Abstract
Objective
To identify the initial chest computed tomography (CT) findings and clinical characteristics associated with the course of coronavirus disease 2019 (COVID-19) pneumonia.
Materials and Methods
Baseline CT scans and clinical and laboratory data of 72 patients admitted with COVID-19 pneumonia (39 men, 46.2 ± 15.9 years) were retrospectively analyzed. Baseline CT findings including lobar distribution, presence of ground glass opacities, consolidation, linear opacities, and lung severity score were evaluated. The outcome event was recovery with hospital discharge. The time from symptom onset to discharge or the end of follow-up (for those remained hospitalized) was recorded. Data were censored in events such as death or discharge without recovery. Multivariable Cox proportional hazard regression was used to explore the association between initial CT, clinical or laboratory findings, and discharge with recovery, whereby hazard ratio (HR) values < 1 indicated a lower rate of discharge at four weeks and longer time until discharge.
Results
Thirty-two patients recovered and were discharged during the study period with a median length of admission of 16 days (range, 9 to 25 days), while the rest remained hospitalized at the end of this study (median, 17.5 days; range, 4 to 27 days). None died during the study period. After controlling for age, onset time, lesion characteristics, number of lung lobes affected, and bilateral involvement, the lung severity score on baseline CT (> 4 vs. ≤ 4 [reference]: adjusted HR = 0.41 [95% confidence interval, CI = 0.18–0.92], p = 0.031) and initial lymphocyte count (reduced vs. normal or elevated [reference]: adjusted HR = 0.14 [95% CI = 0.03–0.60], p = 0.008) were two significant independent factors that influenced recovery and discharge.
Conclusion
Lung severity score > 4 and reduced lymphocyte count at initial evaluation were independently associated with a significantly lower rate of recovery and discharge and extended hospitalization in patients admitted for COVID-19 pneumonia.
Keywords: Coronavirus, 2019-nCoV, COVID-19, Computed tomography, CT, Pneumonia, Clinical course, Discharge, Hospitalization, Recovery, Outcome, Prognosis, Hospital stay, Admission, Prediction
INTRODUCTION
An outbreak of coronavirus disease 2019 (COVID-19) pneumonia, caused by a novel coronavirus (2019-nCoV), has led to a serious pandemic (1). The disease, in some patients, progresses into life-threatening acute respiratory distress syndrome (ARDS). As of February 20, 2020, a total of 75567 cases with laboratory confirmed COVID-19 have been detected in China, of whom 2239 have died and 18644 have been cured. Despite a fatality rate of 2.96%, which is lower than those of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), rapid disease progression and complicated manifestations continue to present great challenges to effective management. Thus, the identification of risk factors related to disease prognosis is valuable to guide treatment implementation and improve utilization of limited resources. A prior study of critically ill patients with COVID-19 showed that increased age, comorbidities and ARDS were associated with increased risk of death (2). It has been reported that the majority (80.9%) of 2019-nCoV infected patients have mild disease, with diverse clinical courses due to the heterogeneity of the affected population (3). However, risk factors that affected the clinical course of patients with COVID-19 pneumonia have yet to be further studied.
Computed tomography (CT) has been proven as a valuable tool in the diagnosis of COVID-19 (4,5). Recent publications have characterized typical CT findings of COVID-19 pneumonia during the acute, progressive and recovery stages (6,7,8). CT findings such as ground glass opacities (GGO) and consolidation were found to correlate with the duration of infection (7). In this regard, an understanding of the characteristics of initial chest CT may help to identify prognostic indicators for COVID-19 (9). The potential associations of age and laboratory test results (i.e., white blood cells, lymphocytes, and C-reactive protein) with disease progression have been demonstrated in severe patients (10). Given the above, the purpose of this study was to identify the initial chest CT findings and clinical characteristics that were linked to the clinical course of COVID-19 pneumonia.
MATERIALS AND METHODS
The Institutional Review Board approved this retrospective study (XJTU1AF2020LSK-011) and patient consent was obtained.
