Abstract
Aim
The prognostic burn index (PBI), which consists of half partial‐thickness burn surface area plus full‐thickness burn surface area and age, has been widely used to predict mortality in Japan. However, the prognostic value of PBI has not been investigated sufficiently. The purpose of the present study is to clinically reevaluate the PBI in severe burn patients.
Methods
Data of 69 severe burn patients admitted to the burn center at Kyorin University Hospital (Tokyo, Japan) from January 2008 to December 2017 were analyzed retrospectively. The primary outcome in this study was in‐hospital mortality.
Results
The overall in‐hospital mortality rate was 34.8%. There were significant differences in age, the presence of inhalation injury, total burned surface area, full‐thickness burn area, burn index, and PBI between survivors and non‐survivors. In logistic regression analysis, PBI was independently associated with mortality, while the presence of inhalation injury was not. A PBI above the threshold of 105 was significantly associated with in‐hospital mortality. The area under the receiver operating characteristic curve for PBI was 0.85 (95% confidence interval, 0.73–0.93).
Conclusion
The PBI could be a good prognostic indicator. A PBI above the threshold of 105 was associated with mortality among severe burn patients treated in burn‐care facilities.
Keywords: Area under curve, burn, inhalation injury, mortality, prognosis
Prognostic burn index was significantly associated with mortality regardless the inhalation injury. Prognostic burn index above the threshold of 105 was associated with in‐hospital mortality.

Introduction
Burns are among the most devastating injuries, and approximately 11 million burn patients require medical attention worldwide annually.1 Although the vast majority of burn patients do not have fatal wounds, severe burns can be fatal despite recent advances in burn care. About 265,000 deaths a year have occurred due to burns throughout the world.2 The prediction of mortality in severe burn patients is crucial, and many types of scoring systems have been developed for predicting mortality.3 Age, burn surface area, and inhalation injury remain fundamental factors for burn prognostication, but relative weighting is different among scoring systems. The scores routinely used worldwide are the revised Baux score,4 Belgian Outcome in Burn Injury (BOBI) score,5 and Abbreviated Burn Severity Index (ABSI).6 However, these scores have not been used in Japan; instead, the prognostic burn index (PBI) has been widely used to predict mortality. The PBI, which consists of half partial‐thickness burn surface area plus full‐thickness burn surface area (burn index: BI) and age, was reported by Yasuda et al. in 1986.7 This score is recommended in the current Japanese Society of Burn Injuries guidelines.8 However, the prognostic value of PBI has not been investigated sufficiently. Moreover, inhalation injury is not considered in the PBI calculation.
The purpose of the present study is to clinically reevaluate the PBI and to investigate the association of inhalation injury with the prognostic value of PBI in severe burn patients.
Methods
Patient selection and outcome
Clinical records of all burn patients admitted to the burn center at Kyorin University Hospital (Tokyo, Japan) from January 2008 to December 2017 were reviewed retrospectively. Severe burn patients with partial‐thickness burn area ≥30% or full‐thickness burn area ≥10% were included. The exclusion criteria were cardiac arrest on arrival and death within 24 h without conventional treatment due to requests from patients’ families. The primary outcome in this study was in‐hospital mortality.
Data collection
Data collected were age, gender, percentage of total burn surface area (TBSA), percentage of partial‐thickness burned area (PTBA), percentage of full‐thickness burned area (FTBA), and presence of inhalation injury with clinical signs and evidence of the bronchoscopy.
Scoring systems
Scoring systems calculated and evaluated were BI, PBI, revised Baux score, BOBI score, and ABSI.
Statistical analysis
Continuous variables were expressed as median and interquartile range, and categorical variables were expressed as numbers with percentages. Continuous variables were compared using Student’s t test or the Mann–Whitney U‐test. Categorical data were compared using the χ2‐test or Fisher’s exact test. Logistic regression analysis was used to estimate the influence of PBI and inhalation injury on mortality. The optimal cut‐off value of PBI for predicting mortality was determined using the Youden index.9 Receiver operating characteristic (ROC) curves were drawn and areas under the curves (AUCs) of ROC curves were calculated for age, BI, PBI, revised Baux score, BOBI score, and ABSI. The ability to predict mortality was assessed according to sensitivity, specificity, positive predictive value, negative predictive value, and AUC.
