ABSTRACT
Under stressful conditions such as energy restriction (ER) and physical activity, the RDA for protein of 0.8 g · kg−1 · d−1 may no longer be an appropriate recommendation. Under catabolic or anabolic conditions, higher protein intakes are proposed to attenuate the loss or increase the gain of whole-body lean mass, respectively. No known published meta-analysis compares protein intakes greater than the RDA with intakes at the RDA. Therefore, we conducted a systematic review and meta-analysis to assess the effects of protein intakes greater than the RDA, compared with at the RDA, on changes in whole-body lean mass. Three researchers independently screened 1520 articles published through August 2018 using the PubMed, Scopus, CINAHL, and Cochrane databases, with additional articles identified in published systematic review articles. Randomized, controlled, parallel studies ≥6 wk long with apparently healthy adults (≥19 y) were eligible for inclusion. Data from 18 studies resulting in 22 comparisons of lean mass changes were included in the final overall analysis. Among all comparisons, protein intakes greater than the RDA benefitted changes in lean mass relative to consuming the RDA [weighted mean difference (95% CI): 0.32 (0.01, 0.64) kg, n = 22 comparisons]. In the subgroup analyses, protein intakes greater than the RDA attenuated lean mass loss after ER [0.36 (0.06, 0.67) kg, n = 14], increased lean mass after resistance training (RT) [0.77 (0.23, 1.31) kg, n = 3], but did not differentially affect changes in lean mass [0.08 (−0.59, 0.75) kg, n = 7] under nonstressed conditions (no ER + no RT). Protein intakes greater than the RDA beneficially influenced changes in lean mass when adults were purposefully stressed by the catabolic stressor of dietary ER with and without the anabolic stressor of RT. The RDA for protein is adequate to support lean mass in adults during nonstressed states. This review was registered at www.crd.york.ac.uk/prospero as CRD 42018106532.
Keywords: fat-free mass, exercise, adults, body composition, health, weight loss
Introduction
The protein RDA (0.8 g · kg−1 · d−1) represents the relative quantity of high-quality protein needed to maintain nitrogen balance in 97.5% of apparently healthy males and females aged 19 y and older (1). However, the protein RDA is not necessarily an appropriate guideline to follow when considering the “optimal” protein (nitrogen) intake to promote morphological, physiological, and health-related changes in skeletal muscle, a component of lean mass. In part, the RDA is not intended to be used to estimate the protein needs for solely lean mass tissues, but rather the whole body. Furthermore, the DRIs do not address energy status [i.e., energy restriction (ER) (1)—which promotes lean mass catabolism] as a potential modifier of protein needs (2). Physical activity levels are acknowledged as a potential modifier; however, insufficient evidence was available to recommend different protein needs (1). Although acute and short-term methods [e.g., nitrogen balance and stable isotope kinetics (3, 4)] are predominantly used to estimate protein requirements and allowances, morphological, physiological, and other health-related outcomes associated with lean mass are highly pertinent.
All human cells require amino acids to function normally, but lean mass, particularly the skeletal muscle component, may be uniquely adaptable to fluctuations in protein intake and stress—anabolic and catabolic (5). Promoting lean mass through either growth, preservation, or loss attenuation ostensibly also promotes higher resting energy expenditures (1), functional movement maintenance (6–8), and healthy glucose control (9–11). Daily protein intakes above the RDA are proposed to support higher lean mass when stressors such as ER and/or physical activity occur (12–15). Several previous meta-analyses reported that consuming higher- compared with lower-protein diets favors changes in lean mass when consumed during periods of stress (16–18). However, these reviews also contained studies prescribing diets containing less than the RDA in the lower protein comparator group. Including groups with prescribed dietary protein intakes below the RDA may skew the effect size toward supporting that higher protein intakes are beneficial for lean mass, when in fact consuming less may be detrimental. A meta-analysis that specifically compares higher protein intakes with the RDA on body composition, especially lean mass, during periods with and without purposeful stressors is needed. Therefore, the purpose of this systematic review and meta-analysis of randomized controlled trials is to compare the effects of consuming greater than the protein RDA with those of consuming the RDA on lean mass changes in adults. We hypothesized that protein intakes greater than the RDA would result in beneficial lean mass changes among all studies combined, regardless of the presence or absence of anabolic and/or catabolic stressors.
Methods
This systematic review and meta-analysis followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses report (19). The procedures for identification, screening, data extraction, and analysis were agreed upon in advance among all authors. The research question was defined by using the PICOS (population, intervention, comparison, outcome, and setting) criteria (Table 1). Details of the methods were documented in a protocol that was registered at the International Prospective Register of Systematic Reviews (PROSPERO, CRD 42018106532) before data analysis.
TABLE 1.
The PICOS criteria for defining the research question1
| Parameter | Description |
|---|---|
| Population | Adults, group mean age ≥19 y |
| Intervention | Groups consuming greater than the protein RDA (>0.85 g · kg−1 · d−1) |
| Comparison | Groups consuming the protein RDA (0.8 ± 0.05 g · kg−1 · d−1) |
| Outcome | Changes in lean or fat-free mass |
| Setting | Randomized controlled trials |
| Research question | What is the effect of consuming greater than the protein RDA compared with the RDA on changes in lean mass in adults? |
The PICOS criteria are taken from Moher et al. (19).
Inclusion criteria
Randomized controlled trials with a parallel study design that were published in English were included. All articles must have had a comparison group with a prescribed diet meeting the protein RDA (0.8 ± 0.05 g · kg−1 · d−1) and an intervention group consuming greater than the RDA (>0.85 g · kg−1 · d−1). When information about the prescribed diet was not available, the actual relative protein intake, as calculated by each article's authors, based on baseline body weight determined eligibility. The dietary intervention periods needed to be ≥6 wk long with no upper limit, with or without ER or exercise training (resistance and/or aerobic exercises). Acceptable forms of body composition measurement included DXA, air-displacement plethysmography, hydrostatic weighing, and total body potassium. Articles were excluded if they were not original research (e.g., reviews of literature); included participants <19 y old; did not have or could not provide lean or fat-free mass change values; or prescribed very-low-energy diets (<800 kcal/d). There was no lower limit for publication date.
Search strategy
Two independent reviewers conducted a systematic search of the literature on 20 December, 2017 using the PubMed, Scopus, CINAHL, and Cochrane databases (JLH and YW). The same search was conducted again on 3 August, 2018 for updates (YW and REB). The search terms and search parameters specific to each database are in Supplemental Table 1. Additional articles were identified through manual searches and reference lists of previously published review articles (16–18, 20–31).
