ABSTRACT
Exosomes are membrane-bound organelles generally secreted by eukaryotic cells that contain mRNAs, microRNAs, and/or proteins. However, recent studies have reported the isolation of these particles from foods such as lemon, ginger, and milk. Owing to their absorption by intestinal cells and further travel via the bloodstream, exosomes can reach distant organs and affect overall health in both infants and adults. The potential role of food-derived exosomes (FDEs) in alleviating diseases, as well as in modulating the gut microbiota has been shown, but the underlying mechanism is still unknown. Moreover, exosomes may provide biocompatible vehicles for the delivery of anti-cancer drugs, such as doxorubicin. Thus, exosomes may allow medical nutritionists and clinicians to develop safe and targeted therapies for the treatment of various pathologies. The present review introduces FDEs and their contents, highlights their role in disease and infant/adult health, and explores their potential use as therapeutic agents.
Keywords: exosome, food, cancer, inflammation, therapy
Introduction
The effect of food, particularly its bioactive components, on human health and disease has been extensively studied over the past few decades (1–3). Exosomes, also known as extracellular vesicles (EVs), are a type of bioactive component recently discovered in foods. Exosomes are small (50–300 nm), membrane-bound organelles secreted by the endosomal pathway of eukaryotic cells that facilitate intercellular communication (4). Their lumens contain mRNA, proteins, microRNAs (miRNAs), and long non-coding RNAs (5). Evidence suggests that mRNAs in target cells can translate into functional proteins (6). Additionally, miRNAs, proteins, and metabolites retain their biological activity in the recipient cells. Proteins and RNAs delivered by exosomes can affect the properties of recipient cells, thereby influencing diverse physiological and pathological functions. Exosomes from fruits, vegetables, and foods derived from animal sources have been successfully isolated, for example, exosomes derived from lemon have a diameter of ≤70 nm and are morphologically similar to mammalian exosomes (7). Food-derived exosomes (FDEs) can be absorbed in the intestine to act locally. Evidence has also indicated that FDEs may be delivered to other organs via blood flow and function distantly in the recipient cells. Although our understanding of the mechanistic details of selective packaging of FDEs and unloading of their cargo, along with the interaction with target cells is incomplete, these molecules have started to gain attention due to their potential roles in controlling physiological and pathological processes and as therapeutic vehicles. Here, we introduce FDEs and summarize the roles of FDEs in human health and disease, and envision their future applications. As FDEs have not been clearly categorized based on their intrinsic features, studies on FDEs often use synonyms such as “nanoparticles,” “exosome-like nanoparticles,” “nanovesicles,” and “nanoshuttle.” In this review, we use the term “food-derived exosomes (FDEs)” to accommodate all the terms mentioned in previous studies, emphasizing that intensive characterization of FDEs would allow their versatile applications.
FDEs
FDEs are a class of EVs found in food, which carry biomolecules for cell-to-cell communication. These small vesicles (50–300 nm) are surrounded by a phospholipid bilayer and are formed in multivesicular bodies in the cells, as intraluminal vesicles (ILVs). The fusion of a multivesicular body with the plasma membrane leads to the secretion of the ILVs to the extracellular environment, therefore becoming “exosomes” (8, 9).
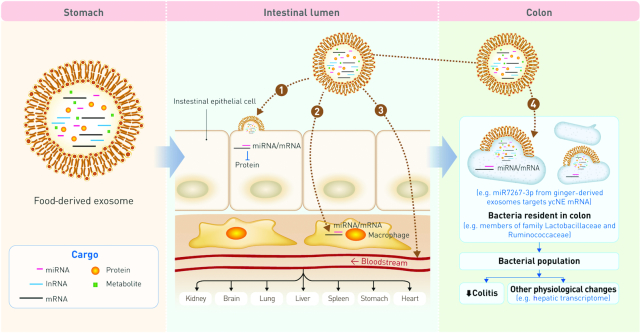
FDEs are exposed to the environment of the digestive tract after food intake. However, the phospholipid bilayer surrounding the FDE protects it from the harsh conditions of the digestive tract, including the acidic environment of the stomach (10) (Table 1). Therefore, bioactive substances (miRNA, mRNA, metabolites etc.) in the lumen of the FDE are not accessible to the degradative enzymes of the gut, ensuring their stability. For instance, curcumin encapsulated in exosomes is 4 times more stable than free curcumin (11); therefore, stable FDEs are efficiently absorbed into the intestinal cells. The endocytosis of FDEs has been observed in both Caco-2 cells (human intestinal epithelial cell line) and CT26 cells (mouse intestinal epithelial cell line). Studies using fluorescence microscopy and flow cytometry have also demonstrated that FDEs can be taken up by intestinal macrophages in the mouse model (12) and by in vitro cultures of human macrophages (13). The FDEs absorbed in the intestine can affect the cellular properties locally, but FDEs also reach distal organs via blood circulation, where they function in host tissues and influence the systemic condition of the whole body. To enter the bloodstream, FDEs must cross the endothelial cell barrier of blood vessels. Studies in human umbilical cord vein endothelial cells (HUVEC), which is an established cell line model of blood vessels, showed that FDEs can be endocytosed by these cells. Moreover, a series of independent experiments described the accumulation of FDEs in several organs including the liver, spleen, brain, intestine, stomach, and lungs (Figure 1).
TABLE 1.
Models of bioavailability and bioactivity of food-derived exosomes
| Method/model | Source | Size (nm) |
|---|---|---|
| Human vascular endothelial cells (HUVEC) (14) | Cow milk | 69 |
| TNO GI1 in vitro model (15) | Cow milk | 200 |
| RAW 264.7 cell line (12) | Ginger, carrot, grape, grapefruit | 700 |
| PKH26-labeled FDE administered in mice by gavage (16) | Ginger | 102.3–998.3 |
| Caco-2 (human intestinal epithelial cell model) (17) | Milk | 30–120 |
| HEK-293 cells (18) | Milk | − |
| Peripheral blood mononuclear cells (18) | Milk | − |
| CT26 mouse intestinal epithelial cell line (19) | Grapes | 380.5 |
| SW480 (colon cancer cell line) (7) | Citrus limon | 50–70 |
| A549 (lung cancer cell line) (7) | Citrus limon | 50–70 |
| LAMA84 (peripheral blood bone marrow cell line) (7) | Citrus limon | 50–70 |
| In vivo tumor xenograft (7) | Citrus limon | 50–70 |
| Mouse model (20) | Broccoli | 18.3–118.2 |
| Human feeding study (20) | Broccoli | 18.3–118.2 |
| Retro-orbital injection of DiR2 -labeled exosomes in mice (14) | Cow milk | 69 |
TNO GI, TNO gastrointestinal model is a computer-simulated multi-compartment system to mimic various regions in the gut.
DiR, dioctadecyl tetramethylindotricarbocyanine iodide is a fluorescent lipophilic dye.
FIGURE 1.
