Summary
Children with hematologic malignancies and hematopoietic stem cell transplant recipients are at high risk for invasive fungal diseases (IFD). There have been an increased number of at-risk children over the past two decades due to improvements in cancer therapies resulting in improved survival of children with high-risk and refractory malignancies. The predominant organisms that cause IFD include Candida spp., Aspergillus spp., and the Mucorales molds. Clinical presentations of IFD vary based on host immune status and the causative organism. Though serum biomarkers such as the galactomannan assay and beta-D-glucan assay have been validated in adults, there are limited data regarding their diagnostic value in children. Thus, the gold standard for IFD diagnosis remains tissue biopsy with histopathological and microbiological evaluation. Treatment of IFD is multimodal and involves anti-fungal drugs, correction of immune dysfunction, and surgical resection when feasible. Pediatric practice regarding IFD is largely extrapolated from data generated in adult patients; in this review we evaluate both primary pediatric studies and guidelines intended for adult patients that are applied to pediatric patients. There remain significant knowledge gaps with respect to the prevention, diagnosis, and treatment of IFD in immunocompromised children, and further research is needed to help guide management decisions.
Keywords: immunocompromised, invasive fungal disease, hematologic malignancies, pediatric, anti-fungal prophylaxis
Introduction
Children with hematologic malignancies and hematopoietic stem cell transplantation (HSCT) recipients are at high risk for infections with a wide range of pathogens due to their immunocompromised status. Specifically, profound neutropenia and severe T-cell defects predispose pediatric hosts to infections with opportunistic fungi, termed invasive fungal diseases (IFD). The leading causes of IFD in children for the past several decades have been yeasts such as Candida species, and molds including Aspergillus species and the Mucorales family (Pana, et al 2017, Steinbach, et al 2012, Wattier, et al 2015, Zaoutis, et al 2005).
IFD are associated with significant morbidity and mortality (Zaoutis, et al 2005) though epidemiologic quantification of incidence is complex. New advances in cancer therapy and supportive care for children undergoing chemotherapy or HSCT have resulted in an increased number of children at risk for IFD (Hale, et al 2010). The advent of improved fungal diagnostic tests have additionally altered the epidemiology of reported IFD (Pana, et al 2017). Institutional variation in diagnostic and supportive care practices impact the incidence of IFD (Groll, et al 2014, Lehrnbecher, et al 2009). Furthermore, recently revised definitions of invasive fungal disease and inconsistencies in the diagnostic criteria make assessment of IFD prevalence difficult (De Pauw, et al 2008, Groll, et al 2014, Pana, et al 2017). Epidemiologic efforts are additionally complicated by the variety of disease phenotypes that comprise IFD, which are dependent upon both pathogen and host immune dysfunction (King, et al 2017).
Symptoms of IFD are more indolent than those of invasive bacterial or viral infections. Pathogens that cause IFD share the potential for hematogenous dissemination of organism resulting in infection of distant tissues. Widely disseminated IFD is extremely difficult to cure in severely immunocompromised patients. Thus, timely diagnosis and treatment of IFD is essential for limiting morbidity and improving survival. However, fungal organisms can be difficult to culture in microbiology laboratories. Adjunctive radiographic imaging, non-culture assays, and serum biomarkers can amplify diagnostic yield (Groll, et al 2014, Huppler, et al 2017, Katragkou, et al 2017). These diagnostic strategies are used to determine the extent of disease dissemination and monitor IFD throughout treatment. Empiric anti-fungal treatment should be initiated promptly when there is suspicion for IFD in a severely immunocompromised patient. However, not all antifungal agents are approved for use in children, and there are limited data regarding appropriate dosing of newer antifungals for various pediatric age groups. Much of pediatric practice is extrapolated from data generated from adult patients.
In this review article, we discuss the risk factors for IFD in children with hematologic malignancies and allogeneic HSCT recipients. We review the epidemiology and clinical presentation of the most common IFD and assess current diagnostic testing methods and treatment regimens, as well as the use of antifungal prophylaxis and environmental control procedures to prevent these infections. Lastly, we identify future directions in the diagnosis, treatment, and prevention of IFD in pediatric patients that will be important subjects of clinical research.
Children at Risk for Invasive Fungal Disease
Risk of IFD is largely mediated by host immune status, though it is also dependent on infectious exposure. IFD are truly opportunistic infections and therefore are extremely rare in patients without significantly impaired immunity. Phagocyte activity and the development of T-cell responses are critical components of the host immune response to fungal infections. Thus, children with hematologic malignancies and allogeneic HSCT recipients carry a particularly high risk for IFD (Barnes and Marr 2007, Fisher, et al 2018).
Acute Leukemia
The treatment of acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) involves recurrent cycles of chemotherapy in order to achieve and maintain disease remission. Acute leukemia is risk-stratified by likelihood of durable remission, and high-risk disease is treated with more intensive chemotherapy. Thus, children with high-risk leukemia undergo periods of profound, prolonged immunosuppression, and specifically myelosuppression, and are therefore at high risk for opportunistic infections (Castagnola, et al 2006, Sung, et al 2009).
Severe neutropenia secondary to intensive chemotherapy has been associated with IFD (Fisher, et al 2018). A multicenter retrospective study of children with AML reported that absolute neutrophil count (ANC) ≤500 cells/ul at the start of a chemotherapy cycle increased the probability of IFD three-fold (Johnston, et al 2013). The exact duration of neutropenia at which risk increases is unknown, but prolonged neutropenia certainly portends an increased risk of IFD (Johnston, et al 2013).
Allogeneic Hematopoietic Stem Cell Transplant
Allogeneic HSCT is the standard of care for many children with hematologic disorders and primary immunodeficiencies. HSCT recipients undergo several phases of immune dysfunction that mediate risk for IFD including pre-engraftment, post-engraftment, and development of graft versus host disease (GVHD). Various host factors and chemotherapeutic agents used during HSCT also impact risk for IFD.
Pre-Engraftment.
Myeloablative conditioning delivered prior to HSCT results in transient bone marrow aplasia. The major IFD risk factor during the pre-engraftment period is prolonged, profound neutropenia. The expected duration of neutropenia following conditioning is dependent upon several factors, including the source and dose of stem cells, but generally lasts several weeks prior to engraftment. During the pre-engraftment period, patients are at significant risk for infectious complications, including IFD (Srinivasan, et al 2013). Similar to patients with leukemia, longer durations of neutropenia increase the risk of IFD.
Post-Engraftment.
After neutrophil engraftment occurs and neutropenia resolves, the risk for IFD is largely mediated by T-cell dysfunction, which persists for months following HSCT. Patients may also receive prophylactic T-cell-suppressive therapy or undergo allograft T-cell depletion to decrease the risk of graft-versus-host disease (GVHD), which may prolong the duration of T-cell dysfunction.
