ABSTRACT
The gut-brain axis (GBA) is a bilateral communication network between the gastrointestinal (GI) tract and the central nervous system. The essential amino acid tryptophan contributes to the normal growth and health of both animals and humans and, importantly, exerts modulatory functions at multiple levels of the GBA. Tryptophan is the sole precursor of serotonin, which is a key monoamine neurotransmitter participating in the modulation of central neurotransmission and enteric physiological function. In addition, tryptophan can be metabolized into kynurenine, tryptamine, and indole, thereby modulating neuroendocrine and intestinal immune responses. The gut microbial influence on tryptophan metabolism emerges as an important driving force in modulating tryptophan metabolism. Here, we focus on the potential role of tryptophan metabolism in the modulation of brain function by the gut microbiota. We start by outlining existing knowledge on tryptophan metabolism, including serotonin synthesis and degradation pathways of the host, and summarize recent advances in demonstrating the influence of the gut microbiota on tryptophan metabolism. The latest evidence revealing those mechanisms by which the gut microbiota modulates tryptophan metabolism, with subsequent effects on brain function, is reviewed. Finally, the potential modulation of intestinal tryptophan metabolism as a therapeutic option for brain and GI functional disorders is also discussed.
Keywords: tryptophan metabolism, serotonin, kynurenine, microbial tryptophan metabolites, gut-brain axis, gut microbiota, irritable bowel syndrome, depression
Introduction
The bidirectional crosstalk between the central nervous system (CNS), enteric nervous system (ENS), and the gastrointestinal (GI) tract is referred to as the gut-brain axis (GBA). Accumulating evidence supports the theory that the gut microbiota plays an important role in the modulation of the GBA by directly or indirectly affecting the production of metabolites (1–3). In line with this notion, disturbances of the gut microbiota have been implicated in functional disorders of the GBA, such as irritable bowel syndrome (IBS) (4, 5) and neuropsychiatric disorders [e.g., autism spectrum disorder (ASD) and major depression] (6–8). Therefore, we previously proposed that the gut microbiota is the brain peacekeeper (9).
Tryptophan is an essential amino acid being utilized for protein synthesis and, thereby, affecting the growth and health of both animals and humans. In addition to serving as a nutrient, accumulating evidence has revealed that changes in the gut microbiota composition affect the GBA by modulating the tryptophan metabolism (10–12). Moreover, products of the tryptophan metabolism, such as serotonin, kynurenines, tryptamine, and indolic compounds, have profound effects on the interaction between gut microbiota and the GBA (13–15). However, due to the complexity of this crosstalk, the mechanistic role of the tryptophan metabolism along the GBA is still being elucidated. In this review, we summarize the potential role of tryptophan and its metabolites as signaling molecules between gut microbiota and brain functions.
Host Tryptophan Metabolism
As an essential amino acid for animals and humans, tryptophan is mainly derived from the diet (16). The digestion of dietary proteins in the small intestine leads to the release of tryptophan, which can be absorbed through intestinal epithelium and enter the bloodstream (17, 18). Unlike other amino acids, tryptophan circulates in the blood and is mainly bound to albumin, while merely 10–20% of tryptophan is free-form in blood circulation. An important physiological function of free tryptophan is the contribution to host protein synthesis (19). The net balance of tryptophan across the portal-drained viscera in animals during the growing period has been thoroughly reviewed previously (20). However, the proportion of free tryptophan being incorporated into proteins varies considerably, which may be attributed to various aspects. On the one hand, tryptophan serves as a sole precursor for the biosynthesis of the neuroendocrine transmitter serotonin and, subsequently, a pineal hormone called melatonin (21); on the other hand, tryptophan in the host can be degraded through the kynurenine pathway (22). In addition, vitamin B6 is an important enzyme cofactor in the tryptophan metabolism (23). The tryptophan availability can be altered in a vitamin B6–dependent manner, as evidenced by an impairment of the tryptophan metabolism after a vitamin B6 deficiency (24).
Host Serotonin Synthesis by Tryptophan
Serotonin (5-hydroxytryptamine) is a monoamine molecule that is synthesized from tryptophan. In the CNS, serotonin acts as a key neurotransmitter that is involved in the modulation of emotional control, food intake, sleep, and pain processing (25). However, central serotonin only accounts for a small proportion of the body's total serotonin. Over 90% of serotonin is located in the GI tract (especially in the hindgut), and serotonin is mainly produced from enterochromaffin cells (ECs) (26). Under normal physiological conditions, peripheral serotonin cannot cross the blood-brain barrier (BBB) (22), pointing to distinct pools of central and peripheral serotonin.
Despite the differences in the availability of serotonin along the GBA, the synthetic processes of serotonin in the CNS and the gut are in a tryptophan hydroxylase (TPH)-dependent manner (Figure 1), as has been thoroughly reviewed (21). TPH exists in 2 isoforms: TPH1 is mainly expressed in the ECs and TPH2 is locally expressed in the brain (25). TPH expressions can be influenced by many factors, such as p-chloroamphetamine (27) and tryptophan excess (28). Interestingly, the dysregulated expression of TPHs is revealed in the psychiatric and GI functional disorders, such as anxiety and IBS (29, 30), suggesting the importance of TPH in the modulation of serotonin availability and its functional consequences.
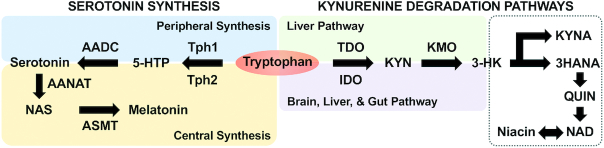
FIGURE 1.
Overview of host tryptophan metabolism via serotonin synthesis and degradation pathway. The key metabolites and enzymes during tryptophan metabolism are presented. AADC, aromatic L-amino acid decarboxylase; AANAT, aralkylamine N-acetyltransfer; ASMT, acetylserotonin O-methyltransferase; IDO, indoleamine 2,3-dioxygenase; KMO, kynurenine 3-monooxygenase; KYN, kynurenine; KYNA, kynurenic acid; NAD, nicotinamide adenine dinucleotides; NAS, N-acetyl-serotonin; QUIN, quinolinic acid; TDO, tryptophan 2,3-dioxygenase; Tph1/2, tryptophan hydroxylase 1/2; 3HANA, 3-hydroxyanthranilic acid; 3-HK, 3-hydroxykynurenine; 5-HTP, 5-hydroxytryptophan.
Host Tryptophan Oxidation Through the Kynurenine Pathway
More than 90% of total tryptophan is oxidized, via the kynurenine pathway, into kynurenine in the liver (31). This kynurenine pathway exerts a primary role in affecting tryptophan availability by the clearance of excess tryptophan. The tryptophan metabolism along the kynurenine pathway is mainly initialized by the induction of either of the rate-limiting enzyme indoleamine-2,3-dioxygenase (IDO) or by tryptophan 2,3-dioxygenase (TDO; Figure 1). While IDO exists in various organs, such as the brain, GI tract, and liver, TDO is almost entirely expressed in the liver (21). IDO can be activated in response to immune stimuli, with interferon-gamma being the most efficient inducer. Among other inflammatory conditions, overexpression of IDO can be observed in the colonic mucosa of patients with inflammatory bowel disease (32). TDO activity is regulated by tryptophan's availability, with its activity being relatively stable, while stress-induced changes in the expression of TDO in the liver are primarily influenced by the activation of the hypothalamic-pituitary-adrenal axis through the action of glucocorticoids (33).
Once kynurenine is produced, it is further metabolized through 2 distinct pathways: kynurenic acid (KYNA) and quinolinic acid (QUIN) pathways (Figure 1) (31). These metabolites, which are produced from the kynurenine pathway and referred to as “kynurenines,” not only are inflammatory mediators, but can also cross the BBB to reach the CNS; therefore, they are regarded as neuromodulators in diverse physiological and pathological processes of brain and GI functional disorders (34). In particular, KYNA is considered as a neuroprotective N-methyl D-aspartate (NMDA) receptor antagonist, while QUIN is regarded as a neurotoxic NMDA receptor agonist (35). The imbalance between the neurotoxic and neuroprotective properties of kynurenines—the KYNA to QUIN ratio, in particular—receives the most attention in patients with brain functional disorders, such as depression (36, 37). Furthermore, QUIN can be converted into niacin and NAD as primary end products (31).
The Gut Microbiota: A Driving Force of the Intestinal Tryptophan Metabolism
Accumulating evidence reveals that the complex commensal microbes that inhabit the mammalian GI tract have versatile impacts on intestinal tryptophan availability (10, 12, 38), thus being considered collectively as a driving force affecting the tryptophan metabolism in the gut.
