Abstract
Extracellular vesicles (EVs), including exosomes, ectosomes and apoptotic vesicles, play an essential role in communication between cells of the innate and adaptive immune systems. Recent studies showed that EVs released after transplantation of allogeneic tissues and organs are involved in the immune recognition and response leading to rejection or tolerance in mice. After skin, pancreatic islet, and solid organ transplantation, donor-derived EVs were shown to initiate direct inflammatory alloresponses by T cells leading to acute rejection. This occurred through presentation of intact allogeneic MHC molecules on recipient antigen presenting cells (MHC cross-dressing) and subsequent activation of T cells via semi-direct allorecognition. On the other hand, some studies have documented the role of EVs in maternal tolerance of fetal alloantigens during pregnancy and immune privilege associated with spontaneous tolerance of liver allografts in laboratory rodents. The precise nature of the EVs, which are involved in rejection or tolerance, and the cells which produce them, is still unclear. Nevertheless, several reports showed that EVs released in the blood and urine by allografts can be used as biomarkers of rejection. This article reviews current knowledge on the contribution of EVs in allorecognition by T cells and discusses some mechanisms underlying their influence on T cell alloimmunity in allograft rejection or tolerance.
1. Introduction
Extracellular vesicles (EVs), including exosomes, microvesicles and apoptotic vesicles, play an essential role in communication between cells, including leukocytes [1]. Recent studies showed that EVs are involved in alloimmunity, leading to rejection or tolerance of allogeneic transplants [2, 3]. In this article, we review recent literature documenting the role of EVs in alloresponses by T cells after transplantation and we discuss the mechanisms by which they can influence alloimmunity towards rejection or tolerance. Additionally, we examine whether analysis of proteins and mRNA of EVs isolated from blood and urine could be used in place of graft biopsies to evaluate the nature of rejection.
2. Role of extracellular vesicles in T cell allorecognition
Activation of T lymphocytes recognizing donor MHC molecules initiates the adaptive immune response leading to rejection or tolerance of allogeneic transplants. T cell allorecognition occurs via two main mechanisms: the direct and the indirect pathways [4–6]. In direct allorecognition, T cells interact with intact allogeneic MHC proteins displayed on the surface of donor cells [7]. In the case of naïve T cells, this process occurs in recipient’s secondary lymphoid organs. The indirect pathway is mediated by T cells recognizing peptides derived from donor MHC and minor antigens, which are processed and presented in association with self-MHC molecules by recipient APCs [7]. In indirect allorecognition, the mechanism by which donor MHC molecules are acquired by host’s APCs is still unclear. While direct alloresponse represents the driving force behind acute allograft rejection, indirect T cell alloreactivity is believed to govern alloantibody production and chronic rejection, a progressive condition associated with graft vasculopathy and tissue fibrosis [8–11]. On the other hand, the role of direct and indirect pathways in allograft tolerance mediated via T cell deletion and/or suppression is poorly understood. However, it is traditionally accepted that CD4+Foxp3+ T cells mediating regulatory tolerance become activated via recognition of alloantigens along with self-MHC class II molecules on recipient effector T cells and/or APCs [12, 13]. In addition to donor cells, some recent studies demonstrate the contribution of extracellular vesicles (EVs) in T cell allorecognition and alloresponse [2, 3]. In this section, we review current knowledge regarding the role of EVs and transfer of allogeneic MHC antigens (MHC cross-dressing) [14–16] in T cell allorecognition and discuss potential mechanisms explaining how these vesicles may influence allograft rejection and tolerance.
2.1. Extracellular vesicles in allograft rejection
Extracellular vesicles or EVs are comprised of a wide array of vesicles divided in three main categories: 1) exosomes produced in the endosomal compartment of resting and activated cells, 2) microvesicles or ectosomes budding from the plasma membrane of activated cells, and 3) apoptotic vesicles (ApoEVs) released during program cells death [1]. EVs are secreted by virtually all cells, including immune cells and are thought to play a key role in intercellular communications [1]. Exosomes and microvesicles carry proteins and RNA (mRNA and miRNA) while ApoEVs also contain DNA. There is accumulating evidence showing that EVs play a pivotal role in the immune system [17]. In 2016, two articles provided direct evidence of the role of EVs in T cell allorecognition and activation after transplantation in laboratory mice. The first study, from our laboratory at MGH, documented that, in contrast to conventional wisdom, passenger leukocytes (dendritic cells) do not leave skin allografts and infiltrate recipient regional lymph nodes soon after transplantation [3]. Instead, we found that, as early as two days post-transplantation, host’s lymph nodes contained many recipient APCs exhibiting vesicles carrying donor MHC class I and II molecules on their cell surface. In the absence of donor cells, it is likely that presentation of donor MHC antigens by these so called “cross-dressed” APCs is responsible for the initiation of T cell alloresponse after skin grafting [3]. In the case of primarily vascularized heart transplants, seminal studies by Larsen et al. have demonstrated the presence of donor passenger leukocytes in the spleen of transplanted mice [18]. In agreement with this, we observed a few (50–100) donor cells in the spleen of heart-transplanted mice. However, we detected over 50,000 recipient APCs cross-dressed with donor MHC in the recipient spleen [3]. The same year, another report from A. Morelli’s laboratory corroborated these findings [2]. In this study, Liu et al. showed that in heart-transplanted mice, efficient passage of donor MHC molecules to recipient conventional DCs (cDCs) depended on the transfer of EVs from donor DCs that migrated from the graft to lymphoid tissues [2]. These EVs exhibited characteristic features of exosomes and were internalized or remained attached to the recipient cDCs. Recipient cDCs that had acquired exosomes became activated and stimulated alloreactive T cells [2]. Collectively, these two studies support the view that recipient APCs displaying allogeneic MHC proteins acquired from EVs, rather than passenger leukocytes, initiate the direct alloresponse leading to acute allograft rejection in skin and heart transplantation.
