Abstract
Optical coherence tomography (OCT) provides excellent image resolution, however OCT optimal acquisition is essential but could be challenging owing to several factors. We sought to assess the quality of OCT pullbacks and identify the causes of suboptimal image acquisition. We evaluated 784 (404 pre-PCI; 380 post-PCI) coronary pullbacks from an anonymized OCT database from our Cardiovascular Imaging Core Laboratory. Imaging of the region-of-interest (ROI—lesion or stented segment plus references) was incomplete in 16.1% pullbacks, caused by pullback starting too proximal (63.7%), inappropriate pullback length (17.1%) and pullback starting too distal (11.4%). The quality of image acquisition was excellent in 36.3% pullbacks; whereas 4% pullbacks were unanalyzable. Pullback quality was most commonly affected by poor blood displacement from inadequate contrast volume (27.4%) or flow (25.6%), followed by artifacts (24.1%). Acquisition mode was ‘High-Resolution’ (54 mm) in 74.4% and ‘Survey’ (75 mm) in 25.6% of cases. The 54 mm mode was associated with incomplete ROI imaging (p = 0.020) and inadequate contrast volume (p = 0.035). We observed a substantial frequency of suboptimal image acquisition and identified its causes, most of which can be addressed with minor modifications during the procedure, ultimately improving patient outcomes.
Keywords: Image acquisition, Optical coherence tomography, Intravascular imaging
Introduction
Intravascular imaging guidance during coronary procedures enables detailed evaluation of the lesion morphology and has been shown to improve procedural and clinical outcomes [1-5]. The utilization of optical coherence tomography (OCT) intravascular imaging has increased, owing in large part to technological advances made during the past few years, in which occlusion technique was replaced by contrast injection only. OCT generates high resolution cross-sectional images of the vessels, allowing for in-depth lesion analysis and plaque characterization [6-8], that aids in vessel preparation and precise stent size selection and deployment [9, 10]. Moreover, OCT has a crucial role in assessment of stent expansion, malapposition and edge dissection [11, 12]. Intravascular OCT imaging involves three steps: optimal image acquisition, image analysis and appropriate interpretation and decision making by the operator based on the obtained images. The first step, image acquisition, is the key to optimal use of OCT, as the remaining steps are dependent on analyzability and quality of the acquired images.
As opposed to other imaging modalities, OCT acquisition requires displacement of blood by injection of contrast media. Insufficient blood clearance during image acquisition can greatly deteriorate the quality of the images [5, 13, 14]. Other factors like a fixed pullback size, inappropriate positioning of the catheter, type of pullback trigger and tortuosity of the vessel can result in inadequacies in OCT image acquisition. These intricacies make the all-important step of Image acquisition quite challenging.
The purpose of our study was to assess the quality of OCT images acquired as part of multicenter clinical trials and identify the specific inadequacies in image acquisition that may impair image analysis and impact clinical decision.
Methods
We utilized an anonymized, retrospective, multicenter, multinational OCT database maintained at the Harrington Heart & Vascular Cardiovascular Imaging Core Laboratory at University Hospitals Cleveland Medical Center, Cleveland, OH, USA and approved by our Institution IRB. All the patients gave informed consent for the original studies and our institution IRB determined that a separate informed consent was not necessary for the purpose of this study. This study was performed in accordance to the 1964 Declaration of Helsinki and its later amendments. A total of 784 pullbacks from closed trials (with already published results) were included in our analysis; none of the trials that were included in the database for this study had pre-specified pullback characteristics, being left to the operator discretion (high resolution vs survey mode, manual vs automatic trigger, manifold vs power injection). Patients with stable angina, unstable angina, or NSTEMI were included. Both pre- and post-PCI OCT images were included and all images were acquired between 2010 and 2017 as per the respective study protocols. Exclusion criteria included: (1) OCT catheter failure, OR (2) other equipment malfunction impacting the image acquisition. Subjects with acute STEMI, urgent PCI, cardiogenic shock, restenosis, stent thrombosis, target left main stenosis, aorto-ostial or diffuse disease, severe angulation or calcification that prevent OCT catheter to be advanced were excluded.
