Abstract
Purpose:
Immune checkpoint blockade has improved outcomes across tumor types; little is known about the efficacy of these agents in rare tumors. We report the results of the (non-pancreatic) neuroendocrine neoplasm cohort of SWOG S1609 Dual Anti-CTLA-4 & Anti-PD-1 blockade in Rare Tumors (DART).
Experimental Design:
We performed a prospective, open-label, multicenter phase 2 clinical trial of ipilimumab plus nivolumab across multiple rare tumor cohorts, with the (non-pancreatic) neuroendocrine cohort reported here. Response assessment by grade was not pre-specified. The primary endpoint was overall response rate (ORR) (RECIST v1.1) (complete response (CR) and partial response (PR)); secondary endpoints included progression-free survival (PFS), overall survival (OS), stable disease >6 months, and toxicity.
Results:
Thirty-two eligible patients received therapy; 18 (56%) had high-grade disease. Most common primary sites were gastrointestinal (47%; N= 15) and lung (19%; N= 6). The overall ORR was 25% (95% confidence interval (CI) 13-64%; CR, 3%, N= 1; PR, 22%, N= 7). Patients with high-grade neuroendocrine carcinoma had an ORR of 44% (8/18 patients) versus 0% in low/intermediate grade tumors (0/14 patients) (p=0.004). The 6-month PFS was 31% (95% CI 19-52%); median OS was 11 months (95% CI 6-∞). The most common toxicities were hypothyroidism (31%), fatigue (28%), and nausea (28%); with alanine aminotransferase (ALT) elevation (9%) as the most common grade 3/4 immune-related adverse event, and no grade 5 events.
Conclusions:
Ipilimumab plus nivolumab demonstrated a 44% ORR in patients with non-pancreatic high-grade neuroendocrine carcinoma, with 0% ORR in low/intermediate grade disease.
Trial Registration:
ClinicalTrials.gov registry: NCT02834013
Keywords: neuroendocrine carcinoma, rare tumors, S1609, DART, ipilimumab, nivolumab
INTRODUCTION
Immune checkpoint blockade has transformed oncology with the potential for durable responses even in patients with metastatic disease. Approved indications for immune checkpoint blockade in rare tumors are limited to Merkel cell carcinoma, cutaneous squamous cancers, and microsatellite-unstable malignancies (1). Rare cancer histologies, collectively representing approximately a quarter of all cancers diagnosed, remain understudied and the efficacy of immune checkpoint blockade in these patient populations is unknown.
Neuroendocrine neoplasms represent a rare histologic subset of tumors with complex classification criteria dependent on the putative organ of origin, precluding a single taxonomy across anatomic sites (2). The World Health Organization (WHO) and European Neuroendocrine Tumour Society (ENETS) have developed a classification scheme reflected in the most recent staging guidelines (Appendix Table 1) and was utilized in our study. Neuroendocrine neoplasms can develop throughout the body, with pancreatic neuroendocrine tumors (PNET) in particular having unique biological and clinical characteristics resulting in additional therapies being utilized for PNETs. Thus, PNETs are assessed in a separate cohort within S1609, currently accruing. Clinical trials for neuroendocrine neoplasms, particularly in high-grade neuroendocrine carcinomas, have been difficult to conduct due to the rarity of the disease, difficulties in precise classification, and a lack of robust predictive biomarkers for therapeutic efficacy. In this study we evaluated the combination of ipilimumab and nivolumab in patients with diverse histologic sites and across tumor grades.
SWOG 1609 DART (Dual Anti-CTLA-4 & Anti-PD-1 blockade in Rare Tumors), a basket immunotherapy trial studying ipilimumab plus nivolumab across multiple cohorts of rare tumor histologic subtypes, was designed to address the question of the efficacy of these agents in these understudied populations. The combination of ipilimumab and nivolumab was selected over nivolumab alone due to the signal-finding nature of this study with small cohorts of rare tumors, with lower-dose ipilimumab chosen to balance tolerability with potential efficacy. The trial is currently open across the United States at 861 sites. We present here the clinical data of the (non-pancreatic) neuroendocrine cohort of SWOG 1609 (S1609) DART.
PATIENTS AND METHODS
The trial was conducted by SWOG, and the investigational agents were provided by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI) under an NCI CRADA agreement with Bristol-Myers Squibb (BMS). All study subjects provided their voluntary, written informed consent using a document approved by the institution’s human subject protection committee. The study was conducted in accordance with the Declaration of Helsinki. The protocol and all amendments were approved by SWOG, the NCI, the NCI central institutional review board (CIRB), and by the regulatory committees at the participating institutions. The Caris analysis of neuroendocrine specimens is IRB exempt as all data were analyzed utilizing de-identified aggregate data.
Rationale for Population:
Rare cancers, for the purposes of this study, were identified typically with an incidence of less than 6 in 100,000 per year (3). Tumor grading was based on 2010 WHO criteria, pathology and grade were determined by review of local pathology reports by the study principal investigators. No central pathology review was performed. This cohort (Cohort 23) of S1609 is comprised of refractory neuroendocrine neoplasms, independent of histologic grade and organ of origin, with the exception of pancreatic neuroendocrine neoplasms, which were stratified to a different cohort due to unique biology and alternate standard of care therapies. Well-differentiated, grade 3 neuroendocrine neoplasms were eligible for this cohort, and microsatellite instability (MSI) status was not available.
Patient Selection:
Eligible patients had (non-pancreatic) neuroendocrine neoplasms, had progressed following at least one line of standard systemic therapy, and did not have an approved or standard therapy available that had been shown to prolong overall survival. At enrollment, patients were required to be 18 years of age or older, have a Zubrod performance status of 0–2, adequate hematologic, hepatic, thyroid, adrenal axis, and renal function, with absolute neutrophil count ≥ 1,000/mcL, platelets ≥ 75,000/mcL, hemoglobin ≥ 8 g/dL, creatinine clearance ≥ 50 mL/min, total bilirubin ≤ 2.0 x institutional upper limit of normal (IULN), AST and ALT ≤ 3.0 x IULN, TSH or free T4 serum ≤ IULN, and normal adrenocorticotropic hormone (ACTH) ≤ IULN. Women of childbearing potential were required to have a negative serum pregnancy test, and subjects were required to practice adequate birth control during protocol participation.
