ABSTRACT
Two core nutrient intake reference values (NRVs) are required for assessing the adequacy and safety of nutrient intakes for population groups: the average requirement (AR) and the tolerable upper level of intake (UL). Applications of such assessments include providing advice to improve intakes, formulating complementary foods, estimating the amounts of nutrients to be added to fortified foods and monitoring changes in intake, and product labeling at the global, national, or regional level. However, there is a lack of unity across country-level organizations in the methodological approach used to derive NRVs, and ARs and ULs are lacking in many compilations, thus limiting the ability to assess nutrient intakes for their population groups. Because physiological requirements vary little across populations globally, and setting reference values requires determining an acceptable level of uncertainty, it is feasible to adapt current recommendations from different sources to harmonize these core reference values. The objective of this review is to demonstrate an approach for harmonizing the NRVs for ARs (here termed “H-ARs”) and ULs (“H-ULs”) that can be applied on a global scale to assessing intakes across populations. The approach incorporates the framework and terminology recommended by reports from the United Nations University, the National Academies of Sciences, Engineering, and Medicine (NASEM), the Institute of Medicine (IOM), and the European Food Safety Authority (EFSA). After reviewing available alternatives, the proposed harmonized values were selected from standards set by EFSA (for Europe) and the IOM (for the United States and Canada), giving priority to those published most recently. Justifications for the proposed values are presented, along with discussion of their limitations. Ideally, these methods should be further reviewed by an international group of experts. Meanwhile, the H-ARs and H-ULs suggested in this review can be used to assess intakes of populations for many applications in global and regional contexts.
Keywords: nutrient reference value, nutrient intake recommendations, nutrient requirement, harmonized average requirement, harmonized upper level
Introduction
Nutrient reference values (NRVs) may be used to assess the adequacy and safety of intakes of population groups, and to design intakes to prevent nutrient inadequacy or excess when planning feeding programs or developing fortified foods. For these purposes, estimates of 2 NRVs are required: the average requirement (AR) and the tolerable upper level of intake (UL) for the population. The AR and UL are considered the core NRVs for evaluating population intakes. In this context, NRVs to cover almost all individuals, such as the RDAs, are not used because they will yield overestimates of inadequacy at the population level.
Two large-scale efforts to set such NRVs are the DRIs developed under the auspices of the Institute of Medicine (IOM) of the US National Academies (1–8) and the dietary reference values set by the European Food Safety Authority (EFSA) (9, 10). The IOM has recently been renamed and incorporated into the National Academies of Science, Engineering, and Medicine (NASEM). Thus all DRI reports through 2011 were published under the IOM, while all subsequent reports are published under NASEM. Here we will continue to refer to the IOM as the source of the DRIs.
Although the WHO/FAO has published recommended nutrient intakes (RNIs) (11), they lack a validated process for deriving either ARs or ULs. This review describes the strengths and limitations of these 3 sets of standards, and proposes a default set of harmonized core NRVs, which may be used widely to assess and plan intakes for population groups on a global scale. We also considered recommendations from the Department of Health in the United Kingdom which include ARs (12). The primary limitation of the UK values, however, is that they were released in 1991 and therefore were not useful to inform the harmonized values reported here. The Codex Alimentarius food labeling guidelines require nutrient reference standards (NRVs) for individual intakes that can be used globally, but these NRVs do not include either ARs or ULs, and thus are not used here (13).
The need for a globally harmonized approach to setting NRVs was recognized some 10 y ago in a 2007 consensus report on International Harmonization of Approaches for Developing Nutrient-Based Dietary Standards in which the current authors, and representatives from the IOM, WHO, and FAO as well as other organizations and countries, were participants (14). However, that report only described a harmonized approach to setting NRVs; it did not set any actual values.
Over the past 2 y, interest in the recommendations of the 2007 consensus report has been revived. An international workshop was convened in 2017 in Rome by NASEM in partnership with the WHO and FAO, with the goal of exploring the evidence for achieving global harmonization of the methodological approaches use to derive NRVs across countries (15). The workshop was followed by a consensus study, also convened by NASEM, which subsequently released its report “Harmonization of Approaches to Nutrient Reference Values” in 2018 (16). That report describes the methods and resources that should be used for setting NRVs, but does not include actual values. It does point out that given the high cost and expertise required to revise or set new recommendations, especially now that systematic reviews have been integrated into the process, an alternative is to evaluate existing NRVs and determine whether they should be kept, updated, or adapted.
However, there also is a need for reference values that can be applied globally to assess population intakes, especially for estimating nutrient gaps that need to be filled by programs. Examples of programs that need global estimates of ARs and ULs include the Food Fortification Initiative, the Global Alliance for Improved Nutrition, which advises on appropriate formulation of complementary foods and monitors the consumption of nutrients in fortified foods; Harvest Plus which needs to estimate target amounts of crop biofortification; and the World Food Program which needs to know the gaps between nutrient requirements and intakes and how to fill them. Currently, the NRVs used by these organizations and the approaches used to derive them are inconsistent. The use of harmonized values would provide these organizations with a common basis for establishing food and nutrition policies and evaluating and comparing the adequacy of nutrient intakes across their target population groups.
Our need to find a solution for the lack of ARs and ULs became acute as a result of our participation in a project, originally in collaboration with WHO, to produce software that will enable users to estimate the prevalence of inadequate and excessive nutrient intakes in population groups, and to estimate the effects of fortification on them. The free software (Intake Monitoring, Assessment and Planning Program; IMAPP) (17) builds on the C-SIDE program for Intake Distribution Estimation, developed by Dr Carriquiry and collaborators at Iowa State University (18), and uses the approach recommended by a report from the IOM to correct for intraindividual variability in nutrient intakes (19).
The authors view the harmonized reference values that follow as an interim measure until a larger consensus can be obtained. We believe that the following issues justify the development and application of harmonizing the 2 core NRVs:
There remains considerable uncertainty about some of the values recommended by the IOM and EFSA, a fact recognized by these organizations in their reports. For many nutrients, AR values do not exist in 1 or both of the reports because of a lack of sufficient evidence, so that an adequate intake (AI) recommendation is made based on the observed intakes of healthy populations. Unfortunately, progress toward updating or revising current values is extremely slow largely because of lack of funding support for the research and for the revision of recommendations. Another problem is that the research methodologies for improving current requirement estimates can be invasive (e.g., requiring isotopes or multiple blood draws), especially for vulnerable population groups (e.g. infants, children, and pregnant women).
Values for some nutrients differ between these reports, adding to uncertainty about the strength of the recommendations, and to the confusion of users who are focused on their application to global issues.
However, it is doubtful whether, after correction for bioavailability of a few nutrients, requirements for the amount of absorbed nutrients differ substantially across regions or the world (14). Most recommendations are quite similar between the 2 organizations and the more recent trend to rely on systematic reviews and to explain clearly the reasons for selecting specific values means that reasons for differences can be understood.
Major public health activities that target population groups (such as food fortification, and formulation of lipid-based and other micronutrient supplements for global distribution), are ongoing. In the absence of harmonized NRVs for populations, current practice is often to recommend amounts of fortification and micronutrient addition without considering the actual nutrient intake gaps in a population group (which requires values for ARs), or estimating the prevalence of potentially excessive intakes (which requires values for ULs). Instead, nutrient adequacy is often inaccurately assessed based on RDAs or RNIs. Because the RDAs and RNIs are recommendations to cover almost all individuals, they will yield overestimates of inadequacy if used to evaluate intakes at the population level.
Many countries, regions, and organizations cannot afford the cost (which easily runs into millions of US dollars for just the systematic reviews) or time to revise their current recommendations or develop new values for ARs and ULs. It is not possible to evaluate and compare the prevalence of inadequate and excessive nutrient intakes of populations within countries, across regions, or across the world, without harmonizing the 2 core NRVs. This (together with harmonized methods for measuring food intake) would seem to be a crucial first step toward enabling such an evaluation.
It is our position that these practical realities justify the creation of a set of core harmonized NRVs for populations. Below we describe our approach and present potential H-ARs and harmonized upper levels (H-ULs).
Current Status of Knowledge
Types of NRVs
Table 1 shows the 4 main types of NRVs that have been typically set, along with the specific terms used by the IOM, EFSA, and WHO/FAO, and most recently, by the NASEM. The terms H-AR and H-UL proposed here are also included in the table. The following definitions are from the IOM (1):
TABLE 1.
Examples of uses and terminology for nutrient reference values1
| Appropriate uses | WHO/FAO | IOM | EFSA | NASEM, 2018 | Harmonization for populations (this paper) | |
|---|---|---|---|---|---|---|
| Average requirement | Estimate prevalence of inadequacy for groups and probability of inadequacy for individuals | Rarely discussed | EAR | AR | AR | H-AR |
| Recommended intake | Use as a target for intake of individuals | RNI | RDA | PRI | RI | |
| Upper level | Estimate prevalence of intakes at risk of being excessive | Rarely discussed | UL | UL | UL | H-UL |
| Adequate intake | Use as a target for intake of individuals | AI | AI | AI |
Adapted from King and Garza (2007) (14). Sources: IOM (1); EFSA (9); WHO/FAO (11); NASEM (16). AI, adequate intake; AR, average requirement; EAR, estimated average requirement; EFSA, European Food Safety Authority; H-AR, harmonized average requirement; H-UL, harmonized upper level; IOM, Institute of Medicine; NASEM, National Academies of Sciences, Engineering and Medicine; PRI, population reference intake; RI, recommended intake; RNI, recommended nutrient intake; UL, safe upper intake level (NASEM)/tolerable upper intake level (IOM and EFSA).