Possible fates of food-derived exosomes (FDEs) and their physiological effects on health and disease. Investigations involving FDEs demonstrate that they can empty their contents, particularly miRNA, in intestinal epithelial cells (1) and intestinal macrophages (2). Moreover, they can travel via the bloodstream to reach target organs e.g. lungs, liver (3). Bacteria in the large intestine can also take up FDEs, and directly or indirectly influence the host's health, such as reducing colitis (4). These findings suggest FDEs as potential candidates for designing nutritional therapies and carriers of synthetic/natural therapeutic agents against various pathological conditions.
Interestingly, FDEs not only transfer their original cargo to the recipient cells but also incorporate into the host delivery system by interacting with endogenous exosomes. Manca et al. (21) labeled milk exosomes with DiR (a fluorescent lipophilic dye) and the miRNAs inside them with ExoGlow. Oral administration of these exosomes in mice showed the accumulation of DiR-labeled exosomes in the liver, whereas miRNAs were accumulated in the brain, kidney, and heart (21, 14). These authors have hypothesized that miRNAs in FDEs may be unloaded into endogenous exosomes, which then carry the cargo to other organs. Moreover, endogenous exosomes may redirect the accumulation of FDEs from one organ to another. For instance, intravenous-injected grapefruit-derived vesicles were accumulated in the liver, but if endogenous exosomes (from peripheral blood) were injected before the injection of grapefruit-derived vesicles, the grapefruit-derived vesicles were accumulated in the lungs, suggesting an interaction between FDEs and endogenous exosomes (22). Further investigation may lead to organ-targeted therapies, particularly therapies for lung disorders.
Another interesting subject of FDE research is their presence in cooked or processed foods. Deep sequencing analyses of pan-fried and pasteurized sirloin, bovine heart, and adrenals have confirmed the presence of miRNA that can potentially target mRNAs in human cells (23). However, whether the stability of miRNAs in cooked or processed food is due to encapsulation by FDEs needs further investigation.
Cancer
The role of FDEs in cancer remains an emerging area of research (Table 2). The uptake of FDEs by cancer cells can modify gene expression and reduce cancer-related phenotypes. For instance, lemon-derived exosomes increase TNF-related apoptosis inducing ligand (TRAIL)–mediated apoptosis, which is associated with reduced angiogenic cytokine secretion (e.g. vascular endothelial growth factor-α, IL-6, and IL-8) (5). Moreover, these FDEs target phosphatidic acid-preferring phospholipase A1 and acetyl-CoA carboxylase α (24, 25), revealing the targetable deregulation for cancer therapies. However, which molecules within FDEs have anti-cancer effects has not been investigated. In breast cancer rat models, for example, camel milk-derived exosomes increased the activity of antioxidant enzymes, including catalase, superoxide dismutase, and glutathione peroxidase, in tumors. This, along with a decrease in inducible nitric oxide synthase expression led to a reduction in tumor growth and metastasis (26). Although the transcript and lipid entities in camel milk-derived exosomes are known (27), the component that induces expression changes in xenografted tumors remains unspecified. It is noteworthy that there is compelling contradictory evidence claiming long-term exposure to bovine milk exosomes as a risk factor for cancer, and is supported by epidemiological studies (28). According to Banikezemi et al. [reviewed in (29)], milk exosomes have also been indirectly associated with the progression of hepatocellular carcinoma and melanoma by targeting miR-155, miR-21, and transforming growth factor-β (TGF-β). Given this discrepancy, in-depth investigations on which specific molecule within milk exosomes leads to cellular and molecular changes are yet to be performed. Further investigation is therefore needed to characterize the intrinsic features of bovine milk exosomes and their safety before using these FDEs in therapies or as drug carriers.
TABLE 2.
Therapeutic potential of food-derived exosomes in treating diseases1
| Origin of FDEs | Drug | Route of administration | Therapeutic potential | Experimental validation |
|---|---|---|---|---|
| Ginger (34) | Doxorubicin | Intravenous | Colon cancer | Colon-26 xenograft |
| Camel milk (26) | N.A. | Media | Breast cancer | MCF-7 |
| Bovine milk (30) | Berry anthocyanidins | Media | Ovarian cancer | OVCA432 |
| Oral gavage | Lung cancer | Mice xenograft | ||
| Media | Breast cancer | MDA-MB-231, MCF-7 | ||
| Media | Pancreatic cancer | PANC1, Mia PaCa2 | ||
| Media | Prostate cancer | PC3, DU145 | ||
| Milk (36) | Paclitaxel | Oral | Lung cancer | Lung xenografts |
| Grapefruit (37) | miR-17 | Nasal | Brain tumor | Mice with GL-26 grafts |
| Ginger (34) | siRNA | Intravenous | Oral cancer | Mice xenograft (KB2cells) |
| Grape (38) | N.A. | Oral | Head and neck cancer oral mucositis | Humans (phase I) |
| Garlic (39) | N.A. | Media | Inflammatory disorders | Mouse macrophages |
| Ginger (40) | siRNA | Oral | Ulcerative colitis, inflammatory bowel disease | FVB3mice |
| Ginseng (41) | N.A. | Topical, oral | Hair growth | Hair follicle organ culture |
| Milk (42) | Curcumin | Media | Anti-cancer drug delivery | Caco-2 cells |
| Grapefruit (33) | Stat3 inhibitor JSI-124 | Intranasal | Glioblastoma | GL-26 implanted C57BL/6J |
| N.A. | Intravenous | Colon | CT26 implanted BALB4/c mice | |
| SW-620 implanted NOD/SCID5 mice | ||||
| Citrus limon (7) | N.A. | Intravenous | CML | NOD/SCID mouse xenograft |
| Grape (43) | N.A. | Oral | Colorectal cancer | Sprague-Dawley rats |
| Lavendar (39) | N.A. | Media | Inflammatory disorders | Mouse macrophages |
| Bovine milk (44) | N.A. | Transdermal | Multiple sclerosis | ? (only hypothesis) |
| Fruit (not specified) (45) | Curcumin | Oral | Colon cancer | Humans (phase I) |
| Breast milk (46) | N.A. | Oral | Lysinuric protein intolerance | Suggestion (Letter to the Editor) |
| Vegetables (not specified) (47) | N.A. | Oral | Kashin-Beck disease | — |
| Apple (48) | N.A. | Media | — | Caco-2 and HEK293 cells |
| Bovine milk (49) | N.A. | Oral gavage | Arthritis | Mouse model |
| Ginger rhizome (39) | N.A. | Media | Inflammatory disorders | Macrophages |
| Bovine milk (50) | N.A. | Oral gavage | Necrotizing enterocolitis | Mouse necrotizing enterocolitis model |
Throughout this table, N.A. stands for not applicable.
KB stands for Keratin forming tumor cell line.
FVB stands for Friend leukemia virus-B susceptible albino mouse strain.
BALB stands for Bagg Albino (inbred mouse strain).
NOD/SCID is a mouse-strain homozygous for combined immune deficiency spontaneous mutation Prkdcscid and lack B- and T-lymphocytes.