Exchange of molecules between cells of the immune system has been known for a long time. Forty years ago, T cells were shown to acquire surface immunoglobulin molecules from B cells [19] and antigens from macrophages [20]. It is now clear that intercellular transfer of proteins and miRNA occurs regularly through cell-cell contact and via vesicles, which are either secreted or exchanged via nanotubes [21]. There is a body of evidence demonstrating that this process is crucial in the initiation and regulation of immunity to microbes and tumors [21]. The transfer of MHC molecules between leukocytes was first reported by Frelinger et al. in 1974 [22]. Acquired peptide-MHC complexes can remain on APCs for as long as 2 days following transfer, providing ample opportunity for T cell activation [23]. Indeed, DCs having acquired allogeneic MHC proteins via cell-cell contact were previously shown to activate alloreactive T cells both in vitro and in vivo via a mechanism referred to as semi direct allorecognition [14, 24, 25]. A few studies have also documented the transfer of MHC class I and II molecules between recipient and donor DCs after solid organ and bone marrow transplantation [15, 26, 27]. First, an elegant study from G. Pettigrew’s laboratory showed that after heart transplantation in mice, recipient DCs acquire MHC molecules from parenchymal cells and simultaneously present them as intact molecules to alloreactive CD8+ T cells (semi-direct presentation) and as peptides to CD4+ T cells (indirect presentation) [26]. Likewise, L. Smyth et al. showed that in skin-grafted mice, MHC-class I acquisition by recipient DCs occurs for at least 1 month following transplantation and was likely the main source of alloantigen that drove CD8+ cytotoxic T cell responses [28]. Collectively, these studies suggest the relevance of a three-cell model of semi-direct allorecognition in which indirectly activated CD4+ T cells provide help for the direct activation of CD8+ T cells (Figure 1A).
Figure 1. MHC cross-dressing can promote interaction between helper or regulatory CD4+ T cells and effector T cells.
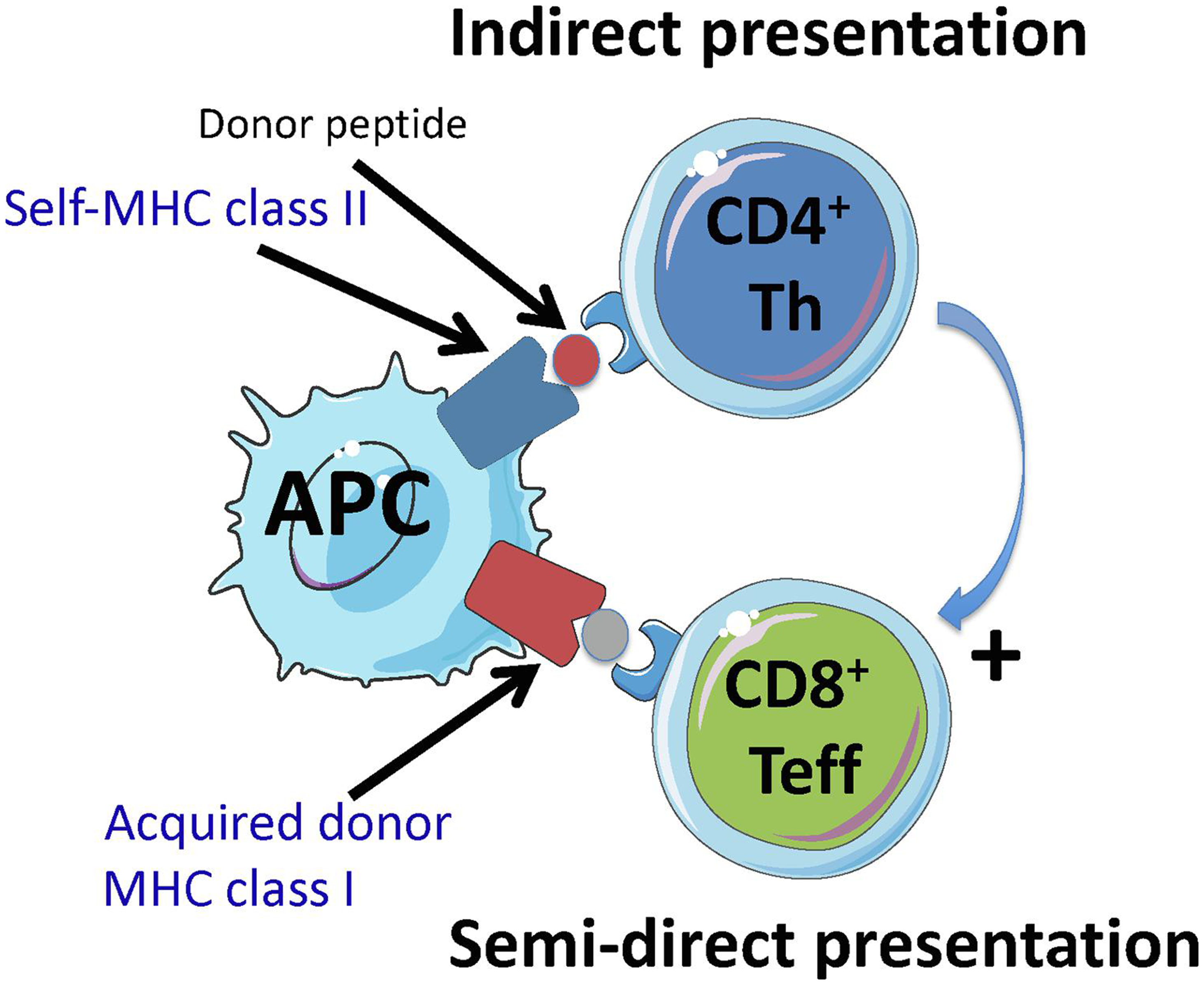
Panel A shows how donor MHC class I cross-dressing of a recipient APC might promote cooperation between CD4+ T helper cells and CD8+ T effector cells by having self-MHC class II + donor peptide (indirect presentation) and donor MHC class I co-presented on the same APC (semi-direct presentation) (three cell cluster).
