Abstract
Water striders have intrigued researchers for centuries from the viewpoints of biology to biomechanics. In this review, we introduce the basic theories and techniques of physics and force measurement for biomechanical research into water striders. Morphological and behavioral traits of water striders are summarized and discussed from biomechanical perspectives, along with comparative study. This integrated review also highlights potential directions for studies on water-walking arthropods, which might inspire future biological and biomechanical research.
Keywords: Water strider, Water repellency, Surface tension, Surface propulsion
INTRODUCTION
The water surface is an intriguing three-phase interface where various important interactions occur. It also functions as habitat for water striders. All water striders belong to the heteropteran infraorder Gerromorpha, i.e., the semi-aquatic bugs (Stys & Kerzhner, 1975; Vargas-Lowman et al., 2019). There are eight families and approximately 1 200 species of water striders, which dwell at the terrestrial-aquatic boundary, including still water, fast running streams, and even the ocean surface (Andersen & Polhemus, 1976). The habitat diversity and remarkable ability to use the water surface makes water striders an excellent model for studies on behavior (Wilcox & Ruckdeschel, 1982), developmental genetics (Armisén et al., 2018), and evolutionary developmental biology (Santos et al., 2015). Water strider morphology and locomotion have also drawn much attention in the fields of surface treatment of materials (Bai et al., 2014), biomechanics (Bush & Hu, 2006), and robotics (Kwak & Bae, 2018).
Water striders have evolved elaborate mechanisms to live on the water surface. Their legs possess a striking ability to repel water (Gao & Jiang, 2004; Wei et al., 2009a), a unique feature named superhydrophobicity (Lafuma & Quéré, 2003; Wang & Jiang, 2007). The water repellency of their legs provides water striders with a remarkable capability to manipulate the water surface. In physics, interactions between matter occur based on forces, i.e., momentum transfer. Force measurement techniques have been developed to fully understand the interactions between water striders and water surfaces.
Both ethological (Fairbairn & Brassard, 1988; Wilcox, 1979) and fluid mechanical research (Hu et al., 2003; Rinoshika, 2012; Steinmann et al., 2018) have been conducted to define the interaction modes of water striders with the water surface. Theoretical analysis has illustrated that the surface tension of water plays a dominant role in various interactions (Keller, 1998), with several experiments confirming other forms of momentum transfer, e.g., capillary waves and vortices (Hu et al., 2003; Rinoshika, 2012; Steinmann et al., 2018).
Among water striders, Aquarius remigis is one of the most common and widespread Gerridae species in North America (Cranston & Sprague, 1961; De La Torre Bueno, 1911; Wilcox, 1979). Most biomechanical studies on water striders have adopted Aquarius remigis for research. However, evolutionary biology requires expanded biomechanical research to cover other species of water striders through comparative study. Comparative approaches may shed light on the underlying biomechanical interactions between morphology, behavior, and the environment of water striders.
It should be noted that this review is written from the point of view of biomechanics. Some biomechanical works referred to in this article serve not only to explain the phenomena, but also to guide the design of bio-inspired robots. Their results can only be used as a reference for biological studies, and some statements remain contentious. Through an integrated review, we attempt to introduce biomechanical methods and results into the field of biology, and to provide inspiration for future research on water-walking arthropods.
FUNDAMENTALS OF BIOMECHANICS IN WATER STRIDERS
Here, we introduce the basic theories and experimental techniques applied in biomechanical studies on water striders. From a biomechanical perspective, both leg morphology and overall locomotion of water striders are of considerable interest to researchers. In theoretical analysis, leg morphology is tightly related to wetting theory, whereas locomotion can be interpreted by force analysis. In experiments, microscale imaging of leg morphology and flow motion is well-summarized in Bush et al. (2007), whereas recent progress in macroscopic force measurement techniques is presented.
Theoretical basis
Water repellency
Water striders are perfectly adapted for life on the water surface, requiring that water does not easily adhere to their legs. For the purpose of quantifying water repellency (hydrophobicity), we will briefly summarize the concepts of surface tension and wetting theory.
Surface tension is a surface force arising from inhomogeneity on the liquid surface. If a liquid molecule is near the surface film, it will experience a force much smaller from above than from below due to the absence of liquid molecules above the surface, resulting in a non-zero net force. On the macroscopic scale, an unbalanced micro force can lead to resistance to surface expansion, namely surface tension, which is proportional to the length of contact line l with coefficient γ.
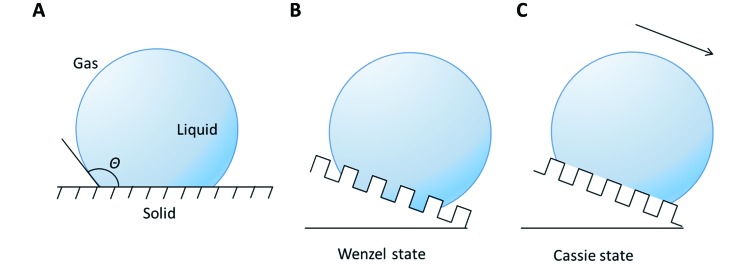
The concept of surface tension can be used to explain the wetting phenomenon. Wetting occurs on a solid-liquid-gas interface, which is usually illustrated using a sketch of a droplet sitting on a solid surface (Figure 1A). The degree of wetting is quantified by the contact angle θ (CA) between the liquid and solid, which is determined by surface tension between substrates as follows (Adamson & Gast, 1997):
1. Schematic of liquid droplet residing on solid surface in different situations.
A: Wetting phenomenon on a flat solid surface with contact angle θ. B: Droplet in contact with a rough surface in Wenzel state. C: Droplet in contact with a rough surface in Cassie state. Both states can result in a high apparent contact angle, but the droplet rolls off more easily under Cassie state (indicated by black arrow). For water strider legs, pillars sketched in (B) and (C) represent protruding leg bristles.
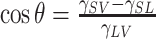 |
1 |
where γSL, γSV, and γLV are the surface tension coefficients of solid and liquid, solid and vapor, and liquid and vapor, respectively. Surface tension coefficient is generally determined by the type of substance, temperature, and pressure.
The superhydrophobic state occurs when the apparent static CA of the solid with water is higher than 150°. The CA of the water strider leg with water is approximately 168° (Gao & Jiang, 2004; Wei et al., 2009a), thus revealing the superhydrophobic nature of water strider legs. To obtain a high CA, it is necessary to take surface roughness into consideration. Two distinct states are identified as superhydrophobic surfaces in common situations: i.e., Wenzel and Cassie states (Wang & Jiang, 2007). In the Wenzel state, surface roughness increases the contact area between the solid and liquid, and water droplets are pinned on the surface (Wenzel, 1936; Figure 1B). In the Cassie state, however, water droplets partially sit on trapped air pockets (Cassie & Baxter, 1944; Figure 1C). This feature results in a significant difference in adhesion properties, whereby water droplets more easily roll off under the Cassie state than under the Wenzel state (Cheng & Rodak, 2005; Lafuma & Quéré, 2003; Patankar, 2004; Wier & McCarthy, 2006).
For identical CA on a flat surface, there exists a critical surface geometry parameter named the slenderness ratio η*, which characterizes the transition point from Cassie to Wenzel state (Zheng et al., 2005). In practical surface contact, a possible transition from Cassie to Wenzel state may occur during water invasion, which should be avoided in most cases (Lafuma & Quéré, 2003; Patankar, 2004; Wang & Jiang, 2007). For water strider legs, impacts with water or natural evaporation on the leg surface can result in a transition from Cassie to Wenzel state (Lafuma & Quéré, 2003), thus posing a threat to water strider mobility on the water surface. Indeed, experiments have shown that water strider legs are in the Cassie state during floatation (Prakash & Bush, 2011), but the repelling side of the leg may transition to the Wenzel state during a stroke (Wei et al., 2009b).
Forces at interface
Water striders must apply various forces to the water surface in order to float and move. In the static state, the forces applied to the superhydrophobic legs can be categorized as buoyancy and surface tension. In the dynamic state, additional factors such as drag, inertia, and viscous friction are included (Bush & Hu, 2006; Hu & Bush, 2010; Koh et al., 2015). When interacting with water, the forces acting on water strider legs can be categorized into (a) propelling force and (b) resistance force. These forces can be further decomposed into vertical and lateral components.
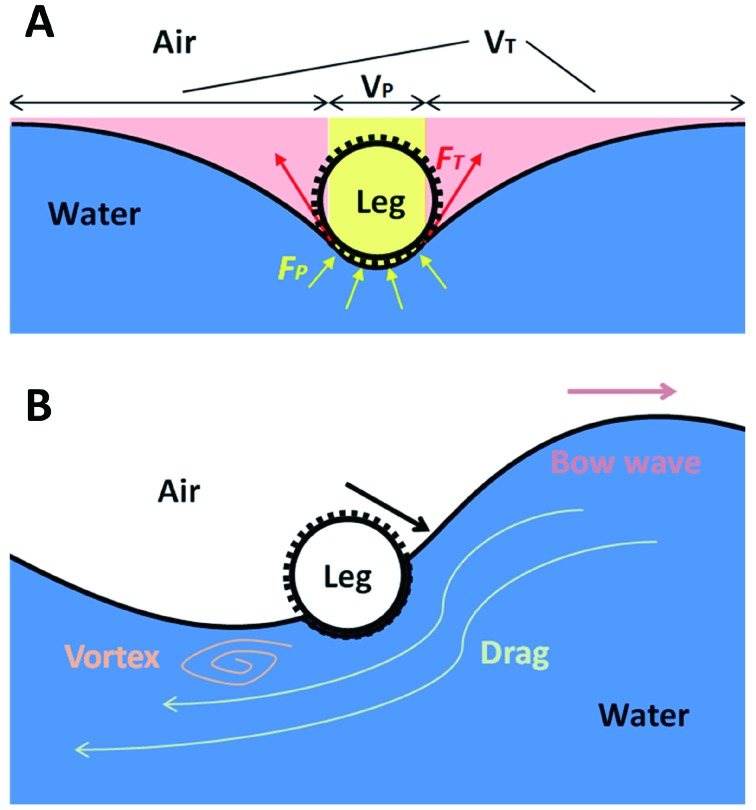
When dealing with vertical forces applied on water strider legs, the additional factors can be neglected, leaving only the two dominate terms: i.e., buoyancy force and surface tension. Archimedes principle states that buoyancy force FP, which is induced by water pressure, equals the weight of water displaced by the object. Consequently, an object denser than water should sink in water. However, in the case of small objects such as water striders, the capillary effect will manifest and the meniscus will form a certain curve when pressed by the object, providing a force large enough for a small object to float (see Figure 2A), namely the vertical component of surface tension force FT. In conclusion, the vertical component of net force between the water strider leg and water is F=FP+FT, in which surface tension force FT plays a dominant role. According to the static theory of Keller (1998), the vertical component of surface tension force FT is equal to the weight of the water with volume VT:
2. Cylinder model schematic of a water strider leg residing on water surface.
A: Static situation. Yellow region represents volume above meniscus in contact with cylinder, VP, associated with water pressure (yellow arrows). Red region represents volume above meniscus not in contact with cylinder, VT, associated with surface tension (red arrows). B: Dynamic situation when motion of fluid cannot be neglected. Waves and vortices are generated during one leg stroke.