Microbial Tryptophan Degradation
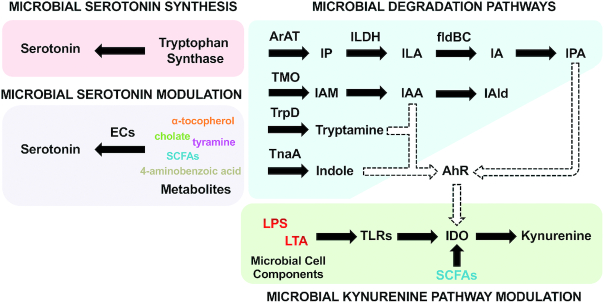
Although the majority of tryptophan derived from ingested protein is absorbed in the small intestine, a certain amount of tryptophan can still reach the large intestine, where it is degraded by a range of commensal microbes (16). Tryptamine, a monoamine that is structurally similar to serotonin, can be generated from the decarboxylation of tryptophan by tryptophan decarboxylases (TrpDs) from commensal bacteria (22). In comparison with conventional mice, germ-free (GF) mice lacking all microorganisms exhibit reduced levels of tryptamine in the gut (39), in line with increased levels of tryptophan in blood circulation (15), suggesting that the gut microbiota participates in the modulation of gut tryptophan decarboxylation. A previous study demonstrated that at least 10% of the human population harbors at least 1 bacterium encoding a TrpD in their gut community (40). In particular, some bacteria belonging to Clostridium, Ruminococcus, Blautia, and Lactobacillus have been identified as being able to convert tryptophan to tryptamine in a TrpD-dependent manner (40).
Tryptophan can also be metabolized by the gut microbiota into indole and its derivatives, such as indole-3-aldehyde (IAld), indole-3-acetic-acid (IAA) and indole-3-propionic acid (IPA; Figure 2) (22). Indole formation from tryptophan occurs through the activation of the enzyme tryptophanase (TnaA), which can be found in many Gram-negative and Gram-positive bacterial species, including Escherichia coli, Clostridium sp., and Bacteroides sp. (41, 42). IAA is formed from indole-acetamide, which is converted from tryptophan by tryptophan monooxygenase of bacteria, such as Clostridium, Bacteroides, and Bifidobacterium (42, 43). In addition, tryptophan can be converted to the intermediate indole-3-lactic acid (ILA) by some bacteria belonging to Lactobacillus and Bifidobacterium through the aromatic amino acid aminotransferase and indolelactic acid dehydrogenase–dependent pathway (44, 45). Furthermore, some bacteria, including Clostridum and Peptostreptococcus, have been reported to convert ILA to IPA in the presence of the phenyllactate dehydratase gene cluster (fldAIBC) (40, 46).
FIGURE 2.
The modulation of tryptophan metabolism by gut microbiota. The key modulatory pathways in the microbial modulation of tryptophan metabolism are presented. AhR, aryl hydrocarbon receptor; ArAT, aromatic amino acid transaminase; ECs, enterochromaffin cells; fldBC, phenyllactate dehydratase; IA, indole acrylic acid; IAA, indole-3-acetic acid; IAld, indole-3-aldehyde; IAM, indole-3-acetamide; IDO, indoleamine 2,3-dioxygenase; ILA, indole-3-lactic acid; ILDH, indolelactic acid dehydrogenase; IP, indole-3-pyruvate; IPA, indolic-3-propionic acid; LPS, lipopolysaccharides; LTA, lipoteichoic acid; SCFA, short-chain fatty acid; TLR, toll-like receptor; TMO, tryptophan-2-monooxygenase; TnaA, tryptophanase; TrpD, tryptophan decarboxylase.
Microbial Modulation of the Serotonin Synthesis Pathway
The modulation of the peripheral serotonin pool by commensal microbiota is revealed in multiple ways. On the one hand, endogenous serotonin in the gut is mainly released from ECs in response to a number of stimuli (26). Previous studies have demonstrated that commensal bacteria—especially spore-forming bacteria of the mouse and human microbiota—promote serotonin biosynthesis in colonic ECs via a metabolite/cell component–dependent mechanism (47, 48). On the other hand, commensal microbiota can directly utilize luminal tryptophan for serotonin synthesis. Several bacteria belonging to Lactococcus, Lactobacillus, Streptococcus, Escherichia coli, and Klebsiella have been reported to be able to produce serotonin by expressing tryptophan synthetase (21). Furthermore, a recent study using GF mice recolonized with specific pathogen–free fecal microbiota revealed that a small but significant amount of serotonin is also produced in the lumen by commensal microbiota from the deconjugation of glucuronide-conjugated serotonin, which is excreted in the gut via the bile duct, through a bacterial enzyme β-glucuronidase–dependent way (49).
Microbial Modulation of the Kynurenine Pathway
The kynurenine pathway is modulated by inflammatory mediators, given that the enzymes within this pathway, especially IDOs, are immunoresponsive (34). It is well known that through the bidirectional crosstalk between the microbiota and immune system, microbial colonization plays an instrumental role in the development of the intestinal immune response of the host (50). Thus, mice lacking gut microbiota exhibit an innate immune system deficiency. In comparison with conventional mice, GF mice show reduced tryptophan degradation through the kynurenine pathway, with higher tryptophan availability and lower kynurenine levels in blood circulation (15, 51). Furthermore, circulating levels of tryptophan and kynurenine are normalized following microbial colonization of mice immediately post-weaning (15).
Innate immunity constitutes the first line of defense against pathogens by responding to pathogen-associated molecular pattern molecules. These microbial molecules are recognized by pattern recognition receptors, including Toll-like receptors (TLRs). Furthermore, dynamic interactions between the gut microbiota and the innate immune system are critical for the gut microbial community and intestinal homeostasis (9). The activation of TLRs by microbial components, such as LPS and lipoteichoic acids, has been identified as a key factor in initiating the tryptophan metabolism through the kynurenine pathway, and the stimulation of TLR-2, TLR-3, TLR-4, TLR-7/8, and TLR-9 has been found to induce the production of kynurenine. Interestingly, only the stimulation of TLR-3 can increase levels of further downstream neuroactive kynurenines, such as KYNA and QUIN, in human peripheral monocytes (52).
In addition, several metabolites produced by gut microbes, such as SCFAs, especially butyrate, are also recognized to modulate the kynurenine pathway (34). Among SCFAs, butyrate is considered as a primary energy source for enterocytes, and also as vital for the down-regulation of intestinal IDO expression via dual mechanisms (53). Firstly, butyrate can reduce signal transducer and activator of transcription 1 (STAT1) expression, leading to the inhibition of the IFN-gamma–dependent phosphorylation of STAT1 and, subsequently, the STAT1-driven transcriptional activity of IDO. Secondly, butyrate downregulates IDO transcription by acting as a histone deacetylase (HDAC) inhibitor (53). By the inhibition of IDO activity, butyrate suppresses kynurenine production from tryptophan (34). SCFAs are the major products from microbial carbohydrate metabolization (54); thus, they point to an important role of microbial carbohydrate metabolism in the modulation of the kynurenine pathway in the gut.
Targeting the Gut Microbiota: A Potential Approach to Balance the Tryptophan Metabolism
Increasing evidence from studies in animal models suggests that the luminal tryptophan can be metabolized by the gut microbiota, which further limits its availability for the host (10, 12, 39). Therefore, the manipulation of the gut microbiota could play a crucial role in determining the tryptophan availability for the host by balancing the microbial tryptophan metabolism and, thereby, serotonin production and tryptophan degradation pathways (Table 1).
TABLE 1.