Average requirement: The average daily nutrient intake that is estimated to meet the requirements of half of the healthy individuals in a particular life stage and gender group.
Recommended intake: The average daily nutrient intake that is sufficient to meet the nutrient requirements of nearly all (97–98%) healthy individuals in a particular life stage and gender group.
Upper level: The highest average daily nutrient intake that is likely to pose no risk of adverse health effects to almost all individuals in the general population.
Adequate intake: The recommended average intake daily intake based on observed or experimentally determined approximations or estimates of nutrient intake by a group (or groups) of apparently healthy people that are assumed to be adequate. It is used when a recommended intake cannot be determined.
Figure 1 illustrates the relations among these NRVs. Importantly, a distribution of both nutrient requirements and nutrient toxicity is assumed. The nutrient review committees convened by IOM and EFSA were asked to estimate this distribution, and typically assumed a normal distribution with the average requirement as the mean and the SD of the requirement as the variance. The requirement distribution is for apparently healthy populations, and is not meant to be used for people who are malnourished or those with diseases that could affect nutrient requirements. For each nutrient, the criterion of adequacy is specified. The criterion, or functional outcome, has typically been the prevention of signs of deficiency, but reduction in the risk of chronic diseases has also been considered for some nutrients.
FIGURE 1.
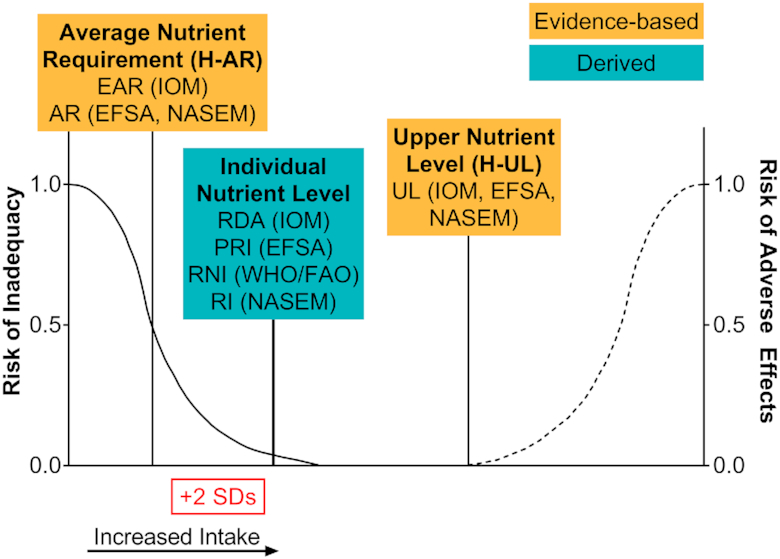
Distribution and terminology for nutrient reference values. IOM (1); EFSA (9); WHO/FAO (11); NASEM (16). AR, average requirement; EAR, estimated average requirement; EFSA, European Food Safety Authority; H-AR, harmonized average requirement; H-UL, harmonized upper level; IOM, Institute of Medicine; NASEM, National Academies of Sciences, Engineering, and Medicine; PRI, population reference intake; RI, recommended intake; RNI, recommended nutrient intake; UL, tolerable upper intake level.
In this context, it is important to distinguish the differences between evaluating the intakes of individuals and evaluating the intakes of population groups. For an individual, the appropriate nutrient intake target should have a low probability of inadequacy. Practically, this recommended intake has been interpreted as an intake that will meet the needs of all but 2.5% of a population group, and is calculated as the average nutrient requirement plus 2 SD of the requirement (see Figure 1). These recommended intake standards should not be used to estimate the prevalence of inadequacy for a population, nor are they appropriate goals when planning intakes for populations.
In this review, the overall approach was to follow the framework recommended in the 2007 report on harmonization (14) and the more recent NASEM report (16), which is based on first deriving the AR [equivalent to the IOM's estimated average requirement (EAR) and EFSA AR], and a UL (equivalent to the IOM and EFSA UL) for the target populations. Because the NASEM report (16) uses the terms AR and UL, we suggest the terms H-AR and H-UL for the harmonized equivalents of these NRVs. We do not use individual intake values [RDAs, population reference intakes (PRIs), RNIs, or recommended intakes] or adequate intake standards (AIs) as harmonized standards for populations because they cannot be used to estimate the prevalence of inadequacy. However, we have estimated H-ARs from AIs for those nutrients where no AR exists, and occasionally examine and compare RNIs, RDAs, and PRIs when choosing the harmonized values.
Limitations of current NRVs
Because the harmonized core NRVs are intended to be used internationally, it would be ideal to use WHO/FAO NRVs for all nutrients. However, as noted above, the WHO/FAO tables (11) are of limited value because they provide only RNI values, equivalent to the RDAs in IOM reports and the PRIs of EFSA, but almost no average values equivalent to the IOM's EARs or EFSA's ARs. Thus, they are not suitable for assessing the prevalence of inadequate intakes of specific nutrients in a population group, which is estimated as the percentage of intakes below the AR, as recommended by the IOM (19), EFSA (9), and the recent NASEM report on harmonization of approaches (16).
WHO/FAO uses the concept of “mean requirement” for vitamin A (11), although the principles underlying the term “mean requirement” differ from those used in the IOM and EFSA reports. Specifically, WHO/FAO's mean requirement for vitamin A was set to cover the requirements of 97.5% of the population. WHO/FAO also provides an EAR (in addition to an RNI) for vitamin B-12 and folate, because these values were taken directly from the IOM report on these vitamins (3). Selenium has a “normative requirement” based on intakes adequate for enzyme activity and stores, which is a different criterion from that used by the IOM to estimate an EAR (4). Thus, except for vitamin B-12 and folate, the WHO/FAO values do not meet the usual criteria for “average” requirements.
There are also limitations associated with some of the IOM and EFSA NRVs. They were developed for populations living in the United States, Canada, and Europe, and thus do not consider the full range of global differences in diets and physical activity that might affect nutrient requirements. More importantly, for 6 nutrients both the IOM and EFSA provide recommended intakes as AIs because there were insufficient data to set an EAR/AR; however, AIs tend to overestimate true mean requirements.
In addition, because of a lack of empirical data, many of the IOM and EFSA NRVs for children and adolescents were extrapolated up from the AIs for infants and/or down from the recommendations (sometimes AIs and sometimes EAR/ARs) for adults. This has caused implausible increases or decreases in the AIs or EARs between adjacent age groups for some nutrients, as well as discrepancies in the ULs. An example includes the revised IOM calcium requirements, which increased from an AI of 260 mg/d at age 7–12 mo to an EAR of 500 mg/d at age 1–3 y (8), making appropriate formulation of complementary foods difficult.
Finally, there is always a degree of uncertainty about true requirements, interindividual variability in these requirements, and estimates of true nutrient intake and bioavailability. Thus, small differences in NRVs from the IOM and EFSA would be expected.
General approach to selecting proposed H-AR values for vitamins and minerals
The following criteria were used to select the harmonized average nutrient values, which are summarized in Table 2. Tables 3 and�4 provide additional information on the process.
TABLE 2.
