Key Points
Question
Are natural and surgical premature menopause (occurring before age 40 years) associated with future development of cardiovascular diseases?
Findings
In a cohort study that included 144 260 postmenopausal women, premature menopause, compared with no premature menopause, was significantly associated with increased risk for a composite outcome that included coronary artery disease, heart failure, aortic stenosis, mitral regurgitation, atrial fibrillation, ischemic stroke, peripheral artery disease, and venous thromboembolism. For natural premature menopause, the hazard ratio was 1.36; for surgical premature menopause, the hazard ratio was 1.87.
Meaning
Natural and surgical premature menopause may be associated with increased risk for a composite of cardiovascular diseases.
Abstract
Importance
Recent guidelines endorse using history of menopause before age 40 years to refine atherosclerotic cardiovascular disease risk assessments among middle-aged women. Robust data on cardiovascular disease risk in this population are lacking.
Objective
To examine the development of cardiovascular diseases and cardiovascular risk factors in women with natural and surgical menopause before age 40 years.
Design, Setting, and Participants
Cohort study (UK Biobank), with adult residents of the United Kingdom recruited between 2006 and 2010. Of women who were 40 to 69 years old and postmenopausal at study enrollment, 144 260 were eligible for inclusion. Follow-up occurred through August 2016.
Exposures
Natural premature menopause (menopause before age 40 without oophorectomy) and surgical premature menopause (bilateral oophorectomy before age 40). Postmenopausal women without premature menopause served as the reference group.
Main Outcomes and Measures
The primary outcome was a composite of incident coronary artery disease, heart failure, aortic stenosis, mitral regurgitation, atrial fibrillation, ischemic stroke, peripheral artery disease, and venous thromboembolism. Secondary outcomes included individual components of the primary outcome, incident hypertension, hyperlipidemia, and type 2 diabetes.
Results
Of 144 260 postmenopausal women included (mean [SD] age at enrollment, 59.9 [5.4] years), 4904 (3.4%) had natural premature menopause and 644 (0.4%) had surgical premature menopause. Participants were followed up for a median of 7 years (interquartile range, 6.3-7.7). The primary outcome occurred in 5415 women (3.9%) with no premature menopause (incidence, 5.70/1000 woman-years), 292 women (6.0%) with natural premature menopause (incidence, 8.78/1000 woman-years) (difference vs no premature menopause, +3.08/1000 woman-years [95% CI, 2.06-4.10]; P < .001), and 49 women (7.6%) with surgical premature menopause (incidence, 11.27/1000 woman-years) (difference vs no premature menopause, +5.57/1000 woman-years [95% CI, 2.41-8.73]; P < .001). For the primary outcome, natural and surgical premature menopause were associated with hazard ratios of 1.36 (95% CI, 1.19-1.56; P < .001) and 1.87 (95% CI, 1.36-2.58; P < .001), respectively, after adjustment for conventional cardiovascular disease risk factors and use of menopausal hormone therapy.
Conclusions and Relevance
Natural and surgical premature menopause (before age 40 years) were associated with a small but statistically significant increased risk for a composite of cardiovascular diseases among postmenopausal women. Further research is needed to understand the mechanisms underlying these associations.
This cohort study uses UK Biobank data to compare the development of cardiovascular diseases (CVD) and cardiovascular risk factors in women with premature (before age 40 years) vs nonpremature natural and surgical menopause.
Introduction
Although cardiovascular disease is the leading cause of death among women in the United States and worldwide,1 sex-specific risk factors for cardiovascular disease in women remain under-recognized and incompletely understood.2 In studies of women who underwent menopause in the 1990s and 2000s, most women in Western countries experienced menopause between age 40 and 58 years, with mean age at menopause of 51 to 52 years.3,4 Up to 10% of women underwent menopause before age 45 years, and 1% experienced menopause before age 40 years.4,5,6 Premature menopause has been associated with increased risk of coronary artery disease (CAD) and, less consistently, with increased risk of stroke.7,8,9,10 Additionally, an analysis from the Women’s Health Initiative found a modest association between earlier menopausal age and increased risk of heart failure.11
Based on these and other data,7,8 recent updates to the cholesterol12 and primary prevention13 guidelines from the American College of Cardiology/American Heart Association endorse using a history of premature menopause (defined as menopause prior to age 40 years) to refine cardiovascular risk assessments and guide statin prescription for asymptomatic women in midlife at intermediate risk of atherosclerotic cardiovascular disease (ASCVD). However, robust data are limited on the development of cardiovascular risk factors among women who have undergone menopause before age 40 and the long-term risk of both ASCVD and nonatherosclerotic cardiovascular diseases in this population.8 Further, data are inconsistent regarding whether cardiovascular disease risk differs between women with natural and surgical premature menopause,14 although recent large studies have not shown a difference.7,10
This analysis used data from the large-scale, observational UK Biobank to examine development of diverse cardiovascular diseases, as well as incident cardiovascular risk factors, in women with natural and surgical menopause before age 40. The risk associated with alternate menopausal age thresholds was also explored.
Methods
Study Cohort
The UK Biobank is a population-based cohort of more than 500 000 adult residents of the United Kingdom recruited between 2006 and 2010.15,16 At the baseline study visit, participants provided informed consent and completed questionnaires about health history (including reproductive history and medication use), lifestyle, and sociodemographic factors and underwent physical assessment and phlebotomy.15 Incident diagnoses were collected from follow-up study visits and linkage to national health records.15 Follow-up occurred through August 2016. The Massachusetts General Hospital institutional review board approved analyses of these data. The statistical analysis plan is available in Supplement 1.
Postmenopausal women who were 40 to 69 years old at enrollment were considered for inclusion. Women who were premenopausal, had unknown menopause status, or had missing or unknown age at menopause were excluded, as were women with prevalent diagnoses of any of the cardiovascular diseases under study and those with congenital heart disease (see the eAppendix in Supplement 2 for relevant International Classification of Diseases codes).
Exposures
Reproductive history, including use of systemic menopausal hormone therapy (MHT), was ascertained at the baseline study visit by participant self-report. In primary analyses, to maintain consistency with recent American College of Cardiology/American Heart Association guidelines, natural premature menopause was defined as menopause occurring before age 40 years without bilateral oophorectomy. Surgical premature menopause was defined as bilateral oophorectomy before age 40 years. Prevalent cardiovascular risk factors were captured from International Classification of Diseases codes and/or self-report.
Outcomes
The primary outcome was a composite of incident CAD, heart failure, aortic stenosis, mitral regurgitation, atrial fibrillation or flutter, ischemic stroke, peripheral artery disease, and venous thromboembolism. Secondary outcomes included incident hypertension, hyperlipidemia, type 2 diabetes, and individual diagnoses included in the composite primary end point. Outcomes were captured from the appearance of a qualifying International Classification of Diseases code in the medical record (eAppendix in Supplement 2). Prior genetic association analyses with this definition of CAD in the UK Biobank showed concordant results compared with those from validated epidemiologic studies.17,18 Ischemic stroke diagnoses were centrally validated by UK Biobank study staff.
Power Analysis and Sample Size Calculation
Sample size was determined by the number of eligible participants in the UK Biobank. Assuming 1 or more incident cardiovascular diagnoses would occur in 5% of women during follow-up, the sample had more than 99% power to detect a hazard ratio of 2.0 for the primary outcome in both natural and surgical premature menopause groups at an α level of .05.