Patients
From January 16 to February 13, 2020, a total of 72 patients (39 men, 46.2 ± 15.9 years) with COVID-19 confirmed by nucleic acid test were identified from three hospitals in Xi'an, Shaanxi province, China. Baseline chest CT and laboratory examination results were collected. All the patients were treated based on a national standard diagnosis and treatment criteria for COVID-19 which includes initiation of antivirals, interferon, Chinese herbal medications, supplemental oxygen as needed and hospitalization (11). The disease type, i.e., uncomplicated illness, mild pneumonia and severe pneumonia was evaluated by the World Health Organization criteria, in which critical ARDS, sepsis or septic shock were considered severe (12). As COVID-19 pneumonia represents a new and rapidly developing outbreak, standards were developed to assess patient recovery and readiness for discharge after treatment (11) and were set as follows: 1) normal body temperature for more than 3 days; 2) significant improvement of respiratory symptoms (such as respiratory rate, hypoxemia or SpO2 values returned to normal range); 3) inflammation of the lungs showed obvious findings of absorption on CT imaging; and 4) two consecutive negative respiratory viral nucleic acid tests (taken at least one day apart).
Image Acquisition
The chest CT scans were acquired by using 16- to 64-multidetector CT scanners (Philips Brilliant 16, Philips Healthcare, Best, Netherlands; GE VCT LightSpeed 64, GE Healthcare, Milwaukee, WI, USA; GE Optima 680, GE Healthcare, Waukesha, WI, USA). The CT parameters were as follows: 120 kVp, current intelligent control (auto mA) of 30–300 mA, and slice thickness reconstructions of 0.6–1.5 mm. All CT scans were performed without intravenous contrast material.
Data Collection and Evaluation
The demographic data, exposure history, clinical symptoms, laboratory findings, and the time from disease onset to hospital visit were obtained from the medical records. The date of disease onset was defined as patients' reported date of symptom onset. The clinical course, i.e., the time from symptom onset to discharge with recovery or the end of follow-up were collected. The CT images were each independently reviewed by two experienced radiologists with 5 years of experience and, if there was a disagreement, the diagnosis would be made in consensus by another expert with more than 10 years of experience in respiratory imaging. As previously reported (8), CT findings including the presence and distribution of GGO, consolidation, linear opacity, pleural effusion and lymphadenopathy were evaluated. We also evaluated the degree of lobar involvement and total lung severity score. Each of the five lung lobes was assessed in detail for degree or area of involvement and assigned a score of 0 for 0% lobe involvement, 1 for 1–25% lobe involvement, 2 for 26–50% lobe involvement, 3 for 51–75% lobe involvement, or 4 for 76–100% lobe involvement according to visual evaluation (8). A total severity score was calculated by summing the five lobe scores (range, 0–20).
Statistical Analysis
The initial chest CT findings and clinical characteristics were compared among the disease severity groups (uncomplicated, mild, and severe). Continuous variables were represented as means and standard deviations or median and range, while categorical variables were expressed as counts and percentages. Chi-square test was used for categorical variables, t test was used for continuous variables, and nonparametric rank test was used for non-conforming variables. The clinical outcome event of interest in this study was patient recovery and hospital discharge. Data were censored in events such as death or discharge without recovery. Univariate Cox proportional hazard regression analysis was first used to explore the association of CT findings, age, sex, and laboratory-testing with the length of hospitalization until the occurrence of the outcome event or the end of follow-up (for those who had not been discharged during the study period). Then, variables with p values < 0.2 in univariate analysis were used to further analyze the associations with the clinical course by multivariable Cox proportional hazard regression analysis. Variables such as total lung severity score, duration between the symptom onset and presentation/CT examination time, and number of lung lobes affected, were collapsed into binary data by grouping them by the median of each data for Cox regression analysis. Because the outcome event for the analysis was defined as recovery and hospital discharge, hazard ratio (HR) values < 1 indicated a lower rate of discharge at 4 weeks and longer time until discharge compared with the reference category. In addition, the univariable unadjusted and multivariable-adjusted Kaplan-Meier curves were used for displaying the cumulative incidence of discharge with recovery for the identified factors. All statistical analyses were performed using SPSS 20.0 (IBM Corp., Armonk, NY, USA) and MedCalc 19.0 (MedCalc Software Ltd., Ostend, Belgium) software. P < 0.05 was considered statistically significant.
RESULTS
Participant Demographics, Clinical Features, and Laboratory Test Results
Three patients had uncomplicated illness, 61 patients had mild disease, and 8 patients had severe disease. Fourteen patients initially had negative nucleic acid tests that were subsequently positive. Patients had been admitted to the hospital for 4 to 27 days (median, 17 days). Thirty-two patients recovered and were discharged during the study period, and the median length of the clinical course was 15.5 days (range, 9 to 25 days). For those who remained hospitalized at the end of this investigation, the median length of hospitalization was 17.5 days (range, 4 to 27 days). No patient died during the study period. Among those with COVID-19, the severe patients were older than patients with mild and uncomplicated illness (P < 0.001). Laboratory testing at initial hospital-visit indicated that 30.6% of patients had low lymphocyte counts (Table 1).
