ABSTRACT
Brain development is markedly affected by prenatal alcohol exposure, leading to cognitive and behavioral problems in the children. Protecting neuronal damage from prenatal alcohol could improve neural connections and functioning of the brain. DHA, a n–3 (ω-3) long-chain PUFA, is involved in the development of neurons. Insufficient concentrations of DHA impair neuronal development and plasticity of synaptic junctions and affect neurotransmitter concentrations in the brain. Alcohol consumption during pregnancy decreases the maternal DHA status and reduces the placental transfer of DHA to the fetus, resulting in less DHA being available for brain development. It is important to know whether DHA could induce beneficial effects on various physiological functions that promote neuronal development. This review will discuss the current evidence for the beneficial role of DHA in protecting against neuronal damage and its potential in mitigating the teratogenic effects of alcohol.
Keywords: nutrition, docosahexaenoic acid, brain development, prenatal alcohol, fetal alcohol spectrum disorder
Introduction
Alcohol is a teratogenic compound that can pass through the placenta, causing damage to the developing embryo. Prenatal alcohol exposure could damage the brain and other organs, resulting in developmental disabilities in the affected children. Collectively, the detrimental effects of alcohol are classified as fetal-alcohol spectrum disorders (FASDs). Due to underreporting and inconsistent definitions worldwide, the true prevalence of FASD is unknown (1), but recent studies reported a prevalence of ∼4% in Canada (2) and 2–5% in the United States (3). It is predicted that the numbers will only continue to increase with improved detection methods and tools for FASD diagnosis.
There is no agreed-upon safe amount of alcohol that can be consumed during pregnancy, and women may be unaware they are pregnant, as pregnancy may occur unexpectedly. Thus, it is possible that if alcohol is consumed in the first few weeks after conception, this could result in FASD. Therefore, the growing fetus, if exposed to any amount of alcohol, may have a disruption in its development. In particular, substantial evidence shows the detrimental impact of prenatal alcohol exposure on the normal process of fetal neurogenesis and brain development, as well as the affected child's cognitive and intellectual abilities. Despite this, there is currently no prophylaxis to prevent FASD. Current public health strategies focus on prevention via education and counselling to the mother on alcohol abstinence. Since maternal nutrition has been shown to play a role in FASD occurrence and severity (4), it is worth exploring nutritional strategies for FASD.
For the developing brain, the n–3 fatty acid DHA (22:6n–3) is accepted as an essential nutrient. DHA is enriched in neuronal membrane phospholipids (5) and has been implicated in multiple brain functions, including neurogenesis and neuronal survival (6–8). With alcohol consumption, DHA concentrations are compromised (see details in the section below entitled “Prenatal alcohol-induced DHA deficiency”) and this interferes with brain development (4, 9, 10). Although consuming alcohol during pregnancy is ill-advised, this review will focus on the association between DHA and prenatal alcohol exposure, while also providing evidence on the potential of DHA as a future therapeutic supplement for preventing or mitigating the teratogenic effects of alcohol on brain development and functions.
Current Status of Knowledge
DHA during pregnancy and brain development
The brain is rich in lipids (∼47.4% of dry matter) with a high concentration of n–3 PUFAs essential for neuronal development (5). DHA, the most abundant PUFA in the brain, accounts for 50% of the total brain lipids and between 60% and 80% of the brain membrane phospholipids (11). DHA is highly esterified to phosphatidylethanolamine and phosphatidylserine (12, 13). The 6 cis double bonds enable the kinking of DHA molecules, which prevents packing of adjacent phospholipid molecules, causing disordered lipid arrangements in membranes necessary to increase fluidity by changing the physical-chemical properties (14). DHA is enriched in the gray matter of the neuronal membrane and is highest in the synaptic membrane (15, 16). It is required for optimal neuronal membrane dynamics for the proper functioning of receptors, ion channels, and proteins involved in the transport of biochemical molecules and synapsis. DHA has also been shown to play a critical role in modulating neurogenesis, myelination, membrane integrity, signal transduction, neurotransmission, and neuroplasticity in neurons (15, 17). Overall, optimal DHA concentrations are required to maintain neuronal membrane structure and function, which is vital for overall neurological development.
The accretion of DHA is high during fetal and neonatal brain-growth spurts and continues up to 2 y of life (5, 17). The third trimester experiences the highest DHA accumulation; however, this slows down during the late infant stages and reaches a plateau during early adulthood (5). This confirms the need for a constant supply of DHA from the fetal stages to early life (5). Although DHA can be supplied via the diet, the brain is fully capable of synthesizing DHA endogenously via the neuroglial cells known as astrocytes (18). These cells are able to begin the initial desaturation and elongation steps from the dietary essential fatty acid α-linolenic acid (ALA; 18:3n–3). Once desaturated and elongated, DHA is released into the surrounding cerebrospinal fluid, where it can then be used for multiple structural and functional roles in the brain (18–20). However, whether the DHA supply can be met solely by endogenous synthesis in vivo remains controversial. This arises from studies tracking the entry of radiolabeled ALA into the rat brain, where it was found to be almost completely metabolized via β-oxidation into aqueous β-oxidation products (21). This indicated that the actual conversion of ALA into DHA was <0.2% (21). Thus, it appears that the brain depends on the exogenous supply of DHA more than endogenous synthesis in the brain.
Consequently, the low endogenous production of DHA renders greater requirements for dietary sources of DHA, particularly during the brain-growth-spurt period during the end of pregnancy and throughout lactation. Current guidelines for the daily intake by pregnant and lactating women recommended by the UN FAO is 300 mg EPA + DHA/d, of which 200 mg should be DHA (22). However, the current average daily intake is ∼100 mg in the United States (23) and Canada (24), which is far lower than the recommended intake. In fact, our recent study targeting Canadian-Manitoban pregnant mothers’ intakes of DHA found that only 16.1% of mothers met adequate intake levels (25). Whether these mothers were consuming alcohol during pregnancy, and therefore any consequential effect on DHA concentrations, is unknown. Ultimately, it is evident that DHA is an important structural component for the growth of the brain and thus must be supplied at optimal amounts.
Effects of DHA on brain physiology and functions
The functions of DHA on brain development and neurocognition have been shown in both rodent and human studies (26, 27). This is thought to involve the interaction of DHA with pathways responsible for the regulation of neurotransmitters (28), neuronal survival (6), and neurotrophins (29, 30), thereby improving cognitive and memory function.
Effects of DHA on neurotransmitters
The actions of neurotransmitters are vital for proper brain development and function. DHA has been implicated in the regulation of many neurobiological systems, such as the glutamatergic system, dopaminergic system, noradrenergic system, and serotonergic system (31–33) and their related receptors. For example, Tang et al. (34) found that maternal DHA deficiency led to a reduction in hippocampal cell proliferation in neonatal female rat pups, in which the brain membrane DHA was positively associated with metabolism and turnover of glutamate and serotonin.
In addition, DHA increases synapsin and glutamate receptor expressions, whereas the lack thereof decreases their protein concentrations in hippocampal neurons (32). γ-Aminobutyric acid (GABA) is an inhibitory neurotransmitter that decreases neuronal excitability, thus reducing neuron activity. DHA inhibits GABA activity by desensitizing GABA receptors through altering lipid bilayer fluidity (35, 36). Another neurotransmitter, dopamine, plays a major role in reward-motivated behavior, motor control, and vasodilation. In a mouse model, DHA feeding partially restored dopaminergic neuronal system activity (37) and increased dopamine concentrations in the hypothalamus (38). Decreased brain DHA also caused a reduction in dopamine receptors in female rats (39). Other studies reported the effects of DHA on increasing neurotransmitters, such as noradrenaline (40) and acetylcholine (41).
Overall, it is believed the mechanistic actions of DHA on neurotransmitters, particularly during brain development, are on their metabolism and regulation (28, 42). These findings underline the importance of DHA in the development and functioning of the brain through the regulation of its neurotransmitters. A deficiency of DHA may impair or reduce neurogenesis and neural communications. Prenatal and postnatal DHA supplementation could improve deficits in memory, social behavior, and other cognitive functions by increasing neurogenesis and modulating neurotransmitter activities (Figure 1).
FIGURE 1.