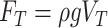 |
2 |
where ρ is the density of water, g is the gravitational acceleration, and VT is the volume above the free meniscus not in contact with the leg (illustrated in Figure 2A in red). Combining equation (2) with Archimedes principle, we can get:
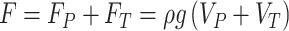 |
3 |
which means that the vertical component of the total force between the leg of a water strider and the water surface is equal to the weight of the water displaced. Although this law is derived in a static situation, it still holds approximately under dynamic processes.
The lateral forces, however, require more careful analysis. Keller (1998) concluded that the lateral force on a partly submerged leg at rest is strictly zero, which cannot explain the propulsion of water striders. Thus, drag and viscous friction should be taken into consideration. These terms arise from the motion and viscosity of flow, which are difficult to calculate. An alternative method is to measure the momentum carried with waves and vortices generated by the leg in motion to estimate the force applied on the leg (Figure 2B).
Force measurement techniques
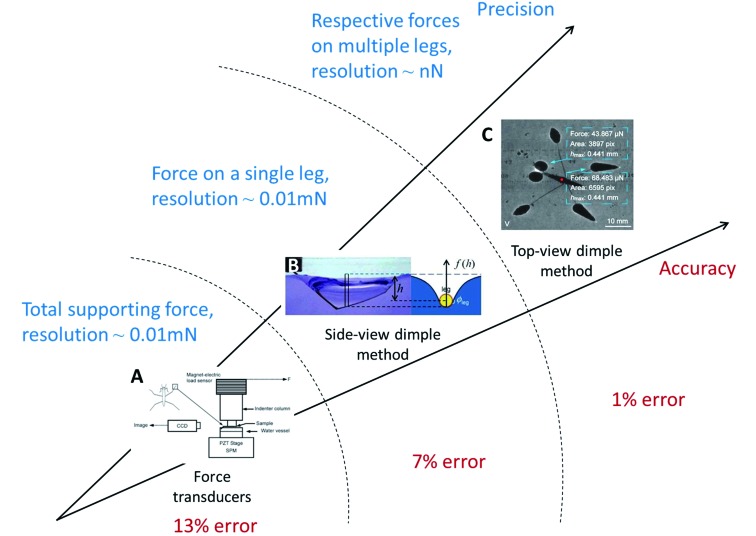
To study various interactions between water strider legs and the water surface, researchers have developed numerous methods to measure the forces applied to the strider by water, as shown in Figure 3. Recent force measurement approaches for water striders have been developed based on both ex vivo and in vivo measurement. Early methods such as force transducers disturb the normal motion of water striders. In contrast, new approaches such as the dimple method have shown unprecedented accuracy and promising application in non-interference research.
3. Classification and comparison of force measurement methods.
A: Force transducers. Reprinted with permission from Langmuir, Wei et al., 2009a, American Chemical Society. B; Side-view dimple method. Reprinted with permission from Langmuir, Feng et al., 2007, American Chemical Society. C: Top-view dimple method. Reprinted with permission from Langmuir, Zheng et al., 2016, American Chemical Society. Measurement technique developed from measurement of total supporting force on striders to simultaneous measurement of all forces on multiple legs, with precision and accuracy improved. Data for precision and accuracy collected from Koh et al. (2015), Perez Goodwyn et al. (2008a), and Zheng et al. (2016).
Force transducers
A general solution to force measurement is to use force sensors. Due to the small size of water striders, it is difficult to attach any kind of force transducer to their legs. The earliest approach fixed a single leg to a transducer while moving the water vessel toward or away from the leg. This ex vivo method can only be used to study the properties (e.g., load-bearing capacity and adhesion) of the leg (Feng et al., 2007; Gao & Jiang, 2004; Shi et al., 2007; Uesugi et al., 2017; Watson et al., 2010; Wei et al., 2009a, 2009b; Xu et al., 2012), but cannot disclose the force in water strider’s normal activities.
An improved version of this approach successfully applied the force transducer on a live water strider, but at the cost of limiting free movement. Based on this method, the specimen is first cooled at 4 ℃ or anesthetized with CO2 to freeze its motion temporarily and is then tethered to a force transducer. Thus, measurement of the total force on the water strider or stroke force on an individual leg is achieved (Perez Goodwyn & Fujisaki, 2007; Perez Goodwyn et al., 2008a; Uesugi et al., 2017), but with relatively low accuracy.
Hu et al. (2003) also deduced the magnitude of total force in one stroke from the acceleration and leaping height of a water strider through analysis of high-speed video footage. Although this method has no impact on the free motion of water striders, it can only be applied to the measurement of total force in a vigorous motion, with low accuracy.
Side-view dimple method
When interacting with the water surface, each individual leg presses water to form a dimple (Denny, 1993; Feng et al., 2007; Gao & Jiang, 2004; Figure 4A). Force transducers follow Newton’s second law F=ma, but the dimple method relies on the updated Archimedes principle F=ρgV, which converts magnitude of force to magnitude of volume. Subsequently, the remaining problem is how to calculate dimple volume. Direct measurement of volume of an irregular dimple is difficult. The general solution is to reconstruct the meniscus based on directly measured parameters, which has led to the development of several distinct approaches established on the same principle.
4. Principle of dimple method.
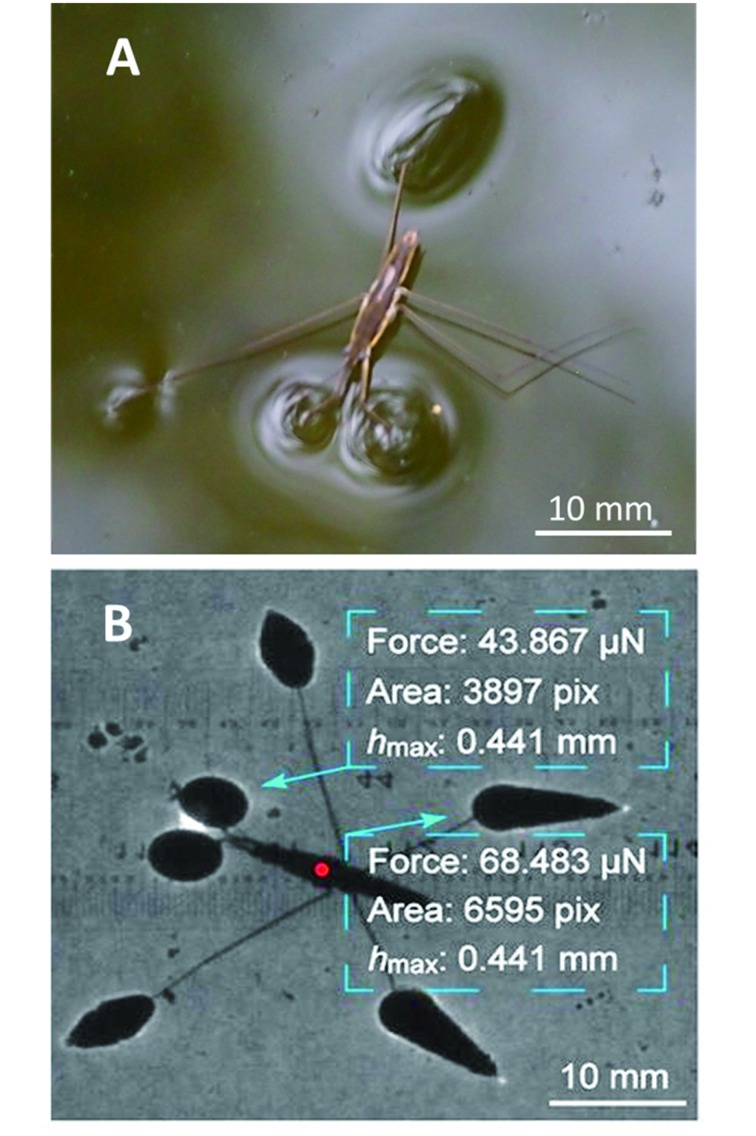
A: Dimples formed by superhydrophobic legs of water strider Aquarius remigis. B: Shadow method applied to floating water strider Aquarius remigis. Reprinted with permission from Langmuir, Zheng et al., 2016, American Chemical Society.
One analytical method is based on volume estimation. An outstanding theoretical analysis by Vella (2008) estimated the supporting force on a bending cylinder of finite length on the water surface as follows:
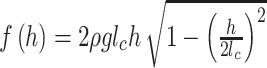 |
4 |
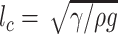 |
5 |
where ρ is the density of water, g is the gravitational acceleration, h is the depth of the center of the cylinder beneath the free surface, and γ is the surface tension of the water.
Total supporting force is obtained by integrating force per unit length along the wetted length in the following equation:
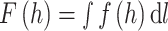 |
6 |
This model was later adopted by Koh et al. (2015) and Yang et al. (2016) to calculate the upward force on a water strider during jumping mode.
This method yields simulations that fit well with experiments on both real water striders and robot striders within 7% error (Koh et al., 2015), showing a great leap from traditional approaches. Moreover, this method can measure force on a single leg without interfering with normal water strider behavior. However, it also largely depends on the accuracy of capturing the side-view of the dimple, and thus during experiment only two dimples at most can be simultaneously captured with one high-speed camera, providing the camera is placed at a perfect viewpoint. To achieve high accuracy and avoid constantly adjusting the position of cameras, there should be a limit to the orientation of the water strider through a narrow width of the vessel.
