Abstract
Objective
To investigate the technical and clinical efficacy of the percutaneous insertion of a biliary metallic stent, and to identify the factors associated with biliary stent dysfunction in patients with malignant duodenobiliary obstruction.
Materials and Methods
The medical records of 70 patients (39 men and 31 women; mean age, 63 years; range, 38−90 years) who were treated for malignant duodenobiliary obstruction at our institution between April 2007 and December 2018, were retrospectively reviewed. Variables found significant by univariate log-rank analysis (p < 0.2) were considered as suitable candidates for a multiple Cox's proportional hazard model.
Results
The biliary stents were successfully placed in all 70 study patients. Biliary stent insertion with subsequent duodenal stent insertion was performed in 33 patients and duodenal stent insertion with subsequent biliary stent insertion was performed in the other 37 study subjects. The median patient survival and stent patency time were 107 days (95% confidence interval [CI], 78–135 days) and 270 days (95% CI, 95–444 days), respectively. Biliary stent dysfunction was observed in 24 (34.3%) cases. Multiple Cox's proportional hazard analysis revealed that the location of the distal biliary stent was the only independent factor affecting biliary stent patency (hazard ratio, 3.771; 95% CI, 1.157–12.283). The median biliary stent patency was significantly longer in patients in whom the distal end of the biliary stent was beyond the distal end of the duodenal stent (median, 327 days; 95% CI, 249–450 days), rather than within the duodenal stent (median, 170 days; 95% CI, 115–225 days).
Conclusion
The percutaneous insertion of the biliary metallic stent appears to be a technically feasible, safe, and effective method of treating malignant duodenobiliary obstruction. In addition, a biliary stent system with a distal end located beyond the distal end of the duodenal stent will contribute towards longer stent patency in these patients.
Keywords: Biliary, Bile duct, Duodenum, Obstruction, Stens
INTRODUCTION
Patients with gastroduodenal and pancreaticobiliary malignancies can develop simultaneous or sequential biliary and duodenal obstructions. As most of these malignancies are unresectable at the time of diagnosis, the treatment options are restricted to palliative management which typically involves stent insertions (1,2,3). The simultaneous insertion of biliary and duodenal metallic stents, or consecutive insertion of these stents has been established as a safe and effective palliative method in this regard (4,5,6,7,8,9,10,11).
As biliary obstruction develops near the papilla in most patients with malignant duodenobiliary obstruction, biliary metallic stents should be placed with one end in the duodenum or in a previously inserted duodenal stent lumen. There have been conflicting opinions in the literature as to whether the biliary stent insertion should be performed before or after duodenal stent insertion in patients with simultaneous biliary and duodenal obstruction (4,5,6,7,8,9,10,11). Several endoscopic studies have suggested that a biliary stent insertion would be more successful when done prior to duodenal stent insertion because of the difficulty either in passing the duodenal stricture with the duodenal stent, or in accessing the papilla through the mesh of the duodenal stent (5,6,8,10). Moreover, if the major duodenal papilla is involved and the duodenal lumen is obstructed due to tumor invasion, it is very difficult to achieve biliary decompression via the endoscopic transpapillary route (4,6,11). In addition, a biliary stent insertion cannot be done endoscopically after a duodenal covered stent insertion. However, the existence of a biliary stent in the duodenal lumen may disrupt the insertion of a duodenal stent, which may then destroy the structure of the biliary stent, causing its dysfunction (7). Other previous studies have also suggested that duodenal stent insertion should be performed prior to biliary stent insertion because biliary stent insertion through the duodenal stent mesh is easier (7,11).
Although endoscopic approach is usually considered as a first choice for the treatment of malignant biliary obstruction, endoscopic insertion of a biliary stent in patients with malignant duodenobiliary obstruction is sometimes very difficult or even impossible due to tight duodenal stricture, pre-existing duodenal stent, or combined obstruction of the duodenal papilla. On the other hand, regardless of combined duodenobiliary obstruction, the presence of a duodenal stent, or timing of biliary stent insertion, percutaneous insertion of a biliary stent is technically successful in most cases. Therefore, percutaneous biliary stent insertion is usually performed in patients with malignant duodenobiliary obstruction.
Thus, the purpose of our present study was to investigate the technical and clinical efficacy of the percutaneous insertion of a biliary metallic stent and identify any factors associated with biliary stent dysfunction in patients with malignant duodenobiliary obstruction.
MATERIALS AND METHODS
Patients
The medical records of 92 consecutive patients with malignant duodenobiliary obstruction, who were treated at our institution between April 2007 and December 2018, were retrospectively reviewed. Patients were included in our study series if they had a duodenobiliary obstruction caused by malignancy that could not be treated surgically, due to unresectability, late tumor-stage, advanced age, or a comorbid condition, and if an endoscopic attempt to drain the obstructed bile duct was unsuccessful due to combined duodenal obstruction or previous duodenal metallic stents. We excluded 22 patients from further analysis either because they had a duodenal stent in the first portion of the duodenum without stent coverage of the duodenal papilla (n = 18), or were lost to follow-up (n =4). The final study cohort therefore comprised 70 patients (39 men and 31 women; mean age, 63 years; range, 38–90 years) with malignant duodenobiliary obstruction. The patient characteristics are presented in Table 1. This study was approved by our Institutional Review Board, which waived the requirement for written informed consent due to the retrospective nature of the analyses. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Table 1. Baseline Demographics and Clinical Data of Study Patients.