H-ARs for protein and vitamins, and minerals1
| Proteins and vitamins | Protein,2 g/kg/d | Vitamin A, μg RAE | Vitamin C,3 mg | Vitamin D, μg | Vitamin E, mg α-tocopherol | Thiamin, mg | Riboflavin, mg | Niacin, mg | Vitamin B-6, mg | Folate,4 μg DFE | Vitamin B-12, μg | Pantothenic acid, mg | Biotin, mg | Choline, mg | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | EFSA | EFSA | EFSA | IOM | IOM | IOM | EFSA | IOM | EFSA | EFSA | IOM | EFSA | EFSA | EFSA | |||
| Life stage5 | |||||||||||||||||
| Infants | |||||||||||||||||
| 7–11 mo | 1.12 | 190 | |||||||||||||||
| Children | |||||||||||||||||
| 1–3 y | 0.79 | 205 | 15 | 10 | 5 | 0.4 | 0.5 | 5 | 0.5 | 90 | 0.7 | 3.2 | 16 | 112 | |||
| 4–6 y | 0.69 | 245 | 25 | 10 | 6 | 0.5 | 0.6 | 6 | 0.6 | 110 | 1 | 3.2 | 20 | 136 | |||
| 7–10 y | 0.75 | 320 | 40 | 10 | 6 | 0.5 | 0.8 | 6 | 0.9 | 160 | 1 | 3.2 | 20 | 200 | |||
| Males | |||||||||||||||||
| 11–14 y | 0.73 | 480 | 60 | 10 | 9 | 0.7 | 1.1 | 9 | 1.2 | 210 | 1.5 | 4 | 28 | 272 | |||
| 15–17 y | 0.71 | 580 | 85 | 10 | 12 | 1 | 1.4 | 12 | 1.5 | 250 | 2 | 4 | 28 | 320 | |||
| 18–24 y | 0.66 | 570 | 90 | 10 | 12 | 1 | 1.3 | 12 | 1.5 | 250 | 2 | 4 | 32 | 320 | |||
| 25–50 y | 0.66 | 570 | 90 | 10 | 12 | 1 | 1.3 | 12 | 1.5 | 250 | 2 | 4 | 32 | 320 | |||
| 51–70 y | 0.66 | 570 | 90 | 10 | 12 | 1 | 1.3 | 12 | 1.5 | 250 | 2 | 4 | 32 | 320 | |||
| >70 y | 0.66 | 570 | 90 | 10 | 12 | 1 | 1.3 | 12 | 1.5 | 250 | 2 | 4 | 32 | 320 | |||
| Females | |||||||||||||||||
| 11–14 y | 0.71 | 480 | 60 | 10 | 9 | 0.7 | 1.1 | 9 | 1.2 | 210 | 1.5 | 4 | 28 | 272 | |||
| 15–17 y | 0.68 | 490 | 75 | 10 | 12 | 0.9 | 1.4 | 11 | 1.3 | 250 | 2 | 4 | 28 | 320 | |||
| 18–24 y | 0.66 | 490 | 80 | 10 | 12 | 0.9 | 1.3 | 11 | 1.3 | 250 | 2 | 4 | 32 | 320 | |||
| 25–50 y | 0.66 | 490 | 80 | 10 | 12 | 0.9 | 1.3 | 11 | 1.3 | 250 | 2 | 4 | 32 | 320 | |||
| 51–70 y | 0.66 | 490 | 80 | 10 | 12 | 0.9 | 1.3 | 11 | 1.3 | 250 | 2 | 4 | 32 | 320 | |||
| >70 y | 0.66 | 490 | 80 | 10 | 12 | 0.9 | 1.3 | 11 | 1.3 | 250 | 2 | 4 | 32 | 320 | |||
| Pregnancy | |||||||||||||||||
| ≤ 18 y | Add 7.2 g/d | 540 | 75 | 10 | 12 | 1.2 | 1.5 | 14 | 1.5 | 520 | 2.2 | 4 | 32 | 384 | |||
| 19–30 y | Add 7.2 g/d | 540 | 80 | 10 | 12 | 1.2 | 1.5 | 14 | 1.5 | 520 | 2.2 | 4 | 32 | 384 | |||
| 31–50y | Add 7.2 g/d | 540 | 80 | 10 | 12 | 1.2 | 1.5 | 14 | 1.5 | 520 | 2.2 | 4 | 32 | 384 | |||
| Lactation | |||||||||||||||||
| 18 y | Add 12.5 g/d | 1020 | 145 | 10 | 16 | 1.2 | 1.7 | 13 | 1.4 | 380 | 2.4 | 5.6 | 36 | 416 | |||
| 19–30 y | Add 12.5 g/d | 1020 | 145 | 10 | 16 | 1.2 | 1.7 | 13 | 1.4 | 380 | 2.4 | 5.6 | 36 | 416 | |||
| 31–50 y | Add 12.5 g/d | 1020 | 145 | 10 | 16 | 1.2 | 1.7 | 13 | 1.4 | 380 | 2.4 | 5.6 | 36 | 416 | |||
| Iron6 | Zinc7 | ||||||||||||||||
| Minerals | Calcium, mg | Phosphorus, mg | Chromium, μg | Copper, μg | Fluoride, mg | Manganese, mg | Molybdenum, μg | Iodine, μg | High absorption, mg | Moderate absorption, mg | Low absorption, mg | Magnesium, mg | Selenium, μg | Refined diet, mg | Semi-refined, mg | Semi-unrefined, mg | Unrefined diet, mg |
| Source | EFSA | IOM | IOM | IOM | EFSA | EFSA | IOM | IOM | EFSA | EFSA | EFSA | IOM | IOM | EFSA | EFSA | EFSA | EFSA |
| Life stage5 | |||||||||||||||||
| Infants | |||||||||||||||||
| 7–11 mo | 8 | 16 | 2.4 | ||||||||||||||
| Children | |||||||||||||||||
| 1–3 y | 390 | 380 | 8.8 | 260 | 0.48 | 0.4 | 13 | 65 | 5 | 10 | 65 | 17 | 3.6 | ||||
| 4–6 y | 680 | 405 | 12 | 340 | 0.8 | 0.8 | 17 | 65 | 5 | 10 | 110 | 23 | 4.6 | ||||
| 7–10 y | 680 | 405 | 12 | 340 | 1.2 | 1.2 | 17 | 65 | 8 | 16 | 110 | 23 | 6.2 | ||||
| Males | |||||||||||||||||
| 11–14 y | 960 | 1055 | 20 | 540 | 1.76 | 1.6 | 26 | 73 | 8 | 12.8 | 25.6 | 200 | 35 | 8.9 | |||
| 15–17 y | 960 | 1055 | 28 | 685 | 2.56 | 2.4 | 33 | 95 | 8 | 12.8 | 25.6 | 340 | 45 | 11.8 | |||
| 18–24 y | 860 | 580 | 28 | 700 | 2.72 | 2.4 | 34 | 95 | 6 | 9.6 | 19.2 | 330 | 45 | 7.5 | 9.3 | 11 | 12.7 |
| 25–50 y | 750 | 580 | 28 | 700 | 2.72 | 2.4 | 34 | 95 | 6 | 9.6 | 19.2 | 350 | 45 | 7.5 | 9.3 | 11 | 12.7 |
| 51–70 y | 750 | 580 | 24 | 700 | 2.72 | 2.4 | 34 | 95 | 6 | 9.6 | 19.2 | 350 | 45 | 7.5 | 9.3 | 11 | 12.7 |
| >70 y | 750 | 580 | 24 | 700 | 2.72 | 2.4 | 34 | 95 | 6 | 9.6 | 19.2 | 350 | 45 | 7.5 | 9.3 | 11 | 12.7 |
| Females | |||||||||||||||||
| 11–14 y | 960 | 1055 | 16.8 | 540 | 1.84 | 1.6 | 26 | 73 | 7 | 11.2 | 22.4 | 200 | 35 | 8.9 | |||
| 15–17 y | 960 | 1055 | 19.2 | 685 | 2.24 | 2.4 | 33 | 95 | 7 | 11.2 | 22.4 | 300 | 45 | 9.9 | |||
| 18–24 y | 860 | 580 | 20 | 700 | 2.32 | 2.4 | 34 | 95 | 7 | 11.2 | 22.4 | 255 | 45 | 6.2 | 7.6 | 8.9 | 10.2 |
| 25–50 y | 750 | 580 | 20 | 700 | 2.32 | 2.4 | 34 | 95 | 7 | 11.2 | 22.4 | 265 | 45 | 6.2 | 7.6 | 8.9 | 10.2 |
| 51–70 y | 750 | 580 | 16 | 700 | 2.32 | 2.4 | 34 | 95 | 6 | 9.6 | 19.2 | 265 | 45 | 6.2 | 7.6 | 8.9 | 10.2 |
| >70 y | 750 | 580 | 16 | 700 | 2.32 | 2.4 | 34 | 95 | 6 | 9.6 | 19.2 | 265 | 45 | 6.2 | 7.6 | 8.9 | 10.2 |
| Pregnancy | |||||||||||||||||
| ≤ 18 y | 860 | 1055 | 23.2 | 785 | 2.32 | 2.4 | 40 | 160 | 7 | 11.2 | 22.4 | 335 | 49 | 7.5 | 8.9 | 10.2 | 11.5 |
| 19–30 y | 860 | 580 | 24 | 800 | 2.32 | 2.4 | 40 | 160 | 7 | 11.2 | 22.4 | 290 | 49 | 7.5 | 8.9 | 10.2 | 11.5 |
| 31–50y | 750 | 580 | 24 | 800 | 2.32 | 2.4 | 40 | 160 | 7 | 11.2 | 22.4 | 300 | 49 | 7.5 | 8.9 | 10.2 | 11.5 |
| Lactation | |||||||||||||||||
| ≤ 18 y | 860 | 1055 | 35.2 | 985 | 2.32 | 2.4 | 35 | 209 | 7 | 11.2 | 22.4 | 300 | 59 | 8.6 | 10 | 11.3 | 13.7 |
| 19–30 y | 860 | 580 | 36 | 1000 | 2.32 | 2.4 | 36 | 209 | 7 | 11.2 | 22.4 | 255 | 59 | 8.6 | 10 | 11.3 | 13.7 |
| 31–50y | 750 | 580 | 36 | 1000 | 2.32 | 2.4 | 36 | 209 | 7 | 11.2 | 22.4 | 265 | 59 | 8.6 | 10 | 11.3 | 13.7 |
Sources of data: IOM (1); EFSA (9). H-ARs for nutrient names in italics are calculated as 0.8 times the AI; using these calculated H-ARs is likely to result in overestimates of the prevalence of inadequacy within a population. DFE, dietary folate equivalent; EFSA, European Food Safety Authority; H-AR, harmonized average requirement; IOM, Institute of Medicine; RAE, retinol activity equivalents.
Protein: Additional protein requirements during pregnancy range from +0.52 g/d (first trimester) to +23 g/d (third trimester); additional protein requirements during lactation range from +15 g/d to +10 g/d (after 6 mo); children's protein requirements vary by year of age; the values in the table are for the approximate midpoint age of each age group: 0.5 y for 7–11 mo, 2 y for 1–3 y, 5 y for 4–6 y, 8 y for 7–10 y, 13 y for 11–14 y, and 16 y for 15–17 y.
Vitamin C requirements during pregnancy are imputed from nonpregnant requirements.
Folate requirements during pregnancy are from IOM.
Mapping IOM and EFSA age groups to H-AR age groups: IOM 4–8 y = 4–6 y and 7–10 y; IOM 9–13 y = 11–14 y; IOM 14–18 y = 15–17 y; IOM 19–30 y = 18–30 y; IOM 31–50 y = 25–50 y; EFSA adult = 31–50 y, 51–70 y, and >70 y; Pregnant: EFSA 18–24 y = ≤18 y and 19–30 y; Pregnant: EFSA ≥25 y = 31–50 y; Lactating: EFSA 18–24 y = ≤18 y and 19–30 y; Lactating: EFSA ≥25 y = 31–50 y.
