ABSTRACT
Human studies have established a positive association between the intake of industrial trans fatty acids and the development of cardiovascular diseases, leading several countries to enact laws that restrict the presence of industrial trans fatty acids in food products. However, trans fatty acids cannot be completely eliminated from the human diet since they are also naturally present in meat and dairy products of ruminant animals. Moreover, bans on industrial trans fatty acids have not yet been instituted in all countries. The epidemiological evidence against trans fatty acids by far overshadows mechanistic insights that may explain how trans fatty acids achieve their damaging effects. This review focuses on the mechanisms that underlie the deleterious effects of trans fatty acids by juxtaposing effects of trans fatty acids against those of cis-unsaturated fatty acids and saturated fatty acids (SFAs). This review also carefully explores the argument that ruminant trans fatty acids have differential effects from industrial trans fatty acids. Overall, in vivo and in vitro studies demonstrate that industrial trans fatty acids promote inflammation and endoplasmic reticulum (ER) stress, although to a lesser degree than SFAs, whereas cis-unsaturated fatty acids are protective against ER stress and inflammation. Additionally, industrial trans fatty acids promote fat storage in the liver at the expense of adipose tissue compared with cis-unsaturated fatty acids and SFAs. In cultured hepatocytes and adipocytes, industrial trans fatty acids, but not cis-unsaturated fatty acids or SFAs, stimulate the cholesterol synthesis pathway by activating sterol regulatory element binding protein (SREBP) 2–mediated gene regulation. Interestingly, although industrial and ruminant trans fatty acids show similar effects on human plasma lipoproteins, in preclinical models, only industrial trans fatty acids promote inflammation, ER stress, and cholesterol synthesis. Overall, clearer insight into the molecular mechanisms of action of trans fatty acids may create new therapeutic windows for the treatment of diseases characterized by disrupted lipid metabolism.
Keywords: elaidic acid, industrial trans fatty acid, ruminant trans fatty acid, inflammation, ER stress, lipid metabolism, cholesterogenesis, cardiometabolic disease
Introduction
trans Fatty acids are unsaturated fatty acids that contain 1 or more unconjugated double bond in the trans configuration. The term trans fats is used to describe triglycerides that are rich in trans fatty acids. Although some trans fatty acids are produced during fermentation in the rumen of ruminant animals, most trans fatty acids are generated during industrial processing through partial hydrogenation of vegetable oils rich in PUFAs. The amount of trans fatty acids in partially hydrogenated vegetable oils can be as high as 60%, with different isoforms of trans-octadecenoic acid (trans 18:1) accounting for 80–90% of the total trans fatty acid content (1–3). Foods containing these industrially produced artificial trans fatty acids carry several benefits including improved texture, better taste, and enhanced shelf life (4–6).
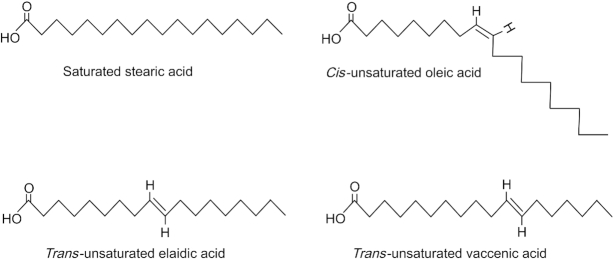
As indicated above, trans fatty acids are defined by the presence of 1 or more unconjugated trans double bond. Fatty acids that contain conjugated trans double bonds, such as conjugated linoleic acid, are considered a separate entity and are only covered briefly in this review. In the trans configuration, the 2 hydrogen atoms around the double bond point in opposite directions, whereas in the cis configuration these hydrogen atoms point in the same direction. In comparison to the cis form, in which the 2 bond angles add up to create a kink in the alkyl chain, in the trans form, the 2 bond angles correct each other, giving rise to a straight chain tertiary structure akin to that of SFAs (Figure 1) (5, 7). Differences in tertiary structure affect crystalline packaging, which in turn influences physicochemical properties such as the melting point. For example, the 18-carbon cis oleic acid is liquid at room temperature, with a melting point of 14°C. By contrast, elaidic acid, which is the trans geometric isomer of oleic acid, has a much higher melting point at 45°C and is solid at room temperature. For comparison, the fully saturated stearic acid has a melting point of 69°C (8). As discussed below, evidence abounds indicating that beyond affecting their geometric isomerization and physicochemical properties, the cis-trans configuration of fatty acids has a major influence on the physiological properties after human consumption.
FIGURE 1.
Structure of the geometric isomers of C18 fatty acids showing elaidic and vaccenic acids with a trans double bond, oleic acid with a cis double bond, and the fully saturated stearic acid.
Evidence on the potential detrimental effects of industrial trans fatty acids first emerged in the 1950s. In 1957 Kummerow and colleagues (9) found that lipid extracts of tissue specimens from 24 human subjects who died of heart disease contained ≤12.2% trans fatty acids in their adipose tissue, 14.4% in the liver, 9.3% in heart tissue, 8.8% in aortic tissue, and 8.8% in atheroma. Subsequent studies showed that the concentration of trans 18:1 and 16:1 fatty acids was 6.8% higher in the adipose tissue of individuals who died of ischemic heart disease compared with individuals who died of other causes (10, 11). In 1990 Mensink and Katan (12) firmly demonstrated the plasma cholesterol-raising effect of industrially produced trans-octadecenoic acids in human volunteers. Several years later Willett and colleagues (13) found in the Nurses’ Health Study that the intake of trans fatty acids from partially hydrogenated vegetable oils, after adjustment for age and total energy intake, was positively associated with the risk of coronary artery disease, with the RR of the highest versus lowest quintile at 1.50 (95% CI: 1.12–2.00, P = 0.001).
The studies that strongly connected the intake of trans fatty acids with cardiovascular derailment were initially abhorred by players in the margarine industry. However, this later fueled more research into the potential atherogenicity of trans fatty acids. Collectively, these studies strongly suggest a causal relation between industrial trans fatty acid consumption and the development of cardiovascular disease in humans (13–18). In response to this finding, a number of countries enacted laws that either restricted or completely banned food companies from incorporating trans fatty acids into their food products. Two independent studies found that New York State counties that had 3 or more years of restrictions of industrial trans fatty acids had an average 7.8% reduction in the incidence of myocardial infarction and a 4.5% reduction in cardiovascular disease mortality rates compared with counties with no such restrictions (19, 20).
In many countries food items such as margarine, crackers, bakery products, cookies, and deep-fried foods were previously loaded with industrial trans fatty acids. Nowadays however, the concentrations of trans fatty acids in these foods are very low, which has resulted in a substantial decline in the intake of industrial trans fatty acids (21–30). For instance, the 2018 Dutch Nutrition Survey reported that in 2018, trans fatty acids only provided ∼0.3% of the daily energy requirement, as opposed to 5–10% several decades ago. However, industrial trans fatty acids persist in our food supply because laws aimed at restricting industrial trans fatty acids have not yet been instituted in every country. In addition, trans fatty acids cannot be completely removed from human diets due to their presence in the meat and dairy products of ruminants. These trans fatty acids, which include trans-vaccenic acid and rumenic acid, are generated through the biohydrogenation of PUFAs in the rumen of these animals (1, 31, 32). Ruminant fats can contain ≤8% of trans fatty acids. Trans-vaccenic acid is the predominant form and represents 50–80% of the total ruminant trans fatty acid intake (29, 32, 33).
As health concerns connected to the consumption of industrial trans fatty acids can be mitigated by removing them from foods, there has been little incentive for researchers to probe mechanistic insights related to trans fatty acids. The end result is a marked imbalance between the vast amount of epidemiological data on trans fatty acids and health outcomes and the limited understanding of the molecular mode of action of trans fatty acids. By collating the mechanistic studies on trans fatty acids, this review aims to improve our understanding of the mechanisms underlying the deleterious effects of trans fatty acids. The focus is on the physiological, metabolic, and molecular pathways affected by trans fatty acids. To achieve this objective, some parallels will be drawn between trans fatty acids and other relevant types of fatty acids. In addition, this review will carefully dissect the argument that ruminant trans fatty acids have contrasting effects to industrial trans fatty acids.
Potential Adverse Outcome Pathways of trans Fatty Acids
Although the majority of studies performed on trans fatty acids are observational, a substantial number of experimental studies have been performed. These studies vary from experiments in cultured cells and animal models to human clinical trials. A number of these studies have provided evidence that certain trans fatty acids influence the regulation of physiological processes such as lipid metabolism, inflammation, oxidative stress, endoplasmic reticulum (ER) stress, autophagy, and apoptosis. The dysregulation of some of these biological pathways by trans fatty acids has been proposed as a potential underlying mechanism that contributes to the negative effects of trans fatty acids on cardiometabolic health (34–36). These potential adverse outcome pathways are further explained in detail in the following sections.
Plasma Cholesterol and Lipoprotein Profile
Cholesterol is transported in the blood mainly as part of LDL and HDL. Smaller amounts of cholesterol are also contained in the triglyceride-rich chylomicrons, VLDL, and their remnant particles. Increased concentrations of LDL cholesterol, non-HDL cholesterol, or apoB-containing particles increase the risk of atherosclerotic cardiovascular disease (37–39).