| Characteristics | Patients (n = 70) |
|---|---|
| Sex (%) | |
| Male | 39 (55.7) |
| Female | 31 (44.3) |
| Age (mean ± SD, years) | 38–90 (63 ± 11.6) |
| Primary cancer (%) | |
| Pancreas cancer | 29 (41.4) |
| Gastric cancer | 21 (30.0) |
| Duodenal cancer | 6 (8.6) |
| Ampulla of Vater cancer | 2 (2.9) |
| Gallbladder cancer | 5 (7.1) |
| Bile duct cancer | 4 (5.7) |
| Other* | 3 (4.3) |
| Level of biliary obstruction (%) | |
| Upper common bile duct | 5 (7.1) |
| Lower common bile duct | 39 (55.7) |
| Whole common bile duct | 26 (37.1) |
| Type of biliary stent (%) | |
| Covered | 63 (90.0) |
| Uncovered | 7 (10.0) |
| Location of distal end of biliary stent (%) | |
| Beyond duodenal stent | 31 (44.3) |
| Within duodenal stent | 39 (55.7) |
| Location of duodenal obstruction† (%) | |
| Type I | 16 (22.9) |
| Type II | 49 (70.0) |
| Type III | 5 (7.1) |
| Timing of duodenal stenting (%) | |
| Before biliary stenting | 37 (52.9) |
| After biliary stenting | 33 (47.1) |
| Type of duodenal stent (%) | |
| Covered | 52 (74.3) |
| Uncovered | 18 (25.7) |
| Approach route for duodenal stenting (%) | |
| Fluoroscopically | 38 (54.3) |
| Endoscopically | 32 (45.7) |
*1 ovarian cancer, 1 lung cancer, 1 ureter cancer, †Type I, proximal to duodenal papilla without involvement of papilla; type II, at second part of duodenum with involvement of papilla; and type III, distal to papilla without involvement of papilla. SD = standard deviation
Technique
A total of five interventional radiologists with 3–22 years of experience performed percutaneous transhepatic biliary drainage (PTBD) and biliary stent insertion. The choice of stent deployment technique and biliary stent type used in each study patient was at the discretion of the treating physician operators. All biliary stent deployments were performed using uncovered (Epic, Boston Scientific, Galway, Ireland; or DB stent, S&G Biotech, Seongnam, Korea) or covered stents (GD stent, TaeWoong Medical, Gimpo, Korea; GD stent with long extension, TaeWoong Medical; ComVi, TaeWoong Medical; or Hercules, S&G Biotech). The covered stents were partially polytetrafluoroethylene (PTFE)-covered nitinol stents with 2 or 3 cm bare extensions at the proximal end to prevent stent migration, tumor overgrowth, and intrahepatic duct occlusion. The GD and Hercules stents were used for the management of extrahepatic biliary obstruction, whereas a ComVi stent was used for a duodenal extension in patients who had previously undergone a duodenal stent insertion. A GD stent with a long extension was used for the initial management of simultaneous duodenobiliary obstruction cases, or for duodenal extensions in patients who had malignant duodenal stricture or had previously undergone duodenal stent insertion. All biliary stents were available in diameters of 8 and 10 mm. Other than the long type GD stent, all other stents were available in lengths of 6, 8, and 10 cm. The long type GD stents were 18 or 23 cm long.
A duodenal stent insertion with subsequent biliary stent insertion was performed in 37 patients in our current series and biliary stent insertion with subsequent duodenal stent insertion was performed in 33 patients (Fig. 1). Duodenal stent insertion was performed either fluoroscopically (n = 38) or endoscopically (n = 32) using covered (n = 52) or uncovered stents (n = 18). A total of 80 duodenal stents was used and all of them completely covered the papilla.
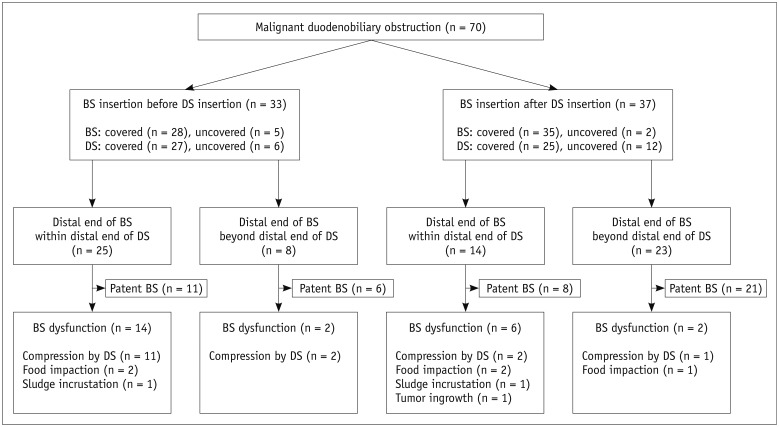
Fig. 1. Flow chart of BS insertion in patients with malignant duodenobiliary obstruction.
BS = biliary stent, DS = duodenal stent
In cases of duodenal obstruction following biliary stent insertion, all duodenal stents completely covered the duodenal ampulla. If the biliary stent did not have a long extension, its distal end was covered by the subsequently inserted duodenal stent. Similarly, in cases of biliary obstruction following a duodenal stent insertion, a percutaneous biliary stent insertion was attempted into the duodenal uncovered stent lumen, the space between the duodenal covered stent and the duodenal wall, the distal duodenum, or the jejunum beyond the distal portion of the duodenal stent. In cases with simultaneous biliary and duodenal obstruction without any previous interventions, a subsequent biliary stent was inserted within several days after the placement of a duodenal stent. If the bile flow through the biliary stent was insufficient due to an uncovered duodenal stent mesh, or extrinsic compression of the covered extension caused by the duodenal covered stent, Balloon Dilation (Boston Scientific) was performed followed by the insertion of an additional biliary stent (ComVi stent, TaeWoong Medical) into the insufficient area.
Prior to catheter removal, the temporary biliary drainage catheter was clamped and left in place for 2–3 days to evaluate stent patency. The position and function of the stent were evaluated by follow-up cholangiography. The catheter was subsequently removed if any free contrast flow through the stent into the duodenum or jejunum was documented or if the serum bilirubin level was markedly decreased or normalized.
