Abstract
Interspecific killing is a primary reason for the low survival rates of some animal species. The giant panda (Ailuropoda melanoleuca) is an altricial eutherian mammal and thus, in comparison to other infants, panda cubs are highly vulnerable, which may significantly influence the selection of breeding sites by females. Here, we used infrared camera traps to monitor giant panda dens for 5.5 years in Foping National Nature Reserve (FNNR) to determine how interspecific factors affect den selection by wild female pandas. Results indicated that Asian black bears (Ursus thibetanus), yellow-throated martens (Martes flavigula), leopard cats (Prionailurus bengalensis), and masked palm civets (Paguma larvata) visited the dens frequently, and the presence of these species negatively influenced den selection by female pandas. Interestingly, the presence of rodents and terrestrial birds appeared to indicate den safety, and female giant pandas were not averse and even preferred dens with a high abundance index of rodents and terrestrial birds. The den suitability index (DSI) was a reliable tool for evaluating whether dens were suitable for female giant pandas to give birth to and rear cubs, with preference for dens with high DSI values. This study increases our understanding of the den selection criteria of female giant pandas and the main threats to the survival of their cubs, thus providing important guidance for the conservation and management of this species.
Keywords: Ailuropoda melanoleuca, Interspecific interference competition, Dens, Den suitability index
INTRODUCTION
As a place for sleeping, hibernating, birthing, and infant-rearing, a den can help animals cope with extreme changes in climate, e.g., low and high temperatures and violent rainstorms, by forming a stable microclimate (Endres & Smith, 1993; Magoun & Copeland, 1998). In addition, dens can help animals avoid threats from natural enemies (Magoun & Copeland, 1998). Interspecific killing is a critical type of interference competition among carnivores, with death from other carnivores a primary reason for the low survival rates in some animal species (Mills & Mills, 2014; Palomares & Caro, 1999; Périquet et al., 2016). For example, studies have indicated that 78.2% of cheetah (Acinonyx jubatus) cubs in dens are killed by lions and other predators, such as domestic dogs (Canis lupus familiaris), secretary birds (Sagittarius serpentarius), and other small carnivorous animals (Mills & Mills, 2014). Therefore, the selection of an appropriate den is essential for the survival and reproduction of animals if they must rest and rear young (Dawson et al., 2005; Laurenson, 1993; Rabinowitz & Pelton, 1986; Reichman & Smith, 1990). Various studies have shown that environmental characteristics, soil type, vegetation characteristics, and distance to a water source are the main criteria by which animals choose suitable dens (Kaneko et al., 2010; Norris et al., 2002; Way et al., 2001). In addition, interspecific interference competition is another important factor affecting the selection of dens (Périquet et al., 2016).
The giant panda (Ailuropoda melanoleuca) is an endangered species, which has evolved many features to adapt to the living environment, particularly the specialized diet (Hu et al., 2017; Nie et al., 2015; Wei et al., 2015, 2019). Unlike other bears, giant pandas do not hibernate and usually sleep wherever they eat in a bamboo forest. Only females use dens to give birth and rear cubs during the breeding season, usually from August and September (Hu, 2001; Schaller et al., 1985; Zhu et al., 2001). Cubs often live in tree hollows or rock dens in the first few months after birth (Schaller et al., 1985). As a specific bamboo specialist, giant pandas, including mothers, spend a considerable amount of time foraging to maintain their daily energy requirements from their low-energy food (Schaller et al., 1985). Therefore, during this period, many sympatric predators may threaten the cubs, including yellow-throated martens (Martes flavigula), leopard cats (Prionailurus bengalensis), and golden eagles (Aquila chrysaetos) (Schaller et al., 1985). Thus, to protect their vulnerable cubs, female giant pandas will select proper dens with certain special characteristics to increase cub safety. Previous studies have indicated that giant pandas in the Qinling Mountains mainly use rock dens, but use tree hollows in the mountains of Sichuan, which contain more old growth forests (Wei et al., 2019; Zhang et al., 2007). In the Qinling Mountains, maternal giant pandas prefer tunnel-type dens with a small entrance and a deep cavity, which may be a deterrent to predators (Zhang et al., 2007).