Statistical analyses were undertaken with StatFlex version 6 (Artech, Osaka, Japan), and JMP 13 (SAS Institute, Cary, NC, USA).
Result
During the study period, 255 burn patients were admitted to Kyorin University Hospital. After selection, 69 severe burn patients were evaluated in the current study (Fig. 1). Table 1 shows the patients’ characteristics. The overall in‐hospital mortality rate was 34.8%. In univariate analysis, there were significant differences in age, the presence of inhalation injury, TBSA, FTBA, BI, and PBI between survivors and non‐survivors. There were no significant differences in the PTBA or gender between the two groups.
Fig. 1.
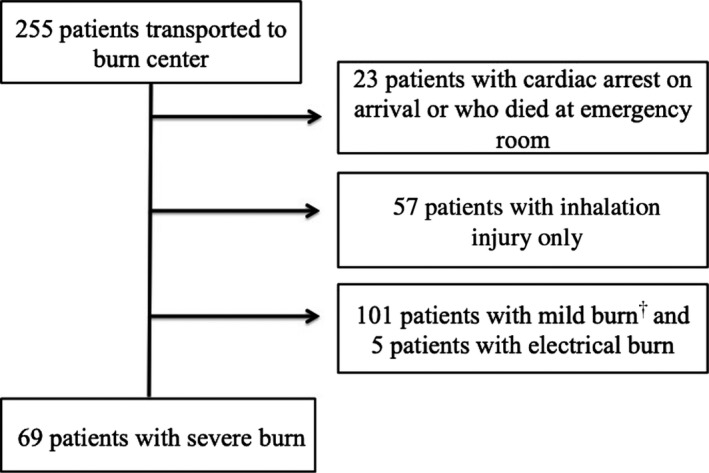
Patient selection for this study. †<30% of partial‐thickness burn area and <10% of full‐thickness burn area.
Table 1.
Characteristics of the study cohort of patients with severe burns
| Variable |
All cases (n = 69) |
Survivors (n = 45) |
Non‐survivors (n = 24) |
P‐value |
|---|---|---|---|---|
| Male, n (%) | 46 (67) | 28 (62) | 18 (75) | ns |
| Presence of inhalation injury, n (%) | 35 (51) | 15 (33) | 20 (83) | <0.0010 |
| Age (years) | 59 (42–70) | 49 (39–69) | 65.5 (52–79.5) | <0.0500 |
| TBSA (%) | 52 (30–80) | 38 (30–57) | 81.5 (57.8–90) | <0.0001 |
| PTBA (%) | 14 (3–30) | 12 (4–32) | 19 (0–24) | ns |
| FTBA (%) | 20 (10–68) | 16 (10–32) | 67.5 (36.5–80) | <0.0001 |
| BI | 33.5 (20–78) | 25 (19–42.5) | 78.5 (47.9–82.3) | <0.0001 |
| PBI | 98 (74–126) | 86 (69.5–106) | 130.3 (105.9–148.3) | <0.0001 |
BI, burn index; FTBA, full‐thickness burn surface area; ns, not significant; PBI, prognostic burn index; PTBA, partial‐thickness burn surface area; TBSA, total burn surface area.
In logistic regression analysis, PBI was independently associated with mortality, whereas the presence of inhalation injury was not (Table 2). In a more detailed evaluation of inhalation injury, we found that there was a significant interaction between the presence of inhalation injury and mortality among burn patients with TBSAs of 21–60% (P < 0.05), whereas there were no significant interactions among those with TBSAs of 1–20%, 61–80%, or 81–100% (Table 3).
Table 2.
Logistic regression analysis for in‐hospital mortality among burn patients (n = 69)
| Variable | Coefficient | Standard error | Wald value | P‐value |
|---|---|---|---|---|
| PBI | −0.05 | 0.01 | 3.32 | 0.009 |
| Inhalation injury | −0.88 | 0.71 | 1.25 | 0.210 |
PBI, prognostic burn index.
Table 3.