Several studies have examined the effect of trans fatty acids on plasma cholesterol concentrations and lipoprotein dynamics. A randomized crossover trial carried out at Wageningen University in 1990 randomly assigned 59 participants to each of 3 isocaloric diets for 3 wk in which 10% of the daily energy was provided by either oleic acid, trans isomers of octadecenoic acid, or palmitic and lauric acid. When compared with the oleic acid diet, the trans fatty acid diet significantly reduced the serum concentrations of HDL cholesterol by 12%, and increased the concentrations of total and LDL cholesterol by 5.8% and 13.9%, respectively. By contrast, the SFA diet did not significantly affect serum HDL cholesterol but significantly raised the concentrations of total and LDL cholesterol by 12.1% and 17.6%, respectively (12). Significant changes in plasma lipoproteins were also recorded in a similar crossover study conducted in 56 participants. Mean serum concentrations of LDL cholesterol were 6.0% higher after an SFA-enriched diet than after a cis-unsaturated fatty acid diet, and 8.4% higher after a trans-unsaturated fatty acid diet. Total serum cholesterol concentrations were 3.2% and 3.4% higher after the saturated and trans-unsaturated diets, respectively, compared with the cis-unsaturated diet. Meanwhile, HDL cholesterol concentrations were 4.1% and 6.8% lower after the saturated and trans-unsaturated diets, respectively, than after the cis-unsaturated diet (40). This unique property of industrial trans fatty acids to simultaneously increase the circulating concentrations of total and LDL cholesterol whilst decreasing the concentrations of HDL cholesterol is believed to result in stronger atherogenicity compared with saturated or cis-unsaturated fatty acids.
Several additional studies examined the ability of industrial trans fatty acids to modulate plasma cholesterol. In a randomized crossover study, 32 healthy men and women were assigned for 4 wk to either a diet providing 9.2% energy as industrial trans fatty acids from partially hydrogenated soybean oil or 12.9% energy as SFAs from palm kernel fat. The trans fatty acid diet decreased serum HDL cholesterol concentrations by 19% yet did not significantly affect LDL cholesterol and triglyceride concentrations in comparison with the SFA diet (41). In another crossover study with each dietary intervention lasting 5 wk, 50 normocholesterolemic men consumed 8% of daily energy as either trans 18:1 or stearic acid. Compared with the stearic acid diet, the trans fatty acid diet resulted in significantly higher plasma concentrations of total (0.22 mM, 4.5%) and LDL cholesterol (0.26 mM, 8.35%), with no significant differences in HDL cholesterol concentrations (42).
Mauger and colleagues (43) reported that the athero-genicity of trans fatty acids may lie in part in their ability to reduce LDL particle size in a dose-dependent manner. Matthan and colleagues (44) have likewise shown in hypercholesterolemic women that the deleterious lipoprotein profile caused by trans fatty acid intake is partly explained by increased apoA1 and decreased apoB100 catabolism. Interestingly, a limited number of studies did not find a significant deleterious effect of trans fatty acid intake on plasma lipid concentrations (45, 46). These discrepant results are potentially due to the use of a different reference or control diet. Additionally, differences in amounts of trans fatty acids consumed may account for some of the conflicting findings, as the effects of trans fatty acids on plasma lipids seem proportional to intake (47).
Studies performed in rodent models support the plasma LDL-raising effect of trans fatty acids. In LDL-receptor knockout mice, provision for 16 wk of a diet enriched with 34.7 g of elaidic acid from partially hydrogenated soybean oil per 100 g/fat increased plasma concentrations of total cholesterol, LDL cholesterol, and triglycerides by 5.5-fold, 3.2-fold, and 3.8-fold, respectively, when compared with an isocaloric diet rich in PUFAs. Concentrations of plasma HDL cholesterol did not differ significantly (48). A similar but independent study performed under the same experimental conditions in the same animal model also resulted in significant increases in plasma concentrations of total cholesterol by 2.1-fold, LDL cholesterol by 1.7-fold, triglycerides by 4.2-fold, and a reduction in HDL cholesterol by 2.3-fold (49). Overall, it can be concluded that the positive association between trans fatty acid intake and cardiovascular disease risk is likely, at least in part, explained by the unfavorable effect of trans fatty acids on plasma LDL cholesterol and the overall lipoprotein profile.
Inflammation
Sterile inflammation describes inflammation that occurs in the absence of an infection and is a symptom of numerous chronic diseases and pathologies, including atherosclerosis. Indeed, in addition to being a lipid-driven process, atherosclerosis is primarily an inflammatory disease characterized by the accumulation of macrophage foam cells in the vascular wall, triggering the secretion of numerous inflammatory mediators and leading to the recruitment of other immune cells (50). One mechanism by which trans fatty acids may promote atherogenesis is by activating inflammation (36). In a randomized controlled trial in healthy men, a diet containing 8% of daily energy from industrial trans fatty acids caused a 3.4-fold increase in plasma concentrations of C-reactive protein (CRP) after 5 wk of intake compared to a control diet with no trans fatty acids (51). In subjects with moderate hypercholesterolemia, the consumption of industrial trans fatty acids from stick margarine at 6.7% of total energy for 32 d significantly increased concentrations of TNFα, IL-1β and IL-6 in peripheral blood mononuclear cells (PBMCs) compared with PUFAs from soybean oil (52). Furthermore, in 2 independent cross-sectional studies, 1 conducted in overweight women (53) and the other in patients with heart disease (54), the intake of foods rich in industrial trans fatty acids positively correlated with plasma concentrations of inflammatory markers such as CRP, TNFα, chemokine (C-C motif) ligand 2 (CCL2), and IL-6, after adjustment for various factors.
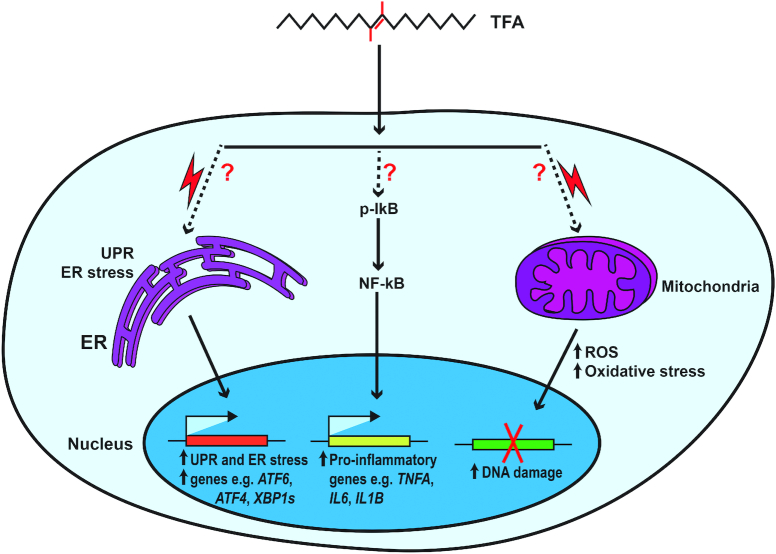
Due to ethical constraints and other challenges connected with human studies, mechanistic probing into the proinflammatory effects of trans fatty acids has been conducted in animal models and via in vitro experiments. Using atherosclerosis-prone LDL-receptor knockout mice, it was shown that compared with mice fed a diet rich in PUFAs, 16 wk of diets enriched with elaidic acid from partially hydrogenated soybean oil significantly increased the release of the inflammatory cytokine IL-6 by ∼1.5-fold (48), as well as the expression of Ccl2, Tnfa, and Il-6 by ≥2-fold in abdominal aortas (49). Secondary to the induction of inflammation, the mice on the trans fatty acid diet showed increased accumulation of activated macrophages in the enlarged atherosclerotic lesions in the aortic intima (48, 49). Another animal study reported that ad libitum feeding of C57BL/6 mice for 16 wk with a trans fatty acid diet, providing ∼13% of energy intake from partially hydrogenated vegetable oil, resulted in a hepatic necroinflammatory phenotype, characterized by a 4.4-fold increase in hepatic Tnfa expression compared with a control diet (55). The proinflammatory effect of trans fatty acids seems robust and transcends specific animal models. In peroxisome proliferator–activated receptor (PPAR) α–deficient mice (56), as well as in mice with full body or liver-specific knockout of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) (57), trans fatty acid-enriched diets enhanced activation of NF-κB and increased hepatic expression of Tnfa, Ccl2, Opn (osteopontin), and macrophage markers (56, 57). A number of in vitro studies have examined the mechanism of action of trans fatty acids using different cell types. Please note that the concentration of fatty acids used in all in vitro studies included in this review ranges from 0.05 to 0.5 mM, with some exceptions, which can be considered physiological, as the total plasma trans fatty acid concentrations in humans can reach as high as 0.6 mM and 0.09 mM in nonfasting (58) and fasting (59) conditions, respectively. At the cellular level, the activation of the transcription factor NF-κB by the industrial trans elaidic and linoelaidic acids but not cis-linoleic acid was demonstrated in human microvesicular endothelial cells. Compared with untreated controls and linoleic acid, 0.05 mM and 0.1 mM concentrations of elaidic or linoelaidic acid resulted in profound activation of NF-κB signaling, demonstrated by an ∼2-fold increase in IκB-α phosphorylation and subsequent elevation in IL-6 concentrations and TNFα expression (60). So far, the induction in NF-κB signaling is the most plausible mechanism by which trans fatty acids stimulate inflammation, even though the experimental studies supporting this hypothesis are very few and do not interrogate this pathway in great detail (Figure 2). In a recent study, Hirata and colleagues showed that in comparison to both control and oleic acid, 12-h incubation with 0.2 mM elaidic acid induced cleavage of caspase 3 leading to enhanced apoptotic cell death in RAW264.7 macrophages. The elaidic acid effect was mediated through hyperactivation of the apoptosis signal-regulating kinase 1 (ASK1)-p38 mitogen-activated protein (MAP) kinase pathway, which is reported to promote inflammatory signal transduction (61).