Wild giant pandas have a sensitive olfactory system (Zhou et al., 2019), and thus, sensing pheromones released by predators may be an important factor by which female giant pandas estimate whether a den is suitable for giving birth or rearing cubs. Therefore, how potential predators affect den selection by giant pandas during the breeding season is an important question. To address this, we used infrared camera traps to monitor giant panda dens for 5.5 years in Foping National Nature Reserve (FNNR) in the Qinling Mountains and to study the main interspecific factors threatening the survival of panda cubs. At the same time, by combining the habitat characteristics of den sites and relative abundance of sympatric companion species in the study area, we explored the main factors involved in den selection by wild giant pandas. These results should improve our understanding of den selection by giant pandas, thus providing a guide for the conservation and management of this vulnerable species.
MATERIALS AND METHODS
Study area
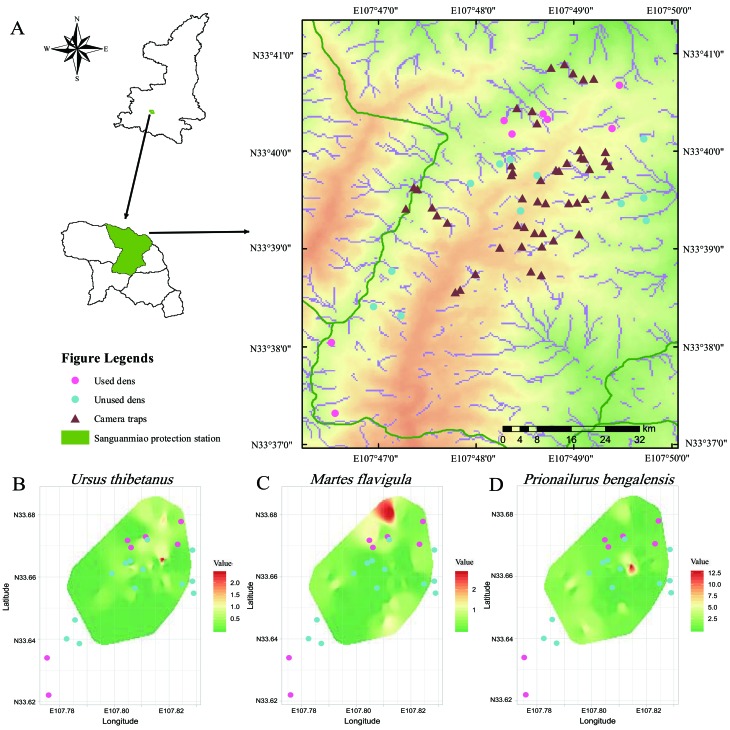
Located in Foping County, Shaanxi Province, China, Foping National Nature Reserve (FNNR) (E107°40′–107°55′, N33°33′–33°46′) covers an area of 292.40 km2, with an altitude range of 980–2 904 m a.s.l. The giant panda is the primary animal protected in this reserve, which contains the highest population density of giant pandas in the world. The third and fourth national surveys estimated that there were approximately 76 and 67 wild giant pandas (excluding cubs) in the reserve, respectively (State Forestry Administration, 2006, 2015). Our study was conducted at the Sanguanmiao protection area, which covers approximately 60 km2 of habitat known to be occupied by more than 20 giant pandas (unpublished data) in the core area of FNNR.
Field investigation and camera-trap survey
Previous surveys documented the location of potential den sites, with a total of 20 dens found in our study area (Ye et al., 2007; Zhang et al., 2007). From July 2012 to November 2018, we monitored and checked the den sites at least two times during the denning season every year. At the same time, infrared cameras (Reconyx Hyperfire HC600, USA) were set up to monitor den visits by giant pandas and sympatric companion species. We had two criteria to determine whether pandas used the dens: (1) Mothers and cubs were observed in the dens during the breeding season through field observations and infrared cameras surveys; (2) Significant fresh signs of giant pandas (e.g., fur, scratch marks, and bedding materials) were found in the dens. Otherwise, the dens were characterized as unused.
Twenty infrared cameras were deployed to monitor the potential den sites, and 49 infrared camera traps were established on the animal trails along the ridge to survey the distribution and relative abundance of sympatric companion species in the study area (Figure 1A). Infrared cameras were mounted on trees 50–60 cm above the ground and 2–3 m from the den or trail range. All cameras were operated 24 h/day and set with no flash but infrared illumination for low-light image capture. When triggered, the camera traps were programmed to capture 10 photos, with a delay of 0.1 s between each photo and no quiet period before the next trigger.
1. Distribution of 20 giant panda dens (including eight maternal dens) and 49 infrared cameras set on animal trails along ridges in Sanguanmiao area (A) and relative abundance index (RAI) of three carnivores (Ursus thibetanus, Martes flavigula, and Prionailurus bengalensis, respectively) captured by 49 infrared cameras (B–D) .