Comparison of mortality for total burn surface area (TBSA) with and without inhalation injury
| TBSA (%) | Mortality (%) | P‐value | |
|---|---|---|---|
| Inhalation injury (+) | Inhalation injury (−) | ||
| 1–20 | 50.0 | 0.0 | 0.09 |
| 21–60 | 31.3 | 4.8 | <0.05 |
| 61–80 | 75.0 | 33.3 | 0.20 |
| 81–100 | 71.4 | 66.7 | 0.87 |
Sensitivity and specificity curves were examined to determine the optimal cutoff value of PBI for predicting mortality, and we found the optimal cutoff value of PBI was 105 (Fig. 2). The PBI value of 105 could significantly predict mortality with a sensitivity of 79% (95% confidence interval [CI], 60–91), a specificity of 73% (95% CI, 59–84), a positive predictive value of 61% (95% CI, 44–76), and a negative predictive value of 87% (95% CI, 73–94).
Fig. 2.
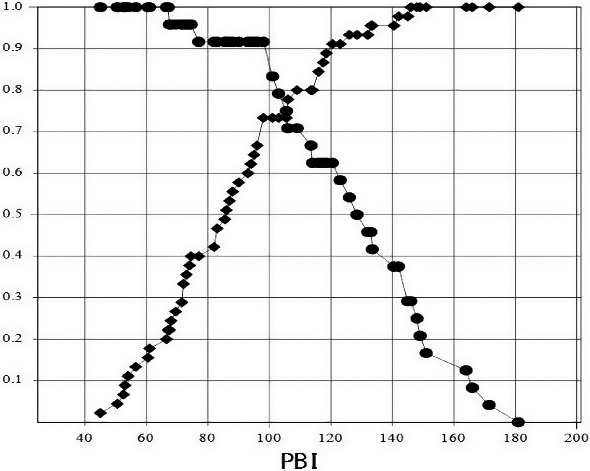
Sensitivity and specificity curves for the prognostic burn index (PBI) associated with hospital mortality among severe burn patients (n = 69). Sensitivity and specificity are reperesented by circle shapes and diamond shapes respectively.
Receiver operating characteristic curves for the ability of age, the BI, and the PBI to predict mortality were examined, and AUCs of the ROC curves were 0.65 (95% CI, 0.51–0.77), 0.79 (95% CI, 0.64–0.88), and 0.85 (95% CI, 0.73–0.93), respectively. Furthermore, the ROC curves for the ability of the PBI, revised Baux score, BOBI score, and ABSI to predict mortality were also examined, and AUCs of the ROC curves were 0.85 (95% CI, 0.73–0.93), 0.90 (95% CI, 0.80–0.95), 0.87 (95% CI, 0.77–0.93), and 0.87 (95% CI, 0.76–0.93), respectively (Table 4). There were no significant differences in the AUCs between the PBI and other scores.
Table 4.
Comparison of area under the receiver operating characteristic curves (AUCs) among scores for the prediction of in‐hospital mortality in burns patients
| AUC | 95% CI | χ2‐test | P‐value | |
|---|---|---|---|---|
| PBI | 0.853 | 0.73–0.93 | ||
| Revised Baux | 0.898 | 0.80–0.95 | 1.26 | 0.26† |
| BOBI | 0.872 | 0.77–0.93 | 0.09 | 0.75† |
| ABSI | 0.866 | 0.76–0.93 | 0.05 | 0.82† |
ABSI, Abbreviated Burn Severity Index; BOBI, Belgian Outcome in Burn Injury; CI, confidence interval.
Compared with prognostic burn index (PBI).
Discussion
In this study, we undertook a retrospective survey over 10 years for all burn patients treated in our burn‐care facility for these. Overall, 255 burn patients were evaluated, and only 69 severe cases were further investigated for analysis. In this sense, the information found in the current study was not a secondary use of so‐called “big data” but real clinical data, which could be greatly beneficial to everyday burn cares in intensive and critical care units. As a result, our study provided two important clinical suggestions: PBI was significantly associated with mortality regardless of complicating inhalation injury among severe burn patients, and a PBI above the threshold of 105 was associated with in‐hospital mortality. There was only one death among 101 excluded milder cases. The patient was in his or her 80s and suffered about 10% of 2nd degree burn (PBI = 92.5), and his or her death was not caused by burn but myocardial infarction. Moreover, even if we use all 170 cases (69 included plus 101 excluded cases) to calculate the optimal cut‐off border, it shifted only slightly around 100 from 105.
