Abstract
Objective
Originator intravenous rituximab is an important rheumatology treatment but is costly, and administration requires several hours. Because biosimilar rituximab may cost less and subcutaneous rituximab requires a shorter visit, both may reduce costs and increase treatment capacity (infusions per year).
Methods
We implemented time‐driven activity‐based costing (TDABC), a method to assess costs and opportunities to increase capacity, throughout the care pathway for 26 patients receiving a total of 30 rituximab infusions. Using the TDABC estimates, we created a base case, which included provider time, salaries, infusion rates and times, and drug formulation, to simulate an induction cycle (two infusions). We varied these parameters in sensitivity analyses and assessed the impact of infusion rates and formulation (biosimilar vs. subcutaneous) on capacity before and after assuming a fixed budget.
Results
The base‐case cost was $19 452; more than 90% was due to drug cost. In sensitivity analyses, varying projected biosimilar cost led to the greatest cost savings ($8,988 per cycle). Faster infusion rates and subcutaneous rituximab increased annual capacity (300% and 800%, respectively). With a fixed budget, subcutaneous rituximab led to a relative increase in capacity over biosimilar rituximab except when biosimilar cost savings relative to originator rituximab exceeded 40%; faster biosimilar infusion rates did not meaningfully affect these findings.
Conclusion
Using TDABC, we demonstrate that rituximab cost is the primary driver of treatment cost, but capacity is largely driven by treatment time. Subcutaneous rituximab leads to higher capacity than biosimilar rituximab across a range of plausible costs; its use in rheumatology should be studied.
INTRODUCTION
In 2016, Medicare spent more than $80 million on rituximab prescribed by rheumatologists for anti–neutrophil cytoplasmic antibody–associated vasculitis, rheumatoid arthritis, systemic lupus erythematosus, and other conditions (1). The typical regimen for remission induction in these conditions consists of two rituximab infusions administered over 2 weeks. Each dose is administered slowly over 3 to 5 hours to reduce the risk of an infusion reaction (2).
Patient access to rituximab is limited by drug and administration costs as well as by the number of infusions per day (ie, capacity) that the health care system can accommodate because of long infusion duration. Limitations in the capacity of health care systems to administer infusions have been previously described and represent a growing area of research 3, 4, 5. Because of the high costs associated with rituximab, many insurers limit access and/or require prior authorization, which contributes substantially to delays in treatment initiation (5). In addition, because the infusion duration limits the number of daily treatments in a given center, scheduling delays are common (5). Identifying opportunities to reduce the cost of rituximab administration and lower the logistical hurdles to administering rituximab may increase infusion center capacity, facilitate patient access, and reduce societal costs.
Three potential changes to rituximab administration may improve patient treatment access. First, subcutaneous rituximab requires only 30 minutes for administration and is approved for subsequent rituximab treatments in oncology but has not been studied in rheumatology 6, 7. Second, biosimilar rituximab is expected to be introduced to the market soon and may be a less expensive but equally effective alternative to originator rituximab 8, 9, 10. Third, a rapid infusion rate protocol may be used safely following the first infusion and would increase capacity by shortening the duration of subsequent treatments 4, 11, 12, 13.
Time‐driven activity‐based costing (TDABC) is a novel method to estimate the costs of resource use and one that is increasingly used across health care specialties 14, 15, 16, 17, 18. TDABC estimates costs by directly accounting for resources (eg, personnel, drug, and space) used and the time spent with each resource. TDABC can therefore be used to identify opportunities to reduce cost and improve administration efficiency (19). TDABC has not previously been used to assess the cost of rituximab administration in rheumatology. We performed a TDABC study of rituximab administration and used the results of this analysis to project the potential impact of using subcutaneous rituximab, of using biosimilar rituximab, and of faster infusion rates on cost and capacity.
MATERIALS AND METHODS
The TDABC method
We considered the base case of induction therapy, which includes two outpatient intravenous rituximab infusions: an initial dose and a faster subsequent dose separated by approximately 2 weeks. The infusions were ordered by an American Board of Internal Medicine–certified rheumatologist, prepared in‐house by a pharmacist, and administered by a registered nurse in an academic tertiary hospital–based infusion center. Oncology infusions are administered in a separate facility. To estimate the cost of this base case, we outlined each step of the infusion process, from physician order to patient discharge from the infusion center, in collaboration with rheumatology providers, staff, and nurses (Supplementary Figure 1).
Observed infusions
We identified the personnel, consumables, and space used in the infusion process for 30 rituximab infusions among 26 unique patients, a convenience sample of prospectively observed patients at the Medical Infusion Clinic (MIC) of Massachusetts General Hospital (MGH). The 30 infusions included 11 first‐time doses and 19 second doses.