FIGURE 2.
Proposed molecular mechanisms of trans fatty acids. ATF4, activating transcription factor 4; ATF6, activating transcription factor 6; ER, endoplasmic reticulum; p-IκB, phosphorylated IκB; ROS, reactive oxygen species; TFA, trans fatty acid; UPR, unfolded protein response; XBP1s, X-box binding protein 1, spliced form; ?, represents unclarified mechanism.
Whereas the above studies point to proinflammatory effects of trans fatty acids, a number of other studies have reported neutral or anti-inflammatory effects of certain trans fatty acids. We recently showed that in angiopoietin-like 4 (ANGPTL4)-deficient C57BL/6 mice, a 7-wk intervention with a high-fat diet supplying ∼14% of calories as trans fatty acids from partially hydrogenated soybean oil did not induce inflammation compared with an isocaloric diet containing SFAs. Mice deficient in ANGPTL4, an endogenous inhibitor of lipoprotein lipase, are unique in that they develop a massive acute phase response upon consumption of a diet rich in SFAs. The SFA-enriched diet markedly increased hepatic expression of serum amyloid A (SAA), haptoglobin, and lipocalin along with a concomitant elevation in the plasma concentrations of these inflammatory markers by ≥50-fold, whereas the trans fatty acid-enriched diet did not (62). Corroborative data were obtained in vitro in RAW264.7 macrophages incubated for 6 h with 0.5 mM fatty acids. Compared with the vehicle control, elaidic acid had a neutral effect or modestly decreased expression of inflammatory markers, whereas palmitic acid and stearic acid induced the expression of inflammatory markers by 2 or more fold, leading to increased protein concentrations of inflammatory and apoptotic markers (62). Accordingly, whether trans fatty acids should be classified as proinflammatory may depend on the reference, e.g., no treatment, cis-unsaturated fatty acids, or SFAs. Notwithstanding these considerations, experimental data from clinical studies, along with animal and cell culture experiments, suggest that the deleterious effects of trans fatty acids may be partly mediated by activating inflammation, especially when compared with cis-unsaturated fatty acids.
ER Stress, Reactive Oxygen Species Production, and Oxidative Stress
At the cellular level, environmental, physiological, and pathological insults can generate varying degrees of stress, such as ER and oxidative stress. ER stress impairs ER function and leads to activation of the unfolded protein response (UPR). The UPR comprises a signaling cascade that promotes cell survival but can also lead to apoptotic cell death (63). High concentrations of reactive oxygen species (ROS) due to an imbalance between ROS production and antioxidant defense can result in oxidative stress and subsequent damage to lipids, DNA, and protein (64, 65).
When present in excessive amounts, certain fatty acids, including trans fatty acids, are known to be injurious to cells due to their modulatory effects on ER stress, ROS production, and oxidative stress (35). In a mouse model of hyperlipidemia, 8 wk of feeding a diet containing 5% elaidic acid significantly increased superoxide production compared with a diet containing 5% oleic acid, concomitant with increased atherosclerotic lesion size and increased NADPH oxidase expression in the aortic vessel wall (66). The authors confirmed the in vivo findings in cultured smooth muscle cells incubated for 24 h with 0.1 mM elaidic acid to further underscore the role of ROS and oxidative stress as mediators of trans fatty acid-induced atherosclerosis (66). In C57Bl6/J mice (67) and Wistar rats (68), the presence of industrial trans fatty acids in the diet resulted in hepatic lipotoxicity characterized by an increase in oxidative stress and a decrease in hepatic antioxidant activity of catalase, superoxide dismutase, and glutathione peroxidase.
Events in the endothelial lining of blood vessels are known to be important in the pathogenesis of vascular diseases. Human umbilical vein endothelial cells incubated for 24 h with elaidic and linoelaidic acid induced apoptosis through increased ROS production and raised the activity of caspase 3 in a dose-dependent manner from 0.1 to 1.0 mM (69). Furthermore, in human microvascular endothelial cells, a 3-h treatment with elaidic and linoelaidic acid at 0.05 mM and 0.1 mM increased superoxide production leading to vascular inflammation through enhanced NF-κB signaling (60). One study explored the possible mechanisms behind the neurotoxic effects of elaidic acid in SH-SY5Y neuroblastoma cells, which were incubated for 24 h with vehicle control or increasing concentrations of elaidic acid ranging from 0.01 mM to 0.8 mM. Along with increased ER stress, elaidic acid concentrations higher than 0.1 mM induced ROS production and decreased the activity of antioxidants, which resulted in oxidative damage and apoptosis (70).
By contrast, in RAW264.7 macrophages treated with 0.5 mM fatty acids for 24 h, elaidic acid showed a similar inability to oleic acid to induce ER stress. Whereas palmitic acid potently induced ER stress characterized by enhanced mRNA and protein expression of several stress markers, including C/EBP homologous protein (CHOP) and spliced activated form of X-box binding protein 1 (XBP1s), elaidic acid had only a modest to no effect (62). Taken together, there is some evidence suggesting that trans fatty acids promote oxidative and ER stress when compared with cis-unsaturated fatty acids, which may contribute to their deleterious effects.
Autophagy
Autophagy describes an adaptive response to stress during which organelles and other intracellular components are degraded in the lysosome as a way of recouping energy substrates for cell survival and maintenance of cell integrity. Autophagy involves a complex signaling cascade ranging from phagophore formation to the proteolytic degradation of engulfed targets by lysosomal proteases (71, 72). Few studies have investigated fatty acids as triggers of autophagy (73). Incubation of primary cardiac myofibroblasts with 0.2 mM and 0.4 mM trans-vaccenic and elaidic acid for 24 h induced autophagy, as shown by autophagosome formation, microtubule-associated protein 1A/1B-light chain (LC) 3B (LC3-β) lipidation, LC3-II formation, and autophagy-related proteins 5-12 (ATG5-12) accumulation, leading to apoptosis, as reflected by elevated concentrations of cleaved caspases 9, 3, 7, and enhanced translocation of Bcl-2-associated X protein (Bax) to the mitochondria (74). These results indicate that trans fatty acids are potent stressors to myofibroblasts culminating in premature apoptosis. A recent study, however, reported contrasting results in U2OS cells stably expressing different biosensor markers of autophagy, UPR, and Golgi stress. In these cells, 6-h incubation with 0.5 mM elaidic or trans-vaccenic acid inhibited autophagy induced by SFAs (75). The suggestion was raised that the lipotoxicity of trans fatty acids may reside in their ability to inhibit the cytoprotective stress response induced by SFAs (75). So far, it is rather difficult to make tangible conclusions from the few available studies. Additional work is needed to clarify the exact role of trans fatty acids in autophagy and the implications for cardiometabolic health.
Molecular Effects of trans Fatty Acids on the Liver
In the liver, an intricate balance exists between the uptake, storage, synthesis, secretion and oxidation of lipids in order to maintain local and systemic lipid homeostasis. Aberrant hepatic lipid metabolism can lead to dyslipidemia and nonalcoholic fatty liver disease (NAFLD), both of which are important risk factors for cardiovascular disorders (76, 77). A number of studies have shown that the liver bears the brunt of trans fatty acid-mediated pathology. In mice, the intake of diets providing 12–20% of energy as industrial trans fatty acid over a period of 7 to 24 wk was shown to promote liver damage, characterized by elevated concentrations of plasma alanine aminotransferase activity and increased plasma concentrations of acute phase proteins, including SAA and haptoglobin (55, 78–80). Furthermore, histological staining of liver slices showed profound fat accumulation in mice fed diets rich in industrial trans fatty acids as well as histological indications of nonalcoholic steatohepatitis and cirrhosis (55, 56, 81, 82). In a number of animal studies, the steatotic liver phenotype was associated with increased expression of genes involved in lipogenesis, including fatty acid synthase (Fasn), acetyl-CoA carboxylase α (Acaca), and sterol regulatory element binding protein (Srebp1) (56, 80, 82). In a recent study, we found that a 7-wk intervention with a diet supplying ∼14% of calories as trans fatty acids from partially hydrogenated soybean oil increased the ratio of liver to gonadal fat mass by ∼2-fold, plasma alanine aminotransferase activity by ∼3-fold, acute phase proteins haptoglobin by ∼10-fold, and SAA by ∼2-fold in comparison with diets enriched in cis-unsaturated fatty acids and SFAs. Additionally, the diet rich in industrial trans fatty acids increased steatosis, hepatic cholesterol concentrations, and fibrosis markers, suggesting enhanced NAFLD (78).