Table 1. Initial Clinical Characteristics of 72 Patients with COVID-19 Pneumonia.
| Characteristic | All Patients (n = 72) | Disease Severity† | |||
|---|---|---|---|---|---|
| Uncomplicated Illness (n = 3) | Mild (n = 61) | Severe (n = 8) | P | ||
| Age (years)‡ | 46.2 ± 15.9 | 35.0 ± 19.2 | 43.8 ± 13.4 | 68.6 ± 15.3 | < 0.001* |
| Male sex | 39 (54.2) | 2 (66.6) | 34 (55.7) | 3 (37.5) | 0.564 |
| Comorbidities | |||||
| Hypertension | 7 (9.7) | 1 (33.3) | 4 (6.6) | 2 (25.0) | 0.094 |
| Diabetes | 2 (2.8) | 0 (0.0) | 2 (3.3) | 0 (0.0) | 0.831 |
| Coronary artery disease | 7 (9.7) | 0 (0.0) | 5 (8.2) | 2 (25.0) | 0.271 |
| Chronic glomerulonephritis | 1 (1.4) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0.913 |
| Exposure history | 0.065 | ||||
| Recent travel to Wuhan | 28 (38.9) | 2 (66.6) | 26 (42.6) | 0 (0.0) | - |
| Contact with infected patient | 31 (43.1) | 1 (33.3) | 23 (37.7) | 7 (87.5) | - |
| Unknown | 13 (18.0) | 0 (0.0) | 12 (19.7) | 1 (12.5) | - |
| Initial symptoms | |||||
| Fever | 65 (90.3) | 2 (66.6) | 55 (90.2) | 8 (100.0) | 0.251 |
| Cough | 34 (47.2) | 1 (33.3) | 28 (45.9) | 5 (62.5) | 0.599 |
| Expectoration | 21 (29.2) | 0 (0.0) | 19 (31.1) | 2 (25.0) | 0.492 |
| Fatigue | 10 (13.9) | 2 (66.6) | 7 (11.5) | 1 (12.5) | 0.095 |
| Chest pain and/or shortness of breath | 7 (9.7) | 0 (0.0) | 6 (9.8) | 1 (12.5) | 0.821 |
| Sore throat | 13 (18.1) | 2 (66.6) | 9 (14.8) | 2 (25.0) | 0.064 |
| Muscle soreness | 7 (9.7) | 0 (0.0) | 6 (9.8) | 1 (12.5) | 0.821 |
| Headache | 4 (5.6) | 0 (0.0) | 4 (6.6) | 0 (0.0) | 0.683 |
| Nausea and/or vomiting | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0.105 |
| Diarrhea | 2 (2.8) | 0 (0.0) | 1 (1.6) | 1 (12.5) | 0.204 |
| No obvious symptoms | 4 (5.6) | 0 (0.0) | 4 (6.6) | 0 (0.0) | 0.683 |
| Initial negative nucleic acid test | 14 (19.4) | 1 (33.3) | 12 (19.7) | 1 (12.5) | 0.734 |
| Initial laboratory testing§ | |||||
| White blood cell count (↓) | 20 (27.8) | 0 (0.0) | 18 (29.5) | 2 (25.0) | 0.528 |
| Lymphocyte count (↓) | 22 (30.6) | 0 (0.0) | 17 (27.9) | 5 (62.5) | 0.068 |
| Percent lymphocytes (↓) | 23 (31.9) | 0 (0.0) | 19 (31.1) | 4 (50.0) | 0.269 |
| Percent neutrophilic granulocyte (↑) | 19 (26.4) | 0 (0.0) | 16 (26.2) | 3 (37.5) | 0.453 |