In light of evidence showing the therapeutic efficacy of FDEs, clinical trials with FDEs have been performed. Exosomes derived from grape berries demonstrated the alleviation of oral mucositis during chemoradiation of head and neck cancer (NCT01668849) (24). FDEs also provide a solution for the transport of anti-cancer compounds as they overcome the limitations of bioavailability, stability, and safety posed by synthetic liposomes and exosomes from non-food sources. Exploiting this, milk exosomes have been utilized to encapsulate anti-cancer anthocyanins or paclitaxel (30, 31, 32). Lung cancer xenografts have shown a significant reduction in growth upon oral administration of anthocyanin- (30) or paclitaxel-loaded milk exosomes (32). Additionally, these exosomes changed ovarian cancer from cisplatin-resistant to cisplatin-sensitive by decreasing the expression of P-glycoprotein (31). The reassembly of grapefruit-derived exosomes carrying a STAT-3 inhibitor, JSI-124, reduced glioblastoma growth in the mouse brain (33). Doxorubicin or Survivin small interfering RNA (siRNA) has been incorporated into reassembled ginger-derived exosomes containing folic acid for targeting colon tumors and papilloma, reducing their volume via increased apoptosis and decreasing their proliferation in mice (34, 35).
In addition to direct empirical evidence, some studies proposed the potential involvement of FDEs in the anti-cancer activity of miRNAs. These studies reported some secreted miRNAs from plants with functions related to cancer. Given that secreted miRNAs are mostly preserved in exosomes, the miRNAs secreted from plant cells may also act through FDEs. The oral administration of plant miR-159 to mice reduced the proliferation of breast cancer xenografts by targeting human T cell factors (51). Despite the presence of exosome-encapsulated plant miR-159 in human sera and its negative correlation with the progression of breast cancer, the food source for miR-159 has not been found. Next-generation sequencing of small RNAs from coconut water also demonstrated the presence of miRNAs that target human genes involved in cancer-related pathways. The same study also successfully isolated and characterized the coconut-water-derived exosomes through dynamic light scattering and fluorescence staining with the phospholipid dye “Dil,” indicating that these FDEs were 100–200 nm in diameter and surrounded by a phospholipid bilayer, similar to mammalian exosomes. However, how many miRNAs in coconut water are associated with exosomes remain unknown (52). Thus, the comprehensive and systemic analysis of biomolecules in FDEs from various sources needs to be performed in the near future.
Inflammation
In addition to cancer, FDEs also affect inflammatory responses, particularly in the intestines. For example, unidentified molecules in grape berry-derived exosomes promote the proliferation of Lgr5+ stem cells in intestinal crypts via Wnt signaling activation, followed by a delay in inflammatory colitis in dextran sulfate sodium (DSS)-induced colitis murine models (43). Sulforaphane in broccoli-derived exosomes also reduces intestinal inflammation in colitis models, and broccoli-derived exosomes activate adenosine monophosphate protein kinase (AMPK) phosphorylation in CD11c+ dendritic cells (20).
From a clinical point of view, ginger-derived exosomes with bioactive gingerol and shogaol could be produced commercially on a large scale. These FDEs are well absorbed by the intestinal mucosa, causing a decrease in proinflammatory cytokines IL-1β and IL-6, and an increase in anti-inflammatory cytokine IL-10. Their efficacy has been experimentally demonstrated in mouse inflammatory bowel disease models and may provide a way forward for the treatment of Crohn's disease and ulcerative colitis in humans (53). Furthermore, the production and reassembly of lipids from ginger-derived exosomes are able to provide CD98-targeted siRNA-loaded ginger nanovesicles. The use of this siRNA carrier requires a dose 10,000 times lower than that of naked siRNA and is less toxic than the commercial “DC-Chol/DOPE” liposome formulation used for treating intestinal inflammation (43). Moreover, Chen et al. reported that the lipid components of ginger-derived exosomes alone inhibit inflammasome formation and assembly (39). Since inflammasome assembly is an essential characteristic of disorders such as diabetes mellitus 2 (54) and Alzheimer's disease (55), ginger-derived exosomes could be useful therapies against these disorders (Table 2).
Intestinal macrophages have been shown to take up FDEs from grapefruit, inhibiting the expression of proinflammatory cytokines such as TNF-α and IL-1β. These grapefruit-derived exosomes can be successfully loaded with methotrexate to delay inflammation in a DSS-induced colitis murine model (33). Human macrophages can also take up bovine milk exosomal miRNAs; however, the targets of these exosomal miRNAs warrant further investigation (56). In addition, shogaols in ginger-derived exosomes have been reported to prevent liver damage caused by alcohol. These FDEs activate Nrf2 via the Toll-like receptor/TIR-domain-containing adaptor-inducing interferon-β (TLR/TRIF) pathway to induce the upregulation of detoxifying enzymes in the liver for protection against reactive oxygen species. These results suggest a medical advantage, as no side effects have been reported (16).
Interestingly, exosomal miRNAs from ginger, grapefruit, and curcumin are preferentially taken up by specific bacterial species in the gut, depending upon their lipid composition. miR-7267 from ginger-derived exosomes was reported to regulate the mRNA and protein expression of ycNE in Lactobacillus rhamnosus. Since metabolites secreted by bacteria may influence other bacterial populations and ultimately the entire gut microbiome, the effects of FDEs on specific bacterial species could subsequently lead to the modulation of the inflammatory response in colitis. The microbiome of human subjects also changes after intake of ginger-derived exosomes (57). However, the correlations between bacterial population changes and physiological effects need to be further investigated before the clinical application of FDEs.
Nervous System and Musculoskeletal Disorders
Along with expanding research on the roles of FDEs in cancer and inflammation, researchers are now studying the effects of FDEs on disorders related to the nervous and musculoskeletal systems. Mutai et al. (58) found that the depletion of exosomes in a milk diet decreased spatial learning and memory by 130%. It also significantly decreased the acoustic startle response in female mice, establishing an association between milk exosomes and neurocognitive performance. These findings are consistent with that of a study reporting the accumulation of milk exosomal miRNAs, such as miR-34-a, in the mouse brain, suggesting that FDEs mediate the transfer of genetic regulators (21). Multiple sclerosis (MS) is a nervous system disorder in which the myelin-oligodendrocyte glycoprotein (MOG) in the neuronal myelin sheath deteriorates, causing nerve impulse abnormalities. Treatment of MS using bovine milk exosomes has been proposed from studies involving murine MS models. The surfaces of bovine milk exosomes contain the protein butyrophilin which shares 50% sequence homology with MOG. Due to this similarity, butyrophilin can be used to induce antigen-specific tolerance (44); however, the current lack of empirical evidence presents a new direction for further investigation.