Statistical Analysis
Baseline continuous variables were compared using analysis of variance or the Kruskal-Wallis test, as appropriate, and categorical variables were compared using the Pearson χ2 test. Cox proportional hazard models were fitted to estimate associations of natural and surgical premature menopause with time to (1) first incident cardiovascular disease diagnosis (primary outcome); (2) each of the 8 individual cardiovascular diseases under study; and (3) incident hypertension, hyperlipidemia, and type 2 diabetes. Women with prevalent hypertension, hyperlipidemia, and type 2 diabetes were excluded from the corresponding incident disease models. Menopause history was included in the models as a categorical, nonordered variable (menopause at age ≥40 years, natural premature menopause, and surgical premature menopause), with menopause at age 40 years or older serving as the reference group. In additional exploratory analyses, hazards associated with alternate age thresholds were compared with a reference group of women with age at menopause of 50 years or older.
All models were adjusted for age at study enrollment, incorporated as both a quadratic and linear term to account for nonlinear effects, and race/ethnicity. Race/ethnicity was systematically ascertained from self-report and adapted into consolidated categories for analysis (because 95.5% of the sample was white, race was dichotomized as white vs nonwhite in models), as both cardiovascular disease risk and premature menopause prevalence vary by race/ethnicity. Models for incident cardiovascular diseases incorporated conventional ASCVD risk factors (systolic blood pressure, non–high-density lipoprotein cholesterol, prevalent type 2 diabetes, body mass index [BMI, incorporated as a continuous variable], and smoking), use of medications (antihypertensive medications, lipid-lowering medications, and MHT), and high-sensitivity C-reactive protein. Models for incident cardiovascular risk factors (hypertension, hyperlipidemia, and type 2 diabetes) were further adjusted for BMI and the other 2 risk factors not under consideration in each model. The normality of continuous covariates was assessed, and C-reactive protein was log-transformed to achieve normality.
For each participant, follow-up began at enrollment and was measured separately for each diagnosis. Time to censoring for each outcome was determined by the date a diagnosis appeared in the medical record or last follow-up. The proportional hazards assumption was tested using Schoenfeld residuals. To interrogate the possibility of bias arising from differential competing risks (death) across groups, Fine-Gray subdistribution hazards were calculated incorporating noncardiovascular death as a competing risk for incident cardiovascular diagnoses and all-cause death as a competing risk in models for incident hypertension, hyperlipidemia, and type 2 diabetes.
In fully adjusted models in the primary analysis, no assumptions were made about missing data, and women with missing covariates were excluded from incident disease models. To interrogate possible nonrandomness of missing laboratory biomarker data, multivariable models for missingness were created using age, sex, type 2 diabetes, smoking status, BMI, systolic blood pressure, and medication use as covariates. Any covariates found to be significantly associated with biomarker missingness were incorporated, along with age and race/ethnicity, in predictive models to impute missing data using the predict() function in R, and these imputed data were used in sensitivity analyses. Other missing covariate data were imputed using predictive models based on age and race/ethnicity.
Differences in hazards between natural vs surgical premature menopause were assessed using the statistical test for heterogeneity. The Cochran-Armitage test assessed trends in cardiovascular disease risk across age at menopause thresholds.
Two-sided P < .05 was considered significant. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Analyses were performed using R version 3.5 (R Foundation for Statistical Computing).
Results
Description of the Study Cohort
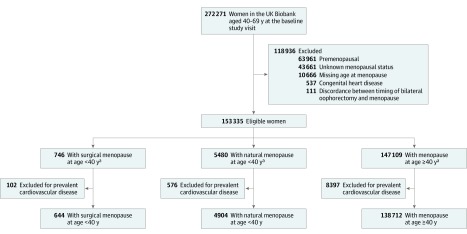
After exclusions, the final study cohort included 144 260 postmenopausal women, of whom 4904 (3.4%) experienced natural menopause before age 40 (natural premature menopause) and 644 (0.4%) experienced surgical menopause before age 40 (surgical premature menopause) (Figure 1). Women were followed up for a median of 7 years (interquartile range, 6.3-7.7; overall range, 0-10). The mean (SD) age of the cohort at enrollment was 59.9 (5.4) years, and 95.5% of the cohort was white. The mean (SD) age at menopause was 50.3 (4.2) years among women without premature menopause, 35.4 (3.9) years among women with natural premature menopause, and 34.2 (4.2) years among women with surgical premature menopause (P < .001). Although differences reached statistical significance, parity was numerically similar (median parity of 2) across groups (Table 1).
Figure 1. Creation of the Study Sample From Women in the UK Biobank.
Postmenopausal women who were aged 40 to 69 years old at study enrollment were considered for inclusion. Exclusion criteria included unknown menopausal status, missing data on age at menopause, and prevalent diagnoses of coronary artery disease, heart failure, aortic stenosis, mitral regurgitation, atrial fibrillation, ischemic stroke, peripheral artery disease, venous thromboembolism, or congenital heart disease.
aThe mean (SD) age at enrollment was 57.4 (7.8) years for the surgical premature menopause group, 58.9 (7.1) years for the natural premature menopause group, and 60.0 (5.4) years for the group without premature menopause.
Table 1. Baseline Characteristics of the Study Cohort.