In conjunction with these studies in mice, several studies have examined the effect of elaidic acid on lipid metabolism in human and murine hepatoma cells. In human Huh-7 cells, compared with oleic acid and nontreated controls, treatment with 0.1 mM elaidic acid for 16 h stimulated SREBP1c-dependent lipid synthesis through enhanced expression of SREBP target genes, leading to an ∼2-fold increase in de novo synthesis of cholesterol and fatty acids (83). Luciferase reporter gene assays indicated that elaidic acid potently induced sterol regulatory element (SRE)-luciferase activity in HEK293 cells, whereas oleic acid inhibited SRE activity (83).
Nielsen and colleagues applied an integrated lipidomics, transcriptomics, and proteomics approach in human HepG2 cells incubated with 0.1 mM fatty acids for 24 h. Elaidic acid, but not oleic or stearic acid, potently increased the expression of key enzymes involved in cholesterol and fatty acid biosynthesis. Relative to oleic acid, elaidic acid upregulated SREBP2 expression by 2.3-fold along with increased expression of several other cholesterogenic genes such as HMG-CoA reductase by 2.9-fold, squalene epoxidase by 3.3-fold, and mevalonate kinase by 2.2-fold (84). We confirmed the findings of Nielsen and colleagues in murine Hepa1–6 hepatocytes treated for 24 h with 0.5 mM fatty acids, and showed an unequivocal role of SREBP2 in mediating the effect of trans fatty acids on lipid/cholesterol metabolism (78). Specifically, we found that elaidic acid potently upregulates the expression of genes involved in cholesterol synthesis and induces the expression and activity of SREBP2. Silencing of Srebp2 by siRNA-mediated knockdown abrogated the cholesterogenic effect of elaidic acid. The Srebp2 activation by elaidic acid is likely due to lowered concentrations of intracellular free cholesterol since cholesterol is a negative regulator of the SREBP signaling pathway. A previous study reported that elaidic acid is a high affinity substrate for the esterification of cholesterol into cholesterol esters (85), which might explain why elaidic acid reduces concentrations of intracellular free cholesterol. In support of this hypothesis, elaidic acid was shown to increase the expression of the cholesterol esterification enzyme, sterol O-acyltransferase 1 (Soat1), by 2.1-fold compared with oleic acid (84). In our study, although elaidic acid significantly lowered intracellular concentrations of free cholesterol by almost half, there was no concomitant increase in concentrations of cholesterol ester compared with the nontreated control (78). Furthermore, silencing of Soat1 did not abrogate the induction of cholesterol synthesis genes by elaidic acid. Accordingly, enhanced cholesterol esterification likely does not explain the increase in SREBP2 activity by elaidic acid. Rather, elaidic acid appears to decrease the sensitivity of SREBP cleavage-activating protein (SCAP) to cholesterol (78). Overall, the cholesterogenic effect of elaidic acid can at least partly be ascribed to its ability to reduce the concentrations of intracellular free cholesterol and decrease the sensitivity of SCAP to cholesterol. This anabolic effect of elaidic acid may contribute to the deleterious effect of industrial trans fatty acids on lipid metabolism.
Molecular Effect of trans Fatty Acids on Adipose Tissue
Compared with the liver, fewer studies have reported the effects of trans fatty acids on adipose tissue. Most human studies that have investigated the effects of trans fatty acids on adipose tissue have focused on conjugated linoleic acids (CLAs). CLAs are a distinct class of naturally occurring trans fatty acids that require their own full review, as done elsewhere (86, 87), and therefore will only be discussed briefly here. In the USA, 10E,12Z CLA is marketed commercially as a natural weight loss supplement due to its reported ability to reduce adipose tissue mass (88, 89). Notwithstanding their potential health benefits, a number of studies have revealed that intake of CLAs may cause negative side effects such as increasing plasma markers of inflammation and oxidative stress (88), liver damage (90), and inflammation in macrophages (91). In mice, diets that contain 10E,12Z CLA remarkably reduced fat mass yet at the same time promoted hyperinsulinemia, adipose tissue inflammation, and liver damage (92–95). Treatment of 3T3-L1 adipocytes with 0.25 mM 10E,12Z CLA for 7 d stimulated lipolysis and fatty acid oxidation, leading to enhanced lipid utilization and decreased triglyceride storage, when compared with 9Z,11E CLA, palmitic acid, or the nontreated control (96). At the molecular level, 10E,12Z CLA reduced the expression of lipogenic genes such as Acaca, diacylglycerol O-acyltransferase 1 and 2 (Dgat1 and Dgat2), by ≥2-fold, and increased the expression of the fat oxidation gene, carnitine palmitoyltransferase 1A (Cpt1a), by ∼3.5-fold compared with the nontreated control. Interestingly, the increased fatty acid oxidation in adipocytes was associated with increased mitochondrial ROS production and a proinflammatory response, characterized by the increased expression of Ccl2 and Il6 (96).
Apart from CLAs, a diet enriched with elaidic acid reduced fat tissue mass in LDL-receptor knockout mice along with an increase in liver mass and liver steatosis (82). Consistent with these findings, and as previously mentioned, feeding wild-type C57BL/6 mice a high-fat diet rich in industrial trans fatty acids reduced adipose tissue mass by ∼30% but increased liver mass by ∼40% compared with isocaloric diets rich in cis-unsaturated fatty acids or SFAs (78). The decrease in adipose tissue mass was accompanied by a strong upregulation of genes involved in fatty acid and cholesterol synthesis in both gonadal and inguinal fat tissue depots (78, 97). The evidence from these studies suggests that diets rich in trans fatty acids cause preferential fat accumulation in the liver at the expense of adipose tissues. The adipose tissue seems to compensate for this anomaly by stimulating de novo lipogenesis. The molecular mediators accounting for the preferential fat trafficking to the liver remain unknown. These intriguing observations warrant further investigation, as any mechanistic insight gained could be utilized to redirect fat towards specific tissues under different physiological states to reduce ectopic fat accumulation and potentially diminish NAFLD.
Mechanisms of Action of Industrial and Ruminant trans Fatty Acids
There is an ongoing debate as to whether industrially produced and naturally produced ruminant trans fatty acids exert the same effects on cardiovascular health. A number of human studies have reported that the harmful effects of trans fatty acids are exclusive to industrial trans fatty acids (1, 31, 98), whereas ruminant trans fatty acids are reported to be innocuous or even beneficial to cardiovascular health (99–101). In contrast, other epidemiological and clinical studies have reported that ruminant trans fatty acids are equally culpable as industrial trans fatty acids in promoting cardiovascular diseases (102–105). Below we will delineate the possible differences between industrial and ruminant trans fatty acids at the molecular level.
With respect to plasma lipoproteins, Gebauer and colleagues compared the effects of isocaloric diets containing 3.3% energy as either stearic acid (control diet), trans-vaccenic acid, or industrial trans fatty acids in a randomized, crossover feeding trial in 106 healthy subjects who were each provided the diets for 24 d. Compared with the control diet, the industrial trans fatty acid diet increased total and LDL cholesterol by 1.9% and 3.4%, respectively, whereas the ruminant trans fatty acid diet resulted in the greatest increase in total and LDL cholesterol by 4.5% and 6.1%, respectively, leading to the conclusion that both vaccenic acid and trans fatty acids derived from partially hydrogenated oil adversely affect LDL cholesterol. Furthermore, the ruminant trans fatty acid diet marginally increased HDL cholesterol by 2.1%, whereas the industrial trans fatty acid diet had no significant effect on HDL cholesterol (105). Brouwer and colleagues conducted a quantitative review in which they examined the effects of industrial and ruminant trans fatty acids in humans. To eliminate differences in control treatments and allow for comparison between the studies, for each study the authors recalculated what the effect of trans fatty acid on lipoprotein would be if they isocalorically replaced cis MUFAs. For each percentage of dietary energy, linear regression analysis showed that industrial and ruminant trans fatty acids both increased the plasma ratio of LDL to HDL cholesterol by 0.055 and 0.038, respectively (104). The above studies demonstrate that when the consumption levels of ruminant and industrial trans fatty acids are matched, they have similar effects on plasma lipoproteins.
Differences in biological mechanisms between ruminant and trans fatty acids have been examined using preclinical models in animals and cultured cells. In 1 study, LDL-receptor knockout mice were fed for 14 wk with a diet containing either 4% partially hydrogenated vegetable shortening providing 1.5% trans fatty acids in the form of elaidic acid, or 15% butter providing 1.5% ruminant trans fatty acids in the form of vaccenic acid. Compared with a control diet that contained no trans fatty acids, the elaidic acid diet increased atherosclerotic plaque formation by ∼5% of the aortic luminal surface area, whereas the vaccenic acid diet increased the plaque size by <1%. When each of the diets was supplemented with 2% cholesterol, the relative atheroprotective effect of vaccenic acid was potentiated as the vaccenic acid diet significantly reduced atherosclerotic plaque formation by 6.2% of the aortic luminal surface area, whereas the elaidic acid diet showed no significant changes in comparison to the control diet (106). Hence, although ruminant and industrial trans fatty acids appear to have similar effects on plasma lipoprotein concentrations in humans, the few studies in animal models suggest a protective effect of ruminant trans fatty acids against cardiovascular disease. This highlights the importance of other potential mechanistic differences with respect to inflammation, ROS production, ER stress, and oxidative stress, as previously discussed.
