ABSTRACT
The Dietary Guidelines for Americans (DGA) provide nutrition advice for Americans >2 y of age. The 2020–2025 DGA proposes a life stage approach, focusing on birth through older adulthood. Limited recommendations for beverages exist except for milk, 100% fruit juice, and alcohol. The goal of this article is to provide a better understanding of the role of beverages in the diet using current scientific evidence. A Medline search of observational studies, randomized controlled trials, and meta-analyses was undertaken using key beverage words. We highlight the role beverages can play as a part of the DGA and considered beverages not traditionally included, such as those that are phytonutrient dense. Our primary consideration for beverage consumption targeted healthy Americans aged ≥2 y. However, with the proposed expansion to the life span for the 2020–2025 DGA, we also reviewed evidence for infants and toddlers from birth to 24 mo. Examples are provided on how minor changes in beverage choices aid in meeting recommended intakes of certain nutrients. Guidance on beverage consumption may aid in development of better consumer products to meet broader dietary advice. For example, beverage products that are nutrient/phytonutrient dense and lower in sugar could be developed as alternatives to 100% juice to help meet the fruit and vegetable guidelines. Although beverages are not meant to replace foods, e.g., it is difficult to meet the requirements for vitamin E, dietary fiber, or essential fatty acids through beverages alone, beverages are important sources of nutrients and phytonutrients, phenolic acids and flavonoids in particular. When considering the micronutrients from diet alone, mean intakes of calcium (in women), potassium, and vitamins A, C, and D are below recommendations and sodium intakes are well above. Careful beverage choices could close these gaps and be considered a part of a healthy dietary pattern.
Keywords: beverages, dietary guidelines, water, milk, juice, coffee, tea, sugar-sweetened beverages, alcohol
Introduction
The Dietary Guidelines for Americans (DGA) provide nutrition advice for Americans who are >2 y of age. The 2020–2025 DGA proposes a life stage approach, focusing on birth through older adulthood (1). The Guidelines are published every 5 y by the USDA, jointly with the US Department of Health and Human Services. The most recent edition, the 2015–2020 DGA, has not extensively considered specific beverage recommendations with the exception of milk, 100% fruit juice, and alcohol (2). The landscape of beverage consumption has changed markedly in recent years. Sugar-sweetened beverage (SSB) intake has fallen by 68 and 45 kcal/d from 1999–2000 to 2009–2010 for youths and adults, respectively (3, 4). The percentage of individuals consuming milk has decreased among all age groups (5). Specialty coffee and tea houses and beverages have become a cultural phenomenon and energy drinks keep growing in popularity (6). Thus, it is an appropriate time to consider how beverages contribute to intakes of essential nutrients with the potential to fill nutrient gaps and provide nonessential phytonutrients with evidence to promote health, as well as consider constituents to limit.
The overall goal of this article is to highlight the role of beverages as sources of nutrients and phytonutrients. We consider the role beverages play as a part of the DGA and consider beverages not traditionally included in the DGA. Our considerations for beverage consumption target healthy Americans aged ≥2 y.
DGA
There are 5 key messages of the 2015–2020 DGA: 1) a focus on healthy eating patterns; 2) dietary shifts that may be needed to achieve such patterns; 3) a focus on variety, nutrient density, and intake amounts; 4) limited calories from added sugars and saturated fats and reduced sodium intake; and 5) a supportive role in adopting a healthy dietary pattern in the home, school, work, and communities. The DGA provides guidance for choosing a healthy dietary pattern with a focus on meeting nutrient needs and preventing (rather than treating) major diet-related chronic diseases. One of the 3 dietary patterns recommended in the DGA is the Healthy US-Style Eating Pattern, which is based on the types of foods Americans typically consume, but in nutrient-dense forms and appropriate amounts [see Appendix 3 of US Department of Health and Human Services and USDA (2)]. The pattern considers 6 food groups (vegetables, fruits, grains, dairy, protein foods, and oils). Of these, the vegetables, fruits, and dairy groups relate to commonly consumed beverages.
Current DGA recommendations for beverages include:
Beverages that are calorie-free, especially water, or that contribute beneficial nutrients, such as fat-free and low-fat milk and 100% juice, should be the primary beverages consumed.
Milk and 100% fruit juice should be consumed within recommended food group amounts and calorie limits.
Coffee, tea, and flavored waters also can be selected, but calories from creams and milks, added sugars, and other additions (e.g., flavorings) should be accounted for within the eating pattern.
Limit caffeine intake to <400 mg/d.
SSBs, such as soft drinks, sports drinks, and fruit drinks that are <100% juice, can contribute excess calories while providing few or no key nutrients. If they are consumed, amounts should be within overall calorie limits and limits for calories from added sugars.
If alcohol is consumed, it should be in moderation—≤1 drink/d for women and ≤2 drinks/d for men—and only by adults of legal drinking age [1 alcoholic drink-equivalent = 14 g (0.6 fl oz) pure alcohol]. Alcohol is contraindicated during pregnancy; when doing activity that requires attention, skill, and coordination; and when using certain pharmaceutical drugs and/or undergoing therapeutic procedures that interact with alcohol.
The 2020–2025 DGA will also consider the importance of beverages as concerns their having a role in achieving nutrient and food group recommendations, a topic that was proposed for public comment (https://www.cnpp.usda.gov/dietary-guidelines).
About three-fourths of the US population falls short of meeting the recommendations for intakes of fruits, vegetables, and dairy (2, 7). Similarly, mean US intakes of potassium, fiber, calcium, and vitamin D are below recommendations (8–11). In addition, US intakes exceed recommendations for added sugars, saturated fats, and sodium with ≥70% of the population aged ≥1 y consuming more than the recommended limits (2). Therefore, the objective of this report is to describe how beverages may close such nutrient gaps or contribute to reductions in chronic disease risk. To meet this objective, this report will review the scientific evidence to date on nutrient content and health benefits/risks of various beverages via a Medline search of observational studies, randomized controlled trials (RCTs), and meta-analyses using key beverage words.
Water
Water is the principal chemical component of the body, making up ∼60% of body weight (12). Water is necessary for normal cellular metabolism as well as elimination of wastes through urination, perspiration, and bowel movements and has a role in temperature maintenance and lubrication of joints. Water consumption allows for the delivery of fluid without calories and provides little nutrient value, with the possible exception of fluoride. Drinking water is a major source of dietary fluoride in the United States. Approximately 74% of the US population receives water with sufficient fluoride for the prevention of dental caries (13). Most bottled waters contain suboptimal concentrations of fluoride, although this can vary (14).
Tap water may contribute to total calcium, magnesium, and sodium intakes. In a mineral analysis from municipal water authorities of 21 major US cities, half of the tap water sources examined contained 8–16% and 6–31% of the RDA for calcium and magnesium, respectively, for adults consuming 2 L/d (15). Furthermore, the role of water hardness as a risk factor for cardiovascular disease (CVD) has been investigated. In a meta-analysis of 7 case-control studies including 44,000 adult subjects, comparing those exposed to the highest concentration with those exposed to the lowest concentration of calcium and magnesium, there was a benefit of calcium intake as well as for magnesium (16). Water softening using a sodium salt is commonly used to reduce water hardness. Domestic water softeners can increase sodium concentrations to >300 mg/L in drinking water (17) and need to be considered to meet population-based sodium recommendations, and also recommendations for sodium-restricted diets.
Most healthy people meet their daily hydration needs by using thirst as a guide. Although there are no exact requirements (12), daily total water requirements increase with age from early infancy (∼0.6 L or 20 oz) through childhood (∼1.7 L or 57 oz) and general daily recommendations for healthy women and men are ∼2.7 L (91 oz) and ∼3.7 L (125 oz) of total water, respectively. These recommendations include fluids from water, other beverages, and food. For older adults, relying solely on thirst may not be sufficient to maintain hydration status. Short-term and long-term fluid intake in response to repeated dehydration stimuli appear to be reduced among older adults over the age of 65 y (18). Many factors may influence fluid intake, including cognitive ability, medication, and incontinence (19). Poor fluid status and dehydration can also alter medication function and effectiveness.
Factors that influence water needs include exercise, environment, and overall health (12). Additional fluid is needed with the loss of sweat that comes with exercise and hot or humid weather. Fluid loss may also occur with a fever, vomiting or diarrhea, or certain medical conditions.
Water as a part of the DGA
The DGA recognizes that water should be the primary beverage consumed to meet fluid needs. In addition:
For most healthy people, thirst should be the guide for fluid needs.
The amount of fluid needed will vary with age, gender, water loss from physical activity, heat exposure, fever, vomiting, or diarrhea.
Milk and Milk Substitutes
DGA recommendations include fat-free and low-fat (1%) dairy, including milk or fortified soy beverages (fortified with calcium, vitamin A, and vitamin D). The DGA recommendation for dairy intake for adults is 3 cup-equivalents/d (710 mL-equivalents/d). The dairy group contributes many nutrients, including high-quality protein, calcium, phosphorus, vitamin A, vitamin D (in products fortified with vitamin D), riboflavin, vitamin B-12, potassium, zinc, choline, magnesium, and selenium. Eight ounces of milk and milk substitutes are good sources of potassium and vitamin A and rich sources of calcium and vitamin D (Table 1; 20).
TABLE 1.