| Characteristic | No. (%) | P Valuea | ||
|---|---|---|---|---|
| Women With Surgical Menopause at Age <40 y (n = 644) | Women With Natural Menopause at Age <40 y (n = 4904) | Women With Menopause at Age ≥40 y (n = 138 712) | ||
| Age, y | 57.4 (7.8) | 58.9 (7.1) | 60.0 (5.4) | <.001 |
| Race/ethnicity | ||||
| White | 613 (95.2) | 4629 (94.4) | 132 476 (95.5) | .001 |
| Asian | 8 (1.2) | 96 (2.0) | 2500 (1.8) | |
| Black | 13 (2.0) | 97 (2.0) | 1606 (1.2) | |
| Mixed | 4 (0.6) | 33 (0.7) | 645 (0.5) | |
| Other | 6 (0.9) | 49 (1.0) | 1485 (1.1) | |
| Parity | 2 (1 to 2) | 2 (1 to 3) | 2 (1 to 2) | .002 |
| No. | 4899 | 138 624 | ||
| History of gestational hypertension/preeclampsia | 4 (0.6) | 36 (0.7) | 912 (0.7) | .80 |
| History of hysterectomy | 585 (90.8) | 2524 (51.5) | 12 505 (9.0) | <.001 |
| No. | 641 | 4888 | 138 600 | |
| Mean age at menopause, y | 34.2 (4.2) | 35.4 (3.9) | 50.3 (4.2) | <.001 |
| Current or former smoking | 332 (51.6) | 2391 (48.8) | 57 187 (41.2) | <.001 |
| Exercise ≥2 times weekly | 120 (50.4) | 1079 (54.2) | 34 980 (52.4) | .23 |
| No. | 238 | 1991 | 66 770 | |
| Alcohol consumption at least weekly | 304 (47.2) | 2645 (54.0) | 88 357 (63.7) | <.001 |
| No. | 4898 | 138 628 | ||
| Body mass index, mean (SD)b | 28.7 (6.0) | 27.9 (5.3) | 27.0 (4.9) | <.001 |
| No. | 629 | 4814 | 136 684 | |
| Systolic blood pressure, mean (SD), mm Hg | 139.2 (21.6) | 139.2 (20.4) | 140.5 (20.3) | <.001 |
| No. | 604 | 4614 | 130 586 | |
| Diastolic blood pressure, mean (SD), mm Hg | 81.7 (11.4) | 81.1 (10.4) | 81.2 (10.4) | .44 |
| No. | 604 | 4614 | 130 588 | |
| Hypertension | 218 (33.9) | 1506 (30.7) | 37 616 (27.1) | <.001 |
| Hyperlipidemia | 98 (15.2) | 809 (16.5) | 16 557 (11.9) | <.001 |
| Type 2 diabetes | 13 (2.0) | 132 (2.7) | 1949 (1.4) | <.001 |
| Chronic kidney disease | 1 (0.2) | 25 (0.5) | 230 (0.2) | <.001 |
| History of any cancer | 151 (23.7) | 707 (14.5) | 14 159 (10.2) | <.001 |
| No. | 638 | 4875 | 138 311 | |
| Medication use at baseline visit | ||||
| Antihypertensive medication | 148 (23.0) | 1110 (22.6) | 26 751 (19.3) | <.001 |
| Cholesterol-lowering medication | 122 (18.9) | 986 (20.1) | 18 962 (13.7) | <.001 |
| Menopausal hormone therapy (current use) | 174 (27.0) | 778 (15.9) | 8504 (6.1) | <.001 |
| Ever use of menopausal hormone therapy | 544 (84.5) | 3490 (71.5) | 61 078 (44.1) | <.001 |
| No. | 4883 | 138 388 | ||
| Age at initiation of menopausal hormone therapy, mean (SD), y | 36.7 (5.9) | 41.6 (6.3) | 48.7 (4.5) | <.001 |
| No. | 533 | 3191 | 56 280 | |
| Duration of prior menopausal hormone therapy use (not including current users), median (range), y | 10 (5 to 19) | 8 (3 to 13) | 5 (2 to 9) | <.001 |
| No. | 392 | 2468 | 48 355 | |
| Prior use of menopausal hormone therapy ≥5 y | 296 (46.0) | 1706 (34.8) | 26 081 (18.8) | <.001 |
| Time from menopause to initiation of menopausal hormone therapy among menopausal hormone therapy users, median (IQR), y | 0 (0 to 2) | 4 (1 to 11) | 0 (−3 to 1) | <.001 |
| No. | 533 | 3191 | 56 280 | |
| Initiation of menopausal hormone therapy >5 y after menopause (% of menopausal hormone therapy users) | 37 (6.9) | 535 (16.8) | 2618 (4.7) | <.001 |
| No. | 533 | 3191 | 56 280 | |
| Cholesterol, mean (SD), mg/dL | ||||
| Total | 233.3 (46.2) | 230.7 (44.6) | 235.4 (43.1) | <.001 |
| No. | 608 | 4560 | 129 396 | |
| HDL | 58.7 (13.6) | 60.7 (14.7) | 63.0 (14.8) | <.001 |
| No. | 556 | 4161 | 117 832 | |
| LDL | 145.9 (36.2) | 142.9 (34.6) | 145.8 (33.5) | <.001 |
| No. | 608 | 4553 | 129 170 | |
| Triglycerides | 146.6 (122.6 to 167.2) | 134.2 (95.4 to 191.1) | 123.7 (90.6 to 173.0) | <.001 |
| No. | 607 | 4560 | 129 322 | |
| Lipoprotein(a), median (IQR), nmol/L | 22.2 (9.3 to 63.9) | 22.8 (10.3 to 64.2) | 23.3 (10.4 to 61.5) | .52 |
| No. | 490 | 3653 | 9228 | |
| High-sensitivity C-reactive protein, median (IQR), mg/L | 2.2 (1.0 to 4.6) | 1.8 (0.9 to 4.0) | 1.4 (0.7 to 2.9) | <.001 |
| No. | 606 | 4555 | 129 170 | |
Abbreviations: HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein.
SI conversion factors: To convert total, HDL, and LDL cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113; and lipoprotein(a) to μmol/L, multiply by 0.0357.
P values were calculated for continuous variables using analysis of variance (normally distributed variables) or the Kruskal-Wallis test (non–normally distributed variables) and using the Pearson χ2 test for categorical variables.
Calculated as weight in kilograms divided by height in meters squared.
Women with natural and surgical premature menopause were more likely than women without premature menopause to have prevalent cardiovascular risk factors, to have ever smoked tobacco, and to have used MHT at enrollment (Table 1). Less than 0.3% of women in all 3 groups initiated MHT after age 60 years.
Primary Outcome
As summarized in Table 2, 5415 women (3.9%) without premature menopause, 292 (6.0%) with natural premature menopause, and 49 (7.6%) with surgical premature menopause developed 1 or more incident cardiovascular diseases during follow-up. The crude incidences of any ASCVD (CAD, ischemic stroke, or peripheral artery disease) was 2143 women (1.5%) without premature menopause, 123 (2.5%) with natural premature menopause, and 24 (3.7%) with surgical premature menopause.
Table 2. Incidence of Cardiovascular Disease and Cardiovascular Risk Factors During the Study Period.
| Women With Menopause at Age ≥40 y (n = 138 712) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Women With Surgical Menopause at Age <40 y (n = 644) | Women With Natural Menopause at Age <40 y (n = 4904) | ||||||||
| Crude Cumulative Incidence, No. (%) | Woman-Years at Risk, No. | Incidence Rate per 1000 Woman-Years (95% CI) | Crude Cumulative Incidence, No. (%) | Woman-Years at Risk, No. | Incidence Rate per 1000 Woman, Years (95% CI) | Crude Cumulative Incidence, No. (%) | Woman-Years at Risk, No. | Incidence Rate per 1000 Woman-Years (95% CI) | |
| Cardiovascular Disease | |||||||||
| Any incident cardiovascular disease diagnosis | 49 (7.6) | 4346 | 11.27 (8.54-14.6) | 292 (6.0) | 33 254 | 8.78 (7.83-9.82) | 5415 (3.9) | 949 268 | 5.70 (5.55-5.86) |
| Coronary artery disease | 20 (3.1) | 4429 | 4.52 (2.94-6.70) | 79 (1.6) | 33 887 | 2.33 (1.87-2.87) | 1312 (0.9) | 961 477 | 1.36 (1.29-1.44) |
| Heart failure | 9 (1.4) | 4458 | 2.02 (1.08-3.54) | 41 (0.8) | 34 033 | 1.20 (0.89-1.60) | 722 (0.5) | 963 530 | 0.75 (0.70-0.80) |
| Aortic stenosis | 4 (0.6) | 4492 | 0.89 (0.36-1.95) | 23 (0.5) | 34 067 | 0.68 (0.45-0.98) | 270 (0.2) | 964 893 | 0.28 (0.25-0.31) |
| Mitral regurgitation | 5 (0.8) | 4486 | 1.11 (0.49-2.28) | 12 (0.2) | 34 086 | 0.35 (0.20-0.58) | 334 (0.2) | 964 628 | 0.35 (0.31-0.38) |
| Atrial fibrillation | 16 (2.5) | 4450 | 3.60 (2.22-5.56) | 102 (2.1) | 33 816 | 3.02 (2.49-3.63) | 2093 (1.5) | 959 120 | 2.18 (2.09-2.28) |
| Ischemic stroke | 3 (0.5) | 4493 | 0.67 (0.24-1.61) | 32 (0.7) | 34 048 | 0.94 (0.67-1.29) | 584 (0.4) | 963 965 | 0.61 (0.56-0.66) |
| Peripheral artery disease | 3 (0.5) | 4485 | 0.67 (0.24-1.61) | 22 (0.4) | 34 069 | 0.65 (0.43-0.94) | 334 (0.2) | 964 671 | 0.35 (0.31-0.38) |
| Venous thromboembolism | 11 (1.7) | 4416 | 2.49 (1.40-4.16) | 57 (1.2) | 33 724 | 1.69 (1.31-2.16) | 989 (0.7) | 959 696 | 1.03 (0.97-1.10) |
| Cardiovascular Risk Factorsa | |||||||||
| Hypertension | 50 (11.7) | 2844 | 17.58 (13.35-22.78) | 251 (7.4) | 22 819 | 11.00 (9.72-12.40) | 5007 (5.0) | 688 074 | 7.28 (7.08-7.48) |
| Hyperlipidemia | 49 (9.0) | 3644 | 13.45 (10.18-17.46) | 208 (5.1) | 27 840 | 7.47 (6.52-8.52) | 4229 (3.5) | 837 466 | 5.05 (4.90-5.20) |
| Type 2 diabetes | 34 (5.4) | 4276 | 7.95 (5.70-10.84) | 177 (3.7) | 32 567 | 5.43 (4.69-6.26) | 2747 (2.0) | 942 955 | 2.91 (2.81-3.02) |
Percentages reflect the proportions of women with incident hypertension, hyperlipidemia, and type 2 diabetes among those without the corresponding prevalent disease diagnosis.