Top-view dimple method
To examine the vortices generated by water striders, Rinoshika (2012) adopted Particle Image Velocimetry (PIV) analysis to obtain the instantaneous velocity field on the water surface. In their experiment, small polystyrene particles were uniformly scattered on the surface film and illuminated by a light source. Surface film images were captured from above by a high-speed camera. Through analysis of surface film images, the PIV system measures particle displacement over a pre-set time interval and exports the velocity field of the surface. This method, however, can only record the velocity field, not the shape of the meniscus.
In a modified optical approach, i.e., synthetic Schlieren method (Moisy et al., 2009), the three-dimensional topology of the meniscus is successfully mapped out. An image of a random dot pattern is placed beneath the vessel, while the camera captures refracted dot pattern images. Based on comparison of the original and refracted patterns, the water surface is reconstructed through displacement field computation. In a recent experiment on capillary waves produced by water striders, this method showed excellent results (Steinmann et al., 2018). With high accuracy, it can simultaneously measure all forces acting on all six legs. It should be noted that a blurry image (because the camera is focused on the bottom of the vessel) of the gerrid along with its shade also appears in the photographs, but Steinmann et al. remarked that this does not seem to jeopardize the accuracy of the result. They also mentioned that this method may not achieve due accuracy for young instar locomotion.
Another newly developed approach is the shadow method (Yin et al., 2016; Zheng et al., 2016), which originates from shadows of water striders under sunlight, as illustrated in Figure 4B. This elegant shadow is the shade of an individual leg refracted by a deformed meniscus, which magnifies the small dimple to a larger shadow. Furthermore, the deformed meniscus acts as a convex lens to concentrate light rays into the bright edge of the shadow, which makes the shadow easily identified by the human eye and image processing software. The shadows can be easily reproduced in the laboratory with a point light source and captured by camera. Surprisingly, the shadow area of a water strider leg exhibits a good linear relationship with the corresponding supporting force, which could significantly simplify experimental procedures in practical applications. This method has been used to analyze grooming (Zheng et al., 2016), floatation (Yin et al., 2016; Zheng et al., 2016), and lateral propulsion (Lu et al., 2018) of water striders, with unprecedented accuracy. It provides a much more direct view than that of the synthetic Schlieren method. It is also worth noting that this method can simultaneously measure and record forces on multiple legs with simple equipment.
MORPHOLOGY AND FUNCTIONS: SUPERHYDROPHOBIC LEGS
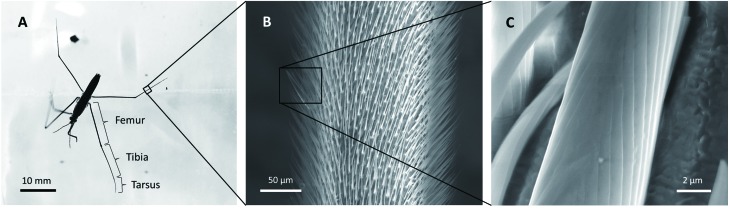
Water striders have three pairs of legs: one pair of forelegs for grabbing and support, and two pairs of long appendages for locomotion. The distal segments of each leg are composed of a femur, tibia, and tarsus, as shown in Figure 5A. The leg is covered by uniformly tilted bristles (Figure 5B), which contain grooves on each (Figure 5C). There are two types of bristles distinguished by their size: i.e., thick and sparse bristles (thorns) used as grooming combs (Finet et al., 2018) and thin and dense bristles that render the legs superhydrophobic (Gao & Jiang, 2004), with their tips bent inward to avoid piercing the water surface. Both types are innervated bristles that can act as mechanoreceptors to sense fluctuations on the water surface (Finet et al., 2018).
5. Hierarchical structure of water strider Aquarius remigis leg .
A: Distal segments of a midleg. B: Uniformly tilted bristles on tibia of hindleg, with tips bent inward. Note: There are two types of bristles distinguished by their size. C: Converging grooves on thick bristle.
In early studies, it was generally believed that secreted wax and hair piles render the insect’s body and legs non-wetting (Caponigro & Eriksen, 1976; Dickinson, 2003; Hu et al., 2003; Spence et al., 1980; Thorpe, 1950; Thorpe & Crisp, 1947, 1949). In 2004, however, Gao & Jiang ( 2004) pointed out that the water CA of wax on water strider legs is 105°, an angle characteristic of insect cuticle wax (Holdgate, 1955), which is not sufficient for superhydrophobicity. They thus proposed that the hierarchical structure of the water strider legs (i.e., presence of dense bristles and grooves) is more significant for a superhydrophobic state. Modelling bristles and grooves as infinitely long rigid cylinders, Feng et al. (2007) demonstrated that the theoretical analysis agrees well with the measured value of the CA, thus suggesting that grooves are essential in generating the superhydrophobicity of water strider legs.
These remarkable works have inspired a new wave of in-depth biomechanical study on the hierarchical structure of water strider legs. The features of legs, bristles, and grooves can be classified into four aspects (Table 1), namely (a) deformation, (b) orientation, (c) dimensions, and (d) spacing. Current topics mainly focus on their roles in maximizing propelling forces and minimizing resistant forces.
1. Morphological traits of adult Aquarius remigis and related functions .
| Morphological trait | Value | Main finding | Function | Reference |
| Data gathered from our scanning electron microscope (SEM) study, Hinton (1976), and Hu & Bush (2010). | ||||
| Deformation | ||||
| Legs | Young’s modulus: ~10 GPa | Adaptive deformation at three joints increases supporting force. | Maximizing propelling forces | Zheng et al., 2009 |
| Flexibility of each segment increases supporting force. | Maximizing propelling forces | Ji et al., 2012 | ||
| Bristles | Young’s modulus: ~10 GPa | Microscale droplets between bristles can be expelled out due to elastic deformation of bristles. | Minimizing resistant forces | Wang et al., 2015 |
| Bristle deformation during lift-up of legs reduces contact area with water, thus reducing detach force. | Minimizing resistant forces | Sun et al., 2018 | ||
| Orientation | ||||
| Legs | Unfixed | Supporting force decreases as stepping angle increases. Penetration occurs at 28°. | Maximizing propelling forces | Feng et al., 2007 |
| Midleg experiences larger stroking force as they extend perpendicularly to direction of motion. | Maximizing propelling forces | Prakash & Bush, 2011 | ||
| Hindleg experiences less drag force as they extend along direction of motion. | Minimizing resistant forces | Prakash & Bush, 2011 | ||
| Bristles | Tilt angle: 30°–50° | Droplets advance more easily toward leg tip, which is linked to a peeling mode during lift-up of legs. | Minimizing resistant forces | Prakash & Bush, 2011 |
| Grooves | Tilt angle: ~10° | Flow slips more easily in direction of grooves. | Minimizing resistant forces | Choi et al., 2006 |
| Dimensions | ||||
| Legs | Length: 1.3–1.7 μm (midleg)
Width: 90–110 μm |
Critical leg aspect ratio l/r (l: wetted length; r: radius of leg) at which supporting force reaches a plateau. | Maximizing propelling forces | Vella, 2008 |
| Bristles | Length: 20–40 μm
Width: 1.5–2 μm |
|||
| Grooves | Depth: ~200 nm
Width: ~400 nm |
|||
| Spacing | ||||
| Legs | Coxa spacing:
Foreleg-midleg: ~5 mm Midleg-hindleg: ~2 mm |
|||
| Bristles | 6–8 μm | Critical bristle spacing at which supporting force reaches a maximum. | Maximizing propelling forces | Xue et al., 2014 |
| Upper limit to maintain Cassie state, and lower limit to prevent bristle collision. | Minimizing resistant forces | Su et al., 2010 | ||
| Grooves | Closely packed | |||
Morphological traits in maximizing propelling forces
For Aquarius remigis, the maximum vertical propelling force per leg without piercing the water surface is ~1.52 mN (15 times body weight), indicating an extraordinary load-bearing capacity (Feng et al., 2007; Gao & Jiang, 2004). In locomotion, the lateral propelling force on one midleg is estimated to be ~0.18 mN (Uesugi et al., 2017). Early calculations treated the leg as an infinitely long rigid cylinder that horizontally resides on the water surface and gave a theoretical vertical propelling force slightly lower than the experimental result (Liu et al., 2007; Vella et al., 2006), suggesting other unconsidered factors at play.
The effect of deformation was not considered until later. Water strider legs are mostly hollow, which results in great flexibility (Wei et al., 2009b). Zheng et al. (2009) and Ji et al. (2012) discovered that the adaptive bending at three joints and elastic deformation of each segment play prominent roles in enhancing vertical propelling force. The flexibility can help the leg adjust to the inclined meniscus (Wei et al., 2009b), thus preventing the tip from piercing the surface (Ji et al., 2012; Koh et al., 2015).
The orientation of legs can be easily related to propelling forces. Feng et al. (2007) revealed that vertical propelling force decreases as the leg stepping angle increases to 28° before penetrating the water surface. Bristles can also contribute to the propelling force in an unexpected way. As demonstrated in Figure 5B, bristles protrude toward the leg tip. This topography leads to a unidirectional adhesion property: i.e., droplets roll off the bristles more easily parallel to the leg than perpendicular to the leg (Prakash & Bush, 2011). Similarly, in regard to orientation of the grooves, liquid flowing perpendicular to the grooves will experience more drag (Choi et al., 2006). As a result, by posing the midleg perpendicular to the direction of motion, the midleg will experience larger drag force when stroking backward, thus in turn generating a larger thrust forward.
The dimensions of the leg have intrigued researchers in terms of leg length. As surface tension is proportional to wetted length, one may expect that the tibia and tarsus should be as long as possible (Su et al., 2010). However, theoretical analysis shows that there is a critical leg aspect ratio l/r (l: wetted length; r: radius of leg) at which vertical propelling force reaches a plateau, whereby the leg will bend greatly to extend above the water surface, leaving the tip unattached to the water (Song et al., 2006; Vella, 2008). Vella (2008) examined several species from the genus Gerris and Aquarius and found that the aspect ratios of their midlegs are below but close to the critical value.
Similarly, calculation with a flat surface model reveals a critical value of bristle spacing when maximizing vertical propelling force, with bristle spacing of Aquarius remigis below but remarkably close to this critical value (Xue et al., 2014).
Morphological traits in minimizing resistant forces
The first attempts to minimize resistant forces arose from the fact that superhydrophobicity may be unnecessary in generating propelling forces. Analyses conducted by Liu et al. (2007), Zheng et al. (2009), and Su et al. (2010) demonstrated that maximum force increases insignificantly with the increase in CA, given that CA is higher than 100°. Shi et al. (2007) used coated gold threads to confirm this finding. For dynamic processes, numerical analysis by Gao & Feng (2011) demonstrated that the propulsive force, as in the static case, is also insensitive to the CA if the surface is hydrophobic.