Targeting the gut microbiota: a potential approach to balance tryptophan metabolism
| Microbial manipulation | Influenced tryptophan metabolism | Model | Design | Findings | Reference |
|---|---|---|---|---|---|
| Antibiotics | Serotonin synthesis | C57BL/B6 mice | Mice were orally treated with an antibiotic mix (ampicillin, neomycin, metronidazole, and vancomycin), whereas the control group received water for 4 weeks | Colonic motility ↓Colonic TPH1 ↓Colonic motility ↓ | Ge et al., 2017 (38) |
| Kynurenine pathway | NIH Swiss mice | Mice were orally treated or were not treated with an antibiotic cocktail (ampicillin, vancomycin, neomycin, metronidazol, and amphotericin-B) for 2 months | Circulating tryptophan ↑Peripheral kynurenine ↓ | Desbonnet et al., 2015 (51) | |
| Preterm piglets | Piglets were orally administered or were not administered with an antibiotic cocktail (ampicillin, gentamycin, and metronidazole) for 4 days | Circulating tryptophan ↑Peripheral kynurenine ↓ | Jiang et al., 2017 (55) | ||
| Microbial degradation | Piglets | Piglets were orally administered or were not administered with an antibiotic cocktail (olaquindox, oxytetracycline calcium, and kitasamycin) for 35 days | Circulating tryptophan ↑Jejunal tryptophan ↓Colonic tryptamine ↑ | Mu et al., 2017 (18) | |
| Colonic tryptamine ↑ | |||||
| Piglets | Piglets were orally administered or were not administered with an antibiotic cocktail (ampicillin, gentamycin, and metronidazole) for 13 days | Fecal indole ↑Fecal indolic compounds ↑ | Gao et al., 2018 (54) | ||
| Pigs | Pigs were orally administered or were not administered with an antibiotic cocktail (olaquindox, oxytetracycline calcium, and kitasamycin) for 35 days | Colonic indole ↑ | Zhang et al., 2017 (56) | ||
| Distal ileal cannulated piglets | Piglets were distal ileal infused with an antibiotic cocktail (ampicillin, gentamycin, and metronidazole), whereas the control group received saline for 25 days | Circulating tryptophan ↓Fecal indolic compounds ↑ | Gao et al., 2018 (12) | ||
| Probiotics | Serotonin synthesis | BALB/C mice | Mice were orally administered or were not administered with Lactobacillus casei 327 | Colonic serotonin ↑Colonic TPH1 ↑Colonic transit ↑ | Hara et al., 2018 (57) |
| Kynurenine pathway | BioBreeding rats | Rats were orally administered or were not administered with Lactobacillus johnsonii cell-free supernatant | Circulating kynurenine ↓Intestinal IDO activity ↓ | Valladares et al., 2013 (58) | |
| Healthy adults | Healthy adults were orally administered or were not administered with Lactobacillus johnsonii for 8 weeks | Circulating tryptophan ↑Circulating kynurenine ↓ | Marcial et al., 2017 (59) | ||
| Sprague-Dawley rats | Rats were orally administered or were not administered with probiotic Bifidobacteria infantis for 14 days | Circulating tryptophan ↑Circulating kynurenine/tryptophan ratio ↓ | Desbonnet et al., 2008 (60) | ||
| Microbial degradation | C57BL mice | Mice were orally administered or were not administered with Lactobacillus reuteri | Circulating tryptophan ↓Peripheral tryptophan metabolites (eg., IAld, IAA, ILA) ↑ | Cervantes-Barragan et al., 2017 (44) | |
| Card9 (-/-) mice | Card9 (-/-) mice were orally administered or were not administered with 3 tryptophan-metabolizing Lactobacillus strains (L. murinus, L. reuteri, and L. taiwanensis) | Intestinal tryptophan metabolite IAA ↓ | Lamas et al., 2016 (62) | ||
| Nutrients | Serotonin synthesis | Piglets | Piglets were fed with sow, formula, or milk for 19 days | Formula vs. sowColonic ECs number ↓Colonic serotonin ↓ | Saraf et al., 2017 (66) |
| GF mice | GF mice colonizing with human gut microbiota and GF mice were fed with polysaccharide-rich diet | Colonic transit ↑Colonic serotonin ↑ | Kashyap et al., 2013 (63) | ||
| GF mice | GF mice colonizing with human gut microbiota compared with GF mice | Colonic serotonin ↑Colonic TPH1↑Colonic transit ↑ | Reigstad et al., 2015 (70) | ||
| Kynurenine pathway | Human primary IEC & IEC cell line | IEC cells were treated or were not treated with commensal bacteria and butyrate | IEC IDO1 expression ↓ | Martin-Gallausiaux et al., 2018 (53) | |
| Microbial degradation | C57BL/6J mice | Mice were fed with high-fat diet, whereas the control group were fed with low-fat diet | Cecal IAA ↓Cecal tryptamine ↓ | Krishnan et al., 2019 (64) | |
| Piglets | Piglets were fed with sow, formula, or milk for 19 days | Formula vs. sow: colonic tryptamine ↑ | Saraf et al., 2017 (66) | ||
| In vitro culture | A tryptophan-utilizing strain isolated from piglet feces was in vitro cultured using glucose or FOS as substrate | FOS: indole ↑ | Wang et al., 2011 (67) | ||
| In vitro culture | A mix of microbial population from pig feces was in vitro cultured in 4 types of carbohydrate sources (sugar beet pulp, rye grass hay, alfalfa hay, and FOS) | FOS: indolic compounds ↓ | Li et al., 2009 (68) | ||
| Pigs | Pigs were fed or were not fed with RS for 100 days | Colonic indolic compounds ↓Colonic tryptamine ↓ | Zhou et al., 2017 (69) | ||
| Cecal cannulated piglets | Cecal cannulated piglets were infused with corn starch, whereas the control group received saline for 19 days | Fecal tryptophan ↑Fecal indolic compounds ↑ | Gao et al., 2019 (10) | ||
| BALB/C & C57BL/6 mice | Mice fed with tryptophan or sugar as an energy source | Colonic IAld ↑ | Zelante et al., 2013 (61) |
BALB/C, albino, laboratory-bred strain; EC, enterochromaffin cell; FOS, fructooligosaccharides; GF, germ-free; IAA, indole-3-acetic-acid; IAld, indole-3-aldehyde; IDO, indoleamine 2,3-dioxygenase; IEC, intestinal epithelial cell; ILA, indole-3-lactic acid; RS, resistant starch; TPH1, tryptophan hydroxylase 1.
Antibiotics
Oral antibiotics are able to reshape the gut microbiota composition and metabolism. Oral treatment with broad-spectrum antibiotics results in the depletion of the gut microbiota and reduced colonic serotonin levels, which, in turn, delay colonic motility in mice (38). As evidenced by the down-regulation of the key synthase TPH1 in the colon (38), this study points to the possible role of commensal microbiota in the modulation of the intestinal serotonin synthesis. Microbial manipulation by antibiotics is reported to affect the kynurenine pathway, as antibiotic-induced microbiota depletion leads to an increased circulating tryptophan availability and reduced metabolism along the peripheral kynurenine pathway in mice (51) and pigs (55). In addition, several studies have shown that antibiotic-induced gut microbial alterations also favor the microbial tryptophan degradation pathway in pigs (54, 56). Along with increased circulating tryptophan levels, the oral administration of antibiotics reduces tryptophan availability in the jejunum and decreases microbial tryptophan decarboxylation activity in the large intestines of pigs (18). Furthermore, oral antibiotic administration increases the levels of indole and indolic compounds in the large intestines of pigs (54, 56). Interestingly, a recent study revealed that the ileal terminal infusion of broad-spectrum antibiotics specifically targeting the large intestinal microbiota results in decreased levels of tryptophan in blood circulation, and enhanced microbial tryptophan degradation, with increased levels of indole in the large intestine (12). Contrary to previous findings (18, 51, 55), the findings in this study (12) point to a distinct role of the large intestinal microbiota in modulating the tryptophan metabolism in response to antibiotic manipulation.
Probiotics
Probiotics, such as bacteria belonging to the genera Lactobacillus and Bifidobacterium, are reported to exert beneficial effects on the tryptophan metabolism (22). On the one hand, as mentioned above, probiotics, such as species within Lactobacillus and Bifidobacterium, can directly convert tryptophan into serotonin (21). On the other hand, some probiotic Lactobacillus strains, such as Lactobacillus casei 327, can indirectly promote colonic serotonin synthesis by increasing TPH1 expression (57). All these ways would affect the peripheral serotonin pool. In addition, several probiotic species are closely involved in the modulation of the kynurenine pathway. In line with increased serum serotonin levels, oral treatment with Lactobacillus johnsonii cell-free supernatant in rats also results in decreased kynurenine levels in the serum, along with reduced intestinal IDO activity (58). A strong trend toward decreased serum levels of kynurenine, along with increased amounts of tryptophan, was observed in humans with the oral administration of Lactobacillus johnsonii for 8 weeks (59). A previous study also revealed that probiotic Bifidobacteria infantis administration in rats results in increased tryptophan levels, with a decreased kynurenine to tryptophan ratio in blood circulation (60). These studies indicate that some probiotic species belonging to Lactobacillus and Bifidobacterium may shift the host tryptophan metabolism by suppressing the kynurenine pathway. Furthermore, some bacteria belonging to Lactobacillus are reported to be able to degrade tryptophan into indolic compounds, such as IAld, ILA, and IAA (44, 61). The oral administration of 3 tryptophan-metabolizing Lactobacillus strains to colitis-susceptible mice promote microbial tryptophan metabolism aryl hydrocarbon receptor (Ah)-dependent signaling, thereby influencing the peripheral tryptophan availability (62). Although the mechanisms whereby the manipulation of the gut microbiota affect the tryptophan metabolism pathways are not yet fully understood, targeting the gut microbiota can be a promising approach for the modulation of the tryptophan metabolism.
Nutrients
Diets are regarded as important factors to shape the microbial tryptophan metabolism (63). For instance, a recent study revealed that a high-fat diet depletes the microbial metabolites IAA and tryptamine in the cecum of mice (64), suggesting that the microbial tryptophan degradation pathway can be attenuated under a high-fat diet. Breast milk not only acts as a sole source of early life nourishment but also contributes to the maturation of gut microbiota in the host (65). Interestingly, a previous study found that formula milk–induced alterations in gut microbiota shift the tryptophan metabolism from serotonin to tryptamine in the neonatal porcine colon (66).
Furthermore, the rate of the microbial tryptophan metabolism can be affected by changes in the luminal availability of nutrients, such as carbohydrates. As evidenced by a previous in vitro study, a strain of a tryptophan-utilizing bacterium isolated from piglet feces uses tryptophan for bacterial protein synthesis with a digestible carbohydrate (glucose) as a substrate, while indigestible carbohydrates [fructooligosaccharides, (FOS)] are a substrate for indole production (67). In addition, increasing carbohydrate availability by adding indigestible carbohydrates, such as FOS and resistant starch, facilitates the carbohydrate metabolism, leading to increased SCFA production while reducing tryptophan degradation and indolic compounds in the large intestine of piglets (68, 69). Consistently, increasing large intestinal carbohydrate availability by cecal starch infusion is demonstrated to suppress the microbial tryptophan degradation, which leads to increased tryptophan levels in the large intestine and further in the serum (10). These studies suggest that increasing carbohydrate availability suppresses microbial tryptophan degradation in the gut, which would further affect the circulating tryptophan pool. In contrast, increasing carbohydrate availability promotes the intestinal serotonin synthesis associated with increased GI transit, as reported by a previous study in mice with oral polysaccharide administration (63). The enhanced production of microbial SCFAs can be involved in this process, given that they have been demonstrated to stimulate the serotonin release in the colonic ECs (70).