EFSA iron requirements for women assume: Premenopausal = 18–50 y, Postmenopausal = >50 y. Three iron requirements are shown, assuming 16%, 10%, and 5% absorption.
Four amounts of zinc requirements are shown, assuming 300 mg phytate/d, 600 mg phytate/d, 900 mg phytate/d, and 1200 mg phytate/d in the diet. EFSA zinc requirements for children assume a mixed diet so no adjustment for phytate; shown as “semi-unrefined diet”.
TABLE 3.
Source of values for H-ARs for vitamins and minerals: IOM compared with EFSA1
| Nutrient | IOM EAR – Children2 | IOM EAR – Adults3 | EFSA AR – Children2 | EFSA AR - Adults3 | H-AR decision | H-AR functional outcome4 |
|---|---|---|---|---|---|---|
| Vitamin A, μg RE/d | 210–630 | 500–625 | 205–580 | 490–570 | Use EFSA | Adequate liver stores |
| Vitamin C, mg/d | 13–63 | 60–75 | 15–85 | 80–90 | Use EFSA | Balance; adequate body pool |
| Vitamin D, μg/d | 10 | 10 | Use IOM | Serum 25-hydroxyvitamin D | ||
| Vitamin E, mg/d | 5–12 | 12 | Use IOM | Prevent peroxide-induced hemolysis | ||
| Thiamin, mg NE/d | 0.4–1.0 | 0.9–1.0 | 0.35 | 0.65 | Use IOM | Normal erythrocyte transketolase activity |
| Riboflavin, mg/d | 0.4–1.1 | 0.9–1.1 | 0.5–1.4 | 1.3 | Use EFSA | Urinary riboflavin excretion |
| Niacin, mg/d | 5–12 | 11–12 | 5.55 | 115 | Use IOM | Excretion of niacin metabolites |
| Vitamin B-6, mg/d | 0.4–1.1 | 1.1–1.4 | 0.5–1.5 | 1.3–1.5 | Use EFSA | Plasma pyridoxal 5-phosphate |
| Vitamin B-12, μg/d | 0.7–2.0 | 2.0 | Use IOM | Maintain hematological status and normal serum B-12 | ||
| Folate, μg DFE/d | 120–330 | 320 | 90–250 | 250 | Use EFSA | Serum and red blood cell folate |
| Calcium, g/d | 500–1100 | 800–1000 | 390–960 | 750–860 | Use EFSA | Factorial approach |
| Phosphorus, g/d | 380–1055 | 580 | Use IOM | Serum phosphate | ||
| Copper, mg/d | 2.60–6.85 | 7.0 | Use IOM | Plasma copper, serum ceruloplasmin, platelet copper | ||
| Molybdenum, μg/d | 13–33 | 34 | Use IOM | Balance studies | ||
| Iodine, μg/d | 65–95 | 95 | Use IOM | Thyroid accumulation and turnover | ||
| Iron, mg/d | 3.0–7.7 | 5.0–8.1 | 5–8 | 6–7 | Use EFSA | Factorial approach |
| Magnesium, mg/d | 65–340 | 255–350 | Use IOM | Balance studies | ||
| Selenium, μg/d | 17–45 | 45 | Use IOM | Plasma glutathione peroxidase activity | ||
| Zinc, mg/d | 2.5–8.5 | 6.8–9.4 | 3.6–11.8 | 6.2–12.7 | Use EFSA | Null balance |
See Table 2 for a summary of the final H-AR values. Sources: IOM (1–8); EFSA (9). AR, average requirement; DFE, dietary folate equivalent; EAR, estimated average requirement; EFSA, European Food Safety Authority; H-AR, harmonized average requirement; IOM, Institute of Medicine; NE, niacin equivalents; RE, retinol equivalents.
Age ranges for children are 1–17 y for EFSA and 1–18 y for IOM.
Does not include requirements for pregnancy or lactation, which are typically higher. H-ARs for pregnancy and lactation are from the same source as H-ARs for adults or adolescents.
Functional outcomes have been abbreviated in this column. For complete information, see IOM (1–8); EFSA (9).
Calculated from the AR of 0.072 mg NE/MJ for thiamin and 1.3 mg/MJ for niacin, assuming 4.2 MJ/d for children (1000 kcal/d) and 8.4 MJ/d for adults (2000 kcal/d).
TABLE 4.
Calculation of H-ARs from AIs1
| Nutrient | IOM AI – Children2 | IOM AI – Adults | EFSA AI – Children2 | EFSA AI – Adults | WHO/FAO RNI – Children | WHO/FAO RNI – Adults | H-AR – Children3 decision | H-AR – Adults3 decision |
|---|---|---|---|---|---|---|---|---|
| Pantothenic acid, mg/d | 2–5 | 5 | 4–5 | 5 | 2–5 | 5 | 3.2–4.0 | 4.0 |
| Biotin, μg/d | 8–25 | 30 | 20–35 | 40 | 8–25 | 30 | 16–28 | 32 |
| Choline, mg/d | 200–550 | 425–550 | 140–400 | 400 | — | — | 112–320 | 320 |
| Chromium, μg/d | 11–35 | 25–35 | — | — | — | — | 8.8–28 | 20–28 |
| Fluoride, mg/d | 0.7–3.0 | 3.0–4.0 | 0.6–3.2 | 2.9–3.4 | — | — | 0.48–2.6 | 2.3–2.7 |
| Manganese, mg/d | 1.2–2.2 | 1.8–2.3 | 0.5–3.0 | 3.0 | — | — | 0.4–2.4 | 2.4 |
Calculations assumed a CV of 12.5%. The H-AR was calculated as 80% of the AI, so that the AI is 2 × CV (25%) greater than the H-AR. See Table 2 for a summary of the final H-AR values. AI, adequate intake; EFSA, European Food Safety Authority; H-AR, harmonized average requirement; IOM, Institute of Medicine; RNI, recommended nutrient intake.
Age ranges for children are 1–17 y for EFSA and 1–18 y for IOM and FAO/WHO.
H-ARs use EFSA values except IOM values for chromium.
For most nutrients, use EFSA's AR values or IOM's EAR values as H-ARs
The harmonized values presented here are derived primarily from the relevant publications by the IOM (2–8), and EFSA (9). EFSA set ARs for 10 nutrients between 2014 and 2017, whereas the IOM EARs for these same nutrients were set between 1998 and 2001 with the exception of the EARs for calcium and vitamin D, which were published in 2011. Some differences between the EARs and ARs for vitamins and minerals, and the decisions about which are accepted as H-ARs, are summarized in Table 3.
The ARs from EFSA were developed most recently and thus are more current than the other standards considered. EFSA also undertook systematic reviews to examine associations between nutrient intake and health outcomes, although this process was limited to a few nutrients because of the resources required. The systematic review process was not formalized at the time that the IOM recommendations were developed, between 1997 and 2005. However, IOM's calcium and vitamin D standards were updated in 2011 and were based on 2 systematic reviews. In general, EFSA's AR values were selected here when IOM and EFSA recommendations are similar. Unfortunately, EFSA provides AR values for only 7 vitamins and 3 minerals, compared to 10 vitamins and 9 minerals from the IOM. FAO/WHO recommendations were considered but not used as they do not provide AR or UL values.
Harmonized values were not selected for all nutrients. Energy requirements are not addressed in detail here because the prevalence of inadequate energy intakes cannot be calculated for a population using the standard EAR cutoff method (19). Energy intake and energy requirements are not independent because individuals with higher requirements usually have higher intakes, and thus 1 of the assumptions underlying the cutoff method is violated. Furthermore, given the difficulty in measuring energy intakes accurately, this evaluation should be supplemented by physiologic measures such as weight for height in children and BMI in adults.
Likewise, we do not address nutrient standards for macronutrient distributions, such as percentage of energy from protein, fat, and carbohydrates, because such standards are not typically used to estimate nutrient adequacy or inadequacy. Other macronutrients, including fatty acids, dietary fiber, and dietary cholesterol, also are not included here, for similar reasons. Although an EAR for carbohydrate intake was set by the IOM (7), it is not used in this context because the low standard (100 g/d for adults, based on brain glucose use) would identify a meaningful prevalence of inadequacy only in populations with severe carbohydrate restriction.
For the 10 nutrients for which EFSA set an AR, the values were similar to corresponding EARs from the IOM. Because the EFSA values are more recent, these values were used for H-ARs. For phosphorus, copper, molybdenum, iodine, magnesium, and selenium, EFSA did not set ARs, so the IOM's EARs are used as H-ARs.