Study Endpoint and Statistical Analysis
The primary study endpoints included the assessment of technical success, complications, successful internal drainage and patient survival. The secondary study endpoint was the assessment of factors influencing biliary stent patency. Technical success was defined as the placement of the biliary stent in an adequate position with no migration. Complications were classified as major or minor in accordance with the guidelines of the Society of Interventional Radiology Standards of Practice Committee (12). Successful internal drainage was defined as the successful removal of the temporary drainage catheter and a decrease in the serum bilirubin level to less than 75% of the pretreatment value within the first month following biliary stent insertion. Patient survival was defined as the time interval between the initial biliary stent insertion and the patient's death or last follow-up. The cutoff date for data analysis was March 31, 2019. Stent occlusion was defined as a radiologically confirmed biliary obstruction with serum bilirubin levels greater than 3 mg/dL, or as any condition requiring repeated interventions to improve the biliary drainage. Stent patency was defined as the time interval between the initial biliary stent insertion and recurrence of obstruction. Patients who had not experienced stent occlusion were censored at the date of the last follow-up or death.
A paired-sample t test was used to compare the pre- and post-stenting serum bilirubin levels. Stent patency and patient survival rates were calculated using the Kaplan-Meier method and differences between the curves were analyzed by the log-rank test. The following variables were included in this analysis: age; gender; underlying malignancy; level of extrahepatic biliary obstruction; type of biliary stent; distal end of the biliary stent location; length of duodenal obstruction; approach route for duodenal stenting; timing of duodenal stenting; and type of duodenal stent. Variables found to be significant by univariate log-rank analysis (p < 0.2) were considered as the candidate variables for analysis by a multiple Cox's proportional hazard model. Variables were selected in a stepwise forward selection manner. Subgroup analysis of patients, who underwent subsequent biliary stent insertion after duodenal stent insertion, was performed. All statistical analyses were performed using SPSS software version 14.0. (SPSS Inc., Chicago, IL, USA), with p values less than 0.05 indicating statistical significance.
RESULTS
Duodenobiliary Obstructions in the Study Cohort
A simultaneous biliary and duodenal obstruction occurred in 23 patients. In the remaining 47 study cases, duodenal obstruction occurred before biliary obstruction in 22 patients and afterwards in 25 patients. The median interval between the onset of the biliary and duodenal obstructions was 65 days (range, 9–420 days).
Technical and Clinical Outcomes of Biliary Stent Insertion
A flow chart of the fate of the 70 patients treated with percutaneous biliary metallic stent insertion for malignant duodenobiliary obstruction is given in Figure 1. Biliary stent deployments were technically successful in all 70 study patients (uncovered stent [n = 7], covered stent [n = 33], covered stent with additional covered stent for duodenal extension [n = 13], long type GD stent [n = 17]) and 83 biliary stents were used in total in this population. In 37 of these cases, a subsequent biliary stent was inserted through the mesh of the duodenal uncovered stent (n = 12) (Fig. 2) or through the space between the duodenal covered stent and the duodenal wall (n = 25). The remaining 33 patients underwent an initial biliary stent insertion without a duodenal stent. This was a transpapillary insertion in which the distal portion of the biliary stent was located from the second part of duodenum to the proximal jejunum. Subsequent duodenal stenting was however performed in all of these cases. In 31 of the 70 patients, the distal portion of the biliary stent was located beyond the distal margin of the duodenal stent (Fig. 2B). In eight of these patients, duodenal stent was inserted after biliary stent insertion. In the remaining 39 patients, the distal portion of the biliary stent was located within the distal margin of the duodenal stent, in the lumen of duodenal uncovered stent (n = 9), or in the space between the duodenal wall and the duodenal uncovered (n = 16) or covered stent (n = 14). Follow-up cholangiographs after the clamp test period (mean, 2.7 days; range, 2–8 days) revealed a patent stent in 57 patients. However, an additional covered stent was necessary to achieve a fluent passage of the contrast media through the biliary stent in five patients due to insufficient expansion of the biliary stent at the mesh of the duodenal uncovered stent (n = 1) or extrinsic compression caused by the duodenal covered stent (n = 4) (Fig. 3). In the remaining eight patients who underwent an additional stent insertion, the drainage catheter could not be removed due to persistent severe narrowing of the biliary stent caused by extrinsic compression of the duodenal stent in seven cases and because of the recurrent food reflux in one patient. Among the 62 patients that showed a patent biliary stent, 61 were free of an external drainage catheter after the follow-up cholangiography. The drainage catheter could not be removed in one patient with a patent stent due to a progressive increase in his serum bilirubin level. Successful internal drainage was thus achieved in 61 (87.1%) of the 70 patients in our current series. The mean serum bilirubin level was 7.2 ± 5.1 mg/dL before drainage and decreased significantly to 1.5 ± 2.1 mg/dL within one month after the biliary stent insertion (p < 0.001).
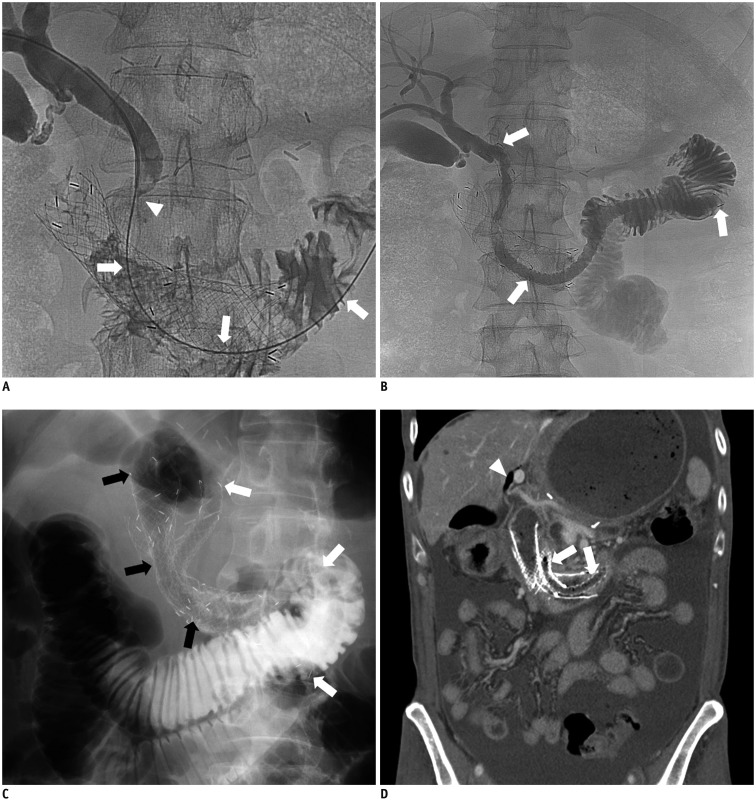
Fig. 2. 61-year-old man with advanced gastric cancer.