Similarly, in murine models of rheumatoid arthritis, it has been demonstrated that the oral intake of bovine milk exosomes containing miR-30-a, miR-223, miR-92-a, β-casein and β-lactoglobulin mRNAs may delay the onset of arthritis (49). This delay was supported by histological evidence of reduced joint inflammation and cartilage deterioration, as well as reduced serum molecular profiles of IL-6 and monocyte chemotactic protein-1 (49). In this regard, the oral intake of milk-derived exosomes by healthy mice led to reduced osteoclastic activity (bone resorption) and an increased number of osteocytes in the bone intertrabecular area (59, 60). These findings need to be further investigated in bone-related disease models such as Osteogenesis Imperfecta and Paget's disease.
In in vitro murine models of C2C12 myoblasts, bovine whey protein exosomes encapsulating miR-30b, miR-149, miR-2881, miR-214, let-7, miR-6520, and miR-16b led to enhanced muscle protein synthesis and increased the diameter of the muscle fiber by modulating the expression of eIF4A, p-Akt (Ser473), and p-AMPK (Thr172) (61). These results may enable the discovery of a clinical solution to alleviate the symptoms of muscular dystrophy and other muscle disorders. Mouse models lacking bovine milk exosomes in their diet showed a decrease in the expression of Rhobtb1 and Socs2 genes in skeletal muscles compared with that of mice fed an exosome-rich diet (62). Although Rhobtb1 is involved in Golgi body maintenance in breast cancer cells (63), Socs2 regulates muscle size via growth hormone signaling (64). Therefore, bovine milk exosomes might be used as supplement additives for improving muscle health and growth. However, FDEs do not accumulate directly in the skeletal muscles, and therefore the FDE-induced gene-expression changes observed in the skeletal muscle may be attributed to cross-communication between this tissue and other organs. Accumulating evidence on the various effects of FDEs on the nervous system and musculoskeletal abnormalities would promote the improvement of therapeutic strategies and trigger clinical trials.
Infant Health and Disease
In addition to growing knowledge on the roles of FDEs in adult disorders, researchers are also exploring their roles in the health and pathological conditions of infants (Table 3). Infants mainly depend on breast milk, especially for the first 6 months (65). Infant formula and colostrum powder based on bovine milk or soy milk are also fed to a large population of infants due to insufficient milk secretion in mothers of preterm infants (66) or other medical or personal reasons (67). Exosomes are found in human breast milk as well as bovine milk and colostrum powder (68). These exosomes resist degradation in the stomach and intestine of preterm and full-term neonates, suggesting a bioactive role of exosomes in ensuring infant health (10). Several studies have revealed the effects of exosomes derived from breast milk on the immune function of infants. The analysis of breast milk-derived exosomes showed that the molecules they contain varies depending upon the maternal allergy status. For example, a lower concentration of mucin-1 was observed in the milk-derived exosomes of sensitized (allergy-induced) mothers compared with the non-sensitized mothers (69). The study also claimed that infants fed breast milk from allergic individuals with a higher concentration of human leukocyte antigen-ABC (HLA-ABC) were likely to develop allergies later, suggesting that allergy-related traits can be transferred from mother to infant via breast milk exosomes. These results further proposed a screening strategy for milk-derived exosomes to prevent allergy development in infants (63). Moreover, human colostrum-derived exosomes also caused an increase in the number of FOXP3 + CD4 + CD25 + T regulatory cells and greater IL-5 secretion in peripheral blood mononuclear cell culture (70). Bovine milk exosomes have an indirect effect on the gut microbiome and the changes observed in the gut microbiome have been correlated with transcriptomic changes of 69 genes in the liver (71). This study may have implications for treating hepatic disorders in infants. Moreover, independent studies have indicated that the same FDEs caused changes in amino acid metabolism and modulated the expression of hepatic amino acid transporters BCAAT1 and BCAAT2 (72). Further studies may provide a solution for low-birth-weight babies and protein accretion in muscles. In an infant animal model, rat milk exosomes could prevent the commonly occurring newborn disease “necrotizing enterocolitis” by increasing proliferating cell nuclear antigen expression and Lgr5+ activity to ensure the proliferation of intestinal epithelial cells (73). However, in these studies, the acting compound in the exosome was not identified.
TABLE 3.
Significance of milk-derived exosomes in infant health and disease1
| Source of milk/recipient | Cargo | Cited significance |
|---|---|---|
| Bovine/cell (74) | mRNA (αs1-casein, αs2-casein, β-casein, κ-casein, β-lactoglobulin) mi-RNA (miR-101, miR-125b, miR-150, miR-223, miR-24–1, miR-93) | Development of gastrointestinal health and immunity |
| Rat/rat intestinal cell (75) | — | Understanding of infant intestinal health |
| Bovine and human colostrum/N.A. (76) | 920 kinds of protein (22% genes for stimuli response) | Useful for infant formulations and other dairy products |
| Bovine milk/N.A. (77) | Proteins for actin cytoskeleton, tight junctions, focal adhesions | Understanding of lactation and immune system |
| Human/child ≤2 y (69) | HLA-ABC2, Mucin-1 | Understanding the transfer of allergic tendencies from mother to child |
| Pig/piglet (78) | miR-200c, miR-21, miR-25–3p, miR-27b, miR-30a, miR-375 | Pig as a model for diseases in breast and immune system development |
| Human/N.A. (79) | miR-17–92 cluster and its paralogs | Understanding of immune system development in newborns |
| Giant panda/N.A. (80) | Let-7, miR-30a, miR-148a dla-miR-1310, dla-miR-2916, dla-miR-319a (from bamboo) | Understanding of the mechanism of neonate development e.g. inner ear, neurodevelopment |
| Tamar wallaby/neonate (81) | Let-7 (f, a, i), miR-204, miR-30, miR-375 | Understanding of developmental events in neonate with reference to lens morphogenesis, nervous system, hormone secretory organs |
| Human/N.A. (5) | Long noncoding RNA (SNHG8, GAS5, ZFAS1) | Understanding the mechanism of neonate allergies, asthma, autoimmune disorders, obesity development |
Throughout this table, N.A. stands for not applicable/not available.
HLA-ABC represents Human Leukocyte Antigen comprising of molecules transcribed from locus A, locus B, and locus C.
Proteomic analysis of bovine milk exosomes revealed the presence of 2107 proteins, most of which were involved in immunological functions such as chemokine signaling, cell receptor signaling, and B cell receptor signaling (77). However, improved methods of milk-exosome isolation have resulted in the observation of a smaller number of proteins (82). A total of 633 proteins with distinct functions have been identified from human milk exosomes (73). Studies have also shown that 575 proteins are differentially expressed between the colostrum and mature milk of bovines and humans, suggesting their role in fulfilling the unique requirements of infants during the different phases of development. Of these proteins, 22% are related to stimuli response, whereas others are involved in ribosome biosynthesis and regulation of the actin cytoskeleton (76). Moreover, protein-coding mRNAs with uncertain functions have been characterized in bovine milk exosomes (83), whereas no such findings have been documented in humans (84).