Two independent physicians, trained and validated at our institution, with more than 3000 OCT pullbacks analyzed each and blinded to any patient information, trial, clinical presentation, were responsible for the analysis of all pullbacks. Each OCT pullback was analyzed in its entirety. The region of interest (ROI) was defined as the stented segment plus the proximal and distal references (5 mm each) in the case of post-PCI pullbacks; or the stented segment co-registered to the pre-PCI pullback, in addition to the references. We documented the frequency of incomplete image acquisition of the ROI and the reasons behind it, such as inappropriate pullback size, and positioning of the OCT catheter.
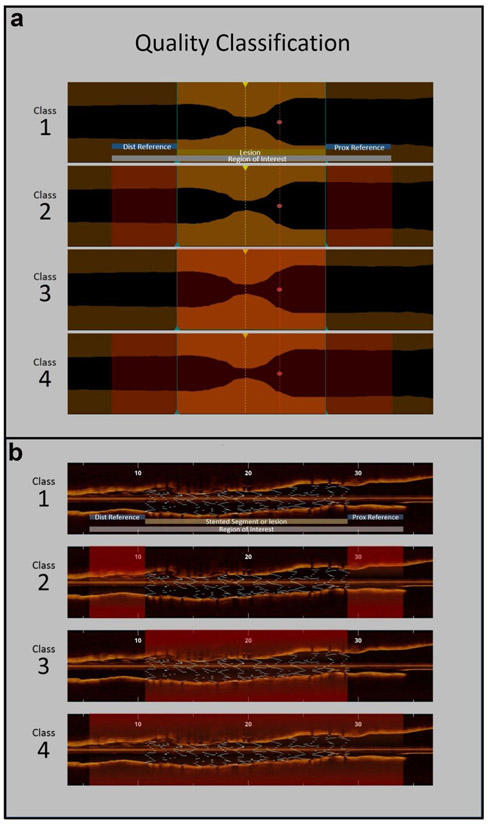
We developed a classification system to objectively assess the quality of each pullback, ultimately grading into Classes 1–4 (Fig. 1), with progressive decline of overall image quality (Class 1 being an excellent quality and Class 4 being mostly unanalyzable). We also documented the reasons for poor image acquisition such as residual blood, artifacts, lack of contrast volume or flow, artifacts and occlusive lesion etc. In addition, we utilized an already published methodology for quality assessment, which was responsible for the FDA approval of OCT and is based on the measurement of analyzable length [15]. In summary clear imaging length (CIL) is the cumulative sum of clear image frames, defined as OCT cross-sections with a visible boundary between the lumen and the vessel wall along a continuous arc of at least 270° around the center of the lumen. CIL was also expressed in percentage relative to the total pullback length. The length of stent visible within the CIL was identified as clear stent length (CSL, in millimeters), which was also expressed in percentage relative to the total stent length.
Fig.1.
Class 1 has excellent quality throughout ROI, other classes have quality issues (shaded red) in reference only (Class 2), stented segment/lesion only (Class 3) or the entire ROI (Class 4). a Pre stent and b post stent
Continuous variables are presented as means and standard deviations. Categorical variables are presented as numbers and percentages. Chi square and binary logistic regression analysis were used for subgroup comparisons between categorical variables and a p = value of < 0.05 was considered significant. All tests were performed using SPSS (IBM) version 25.
Results
A total of 784 pullbacks were included in this study, out of which, 404 (51.5%) were pre-PCI images and 380 (48.5%) were post-PCI. In terms of vessels, left anterior descending (LAD) artery 471 (60.1%) was most common, followed by the right coronary artery 157 (20.0%), left circumflex 134 (17.1%) and diagonal 14 (1.8%). Acquisition mode was ‘High Resolution’ (54 mm pullback length) in 583 (74.4%) and ‘Survey’ (75 mm pullback length) in 201 (25.6%) cases. In terms of access site, 456 (58.2%) were femoral and 328 (41.8%) were radial cases.
Analysis of pullback completeness (Table 1)
Table 1.