Treatment and Monitoring:
Treatment consisted of nivolumab 240mg intravenously (IV) every 2 weeks and ipilimumab 1mg/kg IV every 6 weeks on a continuous schedule, with dose adjustments and brief breaks from therapy specified in the protocol for treatment-related toxicities. Patients were removed from study therapy for disease progression, symptomatic deterioration, treatment delay for any reason >56 days, unacceptable or immune-related toxicity with inability to decrease prednisone to < 10mg daily, or per patient request.
Patients were evaluated with a history and physical, laboratory analyses (complete blood count, comprehensive metabolic panel, thyroid stimulating hormone, free thyroxine, ACTH, cortisol, lipase), and toxicity assessment at least every 6 weeks at the beginning of each cycle. Imaging studies for disease assessment were performed pre-study, week 8, week 16, week 24, and then every 12 weeks until progression.
Statistical Methods and Outcomes:
The primary objective of this Phase II trial was to evaluate the overall response rate (ORR, confirmed complete and partial responses [CR and PR]) by RECIST v1.1 based on local site review. Our objective was to distinguish between a true ORR ≤5% (null hypothesis, as patients had failed all known active therapies) versus ≥ 30% (alternative hypothesis, a potentially clinically meaningful difference in tumor response in refractory solid tumors). A Simon’s two-stage design was used, which required an analysis on the first 6 eligible patients who received protocol therapy. If 1 or more of the 6 patients had a response (confirmed CR or PR), an additional 10 patients were to be accrued. The design specified 2 or more responses out of 16 patients would reject the null hypothesis (one-sided alpha = 13%, power = 87%). This cohort accrued more than 16 patients because, unexpectedly, accrual was faster than expected following the two-week closure notification and several additional patients were enrolled onto incorrect cohorts in S1609 and re-stratified into this cohort after SWOG review of local pathology reports prior to knowledge of clinical benefit or toxicity. Analysis of ORR by tumor grade was not pre-specified. The secondary objectives were to estimate progression-free survival (PFS), overall survival (OS), ORR by immune-related RECIST (iRECIST), PFS by iRECIST, and to assess toxicity.
PFS was measured from the start of protocol therapy to the first date of progression by RECIST v1.1 or death by any cause, with patients last known to be alive without progression censored at the date of last contact. OS was measured from the date of study registration to the date of death by any cause, with patients last known to be alive censored at the date of last contact. PFS and OS estimates were calculated using the Kaplan-Meier method and compared using log-rank tests. Confidence intervals for medians were constructed using the method of Brookmeyer and Crowley (4), and confidence intervals (CI) for point estimates (e.g. 6-month PFS) were calculated using the log-log transformation. CIs for the primary ORR analysis accounted for the two-stage design (5); exact binomial CIs were calculated for subgroups utilizing the R function ‘get_CI’ from the package OneArmPhaseTwoStudy. Fisher’s exact test was used to compare subgroups. All analyses were performed using R version 3.4.3.
RESULTS
Patient Characteristics
Thirty-five patients from 22 National Clinical Trial Network (NCTN) institutions were registered between 4/13/2017-5/25/2018, with 32 patients meeting eligibility criteria and receiving protocol therapy who are summarized in (Table 1). Three patients were excluded from analyses: two who were ineligible one of whom had an ineligible histology, and the other with an inadequate washout period prior to treatment initiation; and one eligible patient refused protocol treatment after giving initial consent. Of the 32 eligible patients who received protocol therapy, the median age was 60 years (range 36-81). The most common sites of primary tumor were lung and small intestine (both n=6). Notably, 18 of the 32 patients (56%) had high-grade carcinoma. The median number of prior lines of therapy was 2 for both the entire cohort as well as for patients with high-grade disease.
Table 1.
Patient Characteristics (Median (min, max) or N (%) reported; N=32 patients).
| Summary | |
|---|---|
| Age | 60.5 (36, 81) |
| Sex | |
| Female | 13 (41) |
| Male | 19 (59) |
| Performance status | |
| 0 | 7 (22) |
| 1 | 24 (75) |
| 2 | 1 (3) |
| Primary site | |
| Appendix* | 1 (3) |
| Cecum* | 1 (3) |
| Cervix | 3 (9) |
| Esophagus* | 1 (3) |
| Lung | 6 (19) |
| Prostate | 2 (6) |
| Rectum* | 4 (12) |
| Small intestine* | 6 (19) |
| Stomach* | 2 (6) |
| Thymus gland | 1 (3) |
| Unknown primary | 5 (16) |
| Ethnicity | |
| Hispanic | 2(6) |
| Not hispanic | 30 (94) |
| Race | |
| White | 25 (78) |
| Black | 6 (19) |
| Asian | 1 (3) |
| Grade | |
| High grade | 18 (56) |
| Intermediate grade | 10 (31) |
| Low grade | 4 (12) |
| Prior lines of therapy | 2 (0, 7) |
Asterisks (*) denotes primary sites included in the GI (non-pancreatic) cohort
Toxicities
Treatment-related adverse events are summarized in Table 2, with 84.4% of patients experiencing an adverse event (AE), and 50% developing a grade 3-4 AE. The most common AEs (across all grades and at least possibly related to treatment) were hypothyroidism (31%), fatigue (28%), nausea (28%), vomiting (25%), aspartate aminotransferase (AST) increase (25%), alkaline phosphatase increase (22%) and anorexia (22%). Six patients experienced Grade 4 events, two with sepsis (6%), two with increased lipase (6%), one with retinopathy (3%), and one with hyperglycemia (3%). Overall, 72% of patients developed an immune-related AE (irAE) of any grade on treatment, with 38% (N= 10) developing grade 3-4 irAEs. The most common irAEs of any grade were hypothyroidism (31%) and AST increase (25%). The most common grade 3-4 irAEs were alanine aminotransferase (ALT) increase (9%) and AST increase, lipase increase, and encephalopathy (all 6%). There were no treatment-related deaths.