The incidence rate of the primary outcome (composite of CAD, heart failure, aortic stenosis, mitral regurgitation, atrial fibrillation, ischemic stroke, peripheral artery disease, and venous thromboembolism) was 5.70/1000 woman-years for women without premature menopause, 8.78/1000 woman-years for natural premature menopause (rate difference, +3.08/1000 woman-years [95% CI, 2.06-4.10] vs women without premature menopause; P < .001), and 11.27 per 1000 woman-years for surgical premature menopause (rate difference, +5.57/1000 woman-years [95% CI, 2.41-8.73] vs women without premature menopause; P < .001).
In analyses of time-to-first cardiovascular diagnosis, both natural premature menopause (hazard ratio [HR], 1.36 [95% CI, 1.19-1.56]; P < .001) and surgical premature menopause (HR, 1.87 [95% CI, 1.36-2.58]; P < .001) were independently associated with incident cardiovascular disease after adjustment for age, race/ethnicity, prevalent type 2 diabetes, ever having smoked, systolic blood pressure, antihypertensive medication use, non–high-density lipoprotein cholesterol, cholesterol-lowering medication use, BMI, C-reactive protein, and ever use of MHT (Table 3). The proportional hazards assumption was satisfied in models for the primary outcome. Complete results for the fully adjusted model for the primary outcome are summarized in eTable 1 in Supplement 2. Results were robust to multiple sensitivity analyses, including models incorporating death as a competing risk, which are summarized in the eResults and eTables 2-11 in Supplement 2.
Table 3. Hazard Ratios for Incident Cardiovascular Disease Diagnoses Associated With Natural and Surgical Premature Menopause (ie, Menopause Before Age 40 Years).
| Sparsely Adjusted Modelsa | Fully Adjusted Modelsb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surgical Premature Menopause | Natural Premature Menopause | P Value for Heterogeneityd | Surgical Premature Menopause | Natural Premature Menopause | P Value for Heterogeneityd | |||||
| Hazard Ratio (95% CI) | P Valuec | Hazard Ratio (95% CI) | P Valuec | Hazard Ratio (95% CI) | P Valuec | Hazard Ratio (95% CI) | P Valuec | |||
| First cardiovascular disease diagnosise | 2.21 (1.66-2.92) | <.001 | 1.60 (1.42-1.80) | <.001 | .04 | 1.87 (1.36-2.58) | <.001 | 1.36 (1.19-1.56) | <.001 | .08 |
| Coronary artery disease | 3.76 (2.42-5.86) | <.001 | 1.81 (1.44-2.28) | <.001 | .004 | 2.52 (1.48-4.29) | <.001 | 1.39 (1.06-1.82) | .02 | .05 |
| Heart failure | 2.74 (1.42-5.29) | .003 | 1.56 (1.14-2.16) | .006 | .14 | 2.57 (1.21-5.47) | .01 | 1.21 (0.81-1.82) | .35 | .08 |
| Aortic stenosis | 3.41 (1.27-9.16) | .02 | 2.48 (1.62-3.80) | <.001 | .56 | 2.91 (0.92-9.15) | .06 | 2.37 (1.47-3.82) | <.001 | .75 |
| Mitral regurgitation | 3.40 (1.41-8.27) | .007 | 0.95 (0.52-1.74) | .87 | .02 | 4.13 (1.69-10.11) | .002 | 0.73 (0.34-1.55) | .41 | .004 |
| Atrial fibrillation | 1.87 (1.14-3.06) | .01 | 1.44 (1.18-1.77) | <.001 | .34 | 1.60 (0.91-2.83) | .11 | 1.25 (1.00-1.58) | .05 | .44 |
| Ischemic stroke | 1.18 (0.38-3.66) | .78 | 1.59 (1.12-2.28) | .01 | .62 | 0.43 (0.06-3.12) | .41 | 1.50 (1.01-2.25) | .04 | .23 |
| Peripheral artery disease | 2.19 (0.70-6.83) | .18 | 1.96 (1.27-3.03) | .002 | .86 | 1.34 (0.33-5.41) | .68 | 1.34 (0.79 -2.26) | .27 | .99 |
| Venous thromboembolism | 2.57 (1.41-4.67) | .002 | 1.68 (1.29-2.20) | <.001 | .20 | 2.73 (1.46-5.14) | .002 | 1.70 (1.27-2.29) | <.001 | .18 |
Sparsely adjusted models are adjusted for age at enrollment and race/ethnicity.
Fully adjusted models are adjusted for age, race/ethnicity, prevalent type 2 diabetes, ever having smoked, systolic blood pressure, use of antihypertensive medication, non–high-density lipoprotein cholesterol, use of cholesterol-lowering medication, body mass index, C-reactive protein, and history of menopausal hormone therapy use.
P values derived from Cox proportional hazards models.
Reflects comparison between hazards associated with natural vs surgical premature menopause using the statistical test of heterogeneity.
Comprised of coronary artery disease, heart failure, aortic stenosis, mitral regurgitation, atrial fibrillation, ischemic stroke, peripheral artery disease, and venous thromboembolism.
Secondary Outcomes
Incidence of Individual Cardiovascular Diseases
The proportional hazards assumption was satisfied in models for all incident cardiovascular diagnoses. After multivariable adjustment, natural premature menopause was independently associated with aortic stenosis (HR 2.37 [95% CI, 1.47-3.82]; P < .001), venous thromboembolism (HR, 1.70 [95% CI, 1.27-2.29]; P < .001), ischemic stroke (HR, 1.50 [95% CI, 1.01-2.25]; P = .04), CAD (HR, 1.39 [95% CI, 1.06-1.82]; P = .02), and atrial fibrillation (HR, 1.25 [95% CI, 1.00-1.58]; P = .05), but not with heart failure (HR, 1.21 [95% CI, 0.81-1.82]; P = .35), mitral regurgitation (HR, 0.73 [95% CI, 0.34-1.55] P = .41), or peripheral artery disease (HR, 1.34 [95% CI, 0.79-2.26]; P = .27) (Table 3).