Photos from the infrared cameras were collected and stored in a folder corresponding to the camera number. The criteria for determining an independent and valid photo were that it should be adjacent to valid photos of the same species on the same camera and the interval time must be at least 30 min (O’Brien et al., 2003). Information on all photos was screened to identify the species of birds and mammals and to exclude interference information. Total number of days worked by an infrared camera at the same location was recorded as the effective working days. The relative abundance index (RAI) was RAI=Pi/N×100, where Pi represents the number of independent effective photos of i species (i = 1, 2,..., 24) that appeared at the same camera site, and N is the number of effective working days of the camera traps (O’Brien et al., 2003). The den suitability index (DSI) was calculated by: DSI=Rpredator/Rcompanion×100, where Rpredator is the total relative abundance of the predators that visited the giant panda dens (predators were carnivores that can kill or harm the pandas or their cubs, including leopards, Asian black bears, yellow-throated martens, leopard cats, and masked palm civets present in the study area); and Rcompanion is the total relative abundance of companion species that appeared in the dens (companion species were animals sympatric with giant pandas that had no predation or competition relationship).
Statistical analysis
All analyses were conducted in R 3.5.2 (R Core Team, 2016), and statistical values are expressed as means±SEM. Wilcoxon signed-rank tests were used to compare the DSI values to estimate whether the den was a suitable selection by the maternal giant panda and to compare the RAI values from our camera traps set on the animal trails along the ridge near the dens between used and unused den sites. ArcGIS 10.5 software (Environmental Systems Research Institute Inc., USA) was used to delineate the distribution patterns of predators in the study area based on the RAIs calculated by the camera-trap data. We used minimal convex polygon method to create areas separately in which dens might be influenced by the three predators (Asian black bears, yellow-throated martens and leopard cats). A 500 m buffer around the polygon was then made according to the length of the giant pandas’ daily maximum movement (Schaller et al., 1985). We used the inverse-distance weighted smoothing method (weight=5) (Shepard, 1968) to generate a heat map of predator RAIs using the R package spatstat (Baddeley et al., 2015). Calculations were based on data obtained from the infrared cameras set on the animal trails along the ridge within the boundary.
RESULTS
Based on continuous field investigations and camera-trap surveys from July 2012 to November 2018, we found that eight of the 20 dens were used by female giant pandas for birthing and rearing cubs. A total of 8 713 days of camera trapping were accumulated in the den sites, with total trapping time on the animal trail along the ridge of 74 283 days over 5.5 years. A total of 732 independent effective photos were taken by the infrared camera traps monitoring the giant panda dens, with 19 species of mammals and birds, belonging to six orders and 14 families, found to visit these dens (See Table 1, Figure 2).
1. List of mammals and birds visiting giant panda dens recorded by camera traps in our study area.
| Species | No. of sites (n) | No. of independent photos (n) | Relative abundance index | Protection category |
| I: National Key Protected Species (Class I); II: National Key Protected Species (Class II); III: Species listed in “The List of Territorial Wildlife of National Protected Beneficial or Valuable Species for Economic and Scientific Research”. T: Endemic to China; LC: Least Concern; EN: Endangered; VU: Vulnerable; NT: Near Threatened; A, B, and C represent species listed in Appendix I, II, and III of CITES, respectively. *: Some rodents (rat) could not be given a species identification and are listed as a separate term. | ||||