Beverage nutrient composition1
| Beverage2 | Kcal | % Energy3 | Protein, g (% DV) | Carbohydrate, g (% DV) | Fat, g (% DV) | SFA, g (% DV) | Fiber, g (% DV) | Sugar, g | Added sugar, g | Caffeine,4 mg | Calcium, mg (% DV) | Sodium, mg (% DV) | Potassium, mg (% DV) | Vitamin A, IU (% DV) | Vitamin C, mg (% DV) | Vitamin D, IU (% DV) | Phytonutrients type, mg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water (8 oz, 237 mL) | 05 | 0 | 0 | 0 | 06 | 06 | 0 | 0 | 0 | 0 | 7 (0) | 07 | 0 | 0 | 0 | 0 | 0 |
| Tea and coffee | |||||||||||||||||
| Black tea, brewed (8 oz) | 05 | 0 | 0 | 0.1 (0.0) | 06 | 06 | 0 | 0 | 0 | 40–74 | 0 | 78 (0.3) | 88 (1.9) | 0 | 0 | 0 | Flavonoid, 116 |
| Black tea, brewed, decaffeinated (8 oz) | 05 | 0 | 0 | 0 | 06 | 06 | 0 | 0 | 0 | 2–5 | 0 | 78 (0.3) | 88 (1.9) | 0 | 0 | 0 | Flavonoid, 57 |
| Black tea, infusion, sweetened (8 oz) | 79 | 4.0 | 0 | 21 (7.0) | 06 | 06 | 0 | 20 | 20 | NA | 0 | 108 (0.4) | 0 | 0 | 0 | 0 | Flavonoid, 84 |
| Green tea, brewed (8 oz) | 05 | 0 | 0 | 0 | 06 | 06 | 0 | 0 | 0 | 25–50 | 0 | 07 | 0 | 0 | 0 | 0 | Flavonoid, 138 |
| Coffee, brewed (8 oz) | 25 | 0 | 0.7 (1.4) | 0.4 (0.1) | 06 | 06 | 0 | 0 | 0 | 95–330 | 5 (0.5) | 27 (0.1) | 124 (2.6) | 0 | 0 | 0 | Phenolic acids, 503 |
| Coffee, brewed, espresso (1 oz) | 25 | 0 | 0.1 (0.2) | 0 | 0.26 (0.3) | 06 | 0 | 0 | 0 | 50–150 | 0 | 07 | 0 | 0 | 0 | 0 | Phenolic acids, NA4 |
| Coffee, sweetened, milk based (8 oz) | 186 | 9.3 | 5.2 (10.4) | 12.6 (4.2) | 3.66 (5.5) | 06 | 0 | 10.7 | 10.7 | 95–330 | 1949 (19.4) | 6810 (3.0) | 458 (9.7) | 0 | 0 | 3 (0.8) | Phenolic acids, NA |
| Coffee, brewed, decaffeinated (8 oz) | 05 | 0 | 0.2 (0.4) | 0 | 06 | 06 | 0 | 0 | 0 | 3–12 | 5 (0.5) | 58 (0.2) | 128 (2.7) | 0 | 0 | 0 | Phenolic acids, 658 |
| 100% fruit & vegetable juices | |||||||||||||||||
| Apple (8 oz) | 110 | 5.5 | 0 | 28 (9.3) | 06 | 06 | 0 | 28 | 0 | 0 | 0 | 3610 (1.6) | 300 (6.4) | 0 | 0 | 0 | Flavonoid, 7 |
| Apple—vitamin C fortified (8 oz) | 101 | 5.1 | 1.0 (2.0) | 25 | 06 | 06 | 0 | 21 | 0 | 0 | 0 | 198 (0.8) | 149 (3.1) | 0 | 7811 (130) | 0 | Flavonoid, 7 |
| Carrot (8 oz) | 70 | 3.5 | 2.0 (4.0) | 14 (4.7) | 06 | 06 | 2 (8) | 11 | 0 | 0 | 0 | 2409 (10.4) | 5909 (12.6) | 19,99911 (400) | 0 | 0 | Carotenoid, 33 |
| Cranberry, unsweetened (8 oz) | 116 | 5.8 | 1.0 (2.0) | 31 (10.3) | 06 | 06 | 0.3 (1) | 31 | 0 | 0 | 20 (2.0) | 58 (0.2) | 195 (4.1) | 0 | 2411 (40) | 0 | Flavonoid, 53 |
| Grapefruit (8 oz) | 101 | 5.1 | 0 | 25 (8.3) | 06 | 06 | 0 | 24 | 0 | 0 | 0 | 3610 (1.6) | 319 (6.7) | 0 | 7811 (130) | 0 | Flavanone, 61 |
| Orange (8 oz) | 110 | 5.5 | 2.0 (4.0) | 26 (8.7) | 06 | 06 | 0 | 22 | 0 | 0 | 19 (1.9) | 0 | 451 (9.7) | 0 | 7211 (120) | 0 | Flavanone, 34 |
| Orange—calcium and vitamin D fortified (8 oz) | 110 | 5.5 | 2.0 (4.0) | 26 (8.7) | 06 | 06 | 0 | 22 | 0 | 0 | 35012(35.0) | 07 | 451 (9.7) | 0 | 7811 (130) | 10111 (25.3) | Flavanone, 34 |
| Tomato (8 oz) | 50 | 2.5 | 2.0 (4.0) | 10 (3.3) | 06 | 06 | 2 (8) | 7 | 0 | 0 | 19 (1.9) | 62911 (27.3) | 379 (8.1) | 4999 (10) | 7211 (120) | 0 | Carotenoid, 22 |
| Tomato, low sodium (8 oz) | 48 | 2.4 | 2.0 (4.0) | 10 (3.3) | 06 | 06 | 2 (8) | 7 | 0 | 0 | 26 (2.6) | 13910 (6.0) | 8869 (18.9) | 40111 (8) | 7211 (120) | 0 | Carotenoid, 22 |
| Dairy | |||||||||||||||||
| Nonfat milk (8 oz) | 91 | 4.6 | 8.89 (17.6) | 12.3 (4.1) | 0.66 (0.9) | 06 | 0 | 12.3 | 0 | 0 | 31611 (31.6) | 13010 (5.7) | 419 (8.9) | 5249 (10.5) | 2.5 (3.3) | 12011 (30.0) | 0 |
| Low-fat milk, 1% (8 oz) | 120 | 6.0 | 10.011 (20.0) | 11.0 (3.7) | 2.56 (3.8) | 1.5 (6.8) | 0 | 15.0 | 0 | 0 | 30011 (30.0) | 12010 (5.2) | 350 (7.4) | 4999 (10.0) | 1.2 (2.0) | 12011 (30.0) | 0 |
| Low-fat milk, 2% (8 oz) | 130 | 6.5 | 8.09 (16.0) | 13.0 (4.3) | 5.0 (7.6) | 3.0 (13.5) | 0 | 12.0 | 0 | 0 | 30011 (30.0) | 12510 (5.4) | 370 (7.9) | 4999 (10.0) | 1.2 (2.0) | 10111 (25.3) | 0 |
| Whole milk, 3.25% (8 oz) | 161 | 8.1 | 8.09 (16.0) | 12.0 (4.0) | 9.0 (13.8) | 5.0 (22.2) | 0 | 12.0 | 0 | 0 | 30011 (30.0) | 12510 (5.4) | 410 (8.7) | 4999 (10.0) | 1.2 (2.0) | 10111 (25.3) | 0 |
| Chocolate milk, 2% (8 oz) | 199 | 10.0 | 1011 (20.0) | 29.0 (9.7) | 5.0 (7.6) | 3.0 (13.5) | 0 | 28.0 | 16 | 5 | 35011 (35.0) | 221 (9.6) | 451 (9.6) | 4999 (10.0) | 0 | 10111 (25.3) | Flavonoid, 3 |
| Milk substitutes | |||||||||||||||||
| Soy milk, plain, unsweetened (8 oz) | 79 | 4.0 | 7.09 (14.0) | 4.0 (1.3) | 4.0 (6.2) | 2.36 (1.0) | 1.0 (4.0) | 1.0 | 0 | 0 | 30011 (30.0) | 8410 (3.7) | 300 (6.4) | 4999 (10.0) | 0 | 12011 (30.0) | Isoflavone, 6 |
| Almond milk, unsweetened (8 oz) | 79 | 4.0 | 1.0 (2.0) | 14.0 (4.7) | 2.56 (3.8) | 0.96 (0.4) | 1.0 (4.0) | 13.0 | 0 | 0 | 45111 (45.1) | 148 (0.6) | 170 (3.6) | 49911 (10.0) | 0 | 10111 (25.3) | NA |
| Coconut milk (8 oz) | 335 | 16.8 | 0 | 9.1 (3.0) | 33 (50.8) | 30.313 (136.5) | 0 | 3.0 | 0 | 0 | 0 | 3910 (1.7) | 0 | 0 | 0 | 0 | NA |
| Rice milk, unsweetened (8 oz) | 113 | 5.7 | 0.7 (1.3) | 22.0 (7.3) | 2.36 (3.5) | 06 | 0.7 (2.8) | 12.7 | 0 | 0 | 28311 (28.3) | 9410 (4.1) | 65 (1.4) | 151 (3.0) | 0 | 2.4 (0.6) | NA |
| Alcohol13 | |||||||||||||||||
| Beer, regular (12 fl oz, 14 g alcohol) | 153 | 7.7 | 1.6 (3.2) | 12.6 (4.2) | 06 | 06 | 0 | 0 | 0 | 0 | 14 (1.4) | 148 (0.6) | 96 (2.0) | 0 | 0 | 0 | Flavonoid, 3 |
| Beer light (12 fl oz, 12 g alcohol) | 103 | 5.2 | 0.9 (1.8) | 5.8 (1.9) | 06 | 06 | 0 | 0.3 | 0 | 0 | 14 (1.4) | 148 (0.6) | 74 (1.6) | 0 | 0 | 0 | NA |
| Wine, red (3.5 oz, 8 g alcohol) | 87 | 4.4 | 0.1 (0.2) | 2.7 (0.9) | 06 | 06 | 0 | 0 | 0 | 0 | 8 (0.8) | 07 | 0 | 0 | 0 | 0 | Flavonoid, 34 |
| Wine, white (3.5 oz, 8 g alcohol) | 84 | 4.2 | 0.1 (0.2) | 2.7 (0.9) | 06 | 06 | 0 | 1 | 0 | 0 | 0 | 07 | 0 | 0 | 0 | 0 | Flavonoid, 2 |
| Distilled beverages, 80 proof (1.5 oz, 14 g alcohol) | 97 | 4.9 | 0 | 0 | 06 | 06 | 0 | 0 | 0 | 0 | 0 | 07 | 1 (0.0) | 0 | 0 | 0 | 0 |
| Limit consumption | |||||||||||||||||
| Ginger ale (7.5 oz) | 80 | 4.0 | 0 | 21 (7.0) | 06 | 06 | 0 | 21 | 42 | 0 | 0 | 4010 (1.7) | 0 | 0 | 0 | 0 | 0 |
| Ginger ale, diet (7.5 oz) | 04 | 0 | 0 | 0 | 06 | 06 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Soda, sweetened, caffeinated (8 oz) | 110 | 5.5 | 0 | 29 (9.7) | 06 | 06 | 0 | 29 | 58 | 22–69 | 0 | 3610 (1.6) | 0 | 0 | 0 | 0 | 0 |
| Energy drinks with caffeine (8 oz) | 130 | 6.5 | 0 | 33 (11.0) | 06 | 06 | 0 | 32 | 64 | 33–400 | 0 | 170 (7.4) | 0 | 0 | 0 | 0 | 0 |
Data from the USDA, Agricultural Research Service, Nutrient Data Laboratory; National Nutrient Database for Standard Reference (20). DV, Daily Value; NA, not available.
One ounce is equivalent to 29.6 mL.
Relative to a 2000-kcal/d diet.
Source: Mitchell et al. (101).
Calorie-free: <5 kcal/serving.
Low fat: ≤3 g/serving or saturated fat–free: <0.5 g/serving.
Sodium-free: <5 mg/serving.
Very low sodium: <35 mg/serving.
Good source: 10–19% DV.
Low sodium: <140 mg/serving.
Rich source: >20% DV.
Exceeds recommended limit (based on 2000-kcal/d diet) (2).
If alcohol is consumed, it should be in moderation—≤1 drink/d for women and ≤2 drinks/d for men—and only by adults of legal drinking age [1 alcoholic drink-equivalent = 14 g (0.6 fl oz) pure alcohol] (2).
Milk intake has been linked to various health outcomes, including a decreased risk of diabetes (low-fat milk only) (21), colon cancer (22, 23), cognitive disorders (24), and stroke (25), and no increased risk of coronary artery disease (CAD) and mortality (26). In a meta-analysis in an adult population, there was no significant association between low-fat or whole milk intake and fatal prostate cancer (27). However, results from a recent meta-analysis of 11 population-based cohort studies in adults reported that whereas intake of total dairy products had no significant impact on increased all-cancer mortality risk, an increase of whole milk (1 serving/d) contributed to elevated prostate cancer mortality risk significantly, with an RR of 1.43 (95% CI: 1.13, 1.81, P = 0.003) (28). Research has linked dairy intake to improved bone health, especially in children and adolescents (29).
The market for plant-based milk substitutes continues to grow, with soy milk among the most popular (30). However, market share for soy milk is decreasing as other plant-based milk substitutes, e.g., almond, cashew, rice, and other plant-based milks, are increasing in popularity (31, 32). Although there is a growing debate on consumer recognition of these products as milk substitutes, the perception of these products is as a replacement for dairy. A consumer study found that the majority of adults surveyed believed alternative milk products are nutritionally equivalent to cow milk (33). The nutrient density of such milk substitutes can vary considerably depending on the raw material used, processing, fortification with vitamins and minerals, and addition of other ingredients such as sugars and oils. Soy milk is currently the only plant-based milk substitute that approximates the protein content of cow milk and is comparable in quality (34). Furthermore, calcium is the only nutrient from milk substitutes to be tested for equivalent bioavailability to cow milk, which was reported to be similar (35). To date, there is insufficient evidence to support health benefits of certain plant-based milk substitutes above those of cow milk.
Whole milk is not included in the DGA recommendations for dairy; dairy fat [2.3%, 1.1%, and 0.1% of saturated, monounsaturated, and polyunsaturated fats, respectively (36)] may not have the health risks associated with other animal fats. Although strong associations between saturated fat intake and occurrence of CVD have been reported (37), a review of observational studies found no relation between milk fat and risk of CVD, CAD, or stroke (38). In the Malmo Diet and Cancer cohort, there was a decreased risk of incident type 2 diabetes with a high intake of high-fat but not for low-fat dairy products (39). In the Observation of Cardiovascular Risk Factors in Luxembourg Study, whole-fat dairy food intake was inversely associated with obesity prevalence (40). In addition, longitudinal evaluation of milk type consumed and weight status in preschoolers found that 1% skim milk drinkers had higher BMI z scores than 2% whole milk drinkers. However, this may reflect the choice whereby parents give overweight/obese children low-fat milk to drink (41). Moreover, a recent controlled clinical study reported that whereas both cheese and butter diets high in dairy SFAs significantly increased LDL cholesterol compared with the effects of carbohydrates, MUFAs, and PUFAs, LDL-cholesterol concentrations were significantly lower in participants consuming a cheese diet than in those consuming a butter diet (42). In sum, the next DGA Committee is encouraged to review the evidence on whole milk and chronic disease risk in the context of achieving nutrient needs and not exceeding calorie needs.