The luminal tryptophan availability is another direct factor influencing the microbial tryptophan metabolism (21, 34). The depletion of host tryptophan resulting from IDO activation or dietary restriction can reduce microbial proliferation, especially bacteria within Lactobacillus (61), some of which are reported to be tryptophan-utilizing bacteria (21, 40). Restoring tryptophan levels by dietary feeding selectively resulted in the expansion of Lactobacillus, which further led to enhancement in the microbial tryptophan metabolism with increased IAld (61). Given that tryptophan would also be absorbed by host directly, the complex crosstalk among the gut microbiota, luminal tryptophan availability, and host tryptophan metabolism requires further investigation.
Tryptophan Metabolism in the Gut-Brain Axis
Increasing evidence points to a prominent role for the gut microbiota in shaping the GBA (9, 71). Alterations in the gut microbiota composition and metabolic activities are closely associated with changes in brain functions and behaviors (2, 12, 51). Recent evidence suggests that tryptophan and relevant metabolites play a central role in the crosstalk between the gut microbiota and brain (Table 2) (22).
TABLE 2.
Tryptophan metabolism and gut microbiota-brain axis
| Tryptophan metabolism | Models | Design | Findings | Reference |
|---|---|---|---|---|
| Serotonin synthesis | GF mice | GF mice were compared with conventional control mice | Anxiety-like behaviors ↑Hippocampal serotonin ↑Circulating tryptophan ↑ | Clarke et al., 2013 (15) |
| GF mice | GF mice were compared with SPF mice | Depressive-like behaviors ↓mPFC & hippocampal serotonin ↑mPFC & hippocampal tryptophan ↑ | Lukic et al., 2019 (11) | |
| Cecal cannulated piglets | Cecal cannulated piglets were infused with corn starch, whereas the control group received saline | Hypothalamic serotonin ↑Serotonin synthetases ↑Circulating tryptophan ↑ | Gao et al., 2019 (10) | |
| Distal ileal cannulated piglets | Distal ileal cannulated piglets were infused with antibiotic cocktail, whereas the control group received saline | Hypothalamic serotonin ↓Serotonin synthetases ↑Circulating tryptophan ↓ | Gao et al., 2018 (12) | |
| C57BL/B6 mice | Mice with induced stress were treated with sodium butyrate, whereas the control group received vehicle | Stress-induced depressive behaviors ↓Stress-induced blood-brain barrier impairment ↓Hippocampal serotonin ↑Hippocampal BDNF↑ | Sun et al., 2016 (72) | |
| ICR mice | ICR mice were treated or were not treated with LPS | Depressive-like behaviors ↑ | Zhu et al., 2015 (73) | |
| Prefrontal cortex serotonin ↓ | ||||
| GF mice | Microbiota-colonized GF mice were compared with GF mice | Neuronal serotonin ↑Neuronal 5-HT4receptor ↑Mucosal serotonin ↑Intestinal transit ↑Proliferation of enteric neuronal progenitors ↑ | De Vadder et al., 2018 (75) | |
| Kynurenine pathway | AIMD mice | AIMD mice were compared with conventional control mice | Cognitive deficits ↑Anxiety-like behaviors ↓Circulating tryptophan ↑Circulating kynurenine ↓ | Desbonnet et al., 2015 (51) |
| Sprague-Dawley rats | Rats with microbiota colonization from depressive patients were compared with control rats | Anxiety-like behaviors ↑Circulating kynurenine ↑Circulating kynurenine/tryptophan ratio ↑ | Kelly et al., 2016 (7) | |
| Sprague-Dawley rats | Rats were orally treated or were not treated with probiotic Bifidobacteria infantis | Depressive properties ↓Circulating tryptophan ↑Colonic tryptamine ↑Circulating kynurenic acid ↑ | Desbonnet et al., 2008 (60) | |
| C57BL/6 mice | Mice were infected or were not infected with Toxoplasma gondiido | Central kynurenine ↑Central kynurenic acid ↑Central quinolinic acid ↑ | Notarangelo et al., 2014 (79) | |
| Wistar rats | High fat diet–induced obese rats were treated with anthocyanins, whereas the control group received vehicle | Neuroinflammation ↓Circulating tryptophan ↑Circulating kynurenic acid ↑ | Marques et al., 2018 (80) | |
| GF mice | GF mice were compared with conventional control mice | Hippocampal miR-294–5p ↑Hippocampal Brd2 ↑Hippocampal Slit3kr ↑ | Moloney et al., 2017 (82) | |
| GF mice | Microbiota-colonized GF mice were compared with GF mice | Anxiety-like behaviors ↓Hippocampal IDO activity ↑ | Clarke et al., 2013 (15) | |
| BALB/C & C57BL/6 mice | Mice with induced stress were orally treated or were not treated with Lactobacillus strains | Intestinal IDO1 expression ↓Circulating kynurenine ↓Stress-induced anxiety-like behavior ↓ | Marin et al., 2017 (83) | |
| Microbial degradation | F344 rats | Rats received an acute cecal injection of indole, whereas the control group received corn oil | Motor activity ↓Eye blinking frequency ↑Dorsal vagal complex c-Fosprotein expression ↑ | Jaglin et al., 2018 (1) |
| GF F344 rats | GF rats colonizing with indole-producing bacteria Escherichia coli were compared with GF rats | Anxiety-like behavior ↑Helplessness ↑Despair behaviors ↑Exploration ↓ | Jaglin et al., 2018 (1) | |
| C57BL/6J mice | Mice with induced antibiotics were treated with microbial tryptophan metabolites (indole, indoxyl-3-sulfate, IPA, and IAld), or the bacterial enzyme tryptophanase | Central neuroinflammation ↓ | Rothhammer et al., 2016 (14) |
AIMD, antibiotic-induced gut microbiota–depleted; BDNF, brain-derived neurotrophic factor; c-Fos, cellular oncogene fos; GF, germ-free; IAld, indole-3-aldehyde; ICR, institute of cancer research; IPA, indole-3-propionic acid; LPS, liposaccharides; mPFC, medial prefrontal cortex; SPF, specific pathogen–free; 5-HT4, 5-hydroxytryptamine 4.
Serotonin and the Gut-Brain Axis
During CNS development, serotonin plays an important role in the modulation of neuronal differentiation and migration, as well as axonal outgrowth, myelination, and synapse formation (21). Since peripheral serotonin cannot pass through the BBB, central serotonin is synthesized from tryptophan, which gets transported through the BBB from blood circulation (25). In comparison with conventional mice, GF mice lacking gut microbiota exhibit enhanced anxiety-like behaviors and higher levels of serotonin in the hippocampus, in line with a higher concentration of the precursor tryptophan in blood circulation (15). These findings suggest a humoral route through which the gut microbiota can influence CNS serotonergic neurotransmission. Recent studies further provide clear evidence that alterations of the gut microbial tryptophan metabolism influence peripheral tryptophan availability, affecting central tryptophan levels and, thereby, leading to changes in the central serotonin metabolism (10–12). Apart from controlling the tryptophan availability, gut microbiota exhibit various other pathways through which they modulate central serotonin synthesis. Thus, the microbial metabolites SCFAs, especially butyrate, which can be transported into blood circulation, are reported to exert neuroprotective effects in stressed mice by increasing the brain serotonin concentration and restoring BBB impairments (72). In addition, inflammatory stimuli, such as LPS, the major components of the outer membrane of Gram-negative bacteria, have been revealed to decrease serotonin levels in the prefrontal cortices of mice (73). This may be due to an LPS-induced immune response, which enforces the kynurenine pathway through the activation of IDO, thereby hampering serotonin synthesis (74).
Interestingly, increased anxiety and an elevated circulating tryptophan availability in GF mice can be restored to normal values following colonization of the gut microbiota, while the increased serotonin levels cannot be normalized in GF mice post-weaning (15). These findings point to a complex network controlling the central serotonin metabolism, involving the serotonin-selective reuptake transporter (SERT) and serotonin turnover into 5-hydroxyindoleacetic acid by monoamine oxidase (MAO) (21). Along with the decrease in the central levels of serotonin and its precursor tryptophan, the antibiotic-induced alterations in the large intestinal microbiota are associated with increased expression of SERT and MAO in the hypothalami of piglets (12). These findings indicate that the enhanced serotonin metabolism, as reflected by increased SERT and MAO expression, may contribute to the decrease in central serotonin levels following gut dysbiosis.
During ENS development, serotonergic neurons are among the first to be present in the ENS, where they impact on neurogenesis and guide the development and survival of neurons (21). A previous study revealed that the colonization of GF mice with microbiota from conventional mice modifies the neuroanatomy of the ENS and enhanced intestinal transit, which is associated with increased neuronal and mucosal serotonin production and the proliferation of enteric neuronal progenitors in the adult intestine (75). The activation of the 5-hydroxytryptamine4 (5-HT4) receptor in the ENS is linked to adult neurogenesis and neuroprotection (76). The pharmacological modulation of the 5-HT4 receptor identifies a mechanistic link between the gut microbiota and maturation of the adult ENS through the serotonin-dependent activation of the 5-HT4 receptor (75).