Pullback completeness and quality analysis
| Variable | n = 784 |
|---|---|
| Pullback completeness analysis | |
| Incomplete stent/lesion, n (%) | 58 (7.4) |
| Incomplete distal reference, n (%) | 99 (12.6) |
| Incomplete proximal reference, n (%) | 30 (3.8) |
| Incomplete region of interest (ROI), n (%) | 126 (16.1) |
| Reason for incomplete pullback | |
| Pullback started too proximal, n (%) | 79 (63.7) |
| Pullback started too distal, n (%) | 14 (11.4) |
| Inappropriate pullback length, n (%) | 21 (17.1) |
| Others, n (%) | 12 (9.5) |
| Pullback quality analysis | |
| Clear image length (CIL), mm, mean (SD) | 49.8 (13.3) |
| Clear image, % (SD) | 84.0 (18.7) |
| Clear stent length (CSL), mm, mean (SD) | 23.2 (10.0) |
| Clear stent, % (SD) | 95.8 (15.5) |
| Classification of pullback image quality | |
| Class 1: excellent quality throughout the pullback, n (%) | 284 (36.3) |
| Class 2: quality issue with the references only, n (%) | 409 (52.2) |
| 2A: minor issue affecting only the reference, n (%) | 278 (35.5) |
| 2B: moderate issue affecting only the reference, n (%) | 92 (11.7) |
| 2C: major issue affecting only the reference, n (%) | 39 (5.0) |
| Class 3: quality issue with the lesion/stented segment only, n (%) | 28 (3.6) |
| 3A: minor issue, n (%) | 6 (0.8) |
| 3B: moderate issue, n (%) | 18 (2.3) |
| 3C: major issue, n (%) | 4 (0.5) |
| Class 4: quality issue with both the lesion/stented segment and references, n (%) | 62 (7.9) |
| 4A: minor issue, n (%) | 6 (0.8) |
| 4B: moderate issue, n (%) | 25 (3.2) |
| 4C: major issue (not analyzable), n (%) | 31 (4.0) |
| Reasons affecting quality | |
| Inadequate contrast volume, n (%) | 128 (27.4) |
| Inadequate contrast flow, n (%) | 120 (25.6) |
| Catheter not flushed, n (%) | 12 (2.6) |
| Artifact (sew-up, out of screen etc.), n (%) | 113 (24.1) |
| Occlusive lesion, n (%) | 7 (1.5) |
The image acquisition of the lesion or stented segment only, excluding the references, was incomplete in 58 (7.4%) of cases; whereas the ROI was incomplete in 126 (16.1%). The most common reason for incomplete ROI imaging, was the pullback starting too proximal 79 (63.7%), thus missing the distal reference, followed by inappropriate pullback length selection (i.e. since the longer pullback length is available, it should have been selected in order to cover the lesion length) (17.1%) and pullback starting too distal 14 (11.4%), thus missing the proximal reference.
Image quality analysis (Table 1)
A total of 284 (36.3%) pullbacks were considered to have excellent quality (Class 1) throughout the pullback, while additional 409 (52.2%) pullbacks had excellent quality in the lesion or stented segment but not in the references (Class 2). On the other hand, 28 (3.6%) had problems in the lesion/ stented segment alone (Class 3) and another 62 (7.9%) of the cases had inadequate quality that affected the ROI (Class 4). The most common quality issue was residual blood due to inadequate contrast volume in 128 (27.4%) cases or contrast flow in 120 (25.6%), followed by artifacts in 113 (24.1%) pullbacks. Quality was affected by catheter not properly flushed in 12 (2.6%) pullbacks. The CIL was found to be 84.0 ± 18.7% and the CSL was noted to be 95.8 ± 15.5%.
Subgroup comparison based on pullback length (Table 2)
Table 2.