Table 2.
Adverse Events at Least Possibly Related to Treatment (n=32 patients)
| Any Grade | Grade 3-5 | |||
|---|---|---|---|---|
| Treatment Related | ||||
| Any | 27 | 84.4% | 16 | 50.0% |
| Serious | 12 | 37.5% | 11 | 34.4% |
| Led to discontinuation | 10 | 31.3% | 8 | 25.0% |
| Led to deatd | 0 | 0.0% | 0 | 0.0% |
| Occurred in ≥5% of patients | ||||
| Fatigue | 9 | 28.1% | 1 | 3.1% |
| Nausea | 9 | 28.1% | 0 | 0.0% |
| Vomiting | 8 | 25.0% | 1 | 3.1% |
| Alkaline phosphatase increased | 7 | 21.9% | 2 | 6.3% |
| Anorexia | 7 | 21.9% | 0 | 0.0% |
| Lymphocyte count decreased | 5 | 15.6% | 1 | 3.1% |
| Platelet count decreased | 5 | 15.6% | 0 | 0.0% |
| Anemia | 4 | 12.5% | 2 | 6.3% |
| Dyspnea | 4 | 12.5% | 1 | 3.1% |
| Generalized muscle weakness | 4 | 12.5% | 0 | 0.0% |
| Weight loss | 4 | 12.5% | 0 | 0.0% |
| Hyperglycemia | 3 | 9.4% | 1 | 3.1% |
| Dizziness | 3 | 9.4% | 0 | 0.0% |
| Dry skin | 3 | 9.4% | 0 | 0.0% |
| Hypoalbuminemia | 3 | 9.4% | 0 | 0.0% |
| Neutrophil count decreased | 3 | 9.4% | 0 | 0.0% |
| White blood cell decreased | 3 | 9.4% | 0 | 0.0% |
| Autoimmune disorder | 2 | 6.3% | 2 | 6.3% |
| Sepsis | 2 | 6.3% | 2 | 6.3% |
| Acute kidney injury | 2 | 6.3% | 1 | 3.1% |
| Endocrine disorders-Other | 2 | 6.3% | 1 | 3.1% |
| Sinusitis | 2 | 6.3% | 1 | 3.1% |
| Blurred vision | 2 | 6.3% | 0 | 0.0% |
| Constipation | 2 | 6.3% | 0 | 0.0% |
| Dry mouth | 2 | 6.3% | 0 | 0.0% |
| Dysgeusia | 2 | 6.3% | 0 | 0.0% |
| Edema limbs | 2 | 6.3% | 0 | 0.0% |
| Fever | 2 | 6.3% | 0 | 0.0% |
| Hypertension | 2 | 6.3% | 0 | 0.0% |
| Hypocalcemia | 2 | 6.3% | 0 | 0.0% |
| Hypokalemia | 2 | 6.3% | 0 | 0.0% |
| Proteinuria | 2 | 6.3% | 0 | 0.0% |
| Skin/subq tissue ds-Other | 2 | 6.3% | 0 | 0.0% |
| Immune-mediated | ||||
| Any | 23 | 71.9% | 12 | 37.5% |
| Hypothyroidism | 10 | 31.3% | 0 | 0.0% |
| AST increased | 8 | 25.0% | 2 | 6.3% |
| Artdralgia | 7 | 21.9% | 1 | 3.1% |
| Diarrhea | 7 | 21.9% | 1 | 3.1% |
| Pruritus | 7 | 21.9% | 0 | 0.0% |
| Rash maculo-papular | 5 | 15.6% | 1 | 3.1% |
| ALT increased | 4 | 12.5% | 3 | 9.4% |
| Lipase increased | 3 | 9.4% | 2 | 6.3% |
| Hyperthyroidism | 3 | 9.4% | 0 | 0.0% |
| Infusion related reaction | 3 | 9.4% | 0 | 0.0% |
| Encephalopathy | 2 | 6.3% | 2 | 6.3% |
| Colitis | 2 | 6.3% | 1 | 3.1% |
| Pancreatitis | 2 | 6.3% | 1 | 3.1% |
| Retinopathy | 1 | 3.1% | 1 | 3.1% |
| Blood bilirubin increased | 1 | 3.1% | 0 | 0.0% |
Outcomes
Among 32 patients, the ORR was 25% (95%CI 13%-42%), with 3% (N= 1) of patients achieving CR and 22% (N= 7) attaining a PR (Table 3, Figure 1A). Altogether, 41% of patients had stable disease with 6% having stable disease >6 months and responses ongoing (Figure 1D). Response rates were similar regardless of organ of origin (Figure 1B). High-grade neuroendocrine carcinoma was present in 56% of patients (N = 18); 31% of patients had intermediate-grade; and 12% had low-grade biology. Within the high-grade neuroendocrine cohort, 44% (95% CI 22%-69% of patients; N = 8) had an objective response, with no responses (95% CI 0%-23%) in the intermediate or low-grade tumors (Figure 1C) (p = 0.004 for ORR in high- versus low/intermediate grade). The overall 6-month PFS rate was 31% (19%, 52%), with a 6-month PFS rate of 44% (27-75%) in high-grade disease versus 14% (4-52%) in low-grade disease. The median PFS is 4 months 95% CI (3, 6) with ongoing responses (Appendix Figure 1) and the median OS is 11 months 95% CI (6,∞) (Appendix Figure 2).
Table 3:
Best Response Summary in 32 Patients with Neuroendocrine Neoplasms
| Response type | All patients (n=32) N(%) | High-grade (n=18) N (%) | Low/Intermediate grade (n=14) N(%) |
|---|---|---|---|
| CR* | 1 (3) | 1 (6) | 0 (0) |
| PR | 7 (22) | 7 (39) | 0 (0) |
| SD > 6 months | 2 (6) | 0 (0) | 2 (14) |
| SD ≤ 6 months | 11 (34) | 3 (17) | 8 (57) |
| PD | 11 (34) | 7 (39) | 4 (29) |
| CR + PR | 8 (25) | 8 (44) | 0 (0) |
| CR + PR + SD > 6 months | 10 (31) | 8 (44) | 2 (14) |
Unconfirmed CR after confirmed PR
Abbreviations: CR = complete response; PR = partial response; SD = stable disease
Figure 1. Waterfall and Swimmer’s plots of tumor measurements.