However, the focus here may not be propelling forces but rather resistant forces. The rigid cylinder model indicated that a larger CA is essential for reducing the vertical resistant force of a leg detaching from the water surface (Lee & Kim, 2009; Su et al., 2010). This was confirmed by Watson et al. (2010), who found that a bristle without grooves exhibits much greater adhesion and weaker resistance to water penetration. Shi et al. (2007) examined fluidic drag on a gold thread surrounded by a layer of oxygen gas and concluded that the air cushion formed by the hierarchical structure reduces fluidic drag on water strider legs. Under a quasi-static process, Wei et al. (2009a, 2009b) measured the force to lift an Aquarius remigis leg from the water surface to be ~20 μN. The detachment force on the water surface is much smaller with a magnitude of ~0.1 μN and can be further reduced to ~0.01 μN with increased lift-up velocity (Sun et al., 2018).
The deformation of bristles plays a crucial part in maintaining the Cassie state, thus reducing resistant forces. Microscale droplets that migrate between bristles can be expelled due to the elastic deformation of bristles, thus preventing transition to the Wenzel state in a humid environment (Wang et al., 2015). Deformation of bristles during leg lift-up can also reduce the water contact area, thus decreasing the detachment force (Sun et al., 2018).
The orientations of legs, bristles, and grooves play vital roles in minimizing resistant forces. For large droplets, the uniform tilt angle of bristles results in a unidirectional adhesion property: i.e., droplets advance more easily toward the leg tip than backward, as the deformation of bristles will resist the backward motion of droplets (Prakash & Bush, 2011; Xu et al., 2012). Similarly, liquid will flow more easily in the direction of grooves (Choi et al., 2006). Consequently, in locomotion, the hindlegs will experience less drag force as they extend along the direction of motion. Additionally, a peeling mode has been observed in water striders when they lift-up their legs, which is directly linked to this unidirectional adhesion property (Prakash & Bush, 2011).
One may assume that there is a critical bristle spacing to minimize resistant force, with small spacing making it easier to form a capillary bridge that sticks adjacent bristles together (Bush et al., 2007). Through sheer calculations, Su et al. (2010) stated that there should be an upper limit of bristle spacing so that a transition to the Cassie state does not occur under medium pressure, as well as a lower limit beyond which the bristles will easily bend so as to contact with adjacent bristles. They claimed that biological surfaces in nature fall into their predicted range.
BEHAVIOR: MECHANICAL INTERACTIONS WITH WATER
Various interactions between water striders and the water surface have been recorded and analyzed. These interactions are very different from terrestrial insect behaviors, thus revealing gerrid adaptations to the semi-aquatic environment and shedding light on bio-inspired aquatic robot design.
Quasi-static interactions
Grooming
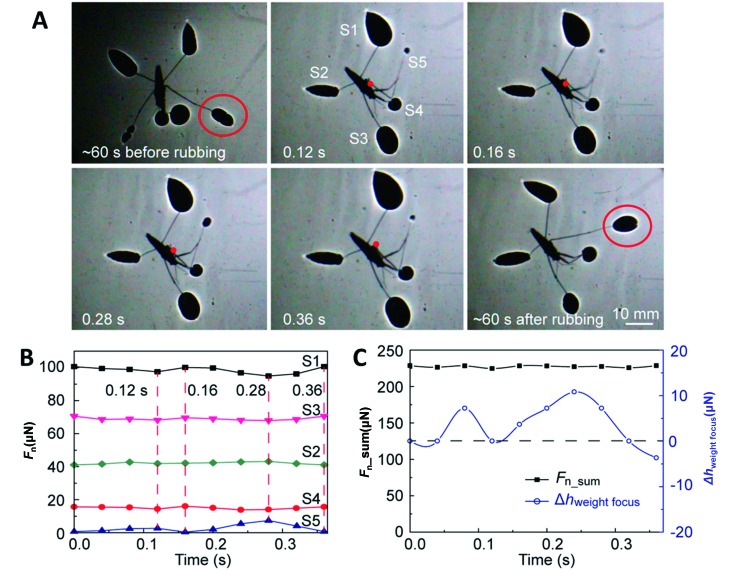
Compared to other interactions between water strider legs and the water surface, grooming behavior is still poorly understood. Bush et al. (2007) mentioned that water striders can groom themselves once wetted. Based on the newly developed shadow method, Yin et al. (2016) and Zheng et al. (2016) demonstrated the force distribution of six legs when a water strider is rubbing its right midleg with its right foreleg (see Figure 6). To maintain balance, the water strider shifts its weight slightly to the right and distributes more supporting force on its left hindleg.
6. Measurement of force distribution and body shift during grooming process of Aquarius remigis .
A: Intuitive display of grooming. Force distribution can be visualized with differences in dimple area. Change in smoothness of shadow edge (indicated by red circle) implies recovery of hydrophobicity after grooming. Note: Weight focus (red dot) is on right side of body. B: Force distribution in grooming. C: Total supporting force and vertical shift of weight focus. Reprinted with permission from Langmuir, Zheng et al., 2016, American Chemical Society.
It has also been suggested that the grooming process enhances water repellency, probably through smearing hydrophobic chemicals onto the legs (Yin et al., 2016; Zheng et al., 2016). Some species of aquatic bugs groom their body and legs, either underwater (Thorpe & Crisp, 1949; Thorpe, 1950) or on land (Kovac & Maschwitz, 1989, 1990; Kovac, 1993). For underwater cases, no secretion is released from the cuticle, and grooming may serve to keep the hairs regularly spaced and to redistribute the air bubbles to wetted regions (Thorpe & Crisp, 1949). For land cases, however, chemicals are secreted from metathoracic glands, which are then rubbed and distributed over the body cuticle by the legs (Kovac & Maschwitz, 1989). Kovac & Maschwitz (1989) found the secretion to be hydrophilic, and thus concluded that hydrophobicity is recovered during grooming due to the clearance of contaminants (mainly microorganisms). Thus, the potential role of grooming in hydrophobicity recovery could result from (a) maintaining regularity of hair, (b) removal of water and other contaminants between hairs, and (c) smearing hydrophobic chemicals on the legs.
Indeed some semi-aquatic insects can excrete liquid waste from their mouthparts (Bush et al., 2007), and anatomical research on the head capsule of Aquarius remigis has indicated a sufficient supply of saliva due to large and functional salivary glands (Cranston & Sprague, 1961). However, excretion of liquid from the mouthparts of water striders has never been identified, not to mention the hydrophobicity of this secretion. Based on our observations, grooming can be subdivided into three modes, namely (a) rubbing the mouthpart with a pair of forelegs, (b) grooming a midleg with an ipsilateral foreleg, and (c) grooming a hindleg with an ipsilateral midleg. If the three modes occur in a sequence, then it may be possible that some chemicals, presumably liquid, are excreted from the mouthpart and passed on to the six legs in a three-step delivery system.
Additionally, water striders can rub their antennas and femur segments. As the antennas and femur segments are usually kept away from water, hydrophobicity may not be a necessary property for them. Therefore, this potential secretion and overall grooming behavior may assume other functions such as maintenance of sensory receptors (Rebora et al., 2019) or as a defense mechanism against disease (Zhukovskaya et al., 2013).
Ripple signaling
Water striders have a sensory system on their legs to detect disturbances on the water surface (Finet et al., 2018; Lawry, 1973; Perez Goodwyn et al., 2009a). The leg near the wave source may receive more intense stimuli, allowing the gerrid to determine the direction of the wave (Murphey, 1971a).
There are generally two kinds of ripple signals received by water striders. One is produced by other insects struggling to free themselves from the water surface (Murphey, 1971a, 1971b; Roh & Gharib, 2019), and the other is produced by the gerrids themselves in reproduction. Aquarius remigis males can produce surface waves of both high frequency (80 to 90 waves/s) and low frequency (3 to 10 waves/s), whereas females only produce low-frequency waves (Wilcox, 1979). The presence or absence of high-frequency wave signal functions as a sole means for males to discriminate adult sex (Wilcox, 1979). Furthermore, mating males produce high-frequency wave signals to repel other harassing males and potentially enhance female foraging efficiency (Wilcox & Stefano, 1991). This function still holds during non-mating seasons, when males use high-frequency wave signals to repel other males and defend food territories (Jabloński & Wilcox, 1996).
Locomotion
Rowing and leaping
Two distinct patterns of lateral propulsion, i.e., rowing and leaping, have been identified in water strider locomotion.
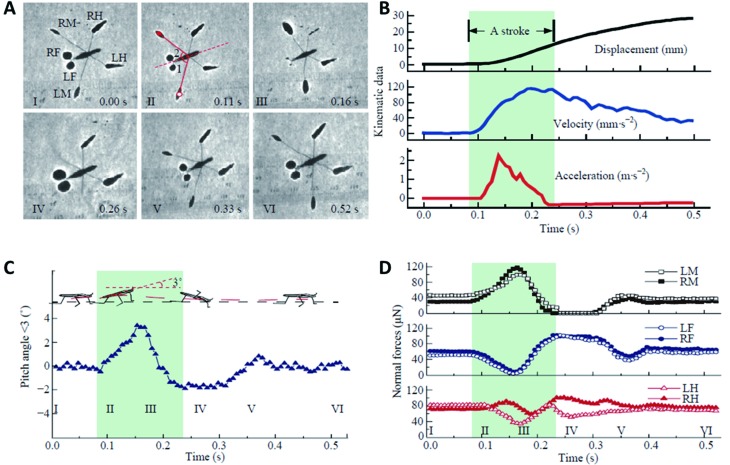
In typical rowing motion, the body of a water strider maintains a constant high position above the water surface. The forelegs and hindlegs serve to stabilize the body posture, while midlegs row against the water surface to gain propulsion, as shown in Figure 7. After a backstroke, the gerrids either directly lift their midlegs off the water surface or drag them along the surface, and then bring them back over the head to their initial positions (Caponigro & Eriksen, 1976). This rowing mode is a short-range motion, presumably to change orientation or for drift compensation.