For nutrients with AIs (and no EAR/ARs), estimate the H-AR
Six nutrients of possible interest did not have ARs set by either the IOM or EFSA: pantothenic acid, biotin, choline, chromium, fluoride, and manganese. However, AIs were available for all 6 nutrients from the IOM, and for all but chromium from EFSA (EFSA did not consider chromium to be an essential nutrient). Although an AI cannot be used to estimate the prevalence of inadequacy, we chose to address this problem by calculating an approximate H-AR for these nutrients. Although it is not rigorous practice to estimate an AR from an AI, we used this approach because it will yield a better estimate of the prevalence of inadequacy than using the unmodified AI. The AIs are almost certainly an overestimate of the recommended intake for individuals (e.g., the RDA) because they represent the average intake of a healthy population. Thus, users should be aware that the calculated H-AR is likely to be an overestimate of the true AR in the group, and may result in an overestimate of the true prevalence of inadequacy. In addition, AIs based on intakes usually rely on data from the United States, Canada, and Europe, which for most nutrients are likely to be somewhat higher than in other regions of the world. Some AIs are based on criteria other than intakes; for example, the EFSA AI for fluoride is derived from an epidemiologic study of the association between fluoridation of water and dental caries. However, even when the AI is experimentally determined, it is assumed, by definition, to approximate the intake of a healthy population. Importantly, most of these 6 nutrients are not known to be of major public health significance, although choline may be an exception. A CV of 12.5% was assumed to reduce the AIs to approximate H-ARs, a similar CV to that used by the IOM and EFSA to increase the AR to the intake recommended for individuals (1.96 times the standard distribution of the AR, added to the AR, should cover 98.5% of the population). Because the SD of requirements is equal to the CV times the mean requirement, the calculated H-AR plus 1.96 (rounded to 2.0) times 12.5%, or 25%, of the H-AR is equal to the AI. Therefore, H-AR = AI/1.25 (see Table 4 for details). To emphasize that the H-ARs calculated from AIs have less certainty than H-ARs set by the IOM or EFSA, these values are italicized in Table 2, and a footnote reminds users that the prevalence of inadequacy may be overestimated when they are used.
Considerations when selecting H-ARs for pregnant or lactating women and for infants
IOM and EFSA both provide 1 recommended intake value for the entire duration of pregnancy, which is also the approach recommended in the 2007 harmonization report (16), and taken here in the development of H-ARs. The circumstances are the same for lactating women.
Because nutrient standards for infants aged <6 mo are almost exclusively expressed as AIs based on the nutrient content of breast milk, rather than ARs, we have not developed H-ARs for infants aged <6 mo. Although it is possible to compare mean intakes to the recommended intakes, no statements can be made about the prevalence of inadequacy within the population. The same is true for older infants (6–11 mo), with the exception of iron, zinc, and protein. EARs and ARs were set by both the IOM and EFSA for these 3 nutrients and may be used to estimate the prevalence of inadequate intakes. The IOM EARs are 6.9 mg/d for iron, 2.5 mg/d for zinc, and 10 g/d for protein (7). The corresponding values for the EFSA ARs are 8 mg/d for iron, 2.4 mg/d for zinc, and 1.12 g · kg−1 · d−1 (6.83 g/d) for protein (9). We chose the more recent EFSA values for the H-ARs in Table 2. Values for infants are not included in the ranges for children shown in Table 3.
Choosing age groups for H-ARs
Because the approaches used by IOM and EFSA include slightly different age groups for their NRVs, it was necessary to choose a new set of age groups for the H-ARs. To retain as much specificity as possible, we selected the more detailed age groups from each. To ensure that all age groups contained a value for the appropriate H-AR, it was sometimes necessary to duplicate an EAR or an AR. For example, EFSA uses 3 age groups for children, 1–3 y, 4–6 y, and 7–10 y, but IOM uses only 2: 1–3 y and 4–8 y. The H-ARs use the 3 EFSA age groups, and when IOM values are chosen for a nutrient, the value for 4–8 y is selected for both 4–6 y and 7–10 y. For older children and adolescents, we also used the EFSA ages for the H-AR age groups. For adults, we used the more detailed IOM ages: 31–50 y, 51–70 y, and >70 y. Likewise, for pregnant and lactating women, we used the 3 IOM age categories: ≤18 y, 19–30 y, and 31–50 y. EFSA provides a single value for these reproductive states, so when EFSA AR values for a nutrient were chosen, the same value was assigned to all 3 of the H-AR pregnancy or lactation ages. The H-AR age groups were also used as H-UL age groups.
Adjustments for bioavailability
A challenge in using harmonized values for requirements is the differing bioavailability of certain nutrients depending on the type of diet that a population consumes. These differences are particularly pronounced for the minerals iron and zinc. As discussed below, we recommend the use of adjustment factors in 1 of 2 ways: either the dietary intakes of these 2 minerals can be adjusted using algorithms that consider the impact of enhancing and inhibiting factors that are simultaneously present in the diet; or if the bioavailability is similar across all diets in the population, the H-AR values themselves can be adjusted. Table 2 gives H-AR values for iron intake assuming 16% absorption, 10% absorption, and 5% absorption. For IMAPP, the user specifies the percent absorption, and the program automatically adjusts the H-AR. Table 2 specifies H-AR values for zinc, corresponding to 4 amounts of zinc absorption from diets that are refined to various degrees. As with iron, the IMAPP user specifies the absorption factors that should be used. Further guidance on appropriate availability factors is provided in references (15) and (16). Thus, it is possible to use harmonized H-ARs for diets with a wide range of mineral bioavailability.
Considerations on H-AR values for specific nutrients
In the case of some nutrients, IOM and EFSA values differ or were expressed in different units.
Protein: Both the IOM and EFSA have specified average protein requirements for various age groups, expressed as g · kg−1 · d−1. These values are based on nitrogen balance studies, plus requirements for growth in children. The IOM has set EARs for protein (9), whereas EFSA has set ARs (11). WHO set an average protein requirement of 0.66 g · kg−1 · d−1 for adults (20). The IOM values range from 0.71 to 0.87 g · kg−1 · d−1 for children through age 18 y, and are 0.66 g · kg−1 · d−1 for adults of all ages. Similarly, the EFSA values range from 0.67 to 0.95 g · kg−1 · d−1 for children through 17 y of age, and are 0.66 g · kg−1 · d−1 for adults. These are requirements for high-biological value proteins, so intakes of protein of poorer quality should be adjusted before calculating the prevalence of inadequacy. Both groups recommend additional protein intake during pregnancy and lactation. Although the values are similar, we have chosen to use the EFSA ARs for H-ARs, because they have been more recently specified than those from the IOM (2012 compared with 2002) or WHO (2007).
Vitamin C: EFSA's recommendations are 15–20 mg/d higher than IOM's (9, 4). Both groups used the criterion of an adequate body pool but in setting the EAR, the IOM balanced plasma and neutrophil concentrations for maximal antioxidant protection with minimal urinary loss. The IOM's EAR was based on a depletion-repletion study (21), which showed that at an intake of 75 mg/d for men, it was possible to achieve 80% saturation of vitamin C in neutrophils, with low urinary losses. EFSA selected instead the daily intake value associated with a plasma concentration of vitamin C of 50 μmol/L and adjusted the value up to compensate for urinary losses. EFSA's AR values are used as H-ARs because the values were based on studies through 2013, whereas the IOM values relied on studies through 2000.
Vitamin D: The IOM's values for vitamin D were revised in 2011 (8), based on new evidence generated after the 1997 report, (2) and in consideration of a range of reports about benefits related to intake that exceeded the AI and, in some cases, the UL. The new recommendations are based on the need to achieve serum 25-hydroxyvitamin D at 40 nmol/L and include an EAR. EFSA concluded that a serum 25-hydroxyvitamin D concentration of 50 nmol/L is a suitable target value but that there was insufficient data to set an AR, setting an AI instead for all population groups. The IOM's EAR is therefore used for the H-AR. It should be noted that the H-ARs assume no sun exposure, so actual intake requirements will vary among populations. Thus, the prevalence of inadequacy is usually based on vitamin D concentration in serum.
Vitamin E: The IOM recommendations for vitamin E (4) have resulted in many reports of a high prevalence of inadequate vitamin E intakes by children in the United States, e.g. 58% of those aged 1–2 y in 1 national survey (22) and 79% of school-age children in another (23), although vitamin E deficiency is stated to be very rare and usually not caused by inadequate intakes. Indeed, the IOM report states that “overt symptoms of deficiency in healthy individuals consuming diets low in vitamin E have never been described” (4). Another relevant consideration is that IOM's EARs apply only to the 2R-stereoisomeric forms of α-tocopherol found in foods, fortified foods, and supplements. Other forms of vitamin E, such as γ-tocopherol (the predominant form in maize, for example) were deemed not to be useful for meeting vitamin E requirements. EFSA determined that there were insufficient data on markers of vitamin E intake, status, and function to derive ARs for vitamin E. However, their AI values for women (11 mg/d) and men (13 mg/d) are similar to the 12 mg/d for adults recommended as the EAR by the IOM. Thus the IOM's EARs are used as H-ARs.
Thiamin and niacin: EFSA expressed both thiamin and niacin ARs as mg/MJ, while the IOM values do not consider energy intake. The IOM committee noted that expressing the thiamin requirements in absolute terms was more useful for predicting biochemical thiamin status, and that no directly relevant studies were found that examined the effect of energy intake on niacin requirements (3). If an energy intake of 4.2 MJ/d for children and 8.4 MJ/d for adults is assumed, then the EFSA values are similar to the lower end of the range of IOM values (see Table 2). For simplicity in estimating inadequacies of population groups, when accurate measures of energy intake are seldom available, we selected the IOM values for both nutrients as H-ARs.
Vitamin B-12: EFSA did not set ARs for vitamin B-12 because they believe that a combination of biomarkers is needed to derive recommendations, yet there is uncertainty about cutoff values for these combined status indicators. They set an AI of 4 μg/d based on observed intakes and associated biomarkers, which is twice the IOM's EAR. Because EFSA did not set an AR, IOM's EAR is used as the H-AR.
