz ID. Germline gain-of-function mutations in AFF4 cause a developmental syndrome functionally linking the super elongation complex and cohesin. Nat Genet 2015;47:338–44. 10.1038/ng.3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Olley G, Ansari M, Bengani H, Grimes GR, Rhodes J, von Kriegsheim A, Blatnik A, Stewart FJ, Wakeling E, Carroll N, Ross A, Park S-M, Bickmore WA, Pradeepa MM, FitzPatrick DR, Deciphering Developmental Disorders Study . BRD4 interacts with NIPBL and BRD4 is mutated in a Cornelia de Lange-like syndrome. Nat Genet 2018;50:329–32. 10.1038/s41588-018-0042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Parenti I, Gervasini C, Pozojevic J, Graul-Neumann L, Azzollini J, Braunholz D, Watrin E, Wendt KS, Cereda A, Cittaro D, Gillessen-Kaesbach G, Lazarevic D, Mariani M, Russo S, Werner R, Krawitz P, Larizza L, Selicorni A, Kaiser FJ. Broadening of cohesinopathies: exome sequencing identifies mutations in ANKRD11 in two patients with Cornelia de Lange-overlapping phenotype. Clin Genet 2016;89:74–81. 10.1111/cge.12564 [DOI] [PubMed] [Google Scholar]
- 62. Parenti I, Teresa-Rodrigo ME, Pozojevic J, Ruiz Gil S, Bader I, Braunholz D, Bramswig NC, Gervasini C, Larizza L, Pfeiffer L, Ozkinay F, Ramos F, Reiz B, Rittinger O, Strom TM, Watrin E, Wendt K, Wieczorek D, Wollnik B, Baquero-Montoya C, Pié J, Deardorff MA, Gillessen-Kaesbach G, Kaiser FJ. Mutations in chromatin regulators functionally link Cornelia de Lange syndrome and clinically overlapping phenotypes. Hum Genet 2017;136:307–20. 10.1007/s00439-017-1758-y [DOI] [PubMed] [Google Scholar]
- 63. Tang H, Guo J, Linpeng S, Wu L. Next generation sequencing identified two novel mutations in NIPBL and a frame shift mutation in CREBBP in three Chinese children. Orphanet J Rare Dis 2019;14:45 10.1186/s13023-019-1022-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Woods SA, Robinson HB, Kohler LJ, Agamanolis D, Sterbenz G, Khalifa M. Exome sequencing identifies a novel ep300 frame shift mutation in a patient with features that overlap Cornelia de Lange syndrome. Am J Med Genet A 2014;164A:251–8. 10.1002/ajmg.a.36237 [DOI] [PubMed] [Google Scholar]
- 65. Yuan B, Pehlivan D, Karaca E, Patel N, Charng W-L, Gambin T, Gonzaga-Jauregui C, Sutton VR, Yesil G, Bozdogan ST, Tos T, Koparir A, Koparir E, Beck CR, Gu S, Aslan H, Yuregir OO, Al Rubeaan K, Alnaqeb D, Alshammari MJ, Bayram Y, Atik MM, Aydin H, Geckinli BB, Seven M, Ulucan H, Fenercioglu E, Ozen M, Jhangiani S, Muzny DM, Boerwinkle E, Tuysuz B, Alkuraya FS, Gibbs RA, Lupski JR. Global transcriptional disturbances underlie Cornelia de Lange syndrome and related phenotypes. J Clin Invest 2015;125:636–51. 10.1172/JCI77435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vasileiou G, Vergarajauregui S, Endele S, Popp B, Büttner C, Ekici AB, Gerard M, Bramswig NC, Albrecht B, Clayton-Smith J, Morton J, Tomkins S, Low K, Weber A, Wenzel M, Altmüller J, Li Y, Wollnik B, Hoganson G, Plona M-R, Cho MT, Thiel CT, Lüdecke H-J, Strom TM, Calpena E, Wilkie AOM, Wieczorek D, Engel FB, Reis A, Deciphering Developmental Disorders Study . Mutations in the BAF-complex subunit DPF2 are associated with Coffin-Siris syndrome. Am J Hum Genet 2018;102:468–79. 10.1016/j.ajhg.2018.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hoyer J, Ekici AB, Endele S, Popp B, Zweier C, Wiesener A, Wohlleber E, Dufke A, Rossier E, Petsch C, Zweier M, Göhring I, Zink AM, Rappold G, Schröck E, Wieczorek D, Riess O, Engels H, Rauch A, Reis A. Haploinsufficiency of ARID1B, a member of the SWI/SNF-A chromatin-remodeling complex, is a frequent cause of intellectual disability. Am J Hum Genet 2012;90:565–72. 