Comparison between sub-groups based on pullback length and access site
| Variable | 54 mm, n = 583, n (%) | 75 mm, n = 201, n (%) | p value | Femoral, n = 456, n (%) |
Radial, n = 328, n (%) | p value |
|---|---|---|---|---|---|---|
| Access—femoral | 375 (64.3) | 81 (40.3) | – | – | – | – |
| Access—radial | 208 (35.7) | 120 (59.7) | – | – | – | – |
| Pullback length (54 mm) | – | – | – | 375 (82.2) | 208 (63.4) | – |
| Pullback length (75 mm) | – | – | – | 81 (17.8) | 120 (36.6) | – |
| Pullback completeness analysis | ||||||
| Incomplete stent/lesion | 55 (9.4) | 3 (1.5) | 0.002 | 38 (8.3) | 20 (6.1) | 0.685 |
| Incomplete distal reference | 84 (14.4) | 15 (7.5) | 0.032 | 68 (14.9) | 31 (9.5) | 0.077 |
| Incomplete proximal reference | 25 (4.3) | 5 (2.5) | 0.300 | 19 (4.2) | 11 (3.4) | 0.722 |
| Incomplete region of interest (ROI) | 106 (18.2) | 20 (10.0) | 0.020 | 85 (18.6) | 41 (12.5) | 0.076 |
| Classification of pullback image quality | ||||||
| Class 1: excellent quality throughout the pullback | 221 (37.9) | 63 (31.5) | 0.379 | 190 (41.7) | 94 (28.7) | 0.001 |
| Class 2: quality issue with the references only | 293 (50.3) | 116 (58.0) | 0.160 | 221 (48.5) | 188 (57.5) | 0.032 |
| Class 3: quality issue with the lesion/ stented segment only | 23 (3.9) | 5 (2.5) | 0.289 | 15 (3.3) | 13 (4.0) | 0.468 |
| Class 4: quality issue with both the lesion/ stented segment and references | 46 (7.9) | 16 (8.0) | 0.767 | 30 (6.6) | 32 (9.8) | 0.098 |
| Reasons affecting quality | ||||||
| Inadequate contrast volume | 102 (30.4) | 26 (19.7) | 0.035 | 73 (29.6) | 55 (24.9) | 0.605 |
| Inadequate contrast flow | 83 (24.7) | 37 (28.0) | 0.698 | 57 (23.1) | 63 (28.5) | 0.236 |
| Catheter not flushed | 7 (2.1) | 5 (3.8) | 0.402 | 5 (2.0) | 7 (3.2) | 0.605 |
| Artifact (sew-up, fold-over, out of screen etc.) | 91 (27.1) | 22 (16.7) | 0.115 | 75 (30.4) | 38 (17.2) | 0.006 |
| Occlusive lesion | 4 (1.2) | 3 (2.3) | 0.616 | 2 (0.8) | 5 (2.3) | 0.282 |
Bold values indicate p < 0.050
A comparison between pullback lengths or modes (adjusted for access site) revealed that 54 mm pullbacks had a significantly higher frequency of incomplete ROI compared to the 75 mm pullbacks (18.2% vs 10%; p = 0.020). Regarding pullback quality, there was a higher frequency of inadequate contrast volume in the 54 mm pullbacks (30.4% vs 19.7%; p = 0.035).
Subgroup comparison based on access site (Table 2)
There was no statistical difference between the femoral and radial access in terms of the completeness of ROI. More pullbacks with Class 1 quality were seen in the case of femoral access (41.7% vs 28.7%; p = 0.001), whereas Class 2 quality pullbacks were more frequent in the case of radial access (48.5% vs 57.5%; p = 0.032). Similar but not statistically significant pattern was seen in the case of Class 3 and 4 quality (Table 2).
Discussion
To the best of our knowledge, in addition to providing a review of parameters and techniques for optimal image acquisition, this is the first study assessing the inadequacies of optical coherence tomography image acquisition using a new objective classification (Table 1; Fig. 1). The analysis revealed a significant number of incomplete and poor quality OCT images, and provided an insight toward understating underlying mechanisms of poor OCT image acquisitions. This in turn, can provide guidance for improvement of OCT image acquisition.
It is worth highlighting that our study included OCT images from varied operators, at different centers, as part of distinct clinical trials, in which specific protocols of image acquisition were followed to generate the best quality of images for offline analysis. Given that the operators were participating in OCT based clinical trials, we considered them experts in this technology. Despite that, we noted a significant frequency of suboptimal images, which leads us to assume that in general practice the prevalence might be even higher, potentially resulting in increased contrast usage, higher radiation exposure and longer procedure time, thereby limiting the overall impact of OCT imaging on PCI.