Kynurenine and the Gut-Brain Axis
As noted above, the kynurenine pathway is the major route of tryptophan degradation. This pathway is of interest not only because it modulates serotonin availability, but also due to the strong evidence implicating the kynurenine pathway in behavioral and cognitive symptoms of neurological disease (31). Thus, dysregulation between serotonin synthesis and the kynurenine pathway influences neuropsychiatric disorders, such as depression (34). Once kynurenine is produced, it can be further metabolized along 2 distinct arms of the pathway, with neuroactive KYNA and QUIN being the major products. KYNA can be neuroprotective against QUIN-induced excitotoxicity, while it can also induce cognitive impairments when excessively elevated (34). Therefore, in addition to limiting the tryptophan availability for central serotonin synthesis, activation of the kynurenine pathway also plays an important role in the modulation of brain functions by producing downstream neurotoxic/neuroprotective metabolites. Furthermore, kynurenine is an Ah-ligand: a receptor that could play vital roles in neuroinflammatory processes, as well as neuropsychiatric disorders (14, 77).
Notably, central kynurenine accumulation results in behavioral, learning, and memory deficits in rodents (78). In contrast, gut microbiota–depleted mice exhibit cognitive deficits, as well as reduced anxiety-like behavior, accompanied by higher levels of tryptophan and lower levels of kynurenine in plasma (51). Furthermore, mice receiving gut microbiota from depressed patients exhibit increased anxiety-like behaviors, in line with higher kynurenine levels and an increased kynurenine to tryptophan ratio in blood circulation (7). Oral administration of the probiotic Bifidobacterium infantis leads to increased circulating tryptophan and KYNA concentrations (60). Similarly, mice infected with Toxoplasma gondiido, an intracellular protozoan parasite, have elevated levels of kynurenine, KYNA, and QUIN in the brain (79). A recent study further reveals that the anthocyanins attenuate neuroinflammation and neurobehavioral changes in rats in response to high fat–induced obesity, by modulating some of the features of diet-induced dysbiosis with an increased abundance of Ruminococcus and decreased abundance of Oscillobacter, which are associated with the increased levels of tryptophan and KYNA (80). These findings indicate that the gut microbiota may affect brain functions through modulation of the kynurenine pathway.
It is well known that various cognitive and behavioral functions are controlled by central micro RNAs (miRNAs) (81). The expressions of miRNAs, such as miR-294–5p, Brd2, and Slit3kr, which regulate the expression of central kynurenine pathway genes, are reported to be increased in the hippocampi of GF mice and can be normalized following microbial colonization (82). These findings suggest that gut microbiota regulate the expression of kynurenine pathway genes in the hippocampus through an miRNA-dependent way. There is also evidence that gut microbiota can modulate the brain kynurenine pathway by directly impacting the activity of its key enzymes. When compared with conventional mice, GF mice show reduced IDO activity, which can be normalized after recolonization with gut microbiota immediately post-weaning (15). Mechanistically, on the one hand, gut microbiota can modulate intestinal immune responses through TLRs, thus affecting the production of cytokines and chemokines (9). These immune factors, especially IFN-gamma, can reach the CNS through blood circulation, subsequently activating the central kynurenine pathway through the stimulation of IDO (74). Moreover, in contrast to serotonin, kynurenine produced from the periphery can cross the BBB (31). On the other hand, circulating SCFAs, such as butyrate, can directly modulate central kynurenine pathways. In more detail, butyrate has been demonstrated to inhibit IDO activity and to modulate the kynurenine pathway in a STAT1/HDAC-dependent manner (53). In addition, as evidenced by a previous study in stressed mice (83), the production of H2O2 by Lactobacillus strains, such as Lactobacillus reuteri, can be protective against the development of despair behaviors by directly inhibiting intestinal IDO1 expression and decreasing the circulating kynurenine levels (83).
Microbial Tryptophan Metabolites and the Gut-Brain Axis
The gut microbiota produces a wide and diverse array of tryptophan metabolites, such as tryptamine and indolic compounds; these are integral parts of the host metabolome, as these metabolites are able to signal locally to the intestinal mucosa and also to distant organs, including the brain (22). This points to the potential role of microbial tryptophan metabolites in communication between gut microbiota and the CNS.
Indole is the main metabolite produced by gut bacteria from tryptophan, through the action of the enzyme TnaA, as mentioned earlier. While indole is a major intercellular signal within the gut microbial ecosystem (84), it also interacts with the intestinal epithelium by promoting tight-junction resistance and the expression of anti-inflammatory cytokines (85). Once absorbed into blood circulation, indole is reported to influence brain function and behavior. Conventional rats receiving an acute cecal injection of indole exhibit a dramatic decrease of motor activity, an increase in eye blinking frequency, and an increase in c-Fos protein expression in the dorsal vagal complex (1). A chronic and moderate overproduction of indole by colonizing GF rats with the indole-producing bacterial species Escherichia coli was further applied in this study. Rats overproducing indole display higher helplessness in the tail suspension test, and enhanced anxiety-like behavior in the novelty, elevated plus maze, and open-field tests (1). This study provides clear evidence that indole plays a critical role along the GBA, and that central indole accumulation could lead to developing anxiety and mood disorders.
Similar to indole, indolic derivatives from tryptophan degradation, such as tryptamine, IAA, and IPA, are known to affect intestinal permeability and host immunity. In particular, IPA is identified as a beneficial metabolite and contributes to the anti‐inflammatory effects of the gut microbiota (86). These indole derivatives are ligands for Ah, which are not only expressed in the GI tract but also in cells of the CNS, including neurons, astrocytes, and microglial cells, where Ah has impacts on neuronal proliferation, differentiation, and survival (87). Since circulating indole derivatives, such as IAA and IPA, can cross the BBB, the mediatory role of indolic metabolites in the context of the GBA is of special interest. In line with this hypothesis, a recent study demonstrated that the microbial indole metabolites of tryptophan, including indole, IAA, and IPA, are able to activate Ah signaling in astrocytes and, thereby, suppress CNS inflammation (14).
The Importance of the Tryptophan Metabolism in Neurogastroenterology
Impaired tryptophan metabolisms have been observed in patients with GI functional disorders (e.g., IBS) (88, 89) and neuropsychiatric diseases (e.g., depression and ASD) (16, 90), pointing to the potential role of the tryptophan metabolism in the pathophysiology of these diseases.
Irritable Bowel Syndrome
IBS is a functional GBA disorder, which is characterized by chronically recurring abdominal pain and changes in bowel habits (91). Brain networks, such as sensorimotor, emotional regulation, salience, and executive control networks, have been identified to be involved in the pathophysiology of symptom-related anxiety, hypervigilance, and visceral hypersensitivity in IBS patients (92). The gut microbiota composition may play an important role in IBS, as the relative abundances of bacterial taxa differ between patients with IBS and healthy controls (HCs) (93). However, to date, only associations from cross-sectional studies have been reported, and a causative role of the gut microbiota in the occurrence of IBS has not been demonstrated. Treatment with antibiotics or probiotics improves some symptoms in IBS patients (5, 94), suggesting a potential link between intestinal microbes and IBS. Furthermore, recent studies report significant differences in fecal microbiota composition and brain connectivity networks and volumes between IBS patients and HCs (4, 95), consistent with a potential relationship between the gut microbial community and brain functions in IBS.
It is suggested that there is a relationship between IBS and an impaired tryptophan metabolism (22). Thus, kynurenine levels and the ratio of kynurenine to tryptophan in plasma were shown to be increased in IBS patients compared to HCs (89), indicating the enhanced activity of the kynurenine pathway in IBS patients. However, in line with the importance of serotonin for the regulation of GI motility, a study including both constipation-predominant and diarrhea-predominant IBS patients reports dysfunctional serotonin synthesis and transport, with decreased expressions of TPH1 and SERT in the colorectal biopsies of IBS patients (29). Interestingly, the changes in serotonin levels differ between IBS subtypes, as serotonin levels in the large intestine are found to be decreased in constipation-predominant IBS, while increased in diarrhea-predominant IBS (88). Furthermore, serotonin receptors, such as the 5-HT3 receptor, are reported to be altered in IBS patients (29). Since the serotonin system can be directly modulated by the gut microbiota (48, 75), further research is warranted investigating whether dysregulation of the serotonin system in IBS patients may be partly due to alterations of the gut microbiota.
Neuropsychiatric Disorders
A number of preclinical studies suggest that the gut microbiota may play a key role in neurodevelopmental and neurodegenerative disorders (5, 8, 96). Furthermore, alterations in the tryptophan metabolism in humans are also linked to a variety of neuropsychiatric conditions, such as depression and ASD (16, 37, 97). A depressive disorder is a debilitating condition and the most common mental illness, affecting more than 300 million people worldwide, with decreased central serotonin availability being a major feature in depression (22), indicating that an altered tryptophan metabolism may be involved in the pathogenesis of depression. Previous studies have revealed that decreased levels of tryptophan and an increased kynurenine to tryptophan ratio in the plasma are closely associated with depression (97, 98). In addition, the ratio of KYNA to QUIN is reported to be decreased in the plasma of depressive patients (37). These alterations in tryptophan metabolism are also correlated with learning impairments and processing speeds in patients with depressive disorder (90), potentially linking the kynurenine pathway with cognitive impairments in patients with depressive disorder. These alterations in the tryptophan metabolism may be driven by the gut microbiota, as evidenced by a previous study revealing that the colonization of GF rats with gut microbiota from depressive patients also induces neurobehavioral changes, with an increased kynurenine to tryptophan ratio in blood circulation (7).
