ABSTRACT
Some evidence indicates that carotenoids may reduce the risk of bladder cancer (BC), but the association is unclear. We conducted a systematic review and meta-analysis of case-control and cohort studies investigating the relation between carotenoid intake or circulating carotenoid concentrations and BC risk in men and women. All relevant epidemiologic studies were identified by a search of PubMed and Scopus databases, and the Cochrane Library from inception to April 2019 with no restrictions. A random-effects model was used to calculate pooled RRs and their 95% CIs across studies for high compared with low categories of intake or circulating concentrations. We also performed a dose-response meta-analysis using the Greenland and Longnecker method and random-effects models. A total of 22 studies involving 516,740 adults were included in the meta-analysis. The pooled RRs of BC for the highest compared with the lowest category of carotenoid intake and circulating carotenoid concentrations were 0.88 (95% CI: 0.76, 1.03) and 0.36 (95% CI: 0.12, 1.07), respectively. The pooled RR of BC for the highest compared with lowest circulating lutein and zeaxanthin concentrations was 0.53 (95% CI: 0.33, 0.84). Dose-response analysis showed that BC risk decreased by 42% for every 1 mg increase in daily dietary β-cryptoxanthin intake (RR: 0.58; 95% CI: 0.36, 0.94); by 76% for every 1 μmol/L increase in circulating concentration of α-carotene (RR: 0.24; 95% CI: 0.08, 0.67); by 27% for every 1 μmol/L increase in circulating concentration of β-carotene (RR: 0.73; 95% CI: 0.57, 0.94); and by 56% for every 1 μmol/L increase in circulating concentrations of lutein and zeaxanthin (RR: 0.44; 95% CI: 0.28, 0.67). Dietary β-cryptoxanthin intake and circulating concentrations of α-carotene, β-carotene, and lutein and zeaxanthin were inversely associated with BC risk. The protocol was registered at PROSPERO as CRD42019133240.
Keywords: carotenoids, α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, lycopene, diet, blood, bladder cancer
Introduction
Carotenoids are a fat-soluble and diverse group of natural pigments of the polyene type (1) and are often present in red, yellow, or orange vegetables and fruit. Carotenoids from natural sources can be classified into 2 groups: hydrocarbons, which do not contain oxygen, such as α-carotene, β-carotene, and lycopene and xanthophylls, which contain oxygen, such as β-cryptoxanthin, lutein, and zeaxanthin (2). α-Carotene and β-carotene are found in high amounts in carrots; β-cryptoxanthin is present in oranges and tangerines, tomatoes contain high amounts of lycopene, and broccoli and spinach contain lutein and zeaxanthin (3). The most commonly studied carotenoids are α-carotene, β-carotene, lycopene, β-cryptoxanthin, lutein, and zeaxanthin because of their abundance in the diet and comparatively high concentrations in plasma (4). Carotenoids are hypothesized to reduce the risk of bladder cancer (BC), most likely due to their capacity to reduce cell proliferation and transformation, inhibit the development of precancerous lesions, regulate gap-junction communication between cells, and scavenge DNA damaging free radicals (5-9). A few epidemiological studies have investigated the relations between the carotenoids and BC risk; however, the results remain inconsistent. One meta-analysis, pooling 4 studies, included studies with the outcome of both BC and other urinary tract cancers and reported an inverse association between total carotenoid intake and the risk of BC (OR: 0.67; 95% CI: 0.55, 0.79) (10), but no meta-analysis has reported an association pooling all studies including the 6 main types of carotenoids and the dose-response association between carotenoids and BC risk. Meanwhile, which carotenoid plays a greater role in reducing BC risk remains unclear. Therefore, we performed a meta-analysis and meta-regression to comprehensively and comparatively assess the associations between carotenoids and the risk of BC.
Methods
This systematic review protocol was registered at PROSPERO as CRD42019133240.
Search strategy
A literature search was performed from inception to April 2019 using the PubMed and Scopus databases and the Cochrane library, without restrictions, using the following search terms: (carotenoid or α-carotene or β-carotene or β-cryptoxanthin or lutein & zeaxanthin or lycopene) and (urinary bladder neoplasms or bladder cancer or bladder neoplasm or bladder tumor or cancer of bladder or tumor of bladder). Moreover, the reference lists from retrieved articles were reviewed to search for further relevant studies. This study was conducted in accordance with the PRISMA (11) and MOOSE guidelines (12).
Eligibility criteria
Studies were included in the meta-analysis if they met the following criteria: 1) population-based epidemiological study design; 2) the exposure of interest was intake of carotenoids or α-carotene or β-carotene or β-cryptoxanthin or lutein and zeaxanthin or lycopene; or circulating concentrations of carotenoids or α-carotene or β-carotene or β-cryptoxanthin or lutein and zeaxanthin or lycopene; 3) the outcome of interest was BC; and 4) OR or RR estimates with 95% CIs were reported. If data was duplicated in >1 study, the study with the largest number of cases was included.
Data extraction
The following data was extracted from each study: the first author's last name, publication year, country where the study was performed, study period, participant sex and age, sample size (cases and controls or cohort size), measure and range of exposure, variables adjusted for in the analysis, and OR or RR estimates with corresponding 95% CIs for the highest versus lowest categories or for each category of carotenoid intake and circulating carotenoid concentrations. The ORs or RRs reflecting the greatest degree of control for potential confounders for use in the main analyses were extracted.
Individual study quality and risk of bias
Individual study quality and risk of bias was assessed using the 9-star Newcastle–Ottawa Scale (13). This is an 8-item instrument allowing the assessment of the patient population and selection, study comparability, follow-up, and the outcome of interest. Interpretation of the scale was performed by awarding points, or “stars,” for high-quality elements. The number of stars was then used to compare study quality in a quantitative manner. The maximum score was 9 points, representing the highest methodological quality. Data extraction was conducted independently by 2 authors (SHW and YNL), with disagreements resolved by consensus.
Assessment of quality of evidence across studies (grading of the evidence)
The quality and strength of the evidence across studies was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (14). Included observational studies started at low-quality evidence by default and then were downgraded or upgraded based on prespecified criteria. Criteria to downgrade included study limitations (weight of studies showed risk of bias according to the Newcastle–Ottawa Scale), inconsistency (substantial unexplained interstudy heterogeneity, P < 0.10), indirectness (presence of factors relating to the population, exposures, and outcomes that limit generalizability), imprecision [95% CIs were wide or overlap no effect (i.e. CIs include RR of 1.0)], and publication bias (significant evidence of small-study effects). Criteria to upgrade included a large size effect (RR >2 or RR <0.5 in the absence of plausible confounders), a dose-response gradient, and attenuation by plausible confounding effects.
Statistical analysis
The measure of effect of interest was the OR or RR and the corresponding 95% CI. As the risk of BC was low, OR from case-control studies approximately estimated RR. The primary outcome was BC risk expressed as RR estimates with corresponding 95% CIs in relation to dietary carotenoid intake or circulating concentrations of carotenoids. We reported all results as RR for simplicity and ease of interpretation. The associations of carotenoid intakes/circulating concentrations of carotenoids with BC risk were estimated using DerSimonian and Laird random-effect (15) models by comparing the highest with the lowest (the referent) category. Heterogeneity among studies was evaluated by the Cochran Q test [considered significant for P values <0.10 (16, 17)] and the I2 parameter, which represented the percentage of total variation across studies that was attributable to heterogeneity rather than to chance (heterogeneity between studies was considered low, moderate, and high according to I2 values of 25%, 50%, and 75%) (18). For 1 study that reported results for participants <65 y and those >65 y separately (19), the 2 RR estimates were combined, and the pooled RR estimate was then included in the meta-analysis. For 7 studies (20-26) that reported results for dietary intake of α-carotene, β-carotene, β-cryptoxanthin, lutein and zeaxanthin, and lycopene separately, the RR estimates from 5 types of carotenoids were combined for each of the 7 studies, and the pooled RR estimates were then included in the meta-analysis to examine the association between dietary total carotenoid intake and the risk of BC. For 1 study (27) that reported results for circulating concentrations of α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene separately, the RR estimates from 6 types of carotenoids were combined, and the pooled RR estimates were then included in the meta-analysis to examine the association between circulating concentrations of total carotenoids and the risk of BC. In addition, we further conducted subgroup analyses for dietary carotenoid intake by sex (men versus women), study design (case-control compared with cohort), origin of participants (Europeans versus Americans), study quality (8 + stars versus 7 stars), respectively, and compared the difference of effect sizes within each subgroup using the method described by Deeks and Altman (28).
