Abstract
Adult male tree shrews vigorously defend against intruding male conspecifics. However, the characteristics of social behavior have not been entirely explored in these males. In this study, male wild-type tree shrews (Tupaia belangeri chinensis) and C57BL/6J mice were first allowed to familiarize themselves with an open-field apparatus. The tree shrews exhibited a short duration of movement (moving) in the novel environment, whereas the mice exhibited a long duration of movement. In the 30 min social preference-avoidance test, target animals significantly decreased the time spent by the experimental tree shrews in the social interaction (SI) zone, whereas experimental male mice exhibited the opposite. In addition, experimental tree shrews displayed a significantly longer latency to enter the SI zone in the second 15 min session (target-present) than in the first 15 min session (target-absent), which was different from that found in mice. Distinct behavioral patterns in response to a conspecific male were also observed in male tree shrews and mice in the first, second, and third 5 min periods. Thus, social behaviors in tree shrews and mice appeared to be time dependent. In summary, our study provides results of a modified social preference-avoidance test designed for the assessment of social behavior in tree shrews. Our findings demonstrate the existence of social avoidance behavior in male tree shrews and prosocial behavior in male mice toward unfamiliar conspecifics. The tree shrew may be a new animal model, which differs from mice, for the study of social avoidance and prosocial behaviors.
Keywords: Social avoidance behavior, Prosocial behavior, Open-field test, Social preference-avoidance test, Social interaction
INTRODUCTION
Social behavior has been widely studied in animals such as fish (Glass et al., 2003), birds (Yoshida et al., 2006), bats (Moga & Moore, 1997), mice (Deboer et al., 2003), rats (Buijs et al., 2019), and monkeys (Hery et al., 1981). In the wild, territorial animals defend their territories against strangers and neighbors (intruders) (Fuchs et al., 1995; Monclús et al., 2014). If this occurs in the laboratory, social behavior is elicited by and directed toward an intruding conspecific (Pryce & Fuchs, 2017; Wang et al., 2013). The patterns of social behaviors in laboratory rodents include social avoidance, approaching, sniffing, genital grooming, following, aggressive chasing, and attacking (Arakawa et al., 2014; Houwing et al., 2019; Toth & Neumann, 2013). Social avoidance and approach behaviors in the laboratory have been widely measured in rodents (Golden et al., 2011; Lukas et al., 2011; Raymond et al., 2019; Toth & Neumann, 2013). Disturbances in social avoidance and approach behaviors can be social dysfunction symptoms of social phobia, autism, depression, schizophrenia, and other neuropsychiatric disorders (Brodkin et al., 2004; Li et al., 2019; Pfaff & Barbas, 2019). Various behavioral tests are used to assess social avoidance and approach behaviors of animals in the laboratory (Bauman et al., 2013; Goodson et al., 2004; Green et al., 2012; Toth & Neumann, 2013). Social behavior is defined here as behaviors expressed toward an intruding conspecific male enclosed in a Plexiglas cylinder (Kaidanovich-Beilin et al., 2011; Lopes, 2014).
As the closest living relatives of primates, tree shrews (Tupaia belangeri chinensis) have been used to establish social defeat models of depression (Fuchs, 2005; Wang et al., 2013). In the wild, tree shrews live singly or in pairs in territories they defend against male intruders (Fuchs et al., 1995). Under laboratory conditions, defensive behavior may involve a brief fighting episode to establish social hierarchy (Wang et al., 2013). The social-defeat stress model in tree-shrews has been studied since 1976 (Holst, 1977; Raab & Storz, 1976), with the chronic psychosocial stress paradigm in male tree shrews also widely used to detect behavioral, endocrinological, immunological, and neurobiological changes in response to psychosocial conflicts (Fischer et al., 1985; Fuchs & Schumacher, 1990; Fuchs et al., 1993; Magariños et al., 1996; Taugner et al., 1980; Wang et al., 2013). Several behavioral tests have been developed to examine social behavior in male tree shrews (Meng et al., 2016; Parésys et al., 2016). Social-defeat stress models in adult male mice and tree-shrews have been widely used in studies of human stress-related disorders (Berton et al., 2006; Pryce & Fuchs, 2017; Wang et al., 2013). However, characteristics of social avoidance and approach behaviors in the laboratory have not been entirely explored in male wild-type tree shrews. In addition, differences in the patterns of social behavior between male tree shrews and mice remain unknown.
In addition to ethical concerns and restrictions, monkey research is costly and time consuming (Yao, 2017). Compared with monkeys, tree shrews are a good model species because they are day-active animals that are small in size (26–40 cm and 110–180 g) and easy to handle and feed, they have a relatively short gestation period (41–55 days), length of puberty (2–6 months), and life span (over 7 years), and are closely related to primates (Zheng et al., 2014). According to molecular phylogenetic studies, tree shrews are more closely related to humans than to mice and rats evolutionarily (Fan et al., 2013, 2019; Xiao et al., 2017). Tree shrews are a good choice as animal models in medical research (Huang et al., 2020; Ni et al., 2018, 2020; Yao, 2017; Zheng et al., 2014). In this present study, we aimed to assess social avoidance and approach behaviors in wild-type tree shrews and mice. In addition, we examined differences in the patterns of social behavior in response to the presence (or absence) of a conspecific male between male wild-type tree shrews and mice.
MATERIAL AND METHODS
Animals
All adult male tree shrews were obtained from a breeding colony at the Animal House Center of the Kunming Institute of Zoology, China. Wild-type tree shrews and C57BL/6J mice (obtained from Chengdu Dossy Experimental Animals, China) were housed in animal facilities (temperature: 24±2 °C; relative humidity: 55%–70%; light intensity 900 lux; sound: <50 dB) under a 12 h light/dark cycle (lights on at 0800h) with ad libitum access to commercial pellet feed and water. All animals were individually housed before and during the experiments. All tree shrews were age- and gender-matched wild-type animals, as were mice. The experiment contexts and procedures in the behavioral tests in the laboratory were almost the same. Experimental animals were defined as any animal bred and reared according to relevant standards and intended to be used in experiments or for other scientific purposes (biomedical research, testing, and teaching) (Basu et al., 1993; Guillen, 2017; Ogden et al., 2016). Here, experimental tree shrews and mice were placed into an open-field box and tracked using EthoVisionTM tracking software (Noldus, Netherlands) in both open-field and social preference-avoidance tests. All animal procedures were performed in accordance with the Institutional Animal Care and Use Committee of Sichuan University (Approval No.: 2018134A). The guidelines used in this experiment were also in accordance with EU standards. According to 3Rs, all efforts were made to minimize animal suffering as well as the number of animals used. All experimental procedures were in compliance with ARRIVE guidelines. Throughout the study, animals were kept and treated according to the guidelines for the care and use of animals in behavioral research as described in Animal Behaviour (Guidelines for the Treatment of Animals in Behavioural Research and Teaching, 2001).
Handling and Habituation
After four weeks of habituation in the laboratory, male tree shrews (n=19, 10 months old) and mice (n=23, 3 months old) were transferred to new cages in a testing room. When considering the lifespan of tree shrews (>7 years) and mice (2–3 years), the ages of the animals used were different. To minimize non-specific stress responses to manipulation, the tree shrews and mice were handled (5 min daily for 14 d) before the behavioral tests. All behavioral tests on tree shrews and mice were performed during the light phase of the cycle similar to the housing room. Before behavioral tests, the tree shrews and mice were given 30 min for adaptation to the testing environment in a new room (light intensity 900 lux; sound: <50 dB). Because tree shrews are susceptible to stress (Fang et al., 2016), they were each transported/carried in their sleeping boxes throughout the behavioral tests to reduce impact. The sleeping boxes (covered and dark) were located outside the cages and were closed with a slider (Supplementary Figure S1). The tree shrews could return to their sleeping box for nocturnal deep sleep. In addition, this sleeping box could be removed from the cages, which made it possible to obtain the tree shrews without any delay for capture.