| Percent monocytes (↑) | 16 (22.2) | 1 (33.3) | 14 (22.9) | 1 (12.5) | 0.715 |
| Hemoglobin (↓) | 10 (13.9) | 0 (0.0) | 9 (14.8) | 1 (12.5) | 0.765 |
| ALT (↑) | 12 (16.7) | 0 (0.0) | 11 (18.0) | 1 (12.5) | 0.676 |
| AST (↑) | 15 (20.8) | 0 (0.0) | 13 (21.3) | 2 (25.0) | 0.643 |
| Creatine kinase (↓) | 6 (8.3) | 1 (33.3) | 5 (8.2) | 0 (0.0) | 0.204 |
| C-reactive protein (↑) | 45 (62.5) | 1 (33.3) | 39 (63.9) | 5 (62.5) | 0.565 |
Unless otherwise indicated, data are reported as number of patients, with percentages in parentheses, n = number of patients. *P < 0.05 means statistical difference, †Types of disease severity were evaluated by World Health Organization criteria (12), ‡Data are reported as mean ± standard derivation, §↑, ↓ represent above and below normal ranges of laboratory results, respectively. Normal ranges of white blood cell count, lymphocyte count, percent lymphocytes, percent neutrophilic granulocyte, percent monocytes, ALT, AST, creatine kinase, and hemoglobin, C-reactive protein were 3.5–9.5 × 109/L, 1.10–3.20 × 109/L, 20–50%, 3.0–10.0%, 40–75%, 115–150 g/L, 7–40 U/L, 13–35 U/L, 40–200 U/L, 0–10 mg/L, respectively. ALT = alanine aminotransferase, AST = aspartate aminotransferase
Chest CT Findings
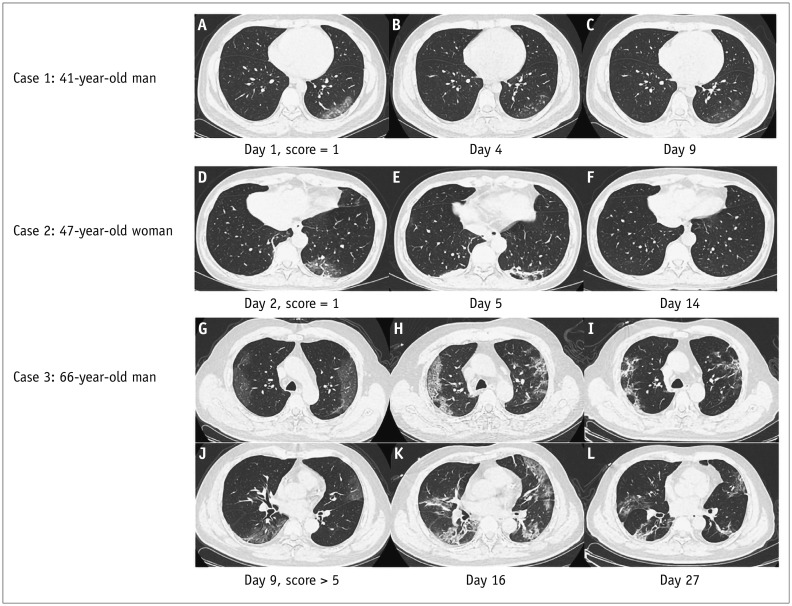
The median interval time between the initial CT and the symptom onset was 3 days, range 1–12 days. Among all patients, 22.2% had GGO, 23.6% had GGO and consolidation, 8.3% exhibited consolidation, 4.2% had GGO and linear opacities, 5.6% had consolidation and linear opacities, and 31.9% had all three signs. 61.1% of patients had involvement of three or more lobes, and 75.0% of patients had bilateral abnormalities. In addition, three patients had a negative initial CT but positive nucleic acid tests with fever or sore throat as the only clinical manifestation. The CT findings are detailed in Table 2. Figure 1 shows the serial CT images of three patients with 41, 47, and 66 years of age, respectively. On follow-up CT, the 66-year-old patient had a higher total severity score than that of the two younger patients (> 5 score vs. 1 score) and did not meet the guideline criteria to be discharged.