We found that the rate of incomplete OCT images (Fig. 2) affecting the ROI was 16.1%. The resulting loss of analyzable length can lead to failure of identification of important pathology in the lesion and the references. In turn, stent planning (in the case of pre-PCI pullbacks) and analysis of stent expansion, malapposition and edge dissection (in the case of post-PCI pullbacks) could be severely impaired [16, 17]. Our data identifies the most common causes of incomplete OCT pullbacks and emphasizes the importance of optimal OCT catheter positioning relative to the lesion and appropriate pullback size selection.
Fig. 2.
A 54 mm pullback (yellow line) where the operator did not capture the ROI (red dash line). A potential 75 mm pullback (blue dash line), and minimal advancement of the OCT catheter would meet the required length to include the complete ROI
Even though two-thirds of pullbacks in our sample had quality issues, only a fraction of these cases were deemed unanalyzable (4%). Nonetheless, the remaining cases had some degree of quality related problems that still affected the image interpretation to variable extent. Our study shows that careful displacement of blood is crucial for optimal image acquisition as about 32% of all images were affected by residual blood, caused by either inadequate contrast volume or flow, resulting in less than ideal image quality (Fig. 3b).
Fig. 3.
Cross-sectional images of an excellent image acquisition (a); residual blood in the catheter (b) and catheter not flushed (c)
The ILUMIEN OPTIS system has two modes for image acquisition: ‘Survey’ mode and ‘High Resolution’ mode. The ‘Survey’ mode provides a rapid pullback of 75 mm over 2.1 s at 180 frames per second (i.e., in 5 frames/mm). The ‘High Resolution’ mode images a 54 mm segment at the same frame rate over 3 s (i.e., 10 frames/mm), having twice the frame density [18]. Our analysis showed that the 54 mm ‘High Resolution’ mode was heavily preferred (74% vs 26%) over the 75 mm ‘Survey’ mode resulting in significant higher frequency of incomplete ROI in the 54-mm pullbacks (18.2% vs 10%; p = 0.02). We assume that the reason for this preference (of 54 mm mode) relates to its name in the software (‘High Resolution’) that suggests greater resolution and better image quality. It is worth mentioning that practically, the resolution and image quality between the two modes are exactly the same, with the main difference being in the frame density and image acquisition time. We believe that for clinical decision making, it is more important to have a complete image of ROI than to have a higher frame density (0.1 mm vs 0.2 mm between the frames) and hence, there might not be enough reason to pursue 54 mm pullbacks in routine clinical practice.
Our sub-group comparison revealed higher frequency of inadequate contrast volume in the 54 mm mode compared to the 75 mm mode (30.4% vs 19.7%; p = 0.035). This finding is explained by the fact that the 54 mm (‘High Resolution’) pullbacks take a longer time to acquire, resulting in more contrast usage (and hence the volume issues) and higher radiation exposure. These factors provide additional basis to favor the 75 mm ‘Survey’ mode for general purpose OCT acquisition.
Based on the literature [2, 13, 19-28], the best practices to avoid residual blood and to achieve excellent image quality include proper engagement of the guide catheter in the coronary artery, avoidance of guide catheters with side holes, optimal synchronization between flushing and OCT catheter pullback (the OCT pullback should not be started before the distal lumen is completely cleared of blood), use of power injections as they are more reliable in providing a blood-free lumen and use of higher-viscosity flush solutions as they provide superior imaging quality compared to the lower-viscosity ones.
We observed a substantial frequency (14% of all cases) of artifacts such as sew-up artifact, out of screen images, fold-over artifact and non-uniform rotational deformity. However, these artifacts only affect a few frames and have a minor effect on the overall quality and analyzability of the pullback. On the other hand, blood in the catheter, which results from failure to flush the catheter before imaging the vessel (Fig. 3c), can affect the entire pullback and in turn have a greater impact on analyzability by blocking the source light [29]. In our study, this completely avoidable phenomenon was seen in a relatively minor number of pullbacks.