In cultured cells, ruminant trans-vaccenic and palmitoleic acid but not elaidic acid potently reduced mRNA expression of TNFA in a dose-dependent manner in both HUVEC and HepG2 cells compared with the vehicle control (107). A similar study by Iwata and colleagues showed that a 3-h treatment of ≤0.1 mM elaidic acid activated NF-κB signaling and increased superoxide production in microvesicular endothelial cells, whereas trans-vaccenic acid showed no such response (60). It was suggested that the anti-inflammatory effect of ruminant trans fatty acids may be due to their property as better ligands for PPARγ. trans-Vaccenic acid was shown to activate the transcriptional activity of PPARγ and/or PPARα in the JCR:LA-cp rat model of dyslipidemia and metabolic syndrome (108–110) and in PBMCs (111). In human PBMCs, incubation with 0.1 mM vaccenic acid for 19 h significantly decreased the intracellular content of IL-2 and TNFα in T-helper cells by 38% and 31%, respectively, compared with the untreated control. The vaccenic acid effect was dependent on PPARγ since the presence of the PPARγ antagonist, T0070907, restored the IL-2 and TNFα-positive T-helper cell population (111). Although the number of studies is limited, the molecular effects of ruminant trans fatty acids may be mediated by PPARs, whereas industrial trans fatty acids regulate transcription via SREBPs, leading to an increase in lipo- and cholesterogenic gene expression. Indeed, in HepG2 cells, elaidic acid increased protein concentrations of enzymes involved in cholesterol synthesis and transport, whereas trans-vaccenic acid did not (112). Similarly, in Hepa1–6 and 3T3-L1 adipocytes, 24-h incubation with 0.5 mM elaidic acid distinctly stimulated activity of SREBP2 leading to induced expression of cholesterol and fatty acid synthesis genes, which was not observed with ruminant trans fatty acids including vaccenic acid (78).
Other pathways, such as cytotoxicity and inflammation, may be similarly regulated by industrial and ruminant trans fatty acids. In RAW264.7 macrophages, both elaidic acid and trans-vaccenic acid induced apoptotic cell death and inflammation (61). In a separate study in RAW264.7 macrophages treated for 24 h with fatty acids, palmitic acid potently induced gene markers of inflammation and ER stress, which could not be reproduced with either industrial or ruminant trans fatty acids (62). With respect to autophagy, a study has juxtaposed the effects of industrial and ruminant trans fatty acids by incubating U2OS cells for 6 h with 0.5 mM elaidic or trans-vaccenic acids, showing that both trans fatty acids similarly inhibited autophagy induced by SFAs (75).
Taken together, evidence from both preclinical and clinical studies show that industrial and ruminant trans fatty acids can behave similarly or differentially, depending on the biological pathway and clinical parameters under investigation. As mentioned earlier, it has been suggested that the alleged positive effects of ruminant trans fatty acids could be due to a relatively low intake compared with industrial trans fatty acids. Nonetheless, the underlying mechanisms for the reported differences are still unclear. From a biochemical perspective, the differences in the source and slight change in the position of the trans double bond may not justify the separate classification of industrial and ruminant fatty acids. However, there are numerous examples of bioactive molecules in which a slight change in molecular structure has a profound impact on the biological properties. Indeed, the position of the trans double bond could impact the extent to which fatty acids are taken up, sensed, metabolized, and incorporated into cellular organelles and membranes. Such potential mechanisms are still poorly delineated and warrant further investigation.
Physiological Relevance of Animal and In Vitro Studies to Humans
The direct relevance of many animal and in vitro studies to humans is certainly debatable. This review has explored mechanistic insights of trans fatty acids from studies in mice and in vitro models. As in humans, mice do not synthesize trans fatty acids endogenously but obtain all trans fatty acids from the diet. Therefore, animal studies can be modeled to mimic the levels of intake in humans, thereby improving the extrapolation of the results to humans. The dosage of trans fatty acids used in the animal experiments described in this review varied from 1.5% to 20% of total energy. At the lower end, this dosage is representative of the physiological levels of intake in humans in countries that do not legally restrict the amounts of industrial trans fatty acids in food. However, even in countries that have restrictive laws, excessive consumption of certain foods could lead to intake levels of trans fatty acids approaching levels reported in the animal experiments. With respect to the in vitro studies, the concentration of fatty acids used ranges from 0.05 mM to 1 mM. The total trans fatty acid concentration in blood plasma can reach as high as 0.6 mM but is expected to be much lower in most individuals and depends on the intake level. The use of trans fatty acid concentrations above 0.5 mM is likely supraphysiological.
Lipoprotein metabolism is well-known to be different between mice and humans. Whereas cholesterol circulates mainly in the form of the proatherogenic LDL particles in humans, in mice cholesterol mainly circulates in the form of HDL (113). In addition, unlike humans, wild-type mice do not develop spontaneous atherosclerosis. Therefore, in this review, discussions on the effect of trans fatty acids on plasma lipoproteins have focused on human studies or in specific mouse models such as the LDL-receptor knockout mice, which carry similar lipoprotein profiles as humans and develop atherosclerosis (114, 115).
Conclusions
Epidemiological studies have indicated that a higher intake of industrial trans fatty acids is associated with an increased risk of cardiovascular disease. Clinical studies in humans have shown that this association is likely explained by an increase in total and LDL cholesterol concentrations and a decrease in HDL cholesterol concentrations by industrial trans fatty acids. Preclinical studies in mice and cultured cells have partly clarified the molecular mechanisms of action of industrial trans fatty acids. In cultured cells, industrial trans fatty acids stimulate inflammation, ER stress, and oxidative stress, although less potently than SFAs. Besides impacting on inflammation and stress-related pathways, industrial trans fatty acids have a profound influence on lipid metabolism. In mice, industrial trans fatty acids promote the preferential storage of fat in the liver at the expense of fat tissue. Studies in cultured liver cells indicate that industrial trans fatty acids stimulate the cholesterol synthesis pathway by activating SREBP2-mediated gene regulation.
Overall, the reported distinct effects of industrial and ruminant trans fatty acids in vitro and in vivo prevents the grouping of all trans fatty acids as a single entity. From an evolutionary perspective, certain animals have conserved the ability to endogenously synthesize certain types of trans fatty acids, rendering it unsurprising that such ruminant trans fatty acids may carry certain benefits. Presently, there is still limited understanding of the mechanism of action of trans fatty acids. Accordingly, further mechanistic studies are required to fully clarify both defined and undefined properties of industrial and ruminant trans fatty acids. Gaining a deeper understanding of the molecular mechanism of action of trans fatty acids may create new therapeutic windows for the treatment of diseases characterized by disrupted lipid metabolism.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—A-BO, SK: conceptualized, wrote, and edited the manuscript; SK: was responsible for the final content of the manuscript; and both authors: read and approved the final manuscript.
Notes
Supported by funding from the Graduate School, Voeding, Levensmiddelentechnologie, Agro-Biotechnologie en Gezondheid (VLAG), from Wageningen University, The Netherlands.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: ACACA, acetyl-CoA carboxylase α; ANGPTL4, angiopoietin-like 4; CCL2, chemokine (C-C motif) ligand 2; CLA, conjugated linoleic acid; CRP, C-reactive protein; ER, endoplasmic reticulum; LC, light chain; NAFLD, nonalcoholic fatty liver disease; PBMC, peripheral blood mononuclear cell; PPAR, peroxisome proliferator–activated receptor; ROS, reactive oxygen species; SAA, serum amyloid A; SCAP, SREBP cleavage-activating protein; SFA, saturated fatty acid; SOAT1, sterol O-acyltransferase 1; SRE, sterol regulatory element; SREBP, sterol regulatory element binding protein; UPR, unfolded protein response.