10.1016/j.ajhg.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Santen GWE, Aten E, Sun Y, Almomani R, Gilissen C, Nielsen M, Kant SG, Snoeck IN, Peeters EAJ, Hilhorst-Hofstee Y, Wessels MW, den Hollander NS, Ruivenkamp CAL, van Ommen G-JB, Breuning MH, den Dunnen JT, van Haeringen A, Kriek M. Mutations in SWI/SNF chromatin remodeling complex gene ARID1B cause Coffin-Siris syndrome. Nat Genet 2012;44:379–80. 10.1038/ng.2217 [DOI] [PubMed] [Google Scholar]
- 69. Tsurusaki Y, Okamoto N, Ohashi H, Kosho T, Imai Y, Hibi-Ko Y, Kaname T, Naritomi K, Kawame H, Wakui K, Fukushima Y, Homma T, Kato M, Hiraki Y, Yamagata T, Yano S, Mizuno S, Sakazume S, Ishii T, Nagai T, Shiina M, Ogata K, Ohta T, Niikawa N, Miyatake S, Okada I, Mizuguchi T, Doi H, Saitsu H, Miyake N, Matsumoto N. Mutations affecting components of the SWI/SNF complex cause Coffin-Siris syndrome. Nat Genet 2012;44:376–8. 10.1038/ng.2219 [DOI] [PubMed] [Google Scholar]
- 70. Hempel A, Pagnamenta AT, Blyth M, Mansour S, McConnell V, Kou I, Ikegawa S, Tsurusaki Y, Matsumoto N, Lo-Castro A, Plessis G, Albrecht B, Battaglia A, Taylor JC, Howard MF, Keays D, Sohal AS, Kühl SJ, Kini U, McNeill A, DDD Collaboration . Deletions and de novo mutations of SOX11 are associated with a neurodevelopmental disorder with features of Coffin–Siris syndrome. J Med Genet 2016;53:152–62. 10.1136/jmedgenet-2015-103393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bramswig NC, Caluseriu O, Lüdecke H-J, Bolduc FV, Noel NCL, Wieland T, Surowy HM, Christen H-J, Engels H, Strom TM, Wieczorek D. Heterozygosity for ARID2 loss-of-function mutations in individuals with a Coffin–Siris syndrome-like phenotype. Hum Genet 2017;136:297–305. 10.1007/s00439-017-1757-z [DOI] [PubMed] [Google Scholar]
- 72. Revenkova E, Focarelli ML, Susani L, Paulis M, Bassi MT, Mannini L, Frattini A, Delia D, Krantz I, Vezzoni P, Jessberger R, Musio A. Cornelia de Lange syndrome mutations in SMC1A or SMC3 affect binding to DNA. Hum Mol Genet 2009;18:418–27. 10.1093/hmg/ddn369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Castronovo P, Gervasini C, Cereda A, Masciadri M, Milani D, Russo S, Selicorni A, Larizza L. Premature chromatid separation is not a useful diagnostic marker for Cornelia de Lange syndrome. Chromosome Res 2009;17:763–71. 10.1007/s10577-009-9066-6 [DOI] [PubMed] [Google Scholar]
- 74. Liu J, Zhang Z, Bando M, Itoh T, Deardorff MA, Clark D, Kaur M, Tandy S, Kondoh T, Rappaport E, Spinner NB, Vega H, Jackson LG, Shirahige K, Krantz ID. Transcriptional dysregulation in NIPBL and cohesin mutant human cells. PLoS Biol 2009;7:e1000119 10.1371/journal.pbio.1000119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mannini L, C Lamaze F, Cucco F, Amato C, Quarantotti V, Rizzo IM, Krantz ID, Bilodeau S, Musio A. Mutant cohesin affects RNA polymerase II regulation in Cornelia de Lange syndrome. Sci Rep 2015;5:16803 10.1038/srep16803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yuen KC, Xu B, Krantz ID, Gerton JL. NIPBL controls RNA biogenesis to prevent activation of the stress kinase PKR. Cell Rep 2016;14:93–102. 10.1016/j.celrep.2015.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mannini L, Menga S, Tonelli A, Zanotti S, Bassi MT, Magnani C, Musio A. SMC1A codon 496 mutations affect the cellular response to genotoxic treatments. Am J Med Genet A 2012;158A:224–8. 10.1002/ajmg.a.34384 [DOI] [PubMed] [Google Scholar]