Table 2. Initial Radiographic Findings of 72 Patients with COVID-19 Pneumonia.
| Characteristic | Total (n = 72) | Disease Severity† | |||
|---|---|---|---|---|---|
| Uncomplicated Illness (n = 3) | Mild (n = 61) | Severe (n = 8) | P | ||
| Lesions | < 0.001* | ||||
| GGO only | 16 (22.2) | 0 (0.0) | 14 (23.0) | 2 (25.0) | - |
| GGO and consolidation | 17 (23.6) | 0 (0.0) | 14 (23.0) | 3 (37.5) | - |
| Consolidation only | 6 (8.3) | 0 (0.0) | 6 (9.8) | 0 (0.0) | - |
| GGO and linear opacity | 3 (4.2) | 0 (0.0) | 3 (4.9) | 0 (0.0) | - |
| Consolidation and linear opacity | 4 (5.6) | 0 (0.0) | 4 (6.6) | 0 (0.0) | - |
| GGO, consolidation and linear opacity | 23 (31.9) | 0 (0.0) | 20 (32.7) | 3 (37.5) | - |
| Without three signs | 3 (4.2) | 3 (100.0) | 0 (0.0) | 0 (0.0) | - |
| Number of lobes affected | < 0.001* | ||||
| 0 | 3 (4.2) | 3 (100.0) | 0 (0.0) | 0 (0.0) | |
| 1 | 15 (20.8) | 0 (0.0) | 13 (21.4) | 2 (25.0) | |
| 2 | 11 (15.3) | 0 (0.0) | 11 (18.0) | 0 (0.0) | |
| 3 | 6 (8.3) | 0 (0.0) | 6 (9.8) | 0 (0.0) | |
| 4 | 13 (18.1) | 0 (0.0) | 11 (18.0) | 2 (25.0) | |
| 5 | 24 (33.3) | 0 (0.0) | 20 (32.8) | 4 (50.0) | |
| More than 2 lobes affected | 44 (61.1) | 0 (0.0) | 38 (62.3) | 6 (75.0) | 0.067 |
| Bilateral involvement | 54 (75.0) | 0 (0.0) | 48 (78.7) | 6 (75.0) | 0.009* |
| Other findings | |||||
| Pleural effusion | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Lymphadenopathy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Total lung severity score | |||||
| Mean ± standard deviation | 4.56 ± 3.14 | 0 | 4.48 ± 2.83 | 6.87 ± 4.05 | 0.004* |
| Median (range) | 4 (0–13) | 0 | 4 (1–12) | 7 (1–13) | - |
Unless otherwise indicated, data are reported as number of patients, with percentages in parentheses, n = number of patients. *P < 0.05 means statistical difference, †Types of disease severity were evaluated by World Health Organization criteria (12). GGO = ground glass opacities
Fig. 1. Chest CT imaging changes of 3 patients with COVID-19 pneumonia during hospitalization.
Case 1: 41-year-old man with history of recent travel to Wuhan presented with fever, respiratory distress for 1 day, and normal lymphocyte count. A. CT on day 1 shows subpleural GGO in LLL. Total severity score is 1. B, C. CT on day 4 and day 9 show extent of GGO slightly decreased. Patient was discharged on day 10 after symptom onset. Case 2: 47-year-old woman with history of recent travel to Wuhan presented with fever, cough, and expectoration for 1 day and normal lymphocyte count. D. CT on day 2 shows subpleural GGO and linear opacities in LLL. Total severity score is 1. E. CT on day 5 shows evolution of GGO into consolidation and linear opacities. Lesions had resolved on CT by day 14. F. Patient was discharged on day 15 after symptom onset. Case 3: 66-year-old man with contact history of infected patient in Wuhan presented with fever and respiratory symptoms for 9 days with reduced lymphocyte count. G, J. CT on day 9 shows subpleural diffuse GGO in bilateral upper and lower lobes. Total severity score was > 5. H, K. CT on day 16 shows progression of GGO into diffuse consolidation and linear opacities. GGO had resolved on CT by day 27 I, L. Patient remained hospitalized. CT = computed tomography, GGO = ground glass opacities, LLL = left lower lobe
Factors Related to the Time Until Discharge with Recovery