Recent RNA sequencing and quantitative real-time PCR analyses have demonstrated that exosomes in the breast milk of mothers of preterm infants carry 21 preterm-specific miRNAs, including miR-1307, which can resist the peculiar degradative environment of the preterm neonate gut (85). This miRNA is expressed in gastric tissues (86) and is involved in cell proliferation (87). Hence, miR-1307 in preterm milk exosomes might have specific roles in the early developmental stages of the gut of preterm infants. Studies on the function of exosomal miRNAs have been widely performed in mammalian animal models, due to the difficulty of conducting studies in human subjects. Studies on porcine milk exosomes demonstrated that miRNAs can be absorbed by the intestinal enterocytes of piglets. According to the Kyoto encyclopedia of genes and genomes pathway analysis, these miRNAs have roles in transcription, immunity, and metabolic processing (88). The uptake of bovine colostrum exosomes containing 7 immune-related miRNAs (miR-24a, miR-30d, miR-93, miR-106a, miR-181, miR-200a, and miR-451) by RAW264.7 cells, induced the overexpression of 3 of these miRNAs (miR106-a, miR181, and miR-451) and the production of cytokines IL-1β and IL-6 (68). Studies on other mammalian models, such as panda and wallaby, demonstrated changes in the miRNA composition of FDEs across different lactation phases according to the requirements of the developing infant. This suggests that the miRNA of these FDEs are signaling molecules transferrable to neonates (80, 81). Together, the results of the above-mentioned studies indicate that the bioactive compounds of FDEs affect infant health, paving the way for their application in therapy and milk-derived formulations.
Challenges
Promising evidence regarding our basic and applied knowledge of FDEs has led to efforts to devise protocols to isolate and engineer them for therapeutic and diet formulations. Although various methods have been used to isolate FDEs, they are generally isolated via ultracentrifugation followed by sucrose gradient centrifugation, which is a time-consuming laborious process, causes aggregation, and requires a large quantity of sample, making it inappropriate for large-scale application. Moreover, there is concern that this method disrupts FDEs, leading to the loss of their biological activity (89). The enrichment of exosomes based on surface biomarkers has also been widely used, particularly for surface CD63 and TSG101 (89), but to the best of our knowledge, the use of this method as an isolation strategy for FDEs is limited currently, because surface markers of FDEs have not been completely defined.
In addition to the identification of surface markers, the biochemical and physical characterization of FDEs including analysis of their morphology, size, charge, and lipids are still needed, particularly for plant-derived exosomes. The size, charge, and surface structure are further related to renal clearance and biodistribution (90). Although lipidomic studies in FDEs have shown cholesterol and sphingomyelin enrichment in exosomal lipid bilayers from mammals and phospholipid enrichment of FDEs from plant sources, an understanding of differences in uptake abilities based on the lipid layer composition of various exosomes is still lacking (91). The characterization of surface features of FDEs including lipid components and other factors may contribute to our understanding of how FDEs determine their target cells. Furthermore, it must be noted that data on edible FDEs from plants do not clearly indicate which particular cell type secretes exosomes and how processing (e.g. cooking) affects exosomal content. In addition, there is not a broadly accepted type of raw material (e.g. freshly obtained compared with market purchased) and therefore it is difficult to compare the results of the different studies. In the future, questions regarding the standardization of isolation and characterization techniques need to be addressed. It is important to gain a consensus among researchers for universally accepted nomenclature and methods.
Generally, studies presenting FDEs as promising therapies are based on mouse models. These models, with shorter generation times and <10% immune system similarity to that of humans, are not sufficient for demonstrating potential long-term effects, particularly in a physiologically relevant environment. Thus, it is necessary to develop improved animal models to understand the long-term effects and to validate the results of in vitro studies. Moreover, clinical trials must be performed to unveil the role of FDEs and estimate the efficacy of FDE-based drug carriers in humans.
Regarding the application of FDEs, bioavailability is also a matter of concern. It is not clear how FDEs are absorbed by cells and efficiently enter the blood circulation, although the passage of FDEs from the gut to the bloodstream has been proven. The targeting of FDEs to specific recipient tissues or cells is another challenge. Currently, determining the preference of an FDE for specific target tissue or cell type is still unresolved. Furthermore, the unpacking of cargo at the final destination of FDEs is not well understood. Therefore, close examination of the absorption, movement, and action of FDEs is required so that the detailed mechanism can be resolved. For clinical applications, it is also important to identify and validate the specific FDE-associated molecule responsible for the therapeutic effect. This will pave the way for the reconstitution of these FDEs by precisely loading them with the relevant miRNA or protein at a higher concentration than those present in natural FDEs. It is also necessary to determine the yield required to impart a physiologically relevant effect, and the duration of the regulatory effect once the cargo is transferred to the target cell. Therefore, standardization procedures to determine dose-dependent efficacies and time gaps between doses, among other factors, need to be established. Stability/shelf life and storage conditions of FDEs from different sources are other issues that need attention. Stability is important if FDEs are used in nutrition supplements, for example, and it includes both physical and biological stability to retain particle size and activity, respectively. Lyophilization can enhance stability in general, but data on shelf life of FDEs after lyophilization is currently lacking. The advancement of our understanding of FDEs together with improvements in the loading techniques may enhance the use of FDE-based therapies, and allow comparative studies on the efficiency of FDE-based and already established therapies, opening a new paradigm in pharmaceuticals.
Conclusions
A growing body of evidence has revealed the effect of FDEs on both physiological and pathological events, and their potential use in treating human diseases and improving health are emerging, offering new therapeutic solutions. The importance of these novel applications is based on their advantages over existing therapeutic options, including market-available drugs, natural exosomes from non-food sources, stem cells, and synthetic liposomes. Promising results from the use of FDEs, directly or with manipulation, in disorders such as cancer, inflammation, gastrointestinal pathologies, and special conditions like pregnancy (92) pave the way for their application. Improvements in technical expertise and a deeper understanding of the mechanistic details will help the medical community provide a safe and efficient tool for resolving public health issues on a broader scale.
ACKNOWLEDGEMENTS
We thank all the members of Ryu's Lab to participate in the discussion for the preparation of this review. The authors' responsibilities were as follows: JM and SR compiled the data. JM, ML, and SR analyzed the literature and drafted the manuscript. All authors have read and approved the final manuscript.
Notes
Supported by Soonchunhyang University Research fund and National Research Foundation grant number 2019M3E5D3073090 of the Bio and Medical Technology Development Program, which is funded by the Ministry of Science, Information, Communications Technology, and Future Planning.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: AMPK, adenosine monophosphate protein kinase, DSS, dextran sodium sulfate; EV, extracellular vesicle; FDE, food-derived exosome; ILV, intralumenal vesicle; miRNA, microRNA; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; siRNA, small interferring RNA.