Milk as a part of the DGA
The DGA dairy recommendation is 3 cups/d (710 mL/d) for a 2000-kcal/d diet. Milk can be considered as a part of meeting the requirements for high-quality protein, calcium, phosphorus, vitamin A, vitamin D, and potassium. In addition:
When choosing milk substitutes, consider the nutrient content.
To be in line with the DGA's recommendation, when milk and milk substitutes are consumed, account for calories from added sugars (e.g., flavored milks) and other additions within the eating pattern.
One Hundred Percent Fruit and Vegetable Juices
The DGA recommends 2 cup equivalents of fruit and 2.5 cup equivalents of vegetables daily. The DGA includes 100% fruit juice in the fruit group as counting toward daily fruit recommendations but recommends limiting 100% fruit juice to 1 cup (237 mL) with the remainder as whole or cut fruit. During 2007–2010 half of the total US population consumed <1 cup of fruit and <1.5 cups of vegetables daily; 76% did not meet fruit intake recommendations and 87% did not meet vegetable intake recommendations (43). Consumption of fruits and vegetables adds nutrients to diets and reduces the risks of heart disease, diabetes, age-related cognitive impairment, some cancers, and all-cause mortality (44–48). Several inverse associations have been reported between fruit and vegetable intake and prospective improvements in anthropometric parameters, and risk of adiposity (49). Specific to 100% fruit juice, a recent review of systematic reviews and meta-analyses in children and adults evaluated the association between juice consumption and various chronic health outcomes and concluded that aside from increased risk of tooth decay in children and small amounts of weight gain in young children and adults, there is no conclusive evidence that consumption of 100% fruit juice has other adverse health effects (50). The authors noted that the meta-analysis on tooth decay was limited in that most of the included studies were cross-sectional and hence were vulnerable to confounding and reverse-causation. The health outcomes included diabetes, CVD, glucose homeostasis, lipid concentrations, and blood pressure. The study also found no significant associations between juice and weight gain in adults, although an analysis of participants in the Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study found an association of 0.22 kg weight gain over 4 y with every serving of 100% fruit juice (240 mL) consumed daily (51). For children, 100% fruit juice consumption was not associated with a BMI z score increase in children aged 7–18 y (52). Drinking 1 serving of 100% fruit juice per day was associated with a small amount of weight gain in children aged 1–6 y (BMI z score change of 0.09 units over 1 y) (52). However, children who consumed ≥1 serving/d of 100% juice also had a greater risk of tooth decay than those with ≤1 serving/d consumption in a meta-analysis of children and adolescents that included 5 cross-sectional and 2 longitudinal studies (53).
Fruit and vegetable juices can be important sources of potassium; vitamins A, C, E, K, and B-6; thiamin; niacin; folate; and choline, as well as potassium, iron, manganese, and fiber (20). In addition, phytonutrients such as flavonoids (anthocyanins, flavonols) and carotenoids, contained in fruit and vegetable juices, have health benefit (54). Hence, fruit and vegetable juices are nutrient dense. For example, 8 oz (237 mL) of many 100% fruit and vegetable juices are good (10–19% daily value) or rich (≥20%) sources of potassium and vitamins A and C (Table 1). Indeed, consumption of 100% fruit juice was associated with improved nutrient intakes across the life span (55–60).
Although 100% fruit and vegetable juices are nutrient dense, sodium needs to be considered, particularly for certain vegetable juices, e.g., tomato. Keeping in mind there is no evidence for an optimal amount and the National Academies of Sciences, Engineering, and Medicine state that there remains insufficient evidence to establish sodium or potassium DRIs for adequacy as Estimated Average Requirements (EARs) and RDAs (61), the WHO guideline on sodium and potassium intake considered to be beneficial for health is a ratio of sodium to potassium of ∼1:1 (62). When considering the FDA definitions of sodium-free (<5 mg/serving), very low sodium (≤35 mg/serving), and low sodium (≤140 mg/serving), almost all commonly consumed beverages fall into one of these categories with perhaps the exceptions of almond milk and certain energy drinks (Table 1).
Although there are no specific guidelines in the current DGA for fruit and vegetable intake for children <2 y of age, the American Academy of Pediatrics (AAP) indicates that there is no nutritional indication to give fruit juice to infants younger than 6 mo and that it is optimal to avoid the use of juice in infants before 1 y of age. The AAP also recognizes that for older children, fruit is to be encouraged, and that up to half of the servings can be provided in the form of 100% fruit juice (but not fruit drinks, which are calorically sweetened beverages with a small percentage of fruit juice), with fruit juice offering no nutritional advantage over whole fruit (63).
One hundred percent fruit and vegetable juices as a part of the DGA
The DGA recommends that at least half of the recommended amount of fruit come from whole fruits. The DGA recognizes that 100% juice can be a part of meeting the recommendation for fruits and vegetables and can be a part of meeting the requirements for potassium, vitamins A and C, as well as fiber. Modeling food intake patterns showed that without 100% fruit juice diets would be substantially lower in vitamin C and potassium than for patterns including fruits plus 100% fruit juice (64). In addition:
Consider 100% juice as a key source of phytonutrients (Table 1) including carotenoids (e.g., orange, carrot, and tomato juice) and phenolic acids (e.g., purple grape, cranberry, and apple juice).
Consider fortified juices for key nutrients, e.g., vitamins C and D and calcium.
Try to avoid introducing juice until the child is a toddler. If juice is introduced, wait until 12 mo and limit consumption to 4–6 oz (118–177 mL).
Choose low-sodium juices.
Coffee and Tea
Coffee
Approximately 75% of the US population aged ≥20 y reported drinking coffee; 49% reported drinking coffee daily (65). Although coffee has very little nutrient content, it contributes ∼5% of the potassium intake in the United States, which is similar to vegetables (excluding potatoes), fruit, and 100% juices (8), and can have low but variable concentrations of fluoride (14). There is evidence that, in healthy adults, moderate coffee consumption (4–5 cups/d or 946–1183 mL/d) has beneficial effects for a number of chronic diseases. A recent review evaluated the evidence from meta-analyses of observational studies and RCTs in adults relating to coffee intake and health outcomes (66). Of 59 unique outcomes examined in 112 meta-analyses of observational studies, coffee was associated with a probable decreased risk of breast, colorectal, colon, endometrial, and prostate cancers; CVD and all-cause mortality; Parkinson disease; and type 2 diabetes. Of 12 unique acute outcomes examined in 9 meta-analyses of RCTs, coffee was associated with a rise in serum lipids, but this result was affected by significant heterogeneity among the study designs and likely by coffee preparation methods. The authors concluded that the robustness of many of the results indicated that coffee can be part of a healthful diet. In part, the beneficial effects could be due to phenolic acids contained in coffee. Most negative impacts of serum lipids are driven by select sterols, including kahweol and cafesterol (67), that are present in percolated or boiled coffee but reduced greatly in paper-filtered drip coffee and espresso preparations (68, 69).
Tea
Brewed tea is a beverage made by hot water infusion of Camellia sinesis leaves. This is not to be confused with herbal “teas” that may contain other botanicals as ingredients. Tea is a major contributor to beverage intake in the US adult population with ∼1 of 3 adults reporting regular consumption on any given day (70). Tea provides few nutrients (∼2% of potassium intake in the United States) (8), although it is considered to be a significant contributor to total fluoride intake (14).
Meta-analyses of RCTs including 13 (71) and 20 (72) studies found that 3 cups (710 mL) of green tea reduced systolic and diastolic blood pressure by ∼2 mm Hg. Similarly, a meta-analysis evaluating black tea consumption on blood pressure (73) reported that 4–5 cups (946–1183 mL) of black tea reduced systolic and diastolic blood pressure by 1.8 and 1.3 mm Hg, respectively. A meta-analysis of 14 cohort studies consisting of 513,804 participants with a median follow-up of 11.5 y reported that an increase of 3 cups/d (710 mL/d) in tea consumption was associated with a 13% decreased risk of stroke (74). Another meta-analysis of 9 studies including 259,267 adult individuals found that those who did not consume green tea had higher risks of CVD, intracerebral hemorrhage, and cerebral infarction than those consuming <1 cup (237 mL) of green tea per day. Those who drank 1–3 cups (237–710 mL) of green tea per day had a reduced risk of myocardial infarction and stroke compared with those who drank <1 cup/d (<237 mL/d). Those who drank ≥4 cups/d (>946 mL/d) had a reduced risk of myocardial infarction compared with those who drank <1 cup/d (<237 mL/d) (75).
The consumption of black tea or green tea has been reported to be associated with a lower risk of diabetes. A meta-analysis of 16 adult cohorts with 37,445 cases of diabetes among 545,517 participants reported a significant linearly inverse association between black tea consumption and diabetes risk. An increase of 2 cups/d (573 mL/d) in tea consumption was associated with a 4.6% reduced risk (95% CI: 0.9, 8.1%) (76). In addition, a dose-response meta-analysis of prospective cohort studies in adults reported that in 18 prospective studies, consisting of 12,221, 11,306, and 55,528 deaths from all cancers, CVD, and all causes, respectively, for all-cancer mortality the RRs for the highest compared with the lowest categories of green tea and black tea consumption were 1.06 (95% CI: 0.98, 1.15) and 0.79 (95% CI: 0.65, 0.97), respectively. For CVD mortality, the RR for the highest compared with the lowest categories of green tea and black tea consumption were 0.67 (95% CI: 0.46, 0.96) and 0.88 (95% CI: 0.77, 1.01), respectively. For all-cause mortality, the RRs for the highest compared with the lowest categories of green tea and black tea consumption were 0.80 (95% CI: 0.68, 0.93) and 0.90 (95% CI: 0.83, 0.98), respectively (77). The dose-response analysis indicated that a 1-cup/d (237-mL/d) increment of green tea consumption was associated with 5% lower risk of CVD mortality and with 4% lower risk of all-cause mortality. Green tea consumption was significantly inversely associated with CVD and all-cause mortality, whereas black tea consumption was significantly inversely associated with all-cancer and all-cause mortality.
Phytonutrients
Green coffee beans contain diverse groups of phenolic compounds, with chlorogenic acids and mixed diesters of caffeic and ferulic acids plus quinic acid being the primary forms (78). Black and green teas are rich sources of monomeric flavan-3-ols as well as complex oxidized flavan-3-ol forms including the theaflavins, thearubigins, and theabrownins (79, 80). Healthful benefits from consuming coffee and tea may be imparted by these phytonutrients, along with caffeine, trigonelline, diterpenes, and soluble fiber (81). Current evidence suggests that both chlorogenic acids and flavan-3-ols are absorbed primarily in the small intestine and appear in the circulation as glucuronide, sulfate, and methylated metabolites (82). Results from in vivo studies in animals and humans report that the phenolic and polyphenolic constituents of coffee and tea have biological activities including antioxidant activities (83–85), increase of fatty acid oxidation and insulin sensitivity (86, 87), and modulation of glucose absorption and utilization (88). In vitro studies have reported on their ability to modulate glucose metabolism (89, 90) as well as to stimulate nitric oxide production and vasodilation (91).
The evidence is accumulating that coffee and tea also have health benefits (see above) and are concentrated sources of dietary phytonutrients. For example, a mean intake of monomeric flavan-3-ols of 124 mg/d, compared with a mean intake of 25 mg/d, was associated with a 51% lower 10-y CAD mortality in the Zutphen Elderly Study (92). This amount is easily achieved with 3 oz (89 mL) of tea compared with two-thirds of a medium apple (93, 94). Although these compounds lack a DRI, their amounts from current intakes of fruits, vegetables, and whole grains fall short of such beneficial effects. Eight ounces (237 mL) of coffee and tea, being major contributors of these phytonutrients, provide amounts exceeding that found in 1 cup of commonly consumed fruits and vegetables (93, 94).