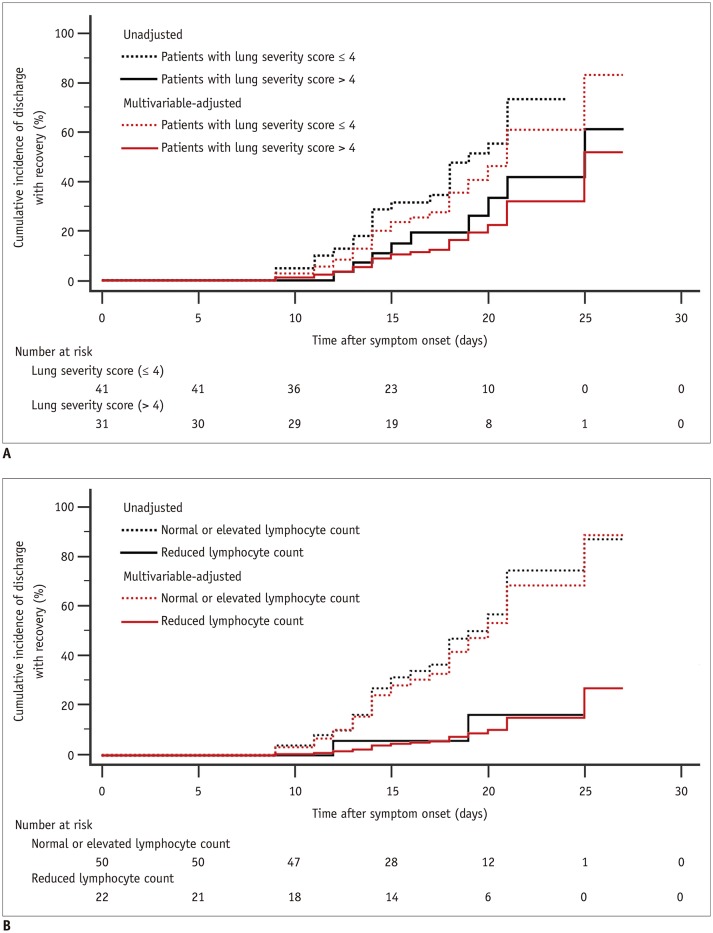
Univariable Cox regression analysis indicated that seven variables (i.e., age, onset time, lesion characteristics, number of lung lobes affected, bilateral involvement, lung severity score, and lymphocyte count) correlated with the length of hospitalization with a p value < 0.20 (Table 3). Multivariable Cox regression further found that the lung severity score and lymphocyte count were independently correlated with the event of discharge with recovery (Table 3). The adjusted HR value of the lung severity score (> 4 vs. ≤ 4 as the reference category) and lymphocyte count (reduced vs. normal or elevated as the reference category) were 0.41 (95% confidence interval [CI], 0.18–0.92; p = 0.031) and 0.14 (95% CI, 0.03–0.60; p = 0.008), respectively. In addition, the unadjusted Kaplan-Meier analysis showed that the cumulative incidences of discharge with recovery in the groups with lung severity score ≤ 4 and in the group with lung severity score > 4 on day 24 were 73.3% and 41.8%, respectively (Fig. 2A). The cumulative incidence of discharge with recovery on day 25 in the group with normal or elevated lymphocyte count and in the group with reduced lymphocyte was 87.4% and 16.3%, respectively (Fig. 2B).
Table 3. Factors Associated with Recovery and Discharge from Hospital at Four Weeks among Patients with COVID-19 in Univariate and Multivariate Cox Regression.
| Variable | Stratification | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR‡ | 95% CI | P | Adjusted HR‡ | 95% CI | P | ||
| Age | > 65 years vs. ≤ 65 years* | 0.34 | 0.08–1.45 | 0.152† | 0.44 | 0.07–2.73 | 0.374 |
| Sex | Female vs. male* | 1.33 | 0.64–2.74 | 0.446 | |||
| Onset time§ | > 3 days vs. ≤ 3* days | 0.59 | 0.29–1.20 | 0.145† | 0.94 | 0.36–2.46 | 0.893 |
| Comorbidities | |||||||
| Hypertension | Yes vs. no* | 1.69 | 0.58–4.94 | 0.334 | |||
| Diabetes | Yes vs. no* | 0.04 | 0–21.74 | 0.318 | |||
| Coronary artery disease | Yes vs. no* | 0.36 | 0.05–2.65 | 0.316 | |||
| Chronic glomerulonephritis | Yes vs. no* | 2.33 | 0.31–17.37 | 0.409 | |||
| Lesions∥ | |||||||
| GGO only | - | 0.60 | 0.15–2.33 | 0.459 | 1.32 | 0.34–5.14 | 0.694 |
| GGO and consolidation | - | 0.56 | 0.15–2.13 | 0.398 | 1.02 | 0.26–3.96 | 0.981 |
| Consolidation only | - | 0.52 | 0.11–2.59 | 0.427 | 0.71 | 0.14–3.55 | 0.681 |
| GGO and linear opacity | - | 0.19 | 0.02–1.83 | 0.149† | 1.60 | 0.11–22.86 | 0.729 |
| Consolidation and linear opacity | - | 0.27 | 0.03–2.62 | 0.260 | 0.29 | 0.03–2.76 | 0.280 |
| GGO, consolidation and linear opacity | - | 0.49 | 0.13-1.83 | 0.291 | 2.44 | 0.54–10.98 | 0.246 |
| Without three signs* | - | - | - | - | |||
| Number of lobes affected | > 2 vs. ≤ 2* | 0.45 | 0.22–0.90 | 0.025† | 0.92 | 0.24–3.53 | 0.899 |
| Bilateral involvement | Yes vs. no* | 0.56 | 0.26–1.18 | 0.126† | 0.76 | 0.29–1.98 | 0.572 |
| Total lung severity score | > 4 vs. ≤ 4* | 0.44 | 0.20–0.98 | 0.045† | 0.41 | 0.18–0.92 | 0.031 |
| Laboratory testing at initial hospital-visit | |||||||
| White blood cell count reduced | Yes vs. no* | 0.95 | 0.45–2.02 | 0.897 | |||
| Lymphocyte count reduced | Yes vs. no* | 0.15 | 0.04–0.63 | 0.010† | 0.14 | 0.03–0.60 | 0.008 |
| Percent lymphocytes reduced | Yes vs. no* | 0.84 | 0.39–1.82 | 0.662 | |||