References
- 1. Milner JA. Molecular targets for bioactive food components. J Nutr. 2004;134(9):2492s–8s. [DOI] [PubMed] [Google Scholar]
- 2. Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131(3s):955s–62s. [DOI] [PubMed] [Google Scholar]
- 3. Percival SS, Bukowski JF, Milner J. Bioactive food components that enhance γδ T cell function may play a role in cancer prevention. J Nutr. 2008;138(1):1–4. [DOI] [PubMed] [Google Scholar]
- 4. Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107(2):102–8. [DOI] [PubMed] [Google Scholar]
- 5. Karlsson O, Rodosthenous RS, Jara C, Brennan KJ, Wright RO, Baccarelli AA, Wright RJ. Detection of long non-coding RNAs in human breastmilk extracellular vesicles: implications for early child development. Epigenetics. 2016;11(10):721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. [DOI] [PubMed] [Google Scholar]
- 7. Raimondo S, Naselli F, Fontana S, Monteleone F, Lo Dico A, Saieva L, Zito G, Flugy A, Manno M, Di Bella MA et al.. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. 2015;6(23):19514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. An Q, van Bel AJ, Huckelhoven R. Do plant cells secrete exosomes derived from multivesicular bodies?. Plant Signal Behav. 2007;2(1):4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanchak MA, Fowke LC. The morphology of multivesicular bodies in soybean protoplasts and their role in endocytosis. Protoplasma. 1987;138(2):173–82. [Google Scholar]
- 10. Tomé-Carneiro J, Fernández-Alonso N, Tomás-Zapico C, Visioli F, Iglesias-Gutierrez E, Dávalos A. Breast milk microRNAs harsh journey towards potential effects in infant development and maturation. Lipid encapsulation can help. Pharmacol Res. 2018;132:21–32. [DOI] [PubMed] [Google Scholar]
- 11. Mandal S. Curcumin, a promising anti-cancer therapeutic its bioactivity and development of drug delivery vehicles.Intl J Drug Res Tech. 2017;6(2):14. [Google Scholar]
- 12. Mu J, Zhuang X, Wang Q, Jiang H, Deng ZB, Wang B, Zhang L, Kakar S, Jun Y, Miller D, Zhang HG. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58(7):1561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lasser C, Alikhani VS, Ekstrom K, Eldh M, Paredes PT, Bossios A, Sjostrand M, Gabrielsson S, Lotvall J, Valadi H. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kusuma RJ, Manca S, Friemel T, Sukreet S, Nguyen C, Zempleni J. Human vascular endothelial cells transport foreign exosomes from cow's milk by endocytosis. Am J Physiol Cell Physiol. 2016;310(10):C800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benmoussa A, Lee CH, Laffont B, Savard P, Laugier J, Boilard E, Gilbert C, Fliss I, Provost P. Commercial dairy cow milk microRNAs resist digestion under simulated gastrointestinal tract conditions. J Nutr. 2016;146(11):2206–15. [DOI] [PubMed] [Google Scholar]
- 16. Zhuang X, Deng ZB, Mu J, Zhang L, Yan J, Miller D, Feng W, McCain CJ, Zhang HG. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shandilya S, Rani P, Onteru SK, Singh D. Small interfering RNA in milk exosomes is resistant to digestion and crosses the intestinal barrier in vitro. J Agric Food Chem. 2017;65(43):9506–13. [DOI] [PubMed] [Google Scholar]
- 18. Baier SR, Nguyen C, Xie F, Wood JR, Zempleni J. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J Nutr. 2014;144(10):1495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, Xiang X, Deng ZB, Wang B, Zhang L. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21(7):1345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deng Z, Rong Y, Teng Y, Mu J, Zhuang X, Tseng M, Samykutty A, Zhang L, Yan J, Miller D. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol Ther. 2017;25(7):1641–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manca S, Upadhyaya B, Mutai E, Desaulniers AT, Cederberg RA, White BR, Zempleni J. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci Rep. 2018;8(1):11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang QL, Zhuang X, Sriwastva MK, Mu J, Teng Y, Deng Z, Zhang L, Sundaram K, Kumar A, Miller D et al.. Blood exosomes regulate the tissue distribution of grapefruit-derived nanovector via CD36 and IGFR1 pathways. Theranostics. 2018;8(18):4912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dever JT, Kemp MQ, Thompson AL, Keller HG, Waksmonski JC, Scholl CD, Barnes DM. Survival and diversity of human homologous dietary microRNAs in conventionally cooked top sirloin and dried bovine tissue extracts. PLoS One. 2015;10(9):e0138275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raimondo S, Saieva L, Cristaldi M, Monteleone F, Fontana S, Alessandro R. Label-free quantitative proteomic profiling of colon cancer cells identifies acetyl-CoA carboxylase alpha as antitumor target of Citrus limon-derived nanovesicles. J Proteomics. 2018;173:1–11. [DOI] [PubMed] [Google Scholar]
- 25. Raimondo S, Naselli F, Fontana S, Manteleone F, Lo Dico A, Saieva L, Zito G, Flugy Pape AM, Manno M, Di Bella MA et al.. Isolation and characterization of Citrus limon L. derived nanovesicles: potential use as antineoplastic agent. Abstracts from the Fourth International Meeting of ISEV, ISEV2015, Washington D.C., USA. J Extracell Vesicles. 2015;4(1): 27783.25967741 [Google Scholar]
- 26. Badawy AA, El-Magd MA, AlSadrah SA. Therapeutic effect of camel milk and its exosomes on MCF7 cells in vitro and in vivo. Integr Cancer Ther. 2018;17(4):1235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yassin AM, Abdel Hamid MI, Farid OA, Amer H, Warda M. Dromedary milk exosomes as mammary transcriptome nano-vehicle: their isolation, vesicular and phospholipidomic characterizations. J Adv Res. 2016;7(5):749–56. [Google Scholar]
- 28. Melnik BC, Schmitz G. Exosomes of pasteurized milk: potential pathogens of Western diseases. J Transl Med. 2019;17(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banikazemi Z, Haji HA, Mohammadi M, Taheripak G, Iranifar E, Poursadeghiyan M, Mordikia A, Rashidi B, Taghizadeh M, Mirzaei H. Diet and cancer prevention: dietary compounds, dietary microRNAs, and dietary exosomes. J Cell Biochem. 2018;119(1):185–96. [DOI] [PubMed] [Google Scholar]
- 30. Munagala R, Aqil F, Jeyabalan J, Agrawal AK, Mudd AM, Kyakulaga AH, Singh IP, Vadhanam MV, Gupta RC. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017;393:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aqil F, Jeyabalan J, Agrawal AK, Kyakulaga AH, Munagala R, Parker L, Gupta R. Exosomal delivery of berry anthocyanidins for the management of ovarian cancer. Food Funct. 2017;8(11):4100–7. [DOI] [PubMed] [Google Scholar]
- 32. Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, Alhakeem SS, Oben K, Munagala R, Bondada S et al.. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine. 2017;13(5):1627–36. [DOI] [PubMed] [Google Scholar]