Article identification and data extraction
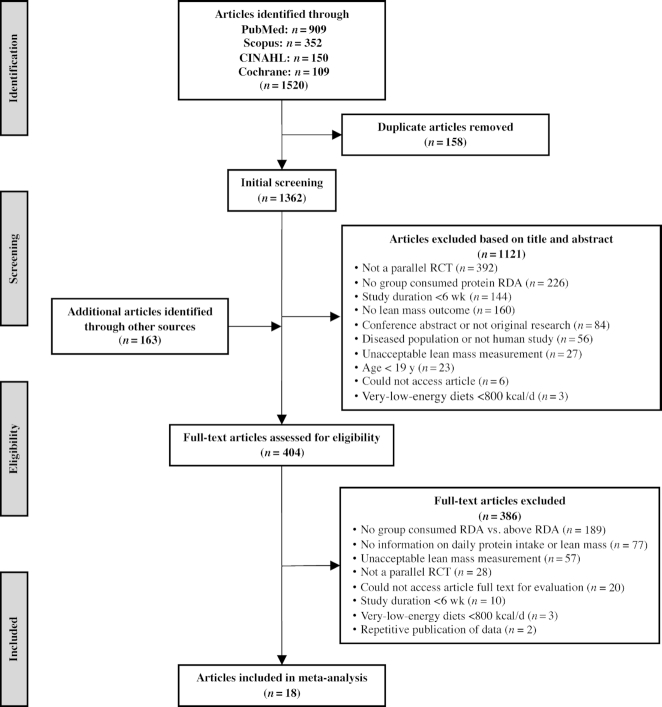
A multiple-pass method was used to identify 1520 articles from the database searches (Figure 1). After duplicates were removed, the first pass involved screening titles and abstracts to identify and exclude clearly irrelevant articles. If there was insufficient information to categorically exclude an article, the full text of the article was reviewed in the second pass. The 2 independent reviewers crosschecked their results after each pass and differences were discussed and reconciled with a third reviewer.
FIGURE 1.
Flowchart of the literature search process. RCT, randomized controlled trial.
Only 6 articles contained the necessary data for inclusion in the meta-analysis that originated from the database searches (32–37). Another 52 authors representing their respective articles were contacted via email to acquire unpublished data to determine their eligibility. Forty articles were excluded because either the authors did not respond, the outcome data were not available, or the articles did not meet the inclusion criteria after information was received. Authors of another 12 articles, 7 from the original database search (38–44) and 5 from external sources (45–49), provided data via email, met the inclusion criteria, and had their articles included in the final meta-analysis for 18 total articles.
The following information was extracted from selected articles independently by both reviewers: first author's last name; publication year; title; body composition assessment method; sample size of each intervention group; mean age, sex ratio (number of females compared with males), and BMI of participants; intervention duration; exercise characteristics and modality; energy and macronutrient intakes; energy status; techniques for dietary control and monitoring compliance; and pre- and postintervention and net changes in whole-body lean mass (post minus pre).
Data synthesis
When articles included multiple treatment arms, each treatment arm was treated as a distinct intervention. For articles including multiple groups that would classify as a comparator group (i.e., groups consuming the RDA), the group most closely matching the treatment was used. For articles including multiple groups that would classify as an intervention group (i.e., groups consuming greater than the RDA), each intervention group was compared with the control group in a separate comparison (32, 33, 35). If an article had multiple time points, only the mean change from pre- to postintervention was retrieved for the outcomes. Only 3 articles had study designs with multiple phases [i.e., controlled feeding and ad libitum feeding phases (32) or weight loss and weight maintenance phases (33, 39)] and each phase was treated as a distinct intervention. Only the controlled feeding (32) and weight maintenance (33) phases qualified based on our criteria and adequate data were only available for the weight loss phase (39).
Fourteen studies included in this review measured body composition using DXA (32, 34–36, 39, 40, 42–49). Two more utilized air displacement plethysmography (37, 41) and 2 used hydrostatic weighing (33, 38) to measure body composition. There were some discrepancies in how lean mass was reported, with 12 articles using the term “lean mass” or “lean body mass,” 5 articles using “fat-free mass,” and 1 article using “muscle mass.” Although precisely these terms are not interchangeable—lean mass may or may not include bone mass, whereas fat-free mass does not include essential fat in the organs and bone—for this review, these terms were considered synonymous, and “lean mass” is used consistently for clarity. Articles that included bone mineral content within lean mass were included in the analyses because bone mineral content only accounts for ∼5% of total lean mass (50); moreover, bone turnover (remodeling) is very slow, requiring a minimum of 4–6 mo (51).
Change value means and SDs for lean mass were extracted when available. Otherwise, change means and change SDs were calculated from pre- and post-intervention values when raw data were provided by an article's authors.
Meta-analyses
Random-effect meta-analyses were conducted in Stata/SE 15.1 software (StataCorp LP) using the metan function, and results are reported as the weighted mean differences (WMDs) and 95% CIs. A positive WMD value was considered a beneficial effect of consuming greater than the RDA on lean mass changes. The SE for mean difference within each comparison was calculated as the squared SEM of the difference using the following formula:
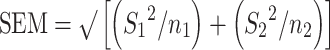 |
(1) |
where S1 and S2 are the SDs for the change means of the intervention and comparator groups, respectively.
Heterogeneity and risk of bias
Heterogeneity was assessed by χ2 and I2 statistics and significance was set at P < 0.05. Risk of bias for each article was assessed using a domain-based evaluation and was independently assessed by the reviewers (Supplemental Table 2). Funnel plots were visually inspected to determine publication bias (Supplemental Figure 1). Sensitivity analyses were performed by removing each comparison one by one. Sensitivity analyses of the influence from removal of each individual study on the WMD and heterogeneity are included in Supplemental Table 3.
Subgroup analyses
Subgroup analyses were determined a priori to elucidate possible modifiers of any observed effects in the overall analysis. Subgroup analyses were performed on changes in lean mass with ER, without ER, with physical activity, and with no physical activity, and the 4 permutations of ER and physical activity statuses (8 subgroup analyses total). All articles with a physical activity component prescribed resistance training (RT). As such, the physical activity subgroups are labeled as being with RT or with no RT. Subgroup analyses were also planned for studies with inclusion criteria that demarcated participants as either >50 or <50 y of age.
Results
Study characteristics
The study characteristics of the 18 parallel randomized controlled trials that met all inclusion criteria, representing 934 participants, are described in Table 2. Four articles each contributed 2 comparisons (32, 33, 35, 40) for 22 total comparisons on changes in lean mass in the overall analysis. Regarding the subanalyses, 14 comparisons were classified as with ER, 8 were with no ER, 3 were with RT, 19 were with no RT, 2 were with ER + RT, 12 were with ER + no RT, 1 was with no ER + RT, and 7 were with no ER + no RT (Table 3). Of the 22 comparisons available in the overall analysis, only 5 comparisons were eligible to be included in the subgroup analysis of older adults whose minimum inclusion age was >50 y. Only 2 comparisons in younger adults qualified for meta-analysis because their maximum inclusion age was <50 y (results not shown).