GVHD.
The risk for IFD is increased in patients with GVHD due to both T-cell dysfunction inherent to the disease and T-cell suppressive therapies used to quell GVHD (Barnes and Marr 2007). Acute GVHD, particularly high-grade GVHD, has been reported as a potential risk factor for IFD in several single-center studies (Hale, et al 2010, Hovi, et al 2000, Srinivasan, et al 2013), as well as one retrospective, multicenter study (Hazar, et al 2019). Similarly, chronic GVHD has been identified as a risk factor for IFD (Castagnola, et al 2018, Hale, et al 2010, Hovi, et al 2000, Kobayashi, et al 2007, Srinivasan, et al 2013). Data are limited with respect to specific therapies for GVHD, however calcineurin inhibitors alone are rarely associated with IFD.
Steroid exposure.
Though any exposure to steroids increases risk of IFD, high-dose steroid treatment (as is commonly used for GVHD) has been described as a risk factor for the development of IFD in several single-center studies (Dvorak, et al 2005, Hol, et al 2014, Hovi, et al 2000, Kobayashi, et al 2007). Prolonged duration (>10 days) of steroid therapy, was also reported to increase risk of IFD (Dvorak, et al 2005). Similar findings have been noted in patients with leukemia (Johnston, et al 2013). However, variations in prescribing among centers limit conclusive quantifications of either dose or duration of steroid therapy that significantly increases risk for IFD (Fisher, et al 2018).
Autologous Hematopoietic Stem Cell Transplantation
Autologous HSCT is utilized for treatment of pediatric cancers including neuroblastoma, central nervous system tumors, and lymphoma. Autologous HSCT enables administration of high doses of chemotherapy to achieve increased tumor penetration by providing stem-cell rescue. Patients who undergo autologous HSCT experience a period of profound neutropenia following conditioning. However, several factors ensure a decreased risk for IFD in comparison to allogeneic HSCT recipients. First, neutrophil engraftment is relatively rapid, usually less than 14 days. Additionally, autologous HSCT has no risk of GVHD and thus no need for immunosuppression or T cell depletion, resulting in minimal T cell dysfunction.
Epidemiology of IFD in Children with Hematologic Malignancy and HSCT Recipients
Defining the incidence of IFD in immunocompromised children is difficult due to institutional practice variations, including the use of antifungal prophylaxis (Lehrnbecher, et al 2009) and reporting of adverse events (Miller, et al 2016). Much of the available epidemiological data are from single center studies (Georgiadou, et al 2012, Hale, et al 2010, Mor, et al 2011) or those focusing specifically on candidiasis or mold infections (Steinbach, et al 2012, Wattier, et al 2015). Additionally, inconsistent use of standardized diagnostic criteria for IFD (De Pauw, et al 2008) makes comparison between studies difficult (Pana, et al 2017).
While the incidence of IFD is low in ALL cohorts (~1%) (Afzal, et al 2009, Inaba, et al 2017), fungal infections have an outsized contribution to infection-related mortality in children with ALL, up to 20% in some reports (O’Connor, et al 2014). IFD during ALL induction therapy has been reported to account for >70% of infection-related mortality (Rubnitz, et al 2004). Rates of IFD in AML cohorts are consistently higher, with a reported incidence of 12–20% (Johnston, et al 2013, Sung, et al 2007a) and accounting for up to 60% of infection-related deaths (Sung, et al 2007a). However, a recent multi-center study in Germany only identified IFD in 3% of AML patients, possibly reflecting the impact of more contemporary supportive care practices in which a majority of patients received antifungal prophylaxis (Bochennek, et al 2016).
Most studies of allogeneic HSCT have reported IFD in ~12–16% of patients (Dvorak, et al 2005, Gomez, et al 2018, Hol, et al 2014, Hovi, et al 2000, Simms-Waldrip, et al 2015). A majority of cases (42–54%) occur in the first 30 days after transplantation, prior to neutrophil engraftment (Castagnola, et al 2006, Cesaro, et al 2017, Hazar, et al 2019). Another large proportion of IFD episodes occur >100 days post-transplant, during which time patients have developed GVHD and consequent T-cell dysfunction (Castagnola, et al 2006, Cesaro, et al 2017, Hazar, et al 2019, Hovi, et al 2000). Autologous transplants have much lower rates of IFD, with the vast majority of infections occurring in the pre-engraftment period (Cesaro, et al 2017, Hovi, et al 2000). A large, multicenter, retrospective study recently reported a one-year cumulative incidence of proven or probable IFD of 0.7% after autologous HSCT (Cesaro, et al 2017). IFD is associated with a significant increase in all-cause mortality among pediatric HSCT recipients (Dvorak, et al 2005), with multiple studies reporting case fatality rates >60% (Gomez, et al 2018, Hovi, et al 2000, Simms-Waldrip, et al 2015).
The microbiology of IFD in children is incompletely reported and available data are primarily derived from single-center studies. Yeast are the predominate pathogens identified in IFD cases in children with hematologic malignancies and HSCT recipients (Hale, et al 2010). Multicenter, prospective studies of candidemia in all pediatric patients, including immunocompetent children, identified Candida albicans is the most commonly reported species isolated (Palazzi, et al 2014, Steinbach, et al 2012). However, non-albicans Candida species occurred more frequently overall than Candida albicans (Palazzi, et al 2014). This is consistent with studies of children with cancer (Castagnola, et al 2006). The majority of invasive mold infections are caused by Aspergillus spp.; (Wattier, et al 2015). The Mucorales molds are the next most commonly reported cause of IFD (Pana, et al 2017) (Otto, et al 2019, Zaoutis, et al 2007). The epidemiology of IFD with other yeasts and molds is not well-defined, though Bartlett and colleagues recently identified non-Candida yeasts in nearly 5% of cases and non-Aspergillus, non-Mucorales molds in >20% of cases (Bartlett, et al 2018).