Personnel costs
We calculated capacity cost rates (CCRs) for each personnel type in 2018 US dollars (USD) per minute using regional and national compensation data, including salary, payroll taxes, and fringe benefits (eg, health insurance) 20, 21, 22. Regional and national averages, rather than actual MGH compensation, were used to achieve generalizability. Rheumatologists were estimated to work 10.5 h/d, whereas all other professionals and staff were estimated to work 8 h/d. Within each workday, we assumed 5% idle or break time for physicians and 10% idle or break time for other personnel. After accounting for 21 days of vacation, 6 days of educational time and sick/personal leave, and 114 days of weekends/holidays there were 224 workdays in a year 16, 23. In the absence of a standardized ratio of nurses to patients in an infusion setting and to provide a conservative estimate, we assumed that all time spent by a patient in an infusion chair could be attributed to the care provided by a nurse. For individuals in a supervisorial role, such as nurse practitioners and nurse managers, we assumed their contribution to the infusion to be one‐fifteenth of the total time patients spent in a chair, in which 15 is the total number of chairs in the MIC. The time spent by a physician ordering rituximab and referring a patient to the infusion center was assumed to be 5 minutes. The estimated time spent by a clerk performing a prior authorization request was based on self‐reported processing times for 15 consecutive rituximab requests.
Drug and nondrug consumables
Medical consumables used at each stage in the preparation and administration of rituximab were costed according to the acquisition cost to MGH, except for drug costs, for which data from the Federal Supply Schedule (FSS) federal contract service were used 24, 25. For the base‐case analysis, we used aggregated data from the MGH Medical Infusion Pharmacy to estimate the preparation time of each dose of rituximab and assumed that all time indicated for preparing and checking the drug by a pharmacy technician and a pharmacist, respectively, was dedicated to the preparation of one dosage of rituximab.
Space costs
Space costs were determined by measuring the square footage of each individual patient care space (eg, chair) in the MIC and calculating the mean time patients spent in the MIC. Construction, rental, and maintenance costs were incorporated into the price per square foot. Space availability was calculated using a 12‐hour Monday through Friday clinical practice (190 800 available minutes in 1 year).
One‐way sensitivity analyses
We performed one‐way sensitivity analyses to assess how assumptions about personnel and drug administration impact TDABC cost estimates. Regarding personnel, we 1) varied the nursing time based on observed face‐to‐face nursing time, 2) varied the nursing time to approximate the nurse's care being split among multiple patients, 3) varied the percentage of nursing time provided by nurse practitioners rather than registered nurses, 4) varied the CCRs in our analysis to reflect the differences in salaries across practice sites, and 5) included the cost of a clerk responsible for prior authorization requirements.
Regarding drug administration, we 1) considered the impact of substituting 60 mg of oral prednisone for 125 mg of intravenous methylprednisolone, which would reduce the chair time because it can be taken at home prior to arriving, therefore saving approximately 30 minutes of chair time while waiting for the steroid to have effect (26); 2) varied the infusion time for the subsequent, shorter dose because faster infusion protocols (approximately 90 minutes vs 120 minutes) have been shown to be safe for subsequent rituximab infusions 4, 11, 12, 13; 3) evaluated the impact of using lower doses of rituximab, which have been found to have similar effectiveness as ordinary doses of rituximab (27); 4) considered the impact of biosimilar rituximab, which is approved by the US Food and Drug Administration and assumed to cost less relative to originator rituximab but is not yet marketed 8, 9, 10, 28; and 5) substituted a subcutaneous injection of rituximab in place of the second infusion and varied the cost estimates relative to the FSS cost for subcutaneous rituximab as listed for oncology indications. Subcutaneous injections are approved for oncology indications and require only a 30‐minute visit (per MGH's oncology practice).
We evaluated the potential impact of variations in cost of space for infusions, ranging from −50% to +50% compared with the base case. We also considered a resource adjustment to account for an adverse reaction ranging from mild, which was observed in 5 of the 30 prospectively observed infusions, to severe anaphylaxis, which is rare (3.2 of 1000) (16); a severe reaction was not observed in this study but was included in analyses because it is resource intensive (29). Given the controversy over shifting subsequent infusion administration from health care facilities to the home, we also evaluated the potential impact of changing the treatment setting (30). Using one‐way analysis results, we also estimated the lowest and highest plausible costs of rituximab administration by combining the extremes of cost associated with key parameter variations.