Open-field test for tree shrews
The open-field test for tree shrews was modified from previous research (Golden et al., 2011; Wang et al., 2013). To detect the spontaneous locomotor behavior of tree shrews, locomotor activities were recorded in the open-field test. Each tree shrew was placed at the end of an open-field box constructed of white Plexiglas (100 cm×40 cm×50 cm) and monitored for 5 min. After each test, the Plexiglas box was cleaned with 75% alcohol solution. During the 5 min, distance and movement in the open-field test were analyzed using the EthoVisionTM tracking software. Total distance and cumulative duration of movement (moving) of tree shrews and mice were measured to detect spontaneous locomotor activity and exploration in the open-field box (Meng et al., 2015).
Open-field test for mice
The open-field test for mice was conducted to evaluate locomotor exploration as described previously (Hefner et al., 2007). The apparatus was a square arena (40 cm×40 cm×35 cm) consisting of a white Plexiglas box. Each mouse was placed in the corner and permitted to explore the apparatus ad libitum for 5 min. After each test, the arena was cleaned with 75% alcohol solution. During the 5 min, distance and movement in the open-field test were analyzed using EthoVisionTM. After familiarization with the open-field apparatus, social avoidance and approach behaviors were measured using the social preference-avoidance test (Toth & Neumann, 2013; Xu et al., 2019).
Social preference-avoidance test for tree shrews
The social preference-avoidance test used for the tree shrews was modified from previous research on mice (Golden et al., 2011). The social preference-avoidance test was assessed using an enclosed Plexiglas box (100 cm×40 cm×50 cm) consisting of a test arena (90 cm×40 cm×50 cm) and target arena (10 cm×40 cm×50 cm). The two arenas were separated by a transparent perforated wall. Each experimental tree shrew was introduced into the test arena and its movement was tracked for two consecutive 15 min sessions. In the first session (no target), there was no naive tree shrew (unfamiliar, species-, age- and gender-matched male tree shrew) in the target arena. In the second session (target), the experimental conditions were identical except that a naive tree shrew was placed in the target arena. Between the two sessions, the experimental tree shrew was removed from the test arena and placed in its home cage for approximately 1 min. After each test, the test box was cleaned with 75% alcohol solution. The two sessions were videotaped and analyzed using EthoVisionTM. The videotaped data recorded under “no target” and “target” conditions were used to determine the activities of the experimental tree shrews in the social interaction (SI) zone (40-cm-wide corridor surrounding target zone; body size in tree shrews: 26–40 cm). Total distance and cumulative duration of movement (moving) of the animals were used to study locomotor activity and exploration in the social preference-avoidance test. In the SI zone, the distance moved, duration of movement (moving time in SI zone), and duration (time spent in SI zone), frequency (number of entrances to SI zone), and latency (time of first entrance into SI zone) were used to evaluate social avoidance and approach behaviors of the animals (Berton et al., 2006; Farrell et al., 2016; Henriques-Alves & Queiroz, 2016). Locomotor activity (total distance and cumulative duration of movement) was assessed throughout the open-field box and SI zone, respectively, to provide an index of general locomotor activity in social contexts when the conspecific target was absent or present. Social preference was defined as a significantly greater investigation time and less latency to enter the SI zone during the second 15 min session (target-present) than during the first 15 min session (target-absent), whereas social avoidance was the opposite.
Social preference-avoidance test for mice
The test box in the social preference-avoidance test was similar to that of the open-field test (40 cm×40 cm×35 cm). The social preference-avoidance test in mice was performed and modified as described previously (Berton et al., 2006; Golden et al., 2011). Briefly, each experimental mouse was placed into the open field and tracked for two consecutive 15 min sessions. During the first session (no target), the open field contained an empty wire mesh cage centered against one wall of the arena. During the second session (target), the experimental conditions were identical except that a social target mouse (unfamiliar C57BL/6J male mouse) was introduced to the wire mesh cage. Between the two sessions, the experimental mouse was removed from the open field and returned to its home cage for approximately 1 min. After each test, the box was cleaned with 75% alcohol solution. The two sessions were videotaped and analyzed using EthoVisionTM. The videotaped data recorded under “no target” and “target” conditions were used to determine the activities of experimental mice in the SI zone (8-cm-wide corridor surrounding target zone; body size in mice: 7–8 cm) (Berton et al., 2006).
Statistical analysis
Statistical analysis was performed using SPSS 22 software (SPSS Inc., USA). All results are expressed as means±standard error of mean (SEM). For normally distributed data, group differences in behavioral measures were tested using one-way analysis of variance (ANOVA), followed by Bonferroni’s post hoc test. Multivariate analysis of covariance followed by Bonferroni’s post hoc test was performed for the distance moved, cumulative duration, time in zone, and frequency in the social preference-avoidance test. Repeated measures ANOVA followed by Bonferroni’s post hoc test was performed for the above parameters in the social preference-avoidance test every 5 min and 1 min. Repeated measures ANOVA followed by Bonferroni’s post hoc test was also used to evaluate the significance of distance moved and cumulative duration in the open-field test every 2.5 min. For data not normally distributed, latency to enter the SI zone was compared with each other using the Wilcoxon signed-rank test or Friedman's test. The effect size was calculated using Cohen’s d. Significance was set at P<0.05.
RESULTS
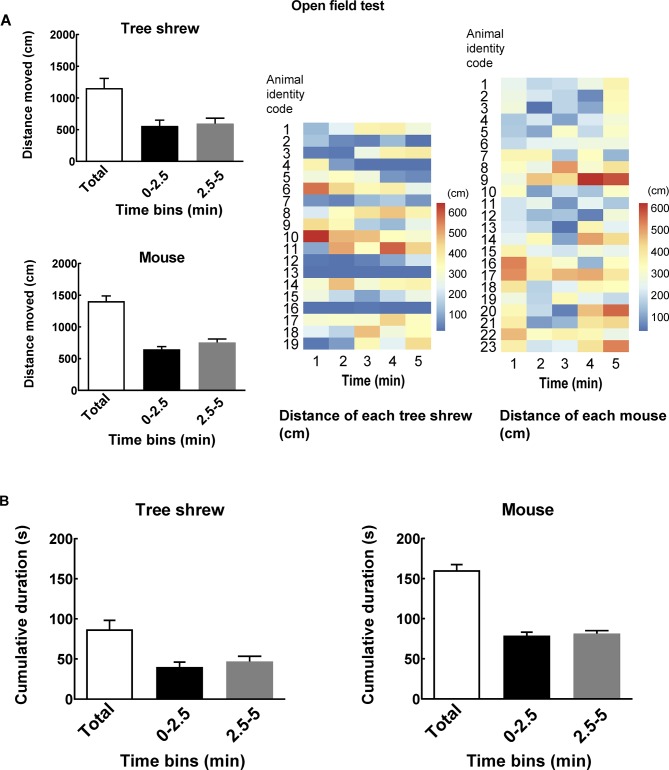
The locomotor activities of wild-type tree shrews and mice were tested for 5 min in the open-field box. Our data showed that the wild-type tree shrews and mice had high locomotor activity in the novel environment (tree shrew: 1 155.04±154.06; mouse: 1 404.53±85.00) (Figure 1A). The heatmaps show locomotor activity expressed as total distance traveled in successive 1 min bins in the open-field test over a 5 min period for each tree shrew and mouse (Figure 1A). The tree shrews exhibited a short duration of movement (moving) in the novel environment (86.95±11.66), whereas the mice exhibited a long duration of movement (160.50±7.34) (Figure 1B). The tree shrews had a higher maximum speed in the open-field test than mice (tree shrew: 165.003±24.974; mouse: 34.023±1.614). However, the tree shrews and mice had similar mean moving speeds in the open-field test (Table 1). After familiarization with the open-field apparatus, the social interaction behaviors of wild-type tree shrews and mice in response to a conspecific male were assessed in the social preference-avoidance test.