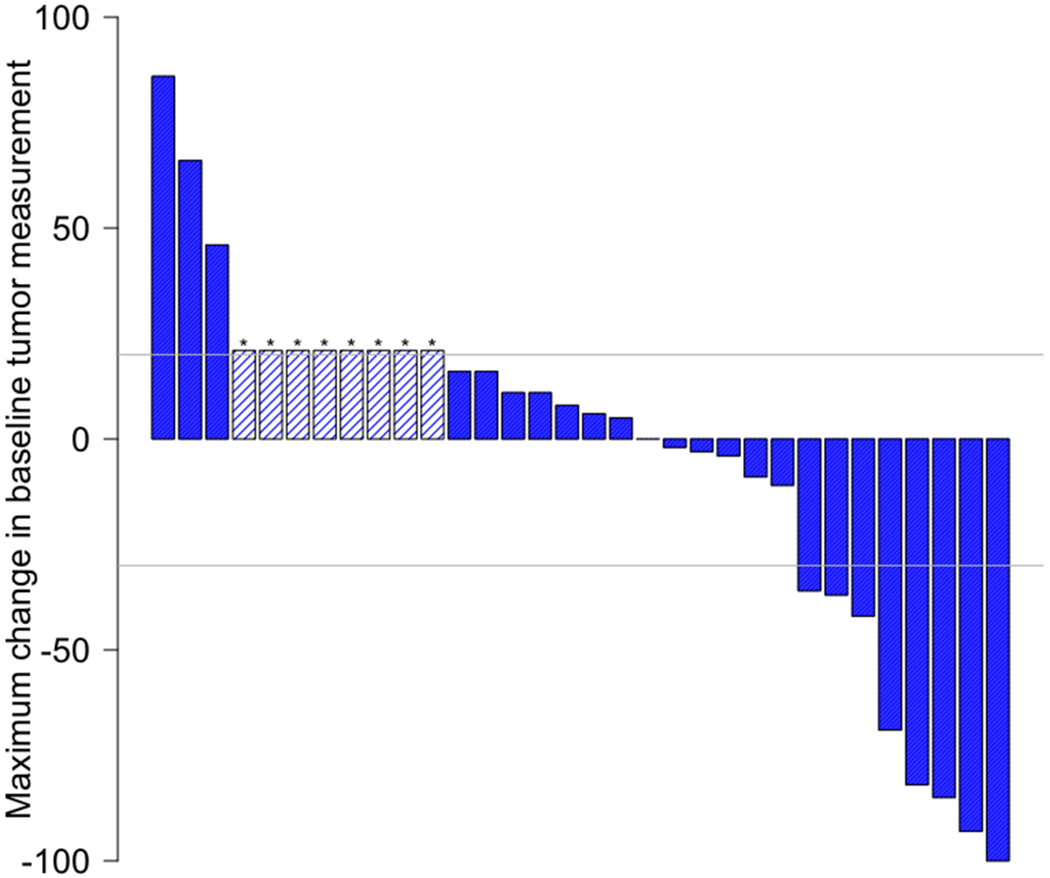
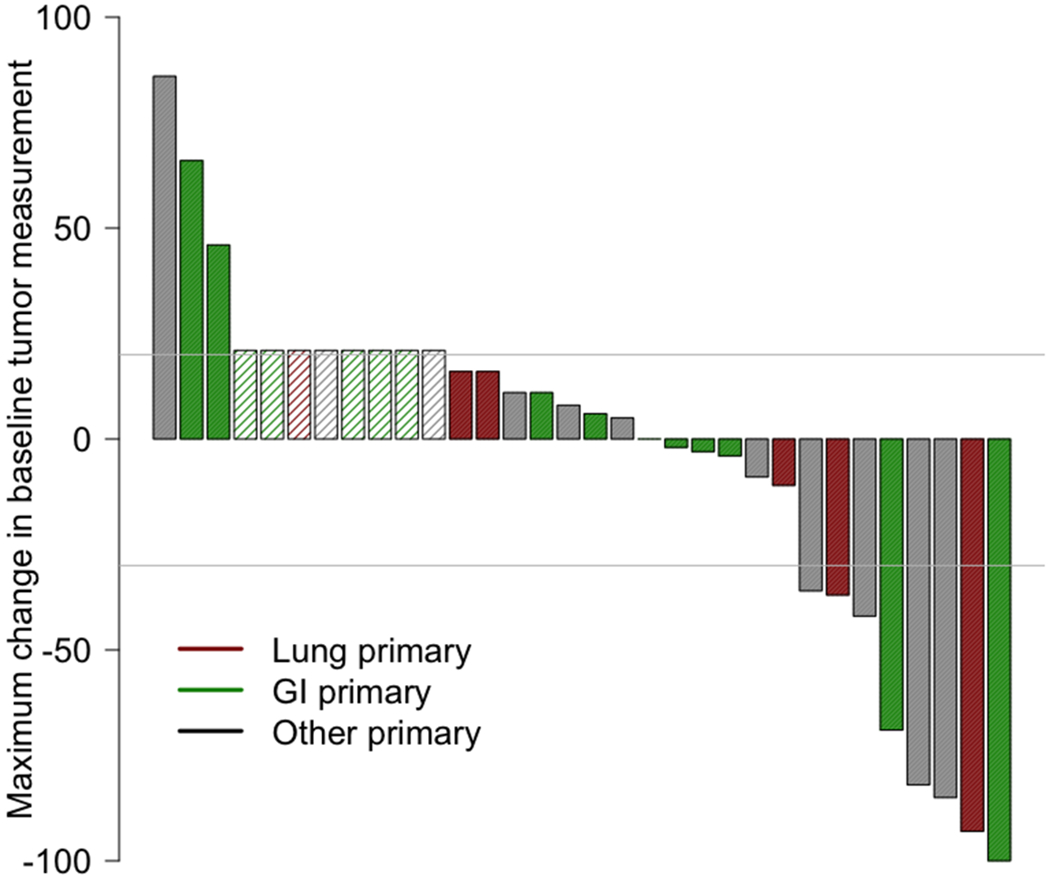
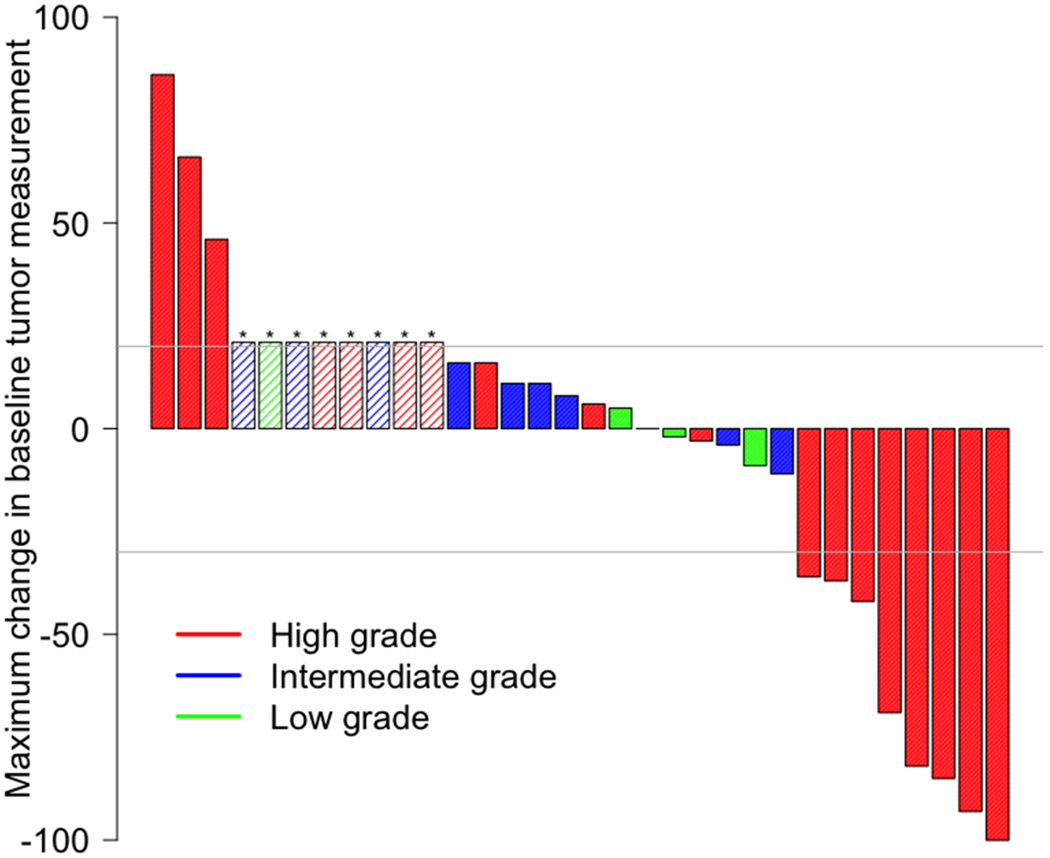
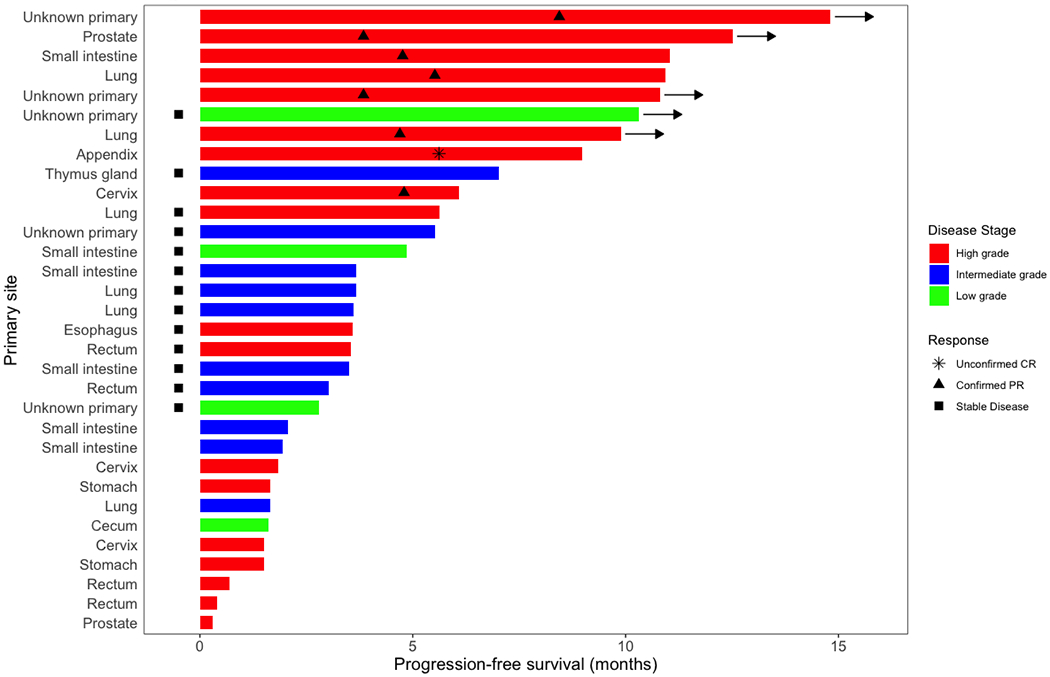
Gray lines at −30% and 20% indicate lines for partial response and progression per RECIST 1.1, respectively. Asterisk (*) and hatched bars in waterfall plots indicate patients who had early clinical progression (N = 3) or new lesions without assessable RECIST changes (N = 5; includes one patient who had new lesions on day 59, but currently remains on study with clinical benefit 326+ days after treatment initiation with confirmed iPR); these patients are shown as 21% increase indicating progression. A) Overall waterfall plot; B) Waterfall plot by primary site; C) Waterfall plot by tumor grade; D) Swimmer’s plot by tumor grade
We also assessed patients with iRECIST. There was only one patient that differed significantly. This patient had intermediate-grade disease of small intestine origin and achieved a confirmed iPR (instead of progressive disease per RECIST v1.1). By RECIST v1.1 this patient had progressed 59 days after treatment initiation, but currently remains on study with clinical benefit 326+ days after treatment initiation with confirmed iPR.