- 33. Wang B, Zhuang X, Deng ZB, Jiang H, Mu J, Wang Q, Xiang X, Guo H, Zhang L, Dryden G et al.. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22(3):522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang M, Xiao B, Wang H, Han MK, Zhang Z, Viennois E, Xu C, Merlin D. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther. 2016;24(10):1783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Z, Wang H, Yin H, Bennett C, Zhang HG, Guo P. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci Rep. 2018;8(1):14644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371(1):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhuang X, Teng Y, Samykutty A, Mu J, Deng Z, Zhang L, Cao P, Rong Y, Yan J, Miller D et al.. Grapefruit-derived nanovectors delivering therapeutic miR17 through an intranasal route inhibit brain tumor progression. Mol Ther. 2016;24(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown JG, inventor.Edible plant exosome ability to prevent oral mucositis associated with chemoradiation treatment of head and neck cancer. United States: Last update checked: 18 April, 2019 (www.clinicaltrials.gov). [Google Scholar]
- 39. Chen X, You Z, Yu J. Exosome-like nanoparticles from ginger rhizomes inhibited NLRP3 inflammasome activation. Mol Pharmaceutics. 2019;16:(6):2690–99. [DOI] [PubMed] [Google Scholar]
- 40. Zhang M, Wang X, Han MK, Collins JF, Merlin D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine (London, England). 2017;12(16):1927–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim JH, inventor Nanoparticle composition for prevention of hair loss and promotion of hair growth. United States: 29 April, 2010. [Google Scholar]
- 42. Vashisht M, Rani P, Onteru SK, Singh D. Curcumin encapsulated in milk exosomes resists human digestion and possesses enhanced intestinal permeability in vitro. Appl Biochem Biotechnol. 2017;183(3):993–1007. [DOI] [PubMed] [Google Scholar]
- 43. Rahimi Ghiasi M, Rahimi E, Amirkhani Z, Salehi R. Leucine-rich repeat-containing G-protein coupled receptor 5 gene overexpression of the rat small intestinal progenitor cells in response to orally administered grape exosome-like nanovesicles. Adv Biomed Res. 2018;7:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mokarizadeh A, Hassanzadeh K, Abdi M, Soraya H, Faryabi MR, Mohammadi E, Ahmadi A. Transdermal delivery of bovine milk vesicles in patients with multiple sclerosis: a novel strategy to induce MOG-specific tolerance. Med Hypotheses. 2015;85(2):141–4. [DOI] [PubMed] [Google Scholar]
- 45. Center JGBC, inventor. Study investigating the ability of plant exosomes to deliver curcumin to normal and colon cancer tissue. USA: Last update checked: 18 April, 2019 (www.clinicaltrials.gov). [Google Scholar]
- 46. Boyd CA, Shennan DB. Breast milk and gene delivery: is lysinuric protein intolerance an exemplar?. Mol Genet Metab. 2010;101(2–3):296. [DOI] [PubMed] [Google Scholar]
- 47. Ning Y, Wang X, Zhang P, Liu A, Qi X, Liu M, Guo X. Dietary exosome-miR-23b may be a novel therapeutic measure for preventing Kashin-Beck disease. Exp Ther Med. 2018;15(4):3680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fujita D, Arai T, Komori H, Shirasaki Y, Wakayama T, Nakanishi T, Tamai I. Apple-derived nanoparticles modulate expression of organic-anion-transporting polypeptide (OATP) 2B1 in Caco-2 cells. Mol Pharm. 2018;15(12):5772–80. [DOI] [PubMed] [Google Scholar]
- 49. Arntz OJ, Pieters BC, Oliveira MC, Broeren MG, Bennink MB, de Vries M, van Lent PL, Koenders MI, van den Berg WB, van der Kraan PM et al.. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol Nutr Food Res. 2015;59(9):1701–12. [DOI] [PubMed] [Google Scholar]
- 50. Li B, Hock A, Wu RY, Minich A, Botts SR, Lee C, Antounians L, Miyake H, Koike Y, Chen Y et al.. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS One. 2019;14(1):e0211431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chin AR, Fong MY, Somlo G, Wu J, Swiderski P, Wu X, Wang SE. Cross-kingdom inhibition of breast cancer growth by plant miR159. Cell Res. 2016;26(2):217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao Z, Yu S, Li M, Gui X, Li P. Isolation of exosome-like nanoparticles and analysis of microRNAs derived from coconut water based on small RNA high-throughput sequencing. J Agric Food Chem. 2018;66(11):2749–57. [DOI] [PubMed] [Google Scholar]
- 53. Zhang M, Viennois E, Prasad M, Zhang Y, Wang L, Zhang Z, Han MK, Xiao B, Xu C, Srinivasan S. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12(5):408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC et al.. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Izumi H, Tsuda M, Sato Y, Kosaka N, Ochiya T, Iwamoto H, Namba K, Takeda Y. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J Dairy Sci. 2015;98(5):2920–33. [DOI] [PubMed] [Google Scholar]
- 57. Teng Y, Ren Y, Sayed M, Hu X, Lei C, Kumar A, Hutchins E, Mu J, Deng Z, Luo C. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe. 2018;24(5):637–52..e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mutai E, Zhou F, Zempleni J. Depletion of dietary bovine milk exosomes impairs sensorimotor gating and spatial learning in C57BL/6 mice. FASEB J. 2017;31(1_suppl):150.4. [Google Scholar]
- 59. Oliveira MC, Arntz OJ, Blaney Davidson EN, van Lent PL, Koenders MI, van der Kraan PM, van den Berg WB, Ferreira AV, van de Loo FA. Milk extracellular vesicles accelerate osteoblastogenesis but impair bone matrix formation. J Nutr Biochem. 2016;30:74–84. [DOI] [PubMed] [Google Scholar]
- 60. Oliveira MC, Di Ceglie I, Arntz OJ, van den Berg WB, van den Hoogen FH, Ferreira AV, van Lent PL, van de Loo FA. Milk-derived nanoparticle fraction promotes the formation of small osteoclasts but reduces bone resorption. J Cell Physiol. 2017;232(1):225–33. [DOI] [PubMed] [Google Scholar]
- 61. Mobley CB, Mumford PW, McCarthy JJ, Miller ME, Young KC, Martin JS, Beck DT, Lockwood CM, Roberts MD. Whey protein-derived exosomes increase protein synthesis and hypertrophy in C2-C12 myotubes. J Dairy Sci. 2017;100(1):48–64. [DOI] [PubMed] [Google Scholar]
- 62. Leiferman A, Shu J, Grove R, Cui J, Adamec J, Zempleni J. A diet defined by its content of bovine milk exosomes and their RNA cargos has moderate effects on gene expression, amino acid profiles and grip strength in skeletal muscle in C57BL/6 mice. J Nutr Biochem. 2018;59:123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McKinnon CM, Mellor H. The tumor suppressor RhoBTB1 controls Golgi integrity and breast cancer cell invasion through METTL7B. BMC Cancer. 2017;17(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vesterlund M, Zadjali F, Persson T, Nielsen ML, Kessler BM, Norstedt G, Flores-Morales A. The SOCS2 ubiquitin ligase complex regulates growth hormone receptor levels. PLoS One. 2011;6(9):e25358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sankar MJ, Sinha B, Chowdhury R, Bhandari N, Taneja S, Martines J, Bahl R. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):3–13. [DOI] [PubMed] [Google Scholar]