1. Total distance and movement provide information about general activity of tree shrews and mice.
A: Total distance moved by tree shrews and mice in box during 5 min open-field test and every 2.5 min (left). Heatmaps show locomotor activity expressed as distance traveled in successive 1 min bins in open-field test over a 5 min period for each tree shrew and mouse (right). B: Total cumulative duration of movement (moving) of tree shrews and mice in open-field box during a 5 min sample period and every 2.5 min. Values are means±SEM (tree shrew: n=19; mouse: n=23).
1. Mean, minimum, and maximum speeds of mice and tree shrews in open-field test (mean±SEM) .
| Minimum (cm/s) | Mean (cm/s) | Maximum (cm/s) | |
| Mouse | 0.014±0.002 | 4.683±0.283 | 34.023±1.614 |
| Tree shrew | 0 | 3.851±0.514 | 165.003±24.974 |
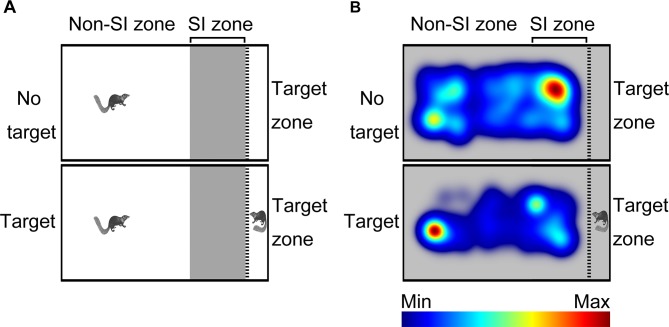
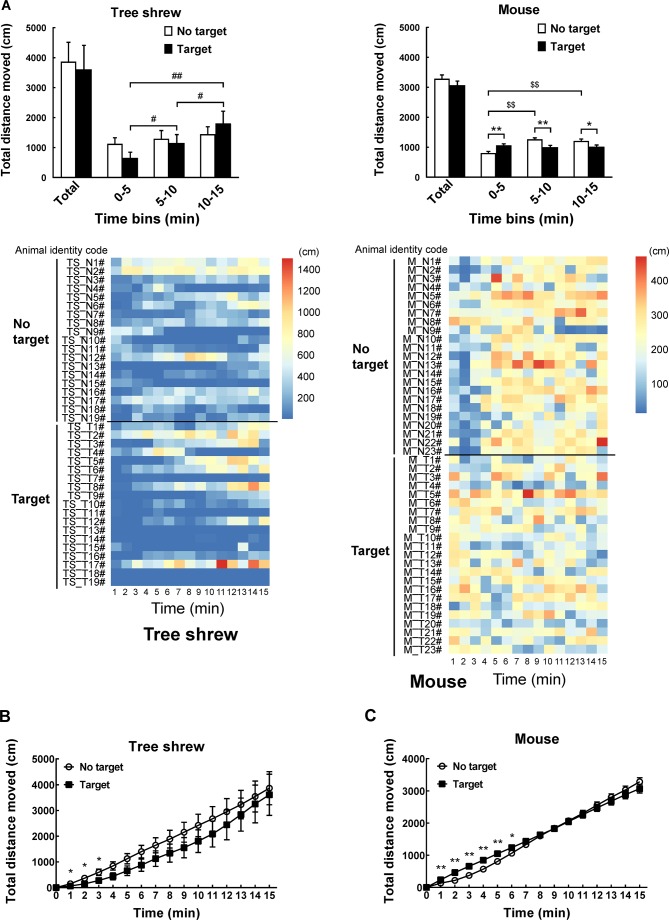
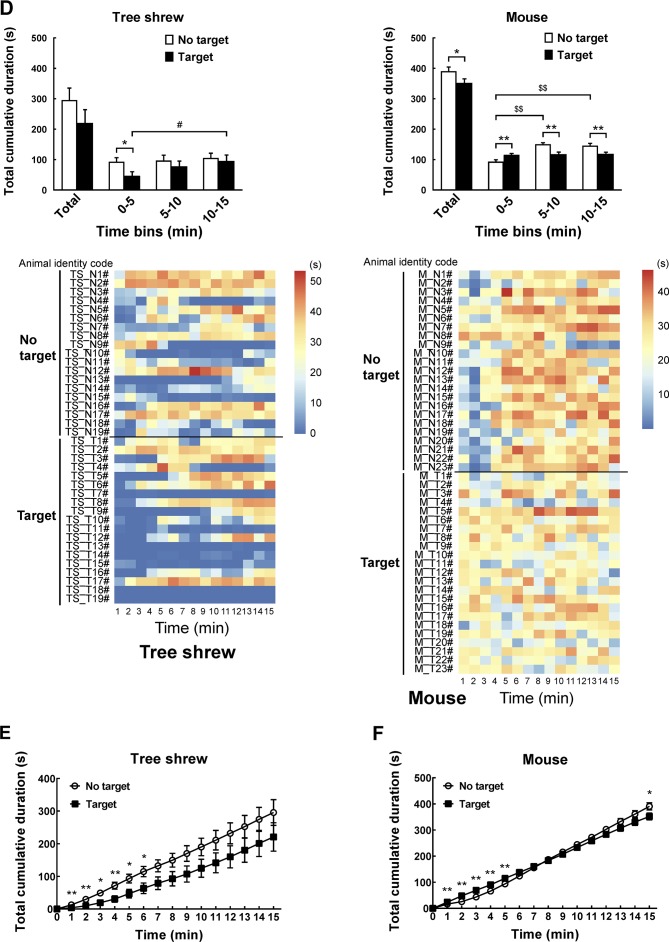
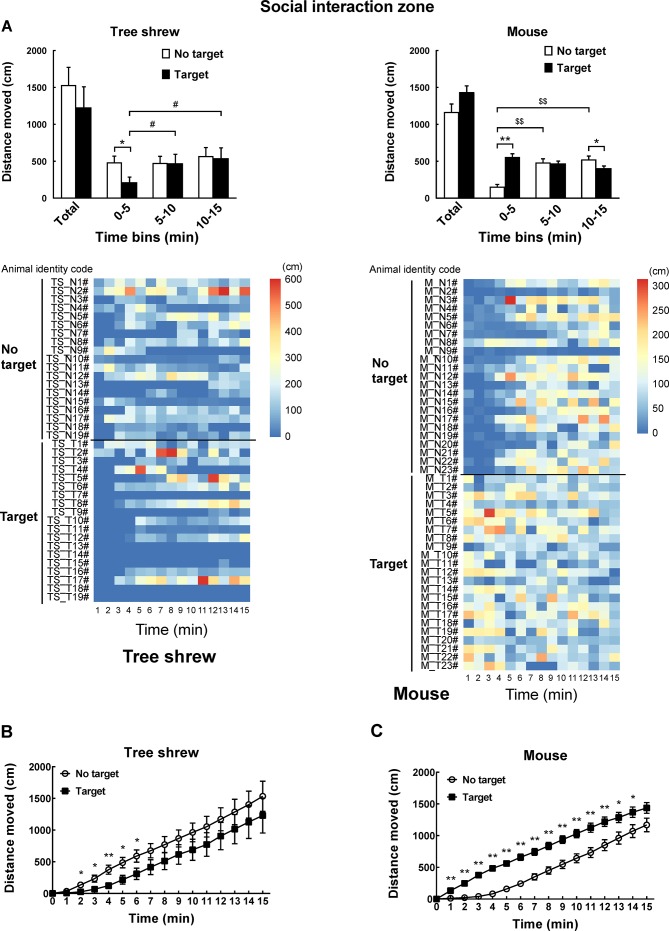
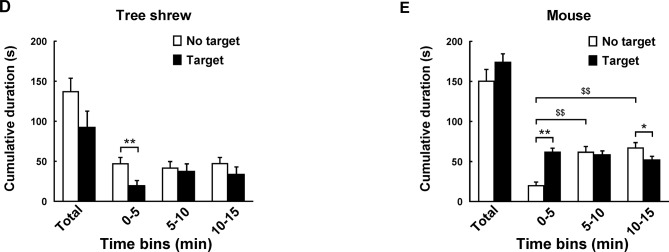
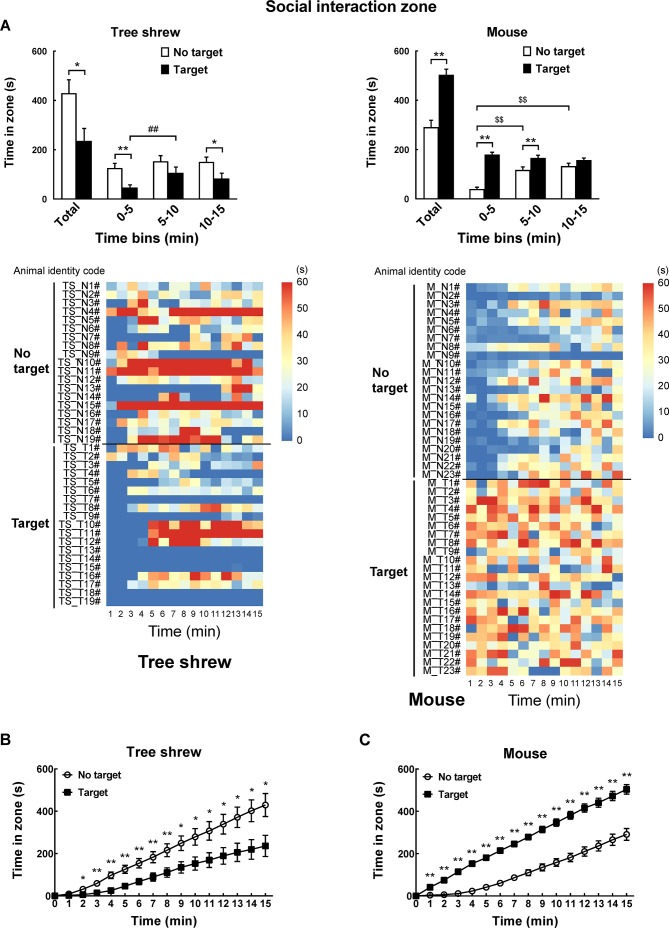
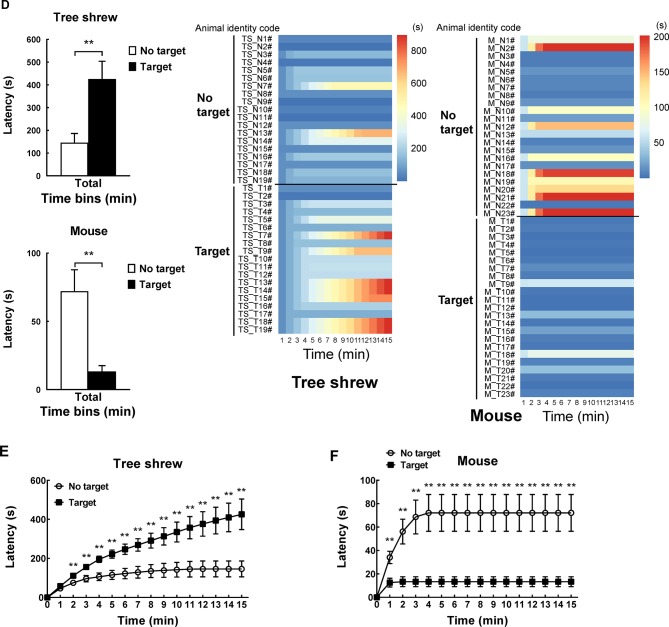
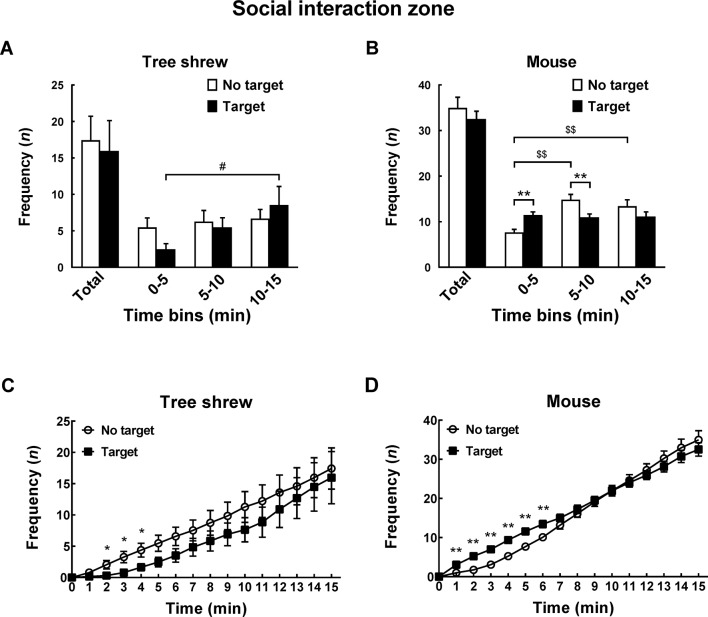
A modified apparatus was used to evaluate the social interaction behaviors of wild-type tree shrews (Figure 2A). Their activities in the social preference-avoidance test were examined when the conspecific target was absent or present during two consecutive sessions (Figure 2B). In the 30 min social preference-avoidance test for tree shrews and mice (first 15 min session: no target; second 15 min session: target-present), there were no significant differences in total distance moved in the compartment between the two consecutive sessions (Figure 3A). However, there were significant differences in total distance moved by the tree shrews in the compartment among target0-5 min (655.64±186.79), target5-10 min (1 151.67±281.08), and target10-15 min (1 802.58±412.06) when the target tree shrews were present (F(2, 35)=7.401, P<0.01;Figure 3A). Significant differences in total distance moved were also found in mice when the target was absent (F(2, 43)=36.269, P<0.01; no target0-5 min (806.32±53.46), no target5-10 min (1 264.22±48.80), and no target10-15 min (1 210.08±65.25); Figure 3A). In addition, there were significant differences in total distance moved by the mice between target-absent and target-present groups in the first, second, and third 5 min periods (Figure 3A). Lower moving distance in mice was found in the first 5 min period and higher moving distance was found in the second and third 5 min periods when the target was absent. Locomotor activity was expressed as total distance traveled and cumulative duration of movement (moving) in successive 1 min bins over the course of the experimental trial for each tree shrew and mouse (Figure 3A, D). In the first 3 min of the 15 min session, tree shrews showed larger moving distance when the target was absent than when the target was present (Figure 3B). Significant differences in total traveling distance were found in mice in the first 6 min of the 15 min session (Figure 3C). Total cumulative duration of movement (moving) in mice significantly decreased when the target animals were present in the compartment during the 30 min sample period (F(1, 44)=4.074, P<0.05; no target (390.58±13.72), target (351.89±13.38);Figure 3D). In accordance with the total distance moved by mice, similar findings were found in total cumulative duration of movement (moving) of mice in the first, second, and third 5 min periods (Figure 3D). Significant differences in total cumulative duration of movement by the tree shrews were found between target-absent and target-present groups in the first 5 min period (no target (93.09±13.23), target (46.90±13.22); Figure 3D). Furthermore, the experimental tree shrews had a lower duration of movement when the target animals were present during the 6 min period (Figure 3E). In the first 5 min of the second 15 min session (target-present), the target animals significantly increased the cumulative duration of movement in mice, whereas the experimental mice (target-present) showed a significant reduction at the end of the session (Figure 3F). There was no significant difference in distance moved in the SI zone by the tree shrews and mice between target-absent and target-present groups during the 30 min test period (Figure 4A). Heatmaps show the locomotor activity expressed as distance moved for each tree shrew and mouse in the SI zone in successive 1 min bins (target-absent and target-present sessions). The experimental tree shrews showed a significant decrease in distance moved