Multiway sensitivity analyses
We focused multiway sensitivity analyses on simultaneously varying the most influential parameters in one‐way analyses (formulation and infusion duration) relevant to the second dose; we assumed that neither the infusion rate nor the formulation (intravenous vs subcutaneous) of the first infusion could be changed because of the risk of infusion reactions. MIC annual capacity (eg, hours per year and infusions per year available for rituximab infusions) was estimated based on a retrospective review of 3 months of scheduling data as well as infusion durations estimated using TDABC.
In multiway sensitivity analyses, we assumed a fixed annual budget and evaluated the impact of varying formulation (ie, intravenous vs subcutaneous), associated drug prices (ie, subcutaneous and biosimilar), and infusion rates on the annual MIC capacity (infusions per year). The fixed budget was estimated by multiplying the current annual number of subsequent rituximab treatments by the costs associated with these infusions, as estimated using TDABC. The costs of biosimilar or subcutaneous rituximab for rheumatology indications are unknown, so we estimated these at $8988 (based on the current originator rituximab FSS cost) and $6355 (based on the oncology indication FSS cost), respectively, and varied these estimates in sensitivity analyses.
We first assessed the differences in annual capacity for subsequent infusions associated with two different treatment strategies for subsequent rituximab doses: 1) biosimilar intravenous rituximab or 2) subcutaneous originator rituximab. We assumed that in addition to potential differences in drug cost, subcutaneous rituximab was associated with reduced personnel, nondrug consumable, and space costs because of its formulation and its administration in a 30‐minute visit. Second, we evaluated how differences in capacity between these two treatment strategies might be impacted by the administration of biosimilar rituximab using a rapid infusion rate protocol.
RESULTS
Base case
The base case of two rituximab infusions (one initial loading dose and one subsequent dose) costs $19 452 in 2018 USD (Table 1). The initial dose costs $9812, whereas the subsequent dose costs $9640. Drug costs accounted for more than 90% of the total first and subsequent infusion costs (92% and 93%, respectively; Supplementary Table 1). Total personnel costs were $770 ($435 for the initial infusion and $335 for the subsequent infusion; Supplementary Table 2), nondrug consumable costs were $56 ($28 for each infusion; Supplementary Table 3), and space costs were $628 ($350 and $278 for the first and subsequent infusions, respectively; Supplementary Table 4).
Table 1.
Base‐case costs (2018 US dollars)
| Infusions | Cost, $ | % of Total |
|---|---|---|
| Long (first) infusion total | ||
| Drug consumables | 8999 | 92 |
| Personnel consumables | 435 | 4 |
| Space consumables | 350 | 4 |
| Nondrug consumables | 28 | 0.3 |
| Total cost | 9812 | 100 |
| Short (second) infusion total | ||
| Drug consumables | 8999 | 93 |
| Personnel consumables | 335 | 3 |
| Space consumables | 278 | 3 |
| Nondrug consumables | 28 | 0.3 |
| Total cost | 9640 | 100 |
| Total base‐case cost | 19 452 | … |
One‐way sensitivity analyses
One‐way sensitivity analyses assessing variations in parameters relevant to rituximab administration led to different estimates of total costs for induction therapy, which consists of two doses (Supplementary Table 5). For instance, varying the estimates to account for MGH salary (cost increases up to $267); shortening the infusion by up to 160 minutes (savings of up to $375); and accounting for nurse practitioner involvement in care (cost increases up to $68), actual nursing time spent providing face‐to‐face care (savings of up to $454), and adverse reactions (cost increases up to $326) minimally changed overall costs (Figure 1). Similarly, changing the premedications required before the infusion from 125 mg of intravenous methylprednisolone to 60 mg of oral prednisone taken before arrival decreased the total cost by $191. Accounting for prior authorization requirements added $4 to the total cost. Administering the second dose of rituximab in a patient's home led to cost savings of $278. Varying (±50%) the cost of space led to minor variations in overall cost (±$314).
Figure 1.