TABLE 2.
Summary of the characteristics for the comparator (RDA) and intervention (>RDA) groups1
| Prescribed diet | Achieved diet | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors (ref) | Group | Duration, wk | ER | RT | n | Age, y | Energy, kcal | Protein, g · kg−1 · d−1 (%) | CHO, % | Fat, % | Energy, kcal | Protein, g · kg−1 · d−1 (%) | CHO, % | Fat, % |
| Porter Starr et al. (37) | RDA | 24 | + | − | 26 | 68.7 ± 1.2 | 500 deficit | 0.8 (15) | 55 | 30 | ∼14582 | 0.77 (∼20)2 | ∼502 | ∼32 |
| >RDA | 24 | + | − | 41 | 67.9 ± 0.8 | 500 deficit | 1.2 (30) | 40 | 30 | ∼14892 | ∼1.1 (∼30)2 | ∼402 | ∼302 | |
| Tang et al. (36) | RDA | 12 | + | − | 21 | 44.8 ± 3.6 | 750 deficit | 0.8 (15) | 60 | 25 | 2273 ± 78 | ∼0.79 (∼14)3 | ∼613 | ∼253 |
| >RDA | 12 | + | − | 22 | 51 ± 2.6 | 750 deficit | 1.4 (25) | 50 | 25 | 2254 ± 75 | ∼1.41 (∼25)3 | ∼503 | ∼253 | |
| Josse et al. (34) | RDA | 16 | + | + | 13 | 26 ± 1 | 750 deficit | (15) | 55 | 30 | 1430 ± 42 | 0.84 ± 0.02 (18 ± 1) | 58 ± 1 | 24 ± 1 |
| >RDA | 16 | + | + | 14 | 30 ± 1 | 750 deficit | (30) | 40 | 30 | 1500 ± 36 | 1.33 ± 0.03 (28 ± 1) | 41 ± 1 | 31 ± 1 | |
| Wycherley et al. (44) | RDA | 12 | + | − | 21 | 45.9 ± 1.8 | ∼1600 | 0.85 (17) | 58 | 25 | 1729 ± 138 | 0.81 ± 0.09 (25.1 ± 0.6) | 51.2 ± 0.8 | 25.1 ± 0.6 |
| >RDA | 12 | + | − | 21 | 47.7 ± 1.7 | ∼1600 | 1.3 (35) | 40 | 25 | 1677 ± 156 | 1.2 ± 0.23 (27 ± 0.7) | 38.3 ± 0.8 | 27 ± 0.7 | |
| Verreijen et al. (43) | RDA | 13 | + | + | 30 | 63 ± 1.1 | 600 deficit | — | — | — | 1662 ± 63 | 0.85 ± 0.04 (18.3 ± 0.7) | 47.8 ± 0.9 | 29.3 ± 0.8 |
| >RDA | 13 | + | + | 30 | 63.7 ± 1.1 | 600 deficit | — | — | — | 1823 ± 103 | 1.11 ± 0.05 (22.9 ± 0.6) | 42.0 ± 1.1 | 29.2 ± 0.7 | |
| Mitchell et al. (48) | RDA | 10 | − | − | 15 | 74.7 ± 1.0 | — | 0.8 | — | 28–31 | 2695 ± 154 | 0.9 ± 0.03 (11.7 ± 0.4) | 56.6 ± 0.7 | 31.7 ± 0.3 |
| >RDA | 10 | − | − | 14 | 73.7 ± 0.9 | — | 1.6 | — | 28–31 | 2779 ± 46 | 1.7 ± 0.03 (20.6 ± 0.4) | 51.1 ± 0.6 | 28.3 ± 0.4 | |
| Bhasin et al. (45) | RDA | 24 | − | − | 21 | 71.3 ± 0.9 | — | 0.8 | — | — | 2423 ± 79 | 0.81 ± 0.02 | — | — |
| >RDA | 24 | − | − | 21 | 73.5 ± 1.2 | — | 1.3 | — | — | 2369 ± 104 | 1.17 ± 0.03 | — | — | |
| Leidy et al. (47) | RDA | 12 | + | − | 25 | 53 ± 3 | 750 deficit | 0.8 (18) | 57 | 25 | 1560 ± 604 | 0.92 ± 0.02 (18.2 ± 0.1)4 | ∼573, 4 | ∼253, 4 |
| >RDA | 12 | + | − | 21 | 46 ± 2 | 750 deficit | 1.4 (30) | 45 | 25 | 1540 ± 604 | 1.52 ± 0.02 (29.5 ± 0.1)4 | ∼443, 4 | ∼263, 4 | |
| Layman et al. (46) | RDA | 12 | + | − | 12 | 50.1 ± 1.1 | 500 deficit | 0.8 | — | <30 | 1659 ± 167 | ∼0.8 (16) | 58 | 26 |
| >RDA | 12 | + | − | 12 | 50.1 ± 1.1 | 500 deficit | 1.6 | — | <30 | 1670 ± 197 | ∼1.5 (30) | 41 | 29 | |
| Wycherley et al. (49) | RDA | 52 | + | − | 33 | 50.2 ± 1.6 | ∼1600 | 0.85 (17) | 58 | 25 | 1731 ± 129 | ∼0.823 (20.4 ± 0.3) | 35.9 ± 0.6 | 27.7 ± 0.6 |
| >RDA | 52 | + | − | 30 | 51.3 ± 1.6 | ∼1600 | 1.3 (35) | 40 | 25 | 1737 ± 198 | ∼1.253 (30.7 ± 0.6) | 47.3 ± 0.7 | 29.8 ± 0.7 | |
| Aldrich et al. (32) | RDA | 8 | + | − | 6 | 51.3 ± 0.9 | ∼800 deficit | 0.8 (15) | 55 | 30 | 1601 ± 8 | 0.8 (∼155) | ∼53.85 | ∼29.55 |
| >RDA (mixed protein) | 8 | + | − | 6 | 49.6 ± 1.4 | ∼800 deficit | 1.52 (30) | 40 | 30 | 1607 ± 2 | 1.52 (∼305) | ∼415 | ∼295 | |
| >RDA (whey protein) | 8 | + | − | 6 | 49.2 ± 0.7 | ∼800 deficit | 1.52 (30) | 40 | 30 | 1601 ± 12 | 1.52 (∼305) | ∼405 | ∼305 | |
| Pal et al. (35) | RDA | 12 | − | − | 25 | 48.4 ± 0.9 | — | — | — | — | 1732 ± 338 | ∼0.823 (15.8 ± 0.7) | 51.5 ± 1.1 | 30.1 ± 0.9 |
| >RDA (casein) | 12 | − | − | 20 | 48.4 ± 0.9 | — | — | — | — | 1607 ± 236 | ∼1.63 (32.9 ± 0.8) | 35.3 ± 1.3 | 29.3 ± 1.0 | |
| >RDA (whey) | 12 | − | − | 25 | 48.4 ± 0.9 | — | — | — | — | 1794 ± 385 | ∼1.593 (31.9 ± 0.8) | 34.9 ± 1.3 | 29.7 ± 0.9 | |
| Layman et al. (39) | RDA | 16 | + | − | 51 | 46 ± 1 | 500 deficit | 0.8 (15) | 55 | 30 | 1482 ± 0 | ∼0.733 (∼155) | ∼595 | ∼265 |
| >RDA | 16 | + | − | 52 | 45.2 ± 1.2 | 500 deficit | 1.6 (30) | 40 | 30 | 1609 ± 0 | ∼1.263 (∼275) | ∼405 | ∼335 | |