in the SI zone in the first 5 min period when the target animals were present (F(2, 35)=3.947, P<0.05; no target (485.92±82.62), target (216.27±69.50)), whereas the experimental mice showed a significant decrease in the distance moved when the target animals were absent (F(2, 43)=43.073, P<0.01; no target (155.93±30.26), target (557.77±43.82);Figure 4A). Experimental tree shrews showed higher motor activity from 2 min to 6 min when the target animals were absent (target-absent vs. target-present; Figure 4B). However, experimental mice showed higher motor activity from 1 min to 14 min when the target animals were present (Figure 4C). Similar findings were found in the cumulative duration of movement (moving) of tree shrews and mice in the SI zone (Figure 4D, E). The presence of target tree shrews significantly decreased time spent by the experimental tree shrews in the SI zone during the second 15 min session, whereas the presence of target mice significantly increased time spent by the experimental mice in the SI zone (tree shrew: F(1, 36)=6.838, P<0.05; no target (429.17±54.27), target (235.89±50.18); mouse:F(1, 44)=34.936, P<0.01; no target (290.85±27.90), target (503.35±22.67);Figure 5A). Compared with the experimental tree shrews when the target animals were absent, tree shrews spent significantly less time in the SI zone in the first and third 5 min periods when the target animals were present (Figure 5A). In contrast, mice spent a significantly longer time in the SI zone in the first and second 5 min periods when the target animals were present (Figure 5A). Heatmaps show the time spent by each tree shrew and mouse in the SI zone over a 30 min period (target-absent and target-present sessions) in successive 1 min bins (Figure 5A). The experimental tree shrews showed a lower cumulative duration spent in the SI zone from 2 min to 15 min when the target animals were present (Figure 5B). However, the experimental mice showed a higher cumulative duration spent in the SI zone during the second 15 min session (target-present) than during the first 15 min session (target-absent; Figure 5C). There were significant differences in latency to enter the SI zone by tree shrews and mice during the 30 min sample period between target-absent and target-present groups (tree shrew: F(1, 36)=10.016, P<0.01; no target (145.74±40.50), target (425.38±78.52); mouse:F(1, 44)=13.052, P<0.01; no target (72.10±15.71), target (13.33±4.22);Figure 5D). Heatmaps show the latency to enter the SI zone by each tree shrew and mouse over the 30 min period (target-absent and target-present sessions) in successive 1 min bins (Figure 5D). The experimental tree shrews demonstrated a significantly longer latency to enter the SI zone in the second 15 min session (target-present) than in the first 15 min session (target-absent; Figure 5E). In contrast, the experimental mice displayed a significantly shorter latency to enter the SI zone when the target mice were present (Figure 5F). Moreover, our data showed no significant differences in frequency of entrances in the SI zone by tree shrews and mice during the 30 min social preference-avoidance test between target-absent and target-present groups (Figure 6A, B). In mice, the frequencies of entrance between the two sessions in the first 5 min period were different from those in the second 5 min period (Figure 6B). Mice demonstrated a lower frequency of entrances into the SI zone when the target animals were absent in the first 5 min period (no target0-5 min (7.65±0.65), target0-5 min (11.43±0.70); Figure 6B). In tree shrews, the target animals significantly decreased the cumulative frequency of entrances in the SI zone from 2 min to 4 min (Figure 6C). However, the cumulative frequency of entrances in mice was significantly increased in the first 6 min of the second session (Figure 6D).
2. Social preference-avoidance test in tree shrews.
A: Schematic of social preference-avoidance test in tree shrews. Social interaction (SI) behaviors were assessed in the compartment. Test arena (SI zone and non-SI zone) and target (novel tree shrew) zone were blocked by perforated Plexiglas partition. Shadow indicates SI zone. B: Heatmaps show cumulative duration spent by wild-type tree shrews throughout compartment during social preference-avoidance test.
3.
3. Behavioral differences in tree shrews and mice between target-absent and target-present trials in social preference-avoidance test.