Panel B shows how simultaneous presentation of intact donor MHC molecules (semi-direct pathway) and self-MHC class II bound to a donor peptide (indirect pathway) or a self-peptide on the same recipient APC can promote interaction between regulatory CD4+ Tregs and effector CD4+ and CD8+ T cells. In this scenario, it is possible that Tregs can suppress effector T cells displaying the same self-MHC class II-peptide complex as recipient APCs (T-T interaction). In this setting, effector T cells are specific for donor MHC while Tregs are not donor specific but interact with self-MHC class II-peptide complexes on activated effector T cells.
A study by Markey et al. has documented the presence of numerous donor cells cross-dressed with recipient MHC class I and II molecules following bone marrow transplantation [27]. Interestingly, donor DCs cross-dressed with recipient MHC antigens disappeared rapidly after myeloablative conditioning whereas after nonmyeloablative conditioning, they persisted along with large numbers of recipient hematopoietic cells [27]. This suggested that only recipient professional APCs had transferred their MHC antigens to donor DCs. In the same study, using a TCR transgenic mouse model (TEa) in which T cells recognize a single MHC-peptide complex (Ab MHC class II bound to MHC class II Eα 52–68 peptide), it was observed that cross-dressed cells enhanced indirect alloresponse by T cells, possibly by increasing the degree of interaction between APCs and CD4+ T cells [27]. This suggests that cross-dressing of donor cells with recipient MHC antigens occurs regularly after bone marrow transplantation, a process that may influence the initiation and perpetuation of GvHD. At the same time, it is likely that recipient APCs are regularly cross-dressed with donor MHC molecules after bone marrow transplantation. The relevance of this phenomenon to rejection of donor bone marrow cells or tolerance of alloantigens acquired via hematopoietic chimerism remains to be investigated.
Our findings, together with those of Morelli’s laboratory, suggest that transfer of donor MHC molecules via EVs is an essential element of the host T cell response to intact donor MHC proteins leading to acute allograft rejection. Indeed, donor EVs being regularly taken up by recipient APCs could represent a major source of donor MHC and other proteins for processing and indirect alloantigen presentation to T cells. However, the contribution of EVs and MHC transfer to indirect alloresponses by T cells and subsequent alloantibody production remains to be evaluated. Little is known about the contribution of EVs to the initiation and perpetuation of chronic allograft rejection. A study by M. Dieudé et al. showed that apoptotic exosome-like vesicles released by endothelial cells triggered the production of anti-perlecan (LG3) auto-antibodies in naïve mice and enhanced their production and allograft inflammation in mice transplanted with an aortic allograft [29]. This suggests that EVs and antigen cross-dressing may participate in graft vasculopathy and chronic rejection of solid organ transplants.
2.2. Extracellular vesicles in allograft tolerance
It is well known that several intrinsic and extrinsic factors govern the nature and magnitude of the immune response induced by a given antigen. The dose, anatomic site of presentation, presence of danger signals, and the nature and state of APC activation are among many factors that control the immunogenicity of a given allogeneic cell or antigen and its ability to drive the immune response towards rejection or tolerance. It is plausible that the immunogenicity of allogeneic EVs follows similar rules in that they can promote allograft rejection or tolerance, depending upon the nature of the vesicles, the APCs that acquire them, and the context of antigen presentation. Thus, EVs should have the potential to be tolerogenic. Support for this comes from studies showing that exosomes released from the intestinal epithelium referred to as tolerosomes can promote tolerance of antigens fed to rats [30, 31]. Likewise, exosomes produced by trophoblasts cells have been associated with feto-maternal tolerance through their expression of FasL [32]. In addition, cross-dressing of leukocytes with HLA-G molecules derived from EVs released by trophoblasts contributes to feto-maternal tolerance [33, 34]. Indeed, during pregnancy, HLA-G molecules are regularly transferred to maternal NK cells, macrophages and T cells of the decidua [35–37]. Finally, Foxp3− T cells being cross-dressed with HLA-G proteins suppress T cell alloresponses as efficiently as classical Foxp3+ Tregs [33, 34].
The factors, which control the tolerogenicity of EVs, are not known. It is possible that EVs act directly on T cells or indirectly via transfer of MHC/peptide complexes and coinhibitory receptors. EVs also carry other molecules capable of influencing T cell activation such as chemokines, cytokines, FasL, miRNA. With regards to alloreactivity, we have previously reported that on their own, allogeneic exosomes expressing MHC class II antigens cannot induce in vitro proliferation and γIFN secretion by naïve T cells [3]. Therefore, it is unlikely that exosomes can serve as bona fide APCs for activation of naïve T cells. This may be due to their suboptimal expression of MHC molecules, their inadequate costimulatory functions and/or their inability to provide certain cytokines produced by APCs such as IL-1. In turn, it is possible that direct interaction of exosomes with T cells results in T cell anergy or exhaustion. Whether this is the case and whether this applies to other larger EVs such as microvesicles and apoptotic EVs remains to be investigated.
Injection of mice with EVs, including exosomes derived from allogeneic immature DCs or Foxp3+ regulatory CD4+ T cells prolonged survival of kidney and cardiac allografts [38–41]. In an autoimmune model, Treg exosomes were shown to suppress inflammatory T cell immunity via transfer of discrete miRNA [42]. However, in transplant models, it remains to be studied whether T cell regulation is mediated directly by certain EVs or through transfer of donor MHC and other molecules to host APCs.