| Campbell et al. (38) | RDA | 12 | − | + | 6 | 66.0 ± 1.4 | — | 0.8 | — | — | — | — | — | — |
| >RDA | 12 | − | + | 6 | 64.0 ± 1.6 | — | 1.62 | — | — | — | — | — | — | |
| Melanson et al. (41) | RDA | 12 | + | − | 41 | 37.9 ± 1.1 | — | — | — | — | — | ∼0.786 | — | — |
| >RDA | 12 | + | − | 36 | 38.8 ± 1.2 | — | — | — | — | — | ∼0.956 | — | — | |
| Claessens et al. (33) | RDA | 12 | − | − | 16 | 46.0 ± 2.2 | — | — | ≥55 | 30 | 1868 ± 142 | ∼0.833 (15.8 ± 0.6) | 62.7 ± 2.4 | 21.2 ± 1.5 |
| >RDA (casein) | 12 | − | − | 14 | 45.4 ± 2.2 | — | (≥25) | — | 30 | 1848 ± 108 | ∼1.853 (34.5 ± 1.3) | 42.3 ± 1.2 | 23.5 ± 1.5 | |
| >RDA (whey) | 12 | − | − | 18 | 44.9 ± 2.0 | — | (≥25) | — | 30 | 1812 ± 103 | ∼1.843 (35.2 ± 1.6) | 42.1 ± 1.1 | 24.3 ± 1.7 | |
| McMillan-Price et al. (40) | RDA (high GI) | 12 | + | − | 27 | 31.8 ± 1.7 | 1400 (F), 1900 (M) | (15) | 55 | 30 | — | ∼0.736 (18 ± 1) | 60 ± 1 | 19 ± 1 |
| >RDA (high GI) | 12 | + | − | 31 | 30.2 ± 1.5 | 1400 (F), 1900 (M) | (25) | 45 | 30 | — | ∼1.086 (28 ± 1) | 42 ± 1 | 27 ± 1 | |
| RDA (low GI) | 12 | + | − | 30 | 30.5 ± 1.4 | 1400 (F), 1900 (M) | (15) | 55 | 30 | — | ∼0.796 (19 ± 0) | 56 ± 1 | 22 ± 1 | |
| >RDA (low GI) | 12 | + | − | 27 | 34.6 ± 1.5 | 1400 (F), 1900 (M) | (25) | 45 | 30 | — | ∼1.056 (26 ± 1) | 40 ± 2 | 29 ± 1 | |
| Reimer et al. (42) | RDA | 12 | − | − | 26 | 40.4 ± 2.7 | — | — | — | — | 1772 ± 102 | 0.84 ± 0.06 (∼12) | 455 | 385 |
| >RDA | 12 | − | − | 22 | 38.7 ± 2.6 | — | — | — | — | 1940 ± 140 | 1.08 ± 0.07 (∼30) | 345 | 36 | |
Values are mean ± SE. CHO, carbohydrate; ER, energy restriction; GI, glycemic index; ref, reference; RT, resistance training.
Estimated using baseline and change values provided in the article.
Estimated protein intake (g · kg−1 · d−1) using week 12 protein intakes (g/d) and baseline body weights.
Dietary intake measured at the end of the 12 wk.
Calculated percentage of intake.
Received individual data.
TABLE 3.
Summary of the lean mass results from the overall and subgroup meta-analyses between the comparator (RDA) and intervention (>RDA) groups1
| n | WMD (95% CI), kg | P value2 | τ2 | I 2, % | χ2 | P value3 | |
|---|---|---|---|---|---|---|---|
| All | 22 | 0.324 (0.011, 0.637) | 0.043 | 0.258 | 51.9 | 43.66 | 0.003 |
| With ER | 14 | 0.361 (0.056, 0.666) | 0.02 | 0.066 | 20.6 | 16.38 | 0.229 |
| With RT | 2 | 0.65 (0.16, 1.14) | 0.009 | 0.000 | 0.0 | 0.92 | 0.338 |
| Without RT | 12 | 0.25 (−0.094, 0.60) | 0.153 | 0.056 | 15.3 | 12.99 | 0.294 |
| Without ER | 8 | 0.23 (−0.44, 0.89) | 0.503 | 0.643 | 73.3 | 26.26 | 0.000 |
| With RT | 1 | — | — | — | — | — | — |
| Without RT | 7 | 0.08 (−0.59, 0.75) | 0.810 | 0.572 | 72.8 | 22.1 | 0.001 |
| With RT | 3 | 0.77 (0.23, 1.31) | 0.005 | 0.044 | 18.4 | 2.45 | 0.294 |
| Without RT | 19 | 0.22 (−0.12, 0.56) | 0.198 | 0.250 | 49.4 | 35.6 | 0.008 |
| Age | |||||||
| Minimum inclusion age >50 y | 5 | 0.91 (0.24, 1.60) | 0.008 | 0.339 | 59.6 | 9.89 | 0.042 |
ER, energy restriction; RT, resistance training; WMD, weighted mean difference.
P value for WMD.
P value for χ2.
Publication dates ranged from 1994 to 2018, study durations ranged from 8 to 52 wk, cohort mean age ± SD ranged from 26 ± 1.0 y to 75 ± 1.0 y, and 3 and 5 comparisons included female and male only participants, respectively. All 18 articles reported some amount of dietary control, with 5 articles indicating that all foods and beverages were provided to the participants, 11 providing a portion, and 2 providing menus and counseling. Dietary intakes were measured via food records (n = 9), menu check-off sheets (n = 2), compliance questionnaires (n = 1), dietary recalls and food records (n = 1), food records and menu checkoff sheets (n = 4), and 1 supervised meal consumption (Supplemental Table 2). In the overall analysis, total protein intakes averaged ∼0.80 g · kg−1 · d−1 in the RDA group and ∼1.30 g · kg−1 · d−1 in the >RDA group and were comparable in the subgroup analyses.