DISCUSSION
Neuroendocrine neoplasms represent a histologically and molecularly heterogeneous constellation of rare cancers that can arise across various organ types. Low- and intermediate-grade well-differentiated neuroendocrine neoplasms overexpress somatostatin receptors, which can be utilized both for functional imaging as well as therapeutic targeting with long-acting somatostatin analogs (6). In contrast, high-grade neuroendocrine carcinomas typically have more aggressive biology and minimal expression of somatostatin receptors and are typically treated with chemotherapy (7). 177Lu-Dotatate has recently shown activity with an 18% response rate for somatostatin-positive (low/intermediate grade) midgut neuroendocrine tumors and has attained Food and Drug Administration approval (8).
To date, immune checkpoint blockade with anti-CTLA-4 and anti-PD-1 has not been prospectively studied broadly across rare tumors, or in combination for neuroendocrine neoplasms. Prior studies of anti-PD-1 directed monotherapy have had limited efficacy across the spectrum of neuroendocrine neoplasms (9),(10),(11),(12),(13). For this trial, the combination of ipilimumab with nivolumab was chosen to maximize response rates in signal finding cohorts relative to monotherapy, and the dose of ipilimumab of 1mg/kg IV every 6 weeks was chosen to minimize toxicity while retaining combinatorial efficacy based on published comparative data in other tumor types (14). In the non-pancreatic neuroendocrine cohort of S1609 reported here, 25% of patients had a response to ipilimumab plus nivolumab, with no difference in ORR relative to organ of origin in our small cohort with a myriad of primary sites of origin (Figure 1B). None of the lung tumors in our cohort were small-cell lung cancer, for which anti-PD-1 and anti-CTLA-4 have previously been investigated (15),(16),(17). Of note, 44% of patients with high-grade neuroendocrine carcinomas had an objective response to therapy (Figure 2). Overall, this regimen resulted in no grade 5 toxicities, a <10% rate of grade 3-4 immune-related colitis and hepatitis, and no reported pneumonitis in this cohort. However, serious treatment-related toxicity occurred in 37.5% of patients and treatment discontinuation due to grade 3-4 toxicities occurred in 31.5% of patients.
With responses across different primary tumor sites and a signal towards improved response in high-grade neuroendocrine carcinomas, a potential predictive biomarker to help select for patients who may derive preferential benefit from immune checkpoint blockade is crucial. Biomarker analyses are underway for patients in the neuroendocrine cohort with a focus on PD-L1 expression by immunohistochemistry, tumor mutational burden, and comprehensive transcriptomic profiling given relevance in other tumor types (18),(19). One prior study found that high-grade gastroenteropancreatic neuroendocrine carcinomas had a higher rate of PD-L1 expression relative to lower-grade tumors, and was associated with poorer survival(20). Poorly-differentiated neuroendocrine carcinoma may also have a higher mutational burden than lower-grade tumors(21,22). Clinically, anecdotal response to anti-PD-1 has been reported in high-mutational burden neuroendocrine carcinoma previously (23).
Additionally, PD-L1 by IHC and tumor mutational burden in both tissue and blood have been associated with improved response to anti-PD-1 therapy across tumor types(18),(24,25),(26). Host factors related to HLA-type, immune status, microbiome and underlying etiology likely play a key role in influencing response to immune checkpoint blockade. To better understand the potential molecular basis for immunotherapeutic response, we assessed TMB and PD-L1 IHC in an independent cohort of neuroendocrine neoplasms not from S1609. While PD-L1 IHC was not different in high-grade versus intermediate/low-grade tumors, high-grade neuroendocrine tumors had a significantly greater rate of high TMB relative to intermediate/low-grade tumors, independent of site of origin (Appendix Table 2). Thus, TMB merits additional evaluation as a biomarker to potentially discriminate response to combinatorial immune checkpoint blockade, which will be specifically assayed in this cohort through whole-exome sequencing. Overexpression of PD-L1 in high-grade neuroendocrine carcinomas of the lung has also been reported (27). The TMB landscape in large cell neuroendocrine tumor of lung (n=353) had been previously investigated and the median TMB found to be higher than in small cell lung cancer and non-small cell lung cancer (28).
Strengths of this study include a broad population of patients across various tumor types representing both academic and community site accrual across the US, and support from the NCI, SWOG, and patient advocacy groups. Weaknesses of this study include its non-randomized nature, small sample size, and heterogenous patient population, which limit outcome comparisons between subgroups. Additionally, central pathology review was not mandated, and grading of tumor was done locally and most often with Ki67 measurement. Local pathology and imaging response assessments were utilized.
As studied in SWOG 1609, a basket rare tumor immunotherapy trial, ipilimumab plus nivolumab has clinical activity in non-pancreatic neuroendocrine neoplasms, in particular high-grade neuroendocrine carcinomas, across numerous primary originating organ sites. Ongoing studies focused on high-grade neuroendocrine carcinoma with rigorous correlative science to better understand host and tumor characteristics of immunotherapeutic response are underway.
Supplementary Material
Statement of Translational Relevance:
SWOG DART S1609 is the first study of combination anti-CTLA-4 (ipilimumab) and anti-PD-1 (nivolumab) across rare tumors, with this cohort focusing on non-pancreatic neuroendocrine neoplasms. Patients with high-grade neuroendocrine carcinoma had a 44% objective response rate (ORR), which could be driven by anti-CTLA-4 in the therapeutic combination in this high tumor mutational burden (TMB) subgroup. Central pathology review, PD-L1 status, and TMB were not available for enrolled patients.