- 78. Gimigliano A, Mannini L, Bianchi L, Puglia M, Deardorff MA, Menga S, Krantz ID, Musio A, Bini L. Proteomic profile identifies dysregulated pathways in Cornelia de Lange syndrome cells with distinct mutations in SMC1A and SMC3 genes. J Proteome Res 2012;11:6111–23. 10.1021/pr300760p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fudenberg G, Imakaev M, Lu C, Goloborodko A, Abdennur N, Mirny LA. Formation of chromosomal domains by loop extrusion. Cell Rep 2016;15:2038–49. 10.1016/j.celrep.2016.04.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Izumi K. Disorders of transcriptional regulation: an emerging category of multiple malformation syndromes. Mol Syndromol 2016;7:262–73. 10.1159/000448747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vergano SS, Deardorff MA. Clinical features, diagnostic criteria, and management of Coffin-Siris syndrome. Am J Med Genet C Semin Med Genet 2014;166C:252–6. 10.1002/ajmg.c.31411 [DOI] [PubMed] [Google Scholar]
- 82. Lin C, Smith ER, Takahashi H, Lai KC, Martin-Brown S, Florens L, Washburn MP, Conaway JW, Conaway RC, Shilatifard A. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol Cell 2010;37:429–37. 10.1016/j.molcel.2010.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sirmaci A, Spiliopoulos M, Brancati F, Powell E, Duman D, Abrams A, Bademci G, Agolini E, Guo S, Konuk B, Kavaz A, Blanton S, Digilio MC, Dallapiccola B, Young J, Zuchner S, Tekin M. Mutations in ANKRD11 cause KBG syndrome, characterized by intellectual disability, skeletal malformations, and macrodontia. Am J Hum Genet 2011;89:289–94. 10.1016/j.ajhg.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gallagher D, Voronova A, Zander MA, Cancino GI, Bramall A, Krause MP, Abad C, Tekin M, Neilsen PM, Callen DF, Scherer SW, Keller GM, Kaplan DR, Walz K, Miller FD. Ankrd11 is a chromatin regulator involved in autism that is essential for neural development. Dev Cell 2015;32:31–42. 10.1016/j.devcel.2014.11.031 [DOI] [PubMed] [Google Scholar]
- 85. Schaaf CA, Misulovin Z, Sahota G, Siddiqui AM, Schwartz YB, Kahn TG, Pirrotta V, Gause M, Dorsett D. Regulation of the Drosophila enhancer of split and invected-engrailed gene complexes by sister chromatid cohesion proteins. PLoS One 2009;4:e6202 10.1371/journal.pone.0006202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kawauchi S, Calof AL, Santos R, Lopez-Burks ME, Young CM, Hoang MP, Chua A, Lao T, Lechner MS, Daniel JA, Nussenzweig A, Kitzes L, Yokomori K, Hallgrimsson B, Lander AD. Multiple organ system defects and transcriptional dysregulation in the Nipbl+/− mouse, a model of Cornelia de Lange Syndrome. PLoS Genet 2009;5:e1000650 10.1371/journal.pgen.1000650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Muto A, Calof AL, Lander AD, Schilling TF. Multifactorial origins of heart and gut defects in nipbl-deficient zebrafish, a model of Cornelia de Lange syndrome. PLoS Biol 2011;9:e1001181 10.1371/journal.pbio.1001181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wu Y, Gause M, Xu D, Misulovin Z, Schaaf CA, Mosarla RC, Mannino E, Shannon M, Jones E, Shi M, Chen W-F, Katz OL, Sehgal A, Jongens TA, Krantz ID, Dorsett D. Drosophila Nipped-B mutants model Cornelia de Lange syndrome in growth and behavior. PLoS Genet 2015;11:e1005655 10.1371/journal.pgen.1005655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tomson BN, D'Amours D, Adamson BS, Aragon L, Amon A. Ribosomal DNA transcription-dependent processes interfere with chromosome segregation. Mol Cell Biol 2006;26:6239–47. 