Heterogeneity and risk of bias
There was significant heterogeneity in the overall analysis on lean mass (χ2 = 43.7, I2 = 51.9%, P = 0.003). The heterogeneity became nonsignificant when only comparisons with ER (χ2 = 16.4, I2 = 20.6%, P = 0.229) and with RT (χ2 = 2.45, I2 = 18.4%, P = 0.294) were analyzed, but remained significant among comparisons with no ER (χ2 = 26.3, I2 = 73.3%, P = <0.001) and no RT (χ2 = 35.6, I2 = 49.4%, P = 0.008).
Four and 14 articles had low and unclear risk of selection bias, respectively, based on the information provided in the articles regarding randomization and allocation concealment (Supplemental Table 2). Eight and 10 articles had low and unclear risk of performance bias, whereas 6 and 12 articles had low and unclear risk of detection bias, respectively. Thirteen, 4, and 1 articles had low, unclear, and high risk of attrition bias, respectively. Nine and 9 articles had low and unclear risk of reporting bias, respectively.
Overall effect of consuming greater than the protein RDA
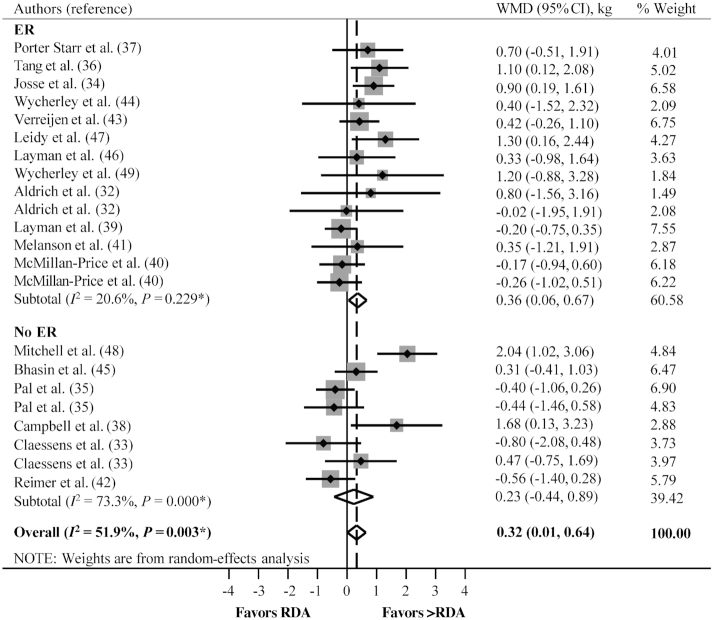
A random-effect analysis of all comparisons showed that consuming greater than the protein RDA benefitted changes in lean mass relative to consuming the RDA (WMD: 0.32 kg; 95% CI: 0.01, 0.64 kg; P = 0.043, n = 22) (Figure 2; Table 3).
FIGURE 2.
The overall and subgroup analyses with ER and with no ER on the effect of consuming greater than the protein RDA compared with the RDA on lean mass changes. *P value for χ2 test for heterogeneity. ER, energy restriction; WMD, weighted mean difference.
Effect of consuming greater than the protein RDA relative to specific stressors
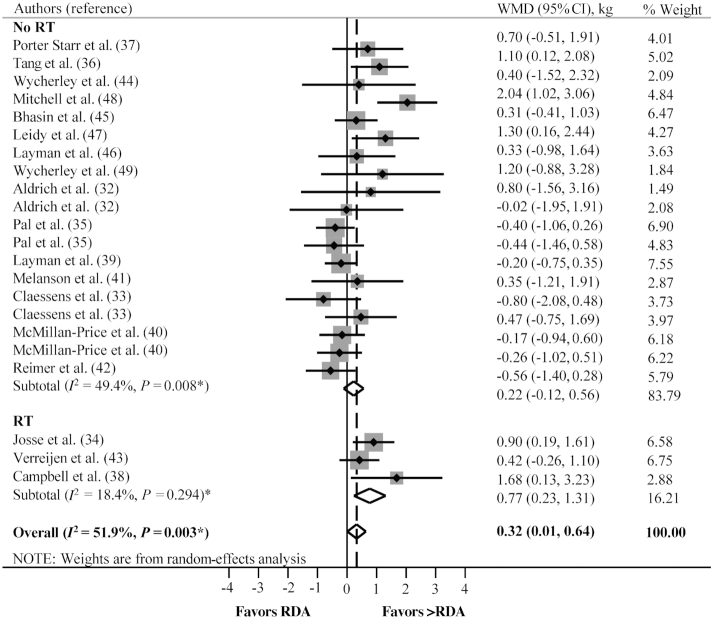
Random-effects analyses of specific subgroups showed that compared with the RDA, consuming greater protein than the RDA attenuated lean mass loss after ER (WMD: 0.36 kg; 95% CI: 0.06, 0.67 kg; P = 0.020, n = 14), but did not influence lean mass change with no ER (WMD: 0.23 kg; 95% CI: −0.44, 0.89 kg; P = 0.503, n = 8) (Figure 2; Table 3). Protein intakes greater than the RDA increased lean mass with RT, relative to no change when the RDA was consumed (WMD: 0.77 kg; 95% CI: 0.23, 1.31 kg; P = 0.005, n = 3), but did not influence changes in lean mass with no RT (WMD: 0.22 kg; 95% CI: −0.12, 0.56 kg; P = 0.198, n = 19) (Figure 3, Table 3).
FIGURE 3.
The overall and subgroup analyses with RT and with no RT on the effect of consuming greater than the protein RDA compared with the RDA on lean mass changes. *P value for χ2 test for heterogeneity. RT, resistance training; WMD, weighted mean difference.