7. Dynamic analysis of water strider Aquarius remigis rowing mode .
A: Shadow variation. B: Kinematic quantities. C: Body pitch angle. D: Force distribution. Forces on midlegs (LM or RM) and on forelegs (LF or RF) compensate for each other, whereas force on hindlegs (LH or RH) varies little. This force tracking technique confirms previous visual observations that forelegs and hindlegs mainly serve as supports (Caponigro & Eriksen, 1976). Reprinted with permission from Journal of Bionic Engineering, Lu et al., 2018, Springer Nature.
In the leaping mode, however, the initial position of the gerrid’s body is much closer to the water surface. During motion, the gerrid springs forward off the surface and lands on the water. Observation shows obvious movements of both midlegs and hindlegs (Bowdan, 1978), although midlegs appear to provide the main propulsion (Rinoshika, 2012). The gerrids retrieve their hindlegs before landing and pull midlegs forward after landing on the surface film (Caponigro & Eriksen, 1976). In lateral propulsion, the leaping mode is a swift movement for both short- and long-distance locomotion, frequently observed in pursuit or escape.
The hydrodynamics of the leg-water interactions are a contentious issue. It was previously assumed that water striders can generate capillary waves dominated by surface tension to transfer momentum (Denny, 1993; Sun & Keller, 2001). However, simple hydrodynamic analysis concluded a minimum velocity of 23 cm/s for a steady surface wave. To produce a surface wave, the gerrid must swing its legs at a velocity faster than the wave it creates, but juvenile water striders are unable to accomplish this task (Denny, 1993). However, Denny’s Paradox has been recently brought into question, for the critical velocity of 23 cm/s may not apply to the unsteady strokes of water striders (Bush & Hu, 2006; Hu & Bush, 2010), as confirmed by further analysis (Closa et al., 2010). Moreover, careful observations by Hu & Bush (2010) put an end to Denny’s Paradox by showing that even an infant water strider can produce both capillary waves and vortices.
Before Denny’s Paradox was questioned, the paradox was perfectly resolved through experiments by Hu et al. (2003). They showed that in lateral propulsion, water striders not only create capillary waves, but also hemispherical dipolar vortices beneath the water surface. They estimated that during the leaping mode. water striders transfer momentum mainly through vortices, a mechanism previously found in the locomotion of birds and fish (Dickinson, 2003). This remarkable result motivated subsequent numerical investigations (Bühler, 2007; Gao & Feng, 2011), but these analyses achieved distinct results. An elaborate experiment concluded that vortices contribute to 64%–90% of the momentum of a water strider (Rinoshika, 2012), with the wave pattern later captured by Steinmann et al. (2018). However, these experiments also have their limits in modelling the real environment, thus leaving a still unanswered open question.
Jumping
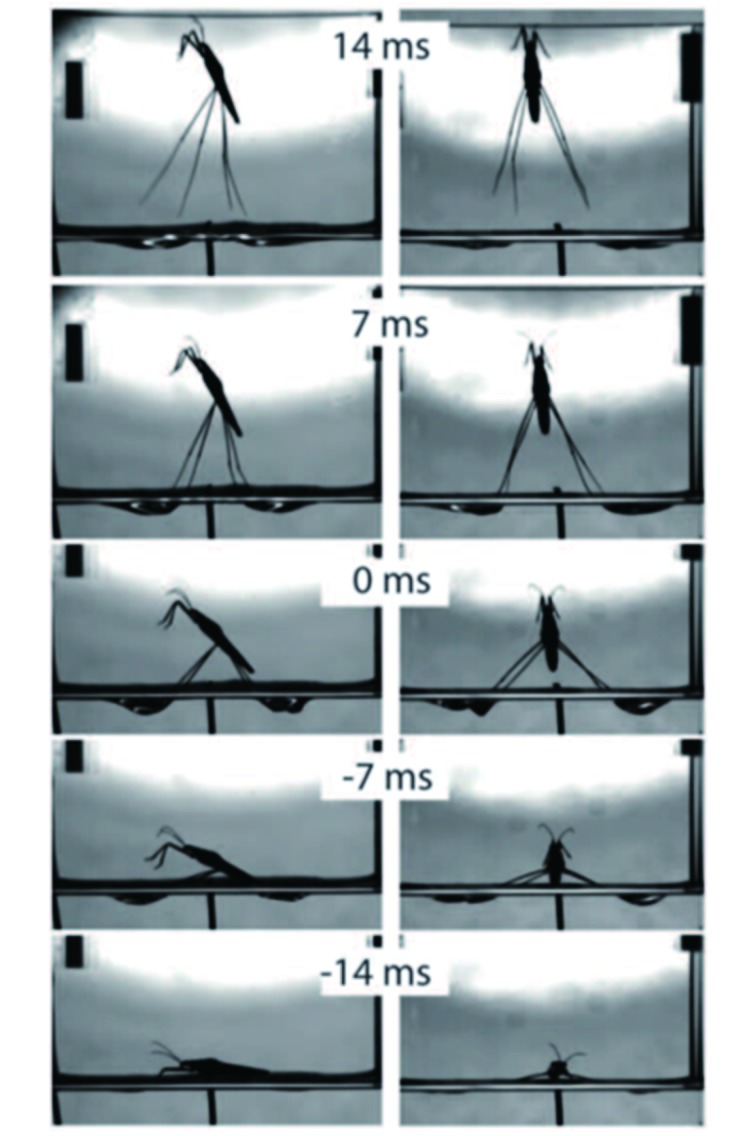
In the jumping mode, the gerrid gradually pulls its midlegs and hindlegs together while synchronously rotating the tibia and tarsus segments of those legs inward, and then disengaging from the surface film (Koh et al., 2015; Yang et al., 2016; Figure 8). On landing, it spreads its legs out to disperse the impact force (Hu & Bush, 2010). Hu and Bush also observed that gerrid legs can penetrate the water surface during landing. When encountering a predator/unwelcomed mate, water striders may jump abruptly, followed by a leaping escape.
8. Vertical jumping of water strider Aquarius paludum. Changes in dimples indicate changes in pressing force .
Reprinted with permission from Science, Koh et al., 2015, American Association for the Advancement of Science.
The source of vertical propulsion was previously attributed to a simple modification on the impact angle of driving force (Hu & Bush, 2010); however, the leg rotation reveals a more profound mechanism. As momentum transfer can only proceed when the leg is in contact with the surface, a short contact time can result in a decrease in the efficiency of momentum transfer. Leg rotation guarantees continuous contact with undistorted water throughout the pushing stage, and thus maximizes momentum transfer (Koh et al., 2015). Analysis has shown that the angular velocity of leg rotation is tuned to optimize the takeoff velocity for Gerris latiabdominis, G. gracilicornis, and A. paludum (Yang et al., 2016). Furthermore, Koh et al. (2015) revealed that the driving force in the pushing stage is just below the critical value of surface breaking.
INTEGRATING MORPHOLOGY, BEHAVIOR, AND ENVIRONMENT: COMPARATIVE DISCUSSION
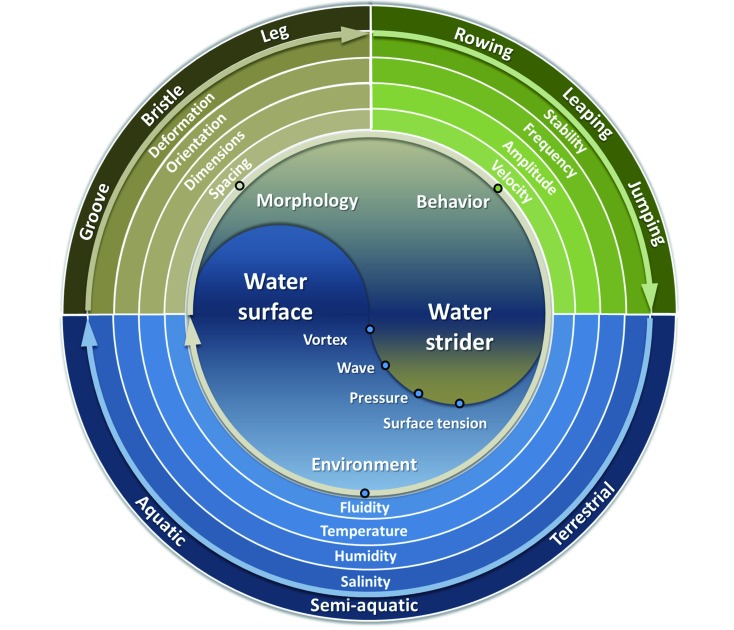
Based on biomechanical study, one may assume every morphological trait of water striders should be optimized. This opinion can simplify research ideas for researchers in biomechanics but may overlook key aspects from an evolutionary point of view. Different species of water striders live in different environments with different temperature, humidity, salinity, and fluidity. These diversities can lead to diverse behavioral and morphological traits from species to species, a key theme in evolutionary biology (Andersen, 1995). Thus, biomechanical studies should not neglect different species or the specific environments in which they live. The optimal approach should be specified to the exact environment instead of a general adaptation to the “water surface”. This perspective also suggests a new trend for biomechanical studies on water striders: (a) identifying specific differences in behavioral or morphological traits between species of water striders through comparative study, and then (b) exploring biomechanical explanations with an integrated view of morphology, behavior, and the environment (Figure 9).
9. New trends for biomechanical studies on water striders: comparative study through an integrated view of morphology, behavior, and the environment.
Latest findings
Several studies advancing this trend have been reported. In biomechanical research, multiple species of water striders were taken into consideration in Vella (2008) and Yang et al. (2016) to illustrate mutual traits shared among species. Pioneering research by Bush et al. (2007) and Hu & Bush (2010) included measurement and flow-visualization experiments on Aquarius remigis, Mesovelia, Hydrometra, Stagnorum, and two species of semi-aquatic bugs. They examined orientation, dimensions, and spacing of legs and bristles as well as dynamic parameters and proposed several dimensionless parameters for comparison. They were the first to introduce the Strouhal number St=fA/V (f: Stroke frequency; A: Stroke amplitude; V: Body velocity) into biomechanical study of water striders to quantify propulsive efficiency. In biological research, two characteristic locomotion gaits have been related to habitat diversity: (a) alternating double tripod gait by Hebridae, Macroveliidae, Hydrometridae, and Mesoveliidae, who exhibit hybrid ground-water surface life, and (b) synchronous rowing gait by Gerridae and Veliidae, who mainly live on open water surfaces (Andersen, 1995; Perez Goodwyn et al., 2009b).