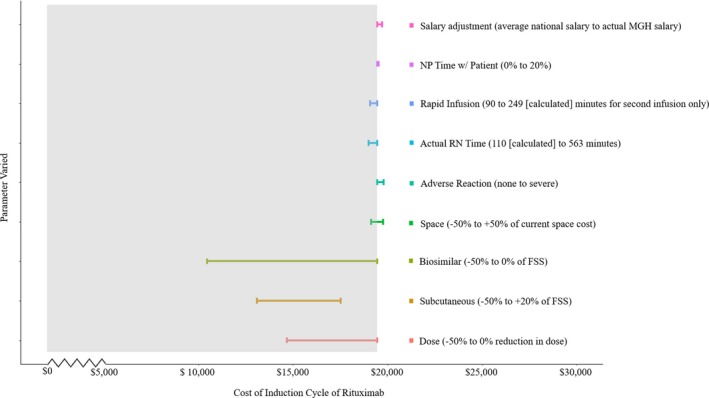
One‐way sensitivity analyses. We varied base‐case assumptions and evaluated the impact of these changes on total cost (2018 US dollars) of rituximab administration (two doses for induction therapy). Mild and severe reactions were assumed to occur at varying rates, and this is reflected in these estimates (mild reactions were observed in 5 of the 30 prospectively observed infusions, whereas severe anaphylaxis was not observed in this study and is rare [3.2 of 1000]). FSS, Federal Supply Schedule; MGH, Massachusetts General Hospital; NP, nurse practitioner; RN, registered nurse.
In contrast, the most impactful variations in cost were those related to drug formulation. Using subcutaneous rather than intravenous rituximab for the second infusion decreased the total cost by up to $3197 (16.4% cost savings) because of potential reductions in drug consumable (savings up to $2668), personnel (savings up to $284), and space costs (savings up to $246). Expected cost savings associated with biosimilar rituximab were also found to substantially reduce the cost of rituximab administration. For instance, if biosimilar rituximab is 50% cheaper than originator rituximab, $8988 would be saved (46.0% cost savings) during induction therapy, assuming other factors remained constant.
Administering oral prednisone prior to the infusion, using a rapid second infusion, and administering biosimilar rituximab at a 50% cost savings led to the lowest TDABC estimate ($9382). Conversely, requiring a prior authorization, assuming that 10% of the patients’ time in clinic is spent with a nurse practitioner, adjusting CCRs to actual MGH salaries, and accounting for a severe adverse reaction led to the highest TDABC estimate ($20 077).
Maximizing infusion center capacity on a fixed budget: a sensitivity analysis
Currently, the MIC administers approximately 130 first infusions and 550 subsequent rituximab infusions per year. Keeping first infusions constant and substituting subcutaneous for intravenous rituximab for subsequent infusions would open an additional 8 hours for infusions each day (Table 2). If all 8 hours were used to administer subsequent doses of rituximab subcutaneously, this would permit 15 additional subsequent treatments each day. Over 1 year, this would increase capacity for second doses by eightfold, adding approximately 4030 subsequent treatments. Alternatively, if this newly available time was used to administer one additional initial dose intravenously each day, five subsequent subcutaneous doses could also be administered each day. Over 1 year, this would increase capacity for first doses by up to 260, a threefold increase, and for second doses by up to 1290, a more than threefold increase.
Table 2.
Projected increases in operational capacity
| No. of Infusions | Hours of Infusions | |||||
|---|---|---|---|---|---|---|
| Overall | First Tx | Second Tx | Overall | First Tx | Second Tx | |
| Baseline infusion capacity | ||||||
| per d a | 3 | 1 | 2 | 11 | 3 | 9 |
| per y b | 680 | 130 | 550 | 2980 | 680 | 2290 |
| Subcutaneous substitution c | ||||||
| Maintain current volume (per d) | 3 | 1 | 2 | 4 | 3 | 1 |
| Newly available capacity (per d) | … | … | … | 8 | … | … |
| All available time for SQ | 15 | 0 | 15 | 8 | 0 | 8 |
| One additional first infusion, rest SQ | 6 | 1 | 5 | 7 | 5 | 2 |
| Newly available capacity (per y) b | ||||||
| All available time for SQ | 4030 | 0 | 4030 | 2020 | 0 | 2020 |
| One additional first infusion, rest SQ | 1550 | 260 | 1290 | 2020 | 1370 | 650 |
| Rapid second infusion substitution | ||||||
| Maintain current volume (per d) | 3 | 1 | 2 | 6 | 3 | 3 |
| Newly available capacity (per d) | … | … | … | 6 | … | … |
| All available time for second infusion | 4 | 0 | 4 | 6 | 0 | 6 |
| One additional first infusion | 1 | 1 | 0 | 5 | 5 | 0 |
| Newly available capacity (per y) b | ||||||
| All available time for second infusion | 980 | 0 | 980 | 1460 | 0 | 1460 |
| One additional first infusion | 260 | 260 | 0 | 1370 | 1370 | 0 |
Abbreviation: SQ; subcutaneous; Tx, treatment.
Number of infusions per day based on infusion center scheduling over a 3‐month period and hours of infusions based on time‐driven activity‐based costing estimates.
Based on the infusion center being open for 261 d/y.
Oncology pricing, with the 30‐minute visit reserved for SQ injection, based on the oncology practice assumed.