This study suggested that PBI was useful for predicting mortality in severe burn patients, consistent with previously published studies. In the present study, PBI was shown to be independently associated with mortality in a logistic regression analysis, and the AUC of the ROC curve for PBI in predicting mortality was 0.85. This value was lower than the previous report, in which Tagami et al.10 reported that the AUC was 0.93. The reason for the difference was speculated that we included only severe burn patients in this study, whereas the previous report used all burn patients including those with slight burns from a “big data” source. Generally speaking, if we recruit a larger number of patients with a wide variety of severity for analyzing data, we expect to obtain better AUC of ROC curves.
The PBI is the sum of half partial‐thickness burn surface area, full‐thickness burn surface area, and age; the presence of inhalation injury is not considered in the calculation of PBI. It is well known that the presence of inhalation injury is significantly associated with mortality,11, 12, 13, 14, 15, 16, 17 however, the present study revealed that inhalation injury was not an independent risk factor for mortality in the multivariate analysis. It could be speculated that patients with severe burns might have a greater risk of fatal outcome regardless of the presence of inhalation injuries, whereas those with mild burns are more sensitive to inhalation injuries. In fact, there was a significant interaction between the presence of inhalation injury and mortality among burn patients with 21–60% TBSA, yet there were no significant interactions among those with TBSA of 61% or more or 1–20% (Table 3). Consistent with our study, a prior study reported no relation between inhalation injury and mortality in larger burns.18 In severe burn patients, the severity of the burn itself, rather than the presence of inhalation, is considered to influence the prognosis. However, mild burn patients have better prognosis with or without inhalation injury.
In the clinical situations for burn treatment, the evaluation of severity and the prediction of mortality could provide useful information during the decision‐making process for severe burn patients. In this study, the threshold of PBI in the association with mortality in severe burn patients was 105. In a previous report, Tagami et al.10 reported that the cut‐off value of PBI in predicting mortality was 85, analyzing nationwide diagnosis procedure combination data. This value was much lower than that of our report, and we think it reasonable that there was a difference between ours and Tagami et al.’s study, because there are a lot of differences in terms of patients’ characteristics and quality of care between the specialized burn‐care facility and other facilities. Furthermore, as stated earlier, deaths from various kinds of causes, other than burn injuries, are included in the big data. Instead, in the current study, we used only severe burn patients and assured all deaths were burn‐related. Therefore, we speculated the cut‐off value of 105 to be helpful for medical staff in burn‐care facilities to make decisions about whether or not to withdraw treatments for severe burn patients.
Although we have shown the usefulness of PBI to predict mortality for burn patients, there are many other scores or indexes. To date, there have been almost no reports about the comparative evaluation between PBI and other scores. In the comparisons of AUCs between PBI and other scores in the present study, the values of the revised Baux score, BOBI score, and ABSI were slightly higher than that of PBI, but the differences were marginal and statistically insignificant. In Japan, the revised Baux score, BOBI score, and ABSI are not common for predicting mortality of burn patients; instead, PBI has been exclusively used, traditionally, because PBI is simple to apply and useful to predict mortality in clinical situations. The usefulness of other scores compared to PBI needs to be further investigated in the future.
Limitations
The present study has several limitations. This was a retrospective study undertaken at a single center, with a relatively small sample size. In order to validate our results, we need multicenter studies with larger study cohorts. Additionally, the severity of inhalation injury was not considered in this study, and patients might be evaluated according to the severity of their inhalation injuries in future studies.
Conclusions
The PBI could be a good prognostic indicator, and a PBI above the threshold of 105 could be associated with mortality among severe burn patients treated in burn‐care facilities.
Disclosure
Approval of the research protocol: This study was carried out according to the guidelines and with the approval of the ethics committee of Kyorin University.
Informed consent: Requirement for informed consent was waived because of the anonymous nature of the data.