Type II duodenal obstruction in this patient had been treated with duodenal uncovered stent 4 months previously.
A. Cholangiogram via right PTBD showing malignant distal common bile duct obstruction (arrowhead). Guide wire was successfully inserted into distal duodenum though mesh of duodenal uncovered stent (white arrows). B. Cholangiogram obtained after placement of long type GD stent (TaeWoong Medical; 10 mm × 23 cm, white arrows) showing good stent position and expansion, as well as good passage of contrast medium to jejunum via stent. Distal end of biliary stent was notably located in proximal jejunum. C. Six weeks after biliary stent (white arrows) placement, another duodenal stent (black arrows) was deployed beside biliary stent due to duodenal stent dysfunction. D. Contrast-enhanced coronal CT image obtained 3 months after insertion of additional duodenal stent showing good expansion of biliary stent (white arrows) and pneumobilia in non-dilated left intrahepatic bile duct (white arrowhead), indicating patent biliary stent. There was no clinical evidence of biliary stent dysfunction up to time of patient's death from disease progression at 167 days after its placement. PTBD = percutaneous transhepatic biliary drainage
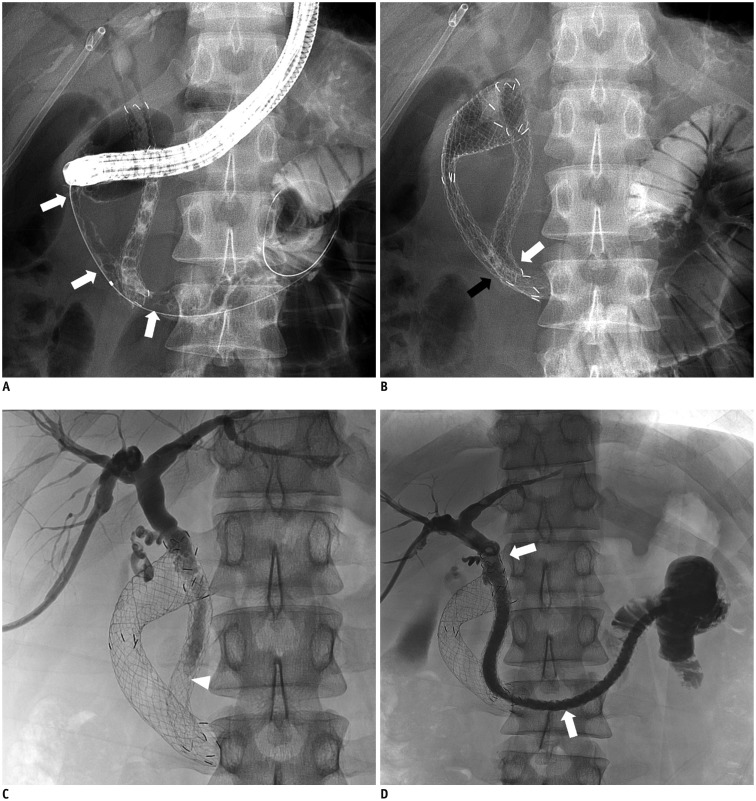
Fig. 3. 31-year-old man with pancreatic cancer. Biliary obstruction in this patient had been treated with covered biliary stent 2 weeks previously.
A. Fluoroscopic image showing type II duodenal stricture (white arrows). Contrast media had refluxed into previously inserted biliary stent. B. Placement of an uncovered duodenal stent (20 mm × 10 cm). Overlap of distal ends of duodenal stent (black arrow) and biliary stent (white arrow) is evident. C. Cholangiogram via right PTBD at one week after duodenal stent placement indicating stasis of contrast medium in common bile duct (arrowhead), suggesting biliary stent malfunction due to extrinsic compression by subsequently inserted duodenal stent. D. Additional long type GD stent (10 mm × 23 cm, white arrows) was deployed through lumen of previous biliary stent into proximal jejunum. Cholangiogram showing good stent position and expansion, as well as good passage of contrast medium to jejunum via subsequent stent.
Procedure-related minor complications arose in three (4.3%) patients, including two cases of self-limiting hemobilia that completely resolved within two days without transfusion and one patient who developed cholangitis which resolved within three days following antibiotic treatment. Major complications occurred in seven (10%) patients. Acute pancreatitis developed immediately after biliary stent insertion in four patients and was successfully treated by conservative management over 1–3 days (mean, 2 days). Perihepatic biloma occurred immediately after temporary drainage catheter removal in two cases and was successfully treated by percutaneous catheter drainage. One patient developed acute cholecystitis at 46 days after a biliary covered stent insertion which was successfully treated by percutaneous transhepatic gallbladder drainage. The overall complication rate was therefore 14.3% (10 of 70 patients).