Furthermore, a dose-response association between carotenoid intake/circulating carotenoid concentrations and BC risk was estimated by the method of Greenland et al. (29) and Orsini et al. (30) to compute slopes from the natural logarithm of the RR across exposure categories. Only studies that reported the number of cases and controls and the RR and its variance estimate for 2 or more quantitative exposure categories were included. The median level of carotenoid intake (mg/d) or circulating carotenoid concentrations (μmol/L) for each category was assigned to each corresponding RR. For the studies not providing the median of the highest category, the intake level was defined as 1.2 times the highest category. (31). The summary RR estimates were obtained from DerSimonian and Laird random-effect (15) models applied to the dose-response slopes. Heterogeneity among studies was evaluated by the Cochran Q test (16, 17) and the I2 parameter (18). An estimated linear dose-response trend was pooled under the assumption of log-linearity of the exposure response association using random effects models for meta-analysis and a P value for the linearity was calculated (30). A potential nonlinear dose-response relation was modeled by using restricted cubic splines with 4 knots percentiles 5%, 35%, 65%, and 95% of the distribution of carotenoid intake/circulating concentrations of carotenoids. A P value for nonlinearity was calculated by testing if the coefficient of the second and third splines were equal to zero.
Publication bias was assessed by constructing funnel plots and 2 formal tests: the Begg-adjusted rank correlation test (32) and the Egger's regression test (33). Furthermore, the trim-and-fill method (34) was used to analyze whether the adjusted OR was significant compared with the original pooling estimates. The meta-analyses were carried out using the statistical package Stata (version 14.2, Stata Corporation).
Results
Literature search
The detailed steps of our literature search are shown in a flow diagram (Figure 1). Briefly, we identified 17 potentially relevant articles concerning dietary carotenoid intake (19-26, 35-43), 4 articles on circulating carotenoid concentrations (27, 44-46), and 2 articles on β-carotene supplementation (42, 47) in relation to risk of BC. A total of 22 studies were included in the meta-analysis. As 1 study (42) reported both dietary carotenoid intake and supplementation of β-carotene intake, it was not only included in studies for dietary carotenoid intake (n = 17), but also included in studies examining supplementation of β-carotene intake (n = 2).
FIGURE 1.
Flowchart of selection of studies for inclusion in the meta-analysis.
Study characteristics
Among the 22 included studies (n = 516,740), 17 were on dietary carotenoid intake and 11 were on total carotenoid intake. Four cohort studies (21, 23, 24, 26) and 7 case-control studies (19, 20, 22, 25, 39-41) involved a total of 10,348 cases (Table 1). Eight (19, 21, 24-26, 39-41) of 11 studies were conducted in the USA and 3 (20, 22, 23) in Europe. Nine (20-26, 39, 43) of 17 studies examining dietary carotenoid intake were on dietary α-carotene intake, 14 (20-26, 35-39, 42, 43) on dietary β-carotene intake, 7 (21-26, 39) on dietary β-cryptoxanthin intake, 8 (20-26, 39) on dietary lutein/zeaxanthin intake, and 8 (20-26, 39) on dietary lycopene intake. Only 2 reports provided results for dietary β-carotene supplement use alone (42, 47). Three nested case-control studies (44-46) and 1 hospital-based case-control study (27) on circulating carotenoid concentrations comprised a total of 1086 cases and 1210 controls; 3 (27, 44, 45) were conducted in the USA and 2 (27, 44) of 3 measured plasma carotenoid concentrations and 1 (45) of 3 measured serum carotenoid concentrations, and 1 (46) was conducted in 10 European countries and measured plasma carotenoid concentrations. Three (27, 45, 46) of 4 studies on circulating carotenoid concentrations were on total carotenoids, α-carotene, β-cryptoxanthin, and lutein and zeaxanthin, and 4 on circulating concentrations of β-carotene and lycopene (Table 1). The quality criteria of the 22 included studies range from 7 to 9 stars. Studies with a lower quality score generally did not select the community controls, but hospital controls or the selected cohort did not represent the community. All 22 included studies adjusted for the important risk factor of cigarette smoking (48).
TABLE 1.
Characteristics of studies on intake/circulating concentrations of carotenoids and bladder cancer risk
| Source | Study design | Location/period | Sex | Age, y | Cases, n | Controls, n | Participants, n | Measure/range of exposure1 | Study quality2 | Adjustment for covariates |
|---|---|---|---|---|---|---|---|---|---|---|
| Dietary intake (μg/d) | ||||||||||
| Risch et al., 1988 (35) | HCC | Canada/1979–1982 | M/F | 35–79 | 826 | 792 | 1618 | β-carotene: 8333 (Q75-Q25) | 8 | Lifetime cigarette consumption, and history of diabetes |
| Other carotenes: 1667 (Q75-Q25) | ||||||||||
| Shibata et al., 1992 (36) | Cohort | USA/1981–1989 | M | 74.9±7.2 | 71 | 24,218 person-years | β-carotene: 4000 (T1), 9200 (T3) | 9 | Age and smoking | |
| Vena et al., 1992 (19) | PCC | USA/1979–1985 | M | 35–90 | 351 | 855 | 1206 | Total carotenoids: (Q1), (Q4) | 8 | Age, calories, smoking, education, total fluid consumption, and sodium |
| Garcia et al., 1999 (20) | HCC | Spain/1983–1986 | M | 64.1 | 495 | 1112 | 1607 | α-carotene: Q4, Q1; β-carotene: Q4, Q1; lycopene: Q4, Q1; lutein: Q4, Q1 | 8 | Smoking status, total pack-years smoked, occupational exposure, total energy intake, vitamin E intake, SFA intake, and intake of other specific carotenoids or other specific flavonoids |
| Bruemmer et al., 1996 (37) | PCC | USA/1987–1990 | M/F | 45–65 | 262 | 405 | 667 | β-carotene: 5842 (T1), 16,330 (T3) | 8 | Age, sex, county, smoking, and calories |