Two recent studies support the view that EVs as well as donor MHC cross-dressing of recipient APCs are involved in spontaneously acquired tolerance of alloantigens. First, W. Burlingham’s laboratory demonstrated the contribution of allogeneic exosomes and MHC antigen cross-dressing in the induction of tolerance to non-inherited maternal antigens (NIMA) [43]. Maternal leukocytes traffic from the mother to the fetus and newborn during pregnancy and breast feeding, respectively [44]. A few maternally derived hematopoietic stem cells and leukocytes (< 1/10,000 cells) are still detected in adults throughout their entire life. Such maternal hematopoietic microchimerism influences the offspring’s immunity against NIMA, including maternal MHC antigens [45–47]. For instance, in patients, kidney allografts expressing NIMA MHC antigens enjoy much longer survival than control transplants expressing unrelated MHC antigens or non-inherited paternal antigens (NIPA) [48]. The question of how very low levels of chimerism (< 1%) in this and other models can impact the host alloimmune responsiveness has been puzzling for decades. A recent paper by Bracamonte et al. has shed some light on this question [43] by showing that the serum of NIMA tolerant mice contained exosomes carrying both NIMA MHC class I and II molecules. In addition, high numbers of dendritic cells cross-dressed with NIMA MHC antigens were detected in tolerant mice but not in non-tolerant mice devoid of microchimerism [43]. Adoptive transfer of allospecific CD4 T cells revealed a “split tolerance” status in mice containing NIMA cross-dressed cells: T cells recognizing intact MHC alloantigens (direct pathway) proliferated, whereas those responding to allopeptide + self-MHC (indirect pathway) were anergic [43]. This study suggests that EVs provide a physiologic link between microchimerism and split tolerance. Another study from A. Thomson laboratory provided evidence for the contribution of donor MHC cross-dressing and semi-direct allorecognition in spontaneous tolerance of mouse liver allografts [49]. Ono et al. showed that intra-graft donor DCs were rapidly replaced with recipient DCs among which 60% cells were cross-dressed with donor MHC class I molecules. These cross-dressed DCs expressed much higher levels of T cell inhibitory PD-L1 and interleukin-10 molecules compared with non-cross-dressed DCs isolated from the graft [49]. The presence of cross-dressed DCs was associated with graft-infiltrating CD8+ T cell expressing PD-1hi and (TIM-3)+ markers of exhaustion [49]. Taken together, these two studies support the view that cross-dressing of recipient APCs with donor MHC molecules, acquired presumably from EVs released by the allograft, can spread and amplify a form of microchimerism leading to regulatory tolerance. It is possible that co-presentation by recipient DCs of self- or donor peptides in the context of self-MHC class II (indirect presentation) and intact donor MHC acquired from donor cells (semi-direct presentation) can promote CD4+Foxp3+ Tregs suppression of effector T cells. At the same time, it is possible that CD4+ Tregs interact directly with activated effector T cells displaying self- or donor peptides bound to self-MHC class II molecules (T-T interaction) (Figure 1B). This three-cell model, reminiscent of linked suppression, could explain why Treg-mediated suppression is donor specific while thymic Tregs (tTregs) are not specific of donor antigens, as recently reviewed by C. Leguern et al. [50].
3. Extracellular vesicles as biomarkers in transplantation
EVs can be isolated from biological fluids, including blood or urine. They are stable and can be stored long-term. Therefore, EV isolation and analysis of their protein and nucleic acid contents is being considered as a way to predict, detect and determine the nature and severity of allograft rejection. Theoretically, this could obviate the need for invasive biopsies. Also, by providing evidence of subclinical rejection this strategy could allow early treatment which would prevent the progression of allograft damage. This section describes some studies supporting the view that EVs can serve as biomarkers in kidney, heart, lung, and pancreatic islet transplantation.
3.1. EVs as biomarkers or renal allograft rejection
Miranda et. al. showed that microvesicles (100–1,000 nm) isolated from urine contain mRNA from all regions of the nephron and collecting duct, making them a potential source of biomarkers for renal disease [51]. Studies have investigated the RNA and proteome of exosomes from urine and plasma to identify potential biomarkers in renal transplant recipients. Some studies aimed to diagnose delayed graft function (DGF), defined as a dialysis requirement during the first week after renal transplantation. This condition occurs in 2–50% of renal transplant cases and is associated with decreased allograft survival and chronic allograft nephropathy [52]. Another study using high-throughput sequencing of the miRNA profile of exosomes in the peripheral blood of kidney transplant recipients, revealed 52 known and 5 conserved exosomal miRNAs specifically expressed in recipients with DGF [53]. Three coexpressed miRNAs, hsa-miR-33a-5p_R-1, hsa-miR-98–5p, and hsa-miR-151a-5p, were highly upregulated in the peripheral blood of kidney graft recipients with DGF [53]. Early detection of DGF may allow for therapeutic intervention and more accurate prediction of graft survival. Alvarez et. al. found that, in patients with DGF, urinary exosomal fractions but not whole unfractionated urine contained high levels of neutrophil gelatinase-associated lipocalin (NGAL), a protein produced in the distal nephron [54]. In fact, NGAL exosomal expression was significantly higher in deceased donor recipients and remained elevated in patients with DGF [54]. This suggests that analysis of urinary exosomes rather than the whole urine may be required to evaluate accurately early graft functions. However, in apparent contrast with this conclusion, the study by Peake et al. showed that urine protein levels of NGAL and IL-18 reflected day 7 post-transplant creatinine reduction ratios (CRR) while the urine exosomal mRNA for these proteins did not [55]. This is probably due to the fact that packaging of mRNA in exosomes is selective and does not always correlate with the presence of mRNA producing selected proteins in the parent cells.