| MAMMALIA | ||||
| Primates | ||||
| I Cercopithecidae | ||||
| 1. Rhinopithecus roxellana | 1 | 2 | 0.02 | I, EN, T, A |
| Carnivora | ||||
| I Ursidae | ||||
| 2. Ursus thibetanus | 1 | 2 | 0.02 | II, VU, A |
| II Ailuropodidae | ||||
| 3. Ailuropoda melanoleuca | 3 | 130 | 1.49 | I, VU, T, A |
| Ⅲ Mustelidae | ||||
| 4. Martes flavigula | 1 | 1 | 0.01 | II, LC, C |
| Ⅳ Viverridae | ||||
| 5. Paguma larvata | 5 | 22 | 0.25 | Ⅲ, LC, C |
| Ⅴ Felidae | ||||
| 6. Prionailurus bengalensis | 1 | 1 | 0.15 | Ⅲ, LC, B |
| Artiodactyla | ||||
| I Suidae | ||||
| 7. Sus scrofa | 2 | 3 | 0.03 | Ⅲ, LC |
| II Cervidae | ||||
| 8. Muntiacus reevesi | 1 | 1 | 0.01 | Ⅲ, LC, T |
| Ⅲ Bovidae | ||||
| 9. Budorcas bedfordi | 3 | 15 | 0.17 | I, VU, T, B |
| 10. Naemorhedus griseus | 1 | 1 | 0.01 | II, VU, A |
| Rodentia | ||||
| I Sciuridae | ||||
| 11. Tamiops swinhoei | 1 | 2 | 0.02 | Ⅲ, LC |
| 12. Sciurotamias davidianus | 9 | 355 | 4.07 | Ⅲ, LC, T |
| 13. Petaurista alborufus | 3 | 25 | 0.29 | Ⅲ, LC, T |
| II Hystricidae | ||||
| 14. Hystrix brachyuran | 2 | 6 | 0.07 | Ⅲ, LC |
| AVES | ||||
| Galliformes | ||||
| I Phasianidae | ||||
| 15. Tragopan temminckii | 5 | 11 | 0.13 | II, LC |
| 16. Chrysolophus pictus | 1 | 2 | 0.02 | II, LC, T |
| Passeriformes | ||||
| I Turdidae | ||||
| 17. Myophonus caeruleus | 3 | 7 | 0.08 | LC |
| 18. Zoothera dauma | 1 | 1 | 0.01 | Ⅲ, LC |
| II Timaliidae | ||||
| 19. Garrulax elliotii | 1 | 1 | 0.01 | Ⅲ, LC, T |
| * Rat | 9 | 144 | 1.65 | Ⅲ, LC |
2. Important sympatric species recorded by infrared camera traps in giant panda dens in Foping National Nature Reserve.
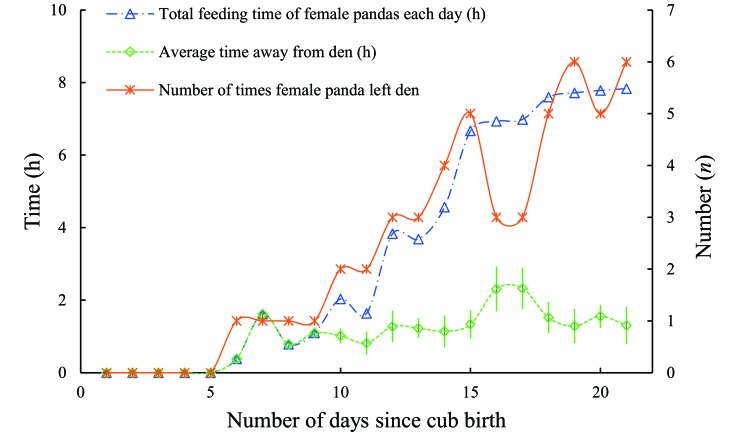
A total of eight birthing or cub-rearing events were recorded in the maternal dens during our survey. One event was well recorded by the infrared camera, lasting 21 days from the birth of a cub by a female giant panda in the den on 11 September until 02 October 2016. The female giant panda did not leave the den for the first five days after the cub was born. However, the time and frequency of the female leaving the den to forage or drink gradually increased from days 5 to 15 after the birth, and then remained stable after 15 days (Figure 3). To ensure the safety of the cubs, the average time that a mother left a den for foraging or drinking was 1.90±0.26 h.
3. Duration of activity of female giant panda during breeding season.
We found that the probability of a den being visited by female giant pandas during the breeding season was negatively correlated with the presence of carnivores in the dens and the vicinity. The RAI of the presence of carnivores in the giant panda dens was 6.83±3.28. Female giant pandas also avoided using dens in areas that had a high RAI of Asian black bears (Wilcoxon rank sum test: W=153, P<0.05) and yellow-throated martens (Wilcoxon rank sum test: W=82.5,P<0.001). However, no obvious differences in the RAI of leopard cats were found between used and unused dens (Wilcoxon rank sum test: W=243,P=0.7347) (Figure 1B–D).
Four ungulate species utilized the giant panda dens for rest and shelter from the rain, including wild boars (Sus scrofa), Chinese muntjacs (Muntiacus reevesi), Chinese gorals (Naemorhedus griseus), and golden takins (Budorcas bedfordi) (Figure 2). In addition, based on the camera-trap photos, golden takins also licked the cliff-face of the dens for salt (three times) and pushed their heads into the dens (four times) (Figure 2). The RAI was 12.45±4.78 for the ungulates, which only appeared in dens unused by the female giant pandas, similar to the carnivores.