| Michaud et al., 1999 (21) | Cohort | USA/1986 | M | 40–75 | 252 | 51,529 person years | α-carotene: 292 (Q1), 1888 (Q5); β-carotene: 2046 (Q1), 9202 (Q5); lycopene: 3415 (Q1), 18,892 (Q5); lutein: 1296 (Q1), 6845 (Q5); β-cryptoxanthin: 10.9 (Q1), 174.8 (Q5) | 8 | Age, pack-years of cigarette smoking, current smoking status, geographic region, total fluid intake, and caloric intake | |
| Wakai et al., 2000 (38) | HCC | Japan/1996–1999 | M/F | 20–99 | 297 | 295 | 592 | β-carotene: 1526 (Q1), 3019 (Q4) | 7 | Smoking and occupational history as a cook |
| Zeegers et al., 2001 (22) | NCC | Dutch/1986–1992 | M/F | 55–69 | 569 | 3123 | 36923 | α-carotene: 190 (Q1), 1570 (Q5); β-carotene: 1430 (Q1), 5710 (Q5); β-cryptoxanthin: 20 (Q1), 480 (Q5); lutein/zeaxanthin: 1340 (Q1), 4580 (Q5); lycopene: 480 (Q1), 2650 (Q5) | 8 | Age, sex, cigarette smoking amount and duration |
| Michaud et al., 2002 (23) | Cohort | Finland/1985–1995 | M | 50–69 | 344 | 27,111 | α-carotene: 111 (Q1), 1333 (Q5); β-carotene: 775 (Q1), 3944 (Q5); lutein/zeaxanthin: 952 (Q1), 1906 (Q5); lycopene: 117 (Q1), 1588 (Q5); β-cryptoxanthin: 2.8 (Q1), 76.9 (Q5) | 7 | Age, duration of smoking, smoking dose, total energy, and trial intervention | |
| Castelao et al., 2004 (39) | PCC | USA/1987–1996 | M/F | Cases: 56.3±7.7 controls: 56.4±8.3 | 1592 | 1592 | 3184 | Total carotenoids: 5146 (Q1), 13,401 (Q5); α-carotene: 424.3 (Q1), 1330.3 (Q5); β-carotene: 2089 (Q1), 6098 (Q5); β-cryptoxanthin: 25 (Q1), 167 (Q5); lutein/zeaxanthin: 748 (Q1), 2127 (Q5); lycopene: 1161 (Q1), 4258 (Q5) | 8 | Education, number of cigarettes smoked per day, number of years of smoking, smoking status in reference year, lifetime use of nonsteroidal anti-inflammatory drugs, and number of years employed as a hairdresser/barber |
| Schabath et al., 2004 (40) | HCC | USA/1999–2003 | M/F | Cases: 63.5±11.5 controls: 62.6±11.5 | 423 | 467 | 890 | Total carotenoids 984 (Q1), 1884 (Q4) | 7 | Age, gender, ethnicity, smoking status, pack-years smoked, and total energy |
| Holick et al., 2005 (24) | Cohort | USA/1980–2000 | F | 30–55 | 237 | 88,796 person- years | α-carotene: 192 (Q1), 1561 (Q5); β-carotene: 1358 (Q1), 8545 (Q5); β-cryptoxanthin: 20 (Q1), 220 (Q5); lutein/zeaxanthin: 1172 (Q1), 11,689 (Q5); lycopene: 844 (Q1), 11,179 (Q5) | 8 | Age, pack-years of cigarette smoking, current smoking, and total caloric intake | |
| García-Closas et al., 2007 (41) | HCC | USA/1998–2001 | M/F | Cases: 65.3±10.2 controls: 64.0±9.9 | 912 | 873 | 1785 | Total carotenoids 612 (Q1), 2175 (Q5) | 7 | Age, gender, region, smoking status and duration of smoking |
| Roswall et al., 2009 (42) | Cohort | Denmark/1993–2006 | M/F | 50–64 | 322 | 55,557 | β-carotene dietary intake (μg/d): 1438 (T1), 4439 (T3) β-carotene supplementation (μg/d): 0 (T1), 8991 (T3) | 9 | Total intake of vitamin C, folate and vitamin E as well as dietary supplemental intake and further for smoking status, smoking duration, smoking intensity, possible cessation and when, passive smoking, and work exposure | |
| Brinkman et al., 2010 (25) | PCC | USA/2000–2003 | M/F | Cases: 62±9.2 controls: 60.7±10.6 | 322 | 239 | 561 | Total carotenoids: 398.26 (Q1) 17932.73 (Q4) α-carotene: 3.4 (Q1), 1105 (Q4); β-carotene: 292 (Q1), 5517 (Q4); β-cryptoxanthin: 0 (Q1), 216 (Q4); lycopene: 0 (Q1), 7746 (Q4); lutein: 0.93 (Q1), 3135 (Q4) | 8 | Age, sex, smoking status, pack years smoked, and total energy intake |
| Wu et al., 2012 (43) | PCC | USA/2001–2004 | M/F | 30–79 | 1087 | 1266 | 2353 | α-carotene: 99 (Q1), 468(Q5); β-carotene: 616 (Q1), 2348 (Q5) | 8 | Gender, age, region, race, Hispanic status, smoking status, usual BMI, and total energy |
| Park et al., 2013 (26) | Cohort | USA/1993–2007 | M/F | 45–75 | 581 | 185,885 | α-carotene: 225 (Q1), 675 (Q4); β-carotene: 1306 (Q1), 3194 (Q4); lycopene: 1921 (Q1), 1920 (Q4); lutein: 755 (Q1), 1774 (Q4); β-cryptoxanthin: 33 (Q1), 196 (Q4) | 9 | Age at cohort entry, ethnicity, and total energy intake, first-degree family history of bladder cancer, employment in a high-risk industry, smoking status, average number of cigarettes, squared average number of cigarettes, number of years smoked, number of years since quitting, interactions of ethnicity with smoking status, average number of cigarettes, and squared average number of cigarettes | |
| Hotaling et al., 2011 (47) | Cohort | USA/2000–2005 | M/F | 50–76 | 319 | 77,050 | Supplementation (μg/d): β-carotene: 0 (Q1), 600 (Q4) | 8 | Gender, age, race/ethnicity, education, family history of bladder cancer, smoking status, pack-years of smoking, fruits and vegetables | |
| Circulating concentrations, nmol/L | ||||||||||
| Helzlsouer et al., 1989 (44) | NCC | USA/1974–1985 | M/F | 11–98 | 35 | 70 | 1053 | β-carotene: 391 (T1), 708 (T3); lycopene: 652 (T1), 950 (T3) | 8 | Smoking and vitamin supplements |
| Nomura et al., 2003 (45) | NCC | Japanese in the USA/1971–1995 | M | 73.2 | 111 | 111 | 2223 | Total carotenoids: 1840 (Q1), 3374 (Q4); α-carotene: 70 (Q1), 152 (Q4); β-carotene: 209 (Q1), 486 (Q4); β-cryptoxanthin: 276 (Q1), 805 (Q4); lycopene: 186 (Q1), 419 (Q4); lutein/zeaxanthin: 590 (Q1), 969 (Q4) | 8 | Pack-years of cigarette smoking in addition to age |
| Hung et al., 2006 (27) | HCC | USA/1993–1997 | M/F | >18 | 84 | 173 | 257 | α-carotene: 390 (Q1), 1180 (Q4); β-carotene 1340 (Q1), 4220 (Q4); β-cryptoxanthin: 510 (Q1), 1640 (Q4); lycopene: 1920 (Q1), 4010 (Q4); lutein: 670 (Q1), 1910 (Q4); zeaxanthin: 340 (Q1), 830 (Q4) | 7 | Age, sex, pack-years and education |
| Ros et al., 2012 (46) | NCC | 10 European countries/1992–2005 | M/F | 25–70 | 856 | 856 | 17123 | Total carotenoids: 1262 (T1), 2313 (T3); α-carotene: 61 (T1), 153 (T3); β-carotene: 289 (T1), 676 (T3); β-cryptoxanthin: 136 (T1), 434 (T3); lycopene: 241 (T1), 532 (T3); lutein: 212 (T1), 427 (T3); zeaxthantin: 52 (T1), 122 (T3) | 8 | Age at blood collection, study center, sex, date of blood collection, time of blood collection, and fasting status and further adjusted for smoking status, duration, and intensity |
Dietary carotenoids includes carotenoids from foods only and total carotenoids includes carotenoids from foods and supplements. Range of exposure indicates the cut points for the highest and lowest categories of daily carotenoids intake.