10.1128/MCB.00693-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bose T, Lee KK, Lu S, Xu B, Harris B, Slaughter B, Unruh J, Garrett A, McDowell W, Box A, Li H, Peak A, Ramachandran S, Seidel C, Gerton JL. Cohesin proteins promote ribosomal RNA production and protein translation in yeast and human cells. PLoS Genet 2012;8:e1002749 10.1371/journal.pgen.1002749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xu B, Sowa N, Cardenas ME, Gerton JL. L-Leucine partially rescues translational and developmental defects associated with zebrafish models of Cornelia de Lange syndrome. Hum Mol Genet 2015;24:1540–55. 10.1093/hmg/ddu565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cukrov D, Newman TAC, Leask M, Leeke B, Sarogni P, Patimo A, Kline AD, Krantz ID, Horsfield JA, Musio A. Antioxidant treatment ameliorates phenotypic features of SMC1A-mutated Cornelia de Lange syndrome in vitro and in vivo. Hum Mol Genet 2018;27:3002–11. 10.1093/hmg/ddy203 [DOI] [PubMed] [Google Scholar]
- 93. Vrouwe MG, Elghalbzouri-Maghrani E, Meijers M, Schouten P, Godthelp BC, Bhuiyan ZA, Redeker EJ, Mannens MM, Mullenders LHF, Pastink A, Darroudi F. Increased DNA damage sensitivity of Cornelia de Lange syndrome cells: evidence for impaired recombinational repair. Hum Mol Genet 2007;16:1478–87. 10.1093/hmg/ddm098 [DOI] [PubMed] [Google Scholar]
- 94. Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 2013;12:931–47. 10.1038/nrd4002 [DOI] [PubMed] [Google Scholar]
- 95. Birch-Machin MA, Bowman A. Oxidative stress and ageing. Br J Dermatol 2016;175(Suppl 2):26–9. 10.1111/bjd.14906 [DOI] [PubMed] [Google Scholar]
- 96. Chamorro Ángel, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol 2016;15:869–81. 10.1016/S1474-4422(16)00114-9 [DOI] [PubMed] [Google Scholar]
- 97. Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell 2012;46:105–10. 10.1016/j.molcel.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 98. Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 2012;149:410–24. 10.1016/j.cell.2012.02.044 [DOI] [PubMed] [Google Scholar]
- 99. Schmelzle T, Hall MN. Tor, a central controller of cell growth. Cell 2000;103:253–62. 10.1016/S0092-8674(00)00117-3 [DOI] [PubMed] [Google Scholar]
- 100. Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 2005;123:569–80. 10.1016/j.cell.2005.10.024 [DOI] [PubMed] [Google Scholar]
- 101. Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol 2004;24:200–16. 10.1128/MCB.24.1.200-216.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pistocchi A, Fazio G, Cereda A, Ferrari L, Bettini LR, Messina G, Cotelli F, Biondi A, Selicorni A, Massa V. Cornelia de Lange syndrome: NIPBL haploinsufficiency downregulates canonical Wnt pathway in zebrafish embryos and patients fibroblasts. Cell Death Dis 2013;4:e866 10.1038/cddis.2013.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Huang J, Nguyen-McCarty M, Hexner EO, Danet-Desnoyers G, Klein PS. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat Med 2012;18:1778–85. 10.1038/nm.2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kline AD, Grados M, Sponseller P, Levy HP, Blagowidow N, Schoedel C, Rampolla J, Clemens DK, Krantz I, Kimball A, Pichard C, Tuchman D. Natural history of aging in Cornelia de Lange syndrome. Am J Med Genet C Semin Med Genet 2007;145C:248–60. 10.1002/ajmg.c.30137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. de Graaf M, Kant SG, Wit JM, Willem Redeker EJ, Eduard Santen GW, Henriëtta Verkerk AJM, Uitterlinden AG, Losekoot M, Oostdijk W. Successful growth hormone therapy in Cornelia de Lange syndrome. J Clin Res Pediatr Endocrinol 2017;9:366–70. 10.4274/jcrpe.4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lee Y, McKinnon PJ. Responding to DNA double strand breaks in the nervous system. Neuroscience 2007;145:1365–74. 10.1016/j.neuroscience.2006.07.026 [DOI] [PubMed] [Google Scholar]