If a rapid infusion protocol were used for all subsequent rituximab infusions, 6 new hours would be available each day for rituximab infusions (Table 2). This newly available time could be used to administer three additional subsequent infusions (at a rapid rate) or one additional initial infusion each day. Over 1 year, this would permit up to 260 additional initial infusions, a threefold increase, or up to 980 additional subsequent infusions, also an approximate threefold increase.
In multiway analyses, we evaluated the impact that varying the cost of biosimilar vs subcutaneous rituximab would have on annual MIC capacity to administer subsequent infusions after assuming a fixed budget. At nearly all variations in the price of subcutaneous and biosimilar intravenous rituximab, subcutaneous use allotted for more subsequent rituximab treatments than if biosimilar intravenous rituximab were used (Figure 2, shades yellow, green, and blue). If the subcutaneous rituximab cost remains the same as it is for oncology indications, biosimilar intravenous rituximab only leads to a relative increase in capacity over subcutaneous use when the cost savings of biosimilar intravenous relative to originator intravenous rituximab meets or exceeds 40%. Our findings were similar when we evaluated the impact that varying these costs would have on capacity to administer first‐time infusions (data not shown).
Figure 2.
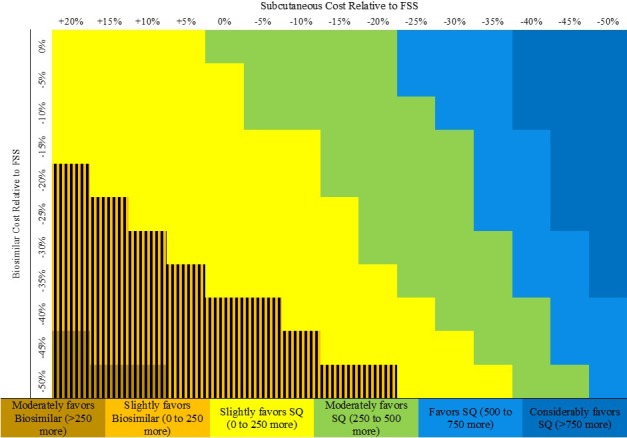
Multiway sensitivity analysis evaluating the relative impact of varying biosimilar rituximab cost vs subcutaneous (SQ) rituximab cost on annual infusion center capacity to administer subsequent rituximab doses. We assumed that the infusion center has a fixed budget of $5 321 950 (includes costs of drug, nondrug consumables, personnel, and space) and evaluated how varying these costs would affect the number of second rituximab doses that can be provided in 1 year. All drug cost savings are relative to the Federal Supply Schedule (FSS) program costs of originator intravenous rituximab. Subcutaneous rituximab requires 30 minutes to administer. The number reflected by the color of each cell represents this difference: number of treatments if subcutaneous rituximab is used − number of treatments if biosimilar rituximab is used. The hatched area reflects the threshold across which biosimilar rituximab is associated with greater capacity when compared with subcutaneous rituximab.
When the potential impact of using a rapid infusion protocol for subsequent infusions is considered in addition to variations in the cost of biosimilar intravenous rituximab, the use of subcutaneous rituximab still maximizes capacity except when the cost savings associated with biosimilar rituximab is at least 35% and the infusion duration is shortened by at least 30 minutes (Supplementary Figure 2).
DISCUSSION
Using TDABC, the total cost of two infusions of rituximab performed as induction therapy for rheumatologic diseases was estimated to be $19 452, of which more than 90% was solely related to the drug cost. Rituximab is known to be an expensive medication that requires substantial resource use for administration, contributing to infusion center delays in access (5). Therefore, TDABC was used to identify the potential cost savings and increased administration capacity that may accompany the adoption of biosimilar rituximab, rapid infusion protocols, and/or subcutaneous rituximab in practice. Although the optimal treatment strategy varied depending on the ultimate costs of infused biosimilar rituximab and subcutaneous rituximab, the subcutaneous formulation consistently led to higher capacity across a wide range of plausible costs.
This study illustrates the potential application of TDABC methodology to estimate costs in rheumatology. Moreover, this study contrasts with prior ones estimating the cost of rituximab induction therapy in rheumatology because it accounts for both drug and nondrug costs (eg, personnel, nondrug consumables, space) by using direct observation of real‐world practice. This investigation therefore extends the findings of prior studies that have evaluated the cost of rituximab and other infusion therapies in rheumatology (31). Previous studies in oncology have used similar costing methods, but rituximab dosing in rheumatology differs from oncology regimens, in which dosage is based on body surface area and the frequency of administrations is higher 7, 32. These results corroborate those of studies conducted outside the United States in oncology settings that concluded that subcutaneous rituximab was less expensive and less time consuming to administer for hematologic malignancies than intravenous rituximab 7, 33.