After multivariable adjustment, surgical premature menopause was independently associated with mitral regurgitation (HR, 4.13 [95% CI, 1.69-10.11]; P = .002), venous thromboembolism (HR, 2.73 [95% CI, 1.46-5.14]; P = .002), heart failure (HR, 2.57 [95% CI, 1.21-5.47]; P = .01), and CAD (HR, 2.52 [95% CI, 1.48-4.29]; P < .001) (Table 3), but not with aortic stenosis (HR, 2.91 [95% CI, 0.92-9.15]; P = .06), atrial fibrillation (HR, 1.60 [95% CI, 0.91-2.83]; P = .11), ischemic stroke (HR, 0.43 [95% CI, 0.06-3.12]; P = .41), or peripheral artery disease (HR, 1.34 [95% CI, 0.33-5.41]; P = .68).
In post hoc models comparing the hazards associated with natural and surgical premature menopause, there was no significant difference between natural and surgical premature menopause for the primary outcome in fully adjusted models (Table 3).
Incorporation of MHT Use in Association Analyses
Associations of premature menopause with incident cardiovascular disease diagnoses remained similar after incorporating ever use of MHT, current MHT use, duration of MHT use, and delayed initiation of MHT 5 or more years after menopause (eTables 12 and 13 in Supplement 2). In a post hoc model to assess whether MHT use for 5 or more years mitigated cardiovascular risk associated with premature menopause, the HR for interaction of natural premature menopause and extended MHT use was 0.92 (95% CI, 0.61-1.37; P = .67), and the HR for interaction of surgical premature menopause and extended MHT use was 0.49 (95% CI, 0.21-1.14; P = .10).
Age at Menopause and Incident Cardiovascular Disease
After multivariable adjustment as above, younger age at menopause remained independently associated with time to first incident cardiovascular diagnosis (HR, 1.02/year of earlier age at menopause [95% CI, 1.01-1.03]; P < .001). Compared with menopause at age 50 years or older, the primary outcome hazard risk progressively increased with lower menopausal age thresholds (P for trend for natural premature menopause < .001, P for trend for surgical premature menopause = 0.03; eTable 14 in Supplement 2). Menopause at age 45 to 49 years, compared with at age 50 years or older, was associated with a modestly elevated hazard of incident CAD after multivariable adjustment (HR, 1.25 [95% CI, 1.08-1.46]; P = .004).
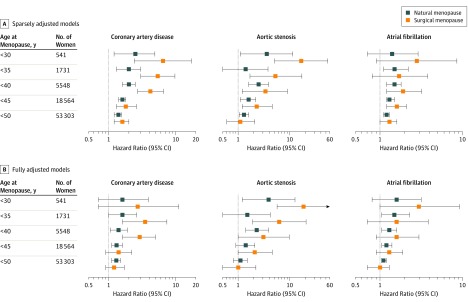
Compared with women experiencing menopause at age 50 years or older, an inverse dose-response relationship was observed between age at menopause and hazard of CAD (P for trend for natural premature menopause = .02, P for trend for surgical premature menopause = .004), aortic stenosis (P for trend for natural premature menopause = .04, P for trend for surgical premature menopause = .004), and, for natural premature menopause, atrial fibrillation (P for trend = .04), with the largest hazards observed among women with menopause before age 30 years (Figure 2; eTable 14 in Supplement 2). In fully adjusted models, large hazards for aortic stenosis were still observed among middle-aged women experiencing natural menopause before age 30 years (HR, 3.56 [95% CI, 1.09-11.63]; P = .03) and surgical menopause before age 30 years (HR, 17.93 [95% CI, 5.44-59.07]; P < .001).
Figure 2. Hazard Ratios and 95% CIs for Coronary Artery Disease, Aortic Stenosis, and Atrial Fibrillation Associated With Different Age at Menopause Thresholds.
Groups are inclusive of all women with age at menopause below the listed cutoff. The reference group for all models is menopause at age 50 years or older. Hazard ratios and 95% CIs are derived from Cox proportional hazard models. A, Sparsely adjusted models are adjusted for age and race/ethnicity. B, Fully adjusted models are adjusted for age, race/ethnicity, prevalent type 2 diabetes, ever having smoked, systolic blood pressure, use of antihypertensive medication, non–high-density lipoprotein cholesterol, use of cholesterol-lowering medication, body mass index, C-reactive protein, and history of menopausal hormone therapy use.
Incident Cardiovascular Risk Factors
The proportional hazards assumption was satisfied in Cox models for incident hypertension and hyperlipidemia. Age-stratified models were fitted in 5-year bins of age at enrollment to satisfy the proportional hazards assumption for type 2 diabetes.
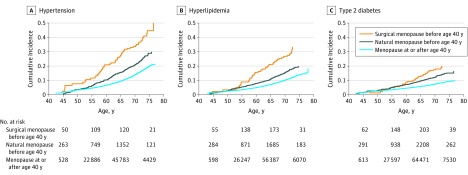
As shown in Figure 3, the incidence of hypertension, hyperlipidemia, and type 2 diabetes was greatest among women with history of surgical premature menopause and lowest among women without premature menopause. In models adjusted for age, race/ethnicity, BMI, and the prevalent hypertension, hyperlipidemia, and type 2 diabetes statuses not under consideration in each model, natural premature menopause was associated with an HR of 1.43 (95% CI, 1.24-1.65; P < .001) and surgical premature menopause with an HR of 1.93 (95% CI, 1.37-2.74; P < .001) for incident hypertension (P for heterogeneity = .11). For incident hyperlipidemia, natural premature menopause was associated with an HR of 1.36 (95% CI, 1.16-1.61; P < .001) and surgical premature menopause with an HR of 2.13 (95% CI, 1.50-3.04; P < .001, P for heterogeneity = .02). For incident type 2 diabetes, greater risk was observed for both premature menopause (HR range, 0.9-1.6 across age strata) and surgical premature menopause (HR range, 1.3-4.7 across age strata) (eTable 15 in Supplement 2). Results were robust to sensitivity analyses, summarized in the eResults and eTables 16-18 in Supplement 2.
Figure 3. Cumulative Incidence of Hypertension, Hyperlipidemia, and Type 2 Diabetes Among Women Without Each Condition at Enrollment.
Women enrolled in the UK Biobank across a 3-decade range of ages. These cumulative incidence graphs depict the probability of developing hypertension, hyperlipidemia, and type 2 diabetes in women followed up to a given age, depicted on the x-axis, among those without each prevalent condition at study enrollment. Women with prevalent hypertension, hyperlipidemia, and type 2 diabetes were excluded from the respective cumulative incidence graph. In addition, at enrollment, 33.9% of women with surgical premature menopause, 30.7% with natural premature menopause, and 27.1% without premature menopause had hypertension; 15.2% with surgical premature menopause, 16.5% with natural premature menopause, and 11.9% without premature menopause had hyperlipidemia; and 2.0% with surgical premature menopause, 2.7% with natural premature menopause, and 1.4% without premature menopause had type 2 diabetes.