Acknowledgements:
The authors wish to thank Ms. Marcia Horn, JD, SWOG Patient Advocate and President/CEO, International Cancer Advocacy Network; Mr. Dion Holmes, Protocol Coordinator, SWOG Operations Office; Dr. Heloisa P. Soares, MD, PhD, University of New Mexico; Dr. Howard Streicher, MD, National Cancer Institute, Investigational Drug Branch, Cancer Therapy Evaluation Program, and Dr. David Arguello, M.D., and Ms. Michelle Winerip, Caris Life Sciences, for their invaluable assistance with this trial.
Funding: This work was supported by National Institutes of Health/National Cancer Institute grant awards CA180888, CA180819, CA180821, CA180820, CA189870, CA180850, CA189873, CA189809, CA180858, CA189830, CA189821, CA180834, CA189971, CA180801, CA189953, CA189856; legacy grant awards CA73590, CA12644; and in part by Bristol-Myers Squibb Company. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Bristol-Myers Squibb Company.
Disclosures: Dr. Kurzrock has research funding from Incyte, Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, Guardant Health, Grifols, Konica Minolta, and OmniSeq, as well as consultant fees from LOXO, X-Biotech, Actuate Therapeutics, Roche and NeoMed. She serves as an advisor to Soluventis. She receives speaker fees from Roche, and also has equity in IDbyDNA, CureMatch, Inc., and Soluventis. Dr. Patel reports grants from Bristol-Myers Squibb, personal fees from Bristol-Myers Squibb, during the conduct of the study; grants from Eli Lilly, Incyte, AstraZeneca/MedImmune, Merck, Pfizer, Roche/Genentech, Xcovery. Fate Therapeutics, Genocea, Iovance, personal fees from AstraZeneca, Illumina, Tempus, Novartis, outside the submitted work. Dr. Chae reports research grants from Abbvie, BMS, Biodesix, Lexent Bio, Freenome; Honoraria/Advisory Boards from Roche/Genentech, AstraZeneca, Foundation Medicine, Counsyl, Guardant Health, Boehringher Ingelheim, Biodesix, Immuneoncia, Lilly Oncology, Merck, and Takeda. Dr. Shah reports grants/research funding from Merck, during the conduct of the study; grants from LOXO Oncology, grants/research funding and personal fees (advisory board) from Eisai, personal fees (advisory board) from Novartis, personal fees (advisory board) from Ignyta, outside the submitted work. Dr. Singh reports personal fees and non-financial support (advisory board) from Eisai Inc, personal fees (advisory board) from Merrimack Pharma, outside the submitted work. Dr. Kasi reports support from NIH/NCI grants CA180888, CA180819, CA180821, CA180820; and in part by Bristol-Myers Squibb Company, during the conduct of the study; grants from TESARO and Halozyme, outside the submitted work. Dr. Al Baghdadi reports personal fees (stock ownership) from Bristol-Myers Squibb, personal fees (Advisory Board) from Bristol-Myers Squibb, during the conduct of the study; personal fees (stock ownership) from Array, Portola, AstraZeneca, Spectrum, Sunesis, Epizyme, Tracon, Seattle Genetics, Celgene, personal fees (Advisory Board) Celgene, Heron, Cardinal Health, and personal fees (Travel expenses for attendance at Advisory Board meetings) from Celgene, Heron, Cardinal Health, outside the submitted work. Dr. Korn reports consulting fees from Merck, ownership interest in and income from Caris Life Sciences. Dr. Ryan reports grants from NCTN/SWOG, during the conduct of the study; grants and personal fees from Pfizer, Eisai, Exelixis, Genentech, Novartis, grants from Argos Therapeutics, Bayer, Bristol-Myers Squibb, Daiichi Sankyo, Glaxo Smith Kline, Janssen, Karyopharm Therapeutics, MabVax, Merck, Morphotek, OSI Pharmaceuticals, Treshold Pharmaceuticals, TRACON pharmaceuticals, outside the submitted work. Dr. Hansel reports personal fees from AstraZeneca (advisory board), Genentech (advisory board), Taris Pharmaceuticals (consultant), grant (PI) from Konica Minolta, outside the submitted work. Dr. Sharon is a full-time employee of the National Cancer Institute (NCI), an arm of the United States government. The NCI negotiated a collaborative research and development agreement (CRADA) with Bristol-Myers Squibb to provide nivolumab and ipilimumab for this clinical trial. Dr. Giles receives consultant fees from and has equity interest in Actuate Therapeutics and Epigene Therapeutics. Drs. Blanke, Chen, Fontaine, Gatalica, Hayward, Matrana, Mayerson, McLeod, Othus, Plets have no COI disclosures to report.