Caffeine
Much of the available evidence on the biological effects of coffee and tea includes an evaluation of both caffeinated and decaffeinated beverages, with both having demonstrated health benefits. However, intake of caffeine has been associated with a number of biological effects, mostly relating to the stimulation of the central and sympathetic nervous system, providing a feeling of alertness after consumption (66, 95–99). The content of caffeine in a serving of coffee is highly variable, depending on the source and type of coffee bean (robusta compared with arabica), the type of roasting (e.g., light, medium, or dark), the coffee-making method (boiling, steeping, filtered, pressure), and the ratio of coffee ground to water, with values ranging from 50 to >300 mg per 8 oz (237 mL) serving (100). Brewed tea has lower caffeine content (15–50 mg/8 oz or 237 mL) (101). Decaffeinated coffee and tea will contain ∼10 mg or less per 8 oz (237 mL) (102).
In the United States, caffeine consumption increases with age and intake is highest among 50- to 64-y-old consumers (226 mg/d). The mean across all age groups is ∼165 mg/d (101). Coffee is the primary source of caffeine (64%; 105.4 mg/d), followed by carbonated soft drinks (17%; 27.9 mg/d) and tea (17%; 27.9 mg/d). Energy drinks contributed <2% to total caffeine intake (2.6 mg/d). The greatest percentage of energy drink consumers were found among 13- to 17- and 18- to 24-y-olds (∼10% of caffeine consumers compared with 4.3% across all age groups). Children (2–12 y) and adolescents (13–17 y) metabolize caffeine more rapidly than adults (101, 103). At 180–200 mg/d, typical caffeine consumption can provide the desired benefit (i.e., mental alertness) with a low risk of adverse side effects such as agitation, anxiety, or sleep disturbance (101, 104). However, concern has been raised that caffeine may cause behavioral issues in children and adolescents (105). A systematic review of RCTs, observational studies, and expert panel guidelines found that high caffeine intakes (e.g., >5 mg · kg body weight–1 · d–1) were associated with an increased risk of anxiety and withdrawal symptoms in children (106). The author concluded that lower contributors of caffeine at <2.5 mg · kg body weight–1 · d–1, equating to 1 or 2 cups of tea or 1 small cup of coffee daily, may benefit cognitive function and sports performance based on adult studies. The author also suggested that caffeinated soft drinks may be less suitable options for children because of the acidity, higher caffeine content, presence of added sugar, and absence of bioactive compounds.
The DGA states that moderate coffee consumption [three to five 8-oz (237 mL) servings per day or ∼400 mg caffeine/d] can be incorporated into healthy eating patterns. Although caffeine is considered a safe substance by the FDA, possible adverse effects on children and adolescents are largely unknown because most research has been conducted in adult populations (107). A recent systematic review evaluated the data on potential adverse effects of caffeine in different demographic groups (adults, pregnant women, adolescents, and children) (108). The authors concluded that the evidence generally supports that consumption of ≤400 mg caffeine/d in healthy adults is not associated with adverse cardiovascular effects, behavioral effects, reproductive and developmental effects, acute effects, or bone status. In addition, consumption of ≤300 mg caffeine/d in healthy pregnant women was generally not associated with adverse reproductive and developmental effects and the available evidence for children and adolescents suggests that <2.5 mg caffeine · kg body weight–1 · d–1 remains an appropriate recommendation. The European Food Safety Authority in their “Scientific Opinion on Caffeine” advised that pregnant women should limit caffeine intake to 200 mg/d (109). The data support that for healthy individuals, lethality may, but does not always, occur after acute consumption of 10 g caffeine, an amount well above what is attainable in coffee and tea beverages. However, the systematic reviews and meta-analyses to date identify a potential research gap in the investigation of caffeine effects at amounts >2.5 mg · kg body weight–1 · d–1 on anxiety in children and at >400 mg/d in adults with pre-existing conditions.
Coffee and tea as a part of the DGA
The DGA recommends limiting caffeine intake to 400 mg/d. Pregnant women are advised to consume no more than 200 mg caffeine/d. In addition:
Although the epidemiological evidence of health benefits is primarily based on brewed products, other coffee and tea products also contain phytonutrients but their contents can vary greatly.
Adolescent and child caffeine consumption should not exceed 2.5 mg · kg body weight–1 · d–1.
Decaffeinated coffee and tea can also serve as healthy beverage choices because the phenolic acids and flavonoids associated with health benefits, although modestly reduced in amounts, are present in these products.
When consuming coffee and tea, account for nutrients and calories from dairy, added sugars, and additions within the overall diet.
Of note, these recommendations are made based on brewed coffee and tea products because of the extent to which broader consumer products including instant and ready-to-drink product forms may have variable polyphenol profiles and/or calories from added sugars and additions. These amounts should be considered when evaluating or developing ready-to-drink or instant products to be included as part of a healthy diet.
Alcohol
Heavy and binge drinking increase the risk of chronic disease (110). A dose-response analysis from a meta-analysis of 84 prospective cohort studies in adults revealed that the lowest risk of CAD mortality occurred with 1–2 drinks/d, but for stroke mortality it occurred with ≤1 drink/d (∼15 g alcohol) (111). A meta-analysis evaluating the effects of alcohol reduction on blood pressure reported that decreasing alcohol intake was associated with a significant reduction in mean systolic and diastolic blood pressures (−3.31 and −2.04 mm Hg, respectively) (112). A dose-response relation was observed between mean percentage of alcohol reduction and mean blood pressure reduction. Low amounts of alcohol intake (<15 g/d) have also been reported to be associated with lower risks of heart disease (111, 113), diabetes (114–116), and dementia (117).
An increased intake of alcohol was also associated with a higher risk of breast cancer with the RR increasing by 7.1% (95% CI: 5.5, 8.7%) (118) and the HR increasing by 4.2% (95% CI: 2.7, 5.8%) (119) for each increase of 10 g alcohol/d. Others have reported that low alcohol intake was related to a higher risk of breast cancer than was little or no alcohol intake, with 1 study reporting intakes of >5 to 15 g/d being related to a 5.9% increase in breast cancer risk (95% CI: 1, 11%) (119). These findings are consistent with another study reporting a significant increase of the order of 4% in the risk of breast cancer at intakes of ≤1 alcoholic drink per day (120).
A pooled analysis of 8 cohort studies in adults reported an increased risk of colorectal cancer that was limited to persons with an alcohol intake of >30 g/d to <45 g/d (RR: 1.16; 95% CI: 0.99, 1.36) and a RR of 1.41 (95% CI: 1.16, 1.72) for those who consumed ≥45 g/d (121). The European Prospective Investigation into Cancer and Nutrition consisted of 347,237 study subjects free of cancer at enrollment and a follow-up averaging 12 y, during which 3759 colorectal cancer cases were observed (122). After adjustment for potential confounding factors, compared with alcohol intakes in women and men of >12 and >24 g alcohol/d, respectively, intakes less than these amounts had an HR of colorectal cancer of 0.87 (95% CI: 0.81, 0.94).
A meta-analysis of 16 prospective cohort studies in adults reported that average beer consumption of ≥1 drink/d (13 g alcohol/d) was associated with an increased risk of lung cancer (RR: 1.23; 95% CI: 1.06, 1.41) (123). This association was observed in both men and women, but only significant in men. An inverse association was observed for both average wine consumption of <1 drink/d and ≥1 drink/d. Average liquor consumption of ≥1 drink/d was found to be associated with increased lung cancer risk in men, with an RR of 1.33 (95% CI: 1.10, 1.62) (123). No association was observed for women. In addition, a pooled analysis of cohort studies reported a slightly greater risk of lung cancer with the consumption of ≥30 g alcohol/d than for no alcohol consumption (men: RR: 1.21; 95% CI: 0.91, 1.61; women: RR: 1.16; 95% CI: 0.94, 1.43) (124).
The HR of all-cause mortality comparing never with light drinkers (0.1–2.9 g alcohol/d) was 1.26 (95% CI: 1.18, 1.35) for women and 1.29 for men (95% CI: 1.10, 1.51) (125). When sources of alcohol were considered, beer use was more strongly related than wine to all-cause mortality for an intake of ≥3 g alcohol/d as compared with lower intakes (0.1–2.9 g alcohol/d). A meta-analysis of 34 prospective studies reported that consumption of alcohol, ≤4 drinks/d (40 g alcohol/d) in men and 2 drinks/d (20 g alcohol/d) in women, was inversely associated with total mortality, with maximum protection of 18% in women and 17% in men. Higher doses of alcohol were associated with increased mortality (126).
When consuming alcohol, it is important to keep in mind the diuretic effect that could lead to dehydration. Alcohol consumption should be within the recommended limits (≤1 drink/d for women and ≤2 drinks/d for men) (2). In addition, alcoholic drinks contain calories. For example, a standard (3.0-oz, 89-mL, 8-g alcohol) glass of red wine contains ∼87 kcal and 12 oz (355 mL, 12 g alcohol) of regular beer lager contain ∼153 kcal (Table 1).
Alcohol as a part of the DGA
The DGA recommends that if alcohol is consumed, it should be in moderation—≤1 drink/d for women and ≤2 drinks/d for men—with consideration for calories and within the limits of healthy eating patterns. Alcoholic beverages should be consumed only by adults of legal drinking age and are contraindicated during pregnancy; when doing activities that require attention, skill, and coordination; and when using certain pharmaceutical drugs and/or undergoing therapeutic procedures that interact with alcohol.
SSBs
SSBs, including soft drinks, non-100% juices, fruit drinks, sports drinks, and energy drinks, can contribute excess calories while providing few or no key nutrients. SSBs are significant sources of added sugars in the diet of US adults, accounting for approximately one-third of added sugar consumption, and are the primary source of added sugar in the diet of children and adolescents (127). In 2011–2014, 6 in 10 youth (63%) and 5 in 10 adults (49%) drank an SSB on a given day (128). On average, US youth consume 143 kcal from SSBs and US adults consume 145 kcal from SSBs on a given day (128). Among adults, consumption of SSBs at least once a day is associated with adverse health consequences, including obesity, type 2 diabetes, and CVD (129–131). In a dose-response meta-analysis of prospective studies in adults, the RR for incident hypertension was 1.08 (95% CI: 1.04, 1.12) for every additional 1 serving/d increase in SSB consumption. The RR for CAD was 1.17 (95% CI: 1.10, 1.24) for every 1 serving/d increase in SSB consumption. There was no significant association between SSB consumption and total stroke for every 1 serving/d increase in SSB consumption (132).
Sports drinks
Sports drinks are designed to help athletes replace water, electrolytes, and energy before and after exercise or competition. In an assessment of the evidence to support claims of improved water absorption during exercise and maintenance of endurance performance, Thompson et al. (133) reported limitations in the scientific evidence. Many of the studies had methodological limitations, such as lack of blinding. Furthermore, most studies were in young male endurance athletes, making difficult translation to other populations, e.g., women, children, and older people.
Energy drinks
Energy drinks are beverages that contain stimulants such as caffeine and are marketed to provide mental and physical stimulation. Energy drinks may also contain sugar or other sweeteners, herbal extracts, taurine, and B vitamins. The International Society of Sports Nutrition recently concluded the primary ergogenic nutrients in most energy drinks appear to be carbohydrate and/or caffeine (134). Energy drinks are particularly popular among adolescents and young adults with nearly two-thirds of teens reporting ever using energy drinks, 31% of 12- to 17-y-olds reporting consuming energy drinks regularly (134), and 5% of high school–age adolescents consuming energy drinks daily (135).
With the rising popularity of energy drinks among these age groups come safety concerns. Caffeine, the most physiologically active ingredient in energy drinks, is generally recognized as safe (GRAS) by the US FDA; however, adverse effects can occur with high intakes, the most common being effects on the cardiovascular and neurological systems (136). Guarana, which contains caffeine in addition to small amounts of theobromine, theophylline, and tannins, also has GRAS status, but when combined with caffeine in an energy drink may lead to caffeine toxicity (137). The position of the AAP is that “stimulant-containing energy drinks have no place in the diets of children and adolescents” (138).