A: Comparison of total distance moved in compartment when target (novel tree shrew or mouse) is absent or present during 30 min sample period (first 15 min session: No target; second 15 min session: Target) and every 5 min. Heatmaps show total distance traveled in successive 1 min bins in social preference-avoidance test over a 30 min period for each tree shrew and mouse. B: In first 3 min of 15 min session, tree shrews demonstrated greater moving distances when target was absent than when target was present. C: Significant differences in total traveling distance in mice were found in first 6 min of 15 min session. D: Total cumulative duration of movement (moving) of tree shrews and mice in compartment during 30 min sample period and every 5 min. Heatmaps show total duration of movement (moving) of tree shrews and mice in successive 1 min bins. E, F: Cumulative duration of movement over time by tree shrews and mice from starting point through 15 min of each session. Values are means±SEM (tree shrew: n=19; mouse: n=23). Significant differences are indicated by *: P<0.05;**: P<0.01;#: P<0.05;##: P<0.01;$: P<0.05;$$: P<0.01 (*: No target vs. target; #: Significantly different among target0-5 min, target5-10 min, and target10-15 min; $: Significantly different among no target0-5 min, no target5-10 min, and no target10-15 min).
4.
4. Behavioral differences in tree shrews and mice between target-absent and target-present trials in social preference-avoidance test.
A: Distance moved in social interaction (SI) zone when target is absent or present during 30 min social preference-avoidance test and every 5 min. Heatmaps show locomotor activity expressed as distance moved by each tree shrew and mouse in SI zone in successive 1 min bins. B: Experimental tree shrews showed higher motor activity from 2 min to 6 min when target animals were absent (target-absent vs. target-present). C: Experimental mice showed higher motor activity from 1 min to 14 min when target animals were present. D, E: Cumulative duration of movement (moving) of tree shrews and mice in SI zone during 30 min sample period and every 5 min. Values are means±SEM (tree shrew: n=19; mouse: n=23). Significant differences are indicated by *: P<0.05;**: P<0.01;#: P<0.05;$$: P<0.01 (*: No target vs. target; #: Significantly different among target0-5 min, target5-10 min, and target10-15 min; $: Significantly different among no target0-5 min, no target5-10 min, and no target10-15 min).
5.
5. Behavioral differences in tree shrews and mice between target-absent and target-present trials.
A: Comparison of time spent by tree shrews and mice in social interaction (SI) zone during 30 min social preference-avoidance test and every 5 min. Heatmaps show time spent by each tree shrew and mouse in SI zone in successive 1 min bins. B: Experimental tree shrews showed lower cumulative duration spent in SI zone from 2 min to 15 min when target animals were present. C: Experimental mice showed higher cumulative duration spent in SI zone during second 15 min session (target-present) than during first 15 min session. D: Latency to enter SI zone of tree shrews and mice during 30 min sample period. Heatmaps show latency to enter SI zone of tree shrews and mice over 30 min period (target-absent and target-present sessions) in successive 1 min bins. E: Experimental tree shrews showed significantly longer latency to enter SI zone in second 15 min session (target-present) than in first 15 min session (target-absent). F: Experimental mice showed significantly shorter latency to enter SI zone when target mice were present. Values are means±SEM (tree shrew: n=19; mouse: n=23). Significant differences are indicated by *: P<0.05;**: P<0.01;##: P<0.01;$$: P<0.01 (*: No target vs. target; #: Significantly different among target0-5 min, target5-10 min, and target10-15 min; $: Significantly different among no target0-5 min, no target5-10 min, and no target10-15 min).
6. Behavioral differences in tree shrews and mice between target-absent and target-present trials.
A, B: Comparison of frequency of entrances in social interaction (SI) zone when target (novel tree shrew or mouse) is absent or present during 30 min social preference-avoidance test and every 5 min. C: In tree shrews, target animals significantly decreased cumulative frequency of entrances in SI zone from 2 min to 4 min. D: Cumulative frequency of entrances of mice significantly increased in first 6 min of second session. Values are means±SEM (tree shrew: n=19; mouse: n=23). Significant differences are indicated by *: P<0.05;**: P<0.01;#: P<0.05;$$: P<0.01 (*: No target vs. target; #: Significantly different among target0-5 min, target5-10 min, and target10-15 min; $: Significantly different among no target0-5 min, no target5-10 min, and no target10-15 min).