Clinical Presentation
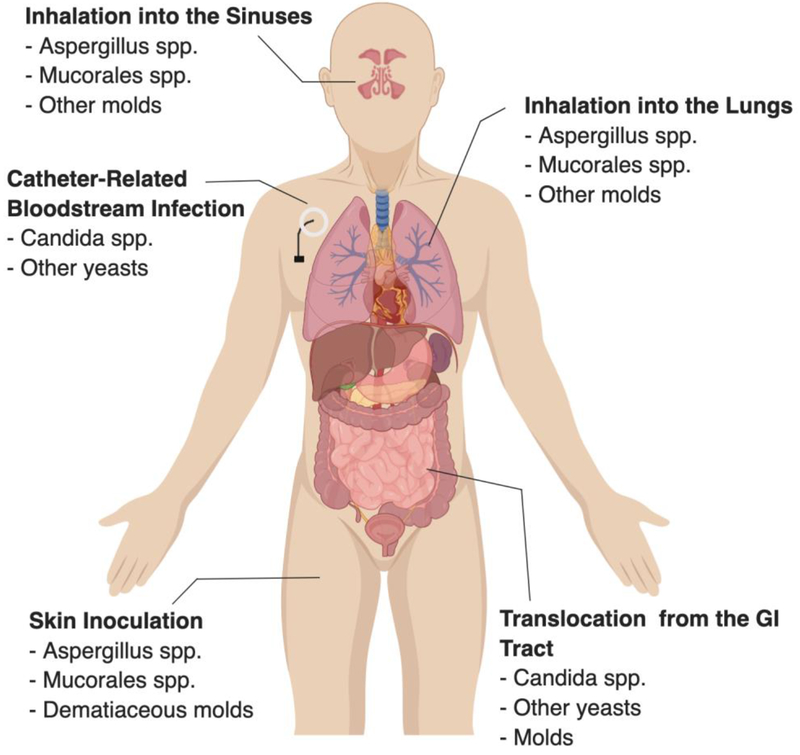
Invasive fungal disease should be considered in any immunocompromised child with prolonged fever (Mor, et al 2011, Musial, et al 1988). Frequently, patients with IFD present with persistent fever despite broad-spectrum antibacterial treatment. The clinical manifestations of IFD are varied and depend on both the causative organism (Table 1) and the mode of acquisition of infection (Figure 1).
Table 1.
Clinical Manifestations, Diagnosis, and Initial Treatment of Invasive Fungal Disease
| Organism | Invasive fungal disease | Diagnostic testing | Treatmenta,b | Common species | |
|---|---|---|---|---|---|
| Yeast | Candida spp. | Bloodstream infection | • Blood culture • Diagnostic imaging to assess for dissemination • Ophthalmologic examc |
• Echinocandin for total of 14 days after clearance of candidemiad • Removal of central venous catheter should be considered |
|
| Disseminated disease | • Blood culture • Diagnostic imaging • Ophthalmologic examc • Tissue biopsy for culture and histopathology |
• Choice of therapy depends on the site of infection • Duration of treatment dependent on treatment of underlying condition • Ocular infections: triazolese • CNS infections: liposomal AmB |
C. albicans C. parapsilosis C. tropicalis C. glabrataf |
||
| Hepatosplenic candidiasis | • Blood culture, • Liver/spleen imaging (US and/or CT) • Tissue biopsy for culture and histopathology |
• Echinocandin or liposomal AmB • Consider corticosteroid therapyg |
|||
| Trichosporon spp. | Disseminated disease | • Blood culture • Tissue biopsy for culture and histopathology |
• Voriconazole +/− liposomal AmB • Removal of central venous catheter should be considered |
T. asahii T. mucoides |
|
| Cryptococcus spp. | Pulmonary disease Bloodstream infection Meningoencephalitis | • Blood culture • Lumbar puncture for CSF analysis and culture • Cryptococcal antigen in serum and CSF • Tissue biopsy for culture and histopathology |
• CNS infections: liposomal AmB plus flucytosine used for induction, then maintenance therapy with fluconazole • Extraneural infections: fluconazole |
C. neoformans C. gattii |
|
| Molds | Aspergillus spp. | Isolated cutaneous nodule | • Serum galactomannan • Serum β-D-glucan • Diagnostic imaging • Tissue biopsy for culture, histopathology, and molecular testing |
• Voriconazole • Surgical resection |
A. fumigatus A. flavus A. terreus A. nigerf A. lentulusf |
| Disseminated or organ-invasive disease | • Serum galactomannan • Serum β-D-glucan • Diagnostic imaging • Tissue biopsy for culture, histopathology, and molecular testing • Bronchoalveolar lavage for culture and galactomannan testing |
• Voriconazole • Surgical resection, if localized disease • Consider G-CSF and/or granulocyte transfusionsh |
|||
| • Fusarium spp. | • Cutaneous disease • Pulmonary disease • Diseminated disease |
• Tissue biopsy for culture, histopathology, and molecular testing • Diagnostic imaging |
Voriconazole • Surgical resection, if localized disease |
F. solani species complex | |
| Mucorales order | Disseminated or organ-invasive disease | • Similar to diagnostic workup for invasive aspergillosisi | • Liposomal AmB • Surgical resection, if localized disease • Consider G-CSF and/or granulocyte transfusionsh |
Rhizopus spp. Mucor spp. Lichthiemia spp. Cunninghamella spp. |
|
Definitive therapy should be tailored based upon final organism identification and susceptibility testing
In addition to antifungal therapy, every attempt at immune reconstitution should be made
For neutropenic patients, eye examination should be done after recovery from neutropenia
Step-down therapy using a triazole antifungal can be performed for clinically stable patients with susceptible isolates and clearance of candidemia
For ocular candidiasis, intravitreal injection of AmB deoxycholate or voriconazole is recommended
Possess intrinsic resistance to triazole antifungals
Short-term treatment with corticosteroids can be considered for patients with persistent high fevers
For patients with potential for neutrophil recovery
Galactomannan and 1,3-β-D-glucan assays are not useful for detecting Mucorales molds
Figure 1.
Common modes of entry for common pathogens seen in invasive fungal disease.
Invasive Candidiasis
Invasive candidiasis should be included on the differential diagnosis for any immunocompromised child with fever or concern for sepsis (King, et al 2017). Generally, invasive candidiasis is conceptualized as one of three conditions: candidemia, fungemia with dissemination, or hepatosplenic candidiasis. Invasive candidiasis is most commonly identified after growth of yeast on blood cultures (Steinbach, et al 2012). Yeast enters the bloodstream after disruption of the gastrointestinal mucosa in neutropenic patients (Nucci and Anaissie 2001). However, acquisition of a bloodstream infection can also occur via a central venous catheter. In contrast to angioinvasive molds, Candida spp. do not infect the respiratory tract after inhalation. Candida spp. colonizes the upper respiratory tract and is often identified in sputum or deep lung cultures, but this represents colonization rather than invasive infection.
Dissemination to almost any organ can occur, including the brain, eyes, lung, liver, spleen, or kidneys. Candida spp. can cause central nervous system (CNS) disease in the form of meningitis, meningoencephalitis, or abscesses – presenting signs may include headache, altered mental status, or seizure (McCarthy, et al 2017). Ocular candidiasis can present as chorioretinitis or endophthalmitis and may result in vision loss (Fierro, et al 2013). Current guidelines from the Infectious Diseases Society of America (IDSA) recommend an ophthalmologic exam for all patients with candidemia (Pappas, et al 2016). Given the higher risk of dissemination, assessment for metastatic foci of candida should occur in all severely immunocompromised patients with candidemia, and disseminated infections require a longer course of treatment than candidemia alone (Pappas, et al 2016).