On comparison based on access site, there was no difference in terms of completeness of pullbacks between femoral and radial sites, however, femoral access seems to have an impact on overall quality of the pullbacks, with a higher frequency of Class 1 quality pullbacks. On the other hand, there was a higher rate of artifacts in the femoral group. Further studies will be needed to confirm these findings.
By providing a detailed insight into the inadequacies of OCT acquisition and identifying the most common causes of suboptimal images, our findings can have significant implications in improving the overall quality and impact of OCT. We re-emphasize a standardized image acquisition protocol/technique based on published guidelines [2] and adequate training of the operator regarding proper catheter positioning, appropriate pullback size selection, and optimal use of contrast. Further studies to correlate image quality with training and experience of the operator, and to assess the impact of improved quality of OCT on procedural decision making and clinical outcomes are needed.
Limitations
Our study has a few limitations. Firstly, it is a retrospective study with its inherent limitations. Secondly, because of the study design, we do not know about the experience and training of the operator with regards to OCT and were unable to correlate the inadequacies in image acquisition with such factors. Third, our database does not include ST-elevation myocardial infarction (STEMI) cases. Fourth, the study does not include images from high risk PCI procedures such as chronic total occlusion (CTO), ostial lesions or left main lesions. Fifth, due to the nature of this study, the OCT acquisition procedure was not controlled, which impact the interpretation of causes of poor image qualities. Lastly, only the Abbott OCT system were included in our study.
Conclusion
Our study identified a significant frequency of cases with suboptimal image acquisition, and its causes, most of which are preventable with minor changes during the procedure. Better education and training on image acquisition will reduce the number of pullbacks necessary to adequately image a vessel, reduce contrast usage and lead to better imaging data quality, ultimately improving procedural decision-making and patient outcomes.
Abbreviations
- OCT
Optical coherence tomography
- LAD
Left anterior descending
- ROI
Region of interest
- PCI
Percutaneous coronary intervention
- CIL
Clear imaging length
- CSL
Clear stent length
- STEMI
ST segment elevation myocardial infarction
- CTO
Chronic total occlusion
Footnotes
Compliance with ethical standards
Conflict of interest Dr. Bezerra receives consulting fees from Abbott Vascular, Inc. Other authors report no relevant conflicts of interest.
Publisher′s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kume T, Uemura S (2018) Current clinical applications of coronary optical coherence tomography. Cardiovasc Interv Ther 33(1):1–10. 10.1007/s12928-017-0483-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, dhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudek D, Falk E, Feldman MD, Fitzgerald P, Garcia-Garcia HM, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CC, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel MA, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Raber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PW, Shimada K, Shinke T, Shite J, Siegel E, Sonoda S, Suter M, Takarada S, Tanaka A, Terashima M, Thim T, Uemura S, Ughi GJ, van Beusekom HM, van der Steen AF, van Es GA, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G, International Working Group for Intravascular Optical Coherence Tomography (2012) Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the international working group for intravascular optical coherence tomography standardization and validation. J Am Coll Cardiol 59(12):1058–1072. 10.1016/j.jacc.2011.09.079 [DOI] [PubMed] [Google Scholar]