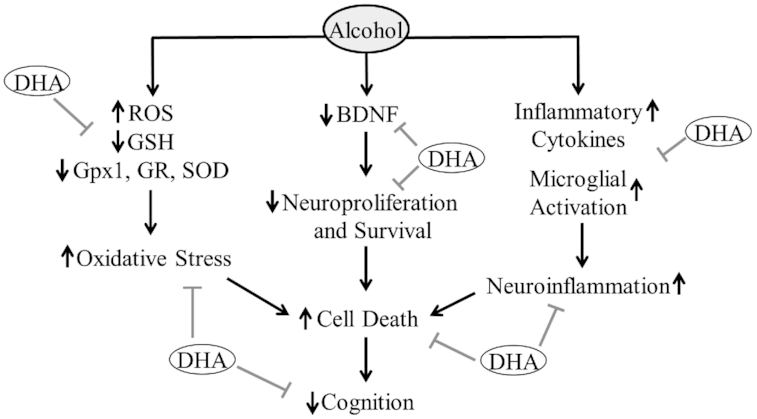
DHA supplementation protects the developing brain against prenatal alcohol exposure. The negative effects of maternal alcohol and/or DHA deficiency on physiological processes will have detrimental consequences on the development or physiological functioning of the offspring's brain. The positive effects of maternal DHA on physiological processes will potentially have beneficial consequences on the development or physiological functioning of the offspring's brain. BDNF, brain-derived neurotrophic factor; Gpx1, glutathione peroxidase-1; GR, glutathione reductase; GSH, reduced glutathione; ROS, reactive oxygen species; SOD, superoxide dismutase.
Effects of DHA on neurotrophins
Neurotrophins, or neurotrophic factors, play vital roles in proper brain development and function. It is also well known that the disruption of neurotrophins or receptor function during development could result in long-term changes in cognition, memory formation, and mood (43). The timing of neurogenesis, survival, and death of neurons is carefully orchestrated in consultation with neurotrophin signaling (43).
While there are several neurotrophins, brain-derived neurotrophic factor (BDNF) is the best characterized (44). BDNF exhibits a unique expression profile throughout the development of the brain, with alterations to expression causing significant and long-term consequences on neuronal cell structure and function (43). This timing is of vital importance, and exposure to toxins or stress during gastrulation and neurulation could influence the expression of these neurotrophins in a timing-dependent manner (20, 43, 45).
BDNF is abundant in the developing brain and induces its biological functions by binding to its high-affinity receptor tropomyosin receptor kinase B (TrkB), which initiates downstream activation of the transcription factor cAMP response element–binding protein (CREB) (20). The activation of CREB turns on the expression of genes involved in neuronal survival (46), neurogenesis (47), and plasticity (48). It is through this mechanism that DHA has been implicated, as several rodent studies have shown that maternal diets high in DHA increase the expression of BDNF, TrkB, and the phosphorylation of CREB (49) in the corresponding offspring. Although it is not specific to DHA, human studies have found n–3 PUFA supplementation to increase BDNF in the peripheral circulation (50, 51), which is thought to be correlated with overall central nervous system expression (52). However, whether DHA supplementation alone during pregnancy renders increased peripheral BDNF in the developing offspring is unknown.
Effects of DHA in neurogenesis and synaptic plasticity
The effects of DHA on neurogenesis, synaptic plasticity, and overall growth of neurons are through its role in regulating neurotransmitters and neurotrophins. Neurogenesis, neurite growth, and synaptic plasticity are important physiological processes that are crucial for brain functions. Although there is limited evidence available, some data support DHA's contributions to these macro-scale processes; however, these are highly mechanistic studies that used only rodent and/or cell culture models. Both in vivo and in vitro evidence showed that DHA stimulates differentiation of neural stem cells of the hippocampus to mature neurons (53, 54), indicating the importance of a constant supply of DHA. DHA's capacity to differentiate stem cells into neurons also involves dendritic neurite sprouting, which has been shown to promote neuronal plasticity (55). When DHA is deficient, neurite growth in hippocampal neurons decreases in the affected fetuses (32, 56). Uptake of DHA into neuronal cells is important for increasing neurite growth in vitro. In human neuroblastoma cells, an increase in the expression of the neuron growth–associated protein-43 promoted neurite outgrowth specifically in response to DHA concentrations (57).
Synaptic plasticity is another factor determining neuronal function, as it is required for long-term potentiation (LTP). LTP of synapses produces long-lasting signal transmission between the neurons, which improves neuronal functioning. Experiments with hippocampal slices showed that DHA supplementation resulted in greater LTP between synapses (58). All of these findings strongly suggest that DHA supplementation is required for both brain development and function through promoting neurogenesis and neurite outgrowth and improving neuronal plasticity. Animal studies that found positive effects of maternal DHA or total n–3 PUFAs on various physiological and functional outcomes in the offspring are shown in Table 1. However, whether human maternal DHA supplementation during pregnancy translates into better cognitive outcomes in the offspring remains controversial.
TABLE 1.
Animal studies showing maternal DHA-mediated benefits on brain physiological and development outcomes of the offspring1
| Study | Animal model | DHA or n–3 PUFA dose | Duration of feeding | Major outcomes |
|---|---|---|---|---|
| Tang et al. (34) | Pregnant Sprague Dawley rats (n = 7/group) and neonatal offspring (n = 6–8/group) | Modified AIN-93G diet with fish oil (20 g/kg; 25% wt/wt DHA of total fatty acids) vs. control diet with soybean oil (50 g/kg) | Throughout gestation (3 wk) | DHA deficiency impaired offspring serotonin/glutamatergic system; also reduced neurogenesis in hippocampus compared with DHA-supplemented group |
| Matsui et al. (33) | Pregnant wild-type/SERT-KO C57BL/6 mice (n = 3–4/group) and offspring (variable; 5–10/group) | Modified AIN-93G diet with 1% ethyl ester DHA (wt/wt of diet) vs. no-DHA control diet | Throughout gestation, lactation and to postnatal day 60–70 | DHA decreased offspring dopamine content in striatum of stressed SERT mice, while improving socialization |
| Balogun et al. (29) | Pregnant C57BL/6 mouse dams (n = 6/group) and offspring (n = 6/group) | Modified AIN-93G diet with 20% fat, high n–3 PUFAs (3.19% DHA wt/wt of total fatty acids) vs. low n–3 PUFA diet (0.39% DHA wt/wt of total fatty acids) | Throughout gestation, lactation, and 16 wk postweaning | High n–3 PUFA diet increased offspring cortical DHA and BDNF at weaning and 16 wk postweaning, which led to increased activation of CREB |
| Bhatia et al. (20) | Pregnant Sprague Dawley rats (variable) and offspring (n = 5–7/group) | Modified AIN-93G diet with 1.2% DHA wt/wt of diet vs. a diet without DHA | Throughout gestation, lactation, and 15 wk postweaning | DHA deficiency decreased offspring brain DHA and BDNF signaling (CREB activation) in frontal cortex, hippocampus, and hypothalamus |
| Cao et al. (32) | Pregnant C57BL/6 mice (unspecified n value) to obtain fetuses at gestational day 18 for ex vivo neuronal cultures (unspecified n value) | Modified AIN-93G diet with DHA (DHASCO; 0.3%, wt/wt diet) vs. no DHA control diet | Fed for 16 d throughout gestation (days 2–18) | DHA supplementation increased fetal synaptic activation, glutamatergic activity; also increased neurite growth |
| Calderon and Kim (56) | Pregnant Sprague Dawley rats (unspecified n value) and fetuses at gestational day 18 for ex vivo neuronal cultures (unspecified n value) | NIH diet with DHA at 2.5 mol% of total fatty acids vs. diets with no DHA | Fed for 16 d throughout gestation (days 2–18) | DHA supplementation increased fetal hippocampal neurite branches in length and number |
BDNF, brain-derived neurotrophic factor; CREB, cAMP response element–binding protein; DHASCO, DHA-rich Single Cell Oil; KO-, knockout; SERT, serotonin transporter.
Effects of DHA on learning and memory functions
As mentioned, an adequate concentration of DHA is necessary for optimal brain function—specifically, perinatal concentrations of DHA are critical for learning and memory functions (59). When DHA is deficient in the young-adult human brain, it results in impairment in learning ability and memory function (60). A 58% decrease in rat brain DHA is associated with impairment in spatial learning (61). However, this deficit may not be permanent, as providing dietary DHA during pregnancy and lactation to mice during this deficient state was shown to improve memory and learning ability (62). DHA also increased rat hippocampus concentrations of Ca2+/calmodulin-dependent protein kinase II, CREB, BDNF, and synapsin 1, which were associated with improvement in short-term memory in rats (63).
In human trials, a follow-up study in children at 39 mo of age showed that, when feeding DHA-enriched infant formula for 1 y, they had better visual and cognitive development compared with those fed a control formula with no added DHA (64). In another randomized controlled trial, supplementation with infant formula containing DHA (0.35%) resulted in an improved Mental Development Index, a measurement of early cognitive and language development, compared with a control group (formula with no DHA) (65); however, no difference between the control and DHA-supplemented group was observed at a 4-y follow-up measurement (66). Furthermore, randomized clinical trials showed DHA supplementation in cereal bars (300 mg/d DHA) and fish oil (2.2 g/d DHA) during pregnancy improved neurocognitive development in infants (67, 68). The results showed improvements in problem solving and hand–eye coordination compared with the placebo group (67, 68). Overall, DHA provision during pregnancy appears to be vital for the proper growth, development, and protection of the fetal brain and, when limited, may cause alterations in brain structure and functions. Therefore, it is conceivable that any insults that may occur with environmental factors, such as alcohol, may have a large impact on fetal brain development, particularly when co-occurring with a DHA deficiency during pregnancy.