These findings have significant implications for the use of rituximab in rheumatology. First, efforts to minimize costs by shifting subsequent rituximab doses out of health care facilities are unlikely to substantially change the cost of rituximab administration because more than 90% of the cost is due to the drug itself. Second, use of subcutaneous rituximab may alleviate bottlenecks in access to treatment while simultaneously reducing costs. However, its use in rheumatology has not been specifically studied, and it is currently not approved for use in rheumatic conditions. Studies evaluating the safety and efficacy of subcutaneous rituximab in rheumatology are therefore warranted. Third, depending on the ultimate cost of biosimilar rituximab, large cost savings may be observed once it is introduced to the market. Fourth, even after accounting for a wide range of potential drug cost savings associated with biosimilar rituximab, subcutaneous formulations with even no or minimal drug cost savings may provide greater financial incentives while simultaneously improving access to treatment. Fifth, several regulatory strategies are currently under consideration to mitigate the impact of drug costs on the health care system. Any of these approaches could have a substantial impact on our findings because the cost of rituximab accounted for more than 90% of total infusion costs. Importantly, variations in drug cost would not necessarily alter our findings regarding capacity when comparing infusion and subcutaneous formulations given the differences in the time required for administration.
At a systems level, there will be some financial winners and losers if the proposed mechanisms to increase infusion center capacity are implemented and they impact revenue streams for practices and systems. The analyses projecting the potential impact of biosimilar rituximab, subcutaneous rituximab, and rapid infusion protocols were conducted from the perspective of the health care system with the intention of identifying opportunities to simultaneously reduce overall costs and improve system efficiency. It is likely that the potential cost savings and increases in capacity that may accompany the use of biosimilar rituximab, subcutaneous formulations, and faster infusion rates would diminish the revenue of practices and health care facilities that administer infusions. First, depending on how subcutaneous formulations are distributed (directly to consumers vs to facilities), hospitals and health care facilities that qualify for the 340B Drug Pricing Program may not benefit from those cost savings (34). Second, it was assumed that newly available time for infusion administration would be used to accommodate patients waiting for rituximab infusions. It is also possible, however, that this time could be occupied by patients awaiting other infusion therapies, therefore attenuating, to some degree, any impact on revenue or off‐setting it completely. The analysis does not account for how the use of subcutaneous rituximab might affect out‐of‐pocket costs, quality of life, and lost wages for patients.
This study has certain limitations. Although regional and national salary information and standard drug cost rebate assumptions were used in the TDABC estimate, the results reflect the practices of a single infusion center in an academic tertiary referral setting. However, the administration of rituximab is largely standardized across institutions, and the majority of the estimated costs in this study were related to the cost of the drug itself. Because it is not feasible to account for every cost associated with health care delivery, the TDABC estimate did not account for the costs associated with ancillary services such as billing and human resources. Projections regarding changes in cost and capacity associated with the use of biosimilar or subcutaneous rituximab are based on assumptions of potential cost differences between these formulations and the originator rituximab and current FSS cost for oncology‐use subcutaneous rituximab, respectively. However, these estimates were varied across a broad range of plausible values to confirm that the results are robust. Finally, although observed data were used for many of the sensitivity analyses, simulated estimates were needed in some scenarios because they were not observed in the study (eg, subcutaneous rituximab, home infusion, and severe infusion reaction).
In conclusion, although the costs associated with rituximab administration are substantial, leveraging biosimilar rituximab, subcutaneous formulations, and rapid infusion protocols may reduce societal costs while expanding access to treatment. Whether use of biosimilar or subcutaneous formulations will be of greater benefit from a societal perspective will depend on the safety and efficacy of biosimilars and ultimately the cost of each.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Wallace had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Wallace, Blumenthal, Choi, Stone, Walensky.
Acquisition of data
Wallace, Harkness.
Analysis and interpretation of data
Wallace, Harkness, Blumenthal, Choi, Stone, Walensky.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank John Cotreau, who managed prior authorization requests for the MGH Division of Rheumatology, Allergy, and Immunology; Traci Powers and the MGH MIC staff, who accommodated us in the MIC; Jacob Soumerai from the MGH Oncology Center and Katie Lafleur from the MGH Pharmacy for providing data on rituximab preparation and subcutaneous administration; and Sean Gilligan from the MGH Division of Rheumatology, Allergy, and Immunology for providing salary and cost data.