Specific morphological traits such as bristle orientation and leg length may have a deep connection to the differences in locomotion traits. Hu & Bush (2010) discovered that Aquarius remigis and water spiders have more tilted bristles compared to tripod walkers, although the underlying mechanism is not fully understood. Research on evolutionary biology of water striders has concluded that midlegs are longer than hindlegs for Gerridae and some Veliidae species due to their adaptation to open water surfaces (Andersen, 1995; Crumière et al., 2016; Khila et al., 2014; Refki et al., 2014; Refki & Khila, 2015). This elongation is correlated with locomotion velocity (Crumière et al., 2016) and largely credited to the elongation of the tibia and tarsus parts of the midleg (Khila et al., 2009), which are the parts in contact with water. The elongation of the midleg may also enhance escape jumping performance of water striders from predators (Armisén et al., 2015).
As to why the rowing gait is better adapted to the water surface compared to the tripod gait, Hu & Bush (2010) proposed that rowing bugs have lower St numbers, indicating higher propulsive efficiencies. Crumière et al. (2016) confirmed that the rowing gait indeed results in less consumed power. Associated data on morphological and dynamic parameters gathered from various species of water striders can be found in Bush et al. (2007), Hu & Bush (2010), and Crumière et al. (2016).
Future developments
Compared to solid ground, friction on the water surface is much smaller and mobility is much higher, thus suggesting a slippery and unstable interface. On this interface, it is more difficult to keep balance or generate thrust. Additionally, predators generally come from above or from the lateral side on land, but on the water surface, predators can also attack from underneath (Armisén et al., 2015), demanding much higher jumping ability. We thus propose two directions that could be of interest to researchers in the field of biology and biomechanics.
Fluidity of water
The water environment on which water striders live may have different parameters such as temperature, humidity, atmospheric pressure, salinity, and fluidity. From a biomechanical perspective, the first four parameters can have a potential influence on surface tension, whereas the last one may affect locomotion traits. However, surface tension and CA vary little with the first four parameters in the natural environment. As a result, we could rule out the influence of the first four parameters and focus on the fluidity of water.
Water striders dwell on still water, running streams, and even ocean surfaces (Andersen & Polhemus, 1976). These environments have different flow speeds and fluctuation amplitudes. Ortega-Jimenez et al. (2017) showed that perturbed water surfaces dominated by waves and bubbles can affect escape jumping performance of Aquarius remigis, especially for newborns. Similar effects were observed by Vinnichenko et al. (2018), who found that water striders cannot stay afloat on constantly renewed surfaces with vertical convection. However, the influence of flow velocity on water strider locomotion has drawn little attention.
Water striders can forage in fast flowing regions of currents where prey capture rates are highest (Junger & Varjú, 1990; Rubenstein, 1984; Wilcox & Ruckdeschel, 1982). For a rich supply of prey, water striders have developed a drift compensation mechanism to keep their position on the stream surface (Fairbairn, 1985). One may thus assume that locomotion velocity and efficiency should be higher for water striders that dwell on fast-running streams. Also, modified morphological traits such as flattened bristles (Perez Goodwyn et al., 2008b) and propelling fans (Santos et al., 2017) may emerge from the underlying selection force of flow velocity, which still demands further research.
Stability of motion
For an asymmetrical locomotion trait, such as tripod gaits, stability of motion may be difficult to maintain, thus consuming more energy in muscle control and body posture adjustment. This statement is supported by Perez Goodwyn et al. (2009b), who studied the modified double tripod gait of Hermatobates weddi and observed a yawing of the body during motion. They suggested that the asymmetrical locomotion gait generates an unbalanced torque on the body, and excessive energy is wasted competing with this torque. It is also interesting to note that grooming is an asymmetrical behavioral trait. One may wonder why water striders can groom their legs effortlessly while maintaining a seemingly unstable posture. A similar discussion about stability can be directed to ripple signaling. Wilcox (1979) documented ripple signals by water striders oscillating forelegs. It would be interesting to discover why water striders can oscillate their forelegs while causing little perturbation at other legs.
The stability of motion may be related to morphological traits such as bristle orientation, leg deformation, leg length, and leg spacing. To avoid slipping during unstable postures, such as grooming, the friction force on the leg tips should be large enough, which could be correlated with the unidirectional adhesion of bristles reported by Prakash & Bush (2011). Generally, for tripod walkers, only the tarsus is in contact with water, and the femur along with tibia forms a two-bar system to support the body. For rowers, however, the femur acts as a one-bar system to support body weight. The stability of these two systems are likely different, which may contribute to the elasticity requirement of the femur. Interestingly, for rowers, the midleg coxa is generally closer to the coxa of the hindleg, whereas for walkers the midleg is at the center of the foreleg coxa and hindleg coxa. This difference in leg spacing could be related to the efficiency and stability of locomotion.
Thus, we propose that stability analysis may raise new questions in water strider research. Stability analysis may involve (a) high-speed cameras that capture the posture of body and leg, (b) force measurement techniques, such as the shadow method, which provide information on forces, derived body center position, and torque, and (c) water strider modelling and numerical analysis.
CONCLUSIONS
In this review, we introduced basic physics theories and newly developed experimental techniques for deeper biomechanical research into water striders. The latest findings include morphological traits (deformation, orientation, dimensions, spacing) related to behavioral traits (grooming, ripple signaling, rowing, leaping, jumping) through analysis of functions (maximizing propelling forces and minimizing resistant forces). Considering that most biomechanical studies on water striders have only focused on a single species, we suggest that a comparative approach would be of assistance in understanding the biomechanics underlying the derived morphological and behavioral traits. We hope this review provides a general scope of biomechanical research on water striders and may inspire future work.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS' CONTRIBUTIONS
Y.T. proposed the ideas. J.Z.M. drafted the manuscript. H.Y.L., X.S.L., and Y.T. revised the manuscript. All authors read and approved the final version of the manuscript.
Funding Statement
This work was supported by the National Natural Science Foundation of China (51425502)
References
- 1.Adamson AW, Gast AP. 1997. Physical Chemistry of Surfaces. 6th ed. New York: John Wiley & Sons, Inc..
- 2.Andersen NM, Polhemus JT. 1976. Water-striders (Hemiptera: Gerridae, Veliidae, etc.). In: Cheng L. Marine Insects. Amsterdam: North-Holland Publ. Co, 187–224.
- 3.Andersen NM Cladistic inference and evolutionary scenarios: Locomotory structure, function, and performance in water striders. Cladistics. 1995;11(3):279–295. doi: 10.1016/0748-3007(95)90016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armisén D, Nagui Refki P, Crumière AJJ, Viala S, Toubiana W, Khila A Predator strike shapes antipredator phenotype through new genetic interactions in water striders. Nature Communications. 2015;6:8153. doi: 10.1038/ncomms9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armisén D, Rajakumar R, Friedrich M, Benoit JB, Robertson HM, Panfilio KA, Ahn SJ, Poelchau MF, Chao H, Dinh H, Doddapaneni HV, Dugan S, Gibbs RA, Hughes DST, Han Y, Lee SL, Murali SC, Muzny DM, Qu JX, Worley KC, Munoz-Torres M, Abouheif E, Bonneton F, Chen T, Chiang LM, Childers CP, Cridge AG, Crumière AJJ, Decaras A, Didion EM, Duncan EJ, Elpidina EN, Favé MJ, Finet C, Jacobs CGC, Cheatle Jarvela AM, Jennings EC, Jones JW, Lesoway MP, Lovegrove MR, Martynov A, Oppert B, Lillico-Ouachour A, Rajakumar A, Refki PN, Rosendale AJ, Santos ME, Toubiana W, van der Zee M, Vargas Jentzsch IM, Lowman AV, Viala S, Richards S, Khila A The genome of the water strider Gerris buenoi reveals expansions of gene repertoires associated with adaptations to life on the water . BMC Genomics. 2018;19:832. doi: 10.1186/s12864-018-5163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai F, Wu JT, Gong GM, Guo L Biomimetic “water strider leg” with highly refined nanogroove structure and remarkable water-repellent performance. ACS Applied Materials & Interfaces. 2014;6(18):16237–16242. doi: 10.1021/am5044054. [DOI] [PubMed] [Google Scholar]
- 7.Bowdan E Walking and rowing in the water strider, Gerris remigis I. A cinematographic analysis of walking . Journal of Comparative Physiology. 1978;123(1):43–49. doi: 10.1007/BF00657342. [DOI] [Google Scholar]
- 8.Bühler O Impulsive fluid forcing and water strider locomotion. Journal of Fluid Mechanics. 2007;573:211–236. doi: 10.1017/S002211200600379X. [DOI] [Google Scholar]
- 9.Bush JWM, Hu DL Walking on water: Biolocomotion at the interface. Annual Review of Fluid Mechanics. 2006;38(1):339–369. doi: 10.1146/annurev.fluid.38.050304.092157. [DOI] [Google Scholar]
- 10.Bush JWM, Hu DL, Prakash M. 2007. The integument of water-walking arthropods: form and function. Advances in Insect Physiology, 34(147): 117–192.
- 11.Caponigro MA, Eriksen CH Surface film locomotion by the water strider, Gerris remigis say . The American Midland Naturalist. 1976;95(2):268–278. doi: 10.2307/2424392. [DOI] [Google Scholar]
- 12.Cassie ABD, Baxter S Wettability of porous surfaces. Transactions of the Faraday Society. 1944;40:546–551. doi: 10.1039/tf9444000546. [DOI] [Google Scholar]
- 13.Cheng YT, Rodak DE Is the lotus leaf superhydrophobic? Applied Physics Letters. 2005;86(14):144101. doi: 10.1063/1.1895487. [DOI] [Google Scholar]
- 14.Choi CH, Ulmanella U, Kim J, Ho CM, Kim CJ Effective slip and friction reduction in nanograted superhydrophobic microchannels. Physics of Fluids. 2006;18(8):087105. doi: 10.1063/1.2337669. [DOI] [Google Scholar]
- 15.Closa F, Chepelianskii AD, Raphaël E Capillary-gravity waves generated by a sudden object motion. Physics of Fluids. 2010;22(5):052107. doi: 10.1063/1.3430004. [DOI] [Google Scholar]
- 16.Cranston FP, Sprague IB A morphological study of the head capsule of Gerris remigis say . Journal of Morphology. 1961;108(3):287–309. doi: 10.1002/jmor.1051080303. [DOI] [Google Scholar]
- 17.Crumière AJJ, Santos ME, Sémon M, Armisén D, Moreira FFF, Khila A Diversity in morphology and locomotory behavior is associated with niche expansion in the semi-aquatic bugs. Current Biology. 2016;26(24):3336–3342. doi: 10.1016/j.cub.2016.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De La Torre Bueno JR The Gerrids of the Atlantic States (Subfamily Gerrinæ) Transactions of the American Entomological Society. 1911;37(3):243–252. [Google Scholar]
- 19.Denny MW. 1993. Air and Water: the Biology and Physics of Life’s Media. Princeton, N.J.: Princeton University Press.