Patient Survival Outcomes
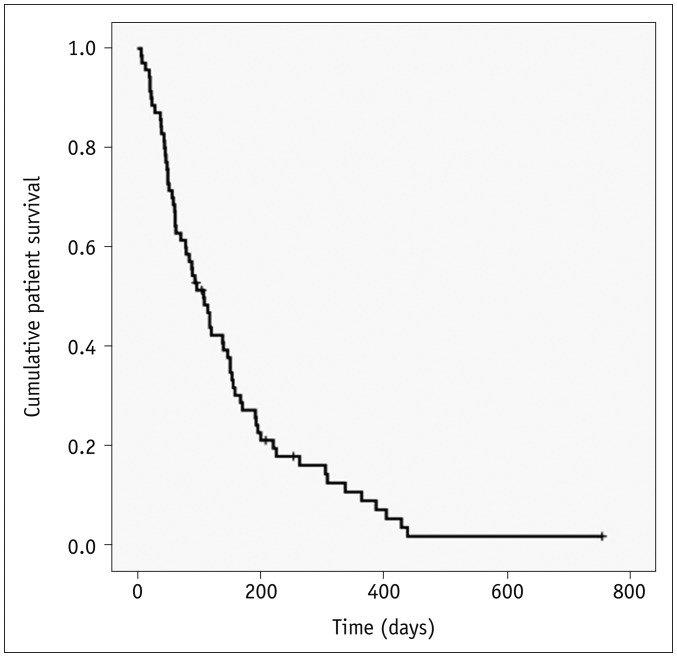
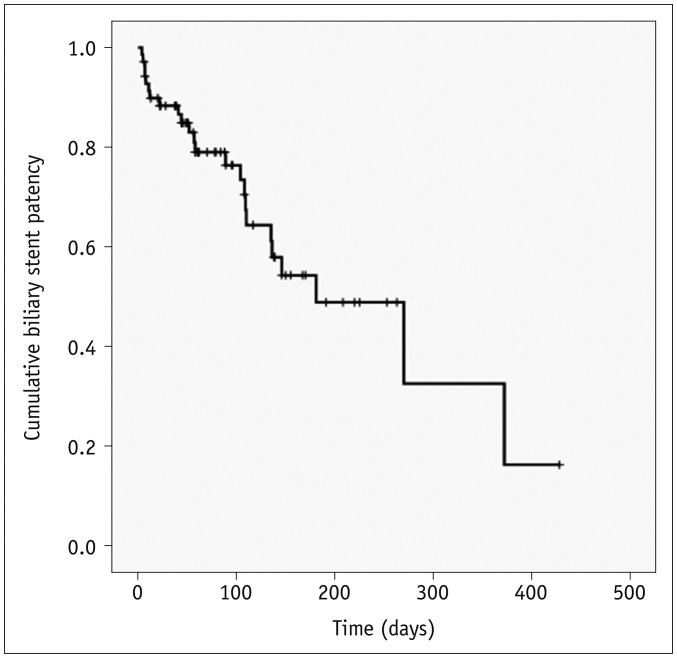
Clinical follow-up information until death or the end of the study was available for all 70 study patients over a duration of 6–754 days (mean, 139 days). Following biliary stent placement, 65 (92.9%) of the 70 study patients died and five (7.1%) remained alive. Kaplan-Meier survival analysis calculated a median survival time of 107 days for the current study series (95% confidence interval [CI], 78–135 days) (Fig. 4).
Fig. 4. Kaplan-Meier curve showing survival outcomes.
Cross hatches indicate censored events.
Biliary Stent Patency and Factors Influencing This
Biliary stent dysfunction was observed in 24 (34.3%) study cases after 87 days. Dysfunction was attributed to extrinsic compression by the duodenal stent in 16 patients, food impaction in five cases, sludge incrustation in two patients, and tumor ingrowth in one individual. Among the 16 patients who developed extrinsic compression caused by their duodenal stent, 10 underwent a subsequent duodenal stent insertion (uncovered duodenal stent [n = 3] and covered duodenal stent [n = 7]) and six underwent the subsequent insertion of a biliary stent (uncovered biliary stent [n = 2] and covered biliary stent [n = 4]). Eight of these 16 patients did not have their temporary drainage catheter removed but among the other eight cases, two were treated by PTBD with subsequent biliary stent insertion and four using endoscopic ultrasound-guided hepatico-esophagostomy (n = 1) or hepatico-gastrostomy (n = 3). In the remaining 10 patients who underwent PTBD, an additional biliary stent insertion could not be performed due to failure of the guidewire passage through the occluded biliary stent in two cases or a poor general condition caused by disease progression in eight patients.
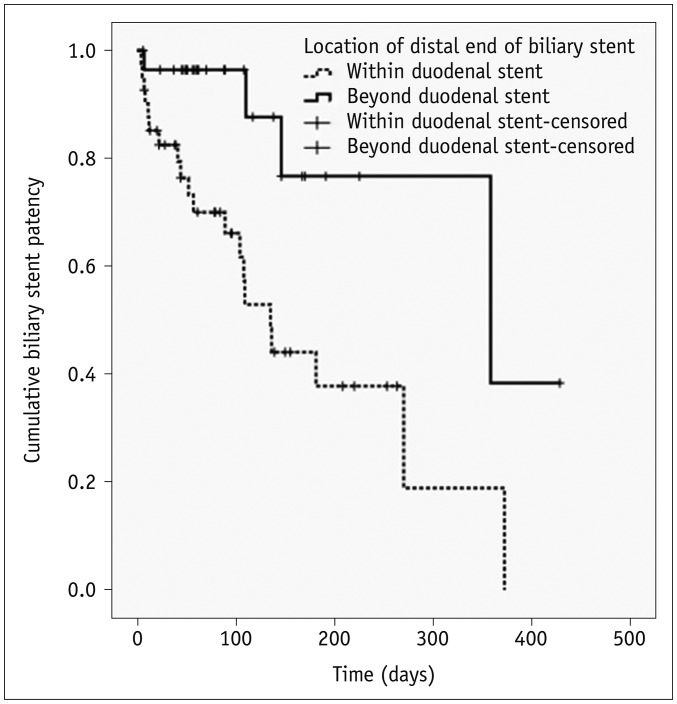
Kaplan-Meier survival analysis indicated that the median stent patency time was 270 days (95% CI, 95–444 days) (Fig. 5). Univariate log-rank analysis revealed that the location of the distal portion of the biliary stent (p = 0.004) and subsequent duodenal stenting (p = 0.176) were potential risk factors for reduced biliary stent patency (Table 2). The multiple Cox's proportional hazard analysis showed that the location of distal end of biliary stent was the only independent predictor of biliary stent patency (hazard ratio, 3.771; 95% CI, 1.157–12.283) (Table 2). The median biliary stent patency rate was significantly longer in patients in whom the distal end of biliary stent was beyond the distal end of the duodenal stent (median, 327 days; 95% CI, 249–405 days), compared with cases in which the distal end of the biliary stent was within the duodenal stent (median, 170 days; 95% CI, 115–225 days) (Fig. 6).