Hepatosplenic candidiasis, also called chronic disseminated candidiasis, is a distinct entity characterized by focal lesions in the liver and spleen (Rammaert, et al 2012). Typical presentations include prolonged fever despite recovery of neutrophil count (Rammaert, et al 2012). Patients usually present with negative blood cultures and no signs of acute disease, and the diagnosis is frequently made radiographically. Diagnostic imaging will reveal focal changes in the liver and spleen, characteristic of hepatosplenic candidiasis (Katragkou, et al 2017). Occasionally, involvement of other abdominal organs or skin occurs. Though the pathogenesis is not well understood, disruption of the mucosal barrier in the gastrointestinal tract is hypothesized to allow for invasion of local vasculature leading to infection of the portal circulation by Candida spp. With the exception of fever, symptoms of hepatosplenic candidiasis often do not occur until immune reconstitution (King, et al 2017), and it is thought that dysfunctional immune responses contribute to symptoms (Rammaert, et al 2012). Fever may be prolonged well beyond immune reconstitution despite resolution of radiographic findings and other symptoms.
Invasive Aspergillosis
The most common site of infection for invasive aspergillosis is the respiratory tract. The primary mode of transmission is inhalation of aerosolized conidia from the environment (Musial, et al 1988). Aspergillus infection can occur in upper and lower respiratory tracts. Pulmonary disease may present with nonspecific symptoms such as cough, dyspnea, and/or chest pain (Müller, et al 2002), although many patients are asymptomatic and diagnoses are made based on incidental finding of pulmonary nodules or other radiographic results (Burgos, et al 2008, King, et al 2017). Rhinosinusitis may present with facial pain, numbness, soft tissue swelling, or eye pain similar to periorbital cellulitis.
Another common site of primary infection is the skin, where fungal spores may be directly inoculated. A history of occlusive dressing or trauma at the site of infection is common (Smith, et al 2018). Cutaneous aspergillosis often presents as a solitary hemorrhagic or necrotic ulcer, though a variety of skin manifestations can occur.
Aspergillus spp. are notable for their ability to cause angioinvasion and subsequently disseminated disease. Dissemination to the brain occurs in immunocompromised children (Müller, et al 2002), resulting in abscesses, meningoencephalitis, or vasculitis. Disseminated aspergillosis may involve the eye, gastrointestinal tract, heart valves, liver, kidneys, or bones (Müller, et al 2002). Cutaneous disease may also be a sign of dissemination, which can manifest as diffuse skin lesions.
Mucormycosis
The clinical features of mucormycosis are similar to aspergillosis, and clinical distinction is rarely possible (Groll, et al 2014). Pulmonary disease and rhinosinusitis are the most common clinical manifestations, but cutaneous disease also occurs. Gastrointestinal mucormycosis can present as an intra-abdominal mass. The clinical presentation of disseminated disease varies based on involved organs.
Diagnostic Testing
Early recognition and diagnosis is paramount in the treatment and control of IFD in immunocompromised hosts. Diagnosis may be challenging, as patients can present with symptoms that are attributed to co-morbidities. Consensus definitions for proven, probable, and possible IFD can guide diagnostic evaluation (Table 2). The approach to diagnosis of IFD relies on histopathology and culture, but is multi-faceted and includes the use of blood cultures, diagnostic imaging, serum biomarkers, and ophthalmologic examination (Table 1). Blood cultures may be useful in yeasts such as Candida spp. or Trichosporon spp. though are of low utility in the diagnosis of invasive mold infections besides those caused by Fusarium spp. (Campigotto, et al 2016).
Table 2.
Criteria for proven and probable invasive fungal disease, excluding endemic mycosesa
| Proven IFD |
Mold • Histopathological, cytopathological or microscopic examination of a sterile specimen in which hyphae are seen with associated tissue damage • Recovery of a mold or “black yeast” by culture of a sterile specimen • Blood culture that yields a mold (e.g., Fusarium spp.)c Yeast • Histopathological, cytopathological or microscopic examination of a sterile specimen showing yeast cells • Recovery of a yeast by culture of a sterile specimen obtained from a site with clinical or radiographic findings consistent with an infectious disease • Blood culture that yields yeast or a yeast-like fungi (e.g. Candida spp., Cryptococcus spp., or Trichosporon spp.) • Presence of cryptococcal antigen in the CSF |
| Probable or Possible IFDb | • Presence of host factorsd • Prolonged, profound neutropenia • Receipt of an allogeneic HSCT • Prolonged use of corticosteroids • Treatment with T-cell immunosuppressants • Presence of a severe inherited immunodeficiency • Presence of clinical criteria consistent with IFD • Radiographic findings of lower respiratory tract fungal disease • Evidence of tracheobronchitis on bronchoscopy • Radiographic evidence of sinonasal disease • Signs of CNS infection on imaging • Evidence of disseminated candidiasis on imaging or examination • Mycological evidence of infection • Presence of mold in sputum, bronchoalveolar lavage fluid, or sinus aspirate sample, either through histopathological analysis or culture • Positive indirect testing for IFD, including positive galactomannan antigen testing for aspergillosis or beta-D-glucan detected in the serum for other IFD |
Adapted from De Pauw, et al 2008
Probable IFD requires the presence of a host factor, a clinical criterion, and a mycological criterion. Cases that meet the criteria for a host factor and a clinical criterion but for which mycological criteria are absent are considered possible IFD.
Blood cultures are rarely positive for molds other than Fusarium spp.
Characteristics by which individuals predisposed to invasive fungal diseases can be recognized
Diagnostic Imaging
The diagnostic process for IFD often begins with radiographic imaging, and the initial imaging modality should be guided by symptoms or clinical manifestations. Diagnostic imaging helps to establish the diagnosis, guide tissue biopsy, and determine extent and site(s) of dissemination (Katragkou, et al 2017).
Candidiasis.
Diagnostic imaging is useful for evaluating dissemination of Candida infection. CNS lesions are best seen by Magnetic resonance imaging (MRI), and the most common findings are nodular enhancing lesions or multiple abscesses (Katragkou, et al 2017). Computed tomography (CT) of the chest is preferred for evaluation of hematogenously disseminated lung disease, which frequently appear as pulmonary nodules.
MRI is superior to CT or ultrasound in the evaluation of hepatosplenic candidiasis (Rammaert, et al 2012). However lesions may not be detected radiographically prior to immune reconstitution (Katragkou, et al 2017). Ultrasound or CT may be useful for surveillance imaging of hepatosplenic candidiasis.
Mold Infections.