| Percent neutrophilic granulocyte increased | Yes vs. no* | 0.80 | 0.35–1.79 | 0.581 | |||
| Percent monocytes increased | Yes vs. no* | 1.18 | 0.56–2.50 | 0.670 | |||
| Hemoglobin reduced | Yes vs. no* | 1.32 | 0.46–3.80 | 0.604 | |||
| ALT increased | Yes vs. no* | 1.24 | 0.51–3.03 | 0.639 | |||
| AST increased | Yes vs. no* | 0.82 | 0.35–1.93 | 0.649 | |||
| Creatine kinase reduced | Yes vs. no* | 1.22 | 0.47–3.19 | 0.686 | |||
| C-reactive protein increased | Yes vs. no* | 0.70 | 0.34–1.42 | 0.317 | |||
*Reference categories, †Variables with p value < 0.20 on univariable analysis were included in multivariable analysis, ‡Because outcome event was discharge with recovery from hospital, HR values < 1 indicated lower rate of discharge with recovery at four weeks and longer time until discharge compared with reference category, §Onset time: duration between symptom onset to initial CT examination time, ∥All lesions are compared with “without the three signs.” CI = confidence interval, HR = hazard ratio
Fig. 2. Kaplan-Meier curves of cumulative incidence of discharge with recovery during follow-up period after symptom onset.
A. Results in patients with lung severity score > 4 group and ≤ 4 group. B. Results for normal or elevated lymphocyte count group and reduced lymphocyte count group.
DISCUSSION
In this paper, we used the baseline CT and clinical features to explore which factors related to the clinical course in patients with COVID-19 pneumonia. Results indicated that older patients may have more severe disease. Higher lung severity score and lower lymphocyte count were found to be independently associated with a significantly lower rate of discharge. These findings support the role of CT imaging and clinical indices at initial hospital-visit for potentially predicting the hospitalization time of patients with COVID-19 pneumonia.
During the observation time, there were 8 patients with severe disease, who were older than patients with mild and uncomplicated illness. This result was consistent with previous data (8). Similar to the SARS-CoV and the MERS-CoV, the 2019-nCoV appears to cause more severe disease in older patients (13,14). Therefore, elderly patients should be closely monitored. Meanwhile, it has been observed that the main manifestations of initial CT in COVID-19 patients were GGO, GGO with consolidation, and bilateral lung involvement. Consistent with prior studies (6,8,15,16), these findings likely correlate pathologically with edema, inflammatory infiltrates, pneumocyte hyperplasia, and organization during an exudative and proliferative acute-phase of COVID-19 pneumonia (17). Among the CT findings, lung injury severity score was found to correlate with the length of clinical course. Results indicated that the cumulative incidence of recovery and hospital discharge in group with lung severity scores ≤ 4 (73.3%) was significantly higher than in those with scores > 4 (61.2%) within the four weeks. One study regarding COVID-19 related acute respiratory disease suggested that interstitial abnormalities seen on CT on admission were significantly correlated with the patients' endpoints, such as discharge, intensive care unit admission, and mechanical ventilation (10). Our study also shows that initial CT findings correlate with the clinical course in patients with COVID-19 pneumonia.