- 3.Prati F, Guagliumi G, Mintz GS, Costa M, Regar E, Akasaka T, Barlis P, Tearney GJ, Jang IK, Arbustini E, Bezerra HG, Ozaki Y, Bruining N, Dudek D, Radu M, Erglis A, Motreff P, Alfonso F, Toutouzas K, Gonzalo N, Tamburino C, Adriaenssens T, Pinto F, Serruys PW, Di Mario C, Expert’s OCTRD (2012) Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J 33(20):2513–2520. 10.1093/eurheartj/ehs095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prati F, Di Vito L, Biondi-Zoccai G, Occhipinti M, La Manna A, Tamburino C, Burzotta F, Trani C, Porto I, Ramazzotti V, Imola F, Manzoli A, Materia L, Cremonesi A, Albertucci M (2012) Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta control’Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention 8(7):823–829. 10.4244/EIJV8I7A125 [DOI] [PubMed] [Google Scholar]
- 5.Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, Choi KB, Shishkov M, Schlendorf K, Pomerantsev E, Houser SL, Aretz HT, Tearney GJ (2002) Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol 39(4):604–609 [DOI] [PubMed] [Google Scholar]
- 6.Sinclair H, Bourantas C, Bagnall A, Mintz GS, Kunadian V (2015) OCT for the identification of vulnerable plaque in acute coronary syndrome. JACC Cardiovasc Imaging 8(2):198–209. 10.1016/j.jcmg.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 7.Jang IK, Tearney GJ, MacNeill B, Takano M, Moselewski F, Iftima N, Shishkov M, Houser S, Aretz HT, Halpern EF, Bouma BE (2005) In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation 111(12):1551–1555. 10.1161/01.CIR.0000159354.43778.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang DH, Halpern EF, Tearney GJ (2002) Characterization of human atherosclerosis by optical coherence tomography. Circulation 106(13):1640–1645 [DOI] [PubMed] [Google Scholar]
- 9.Wijns W, Shite J, Jones MR, Lee SW, Price MJ, Fabbiocchi F, Barbato E, Akasaka T, Bezerra H, Holmes D (2015) Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur Heart J 36(47):3346–3355. 10.1093/eurheartj/ehv367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubo T, Tanaka A, Kitabata H, Ino Y, Tanimoto T, Akasaka T (2012) Application of optical coherence tomography in percutaneous coronary intervention. Circ J 76(9):2076–2083 [DOI] [PubMed] [Google Scholar]
- 11.Chamie D, Bezerra HG, Attizzani GF, Yamamoto H, Kanaya T, Stefano GT, Fujino Y, Mehanna E, Wang W, Abdul-Aziz A, Dias M, Simon DI, Costa MA (2013) Incidence, predictors, morphological characteristics, and clinical outcomes of stent edge dissections detected by optical coherence tomography. JACC Cardiovasc Interv 6(8):800–813. 10.1016/j.jcin.2013.03.019 [DOI] [PubMed] [Google Scholar]
- 12.Attizzani GF, Capodanno D, Ohno Y, Tamburino C (2014) Mechanisms, pathophysiology, and clinical aspects of incomplete stent apposition. J Am Coll Cardiol 63(14):1355–1367. 10.1016/j.jacc.2014.01.019 [DOI] [PubMed] [Google Scholar]
- 13.Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PW, Expert’s OCTRD (2010) Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J 31(4):401–415. 10.1093/eurheartj/ehp433 [DOI] [PubMed] [Google Scholar]
- 14.Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI (2009) Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc Interv 2(11):1035–1046. 10.1016/j.jcin.2009.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon JH, Di Vito L, Moses JW, Fearon WF, Yeung AC, Zhang S, Bezerra HG, Costa MA, Jang IK (2012) Feasibility and safety of the second-generation, frequency domain optical coherence tomography (FD-OCT): a multicenter study. J Invasive Cardiol 24(5):206–209 [PubMed] [Google Scholar]
- 16.Nakamura D, Wijns W, Price MJ, Jones MR, Barbato E, Akasaka T, Lee SW, Patel SM, Nishino S, Wang W, Gopinath A, Attizzani GF, Holmes D, Bezerra HG (2018) New volumetric analysis method for stent expansion and its correlation with final fractional flow reserve and clinical outcome: an ILUMIEN I substudy. JACC Cardiovasc Interv 11(15):1467–1478. 10.1016/j.jcin.2018.06.049 [DOI] [PubMed] [Google Scholar]