Low-calorie sweetened beverages
Low-calorie sweetened (LCS) beverages use sweeteners that have a higher intensity of sweetness per gram than caloric sweeteners such as sucrose and high-fructose corn syrup. The recent AHA science advisory defines LCS beverages to include 6 high-intensity sweeteners currently approved by the US FDA (saccharin, aspartame, acesulfame-K, sucralose, neotame, and advantame), steviol glycoside extracted from the leaves of the stevia plant (Stevia rebaudiana), and monk fruit extract (also known as Siraitia grosvenorii, Swingle fruit, or luo han guo) (139). The 2015–2020 DGA recommends that the daily intake of calories from added sugars not exceed 10% of total calories. Given negligible to no calorie content, LCS beverages could be viewed as SSB alternatives to meet this guideline. In an analysis of household purchases and NHANES dietary intake (140), beverages were the main sources of low-calorie sweeteners and represented 32% of all beverages among adults and 19% among children.
Some observational studies have suggested that LCS beverages may increase certain disease risk factors. A recent meta-analysis including adolescents and adults evaluating the association between consumption of artificially sweetened soda and obesity reported that the pooled RR for obesity in individuals consuming artificially sweetened soda was 1.59 (95% CI: 1.22, 2.08) (141). A meta-analysis of prospective studies in adult cohorts examining the association between LCS beverages and hypertension reported that, in the 4 studies meeting the eligibility criteria (227,254 subjects and 78,177 incident cases of hypertension), the pooled RRs were 1.14 (95% CI: 1.10, 1.18) for highest compared with lowest intake analysis and 1.09 (95% CI: 1.06, 1.11) for every additional 1 serving/d increase in LCS beverage consumption (142). In addition, a meta-analysis of the association between LCS beverages and type 2 diabetes found that higher consumption of noncaloric beverages was associated with a greater incidence of type 2 diabetes, by 25% (95% CI: 18, 33%) and 8% (95% CI: 2, 15%) per 1 serving/d, before and after adjustment for adiposity, respectively (130). The authors indicated publication bias and residual confounding. Prospective longitudinal studies and clinical trials may provide evidence that circumvents the possibility of reverse causation. It was estimated that substituting 1 serving/d of LCS beverages for the same amount of SSBs was associated with 0.47 kg less weight gain over a 4-y period (51). A systematic review and a meta-analysis of trials and prospective cohorts concluded that replacing SSBs with LCS beverages could contribute to a modest weight loss (143, 144). However, existing findings from trials have been criticized for their lack of statistical power, short duration, participants being unblinded to the treatment groups, and conflicts of interest in research funding.
The AHA Nutrition Committee recently reviewed the evidence from observational studies and clinical trials assessing the cardiometabolic outcomes of LCS beverages and concluded that the use of other alternatives to SSBs, with a focus on plain, carbonated, or unsweetened flavored water, should be encouraged (139). They also concluded that with limited evidence on the adverse effects of LCS beverages on health, prolonged consumption of LCS beverages by children is not advised.
SSBs as a part of the DGA
The DGA recognizes that consumption of SSBs (soft drinks, energy drinks, and fruit drinks) should be limited. In addition:
Among individuals who habitually consume SSBs and are habituated to sweet-tasting beverages, replacing SSBs with LCS beverages may provide a first step to reduce SSB consumption. However, replacing SSBs with water or other unsweetened beverages, such as tea and coffee, is strongly encouraged.
Beverages Address Key DGA Messages
A key message of the DGA is healthy eating patterns. An example of a healthy eating pattern is the Healthy US-Style Eating Pattern at the 2000-calorie level (Table 2). The appropriate beverage choices can fit into such a dietary pattern (Table 2). For an understanding of how beverages can be better considered as part of the key messages set forth by the 2015–2020 DGA, commonly consumed beverages were characterized based on calories, macronutrients, caffeine, select minerals and vitamins, and phytonutrients (Table 1).
TABLE 2.
Healthy US-Style Eating Pattern at the 2000-kcal/d level, with daily or weekly amounts from food groups, subgroups, and components1
| Food group | Amount in the 2000-kcal level pattern | 1 cup-eq or 1 oz-eq of food | 1 cup-eq or 1 oz-eq of beverage |
|---|---|---|---|
| Vegetables | |||
| Dark green Red & orange Legumes Starchy Other | 1.5 cup-eq/wk5.5 cup-eq/wk1.5 cup-eq/wk5.5 cup-eq/wk4.5 cup-eq/wk | 1 cup raw or cooked vegetables,2 cups green leafy salad greens,0.5 cup dried vegetables | 1 cup 100% vegetable juice, e.g., tomato, carrot |
| Fruits | 2.5 cup-eq/d | 1 cup fresh fruits, 0.5 cup dried fruits | 1 cup 100% fruit juice, e.g., orange, grapefruit |
| Grains | 6 oz-eq/d | ||
| Whole grains Refined grains | ≥3 oz-eq/d≤3 oz-eq/d | 1 oz dry pasta or rice,1 medium slice bread,0.5 cup cooked rice, pasta, or cereal,1 oz ready-to-eat cereal | NA |
| Dairy | 3 cup-eq/d | 1 cup yogurt,1.5 oz natural cheese, e.g., cheddar,1 oz processed cheese | 1 cup milk or fortified soy milk |
| Protein foods | 5.5 oz-eq/d | ||
| Seafood | 8 oz-eq/wk | 1 oz seafood | |
| Meat, poultry, eggs | 26 oz-eq/wk | 1 oz lean meat or poultry,1 egg | NA |
| Nuts, seeds, soy food products | 5 oz-eq/wk | 0.25 cup cooked beans or tofu,1 tbsp peanut butter,0.25 cup nuts or seeds | |
| Oils | 27 g/d | NA | |
| Limits on calories for other uses (%kcal) | <270 kcal/d (<14%) | ||
1 cup-eq = 237 mL; 1 oz-eq = 30 g; 1 tbsp = 14.8 mL. eq, equivalent; NA, not applicable.
A focus on healthy eating patterns
A variety of beverages fit well into the 3 healthy eating patterns described in the current DGA. As shown in Tables 1 and 2, beverage choices fit into 3 food groups. For example, 8 oz of milk would meet 33% of the daily recommendation for dairy, ∼6 oz (∼177 mL) of 100% fruit juice would meet 30% of the recommendation for the daily intake of fruit, and 6 oz/d of 100% tomato or carrot juice would meet the weekly recommendation for red or orange vegetables. Problems with exceeding sugar and/or sodium intake recommendations may arise with the juice recommendation as with some existing 100% juice products; however, possible advances in food technology could bring this into alignment with the DGA (145).
Dietary shifts that may be needed to achieve such patterns
Modest shifts in beverage choices can help close the gaps between current intakes and dietary recommendations. Below are examples of shifting from SSBs to higher-nutrient-density beverage choices, as well as the impact this has on meeting nutrient recommendations:
Replacing SSBs with water, low-sodium tomato juice, nonfat milk, or unsweetened coffee or tea. Considering the current mean intake of added sugars in the United States for women and men (55 and 62.5 g/d, respectively) (146), which is >20% higher than recommendations (<10% of total calorie intake), substitution of one 8-oz (237 mL) SSB with water (or unsweetened, no-added-dairy coffee or tea) would bring these averages down to 17% and 34% below the recommended limits for men and women, respectively (Figure 1). A substitution of 8 oz (237 mL) of nonfat milk would bring this to 20% and 37% below the recommendation for men and women, respectively. A substitution of 100% low-sodium tomato juice would bring this to 18% and 36% below the recommendation for men and women, respectively.
Replacing SSBs with nonfat milk or low-sodium tomato juice. Mean intakes of potassium, calcium (women only), vitamin A, and vitamin D are below the RDAs (Figure 2A). Substituting 8 oz (237 mL) of SSB with 8 oz (237 mL) of nonfat milk would increase intakes of potassium by 9% of the RDA (both men and women), calcium to 40% and 20% above the RDA (men and women, respectively), vitamin A by 8% and 23% of the RDA (men and women, respectively), and vitamin D by 20% of the RDA (both men and women) (Figure 2). A replacement with 8 oz (237 mL) of low-sodium tomato juice would increase intakes of fiber by ∼8% of current intakes, intake of potassium by ∼19% of the RDA, and vitamin A by 13–18% of the RDA for both men and women (Figure 2B).
Replacing whole milk with nonfat or low-fat milk. One serving of whole milk (8 oz, 237 mL) provides 161 kcal and 5 g SFAs. Given the DGA recommendation that <10% of total calories should come from SFAs, this would account for ∼22% of the recommended limit compared with 0%, 7%, and 14% for nonfat, 1% fat, and 2% fat milk, respectively (Table 1). Thus, saturated fat intake from whole milk ≤3 servings/d falls below the recommended limit but may restrict intake of SFAs as well as discretionary calories from other food sources. Replacing whole milk with nonfat or low-fat milk reduces saturated fat intake from dairy products.
FIGURE 1.
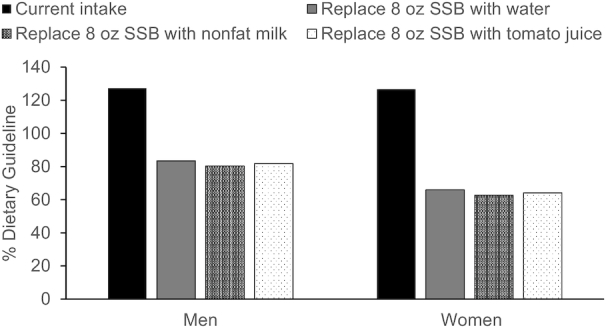
Mean added sugar intake (% Dietary Guideline) for adults when 8 oz (237 mL) of SSB is replaced with 8 oz (237 mL) of water, non/low-fat milk, or tomato juice. Data taken from (146). SSB, sugar-sweetened beverage.
FIGURE 2.
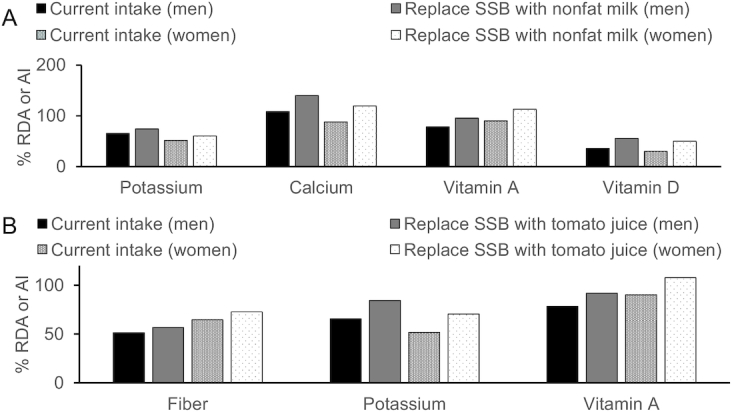
Mean fiber, potassium, calcium, vitamin A, and vitamin D intakes (% RDA or AI) for adults when 8 oz (237 mL) of SSB is replaced with 8 oz (237 mL) of non/low-fat milk (A) or tomato juice (B) (36, 70). AI, Adequate Intake; SSB, sugar-sweetened beverage.
A focus on variety, nutrient density, and intake amounts
Just as with foods, no one beverage will provide all the nutrients and phytonutrients needed for optimal health. And just like foods, variety is the key. Milk and milk substitutes (i.e., soy milk) are rich sources of high-quality protein (except some plant alternative milks) and vitamins A and D. One hundred percent juices are rich in potassium and vitamins A and C while being low in calories, fat, added sugars, and sodium. Although the amount of fiber contained in vegetable juice is relatively low (∼2 g/8 oz or 237 mL), 1 serving/d would increase current intakes in adults by >10%. Coffee, tea, and 100% fruit and vegetable juices are rich sources of phytonutrients. Most beverages listed in Table 1 are low fat (<3 g/serving), with the exception of whole milk and coconut milk, and can be considered as a part of a healthy eating pattern.
Discussion and Conclusions
The DGA was developed to provide guidance in choosing a healthy diet (2). Many Americans fall short of meeting many of these recommendations. The purpose of this report was to highlight the current and potential role of beverages as a part of the DGA. This was done with a consideration of the current scientific evidence as well as the nutrients and phytonutrients that commonly consumed beverages provide, with a focus on nutrient gaps in current intakes.