- 66. Hill PD, Aldag JC, Chatterton RT, Zinaman M. Comparison of milk output between mothers of preterm and term infants: the first 6 weeks after birth. J Hum Lact. 2005;21(1):22–30. [DOI] [PubMed] [Google Scholar]
- 67. Martin CR, Ling PR, Blackburn GL. Review of infant feeding: key features of breast milk and infant formula. Nutrients. 2016;8(5):E279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sun Q, Chen X, Yu J, Zen K, Zhang CY, Li L. Immune modulatory function of abundant immune-related microRNAs in microvesicles from bovine colostrum. Protein Cell. 2013;4(3):197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Torregrosa Paredes P, Gutzeit C, Johansson S, Admyre C, Stenius F, Alm J, Schenynius A, Gabrielsson S. Differences in exosome populations in human breast milk in relation to allergic sensitization and lifestyle. Allergy. 2014;69(4):463–71. [DOI] [PubMed] [Google Scholar]
- 70. Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EP, Schenynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179(3):1969–78. [DOI] [PubMed] [Google Scholar]
- 71. Zhou F, Paz HA, Shu J, Sadri M, Cui J, Fernando SC, Zempleni J. Dietary bovine milk exosomes elicit changes in microbial communities in C57BL/6 mice. Am J Physiol Gastrointest Liver Physiol. 2019;317(5):G618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Leiferman AL, Aguilar A, Mutai E, Adamec J, Zempleni J. Dietary depletion of bovine milk exosomes elicits changes in amino acid metabolism in C57BL/6 mice. FASEB J. 2017;; 31(1_suppl):135.3. [Google Scholar]
- 73. van Herwijnen MJ, Zonneveld MI, Goerdayal S, Nolte-’t Hoen EN, Garssen J, Stahl B, Maarten Altelaar AF, Redgegeld FA, Wauben MH. Comprehensive proteomic analysis of human milk-derived extracellular vesicles unveils a novel functional proteome distinct from other milk components. Mol Cell Proteomics. 2016;15(11):3412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hata T, Murakami K, Nakatani H, Yamamoto Y, Matsuda T, Aoki N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res Commun. 2010;396(2):528–33. [DOI] [PubMed] [Google Scholar]
- 75. Hock A, Miyake H, Li B, Lee C, Ermini L, Koike Y, Chen Y, Maattanen P, Zani A, Pierro A. Breast milk-derived exosomes promote intestinal epithelial cell growth. J Pediatr Surg. 2017;52(5):755–9. [DOI] [PubMed] [Google Scholar]
- 76. Yang M, Song D, Cao X, Wu R, Liu B, Ye W, Wu J, Yue X. Comparative proteomic analysis of milk-derived exosomes in human and bovine colostrum and mature milk samples by iTRAQ-coupled LC-MS/MS. Food Research International (Ottawa, Ont). 2017;92:17–25. [DOI] [PubMed] [Google Scholar]
- 77. Reinhardt TA, Lippolis JD, Nonnecke BJ, Sacco RE. Bovine milk exosome proteome. J Proteomics. 2012;75(5):1486–92. [DOI] [PubMed] [Google Scholar]
- 78. Gu Y, Li M, Wang T, Liang Y, Zhong Z, Wang X, Zhou Q, Chen L, Lang Q, He Z et al.. Lactation-related microRNA expression profiles of porcine breast milk exosomes. PLoS One. 2012;7(8):e43691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q, Zhou X, Wang X, Gao X, Li X. Immune-related microRNAs are abundant in breast milk exosomes. Int J Biol Sci. 2012;8(1):118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ma J, Wang C, Long K, Zhang H, Zhang J, Jin L, Tang Q, Jiang A, Wang X, Tian S et al.. Exosomal microRNAs in giant panda (Ailuropoda melanoleuca) breast milk: potential maternal regulators for the development of newborn cubs. Sci Rep. 2017;7(1):3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Modepalli V, Kumar A, Hinds LA, Sharp JA, Nicholas KR, Lefevre C. Differential temporal expression of milk miRNA during the lactation cycle of the marsupial tammar wallaby (Macropus eugenii). BMC Genomics. 2014;15:1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sedykh SE, Purvinish LV, Monogarov AS, Burkova EE, Grigor'eva AE, Bulgakov DV, Dmitrenok PS, Vlassov VV, Ryabchikova EI, Nevinsky GA. Purified horse milk exosomes contain an unpredictable small number of major proteins. Biochimie Open. 2017;4:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pieters BC, Arntz OJ, Bennink MB, Broeren MG, van Caam AP, Koenders MI, van Lent PL, van den Berg WB, de Vries M, van der Kraan PM et al.. Commercial cow milk contains physically stable extracellular vesicles expressing immunoregulatory TGF-beta. PLoS One. 2015;10(3):e0121123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zempleni J, Aguilar-Lozano A, Sadri M, Sukreet S, Manca S, Wu D, Zhou F, Mutai E. Biological activities of extracellular vesicles and their cargos from bovine and human milk in humans and implications for infants. J Nutr. 2017;147(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kahn S, Liao Y, Du X, Xu W, Li J, Lonnerdal B. Exosomal microRNAs in milk from mothers delivering preterm infants survive in vitro digestion and are taken up by human intestinal cells. Mol Nutr Food Res. 2018;62(11):e1701050. [DOI] [PubMed] [Google Scholar]
- 86. Li SC, Liao YL, Ho MR, Tsai KW, Lai CH, Lin WC. miRNA arm selection and isomiR distribution in gastric cancer. BMC Genomics. 2012;13:(Suppl 1):S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Qiu X, Dou Y. miR-1307 promotes the proliferation of prostate cancer by targeting FOXO3A. Biomed Pharmacother. 2017;88:430–5. [DOI] [PubMed] [Google Scholar]
- 88. Chen T, Xi QY, Ye RS, Cheng X, Qi QE, Wang SB, Shu G, Wang LN, Zhu XT, Jiang QY et al.. Exploration of microRNAs in porcine milk exosomes. BMC Genomics. 2014;15:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. van der Pol E, Boing AN, Gool EL, Nieuwland R. Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J Thromb Haemost. 2016;14(1):48–56. [DOI] [PubMed] [Google Scholar]
- 90. Wang Q, Zhuang X, Mu J, Deng ZB, Jiang H, Zhang L, Xiang X, Wang B, Yan J, Miller D et al.. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4:1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang M, Viennois E, Xu C, Merlin D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers. 2016;4(2):e1134415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Timms K, Mclaughlin J, Day A, Holder B, Westwood M, Forbes K. Cross-kingdom uptake and transfer of exosomal watermelon microRNAs in human intestinal epithelial cells. Abstracts from the Fifth International Meeting of ISEV, ISEV2016, Rotterdam, The Netherlands: J of Extracell Vesicles. 2016;5:31552. [DOI] [PMC free article] [PubMed] [Google Scholar]