Missing Data
In fully adjusted models for incident cardiovascular disease, 21.5% of women had 1 or more missing covariates, with no significant difference in missingness across groups (range, 21.5%-21.8% missingness across the 3 study groups; P = .80). Models using imputed data to replace missing covariates yielded similar results to the primary models (eTable 3 in Supplement 2). Further analyses of data missingness are provided in the eResults in Supplement 2.
Discussion
In a large cohort of postmenopausal women, natural and surgical menopause before age 40 years were associated with a modest but statistically significant increased risk for a composite of cardiovascular diseases, with persistent associations observed after adjustment for conventional cardiovascular risk factors. Although the HRs met statistical significance, absolute risk differences were small given low overall incidence of cardiovascular disease in the study cohort. In secondary analyses, premature menopause was associated with incident CAD, heart failure, aortic stenosis, atrial fibrillation, and venous thromboembolism, as well as incident hypertension, hyperlipidemia, and type 2 diabetes, with greater hazards observed at progressively younger ages at menopause.
The results of this study extend prior findings. In a meta-analysis of 32 studies including 310 000 women, Muka et al7 found elevated risk for CAD among women experiencing menopause at younger than age 45 years vs age 45 years and older but did not find a significant difference between menopause at age 45 to 49 years compared with at 50 years or older. In a meta-analysis of 190 000 women, Roeters van Lennep et al8 detected a significant hazard for ischemic heart disease following menopause at younger than age 40 years but found no difference in stroke risk. The findings of the present study align most closely with a more recent meta-analysis examining incident CAD and stroke vs age at natural menopause, which found significant hazards of CAD and stroke among women with natural menopause between ages 45 and 49 years and larger hazards with progressively earlier age at menopause.19 However, the present study extends these results by more comprehensively adjusting for conventional cardiovascular risk factors and observing persistent independent associations, by finding increased incidence of modifiable cardiovascular disease risk factors, and by testing associations with non-ASCVD cardiovascular diseases. These results may have implications for understanding and mitigating the long-term risk for cardiovascular disease associated with premature menopause.
First, history of premature menopause provides an opportunity for “primordial prevention”—targeted nonpharmacologic strategies aimed at reducing the risk of developing cardiovascular risk factors. In the Framingham Heart Study, higher premenopausal Framingham risk score was associated with earlier age at menopause,20 suggesting premature menopause may serve as a risk signal instead of causally influencing future cardiovascular risk.21 In the present study, however, a greater risk of acquiring cardiovascular risk factors was observed following premature menopause after adjusting for prevalent risk factors. Therefore, premature menopause may not be merely comorbid with conventional cardiovascular risk factors but may separately heighten the likelihood of developing these risk factors.5 By identifying women with premature menopause, allocating resources for lifestyle modification to prevent the onset of modifiable risk factors may yield meaningful clinical returns and requires future study. Additionally, although recent guidelines prompt clinicians to consider menopause to guide statin prescriptions only among women experiencing menopause before age 40,12,13 these results suggest premature menopause should be regarded as a risk continuum from age at onset of younger than 30 years through 45 to 49 years.
Second, the cardiovascular disease risk associated with premature menopause extended beyond ASCVD. Postmenopausal state is associated with increase in cytokines and oxidative stress,22 which may contribute to osteogenesis of valvular interstitial cells.23,24 However, whether this represents the mechanism for the association between premature menopause and aortic stenosis is not known. Furthermore, an association of premature menopause with conventional cardiovascular risk factors was observed, which may have a causal relationship with valvular heart disease as well as CAD according to recent genetic association studies.25 Prior smaller studies described nonsignificant associations between age at menopause and risk of atrial fibrillation, with conflicting directionality of association.26,27 By contrast, in this study, a modest but significant and dose-responsive association between earlier menopause and increased risk of incident atrial fibrillation was present. In addition, the observed risk of venous thromboembolism, independent of MHT use, was similar to findings from the Women’s Health Initiative.28
Third, form of menopause may be associated with differential cardiovascular risk. Although significant differences in risk between natural vs surgical premature menopause did not persist in fully adjusted models, risk differences may stem from differential development of conventional cardiovascular risk factors, and the possibility of reduced statistical power cannot be ruled out. Prior studies suggested that preoperative cardiovascular risk profile dictates future cardiovascular risk in women undergoing oophorectomy,29,30 but baseline cardiovascular risk profiles between natural and surgical premature menopause groups in this study were similar. Although women with surgical menopause had higher rates and longer duration of MHT use, effect estimates were unchanged after adjustment for MHT use. In data collected between 1998 and 2011, rates of elective bilateral oophorectomy at the time of hysterectomy in the United States were declining, but more than one-third of women undergoing hysterectomy still underwent elective bilateral oophorectomy.31,32 The findings of the present study may inform cardiovascular disease risk discussions and surveillance when bilateral oophorectomy is considered, eg, for women with inherited predisposition to ovarian cancer such as BRCA gene mutation carriers.
Limitations
This study has several limitations. First, age at menopause was self-reported and ascertained years after menopause. Women were prompted to indicate when they did not know this information to minimize the risk of misclassification, although 20% of age-eligible women were excluded as a result. Although further residual misclassification is possible, this would be expected to diminish the magnitude of observed associations and bias results toward the null.
Second, data regarding indications for prior bilateral oophorectomy were not available to help clarify risk mechanisms. Sensitivity analyses suggested that observed associations were not driven by cardiovascular risks associated with cancer survivorship.
Third, although the UK Biobank is very large and well-phenotyped, a “healthy participant” selection bias has been noted.33 Similarly, because women were recruited over a 30-year range of ages and women with prevalent cardiovascular diseases were excluded, the sample may be biased toward healthier individuals. While this may underestimate associations, the study’s aim was to assess cardiovascular disease risk among asymptomatic middle-aged women without established cardiovascular disease, in accordance with current guidelines.
Fourth, while data on specific MHT preparations and doses used by participants were not available, multiple analyses argued against a significant mediating role of MHT in observed associations, which is consistent with the findings of 2 contemporary studies.7,10 In addition, because the study sample was more than 95% white, whether the findings generalize to other racial/ethnic groups requires further study.
Conclusions
Natural and surgical premature menopause (before age 40 years) were associated with a small but statistically significant increased risk for a composite of cardiovascular diseases among postmenopausal women. Further research is needed to understand the mechanisms underlying these associations.