Study quality was judged based on the Newcastle–Ottawa Scale (range, 1–9 stars; the higher score indicating higher quality) (13).
NCC study; the number of cases and controls in the group.
F, female; HCC, hospital-based case-control study; M, male; NCC, nested case-control study; PCC, population-based case-control study; Q, quartile/quintile; T, tertile.
Supplemental Table 1 shows a summary of the GRADE assessments for the associations between BC risk and intake of total carotenoids in all studies and in studies stratified by sex (men and women), study design (case-control and cohort), and study quality; intake of 6 different types of carotenoids; circulating concentrations of carotenoids and 6 different types of circulating carotenoid concentrations. The evidence for a lack of harm was rated as very low quality for total sugars and fructose because of downgrades for serious inconsistency and imprecision and low quality for sucrose because of a downgrade for serious imprecision and an upgrade for a significant inverse dose-response gradient.
The quality of evidence was high for circulating concentrations of lutein and zeaxanthin due to no serious risk of bias, inconsistency, indirectness, and imprecision and an upgrade for a dose-response gradient. The quality of evidence was moderate for total carotenoid intake in men due to no serious risk of bias, indirectness, and imprecision and a downgrade for inconsistency and an upgrade for a dose-response gradient. The quality of evidence was low for total carotenoid intake in case-control studies, total carotenoid intake in studies with quality of 8+ stars, total carotenoid intake in studies with quality of 7 stars, β-cryptoxanthin intake, lycopene intake, circulating concentrations of α-carotene, circulating concentrations of β-carotene, circulating concentrations of lycopene, and dietary β-carotene supplementation; or very low for total carotenoid intake, total carotenoid intake in women, total carotenoid intake in cohort studies, α-carotene intake, β-carotene intake, lutein and zeaxanthin intake, circulating concentrations of total carotenoids, and circulating concentrations of β-cryptoxanthin. The reason for the grades of low to very low quality was because evidence was only available from predominantly low‐quality observational studies.
High versus low carotenoid intake or circulating carotenoid concentrations
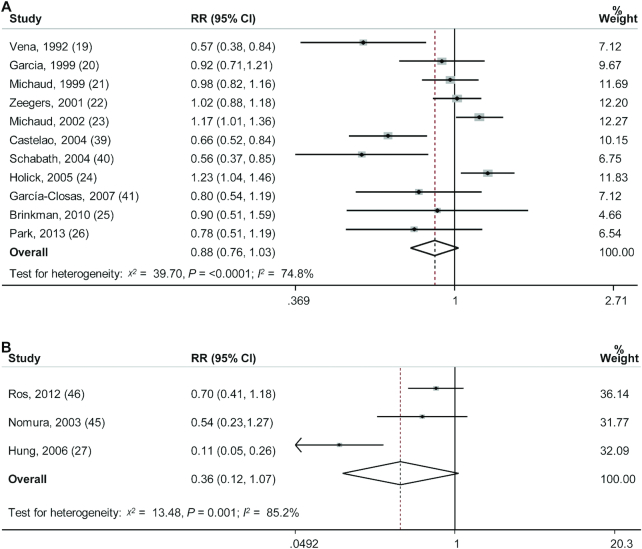
The multivariable-adjusted RRs for each individual study and all studies combined for the highest versus lowest categories of dietary carotenoid intake or circulating carotenoid concentrations are shown in Figure 2. Results from individual studies on dietary total carotenoid intake in relation to BC risk were inconsistent with both inverse and positive associations reported. The association of circulating carotenoid concentrations with BC risk was not statistically significant in 3 individual studies (44-46), but statistically significant in 1 study (27). The adjusted pooled RRs of BC for the highest versus lowest categories of total carotenoid intake and circulating carotenoid concentrations were, respectively, 0.88 (95% CI: 0.76–1.03) and 0.36 (95% CI: 0.12–1.07). Statistically significant heterogeneity was present among studies of total carotenoid intake (χ2 = 39.26, P <0.0001; I2 = 74.5%) and among studies of circulating carotenoid concentrations (χ2 = 13.48, P = 0.001; I2 = 85.2%). The funnel plot for the association between dietary total carotenoid intake and the risk of BC was asymmetric and the Egger test (bias = −13.14, P = 0.07) suggested publication bias (Figure 3), but the Begg test (P = 1.00) did not suggest publication bias. Furthermore, the trimmed-and-filled adjusted RR was the same as the original result. The Begg test (P = 0.60) and Egger test (bias = −9.14, P = 0.47) showed no evidence of publication bias for circulating carotenoid concentrations.
FIGURE 2.
Adjusted RRs of bladder cancer for the highest compared with lowest categories of total carotenoid intake (A; 11 studies, n = 521,015)/circulating carotenoid concentrations (B; 3 studies, n = 1546) in adults. Associations were estimated using DerSimonian and Laird (15) random-effect models comparing the highest with the lowest (the referent) category.
FIGURE 3.
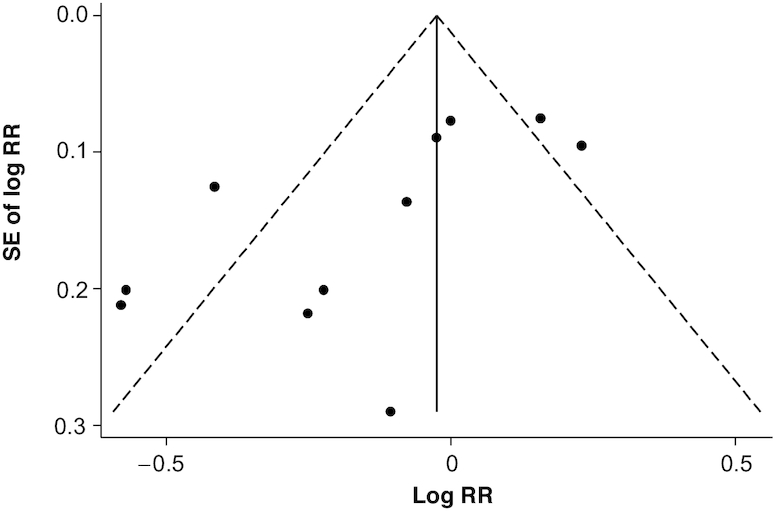
The funnel plot for the association between dietary total carotenoid intake and the risk of bladder cancer (11 studies, n = 521,015) in adults. DerSimonian and Laird random-effect (15) models were used to generate the funnel plot. Log RR: the logarithm of the RR.
Sensitivity and subgroup analyses
Sensitivity and stratified analyses were performed to explore the heterogeneity among studies of carotenoid intake and BC. A sensitivity analysis omitting 1 study at a time and calculating the pooled RRs for the remainder of the studies suggested that the studies by Michaud et al. (23) and Holick et al. (24) substantially influenced the pooled RR. After excluding the 2 studies separately, the inverse association was statistically significant, and the RR for the highest compared with lowest category of total carotenoid intake was 0.85 (95% CI: 0.72, 0.998) after excluding the study by Michaud et al. (23) and 0.85 (95% CI: 0.72, 0.99) after excluding the study by Holick et al. (24). When excluding both studies, the inverse association was statistically significant, and the RR for the highest versus lowest category of total carotenoid intake was 0.81 (95% CI: 0.69, 0.94).