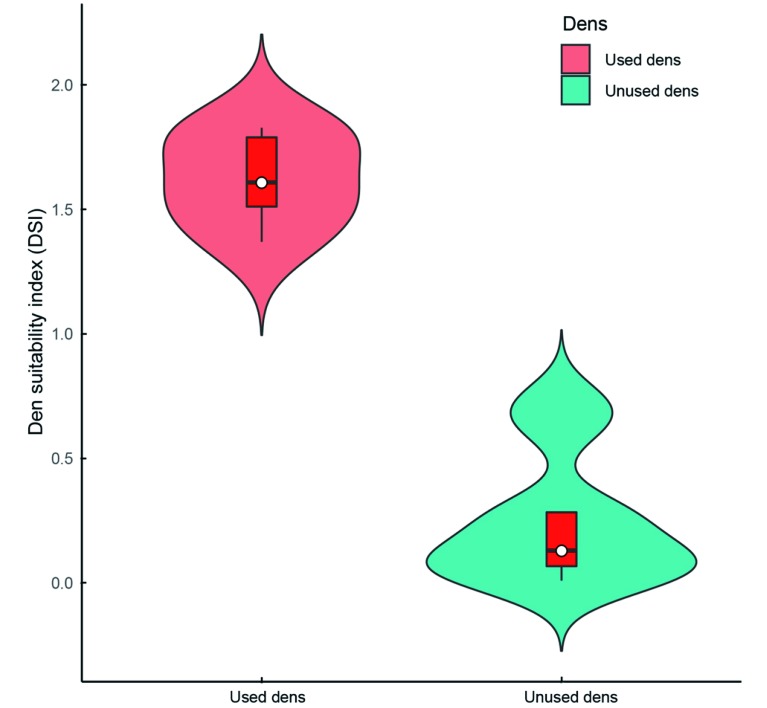
Interestingly, maternal giant pandas were not averse to the activity of rodents or terrestrial birds in or around the dens. The RAI of rodents and terrestrial birds was significantly higher in the used dens (164.91±5.54) than in the unused dens (51.90±17.45). Predators not only kill giant panda cubs but also prey on rodents and terrestrial birds. The frequent occurrence of these animals in and around giant panda dens may indicate the presence of fewer carnivores, and thus better suitability for pandas. The DSI of the used dens was significantly higher than that of the unused dens (Wilcoxon rank sum test: W=45, P<0.001) (Figure 4).
4. Den suitability index (DSI) was significantly different between dens used by female giant pandas and unused dens in Foping National Nature Reserve.
Red box represents distribution and variation of data. White dots represent data median.
DISCUSSION
Our study indicated that female giant pandas selected and used maternal dens dependent on the risk of encountering carnivores, such as Asian black bears, yellow-throated martens, leopard cats, and masked palm civets. Female giant pandas appeared to avoid using dens where carnivores carried out frequent activities. Although we did not observe giant panda cubs being killed by these species in their dens at FNNP, the fact that they acted as predators (Pan et al., 2014; Schaller et al., 1985) and repeatedly visited or approached the maternal dens indicates that predation competition relationships are occurring. Besides these animals, the presence of leopards (Panthera pardus) and some other predators in our study area has been verified previously (Yang et al., 2006). Although they were not direct captured to visit the giant panda dens by our infrared cameras, the potential threat to female giant pandas and their cubs cannot be ruled out. In addition to the threat of predators, competitor species such as golden takins and wild boars can also pose a potential threat to female giant pandas and their cubs in the breeding season (Nie et al., 2019). Giant panda cubs are under threat by both predators or sympatric companion species during the period when their mothers leave the den (Hu, 2001; Laurenson, 1994).
Different species adopt different defensive strategies to avoid killing and interference competition from sympatric species. Maternal brown bears (Ursus arctos), black bears (Ursus americanus), and coyotes (Canis latrans) can recognize and remember several dens where they can move their cubs following disturbance by predators or competitors (Harrison & Gilbert, 1985; Linnell et al., 2000; Way et al., 2001). Other animals can dig multiple openings to dens to allow their young to escape if they are at risk from predators or competitors (Carter et al., 2012; Périquet et al., 2016). Giant panda cubs are more vulnerable than other mammals because they are altricial eutherians (Gittleman, 1994). Giant pandas also cannot create dens (Pan et al., 2014; Schaller et al., 1985), instead only using naturally occurring ones. Their dens are thus very simple, with the placement of some branches or sticks at the entrance (Zhu et al., 2001) to prevent cubs from rolling out, rather than as a defense against predators. Therefore, to improve cub survival rate, other strategies are necessary for maternal giant pandas.