REFERENCES
- 1.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. Journal for ImmunoTherapy of Cancer 2018;6(1):8 doi 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo G, Javed A, Strosberg JR, Jin K, Zhang Y, Liu C, et al. Modified Staging Classification for Pancreatic Neuroendocrine Tumors on the Basis of the American Joint Committee on Cancer and European Neuroendocrine Tumor Society Systems. Journal of Clinical Oncology 2017;35(3):274–80 doi 10.1200/jco.2016.67.8193. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE, Kramer JL, Jemal A. The burden of rare cancers in the United States. CA: A Cancer Journal for Clinicians 2017;67(4):261–72 doi doi: 10.3322/caac.21400. [DOI] [PubMed] [Google Scholar]
- 4.Brookmeyer R, Crowley J. A k-sample median test for censored data. Journal of the American Statistical Association 1982;77(378):433–40. [Google Scholar]
- 5.Koyama T, Chen H. Proper inference from Simon’s two-stage designs. Statistics in Medicine 2008;27(16):3145–54 doi doi: 10.1002/sim.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinke A, Müller H-H, Schade-Brittinger C, Klose K-J, Barth P, Wied M, et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients With Metastatic Neuroendocrine Midgut Tumors: A Report From the PROMID Study Group. Journal of Clinical Oncology 2009;27(28):4656–63 doi 10.1200/jco.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 7.Strosberg JR, Fine RL, Choi J, Nasir A, Coppola D, Chen D- T, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117(2):268–75 doi doi: 10.1002/cncr.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. New England Journal of Medicine 2017;376(2):125–35 doi 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao JC, Strosberg J, Fazio N, Pavel ME, Ruszniewski P, Bergsland E, et al. 1308OActivity & safety of spartalizumab (PDR001) in patients (pts) with advanced neuroendocrine tumors (NET) of pancreatic (Pan), gastrointestinal (GI), or thoracic (T) origin, & gastroenteropancreatic neuroendocrine carcinoma (GEP NEC) who have progressed on prior treatment (Tx). Annals of Oncology 2018;29(suppl_8) doi 10.1093/annonc/mdy293.001. [DOI] [Google Scholar]
- 10.Strosberg JR, Mizuno N, Doi T, Grande E, Delord J- P, Shapira-Frommer R, et al. Pembrolizumab treatment of advanced neuroendocrine tumors: Results from the phase II KEYNOTE-158 study. Journal of Clinical Oncology 2019;37(4_suppl):190- doi 10.1200/JCO.2019.37.4_suppl.190.30523716 [DOI] [Google Scholar]
- 11.Vijayvergia N, Dasari A, Ross EA, Dotan E, Halperin DM, Astsaturov IA, et al. Pembrolizumab (P) monotherapy in patients with previously treated metastatic high grade neuroendocrine neoplasms (HG-NENs). Journal of Clinical Oncology 2018;36(15_suppl):4104- doi 10.1200/JCO.2018.36.15_suppl.4104. [DOI] [Google Scholar]
- 12.Mulvey C, Raj NP, Chan JA, Aggarwal RR, Cinar P, Hope TA, et al. Phase II study of pembrolizumab-based therapy in previously treated extrapulmonary poorly differentiated neuroendocrine carcinomas: Results of Part A (pembrolizumab alone). Journal of Clinical Oncology 2019;37(4_suppl):363- doi 10.1200/JCO.2019.37.4_suppl.363.30576267 [DOI] [Google Scholar]
- 13.Fottner C, Apostolidis L, Ferrata M, Krug S, Michl P, Schad A, et al. A phase II, open label, multicenter trial of avelumab in patients with advanced, metastatic high-grade neuroendocrine carcinomas NEC G3 (WHO 2010) progressive after first-line chemotherapy (AVENEC). Journal of Clinical Oncology 2019;37(15_suppl):4103- doi 10.1200/JCO.2019.37.15_suppl.4103. [DOI] [Google Scholar]
- 14.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. The Lancet Oncology 2017;18(1):31–41 doi 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. New England Journal of Medicine 2018;379(23):2220–9 doi 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 16.Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. The Lancet Oncology 2016;17(7):883–95 doi 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 17.Ott PA, Elez E, Hiret S, Kim D- W, Morosky A, Saraf S, et al. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. Journal of Clinical Oncology 2017;35(34):3823–9 doi 10.1200/jco.2017.72.5069. [DOI] [PubMed] [Google Scholar]
- 18.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Molecular Cancer Therapeutics 2015;14:847–56 doi 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 19.Khagi Y, Kurzrock R, Patel SP. Next generation predictive biomarkers for immune checkpoint inhibition. Cancer and Metastasis Reviews 2017;36(1):179–90 doi 10.1007/s10555-016-9652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim ST HS, Lee S, Ahn S, Lee J, Park SH, Park JO, Lim HY, Kang WK, Kim KM, Park YS. The Impact of PD-L1 Expression in Patients with Metastatic GEP-NETs. J Cancer;7(5):484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vijayvergia N, Boland PM, Handorf E, Gustafson KS, Gong Y, Cooper HS, et al. Molecular profiling of neuroendocrine malignancies to identify prognostic and therapeutic markers: a Fox Chase Cancer Center Pilot Study. British Journal Of Cancer 2016;115:564 doi 10.1038/bjc.2016.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banck MS, Kanwar R, Kulkarni AA, Boora GK, Metge F, Kipp BR, et al. The genomic landscape of small intestine neuroendocrine tumors. The Journal of Clinical Investigation 2013;123(6):2502–8 doi 10.1172/JCI67963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharabi A, Kim SS, Kato S, Sanders PD, Patel SP, Sanghvi P, et al. Exceptional Response to Nivolumab and Stereotactic Body Radiation Therapy (SBRT) in Neuroendocrine Cervical Carcinoma with High Tumor Mutational Burden: Management Considerations from the Center For Personalized Cancer Therapy at UC San Diego Moores Cancer Center. The Oncologist 2017;22:1–7 doi 10.1634/theoncologist.2016-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Molecular Cancer Therapeutics 2017:0386.2017. doi 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khagi Y, Goodman AM, Daniels GA, Patel SP, Sacco AG, Randall JM, et al. Hypermutated Circulating Tumor DNA: Correlation with Response to Checkpoint Inhibitor–Based Immunotherapy. Clinical Cancer Research 2017;23(19):5729–36 doi 10.1158/1078-0432.Ccr-17-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chae YK, Davis AA, Raparia K, Agte S, Pan A, Mohindra N, et al. Association of Tumor Mutational Burden With DNA Repair Mutations and Response to AntiPD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer. Clinical Lung Cancer 2019. doi 10.1016/j.cllc.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Tsuruoka K, Horinouchi H, Goto Y, Kanda S, Fujiwara Y, Nokihara H, et al. PD-L1 expression in neuroendocrine tumors of the lung. Lung Cancer 2017;108:115–20 doi 10.1016/j.lungcan.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Chae YK TK, Chung J, Schrock AB. Genomic alterations (GA) and tumor mutational burden (TMB) in large cell neuroendocrine carcinoma of lung (L-LCNEC) as compared to small cell lung carcinoma (SCLC) as assessed via comprehensive genomic profiling (CGP). 2017; Chicago IL. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