DISCUSSION
In the present study, we evaluated the social behaviors of tree shrews and mice toward unfamiliar conspecifics using the social preference-avoidance test. Compared with the experimental animals in the first 15 min session, the conspecific target animals significantly altered the social behaviors of experimental tree shrews and mice in the second 15 min session. In addition, distinct social interaction patterns in response to a conspecific male were also observed in tree shrews and mice.
The open-field test is commonly used to evaluate locomotion, anxiety, and various alterations in emotional behavior (Seibenhener & Wooten, 2015). Locomotor activity (total distance and cumulative duration of movement) was assessed in the open-field test to provide an index of general locomotor activity in a novel open environment. Tree shrews are susceptible to the surrounding environment (Zheng et al., 2014). As such, the purpose of the open-field test was to allow animals to become familiar with and reduce anxiety to the novel testing environment. According to the measured values of total distance moved in the open-field test every 2.5 min, experimental tree shrews and mice adapted to the novel environment and open-field box. Although mice are nocturnal, high levels of activity were found in the open-field test.
Tree shrews and mice have different living habits. Wild tree shrews often appear in the jungle alone, with scent marking by adult males observed in their territories (Hubrecht & Kirkwood, 2010; Zheng et al., 2014). In addition, tree shrews are mostly monogamous in the wild, which is in agreement with observations made in the laboratory where tree shrews can be effectively maintained in pairs (Hubrecht & Kirkwood, 2010). Although the tree shrews used in the present study were bred in the laboratory, they did not exceed second generation offspring and still retained the habits of wild tree shrews. Male tree shrews in the laboratory defend vigorously against intruding conspecifics when a naturally occurring challenging situation is established under experimental control (Fuchs, 2005; Wang et al., 2011). This may be related to aggressive behavior towards the potential intruder. Males defend their territories, indicating competition for food rights and mating opportunities (Kawamichi & Kawamichi, 1979; van Dongen, 2008). High-quality territories in the wild may have better food sources, nesting sites, or mates (Lopes, 2014). The typical lifestyle of mice in the laboratory is different from that of tree shrews. C57BL mice are non-monogamous social animals, and do not show clear territorial behavior (Hubrecht & Kirkwood, 2010).
The social approach-avoidance test was designed for assessment of approach-avoidance behavior in rats and mice (Golden et al., 2011; Haller & Bakos, 2002; Mikics et al., 2008). Each social approach-avoidance test is composed of two sessions (first session: no target; second session: target present) (Golden et al., 2011). Regardless of the social approach-avoidance test performed under conditions of light or darkness, male mice exhibit prosocial behavior toward unfamiliar conspecifics (Berton et al., 2006; Golden et al., 2011; Kaidanovich-Beilin et al., 2011).
In the present study, we first applied the modified social preference-avoidance test to evaluate social interaction behavior in wild-type tree shrews. Although there were similar behavioral performances in total distance moved and total cumulative duration of movement in the 30 min social preference-avoidance test between tree shrews and mice, distinct behavioral patterns in response to a conspecific male were also observed in tree shrews and mice in the first, second, and third 5 min periods. These findings suggest that social behaviors in tree shrews and mice are time dependent. Moreover, tree shrews demonstrated significantly decreased time spent in the SI zone and prolonged latency to enter the SI zone when the target animals were present. In contrast, mice showed significantly increased time spent in the SI zone and shorter latency to enter the SI zone, similar to previous reports (Berton et al., 2006; Golden et al., 2011). This prosocial behavior in wild-type mice can be suppressed by social defeat stress (Berton et al., 2006). To induce fighting, C57BL/6J mice have been exposed to novel, aggressive CD-1 mice for 10 d; after 10 d of social defeat, social avoidance behavior is induced in susceptible mice (Berton et al., 2006; Golden et al., 2011). Previous study has also demonstrated that rats behave prosocially in the laboratory when they perceive their conspecifics trapped in the restrainer and experiencing nonpainful psychological stress (Bartal et al., 2011). Male tree shrews are territorial animals in the wild and strongly defend against intruding conspecifics (Zheng et al., 2014). In the laboratory, the chronic social defeat stress model is based on exposure of male tree shrews (resident) to each other (intruder) (Fuchs, 2005; Wang et al., 2013). Chronic social defeat stress can induce anhedonia and depression-like behaviors in male tree shrews (Fuchs, 2005; Wang et al., 2013). Previous studies have shown that female and male tree shrews exhibit novel object and location preference in novel location memory tasks (Khani & Rainer, 2012; Nair et al., 2014). Male tree shrews are aggressive, although fights are observed only between individuals of the same sex (Vandenbergh, 1963; Zheng et al., 2014). Male tree shrews may be generally prosocial towards an unfamiliar female conspecific (Schehka et al., 2007). The male-female social interaction test in C57BL/6J mice has been conducted on young females, which elicits prosocial behavior from the male test animal (Bales et al., 2014; Ey et al., 2012). In the present study, experimental wild-type tree shrews showed a significant decrease in sociable activities in the SI zone when target animals were present. These findings further confirm male wild-type tree shrews exhibit social avoidance behavior in the social approach-avoidance test. This may be why it is relatively easy to establish tree shrew models of depression by repeated exposure to episodes of social defeat by an unfamiliar conspecific.
In summary, the social preference-avoidance test was first designed for the assessment of approach-avoidance behaviors in tree shrews. Moreover, male wild-type tree shrews and mice exhibited different characteristics of social behaviors in the social preference-avoidance test. Our study provides further evidence of social avoidance behavior in male tree shrews and prosocial behavior in male mice toward unfamiliar conspecifics. In addition, social behaviors in tree shrews and mice appeared to be dependent. Our results indicate that the tree shrew may be a new animal model to study social avoidance behavior and prosocial behavior.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
R.J.N. was involved in apparatus design, behavioral testing, interpretation of data, and writing the manuscript. Y.T. and X.Y.D. were involved in apparatus design and behavioral testing. L.S.Z. and J.X.W. were involved in data analysis. J.N.Z. was responsible for experimental design. T.L. and X.H.M. were responsible for experimental design and revising the manuscript. All authors read and approved the final version of the manuscript.