A subgroup analysis for dietary total carotenoid intake is shown in Table 2. Although the inverse associations were not different between men and women (P for difference = 0.84), the association for men was statistically significant (RR = 0.85; 95% CI: 0.72, 0.99). The associations appeared to be significantly stronger in case-control studies (0.77; 0.63–0.94) than in cohort studies (1.09; 0.93, 1.27) (P for difference <0.001). Although associations were not statistically significant, they appeared to be stronger in Americans (inverse) than Europeans (positive). The associations were stronger for studies that met more quality criteria (≥8 stars; RR = 0.81; 95% CI: 0.68, 0.95) than the studies that met fewer quality criteria (7 stars; RR = 1.13; 95% CI: 0.95, 1.36). The combined risk estimates of BC risk for the highest versus lowest categories of the intake of 6 dietary carotenoids and circulating carotenoid concentrations are shown in Table 2. Except for circulating concentrations of lutein and zeaxanthin (RR = 0.53; 95% CI, 0.33, 0.84), the inverse associations were not statistically significant for dietary intake and circulating concentrations of α-carotene, β-carotene, β-cryptoxanthin, lutein and zeaxanthin, and lycopene, and for dietary β-carotene supplementation. Except for men (χ2 = 31.07, P < 0.0001; I2 = 71%; P for the Egger test = 0.04), other analyses in Table 2 showed no evidence of publication bias. Furthermore, the Begg test did not show evidence of publication bias (P = 0.72), and the trimmed-and-filled adjusted RR was the same as the original result for men.
TABLE 2.
Pooled risk estimates of bladder cancer risk associated with high versus low carotenoid intake and circulating carotenoid concentrations in adults as defined in each original study: overall and subgroup analysis1
| Test for heterogeneity of RRs | |||||||
|---|---|---|---|---|---|---|---|
| Factors stratified | Studies, n | Participants, n | RR (95% CI) | I2 Parameter, %2 | χ2 | P value | P 3 |
| Dietary intake | |||||||
| Total carotenoids | |||||||
| All studies | 11 | 366,246 | 0.88 (0.76, 1.03) | 74.5 | 39.26 | <0.0001 | |
| Sex | 0.84 | ||||||
| Men | 10 | 172,261 | 0.85 (0.72, 0.99) | 71.0 | 31.07 | <0.0001 | |
| Women | 6 | 193,985 | 0.86 (0.67, 1.09) | 79.6 | 24.47 | <0.0001 | |
| Study design | <0.0001 | ||||||
| Case-control | 7 | 12,925 | 0.77 (0.63, 0.94) | 64.7 | 16.98 | 0.009 | |
| Cohort | 4 | 353,321 | 1.09 (0.93, 1.27) | 56.8 | 6.95 | 0.074 | |
| Origin of the participants | 0.02 | ||||||
| Europeans | 3 | 32,410 | 1.05 (0.92, 1.20) | 39.7 | 3.32 | 0.19 | |
| Americans | 8 | 333,836 | 0.80 (0.64, 1.004) | 76.9 | 30.32 | <0.0001 | |
| Study quality | <0.0001 | ||||||
| 7 stars | 3 | 117,692 | 1.13 (0.95, 1.36) | 51.7 | 4.14 | 0.13 | |
| 8+ stars | 8 | 248,554 | 0.81 (0.68, 0.95) | 63.4 | 19.13 | 0.008 | |
| α-carotene | 9 | 364,699 | 0.94 (0.80, 1.09) | 46.1 | 16.71 | 0.053 | |
| β-carotene | 14 | 447,351 | 0.95 (0.88, 1.03) | 35.9 | 21.85 | 0.08 | |
| β-cryptoxanthin | 7 | 360,758 | 0.90 (0.80, 1.02) | 44.3 | 12.57 | 0.08 | |
| Lutein and zeaxanthin | 8 | 362,365 | 0.91 (0.76, 1.10) | 51.5 | 16.48 | 0.04 | |
| Lycopene | 8 | 362,365 | 0.99 (0.87, 1.12) | 13.2 | 9.22 | 0.32 | |
| Circulating carotenoid concentrations | |||||||
| Total carotenoids | 3 | 2014 | 0.36 (0.12, 1.07) | 85.2 | 13.48 | 0.001 | |
| α-carotene | 3 | 2191 | 0.52 (0.21, 1.27) | 67.1 | 6.08 | 0.048 | |
| β-carotene | 4 | 2296 | 0.67 (0.28, 1.62) | 74.0 | 11.53 | 0.009 | |
| β-cryptoxanthin | 3 | 2191 | 0.64 (0.22, 1.86) | 69.6 | 6.57 | 0.04 | |
| Lutein and zeaxanthin | 3 | 2191 | 0.53 (0.33, 0.84) | 34.0 | 6.06 | 0.19 | |
| Lycopene | 4 | 2296 | 0.76 (0.39, 1.49) | 49.0 | 5.88 | 0.12 | |
| Dietary supplementation | |||||||
| β-carotene | 2 | 132,607 | 0.94 (0.70, 1.26) | 0.0 | 0.1 | 0.75 | |
The associations of carotenoid intakes/circulating carotenoid concentrations with bladder cancer risk were estimated using DerSimonian and Laird random-effect (15) models by comparing the highest with the lowest (the referent) category.
Heterogeneity among studies was evaluated by the Cochran Q test (16, 17) and the I2 parameter, which represents the percentage of total variation across studies that is attributable to heterogeneity rather than chance (49).
P values test for the homogeneity comparison between subgroups.
Dose-response meta-analysis
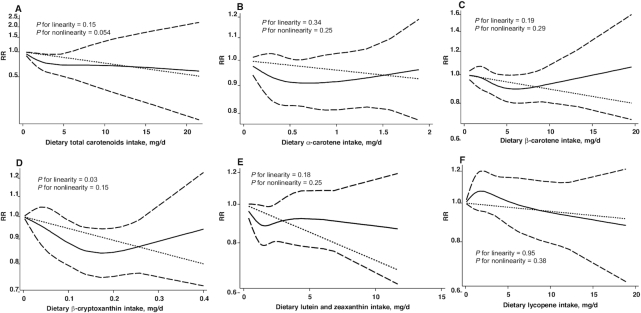
The dose-response relation between dietary carotenoid intake/circulating carotenoid concentrations and BC risk was next assessed. The dose and effect sizes for each individual study are shown in Supplemental Table 2. As only 4 studies reported the number of cases and controls and the RR and its variance estimate for 2 or more quantitative exposure categories of total carotenoid intake, the 4 studies were included in the dose-response analysis. The linear trend between total carotenoid intake and BC risk was not statistically significant (Figure 4A) (P = 0.15). The dataset, Stata code, and output are detailed in Supplemental Table 3.
FIGURE 4.
Dose-response relations between dietary intake of total carotenoids (A; n = 191,744), α-carotene (B; n = 363,092), β-carotene (C; n = 444,126), β-cryptoxanthin (D; n = 360,758), lutein and zeaxanthin (E; n = 360,758), and lycopene (F; n = 360,758) and RRs of bladder cancer in adults. Data were modeled with random-effects restricted cubic spline models with 4 knots (5th, 35th, 65th, and 95th percentiles) and using the Greenland and Longnecker method (29) to estimate the covariances of multivariable-adjusted RRs. Lines with long dashes represent the pointwise 95% CIs for the fitted nonlinear trend (solid line). Lines with short dashes represent the linear trend.