Effects of alcohol on the developing brain
The brain is the most vulnerable organ of the body during prenatal alcohol exposure. In fact, severe cases of FASD are marked by microcephaly and central nervous system impairment. However, only a few human studies are available that assessed the biochemical or molecular alterations induced by prenatal alcohol exposure and these were limited to postnatal brain imaging (69). Therefore, much of the current evidence collected on the molecular impacts of prenatal alcohol is via animal and human in vitro and animal in vivo studies. From this it has been proposed that alcohol-induced neuronal death during the prenatal period is due to oxidative stress (10), neuroinflammation (70), and neurotrophin dysregulation (71).
Proposed prenatal alcohol-induced brain damage: oxidative stress, inflammation, and apoptosis
Effects of alcohol on oxidative stress
The 3 classical methods of alcohol metabolism are via the alcohol dehydrogenase, cytochrome P450-2E1 (CYP2E1), and catalase. These systems render formation of various levels of alcohol-induced oxidative stress. This oxidative stress is in the form of superoxide (O2−•) and hydroperoxyl (HO2•) free radicals, which results in lipid peroxidation, causing membrane disruption, enzymatic dysfunction, endoplasmic reticulum (ER) stress, and chromosomal alterations. When alcohol is metabolized, CYP2E1-mediated alcohol metabolism produces acetaldehyde and hydrogen peroxide, which then interact with copper/iron, producing reactive oxygen species (ROS) in the brain. Acetaldehyde promotes ROS and NO generation through activation of NAD(P)H/xanthine oxidase and NO synthase (72). Aldehyde dehydrogenase converts acetaldehyde to acetate, while producing NAD(H). Excessive decreases in the NAD+ to NAD(H) ratio increases superoxide radicals (73). These free radicals are then neutralized by antioxidative enzymes (glutathione peroxidase, superoxide dismutase, and glutathione reductase) and the molecule reduced glutathione (GSH); however, alcohol reduces the activities of these enzymes while the alcohol-generated ROS depletes the GSH pool (74). This alcohol-mediated oxidative stress leads to neuronal membrane damage by increasing lipid and protein peroxidation (75), disturbing membrane integrity, which ultimately is thought to lead to neuronal death. Overall, in utero alcohol exposure increases ROS concentrations and subsequent oxidative stress, which are thought to be responsible for the subsequent neuroinflammation through activation of inflammatory cytokines (76, 77), which is then followed by neuronal apoptosis in the developing brain (78, 79).
Effects of alcohol on neuroinflammation
Recent reports suggest that neuroimmune activation and inflammation play a role in the alcohol-induced neurotoxicity (80, 81). For example, Terasaki and Schwarz (82) found rat dams fed a low-to-moderate dose of alcohol (blood alcohol: 70 mg/dL; ∼1–2 drinks per sitting for humans) increased fetal hippocampus and cerebral cortex proinflammatory cytokines (TNF-ɑ, IL-5, IL-21) and chemokines [chemokine ligands (CCL) 3, CCL6, CCL9, and the chemokine receptor 2 (CCR2) compared with controls that were not prenatal alcohol-exposed. Additionally, the hippocampus has also been shown to upregulate Toll-IL-1 receptor domain-containing adaptor protein inducing IFN-β (TRIF), TNF-ɑ, and IL-1β in rats after being prenatally exposed to alcohol (80). These alcohol-induced cellular immune cascades have been associated with the activation of microglia, which is thought to be via the activation of Toll-like receptor 4 (TLR4) (81). Other rodent studies have also linked alcohol exposure during the perinatal and neonatal period to microglial activation, the release of cytokines, and later-life cognitive deficits (83, 84). The adult male offspring, exposed to binge-alcohol consumption (blood alcohol: 79 mg/dL) during pre- and early posnatally, had motor coordination and spatial working memory impairments (84). Interestingly, these behavioral effects were associated with an upregulation of proinflammatory signaling (TLR4, NF-κB, p65, caspase-1, and IL-1β), gliosis (glial cell reaction to injury), a reduction in several structural myelin proteins, and ultimately neuronal cell death in both the prefrontal cortex and hippocampus of adult mice exposed to alcohol.
Ultimately, this dysregulated neuronal immune response is associated with neuronal death and later-life cognitive impairment; however, the exact mechanism of the altered regulation and expression of these cytokines remains elusive.
Effects of alcohol on neurotrophins
As a double-edged sword, the underlying processes of neuronal death are also coupled with a reduction in neurogenesis and protection via BDNF. A recent review by Boschen and Klintsova (71) covered extensively the effect of prenatal alcohol exposure on brain neurotrophin regulation during development. The authors found that the effect of alcohol exposure on neurotrophins and their receptor expression in the developing brain was highly dependent on alcohol dose, timing of exposure, route of exposure, and the brain region studied (71), which is in agreement with the severity of FASD as reviewed by Young et al. (4). BDNF, a well-studied neurotrophin in rodent models, has demonstrated downstream signaling changes in various brain regions after prenatal alcohol exposure (70). For example, following alcohol exposure [3 g · kg−1 · d−1 via gastric intubation; equivalent to a moderate alcohol dose similar to the amount reached by women having 1–2 drinks within 1 h (85)] between gestational days 5 and 20, BDNF protein and mRNA were reduced in the rat cortex and hippocampus when assessed on postnatal day (PD) 7–8 (85). Additionally, prenatal alcohol exposure has also been shown to alter concentrations of the TrkB receptor, the high-affinity BDNF receptor, inhibiting the phosphorylation of TrkB on PD 7–8 while leaving the total amount of TrkB unchanged (86). Therefore, it can be postulated that downstream BDNF-induced CREB activation is reduced, rendering a reduction in neurogenesis, LTP, and neuronal protection mechanisms in the fetal brain. However, no studies to date have assessed whether any intervention or treatment can be given to attenuate the effects of alcohol on the developing brain's neurotrophin signaling.
Not only does alcohol have a direct effect on BDNF but it has also been shown to influence BDNF via the widely distributed N-methyl-D-aspartic acid (NMDA) and GABA receptors. The effects of alcohol on the glutamatergic NMDA receptor antagonist and GABAA receptor agonist are well known. These receptors are also affected by neurotrophins, like BDNF, and present a potential area of cross-talk between BDNF and alcohol action (71, 87).
Protective effects of DHA: potential therapeutic during prenatal alcohol exposure?
The effects of prenatal alcohol exposure on the developing offspring's brain have shown the need for targets to help prevent or mitigate FASD. Interestingly, many of the mechanisms described have been shown to be affected by DHA. Thus, in the following sections we will describe what is known about DHA supplementation during prenatal alcohol exposure and the evidence to support its use as a potential therapeutic.
Effects of DHA on alcohol-induced neuronal oxidative stress
Although DHA is not an antioxidant molecule itself, it has been shown to upregulate antioxidant mechanisms. Induction of heme oxygenase-1 promotes antioxidant activity, which has been shown to protect neuronal cells and neuroplasticity (88). In vitro studies using 30 μM DHA was shown to induce heme oxygenase-1 expression by activating protein kinase-B and extracellular signal–regulated kinase pathways and to decrease NO synthesis by reducing NO synthase expression in microglial cells (89). Haorah et al. (72) showed a marked increase in the lipid peroxidation product 4-hydroxynonenal, while decreasing neurofilaments with alcohol exposure. While lipid peroxidation may decrease DHA concentrations, this could be normalized with supplementation or increased intake. This may stabilize neuronal membranes and prevent neuronal cell damage. A study by Patten et al. (90) showed that a fish-oil–enriched maternal diet containing 10% fat (24.6% DHA, %wt/wt) increased the antioxidant GSH, which was accompanied by a reduction in lipid peroxidation in certain brain regions in rat offspring, compared with a diet containing no DHA. In their follow-up study, Patten et al. (90) found that the same amount of DHA supplementation resulted in functional improvement in the prenatal alcohol-exposed brain as evidenced by reversing long-term deficits in synaptic plasticity. These findings suggest that DHA together with antioxidants could protect against alcohol-induced oxidative damage on developing neurons.
Alcohol-induced oxidative stress, via ROS, has also been linked to ER stress in the developing brain (91). As mentioned earlier, alcohol produces substantial ROS by the mitochondria, which impairs redox conditions in the ER (92). This ER stress triggers the unfolded protein response that initially shuts down protein synthesis and, if prolonged, renders the activation of apoptosis (93). Inhibition in protein synthesis causes accumulation or depletion of Ca2+, disrupting Ca2+ homeostasis. This could be due to increased inositol triphosphate receptors (IP3R), ryanodine receptor-1, or the inactivation of sarco/endoplasmic reticulum Ca2+ ATPase could be the underlying mechanism. This is where DHA has been shown to have effects on, by inhibiting the IP3R pathway, preventing Ca2+ depletion, and reducing ER stress (94, 95). Another potential pathway for DHA to inhibit ER stress is via the previously discussed neurotrophin BDNF. BDNF has been shown to suppress ER stress (96), whereas a maternal diet providing 1.25% (wt/wt of total fat) DHA increased BDNF in the brains of offspring (63). Therefore, the DHA-induced BDNF expression and its effect on ER stress in the developing brain should be further explored.