Statistical Analysis Plan
eResults
eTable 1. Complete Results of Fully Adjusted Cox Proportional Hazards Model for Time-to-First Cardiovascular Diagnosis (Primary Outcome)
eTable 2. Sensitivity Analysis: Sub-distribution Hazards for Incident Cardiovascular Disease Derived From Fine-Gray Competing Risk Regression Models, Incorporating Noncardiovascular Death as a Competing Risk
eTable 3. Sensitivity Analysis: Hazard Ratios for Incident Cardiovascular Diagnoses in Postmenopausal Women, With and Without Imputed Data to Replace Missing Covariates in Fully Adjusted Models
eTable 4. Sensitivity Analysis: Hazard Ratios for Incident Cardiovascular Diagnoses in Postmenopausal Women With History of Premature Natural and Surgical Menopause (< 40 Years), Eliminating 772 Women With Incident Heart Failure and 46 Women With Missing Heart Failure Data
eTable 5. Sensitivity Analysis: Hazard Ratios for Incident Cardiovascular Diagnoses in Postmenopausal Women With History of Premature Natural and Surgical Menopause (< 40 Years), Eliminating 15,017 Women With Any Prevalent Cancer at Enrollment and 436 Women With Missing Data on Prevalent Cancer Diagnosis at Enrollment
eTable 6. Sensitivity Analysis: Hazard Ratios for Incident Cardiovascular Diagnoses in Postmenopausal Women With History of Premature Natural and Surgical Menopause (< 40 years), Eliminating 39,281 Women With >1 Year of Menopausal Hormone Therapy Use, 9,456 With Current Menopausal Hormone Therapy Use at Study Enrollment, 4,441 Women With Missing Data on Duration of Prior Menopausal Hormone Therapy Use, and 345 Women With Missing Data on Ever-Use of Menopausal Hormone Therapy
eTable 7. Sensitivity Analysis: Hazards of Incident Cardiovascular Disease Diagnoses Associated With Natural Premature Menopause (i.e., Menopause Before Age 40), Comparing Model Results With and Without Inclusion of 2,524 Women With Prior Hysterectomy in the Group With Natural Premature Menopause
eTable 8. Sensitivity Analysis: Hazard Ratios for Incident Cardiovascular Diagnoses, Stratified by Body Mass Index at Study Enrollment
eTable 9. Sensitivity Analysis: Hazard Ratios for Incident Cardiovascular Diagnoses in Postmenopausal Women Who Were ≥55 Years Old at Study Enrollment
eTable 10. Sensitivity Analysis: Hazard Ratios for Incident Cardiovascular Diagnoses in Postmenopausal Women Who Used Menopausal Hormone Therapy for ≥5 Years Prior to Study Enrollment, Not Including Women Using Menopausal Hormone Therapy at Study Enrollment
eTable 11. Hazard Ratios for Incident Cardiovascular Diagnoses in Postmenopausal Women Compared to a Reference Group of Women Who Were Premenopausal at Study Enrollment
eTable 12. Sensitivity Analysis: Comparison of Models With and Without Adjustment for Ever Use of Menopausal Hormone Therapy
eTable 13. Associations of Use of Menopausal Hormone Therapy With Incident Cardiovascular Outcomes
eTable 14. Hazard Ratios Associated With Different Menopausal Age Thresholds for First Incident Cardiovascular Diagnosis and Eight Separate Cardiovascular Disease Diagnoses, Compared With a Reference Group of Women With Menopause ≥ 50 Years
eTable 15. Incident Development of Hypertension, Hyperlipidemia, and Type 2 Diabetes, Stratified by Age at Study Enrollment
eTable 16. Sensitivity Analysis: Subdistribution Hazards for Incident Hypertension, Hyperlipidemia, and Type 2 Diabetes From Fine-Gray Competing Risk Regression Models, Incorporating Death as a Competing Risk
eTable 17. Sensitivity Analysis: Incident Development of Cardiovascular Risk Factors, Excluding 5,756 Women With Any Incident Cardiovascular Disease Diagnosis
eTable 18. Hazards of Incident Hypertension, Hyperlipidemia, and Type 2 Diabetes by Age at Menopause Compared With Women With Menopause ≥ Age 50
eAppendix. ICD Codes Used to Identify Women With Congenital Heart Disease and to Ascertain Diagnoses of Cardiovascular Risk Factors and Cardiovascular Diseases
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. ; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics 2016 update: a report from the American Heart Association. Circulation. 2016;133(4):2411-2421. doi: 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 2.Bairey Merz CN, Andersen H, Sprague E, et al. Knowledge, attitudes, and beliefs regarding cardiovascular disease in women: the Women’s Heart Alliance. J Am Coll Cardiol. 2017;70(2):123-132. doi: 10.1016/j.jacc.2017.05.024 [DOI] [PubMed] [Google Scholar]
- 3.Zhu D, Chung HF, Pandeya N, et al. Body mass index and age at natural menopause: an international pooled analysis of 11 prospective studies. Eur J Epidemiol. 2018;33(8):699-710. doi: 10.1007/s10654-018-0367-y [DOI] [PubMed] [Google Scholar]
- 4.Shifren JL, Gass ML; NAMS Recommendations for Clinical Care of Midlife Women Working Group . The North American Menopause Society recommendations for clinical care of midlife women. Menopause. 2014;21(10):1038-1062. doi: 10.1097/GME.0000000000000319 [DOI] [PubMed] [Google Scholar]
- 5.Manson JE, Woodruff TK. Reproductive health as a marker of subsequent cardiovascular disease: the role of estrogen. JAMA Cardiol. 2016;1(7):776-777. doi: 10.1001/jamacardio.2016.2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velez MP, Alvarado BE, Rosendaal N, et al. Age at natural menopause and physical functioning in postmenopausal women: the Canadian Longitudinal Study on Aging. Menopause. 2019;26(9):958-965. doi: 10.1097/GME.0000000000001362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muka T, Oliver-Williams C, Kunutsor S, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016;1(7):767-776. doi: 10.1001/jamacardio.2016.2415 [DOI] [PubMed] [Google Scholar]
- 8.Roeters van Lennep JE, Heida KY, Bots ML, Hoek A; collaborators of the Dutch Multidisciplinary Guideline Development Group on Cardiovascular Risk Management after Reproductive Disorders . Cardiovascular disease risk in women with premature ovarian insufficiency: a systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23(2):178-186. doi: 10.1177/2047487314556004 [DOI] [PubMed] [Google Scholar]
- 9.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause. 2012;19(10):1081-1087. doi: 10.1097/gme.0b013e3182517bd0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley SH, Li Y, Tobias DK, et al. Duration of reproductive life span, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J Am Heart Assoc. 2017;6(11):e006713. doi: 10.1161/JAHA.117.006713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall PS, Nah G, Howard BV, et al. Reproductive factors and incidence of heart failure hospitalization in the Women’s Health Initiative. J Am Coll Cardiol. 2017;69(20):2517-2526. doi: 10.1016/j.jacc.2017.03.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285-e350. doi: 10.1016/j.jacc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 13.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dam V, van der Schouw YT, Onland-Moret NC, et al. Association of menopausal characteristics and risk of coronary heart disease: a pan-European case-cohort analysis. Int J Epidemiol. 2019;48(4):1275-1285. doi: 10.1093/ije/dyz016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203-209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klarin D, Zhu QM, Emdin CA, et al. ; CARDIoGRAMplusC4D Consortium . Genetic analysis in UK Biobank links insulin resistance and transendothelial migration pathways to coronary artery disease. Nat Genet. 2017;49(9):1392-1397. doi: 10.1038/ng.3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50(9):1219-1224. doi: 10.1038/s41588-018-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu D, Chung HF, Dobson AJ, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. 2019;4(11):e553-e564. doi: 10.1016/S2468-2667(19)30155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kok HS, van Asselt KM, van der Schouw YT, et al. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47(10):1976-1983. doi: 10.1016/j.jacc.2005.12.066 [DOI] [PubMed] [Google Scholar]