- 107. Falcone G, Mazzola A, Michelini F, Bossi G, Censi F, Biferi MG, Minghetti L, Floridia G, Federico M, Musio A, Crescenzi M. Cytogenetic analysis of human cells reveals specific patterns of DNA damage in replicative and oncogene-induced senescence. Aging Cell 2013;12:312–5. 10.1111/acel.12034 [DOI] [PubMed] [Google Scholar]
- 108. d’Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer 2008;8:512–22. 10.1038/nrc2440 [DOI] [PubMed] [Google Scholar]
- 109. Butterfield DA, Di Domenico F, Swomley AM, Head E, Perluigi M. Redox proteomics analysis to decipher the neurobiology of Alzheimer-like neurodegeneration: overlaps in Down's syndrome and Alzheimer's disease brain. Biochem J 2014;463:177–89. 10.1042/BJ20140772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Di Domenico F, Barone E, Perluigi M, Butterfield DA. The triangle of death in Alzheimer's disease brain: the aberrant cross-talk among energy metabolism, mammalian target of rapamycin signaling, and protein homeostasis revealed by redox proteomics. Antioxid Redox Signal 2017;26:364–87. 10.1089/ars.2016.6759 [DOI] [PubMed] [Google Scholar]
- 111. Butterfield DA, Di Domenico F, Barone E. Elevated risk of type 2 diabetes for development of Alzheimer disease: a key role for oxidative stress in brain. Biochim Biophys Acta 2014;1842:1693–706. 10.1016/j.bbadis.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Reliene R, Fischer E, Schiestl RH. Effect of N-acetyl cysteine on oxidative DNA damage and the frequency of DNA deletions in Atm-deficient mice. Cancer Res 2004;64:5148–53. 10.1158/0008-5472.CAN-04-0442 [DOI] [PubMed] [Google Scholar]
- 113. Arakawa M, Ushimaru N, Osada N, Oda T, Ishige K, Ito Y. N-Acetylcysteine selectively protects cerebellar granule cells from 4-hydroxynonenal-induced cell death. Neurosci Res 2006;55:255–63. 10.1016/j.neures.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 114. Kanno T, Kanno Y, LeRoy G, Campos E, Sun H-W, Brooks SR, Vahedi G, Heightman TD, Garcia BA, Reinberg D, Siebenlist U, O'Shea JJ, Ozato K. BRD4 assists elongation of both coding and enhancer RNAs by interacting with acetylated histones. Nat Struct Mol Biol 2014;21:1047–57. 10.1038/nsmb.2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sokpor G, Xie Y, Rosenbusch J, Tuoc T. Chromatin remodeling BAF (SWI/SNF) complexes in neural development and disorders. Front Mol Neurosci 2017;10:243 10.3389/fnmol.2017.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhang A, Yeung PL, Li C-W, Tsai S-C, Dinh GK, Wu X, Li H, Chen JD. Identification of a novel family of ankyrin repeats containing cofactors for p160 nuclear receptor coactivators. J Biol Chem 2004;279:33799–805. 10.1074/jbc.M403997200 [DOI] [PubMed] [Google Scholar]
- 117. Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, Loh Y-H, Yeo HC, Yeo ZX, Narang V, Govindarajan KR, Leong B, Shahab A, Ruan Y, Bourque G, Sung W-K, Clarke ND, Wei C-L, Ng H-H. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 2008;133:1106–17. 10.1016/j.cell.2008.04.043 [DOI] [PubMed] [Google Scholar]
- 118. Nakamura T, Mori T, Tada S, Krajewski W, Rozovskaia T, Wassell R, Dubois G, Mazo A, Croce CM, Canaani E. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol Cell 2002;10:1119–28. 10.1016/S1097-2765(02)00740-2 [DOI] [PubMed] [Google Scholar]