Ultimately, preventing ER stress could be an underlying mechanism by which DHA reduces ROS production. Interestingly, a recent clinical study showed that when pregnant women were supplemented with a dairy drink containing EPA + DHA (400 mg/d), both mothers and neonates had significantly lower oxidative stress levels compared with a control group that received a regular dairy drink (97). Therefore, preventing or reducing oxidative stress levels by DHA could be a major cellular mechanism through which DHA prevents alcohol-induced neural damage.
Effects of DHA on alcohol-induced neuroinflammation
Alcohol-induced neuronal damage is also linked to neuroinflammation through activation of inflammatory cytokines such as IL-6, TNF-α, and transforming growth factor (76, 77). Resolvin, a bioactive metabolite of DHA, has anti-inflammatory properties (98) that decrease inflammation in microglial cell cultures (99). DHA could directly block microglia-mediated activation of the transcription factor NF-κB, which initiates multiple inflammatory processes (100). DHA also targets cyclooxygenase-2 (COX2) to reduce neuroinflammation (101) by decreasing the formation of the arachidonic acid (C20:4n–6)–derived inflammatory eicosanoids. In 1 study, binge alcohol exposure in rats led to increased aquaporin 4 (AQ4), proinflammatory phospholipase A2 (PLA2), and poly[ADP ribose]polymerase 1 (PARP1) in brain regions with extensive neurodegeneration. In the same study, in vitro supplementation with DHA blocked AQ4, PLA2, and PARP1 in rat hippocampal slices, supporting its anti-inflammatory action for neuronal protection (102). These studies all indicate that DHA supplementation may block alcohol-induced increases in neuroinflammation and neurodegeneration. However, whether DHA supplementation during prenatal alcohol exposure has an effect on cytokines and microglial responses is yet to be determined.
Effects of DHA on alcohol-induced neuronal apoptosis
The loss of neurons with alcohol exposure culminates in memory loss and cognitive decline. The final step of alcohol-induced neurodegeneration involves apoptosis (103). Apoptotic signaling operates by activating either the extrinsic pathway mediated by caspase-8 or the intrinsic (mitochondria-associated) pathway mediated through the apoptosis-inducing factor. The extrinsic pathway goes through a cascade of caspase activation and suppression of B cell lymphoma (Bcl)-2 and Bcl-extra large, whereas the mitochondrial pathway results in DNA damage via PARP activation (104). Both pathways trigger cell death and pave the way for clearance by macrophages and other cell types. Alcohol consumption greatly affects hippocampal growth, development, and life of neuronal cells by apoptosis and neuron cell death (105). In vitro evidence using the hippocampal cultures from rats showed that alcohol-exposed samples had a higher rate of apoptotic death, which was accompanied by decreased DHA-enriched phosphatidylserine accumulation (106). DHA-phosphatidylserine accumulation is known to protect against apoptosis; therefore, the supply of DHA may be critically important with increased alcohol exposure. Another DHA metabolite, neuroprotectin D1, produced through the release of unesterified DHA released by PLA2, is a strong inhibitor of oxidative stress–induced apoptosis and COX2 (107). Neuroprotectin D1 via COX2 exerts neuroprotection through inhibition of proinflammatory signaling and leukocyte infiltration (108).
Collectively, evidence from both in vitro and in vivo studies suggests that DHA supplementation could preserve neuronal functions by improving membrane integrity and synaptic plasticity and prevent alcohol-induced neurodegeneration through reducing oxidative stress, lipid peroxidation, inflammation, and apoptosis. Whether DHA supplementation during prenatal alcohol exposure has an effect on BDNF expression, and whether this is protective against apoptosis in the brain, requires further study.
Prenatal alcohol-induced DHA deficiency
As mentioned earlier, the average pregnant North American woman does not consume enough DHA according to recommendations, which renders low concentrations for the developing fetal brain. Additionally, pregnant women consuming alcohol have decreased maternal intake of n–3 PUFA or DHA foods (109); thus, maternal DHA status and the placental transfer of DHA to the fetus are also decreased (110). May et al. (111) found that South African mothers of children with FASD consumed more total nutrients [protein, vitamin E, C, vitamin B-6, magnesium, phosphorus, EPA, DHA, and docosapentaenoic acid (DPA, C22:5n–3)] compared with their nondrinking controls; however, all mothers were still deficient in these nutrients. Although mothers with FASD children consumed more nutrients, this difference did not lead to any protective or enhanced developmental outcome (109), which may have been due to multiple factors including nutrient absorption and transport or competition with alcohol in the intestine. For instance, placental fatty acid transport is concentration gradient dependent and has a preference towards DHA via fatty acid–binding proteins (110, 112); thus, alcohol may attenuate placental transportation and reduce the supply of DHA to the fetus. For example, an ex vivo experiment found that DHA transport across the human placenta to the developing fetus is decreased when in the presence of alcohol (110). Whether this ex vivo finding also occurs in vivo requires further verification.
Interestingly, this potential reduction in DHA transport to the developing fetal brain via the placenta may account for the observed reduction in brain DHA (113). Experiments on guinea pigs showed that alcohol administration decreased DHA concentrations in fetal brain phospholipids (113). However, whether this finding is true for human feto-placental transport and DHA accumulation in the developing brain requires further elucidation.
Effects of DHA supplementation on pregnancy outcomes and cognitive function: efficacy and effectiveness on the implications for FASD
As described above, the information with regard to the use of DHA as a therapeutic agent during prenatal alcohol exposure is limited, specifically to animal in vivo and in vitro models. Although these studies show its effectiveness against alcohol insult, the question arises as to whether DHA supplementation in human models is effective. No study to date has been done on maternal alcohol consumption and DHA supplementation; therefore, we are limited to clinical trials involving maternal DHA supplementation and their overall effect on pregnancy outcomes, including cognition.
Effects of DHA supplementation on pregnancy outcomes
Many studies have reported the benefits of n–3 PUFA (DHA and EPA) supplementation on pregnancy outcomes (108, 109). According to the 2006 Cochrane review, n–3 PUFA supplementation during gestation resulted in higher birth weight and size in infants (114). In addition, a meta-analysis of randomized clinical trials reported that high-risk pregnancies benefited from supplementation with n–3 PUFAs (115). Furthermore, dietary studies showed that the consumption of n–3 PUFA–rich oily fish (4–5 times/mo) was positively correlated to neonatal weight and head circumference, whereas lean fish consumption (low in n–3 PUFAs) was negatively correlated with these outcomes (116). Therefore, the evidence suggests that supplementation or optimal dietary intake of n–3 PUFAs could potentially improve pregnancy outcomes in women consuming alcohol during pregnancy.
As shown above, most studies used mixtures of n–3 PUFAs and reported beneficial outcomes at the end of pregnancy; only a few studies used DHA alone. A recently published article that re-analyzed 2 clinical trials [Donor Milk for Improved Neurodevelopmental Outcomes (DOMInO) (117) and Kansas University DHA Outcome Study (KUDOS) (118)] showed a significant reduction in the incidence of preterm birth with 800 mg (DOMInO) and 600 mg (KUDOS)/d of DHA supplementation in pregnant women from Australia and the United States (119). A study involving African-American women living in low-income environments, who are at risk of poor nutrition during pregnancy, found that DHA supplementation improved birth weight (120). Additionally, Harris et al. (121) showed that 300 or 600 mg/d of DHA supplementation increased gestational length. Innis and Friesen (122 ) also reported that 400 mg/d of DHA improved visual acuity. Overall, the major findings of these studies were the benefits of prenatal supplementation with n–3 PUFAs or DHA alone on birth weight, length, and, most importantly, gestational length, which are all vital factors for optimal brain growth.
International recommendations for pregnant women and mothers are the same as for the general population (22), which may be suboptimal due to the increased demand for DHA during embryo development (123). The human studies discussed in this review show how n–3 PUFAs, primarily DHA, could alter outcomes of at-risk pregnancies and improve cognitive functions in children (Table 2). More clinical supplementation studies are needed to establish the appropriate dosage for pregnant women, breastfeeding mothers, infant formulas for term and preterm infants, and infants at risk of various disease states.
TABLE 2.