ASDs are neurodevelopmental conditions characterized by social and behavioral impairments. In addition to neurological symptoms, ASD subjects frequently suffer from GI functional abnormalities. According to previous studies (6, 99), the pathogenesis of ASD is suggested to involve an altered gut microbiota. In comparison with HCs, in feces, ASD patients display a reduced Bacteroidetes to Firmicutes ratio; decreased abundances of Alistipes, Bilophila, Dialister, Parabacteroides, and Veillonella; and increased abundances of Collinsella, Corynebacterium, Dorea, and Lactobacillus (6). Microbiota transfer therapy improves GI and autism symptoms in ASD patients, with an increased abundance of Bifidobacterium, Prevotella, and Desulfovibrio (99). In addition, patients with ASD exhibit an altered tryptophan metabolism, with reduced levels of tryptophan and an increased kynurenine to tryptophan ratio in the plasma (16), suggesting a shift of the tryptophan metabolism from serotonin synthesis to the kynurenine pathway in ASD. Finally, a correlation between altered concentrations of tryptophan and serotonin with gut dysbiosis (e.g., Clostridiales) was observed in ASD patients (100), indicating that alterations of tryptophan metabolism in the ASD may be mediated by the gut microbiota.
Conclusions
The understanding of the role of the tryptophan metabolism in the communication between the gut microbiota and CNS is increasing. Studies in animal models have expanded the evidence that the gut microbiota modulates GBA function through an interplay between the immune systems, bacterial metabolites (including SCFAs), and changes in the tryptophan metabolism (Figure 3). However, since the gut microbial communities are different in animals and humans (7, 101), questions remain regarding the relevance of findings in animal studies for the pathogenesis, pathophysiology, and treatment of GBA disorders in humans. Therefore, there is an urgent need for large-scale and highly controlled studies in humans to reveal the causative role of the tryptophan metabolism along the GBA. Further research is likewise needed to investigate whether tryptophan metabolites can act as biomarkers for the diagnosis of GBA disorders. In addition, bacterial tryptophan metabolites can aid in the development of novel therapeutic agents for GBA disorders.
FIGURE 3.
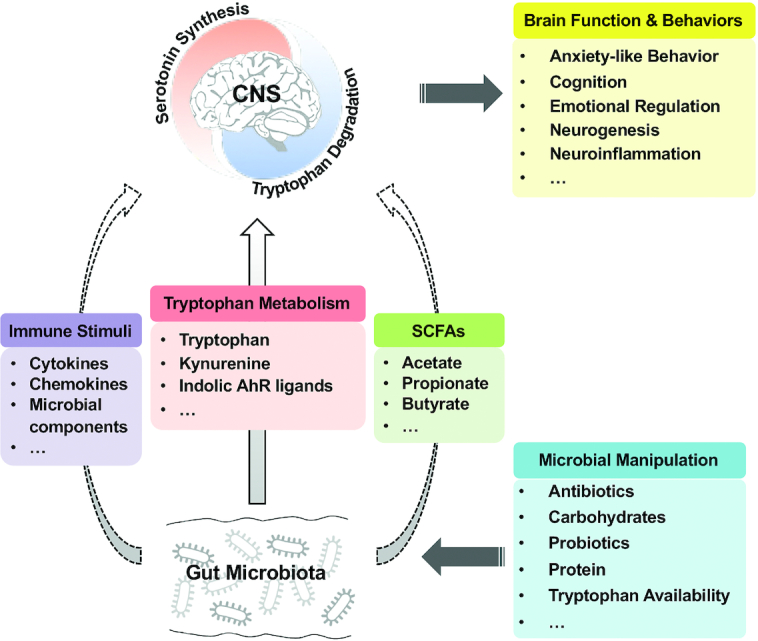
The potential role of tryptophan metabolism in the gut microbiota-brain axis. Manipulations of gut microbiota composition and metabolism by various ways (e.g., antibiotics and probiotics) contribute to the shifts in the central tryptophan metabolism between serotonin synthesis and tryptophan degradation pathways, which thereby influence the brain function and behaviors. The solid arrow indicates the tryptophan metabolism–dependent effects of alterations in gut microbiota on the central tryptophan metabolism; the dashed arrow indicates the tryptophan-independent effects on the central tryptophan metabolism. AhR, aryl hydrocarbon receptor; CNS, central nervous system; SCFA, short-chain fatty acid.
The gut microbiota plays a crucial role in the modulation of the tryptophan metabolism, affecting the balance between the serotonin synthesis and tryptophan degradation pathway (22). Since many factors can influence the gut microbiota composition and metabolism, including diet, antibiotics, and probiotics (Figure 3), the manipulation of the gut microbiota, modulating tryptophan availability, may be a therapeutic option for GBA disorders. It is well recognized that there are differences in microbial compositions in the small and large intestines (101). Thus, the large intestinal microbiota have a greater capacity of tryptophan metabolism than the small intestinal microbiota, with various tryptophan metabolites, such as tryptamine and indolic compounds, being the major products (42, 43). However, to date, the contribution of microbial communities at different intestinal sites to the host tryptophan metabolism is still not clear and requires further investigations by conducting site-specific, microbiota-based interventions.
In conclusion, the tryptophan metabolism plays a central role in the GBA. The major routes of tryptophan metabolism, including serotonin synthesis, the kynurenine pathway, and microbial degradation pathways, are differentially affected in diseases, such as depression and IBS. Since the host tryptophan metabolism is directly and indirectly modulated by the gut microbiota, targeted gut microbiota interventions are promising approaches as therapeutic options for GBA disorders.
ACKNOWLEDGEMENTS
We thank Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality, and Safety Control for support.
The authors’ responsibilities were as follows—KG: wrote the paper; KG and WZ: contributed to the conception of the work; CM, AF, and WZ: revised the paper critically for important content; and all authors: read and approved the final manuscript.
Notes
This study was funded by the National Natural Science Foundation of China (31430082) and the National Key Basic Research Program of China (2013CB127300).
Author disclosures: The authors report no conflicts of interest.
Abbreviations: Ah, aryl hydrocarbon receptor; ASD, autism spectrum disorder; BBB, blood-brain barrier; CNS, central nervous system; EC, enterochromaffin cell; ENS, enteric nervous system; FOS, fructooligosaccharides; GBA, gut-brain axis; GF, germ free; GI, gastrointestinal; HC, healthy control; HDAC, histone deacetylase; IAA, indole-3-acetic acid; IAld, indole-3-aldehyde; IBS, irritable bowel syndrome; IDO, indoleamine 2,3-dioxygenase; ILA, indole-3-lactic acid; IPA, indolic-3-propionic acid; KYNA, kynurenic acid; MAO, monoamine oxidase; miRNA, micro RNA; NMDA, N-methyl D-aspartate; QUIN, quinolinic acid; SERT, serotonin-selective reuptake transporter; STAT1, signal transducer and activator of transcription 1; TDO, tryptophan 2,3-dioxygenase; TLR, Toll-like receptor; TnaA, tryptophanase; TPH, tryptophan hydroxylase; TrpD, tryptophan decarboxylase.