First, female giant pandas spend a substantial amount of time accompanying their cubs. According to our research, females spent the first five days after birth guarding the cub in the den all day. The frequency and amount of time spent away from the den by the female (for eating and drinking) gradually increased with cub growth, but the time spent away was typically no more than two hours. In general, dens near bamboo forests and water are more preferred by female giant pandas as they can save time and energy spent on feeding and drinking (Ye et al., 2007). Previous studies have also demonstrated that maternal giant pandas spend a considerable amount of time in dens with their cubs to protect them from harm during the first three to four months after birth (Pan et al., 2014). Once giant panda cubs can move properly and are able to climb trees, their mothers abandon the dens (Zhu et al., 2001).
The presence of predators and competitors in dens may have a negative influence on den selection by female giant pandas. As a solitary species, the giant panda has a sensitive olfactory system (Zhou et al., 2019), and they may be able to detect the odors of predators and competitors in the environment. Therefore, maternal pandas may select dens that exhibit no predator and competitor activities in order to avoid these animals. Giant pandas also leave scents in their dens that may be detected by potential predators, so they usually do not use the same den for a long time. Previous study has suggested that female giant pandas bring cubs to new dens three to four times in the breeding season (Pan et al., 1988).
Interestingly, maternal giant pandas appear to select dens based on the index of the activity of rodents and terrestrial birds. According to our infrared camera-trap data, leopard cats and yellow-throated martens often preyed on rodents in the unused dens. This suggested that rodent and terrestrial bird activity did not harm the giant panda cubs, and, in fact, the high activities of these species may be indirect evidence of minimal predator presence. Therefore, whether a den is suitable for rearing cubs may be determined by the activity abundance index of rodents and terrestrial birds and the activity index of predators and competitors. The DSI was a reliable index to evaluate whether dens were suitable for female giant pandas to give birth to and rear cubs, with females in the current study selecting dens with a higher DSI index.
In the current framework of ecosystem conservation, it is important to determine how sympatric species affect each other. Most previous studies have focused on the influence of abiotic factors on den selection by giant pandas (Ye et al., 2007; Zhang et al., 2007). Our study further considered the influence of biological factors on the selection of dens from the perspective of interspecies relationships among sympatric species. In fact, the frequent appearance of sympatric predator and competitor species was likely a key factor limiting maternal den selection and thus a challenge for the reproduction of the giant panda. Our study provides a new perspective on the reproductive strategies of giant pandas facing the risk of cubs being killed or disturbed by sympatric predators and competitors. The results of this study enhance our understanding of cub birthing and rearing and the criteria for den selection by wild giant pandas and the main threats to cub survival. This study also provides important guidance for the conservation and management of this endangered species.
SCIENTIFIC FIELD SURVEY PERMISSION INFORMATION
Permission for field surveys in Foping National Nature Reserve was granted by the National Forestry and Grassland Administration and the Foping National Nature Reserve.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
F.W.W. and Y.G.N. designed the study. Y.G.N. and B.W.Z. supervised the study. X.L.L, W.L.Z., H.L.G., and K.G. collected the data. X.L.L., M.W., and W.L.Z. performed data analyses. X.L.L. and W.L.Z. wrote the manuscript. Y.G.N. and B.W.Z. revised the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We gratefully acknowledge the support of the National Forestry and Grassland Administration and Foping National Nature Reserve. We thank Xiao-Lin Wang and Yi-Wen He for field assistance.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31622012) and Key Project of the Chinese Academy of Sciences (QYZDB-SSW-SMC047)
Contributor Information
Bao-Wei Zhang, Email: zhangbw@ahu.edu.cn.
Yong-Gang Nie, Email: nieyg@ioz.ac.cn.
References
- 1.Baddeley A, Rubak E, Turner R. 2015. Spatial Point Patterns: Methodology and Applications with R. London: Chapman and Hall/CRC Press.