Clinical evidence showing DHA-mediated benefits on pregnancy and cognitive development of the offspring1
| Study | Study population and number of participants in the DHA group | DHA dose (other ingredients) | Duration of supplementation | Major outcomes |
|---|---|---|---|---|
| Prenatal supplementation | ||||
| Keenan et al. (120) | Low-income African American pregnant women, n = 43 | 450 mg/d | 6 wk (starting 16–21 wk of gestation) | Increased birth weight and cortisol response compared with soybean oil placebo group |
| Carlson et al. (118) | Pregnant women (8–16 wk of gestation), n = 178 | 600 mg/d (algae oil capsules) | 24–32 wk (or until birth) | Increased gestation duration and infant size compared with soybean/corn oil placebo group |
| Innis and Friesen (122) | Pregnant women, n = 67 | 400 mg/d | 16 wk of gestation to delivery | Improved visual acuity compared with corn/soybean oil placebo group |
| Dunstan et al. (67) | Pregnant women, n = 52 | 2.2 g/d (in fish oil with 1.1 g EPA) | 19 wk (starting 20 wk of gestation) | Improvement in child's eye and hand coordination compared with olive oil placebo group |
| Judge et al. (68) | Pregnant women, n = 14 | 300 mg/d (in cereal bars) | 15 wk (starting 24 wk of gestation) | Improved problem-solving skills at 9 mo old compared with cereal-based placebo group |
| Postnatal supplementation | ||||
| Birch et al. (65) | 5-d-old healthy infants, n = 79 | Ad libitum, 0.36% DHA (with 0.72% AA of total fatty acids, supplemented with iron) | 16 wk | Improved visual acuity and IQ maturation compared with 0.36% iron + DHA group |
AA, arachidonic acid; IQ, intelligence quotient.
Effects of DHA supplementation on pregnancy outcomes during prenatal alcohol exposure
Despite studies showing positive outcomes using DHA supplementation during pregnancy, there are currently no known human studies on the protective effects of DHA supplementation for FASD. A few animal studies, however, showed promising results. In a rodent study, postnatal DHA supplementation (10 g · kg−1 · d−1) via artificial rat milk for 10 d improved behavioral deficits in animals prenatally exposed to alcohol, while improving postnatal vocalizations, playing nature, and anxiety in rats (9). Another study postnatally providing DHA (24.6% of a 10%-fat diet) after prenatal ethanol exposure showed that it increased the antioxidant GSH and reduced lipid peroxidation in the dentate gyrus and cerebellum (10). Thus, DHA can at least partially reverse the negative effects after prenatal alcohol exposure.
Although these studies supplemented DHA postnatally, 1 study found that prenatal supplementation with tuna oil (130 mg DHA/d) increased DHA in the fetal brain of guinea pigs exposed to alcohol and partially restored motor function deficits (124). Therefore, if alcohol is consumed during pregnancy, providing DHA postnatally or prenatally may mitigate the negative effects on the developing brain. Although animal studies provide beneficial effects of DHA, a randomized controlled trial involving alcohol and DHA during pregnancy is yet to be done. However, a clinical study in this regard has ethical ramifications. Thus, future studies should work with an at-risk group in an ethical and respectful manner to determine if mothers are consuming alcohol during pregnancy. This will help establish baseline data for the future follow-up of these alcohol-exposed children and to determine how DHA played a role on their future prognosis.
Controversies related to DHA supplementation and cognitive function
The various effects of maternal and/or postnatal DHA or n–3 PUFA supplementation are summarized in Table 2. As stated previously, the variability in the cognitive and behavioral results makes it difficult to make a conclusive statement on the effects of maternal DHA supplementation on infant brain development. However, many of these studies were conducted in healthy infants, suggesting that DHA may not provide any additional benefits when consumed at higher amounts than normal recommendations. Providing DHA ex utero to increase the concentrations to match those in the womb might be more beneficial for preterm infants (125). Maintaining optimal DHA concentrations in malnourished pregnant women and breastfeeding mothers may provide greater cognitive development in those offspring compared with offspring of mothers who did not meet optimal DHA concentrations. The FASD population is deficient in DHA; thus, there is a strong possibility that DHA supplementation will benefit infants born to pregnant mothers who consume alcohol during pregnancy.
Remaining Knowledge Gaps
Clinical evidence is required to further establish the role of DHA as a therapeutic and/or preventative molecule against prenatal alcohol-induced developmental deficits on the brain. We recently conducted a pilot study on food-intake patterns among pregnant women from a Canadian Indigenous reserve, Point Douglas area, in Winnipeg, Manitoba, Canada. We found that DHA was one of the lowest nutrients consumed in women exposed to alcohol during pregnancy (25). Thus, DHA could benefit pregnant women at high risk of being exposed to alcohol, but we do not know the real status of their DHA concentrations. In this regard, we are currently collecting data to determine the nutritional status of pregnant mothers drinking alcohol in remote First Nation Communities in northern Manitoba. Once we have sufficient information, it will be clearer if DHA may indeed be an important nutrition strategy for those vulnerable mothers. It is of interest to know whether DHA supplementation could reverse some of the cognitive deficits seen in children with FASD. Unfortunately, there are limited human and animal studies showing the potential of DHA to reverse the detrimental effects of alcohol on brain development. Based on earlier evidence, DHA supplementation could easily increase the status of DHA in the brain, but functional improvement may be limited by the extent of the damage caused by prenatal alcohol. However, more research is needed to examine the effect of DHA replenishment on the brain of offspring using rodent models after exposure to alcohol prenatally. This can then be followed up in neonates to adults.
Conclusions
FASD is a pandemic with far-reaching adverse effects on the individual, family, social, and health care systems. DHA requirements may be higher in pregnancy for the optimal development of the fetus. However, directly or indirectly, alcohol may negatively affect DHA intake, absorption, and circulating blood concentrations. Supplementation with DHA necessary for fetal development in pregnant women who may consume alcohol and who have a low DHA intake could mitigate the severe effects of prenatal alcohol exposure. DHA is a potential candidate because it could increase neurogenesis and plasticity, while reducing oxidative stress, inflammation, and apoptosis induced by alcohol exposure in brain. In vitro and in vivo studies with DHA have shown promise, but more clinical studies are needed to validate the efficacy and dose of DHA needed to mitigate the developmental abnormalities due to prenatal alcohol exposure. Overall, global research must focus on early detection, treatment, and rehabilitation of individuals suffering from developmental deficits due to prenatal alcohol exposure.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—BAF and XLL: contributed equally to the literature search, study concept, and writing of the manuscript; MNAE: reviewed and edited the manuscript and provided critical feedback; MS: critically reviewed and edited the manuscript, provided critical feedback, and secured funding; and all authors: read and approved the final manuscript.
Notes
Supported by Research Manitoba, Canada-Israel International Fetal Alcohol Consortium, and Manitoba Liquor and Lotteries Corporation.
Author disclosures: The authors report no conflicts of interest.
BAF and XLL contributed equally to this work.
Abbreviations used: ALA, ɑ-linolenic acid; AQ4, aquaporin 4; Bcl, B cell lymphoma; BDNF, brain-derived neurotrophic factor; CCL, chemokine ligand; CREB, cAMP response element–binding protein; COX2, cyclooxygenase-2; CYP2E1, cytochrome P450-2E1; ER, endoplasmic reticulum; FASD, fetal-alcohol spectrum disorder; GABA, γ-aminobutyric acid; GSH, reduced glutathione; IP3R, inositol triphosphate receptor; LTP, long-term potentiation; NMDA, N-methyl-D-aspartic acid; PARP1, poly[ADP-ribose]polymerase-1; PD, postnatal day; PLA2, phospholipase A2; ROS, reactive oxygen species; TLR4, Toll-like receptor 4; TrkB, tropomyosin receptor kinase B.