Another beneficial use of biomarkers is the non-invasive diagnosis of both antibody-mediated and cell-mediated rejection by analyzing RNA and protein. Sigdel et. al. identified a total of 1018 proteins in unfractionated whole urine and 349 proteins in urinary exosomes. In the urinary exosomes, 11 proteins involved in inflammatory and stress response were more abundant among recipients with acute rejection and three of those proteins were exclusive to that fraction, providing both potential biomarker targets and mechanistic insights [56]. Lim et. al. identified 169 urinary exosomal proteins and found that 46 proteins whose expression was increased in stable kidney transplant recipients while 17 proteins had increased expression in kidney transplant recipients undergoing acute T cell-mediated rejection (TCMR). Further analysis identified two proteins, tetraspanin-1 and hemopexin that were significantly higher in TCMR patients [57]. Another study showed a significant increase in the plasma density of C4d+/CD144+ (an endothelial marker) microvesicles in renal transplant recipients with AMR compared to those with no AMR or healthy subjects. Also, 9 patients who underwent treatment for acute AMR showed a mean 72% decrease in C4d+/CD144+ microvesicle concentration compared with pre-treatment values, suggesting that this marker may also be useful as a surveillance tool for treatment response [58]. In another study, analysis of plasma exosomal RNA from renal transplant recipients also identified 4 genes (gp130, CCL4, TNFα, SH2D1B, CAV1) whose mRNA transcripts were significantly increased among patients with antibody-mediated rejection (AMR) compared with patients with cell-mediated rejection and control groups with no rejection [59].
3.2. EVs as biomarkers of cardiac allograft rejection
The “gold standard” of cardiac allograft rejection remains endomyocardial biopsy despite its invasiveness and a complication rate of approximately 6%. Therefore, identification of biomarkers is essential in cardiac transplantation to offer a safer, non-invasive alternative to biopsy in rejection diagnosis. Kennel et. al. analyzed the exosomal proteome of serum using mass spectrometry-based technology from healthy individuals; patients with heart failure, heart transplant recipients with rejection, and heart transplant recipients without rejection. They found a clustering of three groups using principal component analysis (PCA): healthy controls and heart failure patients; heart recipients without rejection; and heart recipients with acute cellular rejection (ACR) and AMR. Their data also revealed a protein signature that distinguished between heart transplant recipients without rejection and those with ACR or AMR which consisted of 15 proteins. Two proteins were components of the complement activation cascade: C1QA and C1R; 6 proteins were involved in coagulation: FIBA, FIBB, FIBG, FINC, F13A, and TSP1; 3 proteins were Ig sub-fractions: KV302, HV304, HV315; and APOL1, which can induce autophagic cell death [60]. Further work is necessary to determine the utility of this exosomal protein signature and apply it clinically.
3.3. EVs as biomarkers of lung transplant rejection
Exosomes were isolated from serum and bronchoalveolar lavage fluid (BAL) from 30 lung transplant recipients who were stable, had acute rejection, or had bronchiolitis obliterans syndrome (BOS). They investigated the presence of HLA and lung-associated self-antigens (SAgs) and analyzed exosomal micro-RNA (miRNA) contents. Both exosomes from the serum and BAL of recipients with acute rejection or BOS contained donor HLA and SAgs unlike stable lung transplant recipients. Exosomes carrying the SAg, collagen V, were found in the serum of recipients 3 months before acute rejection and 6 months before BOS diagnosis. Exosomes from recipients with acute rejection or BOS contained a specific set of miRNAs unlike stable lung transplant recipients [61]. Further investigation also showed expression of the costimulatory molecules, CD80, CD86, and CD40, and cytokines, CIITA, NF-κB, HIF-1α, IRAK-1, and MyD88, in exosomes isolated from the sera of lung transplant recipients with BOS but not in stable lung transplant recipients [62].
3.4. EVs as biomarkers of pancreatic islet allograft rejection
Naji et. al. used a human-to-mouse xenogeneic pancreatic islet transplant model to quantify the islet transplant exosome in the recipients’ blood using an anti-HLA antibody. They saw a significant decrease in transplant islet signal and changes in the exosomal miRNA and proteomic profiles prior to the appearance of hyperglycemia. These data suggest that exosomes can be used to detect rejection prior to the appearance hyperglycemia. This group also analyzed the donor exosomes from human islet and renal transplant recipients and found tissue specificity and reliable characterization for follow-up periods of 5 years suggesting that exosomes can be used long-term [63].
Taken together, these studies suggest the value of EVs, including exosomes, as biomarkers. Using EVs from blood or urine could obviate the need for invasive biopsies as well as possibly provide evidence of rejection before allograft dysfunction and damage occurs. In addition, effective monitoring could allow for titrating immunosuppression and reduce the harmful effects of these drugs. Further investigation is necessary to further characterize EVs as relevant biomarkers and validate them for clinical practice. Finally, aforementioned studies in feto-maternal and liver transplant tolerance of alloantigens in mice suggest that EVs may also be used as biomarkers of tolerance [43, 49]. However, this remains to be further evaluated in experimental and clinical transplantation.
4. Concluding remarks
EVs contribute to T cell alloimmunity via transfer of donor MHC antigens to recipient APCs thus initiating inflammatory direct responses leading to acute rejection. On the other hand, strong circumstantial evidence has been provided suggesting that EVs and donor MHC cross-dressing are involved in allograft tolerance. However, the precise nature of the EVs, EV-producing cells and the mechanisms by which they influence alloimmunity towards rejection or tolerance remain elusive. We anticipate that answering these questions will lead to the design of novel EV-based therapies in transplantation. Finally, there is accumulating evidence showing that the proteins and mRNA carried by EVs released by the graft in the blood and urine reflects the nature and stage of rejection. Further validation of EVs as biomarkers of rejection or tolerance could obviate the need for invasive biopsies and help adjust immunosuppressive therapy in transplanted patients.
Highlights.
Extracellular vesicles (EVs), including exosomes, ectosomes and apoptotic vesicles, play an essential role in communication between cells of the innate and adaptive immune systems.
Recipient APCs displaying allogeneic MHC proteins acquired from EVs rather than passenger leukocytes initiate the direct alloresponse leading to acute allograft rejection in skin and heart transplantation.
EVs have the potential to facilitate tolerance induction depending upon the nature of the vesicles, the APCs that acquire them and the context of antigen presentation.