Folate: EFSA recommends an AR of 250 μg of dietary folate equivalents (DFEs)/d compared with the IOM's EAR of 320 μg DFE/d. Both organizations based values on the intake required to maintain blood concentrations of folate (and in the case of the IOM, homocysteine) in controlled feeding studies. EFSA did not choose to include the main study used by the IOM because the results disagreed with those from other studies, and instead included an older study showing that lower intakes were sufficient. We use the EFSA AR as the H-AR. Because EFSA did not set an AR for pregnancy, the IOM value (520 μg DFEs/d) was used as the H-AR.
Calcium: EFSA values are slightly lower than those from the IOM. Although the IOM recommendations were revised in 2011, EFSA used these same balance data but added further studies that included calcium supplementation and excluded data from younger adults in whom calcium metabolism is not yet in a steady state. Because of these additional criteria, we chose the EFSA AR as the H-AR for calcium.
Iron: Dietary iron requirements vary depending on inhibitors and enhancers of iron absorption in the same meal. Both the IOM and EFSA committees assumed a high absorption of iron: 18% for the IOM values and 16% for the EFSA values for adults in Table 3; EFSA assumed a lower absorption (10%) for children. As a result, the IOM EARs and the EFSA ARs are similar for adults, whereas the EFSA values for children are somewhat higher. The H-ARs are based on the EFSA ARs. However, as noted earlier, when analyzing diets from countries with less refined diets, a higher H-AR would be appropriate. WHO/FAO estimates that absorption could range from 5% to 15% (11). In Table 2, ARs for iron are shown for 3 general types of diets: low absorption (5%) from an unrefined diet, moderate absorption (10%) from a diet with some meat/fish and moderate phytate, and high absorption (16%) from a diet with higher intake of meat/fish and lower phytate.
Zinc: Zinc requirements vary depending on inhibitors (primarily phytate) in the same meal. EFSA provides ARs for 4 phytate intakes, whereas IOM recommendations provide for 3 intakes. Recommendations tend to be higher from EFSA, especially for infants and young children. For these groups, no adjustment was made for phytate intake. Both organizations used zinc balance/factorial data and EFSA had access to a larger number of publications than the earlier IOM report. The EFSA AR is used as the H-AR for zinc. In Table 2, H-ARs for adults are shown for 4 phytate intakes. Typically, the lowest values (assuming 300 mg phytate/d) would be used for a Western diet, whereas higher values (assuming 900 mg phytate/d) would be used for semi-unrefined diets, and the highest values (assuming 1200 mg phytate/d) for unrefined diets.
General approach to selecting proposed H-UL values for vitamins and minerals
Sources of values for H-ULs
Three sources of values for ULs for vitamin and mineral intakes were considered when setting the H-ULs:
EFSA: UL values were published for vitamins and minerals from 2000 to 2005 (24) and recently summarized (10). Initially, the EFSA committee examined data on adverse effects for 14 vitamins and 20 minerals, but no adequate data to derive a UL was available for many of these nutrients. Ultimately, 8 minerals and 6 vitamins were assigned ULs for most age groups. Table 5 shows EFSA UL values for 13 of these nutrients, omitting boron because this nutrient is not commonly found in food composition tables and thus is not typically examined in dietary intake analyses.
IOM: UL values were set for vitamins and minerals beginning in 1997 and continuing through 2011 (2–8). A complete table of UL values is available online (25). Two IOM publications are available to better understand the ways in which ULs are derived and how they should be interpreted (19, 26).
FAO/WHO: The approach used by FAO/WHO to set upper limits for nutrient intakes has been explained in a document that includes lengthy descriptions and tables showing the approaches of IOM, EFSA, and the United Kingdom (27). However, no ULs were actually set in this document. Rather, ULs are suggested discontinuously throughout the text for each nutrient, often omitting discussion by age group. Elsewhere FAO/WHO has made recommendations regarding appropriate upper amounts of vitamins A, C, B-6, and folate (11). For vitamin A, a maximum of 900 μg/d of retinol for infants and no more than 3000 μg/d during pregnancy was recommended. During lactation, doses up to 60,000 μg retinol equivalents/d (to increase the concentration of the vitamin in breast milk) are safe for the first 6 wk postpartum if the mother is not lactating, and the first 2 mo if she is lactating, after which time the mother is more likely to become pregnant again causing a risk of birth defects from high vitamin A. Also, a maximum of 1000 mg/d of vitamin C and of 100 mg/d of vitamin B-6 for adults was suggested. The maximum suggested supplemental folic acid was 400 μg/d, although the report notes that intakes between 400 and 1000 μg/d would probably be safe (11).
TABLE 5.
Source of values for (H-ULs) for vitamins and minerals: IOM compared with EFSA1
| Nutrient | IOM UL – Children2,3 | IOM UL – Adults3,4 | EFSA UL – Children2,5 | EFSA UL – Adults4,5 | H-UL decision | H-UL adverse effect6 |
|---|---|---|---|---|---|---|
| Vitamin A, μg/d | 600–2800 | 3000 | 800–2600 | 3000 | Use EFSA | Teratogenicity |
| Vitamin C, mg/d | 400–1800 | 2000 | No UL | No UL | Use IOM | Gastrointestinal effects |
| Vitamin D, μg/d | 63–100 | 100 | 50–100 | 100 | Use IOM | High serum calcium |
| Vitamin E, mg/d | 200–800 | 1000 | 100–260 | 300 | Use EFSA | Blood clotting |
| Niacin, mg/d | 10–30 | 35 | 2–8 | 10 | Use EFSA | Flushing |
| Vitamin B-6, mg/d | 30–80 | 100 | 5–20 | 25 | Use EFSA | Neurotoxicity |
| Folate, μg/d | 300–800 | 1000 | 200–800 | 1000 | Use EFSA | Neuropathy if B-12-deficient |
| Choline, g/d | 1.0–3.0 | 3.5 | No UL | No UL | Use IOM | Hypotension, fishy body odor |
| Calcium, g/d | 2.5–3.0 | 2.0–2.5 | No UL | 2.5 | Use IOM | Milk alkali syndrome |
| Phosphorus, g/d | 3.0–4.0 | 3.0–4.0 | No UL | No UL | Use IOM | Elevated serum P |
| Copper, mg/d | 1.0–8.0 | 10.0 | 1.0–4.0 | 5.0 | Use EFSA | Liver function |
| Fluoride, mg/d | 1.3–10.0 | 10.0 | 1.5–7.0 | 7.0 | Use EFSA | Bone fractures |
| Manganese, mg/d | 2.0–9.0 | 11 | No UL | No UL | Use IOM | Blood manganese, neurotoxicity |
| Molybdenum, μg/d | 300–1700 | 2000 | 100–500 | 600 | Use EFSA | Reproductive toxicity |
| Iodine, μg/d | 200–900 | 1100 | 200–500 | 600 | Use EFSA | Changes in thyroid hormones |
| Iron, mg/d | 40–45 | 45 | No UL | No UL | Use IOM | Gastrointestinal distress |
| Magnesium, mg/d | 65–350 | 350 | 250 | 250 | Use EFSA | Mild diarrhea |
| Selenium, μg/d | 90–400 | 400 | 60–250 | 300 | Use EFSA | Selenosis (e.g., loss of hair and nails) |
| Zinc, mg/d | 7–34 | 40 | 7–22 | 25 | Use EFSA | Copper status |
See Table 6 for a summary of the final H-UL values. EFSA, European Food Safety Authority; H-UL, harmonized upper level; IOM, Institute of Medicine; UL, tolerable upper intake level.
Age ranges for children are 1–17 y for EFSA and 1–18 y for IOM.
IOM UL notes: magnesium as pharmacological agent only; vitamin A as preformed vitamin A only; vitamin E as any form of supplemental α-tocopherol; for vitamin E, niacin, and folate, applies only to synthetic forms.
H-ULs for pregnancy and lactation are from the same source as H-ULs for adults or adolescents; EFSA did not set ULs for niacin or copper during pregnancy or lactation.
EFSA UL notes: vitamin A applies to retinol and retinyl esters and does not apply to postmenopausal women as it may not provide an adequate margin of safety; magnesium applies to readily dissociable Mg salts and MgO in food supplements, water, or added to foods and does not apply to children aged 1–3 y; niacin UL applies only to nicotinic acid (ULs for nicotinamide range from 150 to 700 mg/d for children and 900 mg/d for adults).
Selection of proposed values for H-ULs
In choosing values for H-ULs, we considered the protocols followed, the timing of the reviews, and the magnitude of the differences (Table 6). Although the values from FAO/WHO were considered, they were not used directly in setting H-ULs because they did not follow the same protocols as the EFSA and IOM review panels for determining risk of adverse effects. For 13 nutrients, UL values were determined both by EFSA and IOM (see Table 5). For 11 of these nutrients, the recommendations were set during a similar time period: the EFSA recommendations were established in 2000–2005, while the majority of the IOM ULs were set between 1997 and 2001. However, the IOM reviewed and updated the ULs for calcium and vitamin D in 2011. Thus, the IOM values were selected for these 2 nutrients because they are based on a more current literature review. The UL values from IOM and EFSA were similar for most of the other nutrients, but the EFSA values were consistently lower, and therefore more conservative, especially for vitamin E, niacin, vitamin B-6, copper, molybdenum, iodine, and zinc. To better identify any risk of potentially excessive intakes, we chose the ULs set by EFSA for these nutrients. Values from the IOM were used for the remaining nutrients in Table 6 because values from EFSA were not available.
TABLE 6.