Registry and registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
Funding Information
No funding was received.
References
- 1. Peck MD. Epidemiology of burns throughout the world. Part I: distribution and risk factors. Burns 2011; 37: 1087–100. [DOI] [PubMed] [Google Scholar]
- 2. WHO . WHO Health Estimates 2014 summary tables: deaths and global burden of disease, 2014.
- 3. Sheppard NN, Hemington‐Gorse S, Shelley OP, Philip B, Dziewulski P. Prognostic scoring systems in burns: a review. Burns 2011; 37: 1288–95. [DOI] [PubMed] [Google Scholar]
- 4. Osler T, Glance LG, Hosmer DW. Simplified estimates of the probability of death after burn injuries: extending and updating the baux score. J. Trauma 2010; 68: 690–7. [DOI] [PubMed] [Google Scholar]
- 5. Belgian Outcome in Burn Injury Study, G . Development and validation of a model for prediction of mortality in patients with acute burn injury. Br. J. Surg. 2009; 96: 111–7. [DOI] [PubMed] [Google Scholar]
- 6. Tobiasen J, Hiebert JM, Edlich RF. The abbreviated burn severity index. Ann. Emerg. Med. 1982; 11: 260–2. [DOI] [PubMed] [Google Scholar]
- 7. Yasuda K, Henmi H, Yamamoto Y, Mashiko K, Ohtomo Y, Ohtuka T. Nutritional management and assessment on extensively burned patients (in Japanese). Jpn. J. Burn Inj. 1986; 11: 134–8. [Google Scholar]
- 8. Japanese Society for Burn Injuries . Guidelines for the management of burn patients Japanese Society for Burn Injuries, 2015. [cited 25 Jan 2018]. Available from: http://www.jsbiburn.org/members/guideline/pdf/guideline2.pdf (in Japanese).
- 9. Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3: 32–5. [DOI] [PubMed] [Google Scholar]
- 10. Tagami T, Matsui H, Fushimi K, Yasunaga H. Validation of the prognostic burn index: a nationwide retrospective study. Burns 2015; 41: 1169–75. [DOI] [PubMed] [Google Scholar]
- 11. Saffle JR, Davis B, Wiiliams P. Recent outcomes in the treatment of burn injury in the United States: a report from the American Burn Association Patient Registry. J. Burn Care Rehabil. 1995; 16(3 Pt 1): 219–32; discussion 288–9. [DOI] [PubMed] [Google Scholar]
- 12. Raff T, Germann G, Barthold U. Factors influencing the early prediction of outcome from burns. Acta Chir. Plast. 1996; 38: 122–7. [PubMed] [Google Scholar]
- 13. Wolf SE, Rose JK, Desai MH, Mileski JP, Barrow RE, Herndon DN. Mortality determinants in massive pediatric burns. An analysis of 103 children with > or = 80% TBSA burns (> or = 70% full‐thickness). Ann. Surg. 1997; 225: 554–65; discussion 565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryan CM, Schoenfeld DA, Thorpe WP, Sheridan RL, Cassem EH, Tompkins RG. Objective estimates of the probability of death from burn injuries. N. Engl. J. Med. 1998; 338: 362–6. [DOI] [PubMed] [Google Scholar]
- 15. O’Keefe GE, Hunt JL, Purdue GF. An evaluation of risk factors for mortality after burn trauma and the identification of gender‐dependent differences in outcomes. J. Am. Coll. Surg. 2001; 192: 153–60. [DOI] [PubMed] [Google Scholar]
- 16. Muller MJ, Pegg SP, Rule MR. Determinants of death following burn injury. Br. J. Surg. 2001; 88: 583–7. [DOI] [PubMed] [Google Scholar]
- 17. Kobayashi K, Ikeda H, Higuchi R et al Epidemiological and outcome characteristics of major burns in Tokyo. Burns 2005; 31(Suppl 1): S3–11. [DOI] [PubMed] [Google Scholar]
- 18. Barrow RE, Spies M, Barrow LN, Herndon DN. Influence of demographics and inhalation injury on burn mortality in children. Burns 2004; 30: 72–7. [DOI] [PubMed] [Google Scholar]