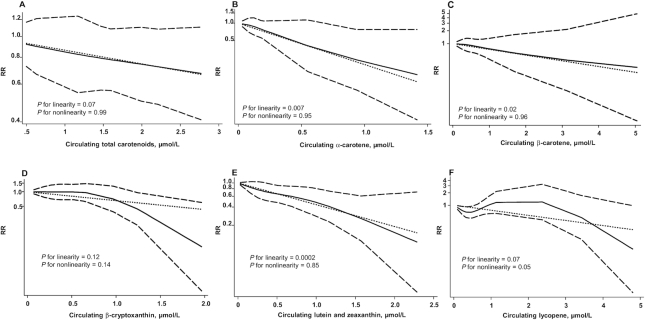
The inverse linear and nonlinear associations were not statistically significant for dietary intake of total carotenoids (Figure 4A), α-carotene (Figure 4B), β-carotene (Figure 4C), lutein and zeaxanthin (Figure 4E), and lycopene (Figure 4F), dietary β-carotene supplementation (Figure 5), and circulating concentrations of total carotenoids (Figure 6A), β-cryptoxanthin (Figure 6D) and lycopene (Figure 6F). However, the inverse linear association was statistically significant for dietary intake of β-cryptoxanthin (RR = 0.58; 95% CI: 0.36, 0.94) (Figure 4D), and circulating concentrations of α-carotene (RR = 0.24; 95% CI: 0.08, 0.67) (Figure 6B), β-carotene (RR = 0.73; 95% CI, 0.57, 0.94) (Figure 6C), and lutein and zeaxanthin (RR = 0.44; 95% CI: 0.28, 0.67) (Figure 6E). These analyses showed no evidence of publication bias.
FIGURE 5.
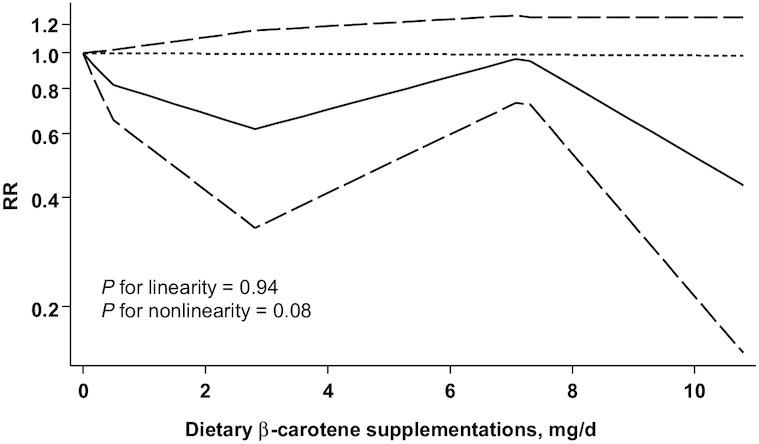
Dose-response relations between β-carotene supplementations and RRs of bladder cancer (n = 132,607) in adults. Data were modeled with random-effects restricted cubic spline models with 4 knots (5th, 35th, 65th, and 95th percentiles) and using the Greenland and Longnecker method (29) to estimate the covariances of multivariable-adjusted RRs. Lines with long dashes represent the pointwise 95% CIs for the fitted nonlinear trend (solid line). Lines with short dashes represent the linear trend.
FIGURE 6.
Dose-response relations between circulating concentrations of total carotenoids (A; n = 1757), α-carotene (B; n = 2191), β-carotene (C; n = 2296), β-cryptoxanthin (D; n = 2191), lutein and zeaxanthin (E; n = 2191), and lycopene (F; n = 2296) and RRs of bladder cancer in adults. Data were modeled with random-effects restricted cubic spline models with 4 knots (5th, 35th, 65th, and 95th percentiles) and using the Greenland and Longnecker method (29) to estimate the covariances of multivariable-adjusted RRs. Lines with long dashes represent the pointwise 95% CIs for the fitted nonlinear trend (solid line). Lines with short dashes represent the linear trend.
Discussion
The findings from this meta-analysis of case-control and prospective cohort studies indicate that the risk of BC decreased by 42% for every 1 mg increase in daily dietary β-cryptoxanthin intake, by 76% for every 1 μmol/L increase in circulating concentrations of α-carotene, by 27% for every 1 μmol/L increase in circulating concentrations of β-carotene, and by 56% for every 1 μmol/L increase in circulating concentrations of lutein and zeaxanthin. Dietary total carotenoid intake was associated with a 15% reduced risk of BC in men. Circulating concentrations of lutein and zeaxanthin was associated with a 47% reduced risk of BC.
In our analysis comparing the highest with the lowest dietary total carotenoid intake, the largest study gave a significant larger RR estimate (23), but specific reasons why the RR from that study was higher are not clear. Therefore, we conducted a sensitivity analysis to estimate the overall RR after excluding this study. The findings showed that the overall risk estimate was statistically significant after the exclusion of this study (15% decreased risk when comparing high with low intake). The study with a larger sample size gave the largest RR and also affected the overall RR; after excluding the study (24), the inverse association was statistically significant (15% decreased risk when comparing high with low intake). After excluding both studies, the inverse association was statistically significant (19% decreased risk when comparing high with low intake). In addition, a subgroup analysis showed that the inverse association was statistically significant in men (0.85; 0.72, 0.99) in case-control studies (0.77; 0.63, 0.95) and in studies that met more quality criteria (≥8 stars; RR = 0.81; 95% CI: 0.68–0.95). Our analysis also suggested that the order of effect on the risk of BC might be α-carotene >lutein and zeaxanthin >β-cryptoxanthin >β-carotene, but we did not find significant results for lycopene in our meta-analysis. Except for total dietary carotenoid intake and the subgroup analysis for men, all other analyses showed no evidence of publication bias. However, the trimmed-and-filled adjusted RR was the same as the original result for the 2 analyses, indicating that the publication bias may not affect the findings from our meta-analysis.
Thus far, biological mechanisms to explain the chemopreventive actions of carotenoids are still being explored. The antimutagenic effects of carotenoids and solvent extracts from fruits and vegetables rich in carotenoids have been reported (50). α-Carotene, β-carotene, lutein, and lycopene increase cellular gap junctional communication, which would improve homeostasis and cell-cell communication, restrict the clonal expansion of initiated cells, and be associated with a reversion of the malignant process (51, 52). Many carotenoids act as antioxidants by decreasing free radicals and reactive oxygen species (51, 53, 54). β-Carotene has been shown to inhibit the early as well as later phases of carcinogenesis and prevent cancer progression by affecting cellular differentiation and proliferation and improving the immune response (53, 55). Evidence shows that in cultured cells, β-carotene (56), lycopene (57), and lutein (58) are able to induce apoptosis which protects against carcinogenesis, tumorigenesis, and cancer (59). Various carotenoids, such as β-carotene and lycopene, have been found to exert efficient protection against aflatoxin B1 mutagenicity, carcinogenicity, and genotoxicity in rats, due to the apparent induction of detoxifying enzymes (60). It has been shown that α-carotene prevents lipid peroxidation, inhibiting the formation and uptake of carcinogens (61). β-Cryptoxanthin exhibited chemopreventive properties by quenching singlet oxygen (62), exhibiting inhibitory activity (63), and stimulating the expression of RB, an antioncogene, and p73 genes (64).
The present study has some strengths. First, our quantitative assessment was based on all available observational studies using a relatively exhaustive search strategy. Second, we performed quantitative syntheses and assessed the quality and strength of the evidence by using the GRADE assessment. Third, all included studies had adjusted for cigarette smoking status, an important risk factor of BC. Fourth, we collected the available studies of the doses and effects of carotenoid intake or circulating concentrations of carotenoids at different strata on BC risk; thus, the dose-response relation was explored comprehensively for the first time. Finally, we were able to conduct subgroup analysis across individual studies due to the large number of included studies.