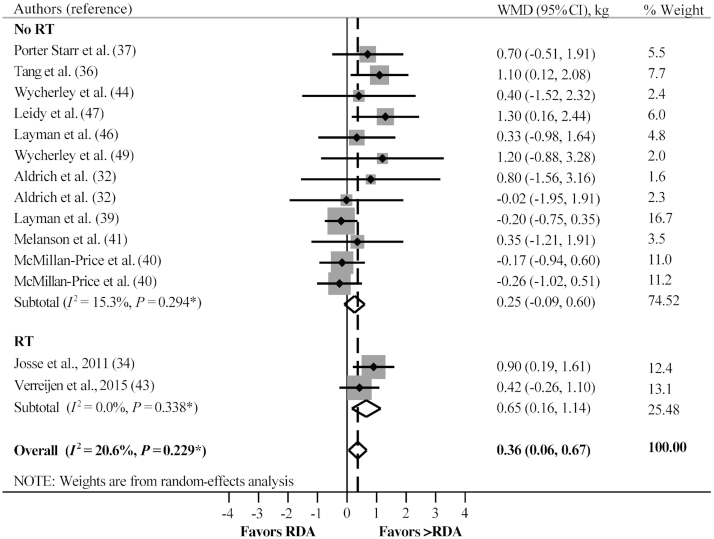
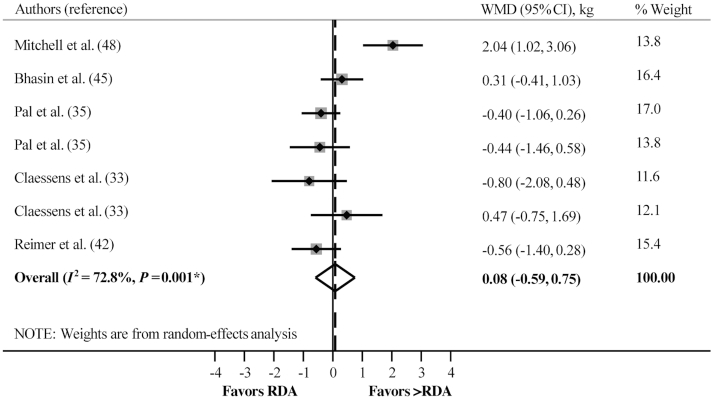
When ≥2 comparisons were available, we performed further subgroup random-effects analyses to delineate any potential effect of consuming greater than the protein RDA on the changes in lean mass induced by ER and/or RT independently or combined. Consuming greater than the protein RDA did not affect lean mass loss with ER + no RT (WMD: 0.25 kg; 95% CI: −0.09, 0.60 kg; P = 0.153, n = 12), but promoted lean mass gain with ER + RT (WMD: 0.65 kg; 95% CI: 0.16, 1.14 kg; P = 0.009, n = 2) (Figure 4; Table 3). With no ER + no RT, chronically consuming greater than the protein RDA, compared with the RDA, did not differentially affect lean mass (WMD: 0.08 kg; 95% CI: −0.59, 0.75 kg; P = 0.810, n = 7) (Figure 5; Table 3).
FIGURE 4.
The effect of consuming greater than the protein RDA compared with the RDA with ER + RT and with ER + no RT on lean mass changes. *P value for χ2 test for heterogeneity. ER, energy restriction; RT, resistance training; WMD, weighted mean difference.
FIGURE 5.
The effect of consuming greater than the protein RDA compared with the RDA in adults without purposeful stressors (with no energy restriction + no resistance training) on lean mass changes. WMD, weighted mean difference. *P value for χ2 test for heterogeneity.
Effect of consuming greater than the protein RDA in older adults
In older adults, consuming greater than the protein RDA compared with the RDA resulted in higher lean mass after the interventions (WMD: 0.91 kg; 95% CI: −0.24, 1.60 kg; P = 0.008, n = 5) (Table 3).
Discussion
This systematic review and meta-analysis of randomized controlled trials was designed and conducted to quantify the overall effect of consuming greater than the protein RDA compared with the RDA on whole-body lean mass among the available literature. In the overall meta-analysis totaling 981 participants from 18 articles that did and did not include purposeful ER or RT, consuming protein in excess of the RDA (∼1.3 g · kg−1 · d−1) influenced beneficial changes in lean mass in adults. In analyses stratified by specific stressors, protein intakes greater than the RDA influenced beneficial changes in lean mass when adults were purposefully stressed by the catabolic stimulus of dietary ER. However, the beneficial effect of consuming protein in excess of the RDA on lean mass was lost in the absence of purposeful stressors—no ER + no RT. Our results suggest that the beneficial effect of protein intakes greater than the RDA on lean mass may only manifest during stressful periods.
Several narrative reviews and perspective articles suggest that consuming greater than the current protein RDA would influence beneficial changes in lean mass that occur during periods with catabolic and anabolic stressors, such as ER or RT, respectively (4, 52, 53). Results from several systematic reviews and meta-analyses scientifically support these hypotheses; however, these articles typically contain comparator groups that consume less than the RDA (16–18). This could feasibly skew the effect sizes to support that consuming greater than the RDA is beneficial for lean mass when in fact consuming less may be detrimental. This systematic review and meta-analysis is the first to use groups that consumed the RDA as the comparator to assess whether consuming protein in excess of the RDA indeed influences beneficial lean mass changes. Results from this study support the results from previous meta-analyses (16–18) that consuming a higher-protein diet favors changes in lean mass that occur in response to ER regardless of RT status (n = 14 comparisons), ER + RT (n = 2), and RT regardless of ER status (n = 3).
Protein requirements and allowances embedded within the DRIs are based on nitrogen balance values obtained in adults consuming adequate energy and high-quality protein sources (54). Under short-term experimental conditions, 0.8 g · kg−1 · d−1 protein would support nitrogen balance in 97.5% of healthy adults (1). Nitrogen balance studies and the subsequent protein reference values are frequently scrutinized (55), with researchers citing well-known limitations of the nitrogen balance methodology (5) resulting in an underestimation of the Estimated Average Requirement and RDA (3). Alternative estimates derived from indicator amino acid oxidation studies indicate that the protein RDA should be close to 1.2 g · kg−1 · d−1 (3). Although indicator amino acid oxidation is a valid technique for estimating individual amino acid requirements, its utility to accurately estimate whole-body protein requirements is unresolved. According to results from the NHANES, American adults consume a mean of ∼1.1 g · kg−1 · d−1 protein (56). If estimates from indicator amino acid oxidation studies were accurate, there would be a high prevalence of protein undernutrition. There are no data to support that such a public health issue exists. Coincidentally, the mean protein intake for the higher-protein group was ∼1.3 g · kg−1 · d−1. On a morphologic scale, our results showed that, in adults not purposefully stressed (no ER + no RT), chronically consuming protein in excess of the RDA during an intervention did not affect changes in lean mass compared with consuming 0.8 g · kg−1 · d−1. These results support that the current RDA is sufficient to retain lean mass, and that higher protein intakes do not influence maintaining lean mass when an individual is not purposefully stressed.