Fig. 5. Kaplan-Meier curve showing biliary stent patency rate.
Cross hatches indicate censored events.
Table 2. Univariate and Multivariate Analysis of Risk Factors Associated with Biliary Stent Patency in Patients with Malignant Duodenobiliary Obstruction.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (year) | 1.168 | 0.516–2.645 | 0.709 | NA | ||
| < 63 | ||||||
| ≥ 63 | ||||||
| Sex | 1.530 | 0.679–3.445 | 0.305 | NA | ||
| Female | ||||||
| Male | ||||||
| Underlying malignancy | 1.476 | 0.542–4.021 | 0.446 | NA | ||
| Periampullary cancer* | ||||||
| Metastatic cancer† | ||||||
| Level of extrahepatic biliary obstruction | ||||||
| Distal CBD | ||||||
| Proximal CBD | 0.531 | 0.229–3.232 | 0.241 | NA | ||
| Whole CBD | 0.382 | 0.049–2.968 | 0.357 | NA | ||
| Type of biliary stent | 2.091 | 0.613–7.137 | 0.239 | NA | ||
| Uncovered | ||||||
| Covered | ||||||
| Location of distal end of biliary stent | 3.788 | 1.292–11.104 | 0.015 | 3.771 | 1.16–12.28 | 0.028 |
| Within duodenal stent | ||||||
| Beyond duodenal stent | ||||||
| Length of duodenal obstruction (mm) | 0.612 | 0.260–1.438 | 0.260 | NA | ||
| < 57 | ||||||
| ≥ 57 | ||||||
| Approach route for duodenal stenting | 0.935 | 0.403–2.166 | 0.875 | NA | ||
| Endoscopy | ||||||
| Fluoroscopy | ||||||
| Timing of duodenal stenting | 0.559 | 0.238–1.313 | 0.182 | 0.990 | 0.39–2.55 | 0.986 |
| Before biliary stenting | ||||||
| After biliary stenting | ||||||
| Type of duodenal stent | 1.724 | 0.694–4.282 | 0.241 | NA | ||
| Uncovered | ||||||
| Covered | ||||||
*Periampullary cancer includes pancreatic cancer, duodenal cancer, ampulla of Vater cancer, gallbladder cancer, and bile duct cancer, †Metastatic cancer includes gastric cancer, lung cancer, ovarian cancer, and ureter cancer. CBD = common bile duct, CI = confidence interval, HR = hazard ratio, NA = not applicable
Fig. 6. Kaplan-Meier curve showing biliary stent patency rate in accordance with location of its distal end.
Patency was found to be significantly improved if biliary stent distal end was located beyond that of duodenal stent rather than within duodenal stent (p = 0.028). Cross hatches indicate censored events.
Subgroup Analysis for Subsequent Biliary Stent Insertion after Duodenal Stent
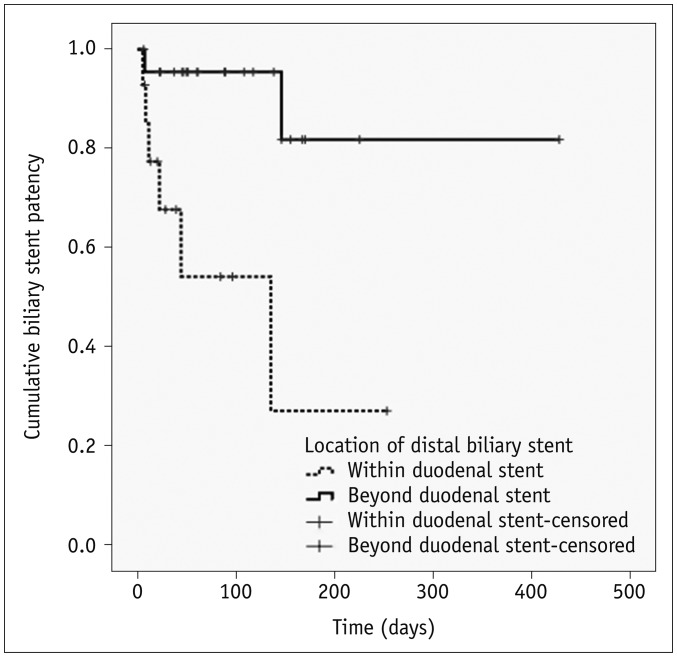
Table 3 and Figure 7 show the results of the subgroup analysis of patients who underwent subsequent biliary stent insertion after duodenal stent insertion. Univariate analysis revealed that the location of the distal portion of the biliary stent was the only potential risk factor for reduced biliary stent patency (hazard ratio, 8.424; 95% CI, 1.632–43.485) (Table 3). The median biliary stent patency rate was significantly longer in patients in whom the distal end of biliary stent was beyond the distal end of the duodenal stent (mean, 370 days; 95% CI, 293–448 days), compared with cases in which the distal end of the biliary stent was within the duodenal stent (mean, 114 days; 95% CI, 45–185 days) (p = 0.003) (Fig. 7).