Funding Statement
This study was supported by the National Natural Science Foundation of China (81671344; 31500859), Major International (Regional) Joint Research Project of the National Natural Science Foundation of China (81920108018), 1.3.5 Project for Disciplines of Excellence, Special Foundation for Brain Research from the Science and Technology Program of Guangdong (2018B030334001), and West China Hospital of Sichuan University (ZY2016103; ZY2016203)
References
- 1.[Anonymous] Guidelines for the treatment of animals in behavioural research and teaching. Animal Behaviour. 2001;61(1):271–275. doi: 10.1006/anbe.2000.1652. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa T, Tanave A, Ikeuchi S, Takahashi A, Kakihara S, Kimura S, Sugimoto H, Asada N, Shiroishi T, Tomihara K, Tsuchiya T, Koide T A male-specific QTL for social interaction behavior in mice mapped with automated pattern detection by a hidden Markov model incorporated into newly developed freeware. Journal of Neuroscience Methods. 2014;234:127–134. doi: 10.1016/j.jneumeth.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Bales KL, Solomon M, Jacob S, Crawley JN, Silverman JL, Larke RH, Sahagun E, Puhger KR, Pride MC, Mendoza SP Long-term exposure to intranasal oxytocin in a mouse autism model. Translational Psychiatry. 2014;4(11):e480. doi: 10.1038/tp.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartal IBA, Decety J, Mason P Empathy and pro-social behavior in rats. Science. 2011;334(6061):1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu P, Masters B, Patel B, Urban O Food safety and inspection service update on food safety of animals derived from biotechnology experiments. Journal of Animal Science. 1993;71(S3):41–42. doi: 10.2527/1993.71suppl_341x. [DOI] [PubMed] [Google Scholar]
- 6.Bauman MD, Iosif AM, Ashwood P, Braunschweig D, Lee A, Schumann CM, Van De Water J, Amaral DG Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Translational Psychiatry. 2013;3(7):e278. doi: 10.1038/tp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 8.Brodkin ES, Hagemann A, Nemetski SM, Silver LM Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Research. 2004;1002(1–2):151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Buijs RM, Ruiz MAG, Hernández RM, Cortés BR The suprachiasmatic nucleus; a responsive clock regulating homeostasis by daily changing the setpoints of physiological parameters. Autonomic Neuroscience. 2019;218:43–50. doi: 10.1016/j.autneu.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Deboer T, Vansteensel MJ, Détári L, Meijer JH Sleep states alter activity of suprachiasmatic nucleus neurons. Nature Neuroscience. 2003;6(10):1086–1090. doi: 10.1038/nn1122. [DOI] [PubMed] [Google Scholar]
- 11.Ey E, Yang M, Katz AM, Woldeyohannes L, Silverman JL, Leblond CS, Faure P, Torquet N, Le Sourd AM, Bourgeron T, Crawley JN Absence of deficits in social behaviors and ultrasonic vocalizations in later generations of mice lacking neuroligin4. Genes, Brain and Behavior. 2012;11(8):928–941. doi: 10.1111/j.1601-183X.2012.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan Y, Huang ZY, Cao CC, Chen CS, Chen YX, Fan DD, He J, Hou HL, Hu L, Hu XT, Jiang XT, Lai R, Lang YS, Liang B, Liao SG, Mu D, Ma YY, Niu YY, Sun XQ, Xia JQ, Xiao J, Xiong ZQ, Xu L, Yang L, Zhang Y, Zhao W, Zhao XD, Zheng YT, Zhou JM, Zhu YB, Zhang GJ, Wang J, Yao YG Genome of the Chinese tree shrew. Nature Communications. 2013;4:1426. doi: 10.1038/ncomms2416. [DOI] [PubMed] [Google Scholar]
- 13.Fan Y, Ye MS, Zhang JY, Xu L, Yu DD, Gu TL, Yao YL, Chen JQ, Lv LB, Zheng P, Wu DD, Zhang GJ, Yao YG Chromosomal level assembly and population sequencing of the Chinese tree shrew genome. Zoological Research. 2019;40(6):506–521. doi: 10.24272/j.issn.2095-8137.2019.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang H, Sun YJ, Lv YH, Ni RJ, Shu YM, Feng XY, Wang Y, Shan QH, Zu YN, Zhou JN High activity of the stress promoter contributes to susceptibility to stress in the tree shrew. Scientific Reports. 2016;6:24905. doi: 10.1038/srep24905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrell MR, Holland FH, Shansky RM, Brenhouse HC Sex-specific effects of early life stress on social interaction and prefrontal cortex dendritic morphology in young rats. Behavioural Brain Research. 2016;310:119–125. doi: 10.1016/j.bbr.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Fischer HD, Heinzeller T, Raab A Gonadal response to psychosocial stress in male tree shrews (Tupaia belangeri) morphometry of testis, epididymis and prostate . Andrologia. 1985;17(3):262–275. doi: 10.1111/j.1439-0272.1985.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs E, Schumacher M Psychosocial stress affects pineal function in the tree shrew (Tupaia belangeri) . Physiology & Behavior. 1990;47(4):713–717. doi: 10.1016/0031-9384(90)90083-g. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs E, Jöhren O, Flügge G Psychosocial conflict in the tree shrew: effects on sympathoadrenal activity and blood pressure. Psychoneuroendocrinology. 1993;18(8):557–565. doi: 10.1016/0306-4530(93)90033-H. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs E, Uno H, Flugge G Chronic psychosocial stress induces morphological alterations in hippocampal pyramidal neurons of the tree shrew. Brain Research. 1995;673(2):275–282. doi: 10.1016/0006-8993(94)01424-G. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs E Social stress in tree shrews as an animal model of depression: an example of a behavioral model of a CNS disorder. CNS Spectrums. 2005;10(3):182–190. doi: 10.1017/S1092852900010038. [DOI] [PubMed] [Google Scholar]
- 21.Glass JD, Grossman GH, Farnbauch L, Dinardo L Midbrain raphe modulation of nonphotic circadian clock resetting and 5-HT release in the mammalian suprachiasmatic nucleus. Journal of Neuroscience. 2003;23(20):7451–7460. doi: 10.1523/JNEUROSCI.23-20-07451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golden SA, Covington III HE, Berton O, Russo SJ A standardized protocol for repeated social defeat stress in mice. Nature Protocols. 2011;6(8):1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodson JL, Lindberg L, Johnson P Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Hormones and Behavior. 2004;45(2):136–143. doi: 10.1016/j.yhbeh.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Green J, Collins C, Kyzar EJ, Pham M, Roth A, Gaikwad S, Cachat J, Stewart AM, Landsman S, Grieco F, Tegelenbosch R, Noldus LPJJ, Kalueff AV Automated high-throughput neurophenotyping of zebrafish social behavior. Journal of Neuroscience Methods. 2012;210(2):266–271. doi: 10.1016/j.jneumeth.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Guillen J. 2017. Laboratory Animals: Regulations and Recommendations for the Care and Use of Animals in Research. 2nd ed. London: Academic Press.
- 26.Haller J, Bakos N Stress-induced social avoidance: a new model of stress-induced anxiety? Physiology & Behavior. 2002;77(2–3):327–332. doi: 10.1016/s0031-9384(02)00860-0. [DOI] [PubMed] [Google Scholar]
- 27.Hefner K, Cameron HA, Karlsson RM, Holmes A Short-term and long-term effects of postnatal exposure to an adult male in C57BL/6J mice. Behavioural Brain Research. 2007;182(2):344–348. doi: 10.1016/j.bbr.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Henriques-Alves AM, Queiroz CM Ethological evaluation of the effects of social defeat stress in mice: beyond the social interaction ratio. Frontiers in Behavioral Neuroscience. 2016;9:364. doi: 10.3389/fnbeh.2015.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hery M, Dusticier G, Faudon M, Barrit MC, Héry F Kinetic study of serotonin metabolism in the suprachiasmatic nucleus of the rat: neuroendocrine incidence (author's transl)] Journal de Physiologie. 1981;77(2–3):497–500. [PubMed] [Google Scholar]
- 30.Holst DV Social stress in tree-shrews: problems, results, and goals. Journal of Comparative Physiology. 1977;120:71–86. doi: 10.1007/BF00617538. [DOI] [Google Scholar]
- 31.Houwing DJ, Heijkoop R, Olivier JDA, Snoeren EMS Perinatal fluoxetine exposure changes social and stress-coping behavior in adult rats housed in a seminatural environment. Neuropharmacology. 2019;151:84–97. doi: 10.1016/j.neuropharm.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 32.Huang ZH, Ni RJ, Luo PH, Zhou JN Distribution of tyrosine-hydroxylase-immunoreactive neurons in the hypothalamus of tree shrews. The Journal of Comparative Neurology. 2020;528(6):935–952. doi: 10.1002/cne.24803. [DOI] [PubMed] [Google Scholar]
- 33.Hubrecht R, Kirkwood J. 2010. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals. 8th ed. Ames: Universities Federation for Animal Welfare, 262–275.