H-ULs for vitamins and minerals1
| Vitamins | Vitamin A,2 μg retinol | Vitamin C, mg | Vitamin D, μg | Vitamin E, mg α-tocopherol | Niacin,3 mg | Vitamin B-6, mg | Folic acid, μg | Choline, mg | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | EFSA | IOM | IOM | EFSA | EFSA | EFSA | EFSA | IOM | |||
| Life stage | |||||||||||
| Infants | |||||||||||
| 0–11 mo | 38 | ||||||||||
| Children | |||||||||||
| 1–3 y | 800 | 400 | 63 | 100 | 2 | 5 | 200 | 1000 | |||
| 4–6 y | 1100 | 650 | 75 | 120 | 3 | 7 | 300 | 1000 | |||
| 7–10 y | 1500 | 650 | 75 | 160 | 4 | 10 | 400 | 1000 | |||
| Males | |||||||||||
| 11–14 y | 2000 | 1200 | 100 | 220 | 6 | 15 | 600 | 2000 | |||
| 15–17 y | 2600 | 1800 | 100 | 260 | 8 | 20 | 800 | 3000 | |||
| 18–50 y | 3000 | 2000 | 100 | 300 | 10 | 25 | 1000 | 3500 | |||
| 51–70 y | 3000 | 2000 | 100 | 300 | 10 | 25 | 1000 | 3500 | |||
| >70 y | 3000 | 2000 | 100 | 300 | 10 | 25 | 1000 | 3500 | |||
| Females | |||||||||||
| 11–14 y | 2000 | 1200 | 100 | 220 | 6 | 15 | 600 | 2000 | |||
| 15–17 y | 2600 | 1800 | 100 | 260 | 8 | 20 | 800 | 3000 | |||
| 18–50 y | 3000 | 2000 | 100 | 300 | 10 | 25 | 1000 | 3500 | |||
| 51–70 y | 2000 | 100 | 300 | 10 | 25 | 1000 | 3500 | ||||
| >70 y | 2000 | 100 | 300 | 10 | 25 | 1000 | 3500 | ||||
| Pregnancy | |||||||||||
| ≤18 y | 3000 | 1800 | 100 | 300 | 25 | 1000 | 3000 | ||||
| 19–30 y | 3000 | 2000 | 100 | 300 | 25 | 1000 | 3500 | ||||
| 31–50 y | 3000 | 2000 | 100 | 300 | 25 | 1000 | 3500 | ||||
| Lactation | |||||||||||
| ≤18 y | 3000 | 1800 | 100 | 300 | 25 | 1000 | 3000 | ||||
| 19–30 y | 3000 | 2000 | 100 | 300 | 25 | 1000 | 3500 | ||||
| 31–50 y | 3000 | 2000 | 100 | 300 | 25 | 1000 | 3500 | ||||
| Minerals | Calcium, mg | Phosphorus, g | Copper, mg | Fluoride,4 mg | Manganese, mg | Molybdenum, mg | Iodine, μg | Iron, mg | Magnesium,5 mg | Selenium, μg | Zinc, mg |
| Source | IOM | IOM | EFSA | EFSA | IOM | EFSA | EFSA | IOM | EFSA | EFSA | EFSA |
| Life stage | |||||||||||
| Infants | |||||||||||
| 0–11 mo | 1500 | 40 | |||||||||
| Children | |||||||||||
| 1–3 y | 2500 | 3 | 1 | 1.5 | 2.0 | 0.1 | 200 | 40 | 60 | 7 | |
| 4–6 y | 2500 | 3 | 2 | 2.5 | 3.0 | 0.2 | 250 | 40 | 250 | 90 | 10 |
| 7–10 y | 2500 | 3 | 3 | 2.5 | 3.0 | 0.25 | 300 | 40 | 250 | 130 | 13 |
| Males | |||||||||||
| 11–14 y | 3000 | 4 | 4 | 5 | 6.0 | 0.4 | 450 | 40 | 250 | 200 | 18 |
| 15–17 y | 3000 | 4 | 4 | 7 | 9.0 | 0.5 | 500 | 45 | 250 | 250 | 22 |
| 18–50 y | 2500 | 4 | 5 | 7 | 11.0 | 0.6 | 600 | 45 | 250 | 300 | 25 |
| 51–70 y | 2000 | 4 | 5 | 7 | 11.0 | 0.6 | 600 | 45 | 250 | 300 | 25 |
| >70 y | 2000 | 3 | 5 | 7 | 11.0 | 0.6 | 600 | 45 | 250 | 300 | 25 |
| Females | |||||||||||
| 11–14 y | 3000 | 4 | 4 | 5 | 6.0 | 0.4 | 450 | 40 | 250 | 200 | 18 |
| 15–17 y | 3000 | 4 | 4 | 7 | 9.0 | 0.5 | 500 | 45 | 250 | 250 | 22 |
| 18–50 y | 2500 | 4 | 5 | 7 | 11.0 | 0.6 | 600 | 45 | 250 | 300 | 25 |
| 51–70 y | 2000 | 4 | 5 | 7 | 11.0 | 0.6 | 600 | 45 | 250 | 300 | 25 |
| >70 y | 2000 | 3 | 5 | 7 | 11.0 | 0.6 | 600 | 45 | 250 | 300 | 25 |
| Pregnancy | |||||||||||
| ≤18 y | 3000 | 3.5 | 7 | 9.0 | 0.6 | 600 | 45 | 250 | 300 | 25 | |
| 19–30 y | 2500 | 3.5 | 7 | 11.0 | 0.6 | 600 | 45 | 250 | 300 | 25 | |
| 31–50 y | 2500 | 3.5 | 7 | 11.0 | 0.6 | 600 | 45 | 250 | 300 | 25 | |
| Lactation | |||||||||||
| ≤18 y | 3000 | 4 | 7 | 9.0 | 0.6 | 600 | 45 | 250 | 300 | 25 | |
| 19–30 y | 2500 | 4 | 7 | 11.0 | 0.6 | 600 | 45 | 250 | 300 | 25 | |
| 31–50 y | 2500 | 4 | 7 | 11.0 | 0.6 | 600 | 45 | 250 | 300 | 25 | |
Sources of data: IOM (1); EFSA (10). EFSA, European Food Safety Authority; H-UL, harmonized upper level; IOM, Institute of Medicine; UL, tolerable upper intake level
Vitamin A applies to retinol and retinyl esters and does not apply to postmenopausal women as it may not provide an adequate margin of safety.
Niacin UL applies only to nicotinic acid in supplements or food fortification (ULs for nicotinamide range from 150 to 700 mg/d for children and 900 mg/d for adults).
Age groups for fluoride for children aged 4–14 y are slightly different: 4–8 y (H-UL = 2.5 mg/d) and 9–14 y (H-UL = 5 mg/d).
Magnesium applies to readily dissociable Mg salts and MgO in food supplements, water, or added to foods and does not apply to children aged 1–3 y.
In examining the methods used by EFSA and the IOM when setting ULs, we did not identify consistent differences. Both groups followed similar risk assessment models when determining ULs, but the selection of the studies to use and the uncertainty factors applied was not always similar. Furthermore, the forms of the nutrient to which the UL applies were not consistent. For example, the EFSA UL for vitamin E for adults is 300 mg/d, whereas the IOM value is 1000 mg/d. The EFSA UL was published in 2003, whereas the UL value from the IOM was published 3 y earlier, in 2000. The EFSA committee relied on a study of adverse effects on blood clotting in humans, and chose a low uncertainty factor of 2, whereas the IOM committee relied on animal studies and used a much higher uncertainty factor of 36. However, the extrapolation from rats to humans still led to a 3.3-fold higher UL from the IOM (1000 mg/d) compared to the UL from EFSA (300 mg/d). Furthermore, the IOM UL applies to all forms of supplemental α-tocopherol, whereas the EFSA UL applies to total intake of vitamin E expressed as α-tocopherol equivalents.
Another example is the UL for niacin, which is 35 mg/d for adults from the IOM and 10 mg/d from EFSA and applies only to intakes from supplements and food fortification. EFSA specifies that the UL applies only to nicotinic acid, and uses an additional uncertainty factor of 3 to adjust for small sample sizes in the studies used. For the same adverse effect (flushing), the IOM set a UL of 35 mg/d, but did not specify the form of supplemental niacin. For both vitamin E and niacin, we chose the lower EFSA value for the H-UL, primarily because the EFSA UL is more conservative, and was set slightly more recently.
Considerations when using H-UL values
As explained in depth by the IOM, the ULs are intakes with a low risk of adverse effects if consumed over a long term. They are not meant to be applied to a single intake (or short periods of intake); the toxicity of a single dose would theoretically be lower than that associated with usual, long-term intake. However, for some nutrients, data on acute doses were used to estimate a UL for chronic intake. The reader should refer to IOM and EFSA publications to better understand the ways in which ULs are derived (24, 26).
For some nutrients, the H-UL applies only to intake from supplements or fortified foods. For example, the vitamin A H-UL applies to retinol and retinyl esters and does not apply to postmenopausal women as it may not provide an adequate margin of safety in relation to the possible decrease in bone density and the risk of bone fracture. Magnesium applies to readily dissociable Mg salts and MgO in food supplements, water, or added to foods and does not apply to children aged 1–3 y. The niacin H-UL applies only to nicotinic acid obtained from dietary supplements, food fortification, or both. Nicotinamide is also used in dietary supplements, but is not known to cause flushing. However, it is important to note that supplement composition tables may not distinguish between nicotinic acid and nicotinamide in supplements, so intakes may reflect both forms of this nutrient.
Published values for ULs state the adverse effect that was used to select the UL values. The adverse effect for each H-UL is shown in Table 5. Usually, the adverse effect is considered the first that would appear if intakes exceed the UL, but additional adverse effects are likely if usual intakes continue to increase above the UL.