References
- 1. Stender S, Astrup A, Dyerberg J. Ruminant and industrially produced trans fatty acids: health aspects. Food Nutr Res. 2008;52:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Enig MG, Pallansch LA, Sampugna J, Keeney M. Fatty acid composition of the fat in selected food items with emphasis on trans components. J Am Oil Chem Soc. 1983;60:1788–95. [Google Scholar]
- 3. Kuhnt K, Baehr M, Rohrer C, Jahreis G. Trans fatty acid isomers and the trans-9/trans-11 index in fat containing foods. Eur J Lipid Sci Technol. 2011;113:1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ginter E, Simko V. New data on harmful effects of trans-fatty acids. Bratisl Lek Listy. 2016;117:251–3. [DOI] [PubMed] [Google Scholar]
- 5. Filip S, Vidrih R. Trans Fatty Acids and Human Health. InTech; 2012. 43–64. [Google Scholar]
- 6. Gotoh N, Kagiono S, Yoshinaga K, Mizobe H, Nagai T, Yoshida A, Beppu F, Nagao K. Study of trans fatty acid formation in oil by heating using model compounds. J Oleo Sci. 2018;67:273–81. [DOI] [PubMed] [Google Scholar]
- 7. Tvrzicka E, Kremmyda LS, Stankova B, Zak A. Fatty acids as biocompounds: their role in human metabolism, health and disease – a review. Part 1: classification, dietary sources and biological functions. Biomed Pap. 2011;155:117–30. [DOI] [PubMed] [Google Scholar]
- 8. Koletzko B, Decsi T. Metabolic aspects of trans fatty acids. Clin Nutr. 1997;16:229–37. [DOI] [PubMed] [Google Scholar]
- 9. Johnston PV, Johnson OC, Kummerow FA. Occurrence of trans fatty acids in human tissue. Science. 1957;126:698–9. [DOI] [PubMed] [Google Scholar]
- 10. Thomas LH, Winter JA, Scott RG. Concentration of 18:1 and 16:1 transunsaturated fatty acids in the adipose body tissue of decedents dying of ischaemic heart disease compared with controls: analysis by gas liquid chromatography. J Epidemiol Community Health. 1983;37:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomas LH, Winter JA, Scott RG. Concentration of transunsaturated fatty acids in the adipose body tissue of decedents dying of ischaemic heart disease compared with controls. J Epidemiol Community Health. 1983;37:22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mensink RP, Katan MB. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N Engl J Med. 1990;323:439–45. [DOI] [PubMed] [Google Scholar]
- 13. Willet WC, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Rosner BA, Sampson LA, Hennekens CH. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet. 1993;341:581–5. [DOI] [PubMed] [Google Scholar]
- 14. Ganguly R, Pierce GN. Trans fat involvement in cardiovascular disease. Mol Nutr Food Res. 2012;56:1090–6. [DOI] [PubMed] [Google Scholar]
- 15. Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–13. [DOI] [PubMed] [Google Scholar]
- 16. Ascherio A, Hennekens CH, Buring JE, Master C, Stampfer MJ, Willett WC. Trans-fatty acids intake and risk of myocardial infarction. Circulation. 1994;89:94–101. [DOI] [PubMed] [Google Scholar]
- 17. Kummerow FA. The negative effects of hydrogenated trans fats and what to do about them. Atherosclerosis. 2009;205:458–65. [DOI] [PubMed] [Google Scholar]
- 18. Ahmed SH, Kharroubi W, Kaoubaa N, Zarrouk A, Batbout F, Gamra H, Najjar MF, Lizard G, Hininger-Favier I, Hammami M. Correlation of trans fatty acids with the severity of coronary artery disease lesions. Lipids Health Dis. 2018;17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brandt EJ, Myerson R, Perraillon MC, Polonsky TS. Hospital admissions for myocardial infarction and stroke before and after the trans-fatty acid restrictions in New York. JAMA Cardiol. 2017;2:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Restrepo BJ, Rieger M. Trans fat and cardiovascular disease mortality: evidence from bans in restaurants in New York. J Health Econ. 2016;45:176–96. [DOI] [PubMed] [Google Scholar]
- 21. Stender S, Dyerberg J, Astrup A. Consumer protection through a legislative ban on industrially produced trans fatty acids in foods in Denmark. Scand J Food Nutr. 2006;50:155–60. [Google Scholar]
- 22. Pérez-Farinós N, Dal Re Saavedra MÁ, Villar Villalba C, Robledo de Dios T. Trans-fatty acid content of food products in Spain in 2015. Gac Sanit. 2016;30:379–82. [DOI] [PubMed] [Google Scholar]
- 23. Kaur G, Cameron-Smith D, Sinclair AJ. Are trans fats a problem in Australia?. Med J Aust. 2012;196:666–7. [DOI] [PubMed] [Google Scholar]
- 24. Roe M, Pinchen H, Church S, Elahi S, Walker M, Farron-Wilson M, Buttriss J, Finglas P. Trans fatty acids in a range of UK processed foods. Food Chem. 2013;140:427–31. [DOI] [PubMed] [Google Scholar]
- 25. Karabulut I. Fatty acid composition of frequently consumed foods in Turkey with special emphasis on trans fatty acids. Int J Food Sci Nutr. 2007;58:619–28. [DOI] [PubMed] [Google Scholar]
- 26. Monge-Rojas R, Colón-Ramos U, Jacoby E, Alfaro T, Tavares do Carmo MDG, Villalpando S, Bernal C. Progress towards elimination of trans-fatty acids in foods commonly consumed in four Latin American cities. Public Health Nutr. 2017;20(13):2440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Remig V, Franklin B, Margolis S, Kostas G, Nece T, Street JC. Trans fats in America: a review of their use, consumption, health implications, and regulation. J Am Diet Assoc. 2010;110:585–92. [DOI] [PubMed] [Google Scholar]
- 28. Zupanič N, Hribar M, Pivk Kupirovič U, Kušar A, Žmitek K, Pravst I. Limiting trans fats in foods: use of partially hydrogenated vegetable oils in prepacked foods in Slovenia. Nutrients. 2018;10:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Craig-Schmidt MC. World-wide consumption of trans fatty acids. Atheroscler Suppl. 2006;7:1–4. [DOI] [PubMed] [Google Scholar]
- 30. Resnik D. Trans fat bans and human freedom. Am J Bioeth. 2010;10:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bendsen NT, Christensen R, Bartels EM, Astrup A. Consumption of industrial and ruminant trans fatty acids and risk of coronary heart disease: a systematic review and meta-analysis of cohort studies. Eur J Clin Nutr. 2011;65:773–83. [DOI] [PubMed] [Google Scholar]
- 32. Ganguly R, Pierce GN. The toxicity of dietary trans fats. Food Chem Toxicol. 2015;78:170–6. [DOI] [PubMed] [Google Scholar]
- 33. Field CJ, Blewett HH, Proctor S, Vine D. Human health benefits of vaccenic acid. Appl Physiol Nutr Metab. 2009;34:979–91. [DOI] [PubMed] [Google Scholar]
- 34. Bassett CMC, McCullough RS, Edel AL, Maddaford TG, Dibrov E, Blackwood DP, Austria JA, Pierce GN. Trans-fatty acids in the diet stimulate atherosclerosis. Metabolism. 2009;58:1802–8. [DOI] [PubMed] [Google Scholar]
- 35. Estadella D, Claudia M, Oller P, De Piano A. Lipotoxicity: effects of dietary saturated and transfatty acids. Mediators of Inflammation. 2014;2013:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zapolska DD, Bryk D, Olejarz W. Trans fatty acids and atherosclerosis – effects on inflammation and endothelial function. J Nutr Food Sci. 2015;5:6. [Google Scholar]
- 37. Palazón-Bru A, Carbayo-Herencia JA, Simarro-Rueda M, Artigao-Ródenas LM, Divisón-Garrote JA, Molina-Escribano F, Ponce-García I, Gil-Guillén VF; GEVA (Group of Vascular Diseases From Albacete). Comparison between non-high-density lipoprotein cholesterol and low-density lipoprotein cholesterol to estimate cardiovascular risk using a multivariate model. J Cardiovasc Nurs. 2018;33:17–23. [DOI] [PubMed] [Google Scholar]
- 38. Ramasamy I. Recent advances in physiological lipoprotein metabolism. Clin Chem Lab Med. 2014;52:1695–727. [DOI] [PubMed] [Google Scholar]
- 39. Chan DC, Watts GF. Apolipoproteins as markers and managers of coronary risk. QJM - Mon J Assoc Physicians. 2006;99:277–87. [DOI] [PubMed] [Google Scholar]
- 40. Zock PL, Katan MB. Hydrogenation alternatives: effects of trans fatty acids and stearic acid versus linoleic acid on serum lipids and lipoproteins in humans. J Lipid Res. 1992;33:399–410. [PubMed] [Google Scholar]
- 41. de Roos NM, Schouten EG, Katan MB. Consumption of a solid fat rich in lauric acids results in a more favourable serum lipid profile in healthy men and women than consumption of a solid fat rich in trans-fatty acids. J Nutr. 2001;131:242–5. [DOI] [PubMed] [Google Scholar]
- 42. Judd JT, Baer DJ, Clevidence BA, Kris-Etherton P, Muesing RA, Iwane M. Dietary cis and trans monounsaturated and saturated FA and plasma lipids and lipoproteins in men. Lipids. 2002;37:123–31. [DOI] [PubMed] [Google Scholar]
- 43. Mauger JF, Lichtenstein AH, Ausman LM, Jalbert SM, Jauhiainen M, Ehnholm C, Lamarche B. Effect of different forms of dietary hydrogenated fats on LDL particle size. Am J Clin Nutr. 2003;78:370–5. [DOI] [PubMed] [Google Scholar]