References
- 1. Roozen S, Peters G-JY, Kok G, Townend D, Nijhuis J, Curfs L. Worldwide prevalence of fetal alcohol spectrum disorders: a systematic literature review including meta-analysis. Alcohol Clin Exp Res. 2016;40:18–32. [DOI] [PubMed] [Google Scholar]
- 2. Flannigan K, Pei J, Stewart M, Johnson A. Fetal alcohol spectrum disorder and the criminal justice system: a systematic literature review. Int J Law Psychiatry. 2018;57:42–52. [DOI] [PubMed] [Google Scholar]
- 3. May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G et al.. Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA. 2018;319:474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young JK, Giesbrecht HE, Eskin MN, Aliani M, Suh M. Nutrition implications for fetal alcohol spectrum disorder. Adv Nutr. 2014;5:675–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr. 1992;120:S129–38. [DOI] [PubMed] [Google Scholar]
- 6. Akbar M, Calderon F, Wen Z, Kim H-Y. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci. 2005;102:10858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao W-N, Hylton NK, Wang J, Chindavong PS, Alural B, Kurtser I, Subramanian A, Mazitschek R, Perlis RH, Haggarty SJ. Activation of WNT and CREB signaling pathways in human neuronal cells in response to the omega-3 fatty acid docosahexaenoic acid (DHA). Mol Cell Neurosci. 2019;99:103386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr. 2007;137:855–9. [DOI] [PubMed] [Google Scholar]
- 9. Wellmann KA, George F, Brnouti F, Mooney SM. Docosahexaenoic acid partially ameliorates deficits in social behavior and ultrasonic vocalizations caused by prenatal ethanol exposure. Behav Brain Res. 2015;286:201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patten AR, Brocardo PS, Christie BR. Omega-3 supplementation can restore glutathione levels and prevent oxidative damage caused by prenatal ethanol exposure. J Nutr Biochem. 2013;24:760–9. [DOI] [PubMed] [Google Scholar]
- 11. Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res. 1985;24:69–176. [DOI] [PubMed] [Google Scholar]
- 12. Rapoport SI, Chang MC, Spector AA. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J Lipid Res. 2001;42:678–85. [PubMed] [Google Scholar]
- 13. Lacombe RJS, Chouinard-Watkins R, Bazinet RP. Brain docosahexaenoic acid uptake and metabolism. Mol Aspects Med. 2018;64:109–34. [DOI] [PubMed] [Google Scholar]
- 14. Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. [DOI] [PubMed] [Google Scholar]
- 15. Tanaka K, Farooqui AA, Siddiqi NJ, Alhomida AS, Ong W-Y. Effects of docosahexaenoic acid on neurotransmission. Biomol Ther (Seoul). 2012;20:152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scott BL, Bazan NG.. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci. 1989;86:2903–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weiser MJ, Butt CM, Mohajeri MH. Docosahexaenoic acid and cognition throughout the lifespan. Nutrients. 2016;8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moore S, Yoder E, Murphy S. Astrocytes, not neurons, produce docosahexaenoic acid (22: 6ω‐3) and arachidonic acid (20: 4ω‐6). J Neurochem. 1991;56(2):518–24. [DOI] [PubMed] [Google Scholar]
- 19. Crawford MA. The role of dietary fatty acids in biology: their place in the evolution of the human brain. Nutr Rev. 1992;50:3–11. [DOI] [PubMed] [Google Scholar]
- 20. Bhatia HS, Agrawal R, Sharma S, Huo Y-X, Ying Z, Gomez-Pinilla F. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PLoS One. 2011;6(12):e28451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Demar JC, Ma K, Chang L, Bell JM, Rapoport SI. alpha-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J Neurochem. 2005;94:1063–76. [DOI] [PubMed] [Google Scholar]
- 22. Food and Agriculture Organization of the United Nations: Astrup AV, Bazine B, Brenna JT, Calder PC, Crawford MA, Dangour A, Donahoo WT, Elmadfa I, Galli C, Gerber M, et al. Fats and fatty acids in human nutrition. Report of an expert consultation. FAO Food Nutr Pap. 2010;91:1–166. [PubMed] [Google Scholar]
- 23. Nochera CL, Goossen LH, Brutus AR, Cristales M, Eastman B. Consumption of DHA + EPA by low-income women during pregnancy and lactation. Nutr Clin Pract. 2011;26:445–50. [DOI] [PubMed] [Google Scholar]
- 24. Jia X, Pakseresht M, Wattar N, Wildgrube J, Sontag S, Andrews M, Subhan FB, McCargar L, Field CJ. Women who take n-3 long-chain polyunsaturated fatty acid supplements during pregnancy and lactation meet the recommended intake. Appl Physiol Nutr Metab. 2015;40:474–81. [DOI] [PubMed] [Google Scholar]
- 25. Dyck KN, Suh M. Intakes of nutrients known for fetal brain development among pregnant women living in downtown and Point Douglas Winnipeg. Winnipeg (Canada): University of Manitoba; 2015. [Google Scholar]
- 26. Luchtman DW, Song C.. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: findings from animal and clinical studies. Neuropharmacology. 2013;64:550–65. [DOI] [PubMed] [Google Scholar]
- 27. McNamara RK, Carlson SE.. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–49. [DOI] [PubMed] [Google Scholar]
- 28. de la Presa-Owens S, Innis SM. Docosahexaenoic and arachidonic acid prevent a decrease in dopaminergic and serotoninergic neurotransmitters in frontal cortex caused by a linoleic and α-linolenic acid deficient diet in formula-fed piglets. J Nutr. 1999;129:2088–93. [DOI] [PubMed] [Google Scholar]
- 29. Balogun KA, Cheema SK.. The expression of neurotrophins is differentially regulated by omega-3 polyunsaturated fatty acids at weaning and postweaning in C57BL/6 mice cerebral cortex. Neurochem Int. 2014;66:33–42. [DOI] [PubMed] [Google Scholar]
- 30. Weiser MJ, Wynalda K, Salem N, Butt CM. Dietary DHA during development affects depression-like behaviors and biomarkers that emerge after puberty in adolescent rats. J Lipid Res. 2015;56:151–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levant B. N-3 (omega-3) polyunsaturated fatty acids in the pathophysiology and treatment of depression: pre-clinical evidence. CNS Neurol Disord Drug Targets. 2013;12:450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cao D, Kevala K, Kim J, Moon H-S, Jun SB, Lovinger D, Kim H-Y. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsui F, Hecht P, Yoshimoto K, Watanabe Y, Morimoto M, Fritsche K, Will M, Beversdorf D. DHA mitigates autistic behaviors accompanied by dopaminergic change in a gene/prenatal stress mouse model. Neuroscience. 2018;371:407–19. [DOI] [PubMed] [Google Scholar]
- 34. Tang M, Zhang M, Cai H, Li H, Jiang P, Dang R, Liu Y, He X, Xue Y, Cao L et al.. Maternal diet of polyunsaturated fatty acid altered the cell proliferation in the dentate gyrus of hippocampus and influenced glutamatergic and serotoninergic systems of neonatal female rats. Lipids Health Dis [Internet]. 2016;15:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bruno MJ, Koeppe RE, Andersen OS. Docosahexaenoic acid alters bilayer elastic properties. Proc Natl Acad Sci. 2007;104:9638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nabekura J, Noguchi K, Witt MR, Nielsen M, Akaike N. Functional modulation of human recombinant gamma-aminobutyric acid type A receptor by docosahexaenoic acid. J Biol Chem. 1998;273:11056–61. [DOI] [PubMed] [Google Scholar]
- 37. Coulombe K, Saint-Pierre M, Cisbani G, St-Amour I, Gibrat C, Giguère-Rancourt A, Calon F, Cicchetti F. Partial neurorescue effects of DHA following a 6-OHDA lesion of the mouse dopaminergic system. J Nutr Biochem. 2016;30:133–42. [DOI] [PubMed] [Google Scholar]
- 38. Jiang L-H, Liang Q-Y, Shi Y. Pure docosahexaenoic acid can improve depression behaviors and affect HPA axis in mice. Eur Rev Med Pharmacol Sci. 2012;16:1765–73. [PubMed] [Google Scholar]
- 39. Davis PF, Ozias MK, Carlson SE, Reed GA, Winter MK, McCarson KE, Levant B. Dopamine receptor alterations in female rats with diet-induced decreased brain docosahexaenoic acid (DHA): interactions with reproductive status. Nutr Neurosci. 2010;13:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mathieu G, Denis S, Langelier B, Denis I, Lavialle M, Vancassel S. DHA enhances the noradrenaline release by SH-SY5Y cells. Neurochem Int. 2010;56:94–100. [DOI] [PubMed] [Google Scholar]
- 41. Minami M, Kimura S, Endo T, Hamaue N, Hirafuji M, Togashi H, Matsumoto M, Yoshioka M, Saito H, Watanabe S et al.. Dietary docosahexaenoic acid increases cerebral acetylcholine levels and improves passive avoidance performance in stroke-prone spontaneously hypertensive rats. Pharmacol Biochem Behav. 1997;58:1123–9. [DOI] [PubMed] [Google Scholar]
- 42. Kodas E, Galineau L, Bodard S, Vancassel S, Guilloteau D, Besnard J-C, Chalon S. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J Neurochem. 2004;89:695–702. [DOI] [PubMed] [Google Scholar]
- 43. Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. 2018;38:579–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci. 2006;110:167–73. [DOI] [PubMed] [Google Scholar]