- 2.Carter A, Luck GW, Wilson BP Ecology of the red fox (Vulpes vulpes) in an agricultural landscape. 1. Den-site selection . Australian Mammalogy. 2012;34(2):145–154. [Google Scholar]
- 3.Dawson RD, Lawrie CC, O’Brien EL The importance of microclimate variation in determining size, growth and survival of avian offspring: experimental evidence from a cavity nesting passerine. Oecologia. 2005;144(3):499–507. doi: 10.1007/s00442-005-0075-7. [DOI] [PubMed] [Google Scholar]
- 4.Endres KM, Smith WP Influence of age, sex, season and availability on den selection by raccoons within the central basin of Tennessee. American Midland Naturalist. 1993;129(1):116–131. doi: 10.2307/2426440. [DOI] [Google Scholar]
- 5.Gittleman JL Are the pandas successful specialists or evolutionary failures? The comparative method can identify distinctive panda traits that require analysis for conservation. BioScience. 1994;44(7):456–464. doi: 10.2307/1312297. [DOI] [Google Scholar]
- 6.Harrison DJ, Gilbert JR Denning ecology and movements of coyotes in Maine during pup rearing. Journal of Mammalogy. 1985;66(4):712–719. doi: 10.2307/1380797. [DOI] [Google Scholar]
- 7.Hu JC. 2001. Research on the Giant Panda. Shanghai: Shanghai Science and Technology Education Press. (in Chinese)
- 8.Hu YB, Wu Q, Ma S, Ma TX, Shan L, Wang X, Nie YG, Ning ZM, Yan L, Xu YF, Wei FW Comparative genomics reveals convergent evolution between the bamboo-eating giant and red pandas. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:1081–1086. doi: 10.1073/pnas.1613870114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaneko Y, Newman C, Buesching CD, Macdonald DW Variations in badger (Meles meles) sett microclimate: differential cub survival between main and subsidiary setts, with implications for artificial sett construction . International Journal of Ecology. 2010;2010:859586. [Google Scholar]
- 10.Laurenson MK Early maternal behavior of wild cheetahs: implications for captive husbandry. Zoo Biology. 1993;12(1):31–43. doi: 10.1002/zoo.1430120106. [DOI] [Google Scholar]
- 11.Laurenson MK High juvenile mortality in cheetahs (Acinonyx jubatus) and its consequences for maternal care . Journal of Zoology. 1994;234(3):387–408. doi: 10.1111/j.1469-7998.1994.tb04855.x. [DOI] [Google Scholar]
- 12.Linnell JDC, Swenson JE, Andersen R, Barnes B How vulnerable are denning bears to disturbance? Wildlife Society Bulletin. 2000;28(2):400–413. [Google Scholar]
- 13.Magoun AJ, Copeland JP Characteristics of wolverine reproductive den sites. The Journal of Wildlife Management. 1998;62(4):1313–1320. doi: 10.2307/3801996. [DOI] [Google Scholar]
- 14.Mills MGL, Mills MEJ Cheetah cub survival revisited: a re-evaluation of the role of predation, especially by lions, and implications for conservation. Journal of Zoology. 2014;292(2):136–141. doi: 10.1111/jzo.12087. [DOI] [Google Scholar]
- 15.Nie YG, Zhou WL, Gao K, Swaisgood RR, Wei FW Seasonal competition between sympatric species for a key resource: implications for conservation management. Biological Conservation. 2019;234:1–6. doi: 10.1016/j.biocon.2019.03.013. [DOI] [Google Scholar]
- 16.Nie YG, Speakman JR, Wu Q, Zhang CL, Hu YB, Xia MH, Yan L, Hambly C, Wang L, Wei W, Wei FW Exceptionally low daily energy expenditure in the bamboo-eating giant panda. Science. 2015;349:171–174. doi: 10.1126/science.aab2413. [DOI] [PubMed] [Google Scholar]
- 17.Norris DR, Theberge MT, Theberge JB Forest composition around wolf (Canis lupus) dens in eastern Algonquin Provincial Park, Ontario . Canadian Journal of Zoology. 2002;80(5):866–872. doi: 10.1139/z02-067. [DOI] [Google Scholar]
- 18.O’Brien TG, Kinnaird MF, Wibisono HT Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Animal Conservation. 2003;6(2):131–139. doi: 10.1017/S1367943003003172. [DOI] [Google Scholar]
- 19.Palomares F, Caro TM Interspecific killing among mammalian carnivores. The American Naturalist. 1999;153(5):492–508. doi: 10.1086/303189. [DOI] [PubMed] [Google Scholar]
- 20.Pan WS, Gao ZS, Lü Z. 1988. The Giant Panda’s Natural Refuge in the Qinling Mountains. Beijing: Peking University Press. (in Chinese)
- 21.Pan WS, Lü Z, Zhu XJ, Wang DJ, Wang H, Long Y, Fu DL, Zhou X. 2014. A Chance for Lasting Survival: Ecology and Behavior of Wild Giant Pandas. Washington: Smithsonian Institution Scholarly Press.