Table 3. Subgroup Univariate Analysis of Risk Factors Associated with Biliary Stent Patency in Patients Who Underwent Subsequent Biliary Stent Insertion after Duodenal Stent Insertion.
| Variable | Univariate | ||
|---|---|---|---|
| HR | 95% CI | P | |
| Age (year) | 0.603 | 0.143–2.537 | 0.490 |
| < 63 | |||
| ≥ 63 | |||
| Sex | 0.712 | 0.170–2.984 | 0.642 |
| Female | |||
| Male | |||
| Underlying malignancy | 1.941 | 0.390–9.666 | 0.418 |
| Periampullary cancer* | |||
| Metastatic cancer† | |||
| Level of extrahepatic biliary obstruction | |||
| Distal CBD | |||
| Proximal CBD | 0.406 | 0.101–1.627 | 0.203 |
| Whole CBD | 0.000 | 0.000 | 0.992 |
| Type of biliary stent | 3.660 | 0.425–31.530 | 0.238 |
| Uncovered | |||
| Covered | |||
| Location of distal end of biliary stent | 8.424 | 1.632–43.485 | 0.011 |
| Within duodenal stent | |||
| Beyond duodenal stent | |||
| Length of duodenal obstruction (mm) | 0.550 | 0.131–2.316 | 0.415 |
| < 57 | |||
| ≥ 57 | |||
| Approach route for duodenal stenting | 0.603 | 0.150–2.430 | 0.477 |
| Endoscopy | |||
| Fluoroscopy | |||
| Type of duodenal stent | 1.183 | 0.281–4.980 | 0.819 |
| Uncovered | |||
| Covered | |||
*Periampullary cancer includes pancreatic cancer, duodenal cancer, ampulla of Vater cancer, gallbladder cancer, and bile duct cancer, †Metastatic cancer includes gastric cancer, lung cancer, ovarian cancer, and ureter cancer.
Fig. 7. Kaplan-Meier curve showing biliary stent patency rate according to location of its distal end in patients who underwent subsequent biliary stent insertion after duodenal stent insertion.
Patency was found to be significantly improved if biliary stent distal end was placed beyond that of duodenal stent rather than within duodenal stent (p = 0.003). Cross hatches indicate censored events.
DISCUSSION
In our present study, the technical success rate (100%), complication rate (14.3%), successful internal drainage rate (87.1%), and median patient survival time (107 days) are very consistent with the results of previous reports on combined biliary and duodenal stent insertion. In those previous studies, the technical success rate ranged from 88–100%, the overall complication rate was in the range of 13–22%, and the median patient survival time ranged from 81–195.5 days (4,5,6,7,8,9,10,11). We found in our current study series that the percutaneous insertion of biliary stents had been technically successful in all patients regardless of the presence of a duodenal stent, type of the duodenal stent, or timing of biliary stent insertion. Moreover, in most cases in our current cohort, previous duodenal stents were found not to perturb the internal contrast flow through a subsequent biliary stent. If contrast flow was disrupted due to insufficient expansion of the biliary stent at the mesh of the duodenal uncovered stent, or at the space between the duodenal covered stent and duodenal wall, an additional biliary stent insertion to expand this restricted portion of the biliary stent was found to be effective in our patients with a duodenal stent in situ.
Biliary stent dysfunction was observed in 24 (34.3%) of our current series of 70 patients after a median period of 87 days, leading to a median stent patency time of 270 days. The main causes of biliary stent dysfunction included extrinsic compression by a duodenal stent and food impaction due to reflux. Previous studies have reported that the obstruction of a biliary stent in patients with a duodenal stent already in situ might cause a heightened duodenobiliary reflux due to severe duodenal invasion and reduced duodenal peristalsis (13). In addition, subsequent duodenal stenting in patients with an existing biliary stent in situ could cause biliary stent dysfunction due to extrinsic compression by the duodenal stent (7).
We found in our current investigation that the location of the distal end of biliary stent was the only independent predictor of biliary stent patency (p = 0.021). Patients in whom the distal end of the biliary stent was located within the distal duodenal stent were found to be at a greater risk (3.771-fold) of stent occlusion than patients with the distal end of biliary stent located beyond the distal duodenal stent. We thus suggest that the distal end of a biliary stent may be more vulnerable to compression by the duodenal stent than other parts and should be located beyond the distal duodenal stent. This could be readily achieved using a conventional percutaneous biliary covered stent with an additional covered extension or a long biliary covered stent, while endoscopic access may be not suitable for positioning the distal end of biliary stent beyond the distal duodenal stent.
Although a long biliary stent did not completely prevent biliary stent occlusion by food reflux, biliary stent occlusion was not significantly influenced by a duodenal obstruction or stent because the distal end of the long biliary stent was usually located in the distal duodenum or proximal jejunum in this study. Also, effective internal drainage could be achieved in most cases because the distal end of the biliary stent system was usually located in the distal duodenum or proximal jejunum. Moreover, we did not observe any dysfunction of this long biliary stent system as a result of tumor ingrowth. Notably, a covered stent using PTFE has been found to be effective in preventing tumor ingrowth (14,15,16,17,18). In a previous study of this same stent type in patients with malignant extrahepatic biliary obstruction, 10 (23.8%) of the 42 patients analyzed had a stent occlusion due to food impaction with biliary sludge (15).
In the subgroup analysis of patients who underwent biliary stent insertion after duodenal stent, we further demonstrated that the location of the distal end of biliary stent was the only potential risk factor of biliary stent patency (p = 0.003). Patients in whom the distal end of the biliary stent was located within the distal duodenal stent were found to be at a greater risk (8.424-fold) of biliary stent occlusion than patients in whom it was located beyond the distal duodenal stent. Thus, we propose that the long biliary stent system is the most important concern when subsequent biliary stent insertion is necessary in patients who already have their duodenal stents. The long biliary covered stent system can be initially used in patients who are presented with a biliary obstruction without a concurrent symptomatic duodenal obstruction. When subsequent duodenal stent insertion is necessary in these patients during the follow-up period, the long biliary covered stent system would not be significantly influenced by the subsequent duodenal stent because the distal end of the covered extension will be located beyond the distal end of the duodenal stent. More importantly, this biliary stent system would not be significantly influenced by the malignant duodenal obstruction itself. In addition, we did not observe any duodenal or jejunal complications associated with the distal end of the biliary stent system in our current patients. Hence, if a combined malignant duodenal stricture is suspected prior to biliary stent insertion, despite the lack of any symptoms of duodenal obstruction, the insertion of a long biliary stent system may be preferable to prevent biliary stent dysfunction due to subsequent duodenal stenting.