- 45. Chan JP, Unger TJ, Byrnes J, Rios M. Examination of behavioral deficits triggered by targeting BDNF in fetal or postnatal brains of mice. Neuroscience. 2006;142:49–58. [DOI] [PubMed] [Google Scholar]
- 46. Wu C-L, Hwang C-S, Yang D-I. Protective effects of brain-derived neurotrophic factor against neurotoxicity of 3-nitropropionic acid in rat cortical neurons. Neurotoxicology. 2009;30:718–26. [DOI] [PubMed] [Google Scholar]
- 47. Zhang Q, Liu G, Wu Y, Sha H, Zhang P, Jia J. BDNF promotes EGF-induced proliferation and migration of human fetal neural stem/progenitor cells via the PI3K/Akt pathway. Molecules. 2011;16:10146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ehrlich DE, Josselyn SA.. Plasticity-related genes in brain development and amygdala-dependent learning. Genes Brain Behav. 2016;15:125–43. [DOI] [PubMed] [Google Scholar]
- 49. Rao JS, Ertley RN, Lee H-J, DeMar JC Jr, Arnold JT, Rapoport SI, Bazinet RP. n-3 Polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. [DOI] [PubMed] [Google Scholar]
- 50. Hadjighassem M, Kamalidehghan B, Shekarriz N, Baseerat A, Molavi N, Mehrpour M, Joghataei MT, Tondar M, Ahmadipour F, Meng GY. Oral consumption of α-linolenic acid increases serum BDNF levels in healthy adult humans. Nutrition. 2015;14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferreira CF, Bernardi JR, Bosa VL, Schuch I, Goldani MZ, Kapczinski F, Salum GA, Dalmaz C, Manfro GG, Silveira PP. Correlation between n-3 polyunsaturated fatty acids consumption and BDNF peripheral levels in adolescents. Lipids Health Dis. 2014;13:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lang UE, Hellweg R, Seifert F, Schubert F, Gallinat J. Correlation between serum brain-derived neurotrophic factor level and an in vivo marker of cortical integrity. Biol Psychiatry. 2007;62:530–5. [DOI] [PubMed] [Google Scholar]
- 53. He C, Qu X, Cui L, Wang J, Kang JX. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc Natl Acad Sci. 2009;106:11370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kitajka K, Sinclair AJ, Weisinger RS, Weisinger HS, Mathai M, Jayasooriya AP, Halver JE, Puskás LG. Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression. Proc Natl Acad Sci. 2004;101:10931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thallmair M, Metz GA, Z'Graggen WJ, Raineteau O, Kartje GL, Schwab ME. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat Neurosci. 1998;1:124–31. [DOI] [PubMed] [Google Scholar]
- 56. Calderon F, Kim H-Y.. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–88. [DOI] [PubMed] [Google Scholar]
- 57. Wu H, Ichikawa S, Tani C, Zhu B, Tada M, Shimoishi Y, Murata Y, Nakamura Y. Docosahexaenoic acid induces ERK1/2 activation and neuritogenesis via intracellular reactive oxygen species production in human neuroblastoma SH-SY5Y cells. Biochim Biophys Acta. 2009;1791:8–16. [DOI] [PubMed] [Google Scholar]
- 58. Fujita S, Ikegaya Y, Nishikawa M, Nishiyama N, Matsuki N. Docosahexaenoic acid improves long-term potentiation attenuated by phospholipase A(2) inhibitor in rat hippocampal slices. Br J Pharmacol. 2001;132:1417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lozada LE, Desai A, Kevala K, Lee J-W, Kim H-Y. Perinatal brain docosahexaenoic acid concentration has a lasting impact on cognition in mice. J Nutr. 2017;147:1624–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stonehouse W, Conlon CA, Podd J, Hill SR, Minihane AM, Haskell C, Kennedy D. DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. Am J Clin Nutr. 2013;97:1134–43. [DOI] [PubMed] [Google Scholar]
- 61. Fedorova I, Hussein N, Baumann MH, Di Martino C, Salem N. An n-3 fatty acid deficiency impairs rat spatial learning in the Barnes maze. Behav Neurosci. 2009;123:196–205. [DOI] [PubMed] [Google Scholar]
- 62. Hiratsuka S, Koizumi K, Ooba T, Yokogoshi H. Effects of dietary docosahexaenoic acid connecting phospholipids on the learning ability and fatty acid composition of the brain. J Nutr Sci Vitaminol. 2009;55:374–80. [DOI] [PubMed] [Google Scholar]
- 63. Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Auestad N, Scott DT, Janowsky JS, Jacobsen C, Carroll RE, Montalto MB, Halter R, Qiu W, Jacobs JR, Connor WE et al.. Visual, cognitive, and language assessments at 39 months: a follow-up study of children fed formulas containing long-chain polyunsaturated fatty acids to 1 year of age. Pediatrics. 2003;112:e177–83. [DOI] [PubMed] [Google Scholar]
- 65. Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol. 2000;42:174–81. [DOI] [PubMed] [Google Scholar]
- 66. Birch EE, Garfield S, Castañeda Y, Hughbanks-Wheaton D, Uauy R, Hoffman D. Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum Dev. 2007;83:279–84. [DOI] [PubMed] [Google Scholar]
- 67. Dunstan JA, Simmer K, Dixon G, Prescott SL. Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93:F45–50. [DOI] [PubMed] [Google Scholar]
- 68. Judge MP, Harel O, Lammi-Keefe CJ. Maternal consumption of a docosahexaenoic acid-containing functional food during pregnancy: benefit for infant performance on problem-solving but not on recognition memory tasks at age 9 mo. Am J Clin Nutr. 2007;85:1572–7. [DOI] [PubMed] [Google Scholar]
- 69. Jacobson SW, Jacobson JL, Molteno CD, Warton CMR, Wintermark P, Hoyme HE, De Jong G, Taylor P, Warton F, Lindinger NM et al.. Heavy prenatal alcohol exposure is related to smaller corpus callosum in newborn MRI scans. Alcohol Clin Exp Res. 2017;41:965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Komada M, Hara N, Kawachi S, Kawachi K, Kagawa N, Nagao T, Ikeda Y. Mechanisms underlying neuro-inflammation and neurodevelopmental toxicity in the mouse neocortex following prenatal exposure to ethanol. Sci Rep. 2017;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Boschen KE, Klintsova AY.. Neurotrophins in the brain: interaction with alcohol exposure during development. Vitam Horm. 2017;104:197–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Haorah J, Ramirez SH, Floreani N, Gorantla S, Morsey B, Persidsky Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med. 2008;45:1542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Velayutham M, Hemann C, Zweier JL. Removal of H2O2 and generation of superoxide radical: role of cytochrome c and NADH. Free Radic Biol Med. 2011;51:160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ojeda L, Nogales F, Murillo L, Carreras O. The role of folic acid and selenium against oxidative damage from ethanol in early life programming: a review. Biochem Cell Biol. 2018;96:178–88. [DOI] [PubMed] [Google Scholar]
- 75. Wang P, Zeng T, Zhang C-L, Gao X-C, Liu Z, Xie K-Q, Chi Z-F. Lipid peroxidation was involved in the memory impairment of carbon monoxide-induced delayed neuron damage. Neurochem Res. 2009;34:1293–8. [DOI] [PubMed] [Google Scholar]
- 76. Abdul-Muneer PM, Chandra N, Haorah J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol. 2015;51:966–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, Zimmerman MC, Chandra N, Haorah J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic Biol Med. 2013;60:282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dikranian K, Qin Y-Q, Labruyere J, Nemmers B, Olney JW. Ethanol-induced neuroapoptosis in the developing rodent cerebellum and related brain stem structures. Dev Brain Res. 2005;155:1–13. [DOI] [PubMed] [Google Scholar]
- 79. Fowler A-K, Thompson J, Chen L, Dagda M, Dertien J, Dossou KSS, Moaddel R, Bergeson SE, Kruman II. Differential sensitivity of prefrontal cortex and hippocampus to alcohol-induced toxicity. PLoS One. 2014;9:e106945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang P, Liu B-Y, Wu M-M, Wei X-Y, Sheng S, You S-W, Shang L-X, Kuang F. Moderate prenatal alcohol exposure suppresses the TLR4-mediated innate immune response in the hippocampus of young rats. Neurosci Lett. 2019;699:77–83. [DOI] [PubMed] [Google Scholar]
- 81. Ahlers KE, Karaçay B, Fuller L, Bonthius DJ, Dailey ME. Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX-dependent neurodegeneration. Glia. 2015;63:1694–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Terasaki LS, Schwarz JM.. Effects of moderate prenatal alcohol exposure during early gestation in rats on inflammation across the maternal-fetal-immune interface and later-life immune function in the offspring. J Neuroimmune Pharmacol. 2016;11:680–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Topper LA, Baculis BC, Valenzuela CF. Exposure of neonatal rats to alcohol has differential effects on neuroinflammation and neuronal survival in the cerebellum and hippocampus. J Neuroinflam. 2015;12:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cantacorps L, Alfonso-Loeches S, Moscoso-Castro M, Cuitavi J, Gracia-Rubio I, López-Arnau R, Escubedo E, Guerri C, Valverde O. Maternal alcohol binge drinking induces persistent neuroinflammation associated with myelin damage and behavioural dysfunctions in offspring mice. Neuropharmacology. 2017;123:368–84. [DOI] [PubMed] [Google Scholar]