- 22.Périquet S, Mapendere C, Revilla E, Banda J, Macdonald DW, Loveridge AJ, Fritz H A potential role for interference competition with lions in den selection and attendance by spotted hyaenas. Mammalian Biology. 2016;81(3):227–234. doi: 10.1016/j.mambio.2015.10.005. [DOI] [Google Scholar]
- 23.R Core Team. 2016. R: A language and environment for statistical computing (R Foundation for Statistical Computing).
- 24.Rabinowitz AR, Pelton MR Day-bed use by raccoons. Journal of Mammalogy. 1986;67(4):766–769. doi: 10.2307/1381145. [DOI] [Google Scholar]
- 25.Reichman OJ, Smith SC Burrows and burrowing behavior by mammals. Current Mammalogy. 1990;2:197–244. [Google Scholar]
- 26.Schaller GB, Hu JC, Pan WS, Zhu J. 1985. The Giant Pandas of Wolong. Chicago, Illinois, USA: University of Chicago Press.
- 27.Shepard D. 1968. A two-dimensional interpolation function for irregularly-spaced data. In: Proceedings of the 1968 23rd ACM National Conference. New York: ACM, 517–524.
- 28.State Forestry Administration. 2006. The 3rd National Survey Report on Giant Panda in China. Beijing, China: Science Press. (in Chinese)
- 29.State Forestry Administration. 2015(2015-02-28). The fourth national survey report on giant panda in China. http://www.forestry.gov.cn/main/72/content-742880.html. (in Chinese)
- 30.Way JG, Auger PJ, Ortega IM, Strauss EG Eastern coyote denning behavior in an anthropogenic environment. Northeast Wildlife. 2001;56:18–30. [Google Scholar]
- 31.Wei FW, Hu YB, Yan L, Nie YG, Wu Q, Zhang ZJ Giant pandas are not an evolutionary cul-de-sac: evidence from multidisciplinary research. Molecular Biology and Evolution. 2015;32(1):4–12. doi: 10.1093/molbev/msu278. [DOI] [PubMed] [Google Scholar]
- 32.Wei FW, Wu Q, Hu YB, Huang GP, Nie YG, Yan L Conservation metagenomics: a new branch of conservation biology. Science China Life Sciences. 2019;62(2):168–178. doi: 10.1007/s11427-018-9423-3. [DOI] [PubMed] [Google Scholar]
- 33.Wei W, Swaisgood RR, Owen MA, Pilfold NW, Han H, Hong MS, Zhou H, Wei FW, Nie YG, Zhang ZJ The role of den quality in giant panda conservation. Biological Conservation. 2019;231:189–196. doi: 10.1016/j.biocon.2018.12.031. [DOI] [Google Scholar]
- 34.Yang CH, Zhang HM, Zhou XP, Wang PY, Wang XM Review of habitat selection in the giant panda (Ailuropoda melanoleuca) . Acta Ecologica Sinica. 2006;26(10):3442–3453. [Google Scholar]
- 35.Ye XP, Yong YG, Yu CQ, Zhang ZW Den selection by the giant panda in Foping Nature Reserve, China. Journal of Natural History. 2007;41(37–40):2529–2536. [Google Scholar]
- 36.Zhang ZJ, Swaisgood RR, Wu H, Li M, Yong YG, Hu JC, Wei FW Factors predicting den use by maternal giant pandas. The Journal of Wildlife Management. 2007;71(8):2694–2698. doi: 10.2193/2006-504. [DOI] [Google Scholar]
- 37.Zhou WL, Nie YG, Hu YB, Swaisgood RR, Zhang YH, Liu DZ, Wei FW Seasonal and reproductive variation in chemical constituents of scent signals in wild giant pandas. Science China Life Sciences. 2019;62(5):648–660. doi: 10.1007/s11427-018-9388-9. [DOI] [PubMed] [Google Scholar]
- 38.Zhu XJ, Lindburg DG, Pan WS, Forney KA, Wang DJ The reproductive strategy of giant pandas (Ailuropoda melanoleuca): infant growth and development and mother-infant relationships . Journal of Zoology. 2001;253(2):141–155. doi: 10.1017/S0952836901000139. [DOI] [Google Scholar]