H-ULs were selected for infants when data were available, but EFSA set a UL only for vitamin D and the only ULs for infants established by the IOM are for vitamins A and D, fluoride, selenium, and zinc. Data were stated to be inadequate for setting other ULs for infants. Where no H-UL is available, IOM states that the only source should be food, given concern about the inability of infants to handle high intakes of nutrients (26). When a H-UL is available for infants, it is important to check that any fortification will not cause the H-ULs to be exceeded for a nutrient.
Conclusions
The harmonized core NRVs for average requirements (H-ARs) and upper levels (H-ULs) proposed here can be used for many applications that depend on population-level estimates of the prevalence of nutrient inadequacies or potentially excessive nutrient intakes. An obvious application is the need to know which nutrients might be included in food fortification or in dietary supplements. This type of information is also needed to design and evaluate a variety of feeding programs and food assistance programs for specific populations, as well as for meals that are provided for groups in institutions such as schools and hospitals. Importantly, national and international food guidance needs to be based on factual information about nutrient adequacies and excesses.
Harmonized values are proposed for a wide variety of nutrients: H-ARs for 25 nutrients, and H-ULs for 19 nutrients. They represent 1 of the largest collections of reference values currently available for evaluating the intakes of populations. Although some assumptions were necessary when selecting the harmonized values, they are based on extensive evaluations conducted over the past 20 y in Europe and the United States and Canada. The use of these consistent reference values across regions and countries will allow a more global comparison of nutrient adequacies and inadequacies, as well as a unique evaluation of possible adverse effects from excessive intakes across population groups.
It should be noted that the confidence in the accuracy of the H-ARs and H-ULs presented here varies widely. H-ARs that are imputed from AIs are likely to have a lower accuracy than those that are based on many years of research, such as protein requirements. Replacing AIs with ARs is the ultimate solution to this challenge. In addition, both H-ARs and H-ULs may vary depending on contextual factors. For example, as noted earlier, the bioavailability of iron and zinc is much lower for unrefined diets than for diets which contain less phytate. Dietary data must be adjusted for these differences in nutrient availability before applying the nutrient standards suggested here. Alternatively, dietary assessment programs may be used to make appropriate adjustment in requirements, as is done for iron and zinc by IMAPP. A comprehensive discussion of the many factors that can alter nutrient requirements (e.g., bioavailability, genetics, interactions, and health status) is presented in references (15) and (16).
The H-ARs and H-ULs can be readily modified to meet regional or local needs, if the user prefers. However, in the past, many countries have not had the resources or expertise to develop or publish the ARs and ULs that are needed to assess the intakes of their population groups. Ideally, the process presented here will assist countries such as these as well as other authoritative bodies to decide whether to accept, adapt, or revise these proposed recommendations rather than start a new, expensive, time-consuming process to derive new requirement and toxicity values.
In the future, it would be important for an international group such as WHO/FAO to review the values proposed here and modify as needed. Another possibility is to expand the harmonized values for populations to include values that cover the requirements of 97.5% of the population and thus could be used to plan the diets of individuals, a topic not covered here. Determining nutrient standards should not be a 1-time process because new discoveries regarding both nutrient requirements and potentially excessive intakes are ongoing. A mechanism for regular reviews and updates of harmonized values should be considered.
ACKNOWLEDGEMENTS
We thank Dr Daniela Hampel [University of California, Davis and the USDA, Agricultural Research Service (ARS), Western Human Nutrition Research Center] for technical support on figure preparation and manuscript formatting. USDA, ARS is an Equal Opportunity Employer. The authors’ responsibilities were as follows—LHA and SPM planned and wrote the manuscript. ALC reviewed the manuscript and provided statistical support and all authors: read and approved the final manuscript.
Notes
No sources of financial support.
Author disclosures: The authors report no conflicts of interest.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: AI, adequate intake; AR, average requirement; DFE, dietary folate equivalent; EAR, estimated average requirement; EFSA, European Food Safety Authority; H-AR, harmonized average requirement; H-UL, harmonized upper level; IMAPP, Intake Monitoring, Assessment, and Planning Program; IOM, Institute of Medicine; NASEM, National Academies of Sciences, Engineering, and Medicine; NRV, nutrient reference value; PRI, population reference intake; RNI, recommended nutrient intake; UL, tolerable upper intake level.
References
- 1. Institute of Medicine. Dietary Reference Intakes. The essential guide to nutrient requirements. Washington (DC): National Academies Press; 2006. [Google Scholar]
- 2. Institute of Medicine. Dietary Reference Intakes for calcium, phosphorus, magnesium, vitamin D and fluoride. Washington (DC): National Academies Press; 1997. [PubMed] [Google Scholar]
- 3. Institute of Medicine. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press; 1998. [PubMed] [Google Scholar]
- 4. Institute of Medicine.Dietary Reference Intakes for vitamin C, vitamin E, selenium and carotenoids. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 5. Institute of Medicine. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 6. Institute of Medicine. Dietary Reference Intakes for water, potassium, sodium, chloride, and sulfate. Washington (DC): National Academies Press; 2004. [Google Scholar]
- 7. Institute of Medicine. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington (DC): National Academies Press; 2005. [Google Scholar]
- 8. Institute of Medicine. Dietary Reference Intakes for calcium and vitamin D. Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- 9. EFSA. Dietary reference values for nutrients. [Internet] Summary report EFSA Supporting Publication; 2017;2017:e15121, [last accessed 2019 July 24]. Available from: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.e15121/epdf. [Google Scholar]
- 10. EFSA, Scientific Committee on Food and Scientific Panel on Dietetic Products, Nutrition and Allergies. [Internet] Summary of tolerable upper intake levels, version 2, August, 2017.EFSA; [cited 2018 Nov 6]. Available from: https://www.efsa.europa.eu/sites/default/files/assets/UL_Summary_tables.pdf. [Google Scholar]
- 11. WHO/FAO. Vitamin and mineral requirements in human nutrition. Second edition. Report of a joint WHO/FAO expert consultation on human vitamin and mineral requirements, 1998. Bangkok (Thailand) Second edition, WHO/FAO; 2004. [Google Scholar]
- 12. Department of Health. Report on health and social subjects, 41. Dietary reference values for food energy and nutrients for the United Kingdom. London: HMSO; 1991. [PubMed] [Google Scholar]
- 13. Codex Alimentarius. International food standards, guidelines on nutrition labelling. CAC/GL2-1985 Geneva: FAO/WHO; 2017. [Google Scholar]
- 14. King JC, Garza C. International harmonization of approaches for developing nutrient-based dietary standards. Supplement to Food Nutr Bull. 2007;28. [Google Scholar]
- 15. National Academy of Science, Engineering, and Medicine. Global harmonization of methodological approaches to nutrient intake recommendations: proceedings of a workshop. Washington (DC): National Academies Press; 2018. [Google Scholar]
- 16. National Academies of Sciences, Engineering, and Medicine. Harmonization of approaches to nutrient reference values: applications to young children and women of reproductive age. Washington (DC: ): National Academies Press; 2018. 10.17226/25148. [DOI] [PubMed] [Google Scholar]
- 17. Iowa State University, Department of Statistics. International Monitoring, Assessment and Planning Program (IMAPP). [Internet] Ames (IA: ): Iowa State University; [cited 2018 Nov 6]. Available from: http://www.side.stat.iastate.edu/imapp.php. [Google Scholar]
- 18. Iowa State University, Department of Statistics. Personal Computer Software for Intake Distribution Estimation (PC-SIDE). [Internet] Ames (IA): Iowa State University; [cited 2018 Nov 6]. Available from: http://www.side.stat.iastate.edu/pc-side.php. [Google Scholar]
- 19. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes: applications in dietary assessment. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 20. FAO/WHO/UNU. Protein and amino acid requirements in human nutrition. WHO Technical Report Series, 935 Geneva: WHO; 2007. [PubMed] [Google Scholar]
- 21. Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J et al.. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci. 1996;93(8):3704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Devaney B, Ziegler P, Pac S, Karwe V, Barr SI. Nutrient intakes of infants and toddlers. J Am Diet Assoc. 2004;104(Suppl 1):S14–21. [DOI] [PubMed] [Google Scholar]
- 23. Suitor CW, Gleason PM. Using dietary reference intake-based methods to estimate the prevalence of inadequate nutrient intake among school-aged children. J Am Diet Assoc. 2002;102:530–6. [DOI] [PubMed] [Google Scholar]
- 24. EFSA Scientific Committee on Food and Scientific Panel on Dietetic Products, Nutrition and Allergies. Tolerable upper intake levels for vitamins and minerals. [Internet] EFSA; 2006; [cited 2018 Nov 6]. Available from: http://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf. [Google Scholar]
- 25. National Academies of Science, Engineering and Medicine, Health and Medicine Division. Dietary Reference Intakes tables and application, [Internet]National Academies of Sciences; [cited 2018 Nov 6]. Available from: http://nationalacademies.org/hmd/Activities/Nutrition/SummaryDRIs/DRI-Tables.aspx. [Google Scholar]
- 26. Institute of Medicine. Dietary Reference Intakes: a risk assessment model for establishing upper intake levels for nutrients. Washington (DC): National Academies Press; 1998. [PubMed] [Google Scholar]
- 27. FAO/WHO. A model for establishing upper levels of intake for nutrients and related substances. [Internet] Geneva (Switzerland): WHO Press; 2006[last accessed 2019 July 24]. Available from: http://www.who.int/ipcs/methods/nra_final.pdf. [Google Scholar]