EVs from blood or urine have the potential to be used as biomarkers to provide evidence of rejection before allograft dysfunction and damage occurs and/or to indicate that a state of tolerance has been achieved.
Funding:
This work was supported by the National Institutes of Health RO1 DK115618 to Gilles Benichou and P01HL018646, P01AI123086, U01AI131470 to Joren C. Madsen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mager ELAS,I, Breakefield XO, Wood MJ, Extracellular vesicles: biology and emerging therapeutic opportunities, Nature reviews. Drug discovery, 12 (2013) 347–357. [DOI] [PubMed] [Google Scholar]
- [2].Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, Sullivan ML, Gibson GA, Watkins SC, Larregina AT, Morelli AE, Donor dendritic cell-derived exosomes promote allograft-targeting immune response, J Clin Invest, 126 (2016) 2805–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Marino J, Babiker M, Crosby Bertorini P, Paster JT, LeGuern C, Germana S, Abdi R, Uehara M, Kim J, Markmann J, Tocco G, Benichou G, Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation, Science Immunology, 1 (2016) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Auchincloss H Jr., Sultan H, Antigen processing and presentation in transplantation, Current Opinion in Immunology., 8 (1996) 681–687. [DOI] [PubMed] [Google Scholar]
- [5].Benichou G, Valujskikh A, Heeger PS, Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice, J Immunol, 162 (1999) 352–358. [PubMed] [Google Scholar]
- [6].Sayegh MH, Watschinger B, Carpenter CB, Mechanisms of T cell recognition of alloantigen. The role of peptides, Transplantation, 57 (1994) 1295–1302. [DOI] [PubMed] [Google Scholar]
- [7].Marino J, Paster J, Benichou G, Allorecognition by T Lymphocytes and Allograft Rejection, Frontiers in immunology, 7 (2016) 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Poggio ED, Clemente M, Riley J, Roddy M, Greenspan NS, Dejelo C, Najafian N, Sayegh MH, Hricik DE, Heeger PS, Alloreactivity in renal transplant recipients with and without chronic allograft nephropathy, Journal of the American Society of Nephrology : JASN, 15 (2004) 1952–1960. [DOI] [PubMed] [Google Scholar]
- [9].Vella JP, Spadafora-Ferreira M, Murphy B, Alexander SI, Harmon W, Carpenter CB, Sayegh MH, Indirect allorecognition of major histocompatibility complex allopeptides in human renal transplant recipients with chronic graft dysfunction, Transplantation, 64 (1997) 795–800. [DOI] [PubMed] [Google Scholar]
- [10].Ciubotariu R, Liu Z, Colovai AI, Ho E, Itescu S, Ravalli S, Hardy MA, Cortesini R, Rose EA, Suciu-Foca N, Persistent allopeptide reactivity and epitope spreading in chronic rejection of organ allografts, J Clin Invest, 101 (1998) 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee RS, Yamada K, Houser SL, Womer KL, Maloney ME, Rose HS, Sayegh MH, Madsen JC, Indirect recognition of allopeptides promotes the development of cardiac allograft vasculopathy, Proc Natl Acad Sci U S A, 98 (2001) 3276–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wood KJ, Sakaguchi S, Regulatory T cells in transplantation tolerance, Nature reviews. Immunology, 3 (2003) 199–210. [DOI] [PubMed] [Google Scholar]
- [13].Waldmann H, Graca L, Cobbold S, Adams E, Tone M, Tone Y, Regulatory T cells and organ transplantation, Seminars in immunology, 16 (2004) 119–126. [DOI] [PubMed] [Google Scholar]
- [14].Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, Lechler RI, A novel pathway of alloantigen presentation by dendritic cells, J Immunol, 173 (2004) 4828–4837. [DOI] [PubMed] [Google Scholar]
- [15].Brown K, Sacks SH, Wong W, Extensive and bidirectional transfer of major histocompatibility complex class II molecules between donor and recipient cells in vivo following solid organ transplantation, FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 22 (2008) 3776–3784. [DOI] [PubMed] [Google Scholar]
- [16].Burlingham WJ, “Cross-Dressing” Becomes Fashionable Among Transplant Recipients, Am J Transplant, 17 (2017) 5–6. [DOI] [PubMed] [Google Scholar]
- [17].Robbins PD, Morelli AE, Regulation of immune responses by extracellular vesicles, Nat Rev Immunol, 14 (2014) 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Larsen CP, Morris PJ, Austyn JM, Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection, J Exp Med, 171 (1990) 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hudson L, Sprent J, Specific adsorption of IgM antibody onto H-2-activated mouse T lymphocytes, J Exp Med, 143 (1976) 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bona C, Robineaux R, Anteunis A, Heuclin C, Astesano A, Transfer of antigen from macrophages to lymphocytes. II. Immunological significance of the transfer of lipopolysaccharide, Immunology, 24 (1973) 831–840. [PMC free article] [PubMed] [Google Scholar]
- [21].Brown K, Fidanboylu M, Wong W, Intercellular exchange of surface molecules and its physiological relevance, Archivum immunologiae et therapiae experimentalis, 58 (2010) 263–272. [DOI] [PubMed] [Google Scholar]
- [22].Frelinger JA, Neiderhuber JE, David CS, Shreffler DC, Evidence for the expression of Ia (H-2-associated) antigens on thymus-derived lymphocytes, J Exp Med, 140 (1974) 1273–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dolan BP, Gibbs KD Jr., Ostrand-Rosenberg S, Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells, J Immunol, 177 (2006) 6018–6024. [DOI] [PubMed] [Google Scholar]