- 44. Matthan NR, Welty FK, Barrett PHR, Harausz C, Dolnikowski GG, Parks JS, Eckel RH, Schaefer EJ, Lichtenstein AH. Dietary hydrogenated fat increases high-density lipoprotein apoA-I catabolism and decreases low-density lipoprotein apoB-100 catabolism in hypercholesterolemic women. Arterioscler Thromb Vasc Biol. 2004;24:1092–7. [DOI] [PubMed] [Google Scholar]
- 45. Judd JT, Baer DJ, Clevidence BA, Muesing RA, Chen SC, Weststrate JA, Meijer GW, Wittes J, Lichtenstein AH, Vilella-Bach M et al.. Effects of margarine compared with those of butter on blood lipid profiles related to cardiovascular disease risk factors in normolipemic adults fed controlled diets. Am J Clin Nutr. 1998;68:768–77. [DOI] [PubMed] [Google Scholar]
- 46. Nestel P, Noakes M, Belling B, McArthur R, Clifton P, Janus E, Abbey M. Plasma lipoprotein lipid and Lp[a] changes with substitution of elaidic acid for oleic acid in the diet. J Lipid Res. 1992;33:1029–36. [PubMed] [Google Scholar]
- 47. Lichtenstein AH, Ausman LM, Jalbert SM, Schaefer EJ. Lipoprotein cholesterol levels. N Engl J Med. 1999;340:1933–40. [DOI] [PubMed] [Google Scholar]
- 48. Machado RM, Nakandakare ER, Quintao ECR, Cazita PM, Koike MK, Nunes VS, Ferreira FD, Afonso MS, Bombo RPA, Machado-Lima A et al.. Omega-6 polyunsaturated fatty acids prevent atherosclerosis development in LDLr-KO mice, in spite of displaying a pro-inflammatory profile similar to trans fatty acids. Atherosclerosis. 2012;224:66–74. [DOI] [PubMed] [Google Scholar]
- 49. Afonso MS, Lavrador MSF, Koike MK, Cintra DE, Ferreira FD, Nunes VS, Castilho G, Gioielli LA, Paula Bombo R, Catanozi S et al.. Dietary interesterified fat enriched with palmitic acid induces atherosclerosis by impairing macrophage cholesterol efflux and eliciting inflammation. J Nutr Biochem. 2016;32:91–100. [DOI] [PubMed] [Google Scholar]
- 50. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. [DOI] [PubMed] [Google Scholar]
- 51. Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79:969–73. [DOI] [PubMed] [Google Scholar]
- 52. Han SN, Leka LS, Lichtenstein AH, Ausman LM, Schaefer EJ, Meydani SN. Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J Lipid Res. 2002;43:445–52. [PubMed] [Google Scholar]
- 53. Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, Willett WC, Hu FB. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr. 2005;135:562–6. [DOI] [PubMed] [Google Scholar]
- 54. Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC. Trans fatty acids and systemic inflammation in heart failure. Am J Clin Nutr. 2004;80:1521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. AJP Gastrointest Liver Physiol. 2008;295:G987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hu X, Tanaka N, Guo R, Lu Y, Nakajima T, Gonzalez FJ, Aoyama T. PPARα protects against trans-fatty-acid-containing diet-induced steatohepatitis. J Nutr Biochem. 2017;39:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Larner DP, Morgan SA, Gathercole LL, Doig CL, Guest P, Weston C, Hazeldine J, Tomlinson JW, Stewart PM, Lavery GG. Male 11-HSD1 knockout mice fed trans-fats and fructose are not protected from metabolic syndrome or nonalcoholic fatty liver disease. Endocrinology. 2016;157:3493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang Q, Zhang Z, Loustalot F, Vesper H, Caudill SP, Ritchey M, Gillespie C, Merritt R, Hong Y, Bowman BA. Plasma trans-fatty acid concentrations continue to be associated with serum lipid and lipoprotein concentrations among US adults after reductions in trans-fatty acid intake. J Nutr. 2017;147:896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vesper HW, Caudill SP, Kuiper HC, Yang Q, Ahluwalia N, Lacher DA, Pirkle JL. Plasma trans-fatty acid concentrations in fasting adults declined from NHANES 1999–2000 to 2009–2010. Am J Clin Nutr. 2017;105:1063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iwata NG, Pham M, Rizzo NO, Cheng AM, Maloney E, Kim F. Trans fatty acids induce vascular inflammation and reduce vascular nitric oxide production in endothelial cells. PLoS One. 2011;6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hirata Y, Takahashi M, Kudoh Y, Kano K, Kawana H, Makide K, Shinoda Y, Yabuki Y, Fukunaga K, Aoki J et al.. Trans-fatty acids promote proinflammatory signaling and cell death by stimulating the apoptosis signal-regulating kinase 1 (ASK1)-p38 pathway. J Biol Chem. 2017;292(20):8174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Oteng A-B, Bhattacharya A, Brodesser S, Qi L, Tan NS, Kersten S. Feeding Angptl4-/- mice trans fat promotes foam cell formation in mesenteric lymph nodes without leading to ascites. J Lipid Res. 2017;58:1100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Han J, Kaufman RJ. The role of ER stress in lipid metabolism and lipotoxicity. J Lipid Res. 2016;57:1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Betteridge JD. What is oxidative stress?. Metab - Clin Exp. 2000;49:3–8. [DOI] [PubMed] [Google Scholar]
- 65. Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Monguchi T, Hara T, Hasokawa M, Nakajima H, Mori K, Toh R, Irino Y, Ishida T, Hirata KI, Shinohara M. Excessive intake of trans fatty acid accelerates atherosclerosis through promoting inflammation and oxidative stress in a mouse model of hyperlipidemia. J Cardiol. 2017;70:121–7. [DOI] [PubMed] [Google Scholar]
- 67. Santos JDB, Mendonça AAS, Sousa RC, Silva TGS, Bigonha SM, Santos EC, Gonçalves RV, Novaes RD. Food-drug interaction: anabolic steroids aggravate hepatic lipotoxicity and nonalcoholic fatty liver disease induced by trans fatty acids. Food Chem Toxicol. 2018;116:360–8. [DOI] [PubMed] [Google Scholar]
- 68. Dhibi M, Brahmi F, Mnari A, Houas Z, Chargui I, Bchir L, Gazzah N, Alsaif MA, Hammami M. The intake of high fat diet with different trans fatty acid levels differentially induces oxidative stress and non alcoholic fatty liver disease (NAFLD) in rats. Nutr Metab (Lond). 2011;8:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zapolska-Downar D, Kosmider A, Naruszewicz M. Trans fatty acids induce apoptosis in human endothelial cells. J Physiol Pharmacol. 2005;56:611–25. [PubMed] [Google Scholar]
- 70. Ma W, Zhao L, Yuan L, Yu H, Wang H, Gong X, Wei F, Xiao R. Elaidic acid induces cell apoptosis through induction of ROS accumulation and endoplasmic reticulum stress in SH-SY5Y cells. Mol Med Rep. 2017;16(6):9337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang J, Nie D. Modulation of autophagy by free fatty acids. Cell Death - Autophagy, Apoptosis and Necrosis. 2015. doi: 10.5772/61484. [Google Scholar]
- 74. Ghavami S, Cunnington RH, Yeganeh B, Davies JJL, Rattan SG, Bathe K, Kavosh M, Los MJ, Freed DH, Klonisch T et al.. Autophagy regulates trans fatty acid-mediated apoptosis in primary cardiac myofibroblasts. Biochim Biophys Acta - Mol Cell Res. 2012;1823:2274–86. [DOI] [PubMed] [Google Scholar]
- 75. Sauvat A, Chen G, Müller K, Tong M, Aprahamian F, Durand S, Cerrato G, Bezu L, Leduc M, Franz J et al.. Trans-fats inhibit autophagy induced by saturated fatty acids. EBioMedicine. 2018;30:261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Faasse S, Braun H, Vos M. The role of NAFLD in cardiometabolic disease: an update. F1000Research. 2018;7:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Abd Alamir M, Goyfman M, Chaus A, Dabbous F, Tamura L, Sandfort V, Brown A, Budoff M. The correlation of dyslipidemia with the extent of coronary artery disease in the multiethnic study of atherosclerosis. J Lipids. 2018;2018:5607349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Oteng A-B, Loregger A, van Weeghel M, Zelcer N, Kersten S. Industrial trans fatty acids stimulate SREBP2-mediated cholesterogenesis and promote non-alcoholic fatty liver disease. Mol Nutr Food Res. 2019;63(19):e1900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Koppe SWP, Elias M, Moseley RH, Green RM. Trans fat feeding results in higher serum alanine aminotransferase and increased insulin resistance compared with a standard murine high-fat diet. Am J Physiol Gastrointest Liver Physiol. 2009;297:G378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Obara N, Fukushima K, Ueno Y, Wakui Y, Kimura O, Tamai K, Kakazu E, Inoue J, Kondo Y, Ogawa N et al.. Possible involvement and the mechanisms of excess trans-fatty acid consumption in severe NAFLD in mice. J Hepatol. 2010;53:326–34. [DOI] [PubMed] [Google Scholar]
- 81. Jeyapal S, Putcha UK, Mullapudi VS, Ghosh S, Sakamuri A, Kona SR, Vadakattu SS, Madakasira C, Ibrahim A. Chronic consumption of fructose in combination with trans fatty acids but not with saturated fatty acids induces nonalcoholic steatohepatitis with fibrosis in rats. Eur J Nutr. 2018;57(6):2171–87. [DOI] [PubMed] [Google Scholar]