Beverages vary in nutrient and caloric content, each with health benefits (and, in the case of SSBs, possible health risks). Apart from hydration, LCS beverages and energy drinks may have limited health benefits and possible adverse effects (147). Although in some instances beverages cannot replace foods, e.g., it is difficult to meet the requirements for vitamin E, dietary fiber, or essential fatty acids through beverages alone, beverages have an important role in our diet being rich sources of nutrients and phytonutrients, phenolic acids and flavonoids in particular. When considering the micronutrients from diet alone, mean intakes of calcium (women), potassium, and vitamins A, C, and D are below recommendations and sodium intakes are well above (148). Modest beverage choices and substitutions could close these gaps. The graphics presented in this report provide select examples on how minor changes in beverage choices can aid in meeting recommended intakes of certain nutrients.
It is also important to encourage product innovation and reformulations that could be used to improve nutrient and/or phytonutrient profiles. For example, guidance is currently limited to 100% juice based on product standards of identity defined by soluble solids which are mostly sugar. As technologies evolve to develop products of improved nutritional profiles (i.e., reduced sugar or increased nutrient density) within dairy, dairy substitutes, fruit and vegetable juice, as well as coffee and tea, these products may not meet the current definitions used in the development of beverage guidance. Evaluation of these products and their incorporation into dietary guidance must consider the nutrient and perhaps phytonutrient density as primary factors which may allow for their consideration as a part of healthy food–based dietary patterns.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the beverage guidelines for children that have been released since the acceptance of this manuscript (https://healthydrinkshealthykids.org/app/uploads/2019/09/HER-HealthyBeverage-consensusStatement.pdf). The authors’ responsibilities were as follows—all authors: were responsible for the design, writing, and final content of the manuscript and read and approved the final manuscript.
Notes
Supported by The Tea Council of the USA (to EJJ).
Author disclosures: MGF is on the scientific advisory board for the Florida Department of Citrus Scientific Research Advisory Committee and has received research funding from Welch's. EJJ is currently employed at Tufts University and Ocean Spray Cooperative. All other authors report no conflicts of interest.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: AAP, American Academy of Pediatrics; CAD, coronary artery disease; CVD, cardiovascular disease; DGA, Dietary Guidelines for Americans; GRAS, generally recognized as safe; LCS, low-calorie sweetened; RCT, randomized controlled trial; SSB, sugar-sweetened beverage.
References
- 1. Tuma PA. Dietary guidelines 2020–2025: update on academy efforts. J Acad Nutr Diet. 2019;119:672–4. [DOI] [PubMed] [Google Scholar]
- 2. US Department of Health and Human Services (US HHS) and USDA. 2015–2020 Dietary Guidelines for Americans. [Internet] 8th ed Washington (DC): US HHS and USDA; December2015; [cited 7 April, 2019]. Available from: http://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 3. Rehm CD, Peñalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999–2012. JAMA. 2016;315:2542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kit BK, Fakhouri THI, Park S, Nielsen SJ, Ogden CL. Trends in sugar-sweetened beverage consumption among youth and adults in the United States: 1999–2010. Am J Clin Nutr. 2013;98:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sebastian RS, Goldman JD, Enns CW, LaComb RP; Food Surveys Research Group. Fluid milk consumption in the United States. What We Eat In America, NHANES 2005–2006. Dietary Data Brief No. 3 Beltsville, MD: Food Surveys Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, USDA; 2010. [Google Scholar]
- 6. Energy drink consumption frequency in the United States in 2016 by age. [Internet] Hamburg, Germany:Statista; 2016; [cited 2 July, 2019]. Available from: https://www.statista.com/statistics/621710/energy-drink-consumption-frequency-in-the-us-by-age/. [Google Scholar]
- 7. Kimmons J, Gillespie C, Seymour J, Serdula M, Blanck HM. Fruit and vegetable intake among adolescents and adults in the United States: percentage meeting individualized recommendations. Medscape J Med. 2009;11:26. [PMC free article] [PubMed] [Google Scholar]
- 8. Hoy M, Goldman J. Potassium intake of the U.S. population: What We Eat In America, NHANES 2009–2010. [Internet] Beltsville, MD: Food Surveys Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, USDA; 2012; [cited 10 March, 2019]. Dietary Data Brief No. 10. Available from: http://ars.usda.gov/Services/docs.htm?docid=19476. [Google Scholar]
- 9. Hoy M, Goldman J. Fiber intake of the U.S. population: What We Eat in America, NHANES 2009–2010. [Internet] Beltsville, MD: Food Surveys Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, USDA; 2014; [cited 10 March, 2019]. Dietary Data Brief No. 12. Available from: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/DBrief/12_fiber_intake_0910.pdf. [Google Scholar]
- 10. Hoy M, Goldman J. Calcium intake of the U.S. population: What We Eat in America, NHANES 2009–2010. [Internet] Beltsville, MD: Food Surveys Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, USDA; 2014; [cited 10 March, 2019]. Dietary Data Brief No. 13. Available from: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/DBrief/13_calcium_intake_0910.pdf. [Google Scholar]
- 11. Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panel on Dietary Reference Intakes for Electrolytes and Water, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board. Water. In: Dietary Reference Intakes for water, potassium, sodium, chloride, and sulfate. Washington (DC): National Academies Press (US); 2005. p. 73–185. [Google Scholar]
- 13. National Center for Chronic Disease Prevention and Health Promotion, Division of Oral Health. Community water fluoridation: 2010 water fluoridation statistics. [Internet] Atlanta, GA: CDC; 2012; [cited 19 November, 2018]. Available from: http://www.cdc.gov/fluoridation/statistics/2010stats.htm. [Google Scholar]
- 14. Cutrufelli R, Pehrsson P, Haytowitz D, Patterson K, Holden J. USDA national fluoride database of selected beverages and foods, release 2. [Internet] Beltsville, MD: Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, Agricultural Research Service, USDA; 2005; [cited 19 November, 2018]. Available from: http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/Fluoride/F02.pdf. [Google Scholar]
- 15. Azoulay A, Garzon P, Eisenberg MJ. Comparison of the mineral content of tap water and bottled waters. J Gen Intern Med. 2001;16:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gianfredi V, Bragazzi NL, Nucci D, Villarini M, Moretti M. Cardiovascular diseases and hard drinking waters: implications from a systematic review with meta-analysis of case-control studies. J Water Health. 2017;15:31–40. [DOI] [PubMed] [Google Scholar]
- 17. US Environmental Protection Agency. Drinking water advisory: consumer acceptability advice and health effects analysis on sodium. [Internet]. Report no. EPA 822-R-03-006 Washington (DC): US Environmental Protection Agency, Office of Water (4304T), Health and Ecological Criteria Division; 2003; [cited 19 November, 2018]. Available from: https://www.epa.gov/sites/production/files/2014-09/documents/support_cc1_sodium_dwreport.pdf. [Google Scholar]
- 18. Kenney WL, Chiu P. Influence of age on thirst and fluid intake. Med Sci Sports Exerc. 2001;33:1524–32. [DOI] [PubMed] [Google Scholar]
- 19. Bernstein M. Nutritional needs of the older adult. Phys Med Rehabil Clin N Am. 2017;28:747–66. [DOI] [PubMed] [Google Scholar]
- 20. Showell P, Pehrsson P. National nutrient database for standard reference, release 28. [Internet] Beltsville, MD: USDA, Agricultural Research Service; 2015; [cited 2 July, 2019]. Available from: http://ars.usda.gov/Services/docs.htm?docid=8964. [Google Scholar]
- 21. Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr. 2013;98:1066–83. [DOI] [PubMed] [Google Scholar]
- 22. Vieira AR, Abar L, Chan DSM, Vingeliene S, Polemiti E, Stevens C, Greenwood D, Norat T. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann Oncol. 2017;28:1788–802. [DOI] [PubMed] [Google Scholar]
- 23. Ralston RA, Truby H, Palermo CE, Walker KZ. Colorectal cancer and nonfermented milk, solid cheese, and fermented milk consumption: a systematic review and meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2014;54:1167–79. [DOI] [PubMed] [Google Scholar]
- 24. Wu L, Sun D. Meta-analysis of milk consumption and the risk of cognitive disorders. Nutrients. 2016;8(12):824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Goede J, Soedamah-Muthu SS, Pan A, Gijsbers L, Geleijnse JM. Dairy consumption and risk of stroke: a systematic review and updated dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc. 2016;5(5):e002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mullie P, Pizot C, Autier P. Daily milk consumption and all-cause mortality, coronary heart disease and stroke: a systematic review and meta-analysis of observational cohort studies. BMC Public Health. 2016;16:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aune D, Navarro Rosenblatt DA, Chan DSM, Vieira AR, Vieira R, Greenwood DC, Vatten LJ, Norat T. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr. 2015;101:87–117. [DOI] [PubMed] [Google Scholar]
- 28. Lu W, Chen H, Niu Y, Wu H, Xia D, Wu Y. Dairy products intake and cancer mortality risk: a meta-analysis of 11 population-based cohort studies. Nutr J. 2016;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dror DK, Allen LH. Dairy product intake in children and adolescents in developed countries: trends, nutritional contribution, and a review of association with health outcomes. Nutr Rev. 2014;72:68–81. [DOI] [PubMed] [Google Scholar]
- 30. Mäkinen OE, Wanhalinna V, Zannini E, Arendt EK. Foods for special dietary needs: non-dairy plant-based milk substitutes and fermented dairy-type products. Crit Rev Food Sci Nutr. 2016;56:339–49. [DOI] [PubMed] [Google Scholar]
- 31. Mintel Group, Ltd. Dairy and non-dairy milk - US - 2018. [Internet] London, England: Mintel Group Ltd; September2018; [cited 19 November, 2018]. Available from: https://store.mintel.com/us-dairy-and-non-dairy-market-report. [Google Scholar]
- 32. Bridges M. Moo-ove over, cow's milk: the rise of plant-based dairy alternatives. Pract Gastroenterol. 2018;42:20–7. [Google Scholar]
- 33. Jackson C, Chen E. “Milk” product labeling in the U.S. New York: Ipsos; 2018; [cited 19 November, 2018]. Available from: https://www.ipsos.com/en-us/news-polls/milk-product-labeling. [Google Scholar]
- 34. Hajirostamloo B. Comparison of nutritional and chemical parameters of soymilk and cow milk. Int J Nutr Food Eng. 2009;3:455–7. [Google Scholar]
- 35. Zhao Y, Martin BR, Weaver CM. Calcium bioavailability of calcium carbonate fortified soymilk is equivalent to cow's milk in young women. J Nutr. 2005;135:2379–82. [DOI] [PubMed] [Google Scholar]
- 36. Food and Nutrition Information Center, National Agricultural Library, USDA. Phytonutrients. [Internet] Beltsville, MD: National Agricultural Library, USDA; [cited 10 March, 2019]. Available from: https://nal.usda.gov/fnic/phytonutrients. [Google Scholar]
- 37. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG et al.. Dietary fats and cardiovascular disease: a Presidential Advisory from the American Heart Association. Circulation. 2017;136:e1–23. [DOI] [PubMed] [Google Scholar]