- [24].Smyth LA, Herrera OB, Golshayan D, Lombardi G, Lechler RI, A novel pathway of antigen presentation by dendritic and endothelial cells: Implications for allorecognition and infectious diseases, Transplantation, 82 (2006) S15–18. [DOI] [PubMed] [Google Scholar]
- [25].Russo V, Zhou D, Sartirana C, Rovere P, Villa A, Rossini S, Traversari C, Bordignon C, Acquisition of intact allogeneic human leukocyte antigen molecules by human dendritic cells, Blood, 95 (2000) 3473–3477. [PubMed] [Google Scholar]
- [26].Harper SJ, Ali JM, Wlodek E, Negus MC, Harper IG, Chhabra M, Qureshi MS, Mallik M, Bolton E, Bradley JA, Pettigrew GJ, CD8 T-cell recognition of acquired alloantigen promotes acute allograft rejection, Proc Natl Acad Sci U S A, 112 (2015) 12788–12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Markey KA, Koyama M, Gartlan KH, Leveque L, Kuns RD, Lineburg KE, Teal BE, MacDonald KP, Hill GR, Cross-dressing by donor dendritic cells after allogeneic bone marrow transplantation contributes to formation of the immunological synapse and maximizes responses to indirectly presented antigen, J Immunol, 192 (2014) 5426–5433. [DOI] [PubMed] [Google Scholar]
- [28].Smyth LA, Lechler RI, Lombardi G, Continuous Acquisition of MHC:Peptide Complexes by Recipient Cells Contributes to the Generation of Anti-Graft CD8+ T Cell Immunity, Am J Transplant, 17 (2017) 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dieude M, Bell C, Turgeon J, Beillevaire D, Pomerleau L, Yang B, Hamelin K, Qi S, Pallet N, Beland C, Dhahri W, Cailhier JF, Rousseau M, Duchez AC, Levesque T, Lau A, Rondeau C, Gingras D, Muruve D, Rivard A, Cardinal H, Perreault C, Desjardins M, Boilard E, Thibault P, Hebert MJ, The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection, Sci Transl Med, 7 (2015) 318ra200. [DOI] [PubMed] [Google Scholar]
- [30].van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M, Intestinal epithelial cells secrete exosome-like vesicles, Gastroenterology, 121 (2001) 337–349. [DOI] [PubMed] [Google Scholar]
- [31].Karlsson M, Lundin S, Dahlgren U, Kahu H, Pettersson I, Telemo E, “Tolerosomes” are produced by intestinal epithelial cells, Eur J Immunol, 31 (2001) 2892–2900. [DOI] [PubMed] [Google Scholar]
- [32].Frangsmyr L, Baranov V, Nagaeva O, Stendahl U, Kjellberg L, Mincheva-Nilsson L, Cytoplasmic microvesicular form of Fas ligand in human early placenta: switching the tissue immune privilege hypothesis from cellular to vesicular level, Mol Hum Reprod, 11 (2005) 35–41. [DOI] [PubMed] [Google Scholar]
- [33].LeMaoult J, Caumartin J, Daouya M, Favier B, Le Rond S, Gonzalez A, Carosella ED, Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells, Blood, 109 (2007) 2040–2048. [DOI] [PubMed] [Google Scholar]
- [34].Brown R, Kabani K, Favaloro J, Yang S, Ho PJ, Gibson J, Fromm P, Suen H, Woodland N, Nassif N, Hart D, Joshua D, CD86+ or HLA-G+ can be transferred via trogocytosis from myeloma cells to T cells and are associated with poor prognosis, Blood, 120 (2012) 2055–2063. [DOI] [PubMed] [Google Scholar]
- [35].Caumartin J, Favier B, Daouya M, Guillard C, Moreau P, Carosella ED, LeMaoult J, Trogocytosis-based generation of suppressive NK cells, The EMBO journal, 26 (2007) 1423–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].HoWangYin KY, Caumartin J, Favier B, Daouya M, Yaghi L, Carosella ED, LeMaoult J, Proper regrafting of Ig-like transcript 2 after trogocytosis allows a functional cell-cell transfer of sensitivity, J Immunol, 186 (2011) 2210–2218. [DOI] [PubMed] [Google Scholar]
- [37].Tilburgs T, Evans JH, Crespo AC, Strominger JL, The HLA-G cycle provides for both NK tolerance and immunity at the maternal-fetal interface, Proc Natl Acad Sci U S A, 112 (2015) 13312–13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Peche H, Renaudin K, Beriou G, Merieau E, Amigorena S, Cuturi MC, Induction of tolerance by exosomes and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model, Am J Transplant, 6 (2006) 1541–1550. [DOI] [PubMed] [Google Scholar]
- [39].Agarwal A, Fanelli G, Letizia M, Tung SL, Boardman D, Lechler R, Lombardi G, Smyth LA, Regulatory T cell-derived exosomes: possible therapeutic and diagnostic tools in transplantation, Front Immunol, 5 (2014) 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yu X, Huang C, Song B, Xiao Y, Fang M, Feng J, Wang P, CD4+CD25+ regulatory T cells-derived exosomes prolonged kidney allograft survival in a rat model, Cell Immunol, 285 (2013) 62–68. [DOI] [PubMed] [Google Scholar]
- [41].Aiello S, Rocchetta F, Longaretti L, Faravelli S, Todeschini M, Cassis L, Pezzuto F, Tomasoni S, Azzollini N, Mister M, Mele C, Conti S, Breno M, Remuzzi G, Noris M, Benigni A, Extracellular vesicles derived from T regulatory cells suppress T cell proliferation and prolong allograft survival, Sci Rep, 7 (2017) 11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC, Wilson MS, MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells, Immunity, 41 (2014) 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bracamonte-Baran W, Florentin J, Zhou Y, Jankowska-Gan E, Haynes WJ, Zhong W, Brennan TV, Dutta P, Claas FH, van Rood JJ, Burlingham WJ, Modification of host dendritic cells by microchimerism-derived extracellular vesicles generates split tolerance, Proc Natl Acad Sci U S A, 114 (2017) 