Imaging for suspected invasive mold infection should be guided by clinical presentation. However, for patients with prolonged fever and no apparent target organ, imaging should focus on the most common sites of disease – the sinuses and lungs. Diagnostic imaging of fungal rhinosinusitis is difficult to interpret in isolation as there are no imaging findings specific to fungal sinus disease. Imaging may be normal and should not be used as the sole diagnostic tool. Bony erosion on CT is consistent with sinusitis, but is a late finding in fungal disease and is often absent on diagnostic imaging (Katragkou, et al 2017). Fungal sinusitis may appear as an absence of enhancement on MRI due to the devascularization of infected tissues (Groppo, et al 2011). MRI has been reported to be more sensitive for detecting early changes seen in fungal rhinosinusitis, though the specificity of CT and MRI are similar (Groppo, et al 2011).
In pulmonary mold infections, CT imaging may reveal pulmonary lesions, including consolidation, cavitation, abscess, or nodules (Katragkou, et al 2017). Imaging does not distinguish between aspergillus and mucormycosis, despite reported association of certain imaging findings with specific organisms. Importantly, classical imaging findings of aspergillosis that are seen in adults (cavitation or air crescent lesions) are not always present in children (Burgos, et al 2008).
Serum biomarkers
Biomarkers are used in conjunction with traditional histopathologic and microbiologic diagnostics in order to improve surveillance and detection of IFD (Huppler, et al 2017). The most frequently used fungal biomarkers are the galactomannan and beta-D-glucan assays. The diagnostic value of these tests differs between adults and children, and data in pediatric populations are relatively limited (Lehrnbecher, et al 2016).
Beta-D-glucan.
The beta-D-glucan assay detects (1–3)-beta-D-glucan, a polysaccharide found within the fungal cell wall (Figure 2) of Aspergillus spp., Candida spp., Fusarium spp., Trichosporon spp., and some endemic. Data from adult patients with hematologic malignancies found that the beta-D-glucan assay has high specificity and positive predictive value as a diagnostic assay, especially when two consecutive test results were positive (Lamoth, et al 2012). There have been few studies to address the utility of the beta-D-glucan assay in children, and the optimal cut-off for a positive test result has not been established (Huppler, et al 2017). Additionally, several medications and blood products can cause false positive results. Of note, the use of the beta-D-glucan assay as a screening tool for IFD in children with fever and neutropenia is not recommended (Groll, et al 2014, Lehrnbecher, et al 2017).
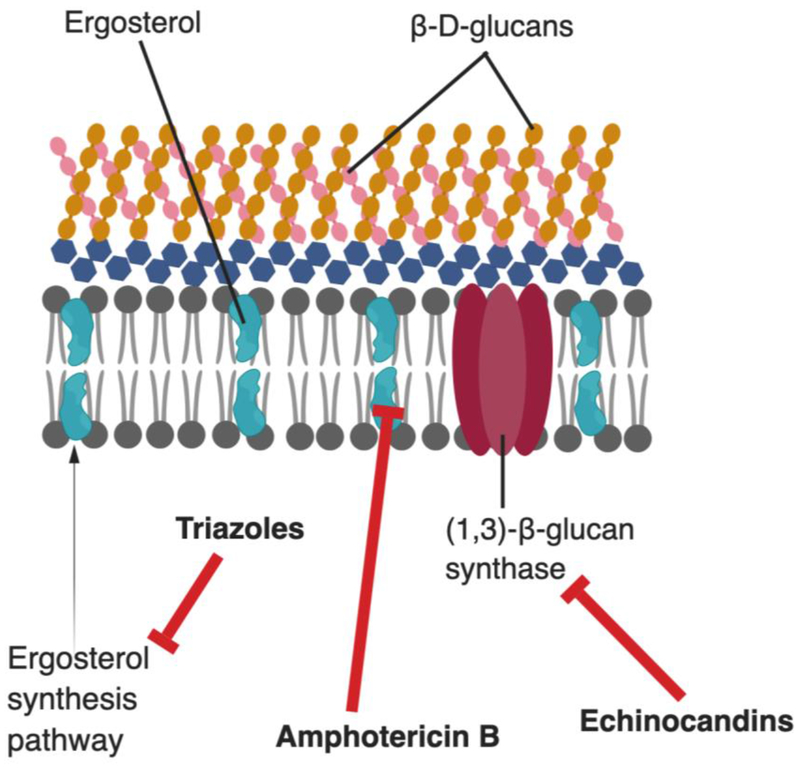
Figure 2.
The fungal cell wall and membrane and the sites of action of common antifungals. Triazole antifungals inhibit the synthesis of ergosterol, a key cell membrane component. Polyene antifungals (such as amphotericin B) act directly on ergosterol and disrupts membrane integrity. Echinocandins prevent the synthesis of (1,3)-β-D-glucan, a key component of the fungal cell wall.
Galactomannan.
The Aspergillus galactomannan assay detects the galactomannan polysaccharide component of Aspergillus spp cell wall. The assay is used both for screening and for diagnostic testing. The sensitivity and specificity depend greatly on the population tested, with the highest sensitivity in patients with hematologic malignancies or HSCT recipients (Arvanitis, et al 2014). A recent meta-analysis of studies evaluating the use of galactomannan detection in pediatric oncology and HSCT revealed variable sensitivity and specificity when the galactomannan assay was used for screening (specificity 50–100%, sensitivity 0–100%) or diagnostic testing (specificity 35–100%, sensitivity 14–100%) (Lehrnbecher, et al 2016). Furthermore, the use of antifungal therapy can decrease the sensitivity of the test and data from adult patients show that the low pretest probability makes it an unreliable surveillance test (Arvanitis, et al 2014, Groll, et al 2014). Expert opinion on the use of galactomannan detection in children is varied: ECIL-4 guidelines recommend consideration of galactomannan assay use for surveillance of children at risk for invasive aspergillosis (Groll, et al 2014), however the international pediatric febrile neutropenia guidelines recommend against routine use (Lehrnbecher, et al 2017).
Histopathology and Culture
Histopathologic analysis and culture of tissue samples are essential for the diagnosis of IFD, and tissue biopsy should be pursued whenever possible. Histopathology enables visualization of fungal elements within tissue and can guide initiation of antifungal therapy or changes to empiric antifungal therapy (Dekio, et al 2015). Histopathology may offer preliminary identification of an invasive mold – for example, Aspergillus spp. are known to have septate hyphae, whereas molds of the Mucorales family are broad and pauci-septate or aseptate. However, a change to more narrow spectrum antifungal treatment should be reserved until definitive identification of pathogen is done by culture or molecular testing.