In addition to the imaging findings, laboratory findings are also important factors to consider (10). This study found that lymphocyte counts correlate with the clinical course of COVID-19. The cumulative incidence of recovery and hospital discharge in patients with normal or elevated lymphocyte count was 87.4% within four weeks, which was significantly higher than that in patients with decreased lymphocyte count (16.3%). These results were consistent with previous reports (10,18). As lymphopenia is an essential characteristic of impaired immunity (19), Huang et al. (20) found lymphopenia in 63% patients and a cytokine storm profile in those who were critically ill. The combination of viral replication in the lower respiratory tract and an aberrant immune response may have an impact on the severity of illness, similar to what has been proven in SARS and MERS (20). Therefore, this study suggests that regular observation of lymphocyte counts in patients with COVID-19 is beneficial for the detection of disease progression and guiding clinical decision-making and adjustment of treatment.
It is worth noting that among the patients who were discharged, 3 had a negative CT but positive nucleic acid tests at presentation and all had uncomplicated illness. These patients initially had fever or sore throat as the only clinical manifestation. Interestingly, all patients had positive findings on their second CT. All three patients were recovered and discharged at the end of follow-up. It is speculated that for these patients, the virus primarily affected the upper respiratory tract instead of the lower respiratory tract and with treatment, their symptoms improved quickly. Therefore, CT scan may correlate with the clinical course by identifying the different patterns and characteristics of the infection.
This paper has some limitations. Firstly, the sample size of the study is small, and larger samples are needed to further clarify the findings. Second, we did not analyze the occurrence of adverse events, such as mechanical ventilation or extracorporeal membrane oxygenation. In the current study, because there was such a small number of adverse events, it was not possible to analyze them. Thus, a follow-up study with more patients may allow for analysis of such events. Third, further follow-up is required to evaluate the outcomes of patients with continued hospitalization status. Fourth, the clinical course may be affected by multiple other factors than the disease course or prognosis itself, such as administrative and logistical factors. Given the fact that COVID-19 is spreading worldwide, the treatment status for patients in epidemic foci may be affected by the administrative and logistical factors due to the large number of patients. However, in non-epidemic areas, the number of patients are far less than that of patients in epidemic areas, the government and hospitals have reliable administrative and logistical support. Therefore, in non-focus areas, the results may have fewer confounding variables and be a better reference for research.
In conclusion, lung severity score > 4 and reduced lymphocyte count at initial evaluation were independently associated with a substantially lower rate of recovery and hospital discharge at four weeks and more extended hospitalization in patients admitted for COVID-19 pneumonia.
Footnotes
This study was funded by National Natural Science Foundation of China (No. 81171317, 81971581, 51706178), Innovation Capability Support Program of Shaanxi (2019TD-018), National Key Research and Development Program of China (2016YFC0100300), the 2011 New Century Excellent Talent Support Plan of the Ministry of Education, China (NCET-11-0438).
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 Feb 24; doi: 10.1016/S2213-2600(20)30079-5. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. [Google Scholar]
- 4.Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020 Feb 19; doi: 10.1148/radiol.2020200432. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH, et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol. 2020;21:494–500. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020 Feb 13; doi: 10.1007/s00330-020-06731-x. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020 Feb 20; doi: 10.1148/radiol.2020200463. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song FX, Shi NN, Shan F, Zhang ZY, Shen J, Lu HZ, et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020 Feb 06; doi: 10.1148/radiol.2020200274. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Xu J, Li H, Cao B. A novel coronavirus (COVID-19) outbreak: a call for action. Chest. 2020 Feb 19; doi: 10.1016/j.chest.2020.02.014. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan WJ, Ni ZY, Yu Hu, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020 Feb 28; doi: 10.1056/NEJMoa2002032. [Epub] [DOI] [Google Scholar]
- 11.Jin YH, Cai L, Cheng ZS, Cheng H, Deng T1, Fan YP, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 is suspected: interim guidance. [Accessed March 13, 2020]. Available at: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
- 13.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang DM, Chamberlain DW, Poutanen SM, Low DE, Asa SL, Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol. 2005;18:1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020 Feb 28; doi: 10.1016/j.jtho.2020.02.010. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Zhou L, Yang Y, Peng W, Wang W, Chen X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8:e11–e12. doi: 10.1016/S2213-2600(20)30071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]