- 17.Raber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, Radu MD, Joner M, Yu B, Jia H, Meneveau N, de la Torre Hernandez JM, Escaned J, Hill J, Prati F, Colombo A, Di Mario C, Regar E, Capodanno D, Wijns W, Byrne RA, Guagliumi G (2018) Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention 14(6):656–677. 10.4244/EIJY18M06_01 [DOI] [PubMed] [Google Scholar]
- 18.Katwal AB, Lopez JJ (2015) Technical considerations and practical guidance for intracoronary optical coherence tomography. Interv Cardiol Clin 4(3):239–249. 10.1016/j.iccl.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 19.Frick K, Michael TT, Alomar M, Mohammed A, Rangan BV, Abdullah S, Grodin J, Hastings JL, Banerjee S, Brilakis ES (2014) Low molecular weight dextran provides similar optical coherence tomography coronary imaging compared to radiographic contrast media. Catheter Cardiovasc Interv 84(5):727–731. 10.1002/ccd.25092 [DOI] [PubMed] [Google Scholar]
- 20.Ozaki Y, Kitabata H, Tsujioka H, Hosokawa S, Kashiwagi M, Ishibashi K, Komukai K, Tanimoto T, Ino Y, Takarada S, Kubo T, Kimura K, Tanaka A, Hirata K, Mizukoshi M, Imanishi T, Akasaka T (2012) Comparison of contrast media and low-molecular-weight dextran for frequency-domain optical coherence tomography. Circ J 76(4):922–927 [DOI] [PubMed] [Google Scholar]
- 21.McCabe JM, Croce KJ (2012) Optical coherence tomography. Circulation 126(17):2140–2143. 10.1161/CIRCULATIONAHA.112.117143 [DOI] [PubMed] [Google Scholar]
- 22.Li X, Villard JW, Ouyang Y, Michalek JE, Jabara R, Sims D, Kemp N, Glynn T, Banas C, Bailey SR, Feldman MD (2011) Safety and efficacy of frequency domain optical coherence tomography in pigs. EuroIntervention 7(4):497–504. 10.4244/EIJV7I4A80 [DOI] [PubMed] [Google Scholar]
- 23.Barlis P, Schmitt JM (2009) Current and future developments in intracoronary optical coherence tomography imaging. EuroIntervention 4(4):529–533 [DOI] [PubMed] [Google Scholar]
- 24.Tearney GJ, Waxman S, Shishkov M, Vakoc BJ, Suter MJ, Freilich MI, Desjardins AE, Oh WY, Bartlett LA, Rosenberg M, Bouma BE (2008) Three-dimensional coronary artery microscopy by intracoronary optical frequency domain imaging. JACC Cardiovasc Imaging 1(6):752–761. 10.1016/j.jcmg.2008.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito T, Date H, Taniguchi I, Hokimoto S, Yamamoto N, Nakamura S, Ishibashi F, Noda K, Oshima S (1999) Evaluation of new 4 French catheters by comparison to 6 French coronary artery images. J Invasive Cardiol 11(1):13–20 [PubMed] [Google Scholar]
- 26.Imola F, Mallus MT, Ramazzotti V, Manzoli A, Pappalardo A, Di Giorgio A, Albertucci M, Prati F (2010) Safety and feasibility of frequency domain optical coherence tomography to guide decision making in percutaneous coronary intervention. EuroIntervention 6(5):575–581. 10.4244/EIJV6I5A97 [DOI] [PubMed] [Google Scholar]
- 27.Takarada S, Imanishi T, Liu Y, Ikejima H, Tsujioka H, Kuroi A, Ishibashi K, Komukai K, Tanimoto T, Ino Y, Kitabata H, Kubo T, Nakamura N, Hirata K, Tanaka A, Mizukoshi M, Akasaka T (2010) Advantage of next-generation frequency-domain optical coherence tomography compared with conventional time-domain system in the assessment of coronary lesion. Catheter Cardiovasc Interv 75(2):202–206. 10.1002/ccd.22273 [DOI] [PubMed] [Google Scholar]
- 28.Regar E, van Ditzhuijzen N, van der Sijde J, Ligthart J, Witberg K, van Soest G, Karanasos A (2016) Identifying stable coronary plaques with OCT technology. Contin Cardiol Educ 2(2):77–88. 10.1002/cce2.27 [DOI] [Google Scholar]
- 29.Huang D, Swanson E, Lin C, Schuman J, Stinson W, Chang W, Hee M, Flotte T, Gregory K, Puliafito C et al. (1991) Optical coherence tomography. Science 254(5035):1178–1181. 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]