- 34.Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR Assessment of social interaction behaviors. Journal of Visualized Experiments. 2011;(48):2473. doi: 10.3791/2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawamichi T, Kawamichi M Spatial organization and territory of three shrews (Tupaia glis) . Animal Behaviour. 1979;27:381–393. doi: 10.1016/0003-3472(79)90173-8. [DOI] [Google Scholar]
- 36.Khani A, Rainer G Recognition memory in tree shrew (Tupaia belangeri) after repeated familiarization sessions . Behavioural Processes. 2012;90(3):364–371. doi: 10.1016/j.beproc.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Li AF, Jing DQ, Dellarco DV, Hall BS, Yang RR, Heilberg RT, Huang CC, Liston C, Casey BJ, Lee FS Role of BDNF in the development of an OFC-amygdala circuit regulating sociability in mouse and human. Molecular Psychiatry. 2019 doi: 10.1038/s41380-019-0422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopes PC When is it socially acceptable to feel sick? Proceedings of the Royal Society B: Biological Sciences. 2014;281(1788):20140218. doi: 10.1098/rspb.2014.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36(11):2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magariños AM, Mcewen BS, Flügge G, Fuchs E Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. Journal of Neuroscience. 1996;16(10):3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng FT, Zhao J, Fang H, Liu YJ The influence of chronic stress on anxiety-like behavior and cognitive function in different human GFAP-ApoE transgenic adult male mice. Stress. 2015;18(4):419–426. doi: 10.3109/10253890.2015.1040986. [DOI] [PubMed] [Google Scholar]
- 42.Meng XL, Shen F, Li CL, Li YH, Wang XW Depression-like behaviors in tree shrews and comparison of the effects of treatment with fluoxetine and carbetocin. Pharmacology Biochemistry and Behavior. 2016;145:1–8. doi: 10.1016/j.pbb.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Mikics E, Tóth M, Varjú P, Gereben B, Liposits Z, Ashaber M, Halász J, Barna I, Farkas I, Haller J Lasting changes in social behavior and amygdala function following traumatic experience induced by a single series of foot-shocks. Psychoneuroendocrinology. 2008;33(9):1198–1210. doi: 10.1016/j.psyneuen.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Moga MM, Moore RY Organization of neural inputs to the suprachiasmatic nucleus in the rat. The Journal of Comparative Neurology. 1997;389(3):508–534. doi: 10.1002/(SICI)1096-9861(19971222)389:3<508::AID-CNE11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 45.Monclús R, Saavedra I, de Miguel J Context-dependent responses to neighbours and strangers in wild European rabbits (Oryctolagus cuniculus) . Behavioural Processes. 2014;106:17–21. doi: 10.1016/j.beproc.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Nair J, Topka M, Khani A, Isenschmid M, Rainer G Tree shrews (Tupaia belangeri) exhibit novelty preference in the novel location memory task with 24-h retention periods . Frontiers in Psychology. 2014;5:303. doi: 10.3389/fpsyg.2014.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ni RJ, Huang ZH, Luo PH, Ma XH, Li T, Zhou JN The tree shrew cerebellum atlas: systematic nomenclature, neurochemical characterization, and afferent projections. The Journal of Comparative Neurology. 2018;526(17):2744–2775. doi: 10.1002/cne.24526. [DOI] [PubMed] [Google Scholar]
- 48.Ni RJ, Wang J, Shu YM, Xu L, Zhou JN Mapping of c-Fos expression in male tree shrew forebrain. Neuroscience Letters. 2020;714:134603. doi: 10.1016/j.neulet.2019.134603. [DOI] [PubMed] [Google Scholar]
- 49.Ogden BE, Pang WY, Agui T, Lee BH Laboratory animal laws, regulations, guidelines and standards in China Mainland, Japan, and Korea. ILAR Journal. 2016;57(3):301–311. doi: 10.1093/ilar/ilw018. [DOI] [PubMed] [Google Scholar]
- 50.Parésys L, Hoffmann K, Froger N, Bianchi M, Villey I, Baulieu EE, Fuchs E Effects of the synthetic neurosteroid: 3β-methoxypregnenolone (MAP4343) on behavioral and physiological alterations provoked by chronic psychosocial stress in tree shrews. International Journal of Neuropsychopharmacology. 2016;19(4):pyv119. doi: 10.1093/ijnp/pyv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfaff D, Barbas H Mechanisms for the approach/avoidance decision applied to autism. Trends in Neurosciences. 2019;42(7):448–457. doi: 10.1016/j.tins.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Pryce CR, Fuchs E Chronic psychosocial stressors in adulthood: studies in mice, rats and tree shrews. Neurobiology of Stress. 2017;6:94–103. doi: 10.1016/j.ynstr.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raab A, Storz H A long term study on the impact of sociopsychic stress in tree-shrews (Tupaia belangeri) on central and peripheral tyrosine hydroxylase activity . Journal of Comparative Physiology. 1976;108(2):115–131. doi: 10.1007/BF02169044. [DOI] [Google Scholar]
- 54.Raymond JS, Wilson BB, Tan O, Gururajan A, Bowen MT Acute alcohol exposure dose-dependently alleviates social avoidance in adolescent mice and inhibits social investigation in adult mice. Psychopharmacology. 2019;236(12):3625–3639. doi: 10.1007/s00213-019-05335-8. [DOI] [PubMed] [Google Scholar]
- 55.Schehka S, Esser KH, Zimmermann E Acoustical expression of arousal in conflict situations in tree shrews (Tupaia belangeri) . Journal of Comparative Physiology A. 2007;193(8):845–852. doi: 10.1007/s00359-007-0236-8. [DOI] [PubMed] [Google Scholar]
- 56.Seibenhener ML, Wooten MC Use of the open field maze to measure locomotor and anxiety-like behavior in mice. Journal of Visualized Experiments. 2015;(96):e52434. doi: 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taugner R, Forssmann WG, Ganten D, Schiller A Studies on the juxtaglomerular apparatus VI. Sympathetic innervation, catecholamines and the renin-angiotensin-system in rats and tree-shrews (Tupaia belangeri) . Cell and Tissue Research. 1980;212(3):375–382. doi: 10.1007/BF00236504. [DOI] [PubMed] [Google Scholar]
- 58.Toth I, Neumann ID Animal models of social avoidance and social fear. Cell and Tissue Research. 2013;354(1):107–118. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- 59.van Dongen WFD Mate guarding and territorial aggression vary with breeding synchrony in golden whistlers (Pachycephala pectoralis) . Naturwissenschaften. 2008;95(6):537–545. doi: 10.1007/s00114-008-0356-1. [DOI] [PubMed] [Google Scholar]
- 60.Vandenbergh JG Feeding, activity and social behavior of the tree shrew, Tupaia glis, in a large outdoor enclosure . Folia Primatologica. 1963;1(3–4):199–207. [Google Scholar]
- 61.Wang J, Zhou QX, Tian M, Yang YX, Xu L Tree shrew models: a chronic social defeat model of depression and a one-trial captive conditioning model of learning and memory. Zoological Research. 2011;32(1):24–30. doi: 10.3724/SP.J.1141.2011.01024. [DOI] [PubMed] [Google Scholar]
- 62.Wang J, Chai AP, Zhou QX, Lv LB, Wang LP, Yang YX, Xu L Chronic clomipramine treatment reverses core symptom of depression in subordinate tree shrews. PLoS One. 2013;8(12):e80980. doi: 10.1371/journal.pone.0080980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao J, Liu R, Chen CS Tree shrew (Tupaia belangeri) as a novel laboratory disease animal model . Zoological Research. 2017;38(3):127–137. doi: 10.24272/j.issn.2095-8137.2017.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu HF, Liu L, Tian YY, Wang J, Li J, Zheng JQ, Zhao HF, He M, Xu TL, Duan SM, Xu H A disinhibitory microcircuit mediates conditioned social fear in the prefrontal cortex. Neuron. 2019;102(3):668–682. doi: 10.1016/j.neuron.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 65.Yao YG Creating animal models, why not use the Chinese tree shrew (Tupaia belangeri chinensis)? . Zoological Research. 2017;38(3):118–126. doi: 10.24272/j.issn.2095-8137.2017.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshida K, McCormack S, España RA, Crocker A, Scammell TE Afferents to the orexin neurons of the rat brain. The Journal of Comparative Neurology. 2006;494(5):845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng YT, Yao YG, Xu L. 2014. Basic Biology and Disease Models of Tree Shrews. Kunming, Yunnan: Science and Technology Press. (in Chinese)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.