Molecular diagnostics
Culture of tissue specimens is the gold standard for identification of fungal pathogens, although contemporary molecular techniques enhance pathogen identification efforts (Larone 2011). Molecular testing allows for the identification of organisms that do not grow in culture and should offer higher sensitivity than other diagnostic techniques (Huppler, et al 2017, Larone 2011). Molecular testing is particularly helpful for more rapid identification of organisms than traditional microbiologic methods, as well as identification of organisms seen on histopathology that do not grow in culture (Chang, et al 2019). One study of invasive mold infection reported increased diagnostic yield from 63% to 96% when PCR testing was utilized (Rickerts, et al 2007). Molecular testing also offers enhanced accuracy; in a recent single-center study fungal sequencing yielded sensitivity of 96.6% and specificity of 98.2% in tissue specimens (Gomez, et al 2017).
However, the utility of molecular assays as screening tests for IFD is unclear. When used as a screening test, serum polymerase chain reaction (PCR) resulted in specificity ranging from 43–85% and sensitivity of 11–80% (Lehrnbecher, et al 2016). When used as a diagnostic test, the specificity was 36–83% and sensitivity was 0–100% (Lehrnbecher, et al 2016). Current guidelines do not recommend routine use of serum PCR for screening or diagnosis of IFD in children (Groll, et al 2014, Lehrnbecher, et al 2017).
Treatment of IFD
The optimal management of IFD includes three factors: antifungal agents, surgical removal when feasible, and an intact immune system. The nature of the underlying diseases in children with hematologic malignancies and HSCT recipients makes the latter very difficult, though every attempt at immune reconstitution should be made in patients with suspected IFD. Appropriate antifungal therapy is the backbone of any therapeutic regimen. However, antifungal therapy is often inadequate for invasive infection as tissue necrosis results in poor penetration of antifungal drugs to the site of infection. Consultation with an infectious diseases specialist with experience treating infections in immunocompromised children is recommended.
Antifungal Therapy
The prompt initiation of empiric antifungal therapy is key in the treatment of children with suspected IFD. The commonly used antifungal agents, including the triazoles, echinocandins, and amphotericin B formulations have varied mechanisms and action and spectrum of activity, as shown in Figure 2 and Table 3, respectively. Empiric therapy can be tailored once a causative pathogen is identified, although specific pathogens are often not defined in IFD. Antifungal susceptibility testing can help guide therapy, though is only routinely performed for Candida spp. (Arendrup, et al 2013). As resistance to antifungal drugs is increasingly recognized, antifungal susceptibility testing has become increasingly important in planning therapy for IFD.
Table 3.
Common antifungal agents, their mechanism, spectrum of activity, and toxicities
| Class | Drugs | Yeast Activity | Mold Activity | Formulations | Toxicity |
|---|---|---|---|---|---|
| Triazolesa | Fluconazole | +++ | None | IV/PO | Voriconazole and Posaconazole: hepatotoxicity, QT prolongationd Voriconazole: photosensitivity, skin cancer, fluorosis, CNS symptoms (delirium, hallucinations) |
| Itraconazole | +++ | + | PO | ||
| Voriconazoleb | +++ | +++c | IV/PO | ||
| Posaconazoleb | +++ | +++ | IV/PO | ||
| Isavuconazole | +++ | +++ | IV/PO | ||
| Echinocandinse | Micafungin Caspofungin | +++ | ++c | IV | Hepatotoxicity |
| Polyenes | Amphotericin B deoxycholate | +++ | +++ | IV | Nephrotoxicityf Infusion reactions |
| Liposomal amphotericin B | +++ | +++ | IV |
Voriconazole is approved for patients aged ≥2 years and posaconazole is approved for patients ≥13 years. Phase 1 trials for isavuconazole are ongoing (ClinicalTrials.gov: NCT 03241550)
Therapeutic drug monitoring is recommended for voriconazole and posaconazole
No activity against Mucorales
Isavuconazole is associated with shortening of the QT interval
For children, there are no relevant pharmacological differences between the echinocandins
Lessened with liposomal formulation
Invasive Candidiasis.
With respect to antifungal treatment for invasive candidiasis, the management principles for pediatric patients are similar to those for adults. There have been few comparative trials evaluating the performance of different antifungal agents in children, and recommendations in children have been extrapolated from adult data. The choice of antifungal agent as well as the duration of therapy is determined by the infecting organism, the extent of dissemination, and the patient’s immune status.
For candidemia, IDSA guidelines recommend initial therapy with an echinocandin (Pappas, et al 2016). One study comparing an echinocandin to fluconazole showed non-inferiority, however there was a trend towards more favorable outcomes with the echinocandin (Reboli, et al 2007). Sub-group analysis of patients with C. albicans demonstrated that echinocandin therapy was more effective than fluconazole, leading to more rapid clearance of positive blood cultures (Reboli, et al 2011). After patients have stabilized and blood cultures are sterilized, many clinicians transition to azole therapy; an international open-label comparative trial of patients treated with echinocandin therapy for 5 days before transitioning to enteral azole therapy did not find a difference in outcomes compared to those treated entirely with echinocandins (Vazquez, et al 2014). Patients should be treated with an antifungal agent for a minimum 14 days after blood cultures have sterilized (Groll, et al 2014, Pappas, et al 2016). In the neutropenic patient, the gastrointestinal tract is the predominant source of infection rather than seeding of a central venous catheter. However, seeding of central catheters is possible as either an initiating or secondary event in neutropenic patients, therefore catheter removal should be considered (Pappas, et al 2016).
Antifungal therapy for disseminated candidiasis is dependent upon the site of dissemination. For hematogenous spread to lungs or abdominal organs (not including hepatosplenic candidiasis), echinocandin therapy is sufficient. However, echinocandins have poor penetration into the vitreous of the eye or the CNS, thus disseminated infections involving the eye or CNS require treatment with amphotericin B or the triazole antifungals (Pappas, et al 2016). The duration of treatment is dependent upon the site of infection, the resolution of symptoms, and correction of predisposing disorders (Groll, et al 2014). In pediatric oncology patients, resolution of neutropenia is essential prior to discontinuation of antifungals.
Initial therapy for hepatosplenic candidiasis should consist of liposomal AmB (LAmB) or an echinocandin, though there are no comparative trials to guide therapy (Pappas, et al 2016). Step-down to oral azole therapy can be done after improvement in symptoms. Patients should be treated until radiographic resolution of lesions, as premature discontinuation of therapy may result in relapse of infection. Given the likely contribution of immune reconstitution or immune dysfunction to symptoms, some have advocated for the use of corticosteroids as adjunctive therapy in those patients for whom fever persists beyond radiographic improvement (Rammaert, et al 2012). However, there have been no systematic analyses of corticosteroid treatment and its role in the treatment of hepatosplenic candidiasis.
Invasive Aspergillosis.