- 38. Huth PJ, Park KM. Influence of dairy product and milk fat consumption on cardiovascular disease risk: a review of the evidence. Adv Nutr. 2012;3:266–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ericson U, Hellstrand S, Brunkwall L, Schulz C-A, Sonestedt E, Wallström P, Gullberg B, Wirfält E, Orho-Melander M. Food sources of fat may clarify the inconsistent role of dietary fat intake for incidence of type 2 diabetes. Am J Clin Nutr. 2015;101:1065–80. [DOI] [PubMed] [Google Scholar]
- 40. Crichton GE, Alkerwi A. Whole-fat dairy food intake is inversely associated with obesity prevalence: findings from the Observation of Cardiovascular Risk Factors in Luxembourg study. Nutr Res. 2014;34:936–43. [DOI] [PubMed] [Google Scholar]
- 41. Scharf RJ, Demmer RT, DeBoer MD. Longitudinal evaluation of milk type consumed and weight status in preschoolers. Arch Dis Child. 2013;98:335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brassard D, Tessier-Grenier M, Allaire J, Rajendiran E, She Y, Ramprasath V, Gigleux I, Talbot D, Levy E, Tremblay A et al.. Comparison of the impact of SFAs from cheese and butter on cardiometabolic risk factors: a randomized controlled trial. Am J Clin Nutr. 2017;105:800–9. [DOI] [PubMed] [Google Scholar]
- 43. Moore LV, Thompson FE. Adults meeting fruit and vegetable intake recommendations—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):709–13. [PMC free article] [PubMed] [Google Scholar]
- 44. Bhupathiraju SN, Wedick NM, Pan A, Manson JE, Rexrode KM, Willett WC, Rimm EB, Hu FB. Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. Am J Clin Nutr. 2013;98:1514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nicklett EJ, Kadell AR. Fruit and vegetable intake among older adults: a scoping review. Maturitas. 2013;75:305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Burch E, Ball L, Somerville M, Williams LT. Dietary intake by food group of individuals with type 2 diabetes mellitus: a systematic review. Diabetes Res Clin Pract. 2018;137:160–72. [DOI] [PubMed] [Google Scholar]
- 47. Mottaghi T, Amirabdollahian F, Haghighatdoost F. Fruit and vegetable intake and cognitive impairment: a systematic review and meta-analysis of observational studies. Eur J Clin Nutr. 2018;72(10):1336–44. [DOI] [PubMed] [Google Scholar]
- 48. Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46:1029–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schwingshackl L, Hoffmann G, Kalle-Uhlmann T, Arregui M, Buijsse B, Boeing H. Fruit and vegetable consumption and changes in anthropometric variables in adult populations: a systematic review and meta-analysis of prospective cohort studies. PloS One. 2015;10:e0140846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Auerbach BJ, Dibey S, Vallila-Buchman P, Kratz M, Krieger J. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pan A, Malik VS, Hao T, Willett WC, Mozaffarian D, Hu FB. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes (Lond). 2013;37(10):1378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Auerbach BJ, Wolf FM, Hikida A, Vallila-Buchman P, Littman A, Thompson D, Louden D, Taber DR, Krieger J. Fruit juice and change in BMI: a meta-analysis. Pediatrics. 2017;139:e20162454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Salas MMS, Nascimento GG, Vargas-Ferreira F, Tarquinio SBC, Huysmans MCDNJM, Demarco FF. Diet influenced tooth erosion prevalence in children and adolescents: results of a meta-analysis and meta-regression. J Dent. 2015;43:865–75. [DOI] [PubMed] [Google Scholar]
- 54. Rodriguez-Casado A. The health potential of fruits and vegetables phytochemicals: notable examples. Crit Rev Food Sci Nutr. 2016;56:1097–107. [DOI] [PubMed] [Google Scholar]
- 55. Nicklas TA, O'Neil CE, Kleinman R. Association between 100% juice consumption and nutrient intake and weight of children aged 2 to 11 years. Arch Pediatr Adolesc Med. 2008;162:557–65. [DOI] [PubMed] [Google Scholar]
- 56. O'Neil CE, Nicklas TA, Rampersaud GC, Fulgoni VL. One hundred percent orange juice consumption is associated with better diet quality, improved nutrient adequacy, and no increased risk for overweight/obesity in children. Nutr Res. 2011;31:673–82. [DOI] [PubMed] [Google Scholar]
- 57. O'Neil CE, Nicklas TA, Zanovec M, Kleinman RE, Fulgoni VL. Fruit juice consumption is associated with improved nutrient adequacy in children and adolescents: the National Health and Nutrition Examination Survey (NHANES) 2003–2006. Public Health Nutr. 2012;15:1871–8. [DOI] [PubMed] [Google Scholar]
- 58. O'Neil CE, Nicklas TA, Zanovec M, Fulgoni VL. Diet quality is positively associated with 100% fruit juice consumption in children and adults in the United States: NHANES 2003–2006. Nutr J. 2011;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O'Neil CE, Nicklas TA, Kleinman R. Relationship between 100% juice consumption and nutrient intake and weight of adolescents. Am J Health Promot. 2010;24:231–7. [DOI] [PubMed] [Google Scholar]
- 60. O'Neil CE, Nicklas TA, Rampersaud GC, Fulgoni VL. 100% orange juice consumption is associated with better diet quality, improved nutrient adequacy, decreased risk for obesity, and improved biomarkers of health in adults: National Health and Nutrition Examination Survey, 2003–2006. Nutr J. 2012;11:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for sodium and potassium. [Internet] Washington (DC): The National Academies Press; 2019; [cited 2 July, 2019]. Available from: https://www.nap.edu/catalog/25353/dietary-reference-intakes-for-sodium-and-potassium. [PubMed] [Google Scholar]
- 62. WHO/FAO. Diet, nutrition and the prevention of chronic diseases. [Internet]. Technical Report Series No. 916 Geneva: WHO; 2003; [cited 19 November, 2018]. Available from: http://www.who.int/dietphysicalactivity/publications/trs916/en/. [Google Scholar]
- 63. Grummer-Strawn LM, Scanlon KS, Fein SB. Infant feeding and feeding transitions during the first year of life. Pediatrics. 2008;122(Suppl 2):S36–42. [DOI] [PubMed] [Google Scholar]
- 64. Britten P, Lyon J, Weaver CM, Kris-Etherton PM, Nicklas TA, Weber JA, Davis CA. MyPyramid food intake pattern modeling for the Dietary Guidelines Advisory Committee. J Nutr Educ Behav. 2006;38:S143–52. [DOI] [PubMed] [Google Scholar]
- 65. Loftfield E, Freedman ND, Dodd KW, Vogtmann E, Xiao Q, Sinha R, Graubard BI. Coffee drinking is widespread in the United States, but usual intake varies by key demographic and lifestyle factors. J Nutr. 2016;146:1762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Grosso G, Godos J, Galvano F, Giovannucci EL. Coffee, caffeine, and health outcomes: an umbrella review. Annu Rev Nutr. 2017;37:131–56. [DOI] [PubMed] [Google Scholar]
- 67. Urgert R, Schulz AG, Katan MB. Effects of cafestol and kahweol from coffee grounds on serum lipids and serum liver enzymes in humans. Am J Clin Nutr. 1995;61:149–54. [DOI] [PubMed] [Google Scholar]
- 68. Gross G, Jaccaud E, Huggett AC. Analysis of the content of the diterpenes cafestol and kahweol in coffee brews. Food Chem Toxicol. 1997;35:547–54. [DOI] [PubMed] [Google Scholar]
- 69. Urgert R, van der Weg G, Kosmeijer-Schuil TG, van de Bovenkamp P, Hovenier R, Katan MB. Levels of the cholesterol-elevating diterpenes cafestol and kahweol in various coffee brews. J Agric Food Chem. 1995;43:2167–72. [Google Scholar]
- 70. LaComb RP, Sebastian RS, Wilkinson EC, Goldman JD. Beverage choices of U.S. adults: What We Eat In America, NHANES 2007–2008. [Internet]. Dietary Data Brief Report No. 6 Beltsville, MD: Food Surveys Research Group, Agricultural Research Service, USDA; 2011; [cited 10 March, 2019]. Available from: http://ars.usda.gov/Services/docs.htm?docid=19476. [Google Scholar]
- 71. Khalesi S, Sun J, Buys N, Jamshidi A, Nikbakht-Nasrabadi E, Khosravi-Boroujeni H. Green tea catechins and blood pressure: a systematic review and meta-analysis of randomised controlled trials. Eur J Nutr. 2014;53:1299–311. [DOI] [PubMed] [Google Scholar]
- 72. Onakpoya I, Spencer E, Heneghan C, Thompson M. The effect of green tea on blood pressure and lipid profile: a systematic review and meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2014;24:823–36. [DOI] [PubMed] [Google Scholar]
- 73. Greyling A, Ras RT, Zock PL, Lorenz M, Hopman MT, Thijssen DHJ, Draijer R. The effect of black tea on blood pressure: a systematic review with meta-analysis of randomized controlled trials. PloS One. 2014;9:e103247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shen L, Song L, Ma H, Jin C, Wang J, Xiang M. Tea consumption and risk of stroke: a dose-response meta-analysis of prospective studies. J Zhejiang Univ Sci B. 2012;13:652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pang J, Zhang Z, Zheng T, Bassig BA, Mao C, Liu X, Zhu Y, Shi K, Ge J, Yang Y et al.. Green tea consumption and risk of cardiovascular and ischemic related diseases: a meta-analysis. Int J Cardiol. 2016;202:967–74. [DOI] [PubMed] [Google Scholar]
- 76. Yang W-S, Wang W-Y, Fan W-Y, Deng Q, Wang X. Tea consumption and risk of type 2 diabetes: a dose-response meta-analysis of cohort studies. Br J Nutr. 2014;111:1329–39. [DOI] [PubMed] [Google Scholar]
- 77. Tang J, Zheng J-S, Fang L, Jin Y, Cai W, Li D. Tea consumption and mortality of all cancers, CVD and all causes: a meta-analysis of eighteen prospective cohort studies. Br J Nutr. 2015;114:673–83. [DOI] [PubMed] [Google Scholar]
- 78. Farah A, Donangelo CM. Phenolic compounds in coffee. Braz J Plant Physiol. 2006;18:23–36. [Google Scholar]
- 79. Menet M-C, Sang S, Yang CS, Ho C-T, Rosen RT. Analysis of theaflavins and thearubigins from black tea extract by MALDI-TOF mass spectrometry. J Agric Food Chem. 2004;52:2455–61. [DOI] [PubMed] [Google Scholar]
- 80. Fernández PL, Martín MJ, González AG, Pablos F. HPLC determination of catechins and caffeine in tea. Differentiation of green, black and instant teas. Analyst. 2000;125:421–5. [DOI] [PubMed] [Google Scholar]
- 81. Farah A. Coffee constituents. In: Chu YF, editor. Coffee: emerging health effects and disease prevention. Oxford, UK: Wiley-Blackwell; 2012. p. 21–58. [Google Scholar]
- 82. Rio DD, Stalmach A, Calani L, Crozier A. Bioavailability of coffee chlorogenic acids and green tea flavan-3-ols. Nutrients. 2010;2:820–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275S–84S. [DOI] [PubMed] [Google Scholar]
- 84. Moura-Nunes N, Perrone D, Farah A, Donangelo CM. The increase in human plasma antioxidant capacity after acute coffee intake is not associated with endogenous non-enzymatic antioxidant components. Int J Food Sci Nutr. 2009;60(Suppl 6):173–81. [DOI] [PubMed] [Google Scholar]
- 85. Natella F, Nardini M, Giannetti I, Dattilo C, Scaccini C. Coffee drinking influences plasma antioxidant capacity in humans. J Agric Food Chem. 2002;50:6211–16. [DOI] [PubMed] [Google Scholar]
- 86. Rodriguez de Sotillo DV, Hadley M, Sotillo JE. Insulin receptor exon 11+/− is expressed in Zucker (fa/fa) rats, and chlorogenic acid modifies their plasma insulin and liver protein and DNA. J Nutr Biochem. 2006;17:63–71. [DOI] [PubMed] [Google Scholar]
- 87. Venables MC, Hulston CJ, Cox HR, Jeukendrup AE. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am J Clin Nutr. 2008;87:778–84. [DOI] [PubMed] [Google Scholar]
- 88. Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr. 2003;78:728–33. [DOI] [PubMed] [Google Scholar]
- 89. Arion WJ, Canfield WK, Ramos FC, Schindler PW, Burger HJ, Hemmerle H, Schubert G, Below P, Herling AW. Chlorogenic acid and hydroxynitrobenzaldehyde: new inhibitors of hepatic glucose 6-phosphatase. Arch Biochem Biophys. 1997;339:315–22. [DOI] [PubMed] [Google Scholar]
- 90. Shimizu M, Kobayashi Y, Suzuki M, Satsu H, Miyamoto Y. Regulation of intestinal glucose transport by tea catechins. BioFactors. 2000;13:61–5. [DOI] [PubMed] [Google Scholar]
- 91. Lorenz M, Urban J, Engelhardt U, Baumann G, Stangl K, Stangl V. Green and black tea are equally potent stimuli of NO production and vasodilation: new insights into tea ingredients involved. Basic Res Cardiol. 2009;104:100–10. [DOI] [PubMed] [Google Scholar]