- 82. Machado RM, Stefano JT, Oliveira CPMS, Mello ES, Ferreira FD, Nunes VS, de Lima VMR, Quintão ECR, Catanozi S, Nakandakare ER et al.. Intake of trans fatty acids causes nonalcoholic steatohepatitis and reduces adipose tissue fat content. J Nutr. 2010;140:1127–32. [DOI] [PubMed] [Google Scholar]
- 83. Shao F, Ford DA. Elaidic acid increases hepatic lipogenesis by mediating sterol regulatory element binding protein-1c activity in HuH-7 cells. Lipids. 2014;49:403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vendel Nielsen L, Krogager TP, Young C, Ferreri C, Chatgilialoglu C, Nørregaard Jensen O, Enghild JJ. Effects of elaidic acid on lipid metabolism in HepG2 cells, investigated by an integrated approach of lipidomics, transcriptomics and proteomics. PLoS One. 2013;8(9):e74283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Neuschwander-Tetri BA, Ford DA, Acharya S, Gilkey G, Basaranoglu M, Tetri LH, Brunt EM. Dietary trans-fatty acid induced NASH is normalized following loss of trans-fatty acids from hepatic lipid pools. Lipids. 2012;47:941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lehnen TE, da Silva MR, Camacho A, Marcadenti A, Lehnen AM. A review on effects of conjugated linoleic fatty acid (CLA) upon body composition and energetic metabolism. J Int Soc Sports Nutr. 2015;12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yang B, Chen H, Stanton C, Ross RP, Zhang H, Chen YQ, Chen W. Review of the roles of conjugated linoleic acid in health and disease. J Funct Foods. 2015;15:314–25. [Google Scholar]
- 88. Risérus U, Basu S, Jovinge S, Fredrikson GN, Ärnlöv J, Vessby B. Supplementation with conjugated linoleic acid causes isomer-dependent oxidative stress and elevated C-reactive protein: a potential link to fatty acid-induced insulin resistance. Circulation. 2002;106:1925–9. [DOI] [PubMed] [Google Scholar]
- 89. Gaullier JM, Halse J, Høivik HO, Syvertsen C, Nurminiemi M, Hassfeld C, Einerhand A, O'Shea M, Gudmundsen O. Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. Br J Nutr. 2007;97:550–60. [DOI] [PubMed] [Google Scholar]
- 90. Ramos R, Mascarenhas J, Duarte P, Vicente C, Casteleiro C. Conjugated linoleic acid-induced toxic hepatitis: first case report. Dig Dis Sci. 2009;54:1141–3. [DOI] [PubMed] [Google Scholar]
- 91. Belda BJ, Lee Y, Vanden Heuvel JP. Conjugated linoleic acids and inflammation: isomer- and tissue-specific responses. Clin Lipidol. 2010;5:699–717. [Google Scholar]
- 92. Poirier H, Shapiro JS, Kim RJ, Lazar MA. Nutritional supplementation with trans-10, cis-12-conjugated linoleic acid induces inflammation of white adipose tissue. Diabetes. 2006;55:1634–41. [DOI] [PubMed] [Google Scholar]
- 93. Terpstra AH, Beynen AC, Everts H, Kocsis S, Katan MB, Zock PL. The decrease in body fat in mice fed conjugated linoleic acid is due to increases in energy expenditure and energy loss in the excreta. J Nutr. 2002;132:940–5. [DOI] [PubMed] [Google Scholar]
- 94. Tsuboyama-Kasaoka N, Takahashi M, Tanemura K, Kim HJ, Tange T, Okuyama H, Kasai M, Ikemoto S, Ezaki O. Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipodystrophy in mice. Diabetes. 2000;49:1534–42. [DOI] [PubMed] [Google Scholar]
- 95. Clément L, Poirier H, Niot I, Bocher V, Guerre-Millo M, Krief S, Staels B, Besnard P. Dietary trans-10, cis-12 conjugated linoleic acid induces hyperinsulinemia and fatty liver in the mouse. J Lipid Res. 2002;43:1400–9. [DOI] [PubMed] [Google Scholar]
- 96. den Hartigh LJ, Han CY, Wang S, Omer M, Chait A. 10E,12Z-conjugated linoleic acid impairs adipocyte triglyceride storage by enhancing fatty acid oxidation, lipolysis, and mitochondrial reactive oxygen species. J Lipid Res. 2013;54:2964–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Collison KS, Maqbool Z, Saleh SM, Inglis A, Makhoul NJ, Bakheet R, Al-Johi M, Al-Rabiah R, Zaidi MZ, Al-Mohanna FA. Effect of dietary monosodium glutamate on trans fat-induced nonalcoholic fatty liver disease. J Lipid Res. 2009;50:1521–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ascherio A, Hennekens CH, Buring JE, Master C, Stampfer MJ, Willett WC. Trans-fatty acids intake and risk of myocardial infarction. Circulation. 1994;89:94–101. [DOI] [PubMed] [Google Scholar]
- 99. Jakobsen MU, Overvad K, Dyerberg J, Heitmann BL. Intake of ruminant trans fatty acids and risk of coronary heart disease. Int J Epidemiol. 2008;37:173–82. [DOI] [PubMed] [Google Scholar]
- 100. Bolton-Smith C, Woodward M, Fenton S, Brown CA. Does dietary trans fatty acid intake relate to the prevalence of coronary heart disease in Scotland?. Eur Hear J. 1996;17:837–45. [DOI] [PubMed] [Google Scholar]
- 101. Gebauer SK, Chardigny JM, Jakobsen MU, Lamarche B, Lock AL, Proctor SP, Baer DJ. Effects of ruminant trans fatty acids on cardiovascular disease and cancer: a comprehensive review of epidemiological, clinical, and mechanistic studies. Adv Nutr. 2011;2:332–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Oomen CM, Ocké MC, Feskens EJM, Van Erp-Baart MAJ, Kok FJ, Kromhout D. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population-based study. Lancet. 2001;357:746–51. [DOI] [PubMed] [Google Scholar]
- 103. Motard-Bélanger A, Charest A, Grenier G, Paquin P, Chouinard Y, Lemieux S, Couture P, Lamarche B. Study of the effect of trans fatty acids from ruminants on blood lipids and other risk factors for cardiovascular disease. Am J Clin Nutr. 2008;87:593–9. [DOI] [PubMed] [Google Scholar]
- 104. Brouwer IA, Wanders AJ, Katan MB. Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans - a quantitative review. PLoS One. 2010;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gebauer SK, Destaillats F, Dionisi F, Krauss RM, Baer DJ. Vaccenic acid and trans fatty acid isomers from partially hydrogenated oil both adversely affect LDL cholesterol: a double-blind, randomized controlled trial. Am J Clin Nutr. 2015;102:1339–46. [DOI] [PubMed] [Google Scholar]
- 106. Bassett CMC, Edel AL, Patenaude AF, McCullough RS, Blackwood DP, Chouinard PY, Paquin P, Lamarche B, Pierce GN. Dietary vaccenic acid has antiatherogenic effects in LDLr-/- mice. J Nutr. 2010;140:18–24. [DOI] [PubMed] [Google Scholar]
- 107. Da Silva MS, Julien P, Bilodeau JF, Barbier O, Rudkowska I. Trans fatty acids suppress TNF-α-induced inflammatory gene expression in endothelial (HUVEC) and hepatocellular carcinoma (HepG2) cells. Lipids. 2017;52:315–25. [DOI] [PubMed] [Google Scholar]
- 108. Wang Y, Jacome-Sosa MM, Ruth MR, Lu Y, Shen J, Reaney MJ, Scott SL, Dugan MER, Anderson HD, Field CJ et al.. The intestinal bioavailability of vaccenic acid and activation of peroxisome proliferator-activated receptor-α and -γ in a rodent model of dyslipidemia and the metabolic syndrome. Mol Nutr Food Res. 2012;56:1234–46. [DOI] [PubMed] [Google Scholar]
- 109. Ruth MR, Wang Y, Yu HM, Goruk S, Reany MJ, Proctor SD, Vine DF, Field CJ. Vaccenic and elaidic acid modify plasma and splenocyte membrane phospholipids and mitogen-stimulated cytokine production in obese insulin resistant JCR: LA-cp rats. Nutrients. 2010;2:181–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jacome-Sosa MM, Borthwick F, Mangat R, Uwiera R, Reaney MJ, Shen J, Quiroga AD, Jacobs RL, Lehner R, Proctor SD. Diets enriched in trans-11 vaccenic acid alleviate ectopic lipid accumulation in a rat model of NAFLD and metabolic syndrome. J Nutr Biochem. 2014;25:692–701. [DOI] [PubMed] [Google Scholar]
- 111. Jaudszus A, Jahreis G, Schlörmann W, Fischer J, Kramer R, Degen C, Rohrer C, Roth A, Gabriel H, Barz D et al.. Vaccenic acid-mediated reduction in cytokine production is independent of c9,t11-CLA in human peripheral blood mononuclear cells. Biochim Biophys Acta - Mol Cell Biol Lipids. 2012;1821:1316–22. [DOI] [PubMed] [Google Scholar]
- 112. Krogager TP, Nielsen LV, Kahveci D, Dyrlund TF, Scavenius C, Sanggaard KW, Enghild JJ. Hepatocytes respond differently to major dietary trans fatty acid isomers, elaidic acid and trans-vaccenic acid. Proteome Sci. 2015;13:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zadelaar S, Kleemann R, Verschuren L, De Vries-Van Der Weij J, Van Der Hoorn J, Princen HM, Kooistra T. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol. 2007;27:1706–21. [DOI] [PubMed] [Google Scholar]
- 114. Veseli BE, Perrotta P, De Meyer GRA, Roth L, van der Donckt C, Martinet W, De Meyer GRY. Animal models of atherosclerosis. Eur J Pharmacol. 2017;816:3–13. [DOI] [PubMed] [Google Scholar]
- 115. Getz GS, Reardon CA. Do the Apoe-/- and Ldlr-/- mice yield the same insight on atherogenesis?. Arterioscler Thromb Vasc Biol. 2016;36:1734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]