This review is subject to the standard limitations of systematic reviews and meta-analyses such as the selection biases and discrepancies in experimental design. In an effort to address these concerns, 3 separate reviewers searched multiple databases and conducted manual searches of relevant systematic reviews and meta-analyses. Some unpublished data were also retrieved from authors through email: 24 responses from 52 requests (46% response rate). A funnel plot analysis was used to visually inspect publication bias. In the overall analysis, there was significant heterogeneity among the comparisons, which could affect the findings. Anticipating this potential limitation, a priori subgroup analyses were identified before searching the literature, which reduced the I2 statistic in 2 of the 4 subgroups to <50%. The lack of data from RT literature that utilized the protein RDA limited our ability to quantify the independent effect of RT with or without ER (n = 3) with confidence. This review also only included 5 articles whose participants were greater than 50 y (mean age >60 y). Protein intake as it relates to aging adults and skeletal muscle quantity and performance is particularly important (45, 53), but the varied experimental designs in the 5 articles resulted in 4 iterations of energy balance and RT, which precluded aggregating the articles separately. More research is needed to document the impact of protein intake on skeletal muscle size, metabolic quality, and function, along with functional measures of daily living. At present, insufficient information exists to assess dietary protein adequacy compared with “optimal” sufficiency based on tissue-specific and health-related outcomes for older adults. The duration of the studies may be another limitation of this meta-analysis, ranging from 8 wk to 52 wk (mode: 12 wk). It is possible that the body composition techniques used to assess lean body mass changes are not precise enough to document a potential effect of protein quantity over the study durations, especially at lesser lengths. This would favor a null effect and may be more relevant in the subgroup analysis without apparent stressors that influence changes in lean mass. Caution is warranted to not over-generalize the results beyond the scope of the durations presented in this study.
The results of this systematic review and meta-analysis indicate that the RDA for protein adequately meets the needs of adults to maintain lean mass when they are not purposely stressed. Protein intakes greater than the RDA are shown to augment beneficial changes in lean mass over time when adults purposefully experience catabolic stressors, specifically weight loss. These findings underscore the need to update the DRIs for protein of the general population with consideration given to the energy and physical activity status of adults.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—JLH, YW, and WWC: designed the research; JLH, YW, and REB: conducted the research; JLH: analyzed the data and wrote the manuscript with editorial assistance from YW, REB, and WWC; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–3 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
JLH and YW contributed equally to this work.
Abbreviations used: ER, energy restriction; RT, resistance training; WMD, weighted mean difference.
References
- 1. Institute of Medicine. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Washington (DC): National Academies Press; 2005. [Google Scholar]
- 2. Scrimshaw NS, Schürch B; International Dietary Energy Consultancy Group. Protein-energy interactions: proceedings of an I/D/E/C/G workshop, held in Waterville Valley, NH, USA, October 21 to 25, 1991. Lausanne, Switzerland: Nestlé Foundation; 1992. [Google Scholar]
- 3. Elango R, Ball RO, Pencharz PB. Individual amino acid requirements in humans: an update. Curr Opin Clin Nutr Metab Care. 2008;11(1):34–9. [DOI] [PubMed] [Google Scholar]
- 4. Traylor DA, Gorissen SHM, Phillips SM. Perspective: protein requirements and optimal intakes in aging: are we ready to recommend more than the recommended daily allowance?. Adv Nutr. 2018;9(3):171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Waterlow JC. The mysteries of nitrogen balance. Nutr Res Rev. 1999;12(1):25–54. [DOI] [PubMed] [Google Scholar]
- 6. Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127(5):998S–1003S. [DOI] [PubMed] [Google Scholar]
- 7. Beasley JM, Shikany JM, Thomson CA. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr Clin Pract. 2013;28(6):684–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campbell WW, Leidy HJ. Dietary protein and resistance training effects on muscle and body composition in older persons. J Am Coll Nutr. 2007;26(6):696S–703S. [DOI] [PubMed] [Google Scholar]
- 9. Phillips SM, Zemel MB. Effect of protein, dairy components and energy balance in optimizing body composition. Nestle Nutr Inst Workshop Ser. 2011;69:97–108.; discussion 108–13. [DOI] [PubMed] [Google Scholar]
- 10. Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol (1985). 2005;99(1):338–43. [DOI] [PubMed] [Google Scholar]
- 11. Karpe F, Ehrenborg EE. PPARδ in humans: genetic and pharmacological evidence for a significant metabolic function. Curr Opin Lipidol. 2009;20(4):333–6. [DOI] [PubMed] [Google Scholar]
- 12. Phillips SM. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med. 2014;44(Suppl 1):S71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tipton KD, Phillips SM. Dietary protein for muscle hypertrophy. Nestle Nutr Inst Workshop Ser. 2013;76:73–84. [DOI] [PubMed] [Google Scholar]
- 14. Phillips SM. Higher dietary protein during weight loss: muscle sparing?. Obesity (Silver Spring). 2018;26(5):789. [DOI] [PubMed] [Google Scholar]
- 15. Phillips SM. Higher protein during an energy deficit: muscle's guardian and fat's enemy?. Med Sci Sports Exerc. 2008;40(3):503–4. [DOI] [PubMed] [Google Scholar]
- 16. Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression. Am J Clin Nutr. 2006;83(2):260–74. [DOI] [PubMed] [Google Scholar]
- 17. Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96(6):1281–98. [DOI] [PubMed] [Google Scholar]
- 18. Kim JE, O'Connor LE, Sands LP, Slebodnik MB, Campbell WW. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta-analysis. Nutr Rev. 2016;74(3):210–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 20. Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96(6):1454–64. [DOI] [PubMed] [Google Scholar]
- 21. Finger D, Goltz FR, Umpierre D, Meyer E, Rosa LH, Schneider CD. Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sports Med. 2015;45(2):245–55. [DOI] [PubMed] [Google Scholar]
- 22. Garrow JS, Summerbell CD. Meta-analysis: effect of exercise, with or without dieting, on the body composition of overweight subjects. Eur J Clin Nutr. 1995;49(1):1–10. [PubMed] [Google Scholar]
- 23. Miller PE, Alexander DD, Perez V. Effects of whey protein and resistance exercise on body composition: a meta-analysis of randomized controlled trials. J Am Coll Nutr. 2014;33(2):163–75. [DOI] [PubMed] [Google Scholar]
- 24. Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, Aragon AA, Devries MC, Banfield L, Krieger JW. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naclerio F, Larumbe-Zabala E. Effects of whey protein alone or as part of a multi-ingredient formulation on strength, fat-free mass, or lean body mass in resistance-trained individuals: a meta-analysis. Sports Med. 2016;46(1):125–37. [DOI] [PubMed] [Google Scholar]