- 21.Scott NS. Understanding hormones, menopause, and heart failure: still a work in progress. J Am Coll Cardiol. 2017;69(20):2527-2529. doi: 10.1016/j.jacc.2017.03.561 [DOI] [PubMed] [Google Scholar]
- 22.Paik JK, Kim JY, Kim OY, et al. Circulating and PBMC Lp-PLA2 associate differently with oxidative stress and subclinical inflammation in nonobese women (menopausal status). PLoS One. 2012;7(2):e29675. doi: 10.1371/journal.pone.0029675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Peña-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52(10):843-850. doi: 10.1016/j.jacc.2008.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Branchetti E, Sainger R, Poggio P, et al. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler Thromb Vasc Biol. 2013;33(2):e66-e74. doi: 10.1161/ATVBAHA.112.300177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nazarzadeh M, Pinho-Gomes AC, Smith Byrne K, et al. Systolic blood pressure and risk of valvular heart disease: a mendelian randomization study. JAMA Cardiol. 2019;4(8):788-795. doi: 10.1001/jamacardio.2019.2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong JA, Rexrode KM, Sandhu RK, Moorthy MV, Conen D, Albert CM. Menopausal age, postmenopausal hormone therapy and incident atrial fibrillation. Heart. 2017;103(24):1954-1961. doi: 10.1136/heartjnl-2016-311002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magnani JW, Moser CB, Murabito JM, et al. Age of natural menopause and atrial fibrillation: the Framingham Heart Study. Am Heart J. 2012;163(4):729-734. doi: 10.1016/j.ahj.2012.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canonico M, Plu-Bureau G, O’Sullivan MJ, et al. Age at menopause, reproductive history, and venous thromboembolism risk among postmenopausal women: the Women’s Health Initiative hormone therapy clinical trials. Menopause. 2014;21(3):214-220. doi: 10.1097/GME.0b013e31829752e0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howard BV, Kuller L, Langer R, et al. ; Women’s Health Initiative . Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women’s Health Initiative observational study. Circulation. 2005;111(12):1462-1470. doi: 10.1161/01.CIR.0000159344.21672.FD [DOI] [PubMed] [Google Scholar]
- 30.Appiah D, Schreiner PJ, Bower JK, Sternfeld B, Lewis CE, Wellons MF. Is surgical menopause associated with future levels of cardiovascular risk factor independent of antecedent levels? the CARDIA study. Am J Epidemiol. 2015;182(12):991-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asante A, Whiteman MK, Kulkarni A, Cox S, Marchbanks PA, Jamieson DJ. Elective oophorectomy in the United States: trends and in-hospital complications, 1998-2006. Obstet Gynecol. 2010;116(5):1088-1095. doi: 10.1097/AOG.0b013e3181f5ec9d [DOI] [PubMed] [Google Scholar]
- 32.Mahal AS, Rhoads KF, Elliott CS, Sokol ER. Inappropriate oophorectomy at time of benign premenopausal hysterectomy. Menopause. 2017;24(8):947-953. doi: 10.1097/GME.0000000000000875 [DOI] [PubMed] [Google Scholar]
- 33.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026-1034. doi: 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Analysis Plan
eResults
eTable 1. Complete Results of Fully Adjusted Cox Proportional Hazards Model for Time-to-First Cardiovascular Diagnosis (Primary Outcome)
eTable 2. Sensitivity Analysis: Sub-distribution Hazards for Incident Cardiovascular Disease Derived From Fine-Gray Competing Risk Regression Models, Incorporating Noncardiovascular Death as a Competing Risk
eTable 3. Sensitivity Analysis: Hazard Ratios for Incident Cardiovascular Diagnoses in Postmenopausal Women, With and Without Imputed Data to Replace Missing Covariates in Fully Adjusted Models
eTable 4. Sensitivity Analysis: Hazard Ratios for Incident Cardiovascular Diagnoses in Postmenopausal Women With History of Premature Natural and Surgical Menopause (< 40 Years), Eliminating 772 Women With Incident Heart Failure and 46 Women With Missing Heart Failure Data
eTable 5. Sensitivity Analysis: Hazard Ratios for Incident Cardiovascular Diagnoses in Postmenopausal Women With History of Premature Natural and Surgical Menopause (< 40 Years), Eliminating 15,017 Women With Any Prevalent Cancer at Enrollment and 436 Women With Missing Data on Prevalent Cancer Diagnosis at Enrollment
eTable 6. Sensitivity Analysis: Hazard Ratios for Incident Cardiovascular Diagnoses in Postmenopausal Women With History of Premature Natural and Surgical Menopause (< 40 years), Eliminating 39,281 Women With >1 Year of Menopausal Hormone Therapy Use, 9,456 With Current Menopausal Hormone Therapy Use at Study Enrollment, 4,441 Women With Missing Data on Duration of Prior Menopausal Hormone Therapy Use, and 345 Women With Missing Data on Ever-Use of Menopausal Hormone Therapy
eTable 7. Sensitivity Analysis: Hazards of Incident Cardiovascular Disease Diagnoses Associated With Natural Premature Menopause (i.e., Menopause Before Age 40), Comparing Model Results With and Without Inclusion of 2,524 Women With Prior Hysterectomy in the Group With Natural Premature Menopause
eTable 8. Sensitivity Analysis: Hazard Ratios for Incident Cardiovascular Diagnoses, Stratified by Body Mass Index at Study Enrollment
eTable 9. Sensitivity Analysis: Hazard Ratios for Incident Cardiovascular Diagnoses in Postmenopausal Women Who Were ≥55 Years Old at Study Enrollment
eTable 10. Sensitivity Analysis: Hazard Ratios for Incident Cardiovascular Diagnoses in Postmenopausal Women Who Used Menopausal Hormone Therapy for ≥5 Years Prior to Study Enrollment, Not Including Women Using Menopausal Hormone Therapy at Study Enrollment
eTable 11. Hazard Ratios for Incident Cardiovascular Diagnoses in Postmenopausal Women Compared to a Reference Group of Women Who Were Premenopausal at Study Enrollment
eTable 12. Sensitivity Analysis: Comparison of Models With and Without Adjustment for Ever Use of Menopausal Hormone Therapy
eTable 13. Associations of Use of Menopausal Hormone Therapy With Incident Cardiovascular Outcomes
eTable 14. Hazard Ratios Associated With Different Menopausal Age Thresholds for First Incident Cardiovascular Diagnosis and Eight Separate Cardiovascular Disease Diagnoses, Compared With a Reference Group of Women With Menopause ≥ 50 Years
eTable 15. Incident Development of Hypertension, Hyperlipidemia, and Type 2 Diabetes, Stratified by Age at Study Enrollment
eTable 16. Sensitivity Analysis: Subdistribution Hazards for Incident Hypertension, Hyperlipidemia, and Type 2 Diabetes From Fine-Gray Competing Risk Regression Models, Incorporating Death as a Competing Risk
eTable 17. Sensitivity Analysis: Incident Development of Cardiovascular Risk Factors, Excluding 5,756 Women With Any Incident Cardiovascular Disease Diagnosis
eTable 18. Hazards of Incident Hypertension, Hyperlipidemia, and Type 2 Diabetes by Age at Menopause Compared With Women With Menopause ≥ Age 50
eAppendix. ICD Codes Used to Identify Women With Congenital Heart Disease and to Ascertain Diagnoses of Cardiovascular Risk Factors and Cardiovascular Diseases