- 92. Arts IC, Hollman PC, Bueno De Mesquita HB, Feskens EJ, Kromhout D. Dietary catechins and epithelial cancer incidence: the Zutphen elderly study. Int J Cancer. 2001;92:298–302. [DOI] [PubMed] [Google Scholar]
- 93. Haytowitz DB, Wu X, Bhagwat S. USDA database for the flavonoid content of selected foods. [Internet]. Release 3.3 Beltsville, MD: USDA, Agricultural Research Service; 2018; [cited 27 October, 2019]. Available from: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav3.3.pdf. [Google Scholar]
- 94. Bhagwat S, Haytowitz D, Holden J. USDA database for the isoflavone content of selected foods. [Internet]. Release 2.0 Beltsville, MD: USDA, Agricultural Research Service; 2008; [cited 2 July, 2019]. Available from: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/isoflav/Isoflav_R2.pdf. [Google Scholar]
- 95. Cappelletti S, Piacentino D, Daria P, Sani G, Aromatario M. Caffeine: cognitive and physical performance enhancer or psychoactive drug?. Curr Neuropharmacol. 2015;13:71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Owens JA, Mindell J, Baylor A. Effect of energy drink and caffeinated beverage consumption on sleep, mood, and performance in children and adolescents. Nutr Rev. 2014;72(Suppl 1):65–71. [DOI] [PubMed] [Google Scholar]
- 97. Acheson KJ, Zahorska-Markiewicz B, Pittet P, Anantharaman K, Jéquier E. Caffeine and coffee: their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am J Clin Nutr. 1980;33:989–97. [DOI] [PubMed] [Google Scholar]
- 98. Rogers NL, Dinges DF. Caffeine: implications for alertness in athletes. Clin Sports Med. 2005;24:e1–13., x–xi. [DOI] [PubMed] [Google Scholar]
- 99. Spiller M. The chemical components of coffee. In: Spiller GA, editor. Caffeine. Boca Raton, FL: CRC Press LLC; 1998. p. 94–158. [Google Scholar]
- 100. Ludwig IA, Mena P, Calani L, Cid C, Del Rio D, Lean MEJ, Crozier A. Variations in caffeine and chlorogenic acid contents of coffees: what are we drinking?. Food Funct. 2014;5(8):1718–26. [DOI] [PubMed] [Google Scholar]
- 101. Mitchell DC, Knight CA, Hockenberry J, Teplansky R, Hartman TJ. Beverage caffeine intakes in the U.S. Food Chem Toxicol. 2014;63:136–42. [DOI] [PubMed] [Google Scholar]
- 102. Gonzalez de Mejia E, Ramirez-Mares MV. Impact of caffeine and coffee on our health. Trends Endocrinol Metab. 2014;25(10):489–92. [DOI] [PubMed] [Google Scholar]
- 103. Branum AM, Rossen LM, Schoendorf KC. Trends in caffeine intake among U.S. children and adolescents. Pediatrics. 2014;133:386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fulgoni VL, Keast DR, Lieberman HR. Trends in intake and sources of caffeine in the diets of US adults: 2001–2010. Am J Clin Nutr. 2015;101:1081–7. [DOI] [PubMed] [Google Scholar]
- 105. Richards G, Smith A. Caffeine consumption and self-assessed stress, anxiety, and depression in secondary school children. J Psychopharmacol. 2015;29:1236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ruxton CHS. The suitability of caffeinated drinks for children: a systematic review of randomised controlled trials, observational studies and expert panel guidelines. J Hum Nutr Diet. 2014;27:342–57. [DOI] [PubMed] [Google Scholar]
- 107. Temple JL. Caffeine use in children: what we know, what we have left to learn, and why we should worry. Neurosci Biobehav Rev. 2009;33:793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wikoff D, Welsh BT, Henderson R, Brorby GP, Britt J, Myers E, Goldberger J, Lieberman HR, O'Brien C, Peck J et al.. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxicol. 2017;109:585–648. [DOI] [PubMed] [Google Scholar]
- 109. EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the safety of caffeine. EFSA J. 2015;13(5):4102. [Google Scholar]
- 110. Rehm J, Gmel GE, Gmel G, Hasan OSM, Imtiaz S, Popova S, Probst C, Roerecke M, Room R, Samokhvalov AV et al.. The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction. 2017;112:968–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2001;38:1112–17. [DOI] [PubMed] [Google Scholar]
- 113. Padilla H, Michael Gaziano J, Djoussé L. Alcohol consumption and risk of heart failure: a meta-analysis. Phys Sports Med. 2010;38:84–9. [DOI] [PubMed] [Google Scholar]
- 114. Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, Rehm J. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009;32:2123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Beulens JWJ, van der Schouw YT, Bergmann MM, Rohrmann S, Schulze MB, Buijsse B, Grobbee DE, Arriola L, Cauchi S, Tormo M-J et al.. Alcohol consumption and risk of type 2 diabetes in European men and women: influence of beverage type and body size. The EPIC-InterAct study. J Intern Med. 2012;272:358–70. [DOI] [PubMed] [Google Scholar]
- 116. Koppes LLJ, Dekker JM, Hendriks HFJ, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care. 2005;28:719–25. [DOI] [PubMed] [Google Scholar]
- 117. Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. 2009;17:542–55. [DOI] [PubMed] [Google Scholar]
- 118. Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW, Coates RJ, Liff JM, Talamini R, Chantarakul N et al.. Alcohol, tobacco and breast cancer – collaborative reanalysis of individual data from 53 epidemiological studies, including 58 515 women with breast cancer and 95 067 women without the disease. Br J Cancer. 2002;87:1234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Romieu I, Scoccianti C, Chajès V, de Batlle J, Biessy C, Dossus L, Baglietto L, Clavel-Chapelon F, Overvad K, Olsen A et al.. Alcohol intake and breast cancer in the European prospective investigation into cancer and nutrition. Int J Cancer. 2015;137:1921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Seitz HK, Pelucchi C, Bagnardi V, La Vecchia C. Epidemiology and pathophysiology of alcohol and breast cancer: update 2012. Alcohol. 2012;47:204–12. [DOI] [PubMed] [Google Scholar]
- 121. Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S et al.. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140:603–13. [DOI] [PubMed] [Google Scholar]
- 122. Aleksandrova K, Pischon T, Jenab M, Bueno-de-Mesquita HB, Fedirko V, Norat T, Romaguera D, Knüppel S, Boutron-Ruault M-C, Dossus L et al.. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med. 2014;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Chao C. Associations between beer, wine, and liquor consumption and lung cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2007;16:2436–47. [DOI] [PubMed] [Google Scholar]
- 124. Freudenheim JL, Ritz J, Smith-Warner SA, Albanes D, Bandera EV, van den Brandt PA, Colditz G, Feskanich D, Goldbohm RA, Harnack L et al.. Alcohol consumption and risk of lung cancer: a pooled analysis of cohort studies. Am J Clin Nutr. 2005;82:657–67. [DOI] [PubMed] [Google Scholar]
- 125. Ferrari P, Licaj I, Muller DC, Kragh Andersen P, Johansson M, Boeing H, Weiderpass E, Dossus L, Dartois L, Fagherazzi G et al.. Lifetime alcohol use and overall and cause-specific mortality in the European Prospective Investigation into Cancer and nutrition (EPIC) study. BMJ Open. 2014;4:e005245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–45. [DOI] [PubMed] [Google Scholar]
- 127. Drewnowski A, Rehm CD. Consumption of added sugars among US children and adults by food purchase location and food source. Am J Clin Nutr. 2014;100:901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion. Get the facts: sugar-sweetened beverages and consumption. [Internet] Atlanta, GA: CDC; 2017; [cited 10 March, 2019]. Available from: https://www.cdc.gov/nutrition/data-statistics/sugar-sweetened-beverages-intake.html. [Google Scholar]
- 129. Malik VS, Popkin BM, Bray GA, Després J-P, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Imamura F, O'Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lundeen EA, Park S, Pan L, Blanck HM. Daily intake of sugar-sweetened beverages among US adults in 9 states, by state and sociodemographic and behavioral characteristics, 2016. Prev Chronic Dis. 2018;15:180335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xi B, Huang Y, Reilly KH, Li S, Zheng R, Barrio-Lopez MT, Martinez-Gonzalez MA, Zhou D. Sugar sweetened beverages and risk of hypertension and CVD: a dose-response meta-analysis. Br J Nutr. 2015;113:709–17. [DOI] [PubMed] [Google Scholar]
- 133. Thompson M, Heneghan C, Cohen D. How valid is the European Food Safety Authority's assessment of sports drinks?. BMJ. 2012;345:e4753. [DOI] [PubMed] [Google Scholar]
- 134. Campbell B, Wilborn C, La Bounty P, Taylor L, Nelson MT, Greenwood M, Ziegenfuss TN, Lopez HL, Hoffman JR, Stout JR et al.. International Society of Sports Nutrition position stand: energy drinks. J Int Soc Sports Nutr. 2013;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Brener N, Merlo C, Eaton D, Kann L, Park S, Blanck H. Beverage consumption among high school students—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(23):778–80. [PubMed] [Google Scholar]
- 136. Ali F, Rehman H, Babayan Z, Stapleton D, Joshi D-D. Energy drinks and their adverse health effects: a systematic review of the current evidence. Postgrad Med. 2015;127:308–22. [DOI] [PubMed] [Google Scholar]
- 137. Duchan E, Patel ND, Feucht C. Energy drinks: a review of use and safety for athletes. Phys Sports Med. 2010;38:171–9. [DOI] [PubMed] [Google Scholar]
- 138. Committee on Nutrition and the Council on Sports Medicine and Fitness. Sports drinks and energy drinks for children and adolescents: are they appropriate?. Pediatrics. 2011;127:1182–9. [DOI] [PubMed] [Google Scholar]
- 139. Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Després J-P, Hu FB, Kris-Etherton PM, Otten JJ, Towfighi A, Wylie-Rosett J et al.. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation. 2018;138:e126–40. [DOI] [PubMed] [Google Scholar]
- 140. Piernas C, Ng SW, Popkin B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr Obes. 2013;8:294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ruanpeng D, Thongprayoon C, Cheungpasitporn W, Harindhanavudhi T. Sugar and artificially sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM Int J Med. 2017;110:513–20. [DOI] [PubMed] [Google Scholar]
- 142. Kim Y, Je Y. Prospective association of sugar-sweetened and artificially sweetened beverage intake with risk of hypertension. Arch Cardiovasc Dis. 2016;109:242–53. [DOI] [PubMed] [Google Scholar]
- 143. Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr. 2014;100:765–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Borges MC, Louzada ML, de Sá TH, Laverty AA, Parra DC, Garzillo JMF, Monteiro CA, Millett C. Artificially sweetened beverages and the response to the global obesity crisis. PLOS Med. 2017;14:e1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Anderson CAM, Thorndike AN, Lichtenstein AH, Van Horn L, Kris-Etherton PM, Foraker R, Spees C. Innovation to create a healthy and sustainable food system: a science advisory from the American Heart Association. Circulation. 2019;139:e1025–32. [DOI] [PubMed] [Google Scholar]
- 146. Epidemiology and Genomics Research Program. Usual daily intake of added sugars. [Internet] Bethesda, MD: NIH, National Cancer Institute, Division of Cancer Control and Population Sciences; 2018; [cited 10 March, 2019]. Available from: https://epi.grants.cancer.gov/diet/usualintakes/pop/2007-10/table_a40.html. [Google Scholar]
- 147. Ehlers A, Marakis G, Lampen A, Hirsch-Ernst KI. Risk assessment of energy drinks with focus on cardiovascular parameters and energy drink consumption in Europe. Food Chem Toxicol. 2019;130:109–21. [DOI] [PubMed] [Google Scholar]
- 148. USDA Agricultural Research Service. Nutrient intakes from food and beverages: mean amounts consumed per individual, by gender and age, in the United States, 2015–2016. What We Eat in America, NHANES 2015–2016. Beltsville, MD: USDA Agricultural Research Service; 2018. [Google Scholar]