References
- 1. Jaglin M, Rhimi M, Philippe C, Pons N, Bruneau A, Goustard B, Dauge V, Maguin E, Naudon L, Rabot S. Indole, a signaling molecule produced by the gut microbiota, negatively impacts emotional behaviors in rats. Front Neurosci. 2018;12:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frohlich EE, Farzi A, Mayerhofer R, Reichmann F, Jacan A, Wagner B, Zinser E, Bordag N, Magnes C, Frohlich E et al.. Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota-brain communication. Brain Behav Immun. 2016;56:140–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. [DOI] [PubMed] [Google Scholar]
- 4. Labus JS, Hollister EB, Jacobs J, Kirbach K, Oezguen N, Gupta A, Acosta J, Luna RA, Aagaard K, Versalovic J et al.. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome. 2017;5(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin FP, Cominetti O, Welsh C, Rieder A et al.. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(2):448–59. [DOI] [PubMed] [Google Scholar]
- 6. Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, Jousson O, Leoncini S, Renzi D, Calabro A et al.. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017;5(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kelly JR, Borre Y, Ciaran OB, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G et al.. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–18. [DOI] [PubMed] [Google Scholar]
- 8. Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J et al.. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–94. [DOI] [PubMed] [Google Scholar]
- 9. Mu C, Yang Y, Zhu W. Gut microbiota: the brain peacekeeper. Front Microbiol. 2016;7:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao K, Pi Y, Mu CL, Farzi A, Liu Z, Zhu WY. Increasing carbohydrate availability in the hindgut promotes hypothalamic neurotransmitter synthesis: aromatic amino acids linking the microbiota-brain axis. J Neurochem. 2019;149(5):641–59. [DOI] [PubMed] [Google Scholar]
- 11. Lukic I, Getselter D, Koren O, Elliott E. Role of tryptophan in microbiota-induced depressive-like behavior: evidence from tryptophan depletion study. Front Behav Neurosci. 2019;13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao K, Pi Y, Mu CL, Peng Y, Huang Z, Zhu WY. Antibiotics-induced modulation of large intestinal microbiota altered aromatic amino acid profile and expression of neurotransmitters in the hypothalamus of piglets. J Neurochem. 2018;146(3):219–34. [DOI] [PubMed] [Google Scholar]
- 13. Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann NY Acad Sci. 2018;1420(1):5–25. [DOI] [PubMed] [Google Scholar]
- 14. Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M et al.. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18(6):666–73. [DOI] [PubMed] [Google Scholar]
- 16. Kaluzna-Czaplinska J, Gatarek P, Chirumbolo S, Chartrand MS, Bjorklund G. How important is tryptophan in human health?. Crit Rev Food Sci Nutr. 2019;59(1):72–88. [DOI] [PubMed] [Google Scholar]
- 17. Yu M, Mu C, Yang Y, Zhang C, Su Y, Huang Z, Yu K, Zhu W. Increases in circulating amino acids with in-feed antibiotics correlated with gene expression of intestinal amino acid transporters in piglets. Amino Acids. 2017;49(9):1587–99. [DOI] [PubMed] [Google Scholar]
- 18. Mu C, Yang Y, Yu K, Yu M, Zhang C, Su Y, Zhu W. Alteration of metabolomic markers of amino-acid metabolism in piglets with in-feed antibiotics. Amino Acids. 2017;49(4):771–81. [DOI] [PubMed] [Google Scholar]
- 19. Le Floc'h N, Otten W, Merlot E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids. 2011;41(5):1195–205. [DOI] [PubMed] [Google Scholar]
- 20. Wu G, Bazer FW, Dai Z, Li D, Wang J, Wu Z. Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci. 2014;2:387–417. [DOI] [PubMed] [Google Scholar]
- 21. O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. [DOI] [PubMed] [Google Scholar]
- 22. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–24. [DOI] [PubMed] [Google Scholar]
- 23. Mesripour A, Hajhashemi V, Kuchak A. Effect of concomitant administration of three different antidepressants with vitamin B6 on depression and obsessive compulsive disorder in mice models. Res Pharm Sci. 2017;12(1):46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ciorba MA. Kynurenine pathway metabolites: relevant to vitamin B-6 deficiency and beyond. Am J Clin Nutr. 2013;98(4):863–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Israelyan N, Margolis KG.. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol Res. 2018;132:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mawe GM, Hoffman JM.. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanders-Bush E, Bushing JA, Sulser F. Long-term effects of p-chloroamphetamine on tryptophan hydroxylase activity and on the levels of 5-hydroxytryptamine and 5-hydroxyindole acetic acid in brain. Eur J Pharmacol. 1972;20(3):385–8. [DOI] [PubMed] [Google Scholar]
- 28. Grahame-Smith DG. Studies in vivo on the relationship between brain tryptophan, brain 5-HT synthesis and hyperactivity in rats treated with a monoamine oxidase inhibitor and L-tryptophan. J Neurochem. 1971;18(6):1053–66. [DOI] [PubMed] [Google Scholar]
- 29. Kerckhoffs AP, ter Linde JJ, Akkermans LM, Samsom M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302(9):G1053–60. [DOI] [PubMed] [Google Scholar]
- 30. Donner NC, Johnson PL, Fitz SD, Kellen KE, Shekhar A, Lowry CA. Elevated tph2 mRNA expression in a rat model of chronic anxiety. Depress Anxiety. 2012;29(4):307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: tryptophan's metabolites in exercise, inflammation, and mental health. Science. 2017;357(6349):eaaf9794. [DOI] [PubMed] [Google Scholar]
- 32. Wolf AM, Wolf D, Rumpold H, Moschen AR, Kaser A, Obrist P, Fuchs D, Brandacher G, Winkler C, Geboes K et al.. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol. 2004;113(1):47–55. [DOI] [PubMed] [Google Scholar]
- 33. O'Farrell K, Harkin A.. Stress-related regulation of the kynurenine pathway: relevance to neuropsychiatric and degenerative disorders. Neuropharmacology. 2017;112(Pt B):307–23. [DOI] [PubMed] [Google Scholar]
- 34. Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2017;112(Pt B):399–412. [DOI] [PubMed] [Google Scholar]
- 35. Pierozan P, Biasibetti H, Schmitz F, Avila H, Parisi MM, Barbe-Tuana F, Wyse AT, Pessoa-Pureur R. Quinolinic acid neurotoxicity: differential roles of astrocytes and microglia via FGF-2-mediated signaling in redox-linked cytoskeletal changes. Biochim Biophys Acta. 2016;1863(12):3001–14. [DOI] [PubMed] [Google Scholar]
- 36. Savitz J, Drevets WC, Wurfel BE, Ford BN, Bellgowan PS, Victor TA, Bodurka J, Teague TK, Dantzer R. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav Immun. 2015;46:55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu H, Ding L, Zhang H, Mellor D, Wu H, Zhao D, Wu C, Lin Z, Yuan J, Peng D. The metabolic factor kynurenic acid of kynurenine pathway predicts major depressive disorder. Front Psychiatry. 2018;9:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ge X, Ding C, Zhao W, Xu L, Tian H, Gong J, Zhu M, Li J, Li N. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J Transl Med. 2017;15(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marcobal A, Kashyap PC, Nelson TA, Aronov PA, Donia MS, Spormann A, Fischbach MA, Sonnenburg JL. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J. 2013;7(10):1933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Williams BB, Van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, Taketani M, Ishihara A, Kashyap PC, Fraser JS, Fischbach MA. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16(4):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee JH, Lee J.. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev. 2010;34(4):426–44. [DOI] [PubMed] [Google Scholar]
- 42. Smith EA, Macfarlane GT.. Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Bacteriol. 1996;81(3):288–302. [DOI] [PubMed] [Google Scholar]
- 43. Roager HM, Licht TR.. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9(1):3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman J, Cortez VS, Caparon MG, Donia MS, Gilfillan S et al.. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8alphaalpha(+) T cells. Science. 2017;357(6353):806–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aragozzini F, Ferrari A, Pacini N, Gualandris R. Indole-3-lactic acid as a tryptophan metabolite produced by Bifidobacterium spp. Appl Environ Microbiol. 1979;38(3):544–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wlodarska M, Luo C, Kolde R, d'Hennezel E, Annand JW, Heim CE, Krastel P, Schmitt EK, Omar AS, Creasey EA et al.. Indoleacrylic acid produced by commensal Peptostreptococcus species suppresses inflammation. Cell Host Microbe. 2017;22(1):25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mandic AD, Woting A, Jaenicke T, Sander A, Sabrowski W, Rolle-Kampcyk U, von Bergen M, Blaut M. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Sci Rep. 2019;9(1):1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hata T, Asano Y, Yoshihara K, Kimura-Todani T, Miyata N, Zhang XT, Takakura S, Aiba Y, Koga Y, Sudo N. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS One. 2017;12(7):e0180745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Desbonnet L, Clarke G, Traplin A, O'Sullivan O, Crispie F, Moloney RD, Cotter PD, Dinan TG, Cryan JF. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun. 2015;48:165–73. [DOI] [PubMed] [Google Scholar]
- 52. Orhan F, Bhat M, Sandberg K, Stahl S, Piehl F, Karolinska Schizophrenia Project consortium, Svensson C, Erhardt S, Schwieler L. Tryptophan metabolism along the kynurenine pathway downstream of Toll-like receptor stimulation in peripheral monocytes. Scand J Immunol. 2016;84(5):262–71. [DOI] [PubMed] [Google Scholar]
- 53. Martin-Gallausiaux C, Larraufie P, Jarry A, Beguet-Crespel F, Marinelli L, Ledue F, Reimann F, Blottiere HM, Lapaque N. Butyrate produced by commensal bacteria down-regulates indolamine 2,3-dioxygenase 1 (IDO-1) expression via a dual mechanism in human intestinal epithelial cells. Front Immunol. 2018;9:2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gao K, Pi Y, Peng Y, Mu CL, Zhu WY. Time-course responses of ileal and fecal microbiota and metabolite profiles to antibiotics in cannulated pigs. Appl Microbiol Biotechnol. 2018;102(5):2289–99. [DOI] [PubMed] [Google Scholar]