Dr. Wallace's work was supported by the NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants K23‐AR‐073334 and L30‐AR‐070520) and the Rheumatology Research Foundation (Scientist Development Award). Dr. Walensky's work was supported by a Steven and Deborah Gorlin Massachusetts General Hospital Research Scholar Award from the Massachusetts General Hospital Executive Committee on Research.
Zachary S. Wallace, MD, MSc, Kimberly G. Blumenthal, MD, MSc, Hyon K. Choi, MD, DrPH, John H. Stone, MD, MPH, Rochelle P. Walensky, MD, MPH: Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts; 2Tyler Harkness, BS: Massachusetts General Hospital, Boston, Massachusetts.
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. Centers for Medicare and Medicaid Serves . Dashboard. URL: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Geographic-Variation/GV_Dashboard.html
- 2. RITUXAN (rituximab) [package insert]. San Francisco: Genentech, Inc.; 2019. [Google Scholar]
- 3. Sehn LH, Donaldson J, Filewich A, Fitzgerald C, Gill KK, Ruzner N, et al. Rapid infusion rituximab in combination with corticosteroid‐containing chemotherapy or as maintenance therapy is well tolerated and can safely be delivered in the community setting. Blood 2007;109:4171–3. [DOI] [PubMed] [Google Scholar]
- 4. Swan JT, Zaghloul HA, Cox JE, Murillo JR Jr. Use of a pharmacy protocol to convert standard rituximab infusions to rapid infusion shortens outpatient infusion clinic visits. Pharmacotherapy 2014;34:686–94. [DOI] [PubMed] [Google Scholar]
- 5. Wallace ZS, Harkness T, Fu X, Stone JH, Choi HK, Walensky RP. Treatment delays associated with prior authorization for infusible medications: a cohort study. Arthritis Care Res (Hoboken) 2019. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McBride A, Balu S, Campbell K, MacDonald K, Abraham I. Subcutaneous versus intravenous rituximab in non‐Hodgkin lymphoma treated with R‐CHOP: economic modeling for the US [abstract]. Blood 2018;132 Suppl 1:4776. [Google Scholar]
- 7. De Cock E, Kritikou P, Sandoval M, Tao S, Wiesner C, Carella AM, et al. Time savings with rituximab subcutaneous injection versus rituximab intravenous infusion: a time and motion study in eight countries. PLoS One 2016;11:e0157957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kay J, Schoels MM, Dorner T, Emery P, Kvien TK, Smolen JS, et al. Consensus‐based recommendations for the use of biosimilars to treat rheumatological diseases. Ann Rheum Dis 2018;77:165–74. [DOI] [PubMed] [Google Scholar]
- 9. Shim SC, Bozic‐Majstorovic L, Berrocal Kasay A, el‐Khouri EC, Irazoque‐Palazuelos F, Cons Molina FF, et al. Efficacy and safety of switching from rituximab to biosimilar CT‐P10 in rheumatoid arthritis: 72‐week data from a randomized phase 3 trial. Rheumatology (Oxford) 2019;58:2193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smolen JS, Cohen SB, Tony HP, Scheinberg M, Kivitz A, Balanescu A, et al. A randomised, double‐blind trial to demonstrate bioequivalence of GP2013 and reference rituximab combined with methotrexate in patients with active rheumatoid arthritis. Ann Rheum Dis 2017;76:1598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pritchard CH, Greenwald MW, Kremer JM, Gaylis NB, Rigby W, Zlotnick S, et al. Safety of infusing rituximab at a more rapid rate in patients with rheumatoid arthritis: results from the RATE‐RA study. BMC Musculoskelet Disord 2014;15:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wallace G, Myers KC, Davies SM, Teusink A, Jodele S. Rapid rituximab infusion is safe in paediatric and young adult patients with non‐malignant indications. Br J Haematol 2016;173:480–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al Zahrani A, Ibrahim N, al Eid A. Rapid infusion rituximab changing practice for patient care. J Oncol Pharm Pract 2009;15:183–6. [DOI] [PubMed] [Google Scholar]
- 14. Kaplan RS, Anderson SR. Time‐driven activity‐based costing. Harv Bus Rev 2004;82:131–8, 150. [PubMed] [Google Scholar]
- 15. Daniels KM, Lappi MD, Sporn SF, Caillouette CN, Heald R, Meara JG. Assessing the cost of prophylactic antibiotic use after cleft lip and lip adhesion procedures. J Healthc Manag 2016;61:282–9. [PubMed] [Google Scholar]