- 85. Schambra UB, Goldsmith J, Nunley K, Liu Y, Harirforoosh S, Schambra HM. Low and moderate prenatal ethanol exposures of mice during gastrulation or neurulation delays neurobehavioral development. Neurotoxicol Teratol. 2015;51:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Feng M-J, Yan S-E, Yan Q-S. Effects of prenatal alcohol exposure on brain-derived neurotrophic factor and its receptor tyrosine kinase B in offspring. Brain Res. 2005;1042:125–32. [DOI] [PubMed] [Google Scholar]
- 87. Brünig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur J Neurosci. 2001;13:1320–8. [DOI] [PubMed] [Google Scholar]
- 88. Le WD, Xie WJ, Appel SH. Protective role of heme oxygenase-1 in oxidative stress-induced neuronal injury. J Neurosci Res. 1999;56:652–8. [DOI] [PubMed] [Google Scholar]
- 89. Lu D-Y, Tsao Y-Y, Leung Y-M, Su K-P. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for ω-3 fatty acids. Neuropsychopharmacology. 2010;35:2238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Patten AR, Sickmann HM, Dyer RA, Innis SM, Christie BR. Omega-3 fatty acids can reverse the long-term deficits in hippocampal synaptic plasticity caused by prenatal ethanol exposure. Neurosci Lett. 2013;551:7–11. [DOI] [PubMed] [Google Scholar]
- 91. Ke Z, Wang X, Liu Y, Fan Z, Chen G, Xu M, Bower KA, Frank JA, Li M, Fang S et al.. Ethanol induces endoplasmic reticulum stress in the developing brain. Alcohol Clin Exp Res. 2011;35:1574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yang F, Luo J.. Endoplasmic reticulum stress and ethanol neurotoxicity. Biomolecules. 2015;5:2538–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Joshi AU, Kornfeld OS, Mochly-Rosen D. The entangled ER-mitochondrial axis as a potential therapeutic strategy in neurodegeneration: a tangled duo unchained. Cell Calcium. 2016;60:218–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Begum G, Harvey L, Dixon CE, Sun D. ER stress and effects of DHA as an ER stress inhibitor. Transl Stroke Res. 2013;4:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Begum G, Kintner D, Liu Y, Cramer SW, Sun D. DHA inhibits ER Ca2+ release and ER stress in astrocytes following in vitro ischemia. J Neurochem. 2012;120:622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen G, Fan Z, Wang X, Ma C, Bower KA, Shi X, Ke Z-J, Luo J. Brain-derived neurotrophic factor suppresses tunicamycin-induced upregulation of CHOP in neurons. J Neurosci Res. 2007;85:1674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kajarabille N, Hurtado JA, Peña-Quintana L, Peña M, Ruiz J, Diaz-Castro J, Rodríguez-Santana Y, Martin-Alvarez E, López-Frias M, Soldado O et al.. Omega-3 LCPUFA supplement: a nutritional strategy to prevent maternal and neonatal oxidative stress. Matern Child Nutr. 2017;13:e12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang MJ, Spite M.. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr. 2012;32:203–27. [DOI] [PubMed] [Google Scholar]
- 99. Rey C, Nadjar A, Buaud B, Vaysse C, Aubert A, Pallet V, Layé S, Joffre C. Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav Immun. 2016;55:249–59. [DOI] [PubMed] [Google Scholar]
- 100. De Smedt-Peyrusse V, Sargueil F, Moranis A, Harizi H, Mongrand S, Layé S. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J Neurochem. 2008;105:296–307. [DOI] [PubMed] [Google Scholar]
- 101. Bazinet RP, Layé S.. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–85. [DOI] [PubMed] [Google Scholar]
- 102. Tajuddin N, Moon K-H, Marshall SA, Nixon K, Neafsey EJ, Kim H-Y, Collins MA. Neuroinflammation and neurodegeneration in adult rat brain from binge ethanol exposure: abrogation by docosahexaenoic acid. PLoS One. 2014;9(7):e101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Goldowitz D, Lussier AA, Boyle JK, Wong K, Lattimer SL, Dubose C, Lu L, Kobor MS, Hamre KM. Molecular pathways underpinning ethanol-induced neurodegeneration. Front Genet. 2014;5:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Quillinan N, Herson PS, Traystman RJ. Neuropathophysiology of brain injury. Anesthesiol Clin. 2016;34:453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010;20(5):596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Akbar M, Baick J, Calderon F, Wen Z, Kim H-Y. Ethanol promotes neuronal apoptosis by inhibiting phosphatidylserine accumulation. J Neurosci Res. 2006;83:432–40. [DOI] [PubMed] [Google Scholar]
- 107. Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer's disease. J Lipid Res. 2009;50(Suppl):S400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. May PA, Hamrick KJ, Corbin KD, Hasken JM, Marais A-S, Blankenship J, Hoyme HE, Gossage JP. Maternal nutritional status as a contributing factor for the risk of fetal alcohol spectrum disorders. Reprod Toxicol. 2016;59:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Haggarty P, Abramovich DR, Page K. The effect of maternal smoking and ethanol on fatty acid transport by the human placenta. Br J Nutr. 2002;87:247–52. [DOI] [PubMed] [Google Scholar]
- 111. May PA, Hamrick KJ, Corbin KD, Hasken JM, Marais A-S, Brooke LE, Blankenship J, Hoyme HE, Gossage JP. Dietary intake, nutrition, and fetal alcohol spectrum disorders in the Western Cape Province of South Africa. Reprod Toxicol. 2014;46:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Campbell FM, Gordon MJ, Dutta-Roy AK. Placental membrane fatty acid-binding protein preferentially binds arachidonic and docosahexaenoic acids. Life Sci. 1998;63:235–40. [DOI] [PubMed] [Google Scholar]
- 113. Burdge GC, Postle AD.. Effect of maternal ethanol consumption during pregnancy on the phospholipid molecular species composition of fetal guinea-pig brain, liver and plasma. Biochim Biophys Acta. 1995;1256:346–52. [DOI] [PubMed] [Google Scholar]
- 114. Makrides M, Duley L, Olsen SF. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre-eclampsia or intrauterine growth restriction. Cochrane Database Syst Rev. 2006;19:(3):CD003402. [DOI] [PubMed] [Google Scholar]
- 115. Horvath A, Koletzko B, Szajewska H. Effect of supplementation of women in high-risk pregnancies with long-chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Br J Nutr. 2007;98:253–9. [DOI] [PubMed] [Google Scholar]
- 116. Le Donne M, Alibrandi A, Vita R, Zanghì D, Triolo O, Benvenga S. Does eating oily fish improve gestational and neonatal outcomes? Findings from a Sicilian study. Women Birth. 2016;29:e50–7. [DOI] [PubMed] [Google Scholar]
- 117. Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P; DOMInO Investigative Team. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA. 2010;304:1675–83. [DOI] [PubMed] [Google Scholar]
- 118. Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, Georgieff MK, Markley LA, Kerling EH, Shaddy DJ. DHA supplementation and pregnancy outcomes. Am J Clin Nutr. 2013;97:808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yelland LN, Gajewski BJ, Colombo J, Gibson RA, Makrides M, Carlson SE. Predicting the effect of maternal docosahexaenoic acid (DHA) supplementation to reduce early preterm birth in Australia and the United States using results of within country randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2016;112:44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Keenan K, Hipwell A, McAloon R, Hoffmann A, Mohanty A, Magee K. The effect of prenatal docosahexaenoic acid supplementation on infant outcomes in African American women living in low-income environments: a randomized, controlled trial. Psychoneuroendocrinology. 2016;71:170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Harris MA, Reece MS, McGregor JA, Wilson JW, Burke SM, Wheeler M, Anderson JE, Auld GW, French JI, Allen KGD. The effect of omega-3 docosahexaenoic acid supplementation on gestational length: randomized trial of supplementation compared to nutrition education for increasing n-3 intake from foods. Biomed Res Int. 2015;2015:123078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Innis SM, Friesen RW. Essential n-3 fatty acids in pregnant women and early visual acuity maturation in term infants. Am J Clin Nutr. 2008;87:548–57. [DOI] [PubMed] [Google Scholar]
- 123. Koletzko B, Cetin I, Brenna JT.. Perinatal Lipid Intake Working Group; Child Health Foundation; Diabetic Pregnancy Study Group; European Association of Perinatal Medicine; European Association of Perinatal Medicine; European Society for Clinical Nutrition and Metabolism; European Society for Paediatric Gastroenterology, Hepatology and Nutrition, Committee on Nutrition, et al. Dietary fat intakes for pregnant and lactating women. Br J Nutr. 2007;98:873–7. [DOI] [PubMed] [Google Scholar]
- 124. Burdge GC. The role of docosahexaenoic acid in brain development and fetal alcohol syndrome. Biochem Soc Trans. 1998;26:246–52. [DOI] [PubMed] [Google Scholar]
- 125. Makrides M. DHA supplementation during the perinatal period and neurodevelopment: do some babies benefit more than others?. Prostaglandins Leukot Essent Fatty Acids. 2013;88:87–90. [DOI] [PubMed] [Google Scholar]