Our study also has several limitations. First, despite the inclusion of large, high-quality cohorts and case-control studies, the inability to rule out residual confounding is a limitation inherent in all observational studies and a reason that observational studies start at low quality by GRADE. Inadequate control for confounders may bias the results in either direction, toward overestimation or underestimation of risk estimates. Although most studies adjusted for other known risk factors for BC, residual or unknown confounding cannot be excluded as a potential explanation for the observed findings. A second limitation is that our results are likely to be affected by some degree of misclassification of exposure, which can lead to an inaccurate RR estimate. The inverse associations between circulating total carotenoid concentration and BC were statistically significant but not significant for those between carotenoid intake and BC. This may be because of measurement errors in the assessment of carotenoid intake, leading to an attenuation of the observed association between carotenoid intake and BC risk. Third, heterogeneity may be introduced because of methodological differences among studies, including different ranges of exposure. Finally, in a meta-analysis of published studies, publication bias could be of concern because small studies with null results tend not to be published. The publication bias did not completly exclude for total dietary carotenoid intake and the subgroup analysis for men due to the inconsistency between different tests for publication bias.
Conclusions
In summary, findings from this meta-analysis of case-control and prospective cohort studies indicate that daily dietary β-cryptoxanthin intake is inversely associated with risk of BC, and total dietary carotenoid intake is inversely associated with BC risk in men. Circulating concentrations of α-carotene, β-carotene, and lutein and zeaxanthin are inversely associated with the risk of BC. The findings from these observational studies need to be confirmed in large randomized clinical trials of carotenoid supplementation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—SW: contributed to the conception, design of the systematic review and meta-analysis, writing, and the final content; SW and YL: contributed to the acquisition and analysis of the data; and all authors: interpreted the results, drafted the manuscript, provided critical revisions of the meta-analysis, and read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
References
- 1. Landrum JT. Carotenoids: physical, chemical, and biological functions and properties. Boca Raton (FL): CRC Press; 2010. [Google Scholar]
- 2. Paiva SA, Russell RM.. Beta-carotene and other carotenoids as antioxidants. J Am Coll Nutr. 1999;18:426–33. [DOI] [PubMed] [Google Scholar]
- 3. Goldbohm RA, Brants HA, Hulshof KF, van den Brandt PA. The contribution of various foods to intake of vitamin A and carotenoids in The Netherlands. Int J Vitam Nutr Res. 1998;68:378–83. [PubMed] [Google Scholar]
- 4. Mangels AR, Holden JM, Beecher GR, Forman MR, Lanza E. Carotenoid content of fruits and vegetables: an evaluation of analytic data. J Am Diet Assoc. 1993;93:284–96. [DOI] [PubMed] [Google Scholar]
- 5. Duthie SJ, Ma A, Ross MA, Collins AR. Antioxidant supplementation decreases oxidative DNA damage in human lymphocytes. Cancer Res. 1996;56:1291–5. [PubMed] [Google Scholar]
- 6. Rauscher R, Edenharder R, Platt KL. In vitro antimutagenic and in vivo anticlastogenic effects of carotenoids and solvent extracts from fruits and vegetables rich in carotenoids. Mutat Res. 1998;413:129–42. [DOI] [PubMed] [Google Scholar]
- 7. Toma S, Albanese E, Palumbo R, Nicolo G, Morerio C, Magiante PE, Bonatti S, Cancedda R, Santi L. In vitro effects of beta-carotene on human oral keratinocytes from precancerous lesions and squamous carcinoma. Anticancer Drugs. 1991;2:581–9. [DOI] [PubMed] [Google Scholar]
- 8. Collins AR. Carotenoids and genomic stability. Mutat Res. 2001;475:21–8. [DOI] [PubMed] [Google Scholar]
- 9. Schabath MB, Grossman HB, Delclos GL, Hernandez LM, Day RS, Davis BR, Lerner SP, Spitz MR, Wu X. Dietary carotenoids and genetic instability modify bladder cancer risk. J Nutr. 2004;134:3362–9. [DOI] [PubMed] [Google Scholar]
- 10. Tang JE, Wang RJ, Zhong H, Yu B, Chen Y. Vitamin A and risk of bladder cancer: a meta-analysis of epidemiological studies. World J Surg Oncol. 2014;12:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 13. Wells GA, Shea B, O'Connell D. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Internet]. Ottawa (Canada): Dept of Epidemiology and Community Medicine, University of Ottawa; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Updated 2018. Accessed 2018 Nov 6. [Google Scholar]
- 14. Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group [Internet]. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Available from: https://gdt.gradepro.org/app/handbook/handbook.html#h.svwngs6pm0f2. Updated October 2013. Accessed 2019 Aug 29. [Google Scholar]
- 15. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 16. Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess. 2003;7:1–76. [PubMed] [Google Scholar]
- 17. Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2:121–45. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vena JE, Graham S, Freudenheim J, Marshall J, Zielezny M, Swanson M, Sufrin G. Diet in the epidemiology of bladder cancer in western New York. Nutr Cancer. 1992;18:255–64. [DOI] [PubMed] [Google Scholar]
- 20. Garcia R, Gonzalez CA, Agudo A, Riboli E. High intake of specific carotenoids and flavonoids does not reduce the risk of bladder cancer. Nutr Cancer. 1999;35:212–4. [DOI] [PubMed] [Google Scholar]
- 21. Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci EL. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst. 1999;91:605–13. [DOI] [PubMed] [Google Scholar]
- 22. Zeegers MP, Goldbohm RA, van den Brandt PA. Are retinol, vitamin C, vitamin E, folate and carotenoids intake associated with bladder cancer risk? Results from The Netherlands Cohort Study. Br J Cancer. 2001;85:977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Michaud DS, Pietinen P, Taylor PR, Virtanen M, Virtamo J, Albanes D. Intakes of fruits and vegetables, carotenoids and vitamins A, E, C in relation to the risk of bladder cancer in the ATBC cohort study. Br J Cancer. 2002;87:960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holick CN, De Vivo I, Feskanich D, Giovannucci E, Stampfer M, Michaud DS. Intake of fruits and vegetables, carotenoids, folate, and vitamins A, C, E and risk of bladder cancer among women (United States). Cancer Causes Control. 2005;16:1135–45. [DOI] [PubMed] [Google Scholar]
- 25. Brinkman MT, Karagas MR, Zens MS, Schned A, Reulen RC, Zeegers MP. Minerals and vitamins and the risk of bladder cancer: results from the New Hampshire Study. Cancer Causes Control. 2010;21:609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park SY, Ollberding NJ, Woolcott CG, Wilkens LR, Henderson BE, Kolonel LN. Fruit and vegetable intakes are associated with lower risk of bladder cancer among women in the Multiethnic Cohort Study. J Nutr. 2013;143:1283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hung RJ, Zhang ZF, Rao JY, Pantuck A, Reuter VE, Heber D, Lu QY. Protective effects of plasma carotenoids on the risk of bladder cancer. J Urol. 2006;176:1192–7. [DOI] [PubMed] [Google Scholar]