- 16. Blumenthal KG, Li Y, Banerji A, Yun BJ, Long AA, Walensky RP. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract 2018;6:1019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Defourny N, Perrier L, Borras JM, Coffey M, Corral J, Hoozee S, et al. National costs and resource requirements of external beam radiotherapy: a time‐driven activity‐based costing model from the ESTRO‐HERO project. Radiother Oncol 2019;138:187–94. [DOI] [PubMed] [Google Scholar]
- 18. Tseng P, Kaplan RS, Richman BD, Shah MA, Schulman KA. Administrative costs associated with physician billing and insurance‐related activities at an academic health care system. JAMA 2018;319:691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaplan RS, Witkowski M, Abbott M, Guzman AB, Higgins LD, Meara JG, et al. Using time‐driven activity‐based costing to identify value improvement opportunities in healthcare. J Healthc Manag 2014;59:399–412. [PubMed] [Google Scholar]
- 20. US Department of Labor, US Bureau of Labor Statistics . Healthcare occupations. URL: https://www.bls.gov/ooh/healthcare/home.htm
- 21. Medical Group Management Association . MGMA 2016 physician compensation and production report. Englewood (CO): Medical Group Management Association; 2016. [Google Scholar]
- 22. US Department of Labor, US Bureau of Labor Statistics . Occupational employment and wages in Boston‐Cambridge‐Nashua — May 2018. URL: https://www.bls.gov/regions/new-england/news-release/occupationalemploymentandwages_boston.htm
- 23. McLaughlin N, Burke MA, Setlur NP, Niedzwiecki DR, Kaplan AL, Saigal C, et al. Time‐driven activity‐based costing: a driver for provider engagement in costing activities and redesign initiatives. Neurosurg Focus 2014;37:E3. [DOI] [PubMed] [Google Scholar]
- 24. Levinson DR. Medicaid drug price comparisons: average manufacturer price to published prices. Washington (DC): US Department of Health and Human Services; 2005. [Google Scholar]
- 25. US Department of Veteran Affairs . Office of procurement, acquisition and logistics (OPAL). URL: https://www.va.gov/opal/nac/fss/pharmPrices.asp
- 26. Carter JD, Zarabadi SA, Ricca LR, McNeil A, Valeriano‐Marcet J, Vasey FB, et al. A safety analysis of oral prednisone as a pretreatment for rituximab in rheumatoid arthritis. Clin Rheumatol 2012;31:1605–10. [DOI] [PubMed] [Google Scholar]
- 27. Bredemeier M, de Oliveira FK, Rocha CM. Low‐ versus high‐dose rituximab for rheumatoid arthritis: a systematic review and meta‐analysis. Arthritis Care Res (Hoboken) 2014;66:228–35. [DOI] [PubMed] [Google Scholar]
- 28. Mulcahy AW, Predmore Z, Mattke S. The cost savings potentional of biosimilar drugs in the United States. URL: https://www.rand.org/content/dam/rand/pubs/perspectives/PE100/PE127/RAND_PE127.pdf RAND Corporation. 2014.
- 29. Banerji A, Rudders S, Clark S, Wei W, Long AA, Camargo CA Jr. Retrospective study of drug‐induced anaphylaxis treated in the emergency department or hospital: patient characteristics, management, and 1‐year follow‐up. J Allergy Clin Immunol Pract 2014;2:46–51. [DOI] [PubMed] [Google Scholar]
- 30. American College of Rheumatology . Position statement: patient saftey and site of service for biologies. 2017. URL: https://www.rheumatology.org/Portals/0/Files/Biologics-Patient-Safety-and-site-of-Service.pdf
- 31. Schmier J, Ogden K, Nickman N, Halpern MT, Cifaldi M, Ganguli A, et al. Costs of providing infusion therapy for rheumatoid arthritis in a hospital‐based infusion center setting. Clin Ther 2017;39:1600–17. [DOI] [PubMed] [Google Scholar]
- 32. Franken MG, Kanters TA, Coenen JL, de Jong P, Koene HR, Lugtenburg PJ, et al. Potential cost savings owing to the route of administration of oncology drugs: a microcosting study of intravenous and subcutaneous administration of trastuzumab and rituximab in the Netherlands. Anticancer Drugs 2018;29:791–801. [DOI] [PubMed] [Google Scholar]
- 33. Mihajlovic J, Bax P, van Breugel E, Blommestein HM, Hoogendoorn M, Hospes W, et al. Microcosting study of rituximab subcutaneous injection versus intravenous infusion. Clin Ther 2017;39:1221–32. [DOI] [PubMed] [Google Scholar]
- 34. Health Resources and Services Administeration . 340B drug pricing program. 2019. URL: https://www.hrsa.gov/opa/index.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