- 20.Dickinson M How to walk on water. Nature. 2003;424(6949):621–622. doi: 10.1038/424621a. [DOI] [PubMed] [Google Scholar]
- 21.Fairbairn DJ A test of the hypothesis of compensatory upstream dispersal using a stream-dwelling waterstrider, Gerris remigis say . Oecologia. 1985;66(1):147–153. doi: 10.1007/BF00378567. [DOI] [PubMed] [Google Scholar]
- 22.Fairbairn DJ, Brassard J Dispersion and spatial orientation of Gerris remigis in response to water current: a comparison of pre- and post-diapause adults . Physiological Entomology. 1988;13(2):153–164. doi: 10.1111/j.1365-3032.1988.tb00919.x. [DOI] [Google Scholar]
- 23.Feng XQ, Gao XF, Wu ZN, Jiang L, Zheng QS Superior water repellency of water strider legs with hierarchical structures: experiments and analysis. Langmuir. 2007;23(9):4892–4896. doi: 10.1021/la063039b. [DOI] [PubMed] [Google Scholar]
- 24.Finet C, Decaras A, Armisén D, Khila A The achaete–scute complex contains a single gene that controls bristle development in the semi-aquatic bugs. Proceedings of the Royal Society B: Biological Sciences. 2018;285(1892):20182387. doi: 10.1098/rspb.2018.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao P, Feng JJ A numerical investigation of the propulsion of water walkers. Journal of Fluid Mechanics. 2011;668:363–383. doi: 10.1017/S0022112010004763. [DOI] [Google Scholar]
- 26.Gao XF, Jiang L Water-repellent legs of water striders. Nature. 2004;432(7013):36. doi: 10.1038/432036a. [DOI] [PubMed] [Google Scholar]
- 27.Hinton HE Plastron respiration in bugs and beetles. Journal of Insect Physiology. 1976;22(11):1529–1550. doi: 10.1016/0022-1910(76)90221-3. [DOI] [Google Scholar]
- 28.Holdgate MW The wetting of insect cuticles by water. Journal of Experimental Biology. 1955;32:591–617. [Google Scholar]
- 29.Hu DL, Chan B, Bush JWM The hydrodynamics of water strider locomotion. Nature. 2003;424(6949):663–666. doi: 10.1038/nature01793. [DOI] [PubMed] [Google Scholar]
- 30.Hu DL, Bush JWM The hydrodynamics of water-walking arthropods. Journal of Fluid Mechanics. 2010;644:5–33. doi: 10.1017/S0022112009992205. [DOI] [Google Scholar]
- 31.Jabloński PG, Wilcox RS Signalling asymmetry in the communication of the water strider Aquarius remigis in the context of dominance and spacing in the non-mating season . Ethology. 1996;102(3):353–359. [Google Scholar]
- 32.Ji XY, Wang JW, Feng XQ Role of flexibility in the water repellency of water strider legs: Theory and experiment. Physical Review E. 2012;85(2):021607. doi: 10.1103/PhysRevE.85.021607. [DOI] [PubMed] [Google Scholar]
- 33.Junger W, Varjú D Drift compensation and its sensory basis in waterstriders (Gerris paludum F.) . Journal of Comparative Physiology A. 1990;167(3):441–446. [Google Scholar]
- 34.Keller JB Surface tension force on a partly submerged body. Physics of Fluids. 1998;10(11):3009–3010. doi: 10.1063/1.869820. [DOI] [Google Scholar]
- 35.Khila A, Abouheif E, Rowe L Evolution of a novel appendage ground plan in water striders is driven by changes in the Hox Gene Ultrabithorax . PLoS Genetics. 2009;5(7):e1000583. doi: 10.1371/journal.pgen.1000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khila A, Abouheif E, Rowe L Comparative functional analyses of ultrabithorax reveal multiple steps and paths to diversification of legs in the adaptive radiation of semi-aquatic insects. Evolution. 2014;68(8):2159–2170. doi: 10.1111/evo.12444. [DOI] [PubMed] [Google Scholar]
- 37.Koh JS, Yang E, Jung GP, Jung SP, Son JH, Lee SI, Jablonski PG, Wood RJ, Kim HY, Cho KJ Jumping on water: Surface tension-dominated jumping of water striders and robotic insects. Science. 2015;349(6247):517–521. doi: 10.1126/science.aab1637. [DOI] [PubMed] [Google Scholar]
- 38.Kovac D, Maschwitz U Secretion-grooming in the water bug Plea minutissima: a chemical defence against microorganisms interfering with the hydrofuge properties of the respiratory region . Ecological Entomology. 1989;14(4):403–411. doi: 10.1111/j.1365-2311.1989.tb00942.x. [DOI] [Google Scholar]
- 39.Kovac D, Maschwitz U Secretion-grooming in aquatic beetles (Hydradephaga): A chemical protection against contamination of the hydrofuge respiratory region. Chemoecology. 1990;1(3–4):131–138. [Google Scholar]
- 40.Kovac D A quantitative analysis of secretion-grooming behaviour in the water bug Plea minutissima leach (Heteroptera, Pleidae): Control by abiotic factors. Ethology. 1993;93(1):41–61. [Google Scholar]
- 41.Kwak B, Bae J Locomotion of arthropods in aquatic environment and their applications in robotics. Bioinspiration & Biomimetics. 2018;13(4):041002. doi: 10.1088/1748-3190/aab460. [DOI] [PubMed] [Google Scholar]
- 42.Lafuma A, Quéré D Superhydrophobic states. Nature Materials. 2003;2(7):457–460. doi: 10.1038/nmat924. [DOI] [PubMed] [Google Scholar]
- 43.Lawry JV Jr A scanning electron microscopic study of mechanoreceptors in the walking legs of the water strider, Gerris remigis . Journal of Anatomy. 1973;116:25–30. [PMC free article] [PubMed] [Google Scholar]
- 44.Lee DG, Kim HY The role of superhydrophobicity in the adhesion of a floating cylinder. Journal of Fluid Mechanics. 2009;624:23–32. doi: 10.1017/S002211200900593X. [DOI] [Google Scholar]
- 45.Liu JL, Feng XQ, Wang GF Buoyant force and sinking conditions of a hydrophobic thin rod floating on water. Physical Review E. 2007;76:066103. doi: 10.1103/PhysRevE.76.066103. [DOI] [PubMed] [Google Scholar]
- 46.Lu HY, Zheng YL, Yin W, Tao DS, Pesika N, Meng YG, Tian Y Propulsion principles of water striders in sculling forward through shadow method. Journal of Bionic Engineering. 2018;15(3):516–525. doi: 10.1007/s42235-018-0042-8. [DOI] [Google Scholar]
- 47.Moisy F, Rabaud M, Salsac K A synthetic Schlieren method for the measurement of the topography of a liquid interface. Experiments in Fluids. 2009;46(6):1021–1036. doi: 10.1007/s00348-008-0608-z. [DOI] [Google Scholar]
- 48.Murphey RK Sensory aspects of the control of orientation to prey by the waterstrider, Gerris remigis . Zeitschrift für Vergleichende Physiologie. 1971a;72(2):168–185. doi: 10.1007/BF00297820. [DOI] [Google Scholar]
- 49.Murphey RK Motor control of orientation to prey by the waterstrider, Gerris remigis . Zeitschrift für Vergleichende Physiologie. 1971b;72(2):150–167. doi: 10.1007/BF00297819. [DOI] [Google Scholar]
- 50.Ortega-Jimenez VM, von Rabenau L, Dudley R Escape jumping by three age-classes of water striders from smooth, wavy and bubbling water surfaces. The Journal of Experimental Biology. 2017;220(15):2809–2815. doi: 10.1242/jeb.157172. [DOI] [PubMed] [Google Scholar]
- 51.Patankar NA Transition between Superhydrophobic states on rough surfaces. Langmuir. 2004;20:7097–7102. doi: 10.1021/la049329e. [DOI] [PubMed] [Google Scholar]
- 52.Perez Goodwyn PJ, Fujisaki K Sexual conflicts, loss of flight, and fitness gains in locomotion of polymorphic water striders. Entomologia Experimentalis et Applicata. 2007;124(3):249–259. doi: 10.1111/j.1570-7458.2007.00571.x. [DOI] [Google Scholar]
- 53.Perez Goodwyn PJ, Wang JT, Wang ZJ, Ji AH, Dai ZD, Fujisaki K Water striders: The biomechanics of water locomotion and functional morphology of the hydrophobic surface (Insecta: Hemiptera-Heteroptera) Journal of Bionic Engineering. 2008a;5(2):121–126. doi: 10.1016/S1672-6529(08)60015-3. [DOI] [Google Scholar]
- 54.Perez Goodwyn PJ, Voigt D, Fujisaki K Skating and diving: Changes in functional morphology of the setal and microtrichial cover during ontogenesis in Aquarius paludum fabricius (Heteroptera, Gerridae) . Journal of Morphology. 2008b;269(6):734–744. doi: 10.1002/jmor.10619. [DOI] [PubMed] [Google Scholar]
- 55.Perez Goodwyn P, Katsumata-Wada A, Okada K Morphology and neurophysiology of tarsal vibration receptors in the water strider Aquarius paludum (Heteroptera: Gerridae) . Journal of Insect Physiology. 2009a;55(9):855–861. doi: 10.1016/j.jinsphys.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Perez Goodwyn P, Maezono Y, Takamatsu H, Fujisaki K Semiaquatic Heteroptera locomotion: coral treaders (Hermatobates weddi, Hermatobatidae), sea skaters (Halovelia septentrionalis, Veliidae), and water striders (Metrocoris histrio, Gerridae) Usual and unusualgaits . Hydrobiologia. 2009b;630(1):219–229. doi: 10.1007/s10750-009-9794-9. [DOI] [Google Scholar]
- 57.Prakash M, Bush JWM Interfacial propulsion by directional adhesion. International Journal of Non-Linear Mechanics. 2011;46(4):607–615. doi: 10.1016/j.ijnonlinmec.2010.12.003. [DOI] [Google Scholar]
- 58.Rebora M, Salerno G, Piersanti S, Michels J, Gorb S Structure and biomechanics of the antennal grooming mechanism in the southern green stink bug Nezara viridula . Journal of Insect Physiology. 2019;112:57–67. doi: 10.1016/j.jinsphys.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Refki PN, Armisén D, Crumière AJJ, Viala S, Khila A Emergence of tissue sensitivity to Hox protein levels underlies the evolution of an adaptive morphological trait. Developmental Biology. 2014;392(2):441–453. doi: 10.1016/j.ydbio.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Refki PN, Khila A Key patterning genes contribute to leg elongation in water striders. EvoDevo. 2015;6(1):14. doi: 10.1186/s13227-015-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rinoshika A Vortical dynamics in the wake of water strider locomotion. Journal of Visualization. 2012;15(2):145–153. doi: 10.1007/s12650-011-0117-7. [DOI] [Google Scholar]
- 62.Roh C, Gharib M Honeybees use their wings for water surface locomotion. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(49):24446–24451. doi: 10.1073/pnas.1908857116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rubenstein DI Resource acquisition and alternative mating strategies in water striders. American Zoologist. 1984;24(2):345–353. doi: 10.1093/icb/24.2.345. [DOI] [Google Scholar]
- 64.Santos ME, Berger CS, Refki PN, Khila A Integrating evo-devo with ecology for a better understanding of phenotypic evolution. Briefings in Functional Genomics. 2015;14(6):384–395. doi: 10.1093/bfgp/elv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santos ME, Le Bouquin A, Crumière AJJ, Khila A Taxon-restricted genes at the origin of a novel trait allowing access to a new environment. Science. 2017;358(6361):386–390. doi: 10.1126/science.aan2748. [DOI] [PubMed] [Google Scholar]
- 66.Shi F, Niu J, Liu J, Liu F, Wang Z, Feng XQ, Zhang X Towards understanding why a superhydrophobic coating is needed by water striders. Advanced Materials. 2007;19(17):2257–2261. doi: 10.1002/adma.200700752. [DOI] [Google Scholar]
- 67.Song YS, Suhr SH, Sitti M. 2006. Modeling of the supporting legs for designing biomimetic water strider robots. In: Proceedings of the IEEE International Conference on Robotics and Automation. Orlando, Florida, USA: IEEE, 2303–2310.