Our present study had several limitations of note including its retrospective design and restriction of the study population to a single center, which can affect the more general applicability of the findings. However, our current cohort is the largest of its type to be investigated to date. An additional limitation was that the median survival of our patients was only 107 days, which restricted the period over which we could observe stent patency. The relatively short life expectancy of our study patients was likely related to the severity of the underlying diseases. Finally, since differences among the metallic stents may have influenced the outcomes, additional prospective comparisons are needed to confirm our results.
In conclusion, the percutaneous insertion of a biliary metallic stent appears to be a technically feasible, safe, and effective method for treating a malignant duodenobiliary obstruction. Moreover, a biliary stent system with a duodenal extension beyond the distal end of the duodenal stent will be more effective in these patients.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Baron TH. Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med. 2001;344:1681–1687. doi: 10.1056/NEJM200105313442206. [DOI] [PubMed] [Google Scholar]
- 2.Jung GS, Song HY, Kang SG, Huh JD, Park SJ, Koo JY, et al. Malignant gastroduodenal obstructions: treatment by means of a covered expandable metallic stent-initial experience. Radiology. 2000;216:758–763. doi: 10.1148/radiology.216.3.r00au05758. [DOI] [PubMed] [Google Scholar]
- 3.Lee BH, Choe DH, Lee JH, Kim KH, Chin SY. Metallic stents in malignant biliary obstruction: prospective long-term clinical results. AJR Am J Roentgenol. 1997;168:741–745. doi: 10.2214/ajr.168.3.9057527. [DOI] [PubMed] [Google Scholar]
- 4.Akinci D, Akhan O, Ozkan F, Ciftci T, Ozkan OS, Karcaaltincaba M, et al. Palliation of malignant biliary and duodenal obstruction with combined metallic stenting. Cardiovasc Intervent Radiol. 2007;30:1173–1177. doi: 10.1007/s00270-007-9045-2. [DOI] [PubMed] [Google Scholar]
- 5.Katsinelos P, Kountouras J, Germanidis G, Paroutoglou G, Paikos D, Lazaraki G, et al. Sequential or simultaneous placement of self-expandable metallic stents for palliation of malignant biliary and duodenal obstruction due to unresectable pancreatic head carcinoma. Surg Laparosc Endosc Percutan Tech. 2010;20:410–415. doi: 10.1097/SLE.0b013e3182001f26. [DOI] [PubMed] [Google Scholar]
- 6.Kim KO, Kim TN, Lee HC. Effectiveness of combined biliary and duodenal stenting in patients with malignant biliary and duodenal obstruction. Scand J Gastroenterol. 2012;47:962–967. doi: 10.3109/00365521.2012.677956. [DOI] [PubMed] [Google Scholar]
- 7.Lee E, Gwon DI, Ko GY, Sung KB, Yoon HK, Shin JH, et al. Percutaneous biliary covered stent insertion in patients with malignant duodenobiliary obstruction. Acta Radiol. 2015;56:166–173. doi: 10.1177/0284185114523267. [DOI] [PubMed] [Google Scholar]
- 8.Maire F, Hammel P, Ponsot P, Aubert A, O'Toole D, Hentic O, et al. Long-term outcome of biliary and duodenal stents in palliative treatment of patients with unresectable adenocarcinoma of the head of pancreas. Am J Gastroenterol. 2006;101:735–742. doi: 10.1111/j.1572-0241.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 9.Moon JH, Choi HJ, Ko BM, Koo HC, Hong SJ, Cheon YK, et al. Combined endoscopic stent-in-stent placement for malignant biliary and duodenal obstruction by using a new duodenal metal stent (with videos) Gastrointest Endosc. 2009;70:772–777. doi: 10.1016/j.gie.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Profili S, Feo CF, Meloni GB, Strusi G, Cossu ML, Canalis GC. Combined biliary and duodenal stenting for palliation of pancreatic cancer. Scand J Gastroenterol. 2003;38:1099–1102. doi: 10.1080/00365520310005532. [DOI] [PubMed] [Google Scholar]
- 11.Zhao L, Xu H, Zhang Y. Palliation double stenting for malignant biliary and duodenal obstruction. Exp Ther Med. 2016;11:348–352. doi: 10.3892/etm.2015.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 13.Hamada T, Nakai Y, Isayama H, Sasaki T, Kogure H, Kawakubo K, et al. Duodenal metal stent placement is a risk factor for biliary metal stent dysfunction: an analysis using a time-dependent covariate. Surg Endosc. 2013;27:1243–1248. doi: 10.1007/s00464-012-2585-9. [DOI] [PubMed] [Google Scholar]
- 14.Gwon DI, Ko GY, Kim JH, Yoon HK, Lee IS, Kim KA, et al. A comparative analysis of PTFE-covered and uncovered stents for palliative treatment of malignant extrahepatic biliary obstruction. AJR Am J Roentgenol. 2010;195:W463–W469. doi: 10.2214/AJR.10.4658. [DOI] [PubMed] [Google Scholar]
- 15.Gwon DI, Ko GY, Kim JW, Ko HK, Yoon HK, Sung KB. Double-stent system with long duodenal extension for palliative treatment of malignant extrahepatic biliary obstructions: a prospective study. Korean J Radiol. 2018;19:230–236. doi: 10.3348/kjr.2018.19.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han YM, Kwak HS, Jin GY, Lee SO, Chung GH. Treatment of malignant biliary obstruction with a PTFE-covered self-expandable nitinol stent. Korean J Radiol. 2007;8:410–417. doi: 10.3348/kjr.2007.8.5.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JW, Gwon D, Han YM, Won JH, Hong HP, Ko GY, et al. A prospective, multicenter study of a double stent system for palliative treatment of malignant extrahepatic biliary obstructions. Acta Radiol. 2015;56:1209–1215. doi: 10.1177/0284185114550702. [DOI] [PubMed] [Google Scholar]
- 18.Schoder M, Rossi P, Uflacker R, Bezzi M, Stadler A, Funovics MA, et al. Malignant biliary obstruction: treatment with ePTFE-FEP-covered endoprostheses initial technical and clinical experiences in a multicenter trial. Radiology. 2002;225:35–42. doi: 10.1148/radiol.2251011744. [DOI] [PubMed] [Google Scholar]