Treatment for aspergillosis should commence once there is suspicion for invasive disease, and voriconazole is the drug of choice for primary treatment (Patterson, et al 2016). Voriconazole is associated with improved survival and fewer side effects when compared to amphotericin B deoxycholate for treatment of aspergillosis (Herbrecht, et al 2002). LAmB and the other lipid formulations have also been used successfully for the treatment of invasive aspergillosis (Cornely, et al 2007a). Caspofungin can also be used as an alternative agent, though comparative trials are lacking (Patterson, et al 2016).
Combination therapies including AmB or mold-active azoles with echinocandins have been reported to be beneficial for treatment of invasive aspergillosis. A recent international randomized trial of adult patients with hematologic malignancies and HSCT recipients with aspergillosis compared voriconazole monotherapy versus combination therapy of voriconazole and anidulafungin (Marr, et al 2015). Treatment with combination therapy was associated with a decrease in mortality after 6 weeks of treatment (19.3% versus 27.5%, p= 0.087). Though the decrease in mortality was not statistically significant, the authors concluded that it was clinically significant. Therapeutic drug monitoring was not performed in this study, thus it is possible that patients randomized to voriconazole alone may have had sub-therapeutic drug levels. Current guidelines suggest that combination therapy of voriconazole and an echinocandin can be utilized for severe disease (Patterson, et al 2016). Our practice is to utilize combination therapy initially with step-down to voriconazole monotherapy after clinical improvement, immune reconstitution, and achievement of appropriate serum voriconazole levels.
Importantly, triazole-resistant Aspergillus is an emerging threat especially in Western European countries. Multi- and pan-azole resistant isolates of A. fumigatus, the most common cause of invasive aspergillosis, have been detected on nearly every continent (Verweij, et al 2015). Resistance appears to be environmentally-acquired since resistant isolates have been cultured from azole-naïve patients. Outcomes associated with invasive azole-resistant Aspergillus are poor; a recent retrospective cohort study found an excess mortality of 25% in patients with voriconazole-resistant isolates (Lestrade, et al 2019). Recommendations from an international expert panel include empiric treatment with LAmB or combination therapy with a triazole and an echinocandin in regions with >10% triazole resistance (Verweij, et al 2015).
Treatment for invasive aspergillosis should be continued for a minimum of 6–12 weeks, with the final duration of therapy determined by the resolution of infection and underlying immune deficit (Groll, et al 2014). Serial radiographic imaging is necessary to document resolution of pulmonary findings or other visceral involvement.
Mucormycosis.
There have been few randomized controlled trials comparing the effectiveness of antifungal agents in the treatment of mucormycosis, and most published data is derived from case series or case reports. This is in part because few antifungals have activity against the Mucorales fungi (Table 3). LAmB remains the cornerstone medical treatment for mucormycosis.
Early initiation of AmB is associated with improved survival in cases of mucormycosis (Chamilos, et al 2008). The lipid formulations of AmB had been shown to be safe in children, and higher doses can be given with minimal toxicity (Wiley, et al 2005). Pediatric dosing is largely extrapolated from adult trials. The European Society for Clinical Microbiology and Infectious Diseases and European Confederation of Medical Mycology joint clinical guidelines recommend LAmB dosing of ≥5 mg/kg/day for most cases of mucormycosis, and up to 10 mg/kg/day for CNS involvement (Cornely, et al 2014b).
Posaconazole, a broad-spectrum triazole, also has activity against most Mucorales fungi and is generally better tolerated than LAmB. Posaconazole is safe and well tolerated in children (Doring, et al 2012), though dosing data for children ≤ 13 years of age are limited. The successful use of posaconazole as salvage therapy has been documented in both adult and pediatric patients with mucormycosis (Greenberg, et al 2006, van Burik, et al 2006).
The triazole drug isavuconazole has activity against Mucorales molds, and a recent single-arm, open label trial in adults showed that isavuconazole had similar all-cause mortality to LAmB (Marty, et al 2016). The safety and efficacy of isavuconazole in pediatric patients have not been established, but isavuconazole has been used successfully to treat mucormycosis in children (Barg, et al 2018). A clinical trial is ongoing to determine the optimal delivery of isavuconazole in pediatric patients (ClinicalTrials.gov: NCT 03241550).
There have been limited published data regarding the efficacy of combination therapy for treatment of mucormycosis in children. A recent retrospective analysis comparing combination therapy versus monotherapy for the initial treatment of mucormycosis in adults with hematologic malignancies found no significant differences in 6-week mortality (Kyvernitakis, et al 2016). Regardless, reviews of published cases of pediatric mucormycosis show that combination therapy is frequently used (Otto, et al 2019, Zaoutis, et al 2007). As with invasive aspergillosis, the duration of therapy is guided by resolution of infection and correction of immune dysfunction.
Therapeutic Drug Monitoring.
Patients with IFD often have comorbid conditions that affect the pharmacokinetics and pharmacodynamics of antifungal medications (Ullmann, et al 2018). Therapeutic drug monitoring (TDM) has been shown to improve the efficacy of treatment with voriconazole while decreasing the drug discontinuation due to adverse outcomes (Park, et al 2012). Similar findings have been reported with the use of TDM with posaconazole, particularly for patients receiving the posaconzaole suspension (Groll, et al 2014). Pediatric guidelines recommend the use of TDM with triazole antifungals, with a target trough level of 1.0–5.0 mg/L for voriconazole and ≥0.5 mg/L for posaconazole (Groll, et al 2014).
Surgical Resection
The odds of therapeutic success and resolution of infection are maximized when surgical resection is combined with medical management (Cornely, et al 2014b). A significant reduction in mortality has been noted when aggressive surgical resection is performed in cases of pulmonary and sinus disease (Cesaro, et al 2014). Therefore, surgical intervention should be considered for accessible lesions that can be resected with clean margins. However, for infections that are widely disseminated or will require radical resections, reduction of fungal burden must be weighed with the potential for surgical morbidity.
Correction of Immune Dysfunction
Correction of a patient’s immune dysfunction is critical to successful treatment of IFD . When possible, immunosuppressive therapy (including corticosteroids) should be minimized.
Neutrophil recovery has been linked with improved outcomes in IFD, and the use of granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) as adjunctive therapy is hypothesized to be beneficial by hastening neutrophil recovery (Cornely, et al 2014a). A large meta-analysis evaluating the use of prophylactic colony stimulating factors in adults and children receiving chemotherapy or undergoing HSCT did not show a mortality benefit but reported a decreased rate of infection due to all causes, including IFD (Sung, et al 2007b).
High-dose granulocyte transfusions are also hypothesized to have benefit by ameliorating the severity and duration of neutropenia, though efficacy data has been inconclusive. A systematic review of randomized controlled trials for granulocyte transfusions concluded that there is insufficient evidence to determine whether granulocyte transfusions impact all-cause mortality, largely due to high risk of bias and ambiguous outcome measurements (Estcourt, et al 2016). However, granulocyte transfusions should be considered for transient supportive therapy for patients in whom neutrophil recovery is expected (West, et al 2017).