- 28. Deeks JJ, Altman DG. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, eds. Systematic reviews in health care: meta-analysis in context. 2nd ed London: BMJ Publication Group; 2001. pp. 285–312. [Google Scholar]
- 29. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- 30. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4:218–28. [DOI] [PubMed] [Google Scholar]
- 32. Begg CB, Mazumdar M.. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 33. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research ed). 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 35. Risch HA, Burch JD, Miller AB, Hill GB, Steele R, Howe GR. Dietary factors and the incidence of cancer of the urinary bladder. Am J Epidemiol. 1988;127:1179–91. [DOI] [PubMed] [Google Scholar]
- 36. Shibata A, Paganini-Hill A, Ross RK, Henderson BE. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: a prospective study. Br J Cancer. 1992;66:673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bruemmer B, White E, Vaughan TL, Cheney CL. Nutrient intake in relation to bladder cancer among middle-aged men and women. Am J Epidemiol. 1996;144:485–95. [DOI] [PubMed] [Google Scholar]
- 38. Wakai K, Takashi M, Okamura K, Yuba H, Suzuki K, Murase T, Obata K, Itoh H, Kato T, Kobayashi M et al.. Foods and nutrients in relation to bladder cancer risk: a case-control study in Aichi Prefecture, Central Japan. Nutr Cancer. 2000;38:13–22. [DOI] [PubMed] [Google Scholar]
- 39. Castelao JE, Yuan JM, Gago-Dominguez M, Skipper PL, Tannenbaum SR, Chan KK, Watson MA, Bell DA, Coetzee GA, Ross RK et al.. Carotenoids/vitamin C and smoking-related bladder cancer. Int J Cancer. 2004;110:417–23. [DOI] [PubMed] [Google Scholar]
- 40. Schabath MB, Grossman HB, Delclos GL, Hernandez LM, Day RS, Davis BR, Lerner SP, Spitz MR, Wu X. Dietary carotenoids and genetic instability modify bladder cancer risk. J Nutr. 2004;134:3362–9. [DOI] [PubMed] [Google Scholar]
- 41. Garcia-Closas R, Garcia-Closas M, Kogevinas M, Malats N, Silverman D, Serra C, Tardon A, Carrato A, Castano-Vinyals G, Dosemeci M et al.. Food, nutrient and heterocyclic amine intake and the risk of bladder cancer. Eur J Cancer. 2007;43:1731–40. [DOI] [PubMed] [Google Scholar]
- 42. Roswall N, Olsen A, Christensen J, Dragsted LO, Overvad K, Tjonneland A. Micronutrient intake and risk of urothelial carcinoma in a prospective Danish cohort. Eur Urol. 2009;56:764–70. [DOI] [PubMed] [Google Scholar]
- 43. Wu JW, Cross AJ, Baris D, Ward MH, Karagas MR, Johnson A, Schwenn M, Cherala S, Colt JS, Cantor KP et al.. Dietary intake of meat, fruits, vegetables, and selective micronutrients and risk of bladder cancer in the New England region of the United States. Br J Cancer. 2012;106:1891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Helzlsouer KJ, Comstock GW, Morris JS. Selenium, lycopene, alpha-tocopherol, beta-carotene, retinol, and subsequent bladder cancer. Cancer Res. 1989;49:6144–8. [PubMed] [Google Scholar]
- 45. Nomura AM, Lee J, Stemmermann GN, Franke AA. Serum vitamins and the subsequent risk of bladder cancer. J Urol. 2003;170:1146–50. [DOI] [PubMed] [Google Scholar]
- 46. Ros MM, Bueno-de-Mesquita HB, Kampman E, Aben KK, Buchner FL, Jansen EH, van Gils CH, Egevad L, Overvad K, Tjonneland A et al.. Plasma carotenoids and vitamin C concentrations and risk of urothelial cell carcinoma in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2012;96:902–10. [DOI] [PubMed] [Google Scholar]
- 47. Hotaling JM, Wright JL, Pocobelli G, Bhatti P, Porter MP, White E. Long-term use of supplemental vitamins and minerals does not reduce the risk of urothelial cell carcinoma of the bladder in the VITamins And Lifestyle study. J Urol. 2011;185:1210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Al-Zalabani AH, Stewart KF, Wesselius A, Schols AM, Zeegers MP. Modifiable risk factors for the prevention of bladder cancer: a systematic review of meta-analyses. Eur J Epidemiol. 2016;31:811–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Higgins JP, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 50. Rauscher R, Edenharder R, Platt KL. In vitro antimutagenic and in vivo anticlastogenic effects of carotenoids and solvent extracts from fruits and vegetables rich in carotenoids. Mutat Res. 1998;413:129–42. [DOI] [PubMed] [Google Scholar]
- 51. Schalch W. Carotenoids in the retina–a review of their possible role in preventing or limiting damage caused by light and oxygen. EXS. 1992;62:280–98. [DOI] [PubMed] [Google Scholar]
- 52. Zhang LX, Cooney RV, Bertram JS. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/2 cells: relationship to their cancer chemopreventive action. Carcinogenesis. 1991;12:2109–14. [DOI] [PubMed] [Google Scholar]
- 53. Gerster H. Anticarcinogenic effect of common carotenoids. Int J Vitam Nutr Res. 1993;63:93–121. [PubMed] [Google Scholar]
- 54. Burton GW, Ingold KU.. beta-Carotene: an unusual type of lipid antioxidant. Science. 1984;224:569–73. [DOI] [PubMed] [Google Scholar]
- 55. Teicher BA, Schwartz JL, Holden SA, Ara G, Northey D. In vivo modulation of several anticancer agents by beta-carotene. Cancer Chemother Pharmacol. 1994;34:235–41. [DOI] [PubMed] [Google Scholar]
- 56. Palozza P, Serini S, Torsello A, Di NF, Maggiano N, Ranelletti FO, Wolf FI, Calviello G. Mechanism of activation of caspase cascade during beta-carotene-induced apoptosis in human tumor cells. Nutr Cancer. 2003;47:76–87. [DOI] [PubMed] [Google Scholar]
- 57. Nara E, Hayashi H, Kotake M, Miyashita K, Nagao A. Acyclic carotenoids and their oxidation mixtures inhibit the growth of HL-60 human promyelocytic leukemia cells. Nutr Cancer. 2001;39:273–83. [DOI] [PubMed] [Google Scholar]
- 58. Sumantran VN, Zhang R, Lee DS, Wicha MS. Differential regulation of apoptosis in normal versus transformed mammary epithelium by lutein and retinoic acid. Cancer Epidemiol Biomarkers Prev. 2000;9:257–63. [PubMed] [Google Scholar]
- 59. Lowe SW, Lin AW.. Apoptosis in cancer. Carcinogenesis. 2000;21:485–95. [DOI] [PubMed] [Google Scholar]
- 60. Gradelet S, Le Bon AM, Berges R, Suschetet M, Astorg P. Dietary carotenoids inhibit aflatoxin B1-induced liver preneoplastic foci and DNA damage in the rat: role of the modulation of aflatoxin B1 metabolism. Carcinogenesis. 1998;19:403–11. [DOI] [PubMed] [Google Scholar]
- 61. Murakoshi M, Nishino H, Satomi Y, Takayasu J, Hasegawa T, Tokuda H, Iwashima A, Okuzumi J, Okabe H, Kitano H et al.. Potent preventive action of alpha-carotene against carcinogenesis: spontaneous liver carcinogenesis and promoting stage of lung and skin carcinogenesis in mice are suppressed more effectively by alpha-carotene than by beta-carotene. Cancer Res. 1992;52:6583–7. [PubMed] [Google Scholar]
- 62. Di MP, Devasagayam TP, Kaiser S, Sies H. Carotenoids, tocopherols and thiols as biological singlet molecular oxygen quenchers. Biochem Soc Trans. 1990;18:1054–6. [DOI] [PubMed] [Google Scholar]
- 63. Tsushima M, Maoka T, Katsuyama M, Kozuka M, Matsuno T, Tokuda H, Nishino H, Iwashima A. Inhibitory effect of natural carotenoids on Epstein-Barr virus activation activity of a tumor promoter in Raji cells. A screening study for anti-tumor promoters. Biol Pharm Bull. 1995;18:227–33. [DOI] [PubMed] [Google Scholar]
- 64. Nishino H, Tokuda H, Murakoshi M, Satomi Y, Masuda M, Onozuka M, Yamaguchi S, Takayasu J, Tsuruta J, Okuda M et al.. Cancer prevention by natural carotenoids. Biofactors. 2000;13:89–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.