- 26. Nissen SL, Sharp RL. Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. J Appl Physiol. 2003;94(2):651–9. [DOI] [PubMed] [Google Scholar]
- 27. Pasiakos SM, McLellan TM, Lieberman HR. The effects of protein supplements on muscle mass, strength, and aerobic and anaerobic power in healthy adults: a systematic review. Sports Med. 2015;45(1):111–31. [DOI] [PubMed] [Google Scholar]
- 28. Schoenfeld BJ, Aragon AA, Krieger JW. The effect of protein timing on muscle strength and hypertrophy: a meta-analysis. J Int Soc Sport Nutr. 2013;10(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwingshackl L, Hoffmann G. Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta-analysis. Nutr J. 2013;12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas DK, Quinn MA, Saunders DH, Greig CA. Protein supplementation does not significantly augment the effects of resistance exercise training in older adults: a systematic review. J Am Med Dir Assoc. 2016;17(10):959.e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bergia RE III, Hudson JL, Campbell WW. Effect of whey protein supplementation on body composition changes in women: a systematic review and meta-analysis. Nutr Rev. 2018;76(7):539–51. [DOI] [PubMed] [Google Scholar]
- 32. Aldrich ND, Reicks MM, Sibley SD, Redmon JB, Thomas W, Raatz SK. Varying protein source and quantity do not significantly improve weight loss, fat loss, or satiety in reduced energy diets among midlife adults. Nutr Res. 2011;31(2):104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Claessens M, van Baak MA, Monsheimer S, Saris WH. The effect of a low-fat, high-protein or high-carbohydrate ad libitum diet on weight loss maintenance and metabolic risk factors. Int J Obes (Lond). 2009;33(3):296–304. [DOI] [PubMed] [Google Scholar]
- 34. Josse AR, Atkinson SA, Tarnopolsky MA, Phillips SM. Increased consumption of dairy foods and protein during diet- and exercise-induced weight loss promotes fat mass loss and lean mass gain in overweight and obese premenopausal women. J Nutr. 2011;141(9):1626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pal S, Ellis V, Dhaliwal S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br J Nutr. 2010;104(5):716–23. [DOI] [PubMed] [Google Scholar]
- 36. Tang M, Armstrong CL, Leidy HJ, Campbell WW. Normal vs. high-protein weight loss diets in men: effects on body composition and indices of metabolic syndrome. Obesity (Silver Spring). 2013;21(3):E204–10. [DOI] [PubMed] [Google Scholar]
- 37. Porter Starr KN, Pieper CF, Orenduff MC, McDonald SR, McClure LB, Zhou R, Payne ME, Bales CW. Improved function with enhanced protein intake per meal: a pilot study of weight reduction in frail, obese older adults. J Gerontol A Biol Sci Med Sci. 2016;71(10):1369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Campbell WW, Crim MC, Young VR, Evans WJ. Increased energy requirements and changes in body composition with resistance training in older adults. Am J Clin Nutr. 1994;60(2):167–75. [DOI] [PubMed] [Google Scholar]
- 39. Layman DK, Evans EM, Erickson D, Seyler J, Weber J, Bagshaw D, Griel A, Psota T, Kris-Etherton P. A moderate-protein diet produces sustained weight loss and long-term changes in body composition and blood lipids in obese adults. J Nutr. 2009;139(3):514–21. [DOI] [PubMed] [Google Scholar]
- 40. McMillan-Price J, Petocz P, Atkinson F, O'Neill K, Samman S, Steinbeck K, Caterson I, Brand-Miller J. Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Arch Intern Med. 2006;166(14):1466–75. [DOI] [PubMed] [Google Scholar]
- 41. Melanson KJ, Summers A, Nguyen V, Brosnahan J, Lowndes J, Angelopoulos TJ, Rippe JM. Body composition, dietary composition, and components of metabolic syndrome in overweight and obese adults after a 12-week trial on dietary treatments focused on portion control, energy density, or glycemic index. Nutr J. 2012;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reimer RA, Willis HJ, Tunnicliffe JM, Park H, Madsen KL, Soto-Vaca A. Inulin-type fructans and whey protein both modulate appetite but only fructans alter gut microbiota in adults with overweight/obesity: a randomized controlled trial. Mol Nutr Food Res. 2017;61(11):1700484. [DOI] [PubMed] [Google Scholar]
- 43. Verreijen AM, Verlaan S, Engberink MF, Swinkels S, de Vogel-van den Bosch J, Weijs PJ. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr. 2015;101(2):279–86. [DOI] [PubMed] [Google Scholar]
- 44. Wycherley TP, Buckley JD, Noakes M, Clifton PM, Brinkworth GD. Comparison of the effects of weight loss from a high-protein versus standard-protein energy-restricted diet on strength and aerobic capacity in overweight and obese men. Eur J Nutr. 2013;52(1):317–25. [DOI] [PubMed] [Google Scholar]
- 45. Bhasin S, Apovian CM, Travison TG, Pencina K, Moore LL, Huang G, Campbell WW, Li Z, Howland AS, Chen R et al.. Effect of protein intake on lean body mass in functionally limited older men: a randomized clinical trial. JAMA Intern Med. 2018;178(4):530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, Christou DD. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133(2):411–17. [DOI] [PubMed] [Google Scholar]
- 47. Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring). 2007;15(2):421–9. [DOI] [PubMed] [Google Scholar]
- 48. Mitchell CJ, Milan AM, Mitchell SM, Zeng N, Ramzan F, Sharma P, Knowles SO, Roy NC, Sjodin A, Wagner KH et al.. The effects of dietary protein intake on appendicular lean mass and muscle function in elderly men: a 10-wk randomized controlled trial. Am J Clin Nutr. 2017;106(6):1375–83. [DOI] [PubMed] [Google Scholar]
- 49. Wycherley TP, Brinkworth GD, Clifton PM, Noakes M. Comparison of the effects of 52 weeks weight loss with either a high-protein or high-carbohydrate diet on body composition and cardiometabolic risk factors in overweight and obese males. Nutr Diabetes. 2012;2:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heymsfield S. Human body composition. Champaign, IL: Human Kinetics; 2005. [Google Scholar]
- 51. Heaney RP. The bone‐remodeling transient: implications for the interpretation of clinical studies of bone mass change. J Bone Miner Res. 1994;9(10):1515–23. [DOI] [PubMed] [Google Scholar]
- 52. Paddon-Jones D, Campbell WW, Jacques PF, Kritchevsky SB, Moore LL, Rodriguez NR, van Loon LJ. Protein and healthy aging. Am J Clin Nutr. 2015;101(6):1339s–45s. [DOI] [PubMed] [Google Scholar]
- 53. Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, Wolfe RR. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance?. J Gerontol A Biol Sci Med Sci. 2013;68(6):677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Scrimshaw NS. Criteria for valid nitrogen balance measurement of protein requirements. Eur J Clin Nutr. 1996;50(Suppl 1):S196–7. [PubMed] [Google Scholar]
- 55. Wolfe RR, Miller SL. The recommended dietary allowance of protein: a misunderstood concept. JAMA. 2008;299(24):2891–3. [DOI] [PubMed] [Google Scholar]
- 56. USDA. Nutrient intakes from food: mean amounts consumed per individual, by gender and age, What We Eat in America, NHANES 2009–2010. Beltsville, MD: Agricultural Research Service; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.