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dutta P, Burlingham WJ, Tolerance to noninherited maternal antigens in mice and humans, Current opinion in organ transplantation, 14 (2009) 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dutta P, Molitor-Dart M, Bobadilla JL, Roenneburg DA, Yan Z, Torrealba JR, Burlingham WJ, Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice, Blood, 114 (2009) 3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Molitor-Dart ML, Andrassy J, Haynes LD, Burlingham WJ, Tolerance induction or sensitization in mice exposed to noninherited maternal antigens (NIMA), Am J Transplant, 8 (2008) 2307–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Akiyama Y, Caucheteux SM, Vernochet C, Iwamoto Y, Tanaka K, Kanellopoulos-Langevin C, Benichou G, Transplantation tolerance to a single noninherited MHC class I maternal alloantigen studied in a TCR-transgenic mouse model, J Immunol, 186 (2011) 1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Burlingham WJ, Grailer AP, Heisey DM, Claas FH, Norman D, Mohanakumar T, Brennan DC, de Fijter H, van Gelder T, Pirsch JD, Sollinger HW, Bean MA, The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors, The New England journal of medicine, 339 (1998) 1657–1664. [DOI] [PubMed] [Google Scholar]
- [49].Ono Y, Perez-Gutierrez A, Nakao T, Dai H, Camirand G, Yoshida O, Yokota S, Stolz DB, Ross MA, Morelli AE, Geller DA, Thomson AW, Graft-infiltrating PD-L1(hi) cross-dressed dendritic cells regulate antidonor T cell responses in mouse liver transplant tolerance, Hepatology, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].LeGuern C, Germana S, On the elusive TCR specificity of thymic regulatory T cells, Am J Transplant, 19 (2019) 15–20. [DOI] [PubMed] [Google Scholar]
- [51].Miranda KC, Bond DT, McKee M, Skog J, Paunescu TG, Da Silva N, Brown D, Russo LM, Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease, Kidney Int, 78 (2010) 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Schroppel B, Legendre C, Delayed kidney graft function: from mechanism to translation, Kidney Int, 86 (2014) 251–258. [DOI] [PubMed] [Google Scholar]
- [53].Wang J, Li X, Wu X, Wang Z, Zhang C, Cao G, Yan T, Expression Profiling of Exosomal miRNAs Derived from the Peripheral Blood of Kidney Recipients with DGF Using High-Throughput Sequencing, Biomed Res Int, 2019 (2019) 1759697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Alvarez S, Suazo C, Boltansky A, Ursu M, Carvajal D, Innocenti G, Vukusich A, Hurtado M, Villanueva S, Carreno JE, Rogelio A, Irarrazabal CE, Urinary exosomes as a source of kidney dysfunction biomarker in renal transplantation, Transplant Proc, 45 (2013) 3719–3723. [DOI] [PubMed] [Google Scholar]
- [55].Peake PW, Pianta TJ, Succar L, Fernando M, Pugh DJ, McNamara K, Endre ZH, A comparison of the ability of levels of urinary biomarker proteins and exosomal mRNA to predict outcomes after renal transplantation, PLoS One, 9 (2014) e98644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sigdel TK, Ng YW, Lee S, Nicora CD, Qian WJ, Smith RD, Camp DG 2nd, Sarwal MM, Perturbations in the urinary exosome in transplant rejection, Front Med (Lausanne), 1 (2014) 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lim JH, Lee CH, Kim KY, Jung HY, Choi JY, Cho JH, Park SH, Kim YL, Baek MC, Park JB, Kim YH, Chung BH, Lee SH, Kim CD, Novel urinary exosomal biomarkers of acute T cell-mediated rejection in kidney transplant recipients: A cross-sectional study, PLoS One, 13 (2018) e0204204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tower CM, Reyes M, Nelson K, Leca N, Kieran N, Muczynski K, Jefferson JA, Blosser C, Kukla A, Maurer D, Chandler W, Najafian B, Plasma C4d+ Endothelial Microvesicles Increase in Acute Antibody-Mediated Rejection, Transplantation, 101 (2017) 2235–2243. [DOI] [PubMed] [Google Scholar]
- [59].Zhang H, Huang E, Kahwaji J, Nast CC, Li P, Mirocha J, Thomas DL, Ge S, Vo AA, Jordan SC, Toyoda M, Plasma Exosomes From HLA-Sensitized Kidney Transplant Recipients Contain mRNA Transcripts Which Predict Development of Antibody-Mediated Rejection, Transplantation, 101 (2017) 2419–2428. [DOI] [PubMed] [Google Scholar]
- [60].Kennel PJ, Saha A, Maldonado DA, Givens R, Brunjes DL, Castillero E, Zhang X, Ji R, Yahi A, George I, Mancini DM, Koller A, Fine B, Zorn E, Colombo PC, Tatonetti N, Chen EI, Schulze PC, Serum exosomal protein profiling for the non-invasive detection of cardiac allograft rejection, J Heart Lung Transplant, 37 (2018) 409–417. [DOI] [PubMed] [Google Scholar]
- [61].Gunasekaran M, Xu Z, Nayak DK, Sharma M, Hachem R, Walia R, Bremner RM, Smith MA, Mohanakumar T, Donor-Derived Exosomes With Lung Self-Antigens in Human Lung Allograft Rejection, Am J Transplant, 17 (2017) 474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gunasekaran M, Sharma M, Hachem R, Bremner R, Smith MA, Mohanakumar T, Circulating Exosomes with Distinct Properties during Chronic Lung Allograft Rejection, J Immunol, 200 (2018) 2535–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Vallabhajosyula P, Korutla L, Habertheuer A, Yu M, Rostami S, Yuan CX, Reddy S, Liu C, Korutla V, Koeberlein B, Trofe-Clark J, Rickels MR, Naji A, Tissue-specific exosome biomarkers for noninvasively monitoring immunologic rejection of transplanted tissue, J Clin Invest, 127 (2017) 1375–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]