- 55. Jiang P, Trimigno A, Stanstrup J, Khakimov B, Viereck N, Engelsen SB, Sangild PT, Dragsted LO. Antibiotic treatment preventing necrotising enterocolitis alters urinary and plasma metabolomes in preterm pigs. J Proteome Res. 2017;16(10):3547–57. [DOI] [PubMed] [Google Scholar]
- 56. Zhang C, Yu M, Yang Y, Mu C, Su Y, Zhu W. Differential effect of early antibiotic intervention on bacterial fermentation patterns and mucosal gene expression in the colon of pigs under diets with different protein levels. Appl Microbiol Biotechnol. 2017;101(6):2493–505. [DOI] [PubMed] [Google Scholar]
- 57. Hara T, Mihara T, Ishibashi M, Kumagai T, Joh T. Heat-killed Lactobacillus casei subsp. casei 327 promotes colonic serotonin synthesis in mice. J Funct Foods. 2018;47:585–9. [Google Scholar]
- 58. Valladares R, Bojilova L, Potts AH, Cameron E, Gardner C, Lorca G, Gonzalez CF. Lactobacillus johnsonii inhibits indoleamine 2,3-dioxygenase and alters tryptophan metabolite levels in BioBreeding rats. FASEB J. 2013;27(4):1711–20. [DOI] [PubMed] [Google Scholar]
- 59. Marcial GE, Ford AL, Haller MJ, Gezan SA, Harrison NA, Cai D, Meyer JL, Perry DJ, Atkinson MA, Wasserfall CH et al.. Lactobacillus johnsonii N6.2 modulates the host immune responses: a double-blind, randomized trial in healthy adults. Front Immunol. 2017;8:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164–74. [DOI] [PubMed] [Google Scholar]
- 61. Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D'Angelo C, Massi-Benedetti C, Fallarino F et al.. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–85. [DOI] [PubMed] [Google Scholar]
- 62. Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM et al.. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22(6):598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kashyap PC, Marcobal A, Ursell LK, Larauche M, Duboc H, Earle KA, Sonnenburg ED, Ferreyra JA, Higginbottom SK, Million M et al.. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology. 2013;144(5):967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Krishnan S, Ding Y, Saeidi N, Choi M, Sridharan GV, Sherr DH, Yarmush ML, Alaniz RC, Jayaraman A, Lee K. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2019;28(12):3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zivkovic AM, German JB, Lebrilla CB, Mills DA. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4653–8.. It should be 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Saraf MK, Piccolo BD, Bowlin AK, Mercer KE, LeRoith T, Chintapalli SV, Shankar K, Badger TM, Yeruva L. Formula diet driven microbiota shifts tryptophan metabolism from serotonin to tryptamine in neonatal porcine colon. Microbiome. 2017;5(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang Z, Mao SY, Zhu WY. Carbohydrate utilization of an indole-producing bacterium. J Nanjing Agricultural Univ. 2011;34(3):140–2. [Google Scholar]
- 68. Li C-Y, Liu J-X, Wang Y-Z, Wu Y-M, Wang J-K, Zhou Y-Y. Influence of differing carbohydrate sources on l-tryptophan metabolism by porcine fecal microbiota studied in vitro. Livest Sci. 2009;120(1):43–50. [Google Scholar]
- 69. Zhou L, Fang L, Sun Y, Su Y, Zhu W. Effects of a diet high in resistant starch on fermentation end-products of protein and mucin secretion in the colons of pigs. Starch. 2017;69(7-8):1600032. [Google Scholar]
- 70. Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29(4):1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6(2):133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sun J, Wang F, Hong G, Pang M, Xu H, Li H, Tian F, Fang R, Yao Y, Liu J. Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci Lett. 2016;618:159–66. [DOI] [PubMed] [Google Scholar]
- 73. Zhu L, Wei T, Gao J, Chang X, He H, Miao M, Yan T. Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neurosci Lett. 2015;606:1–6. [DOI] [PubMed] [Google Scholar]
- 74. Brooks AK, Lawson MA, Rytych JL, Yu KC, Janda TM, Steelman AJ, McCusker RH. Immunomodulatory factors galectin-9 and interferon-gamma synergize to induce expression of rate-limiting enzymes of the kynurenine pathway in the mouse hippocampus. Front Immunol. 2016;7:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. De Vadder F, Grasset E, Manneras Holm L, Karsenty G, Macpherson AJ, Olofsson LE, Backhed F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci U S A. 2018;115(25):6458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29(31):9683–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nguyen NT, Nakahama T, Le DH, Van Son L, Chu HH, Kishimoto T. Aryl hydrocarbon receptor and kynurenine: recent advances in autoimmune disease research. Front Immunol. 2014;5:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pocivavsek A, Thomas MA, Elmer GI, Bruno JP, Schwarcz R. Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology (Berl). 2014;231(14):2799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Notarangelo FM, Wilson EH, Horning KJ, Thomas MA, Harris TH, Fang Q, Hunter CA, Schwarcz R. Evaluation of kynurenine pathway metabolism in Toxoplasma gondii-infected mice: implications for schizophrenia. Schizophr Res. 2014;152(1):261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Marques C, Fernandes I, Meireles M, Faria A, Spencer JPE, Mateus N, Calhau C. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci Rep. 2018;8(1):11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Malmevik J, Petri R, Knauff P, Brattas PL, Akerblom M, Jakobsson J. Distinct cognitive effects and underlying transcriptome changes upon inhibition of individual miRNAs in hippocampal neurons. Sci Rep. 2016;6:19879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moloney GM, O'Leary OF, Salvo-Romero E, Desbonnet L, Shanahan F, Dinan TG, Clarke G, Cryan JF. Microbial regulation of hippocampal miRNA expression: implications for transcription of kynurenine pathway enzymes. Behav Brain Res. 2017;334:50–4..It should be 82. [DOI] [PubMed] [Google Scholar]
- 83. Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, Wu M, Overall CC, Kipnis J, Gaultier A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep. 2017;7:43859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee JH, Wood TK, Lee J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 2015;23(11):707–18. [DOI] [PubMed] [Google Scholar]
- 85. Whitfield-Cargile CM, Cohen ND, Chapkin RS, Weeks BR, Davidson LA, Goldsby JS, Hunt CL, Steinmeyer SH, Menon R, Suchodolski JS et al.. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes. 2016;7(3):246–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jennis M, Cavanaugh CR, Leo GC, Mabus JR, Lenhard J, Hornby PJ. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol Motil. 2018;30(2):e13178. [DOI] [PubMed] [Google Scholar]
- 87. Juricek L, Coumoul X.. The aryl hydrocarbon receptor and the nervous system. Int J Mol Sci. 2018;19(9):2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Manocha M, Khan WI. Serotonin and GI disorders: an update on clinical and experimental studies. Clin Transl Gastroenterol. 2012;3:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Clarke G, McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG. A distinct profile of tryptophan metabolism along the kynurenine pathway downstream of Toll-like receptor activation in irritable bowel syndrome. Front Pharmacol. 2012;3:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhou Y, Zheng W, Liu W, Wang C, Zhan Y, Li H, Chen L, Ning Y. Cross-sectional relationship between kynurenine pathway metabolites and cognitive function in major depressive disorder. Psychoneuroendocrinology. 2019;101:72–9. [DOI] [PubMed] [Google Scholar]
- 91. Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;150(6):1262–79. [DOI] [PubMed] [Google Scholar]
- 92. Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol. 2015;12(10):592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhuang X, Xiong L, Li L, Li M, Chen M. Alterations of gut microbiota in patients with irritable bowel syndrome: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32(1):28–38. [DOI] [PubMed] [Google Scholar]
- 94. Zeber-Lubecka N, Kulecka M, Ambrozkiewicz F, Paziewska A, Goryca K, Karczmarski J, Rubel T, Wojtowicz W, Mlynarz P, Marczak L et al.. Limited prolonged effects of rifaximin treatment on irritable bowel syndrome-related differences in the fecal microbiome and metabolome. Gut Microbes. 2016;7(5):397–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Labus JS, Osadchiy V, Hsiao EY, Tap J, Derrien M, Gupta A, Tillisch K, Le Neve B, Grinsvall C, Ljungberg M et al.. Evidence for an association of gut microbial Clostridia with brain functional connectivity and gastrointestinal sensorimotor function in patients with irritable bowel syndrome, based on tripartite network analysis. Microbiome. 2019;7(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yu M, Jia H, Zhou C, Yang Y, Zhao Y, Yang M, Zou Z. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J Pharm Biomed Anal. 2017;138:231–9. [DOI] [PubMed] [Google Scholar]
- 97. Messaoud A, Mensi R, Douki W, Neffati F, Najjar MF, Gobbi G, Valtorta F, Gaha L, Comai S. Reduced peripheral availability of tryptophan and increased activation of the kynurenine pathway and cortisol correlate with major depression and suicide. World J Biol Psychiatry. 2018;5:1–9. [DOI] [PubMed] [Google Scholar]
- 98. Hestad KA, Engedal K, Whist JE, Farup PG. The relationships among tryptophan, kynurenine, indoleamine 2,3-dioxygenase, depression, and neuropsychological performance. Front Psychol. 2017;8:1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S et al.. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Luna RA, Oezguen N, Balderas M, Venkatachalam A, Runge JK, Versalovic J, Veenstra-VanderWeele J, Anderson GM, Savidge T, Williams KC. Distinct microbiome-neuroimmune signatures correlate with functional abdominal pain in children with autism spectrum disorder. Cell Mol Gastroenterol Hepatol. 2017;3(2):218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mu C, Yang Y, Su Y, Zoetendal EG, Zhu W. Differences in microbiota membership along the gastrointestinal tract of piglets and their differential alterations following an early-life antibiotic intervention. Front Microbiol. 2017;8:797. [DOI] [PMC free article] [PubMed] [Google Scholar]