- 68.Spence JR, Spence DH, Scudder GGE Submergence behavior in Gerris: Underwater basking. American Midland Naturalist. 1980;103(2):385–391. doi: 10.2307/2424638. [DOI] [Google Scholar]
- 69.Steinmann T, Arutkin M, Cochard P, Raphaël E, Casas J, Benzaquen M Unsteady wave pattern generation by water striders. Journal of Fluid Mechanics. 2018;848:370–387. doi: 10.1017/jfm.2018.365. [DOI] [Google Scholar]
- 70.Stys P, Kerzhner I The rank and nomenclature of higher taxa in recent Heteroptera. Acta Entomologica Bohemoslovaca. 1975;72:65–79. [Google Scholar]
- 71.Su YW, Ji BH, Huang YG, Hwang KC Nature’s design of hierarchical superhydrophobic surfaces of a water strider for low adhesion and low-energy dissipation. Langmuir. 2010;26(24):18926–18937. doi: 10.1021/la103442b. [DOI] [PubMed] [Google Scholar]
- 72.Sun PY, Zhao MR, Jiang JL, Zheng YL The study of dynamic force acted on water strider leg departing from water surface. AIP Advances. 2018;8(1):015228. doi: 10.1063/1.5012578. [DOI] [Google Scholar]
- 73.Sun SM, Keller JB Capillary-gravity wave drag. Physics of Fluids. 2001;13(8):2146–2151. doi: 10.1063/1.1384889. [DOI] [Google Scholar]
- 74.Thorpe WH, Crisp DJ Studies on plastron respiration: I. The biology of Aphelocheirus [hemiptera, Aphelocheiridae (naucoridae)] and the mechanism of plastron retention. Journal of Experimental Biology. 1947;24 doi: 10.1242/jeb.24.3-4.227. [DOI] [PubMed] [Google Scholar]
- 75.Thorpe WH, Crisp DJ Studies on plastron respiration. Part IV. Plastron respiration in the Coleoptera. Journal of Experimental Biology. 1949;26:219–260. doi: 10.1242/jeb.26.3.219. [DOI] [PubMed] [Google Scholar]
- 76.Thorpe WH Plastron respiration in aquatic insects. Biological Reviews. 1950;25(3):344–390. doi: 10.1111/j.1469-185X.1950.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 77.Uesugi K, Mayama H, Morishima K. 2017. Direct measurement of propelling force of water strider. In: Proceedings of International Symposium on Micro-NanoMechatronics and Human Science (MHS). Nagoya: IEEE, 1–5.
- 78.Vargas-Lowman A, Armisen D, Burguez Floriano CF, da Rocha Silva Cordeiro I, Viala S, Bouchet M, Bernard M, Le Bouquin A, Santos ME, Berlioz-Barbier A, Salvador A, Figueiredo Moreira FF, Bonneton F, Khila A Cooption of the pteridine biosynthesis pathway underlies the diversification of embryonic colors in water striders. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(38):19046–19054. doi: 10.1073/pnas.1908316116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vella D, Lee DG, Kim HY The load supported by small floating objects. Langmuir. 2006;22(14):5979–5981. doi: 10.1021/la060606m. [DOI] [PubMed] [Google Scholar]
- 80.Vella D Floating objects with finite resistance to bending. Langmuir. 2008;24(16):8701–8706. doi: 10.1021/la800245k. [DOI] [PubMed] [Google Scholar]
- 81.Vinnichenko NA, Plaksina YY, Baranova KM, Pushtaev AV, Uvarov AV Mobility of free surface in different liquids and its influence on water striders locomotion. Environmental Fluid Mechanics. 2018;18(5):1045–1056. doi: 10.1007/s10652-018-9577-9. [DOI] [Google Scholar]
- 82.Wang QB, Yao X, Liu H, Quéré D, Jiang L Self-removal of condensed water on the legs of water striders. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(30):9247–9252. doi: 10.1073/pnas.1506874112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang S, Jiang L Definition of superhydrophobic states. Advanced Materials. 2007;19(21):3423–3424. doi: 10.1002/adma.200700934. [DOI] [Google Scholar]
- 84.Watson GS, Cribb BW, Watson JA Experimental determination of the efficiency of nanostructuring on non-wetting legs of the water strider. Acta Biomaterialia. 2010;6(10):4060–4064. doi: 10.1016/j.actbio.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 85.Wei PJ, Chen SC, Lin JF Adhesion forces and contact angles of water strider legs. Langmuir. 2009a;25(3):1526–1528. doi: 10.1021/la803223r. [DOI] [PubMed] [Google Scholar]
- 86.Wei PJ, Shen YX, Lin JF Characteristics of water strider legs in hydrodynamic situations. Langmuir. 2009b;25(12):7006–7009. doi: 10.1021/la900185a. [DOI] [PubMed] [Google Scholar]
- 87.Wenzel RN Resistance of solid surfaces to wetting by water. Industrial & Engineering Chemistry. 1936;28(8):988–994. [Google Scholar]
- 88.Wier KA, McCarthy TJ Condensation on ultrahydrophobic surfaces and its effect on droplet mobility: Ultrahydrophobic surfaces are not always water repellant. Langmuir. 2006;22(6):2433–2436. doi: 10.1021/la0525877. [DOI] [PubMed] [Google Scholar]
- 89.Wilcox RS Sex discrimination in Gerris remigis: Role of a surface wave signal . Science. 1979;206(4424):1325–1327. doi: 10.1126/science.206.4424.1325. [DOI] [PubMed] [Google Scholar]
- 90.Wilcox RS, Ruckdeschel T Food threshold territoriality in a water strider (Gerris remigis) . Behavioral Ecology and Sociobiology. 1982;11(2):85–90. doi: 10.1007/BF00300096. [DOI] [Google Scholar]
- 91.Wilcox RS, Di Stefano J Vibratory signals enhance mate-guarding in a water strider (Hemiptera: Gerridae) Journal of Insect Behavior. 1991;4(1):43–50. doi: 10.1007/BF01092550. [DOI] [Google Scholar]
- 92.Xu L, Yao X, Zheng YM Direction-dependent adhesion of water strider’s legs for water-walking. Solid State Sciences. 2012;14(8):1146–1151. doi: 10.1016/j.solidstatesciences.2012.05.029. [DOI] [Google Scholar]
- 93.Xue YH, Yuan HJ, Su WD, Shi YP, Duan HL Enhanced load-carrying capacity of hairy surfaces floating on water. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2014;470(2165):20130832. doi: 10.1098/rspa.2013.0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang E, Son JH, Lee S, Jablonski PG, Kim HY Water striders adjust leg movement speed to optimize takeoff velocity for their morphology. Nature Communications. 2016;7(1):13698. doi: 10.1038/ncomms13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yin W, Zheng YL, Lu HY, Zhang XJ, Tian Y Three-dimensional topographies of water surface dimples formed by superhydrophobic water strider legs. Applied Physics Letters. 2016;109(16):163701. doi: 10.1063/1.4964788. [DOI] [Google Scholar]
- 96.Zheng QS, Yu Y, Zhao ZH Effects of hydraulic pressure on the stability and transition of wetting modes of superhydrophobic surfaces. Langmuir. 2005;21(26):12207–12212. doi: 10.1021/la052054y. [DOI] [PubMed] [Google Scholar]
- 97.Zheng QS, Yu Y, Feng XQ The role of adaptive-deformation of water strider leg in its walking on water. Journal of Adhesion Science and Technology. 2009;23(3):493–501. doi: 10.1163/156856108X379155. [DOI] [Google Scholar]
- 98.Zheng YL, Lu HY, Yin W, Tao DS, Shi LC, Tian Y Elegant shadow making tiny force visible for water-walking arthropods and updated Archimedes’ principle. Langmuir. 2016;32(41):10522–10528. doi: 10.1021/acs.langmuir.6b02922. [DOI] [PubMed] [Google Scholar]
- 99.Zhukovskaya M, Yanagawa A, Forschler B Grooming behavior as a mechanism of insect disease defense. Insects. 2013;4(4):609–630. doi: 10.3390/insects4040609. [DOI] [PMC free article] [PubMed] [Google Scholar]