Prevention of Invasive Fungal Disease
Given the morbidity and mortality associated with IFD, significant emphasis has been placed on infection prevention. A multi-faceted approach is needed and involves both limiting exposure through environmental control as well as prophylaxis with antifungal drugs.
Environmental Control Measures
Environmental exposure to aerosolized fungal spores or plants and soil can occur both outdoors and in the hospital setting, and is associated with IFD (Pagano, et al 2017). The development of a protective environment and the utilization of high-efficiency particulate air (HEPA) filtration or laminar air-flow reduce nosocomial exposure to fungal spores (Hahn, et al 2002).
Hospital construction or renovation causes significant dust contamination and dispersal of spores (Kanamori, et al 2015). Infection prevention efforts should focus on decreasing airborne fungal spores and maintaining an active surveillance system for construction-related IFD (Kanamori, et al 2015).
Primary Antifungal Prophylaxis
Antifungal prophylaxis should be considered in patients at high risk for developing IFD. While institutional practices vary, patients that should receive antifungal prophylaxis include those with AML, relapsed leukemia, and allogeneic HSCT recipients (Groll, et al 2014, Science, et al 2014). Data are limited regarding the use of primary antifungal prophylaxis in other populations, though some advocate for consideration of antifungal prophylaxis in all patients with high-risk leukemias (Groll, et al 2014).
Allogeneic HSCT transplant recipients should receive antifungal prophylaxis during the neutropenic phase pre-engraftment (Groll, et al 2014, Science, et al 2014). Fluconazole is commonly utilized for this indication. In two randomized controlled trials enrolling patients ≥12 years old, fluconazole prophylaxis reduced the rate of IFD in HSCT recipients (Goodman, et al 1992, Slavin, et al 1995). A meta-analysis of the use of mold-active prophylaxis compared to fluconazole showed decreased mortality related to IFD, but had no effect on overall mortality and was associated with increased adverse effects (Ethier, et al 2012). Therefore, fluconazole is currently the recommended agent (Science, et al 2014). A trial in adult HSCT recipients, which also enrolled a small number of children, showed that micafungin prophylaxis was superior to fluconazole (van Burik, et al 2004). Echinocandins are well-tolerated and can be utilized as alternative antifungal prophylactic agents in HSCT recipients pre-engraftment.
Given the higher risk for IFD in the setting of GVHD and related immunosuppressive therapies used to treat GVHD (in particular corticosteroids), prophylaxis against both yeast and mold is recommended (Groll, et al 2014, Science, et al 2014). Specific drug recommendations vary based on patient age. Echinocandins can be utilized in infants and children (van Burik, et al 2004). Voriconazole has been utilized successfully as antifungal prophylaxis in HSCT recipients ≥2 years of age (Marks, et al 2011). For children ≥13 years of age, posaconazole can be utilized as antifungal prophylaxis. An international randomized, double-blind trial comparing posaconazole to fluconazole prophylaxis for HSCT recipients with GVHD showed that use of oral posaconazole suspension (with TDM) was as effective as fluconazole in the prevention of all fungal infections but was superior to fluconazole in preventing invasive aspergillosis and in reducing the rate of death attributable to IFD (Ullmann, et al 2007). However, the role of posaconazole as anti-fungal prophylaxis in children with GVHD has yet to be comprehensively studied.
With respect to pediatric patients with AML and relapsed ALL, fluconazole is currently the recommended agent for antifungal prophylaxis (Science, et al 2014). A meta-analysis of adult oncology patients revealed that fluconazole prophylaxis resulted in decreased IFD in populations with a high incidence of IFD (Kanda, et al 2000). A subsequent randomized, controlled trial of posaconazole prophylaxis resulted in lower rates of proven or probable IFD along with reduced fungal-related and overall mortality when compared to fluconazole or itraconazole prophylaxis (Cornely, et al 2007b). An open-label randomized trial comparing caspofungin to fluconazole for prevention of IFD in children with AML (Children’s Oncology Group ACCL0933) recently completed enrollment and results of this trial may influence future recommendations regarding antifungal prophylaxis for pediatric patients. Additionally, as dosing strategies for triazoles in young children become better defined, the opportunity to provide prophylaxis against mold infection in all high-risk pediatric patients will likely prompt a review of current recommendations. Certainly for older pediatric patients with leukemia who are at risk for IFD, prophylaxis with posaconazole should be considered.
Secondary Antifungal Prophylaxis
Secondary antifungal prophylaxis occurs after a patient has resolution of IFD but is entering a subsequent high-risk period. Antifungal prophylaxis after treatment of IFD has been used successfully in HSCT to reduce the risk of developing new or recurrent IFD (Cordonnier, et al 2011),(Maziarz, et al 2017). Secondary prophylaxis has been shown to be safe and effective in children, and has resulted in greatly decreased fungal-related mortality (Azik, et al 2015, Dvorak, et al 2005). The choice of antifungal agent for secondary prophylaxis should be tailored to a patient’s underlying disease, prior IFD, and clinical status. History of previously treated IFD should not be considered an absolute contraindication to proceeding with further chemotherapy or HSCT.
Rare and Emerging Fungal Pathogens
Widespread use of antifungal prophylaxis has improved outcomes for immunocompromised patients, but has resulted in shifting epidemiology of IFD. Improved diagnostic methods additionally have enabled identification of rare human pathogens, many of which are resistant to the antifungal agents used routinely as prophylaxis. A full discussion of rare and emerging fungal pathogens is outside the scope of this review, but clinicians should be aware of hyaline molds, such as Fusarium spp. and Scedosporium spp., which may present similarly to aspergillosis or mucormycosis, though tend to be highly resistant to most anti-fungal agents (Nucci and Anaissie 2007). Dematiaceous molds, called phaeohyphomycosis, and opportunistic yeasts such as Cryptococcus spp. and Trichosporon spp. may also cause infections in children with hematologic malignancies or following HSCT. Of note, fungal species that are not exclusively opportunistic may cause more severe or disseminated disease in immunocompromised patients. These include endemic mycoses caused by dimorphic fungi, such as Histoplasma spp. and Blastomyces spp.
Future Directions
Much remains to be learned about IFD in children. Development of risk stratification algorithms for high-risk populations will help guide decision-making for diagnostic testing and empiric treatment. Further epidemiological research is needed to better understand the incidence of IFD given the increased use of antifungal prophylaxis.
A better understanding of the validity and usefulness of serum biomarkers and molecular testing for IFD in children is needed. Two active studies, Fungal Biomarkers for Diagnosis and Response to Therapy for Pediatric Candidemia (BIOPIC, ClinicalTrials.gov: NCT 2220790) and Non-Invasive Diagnosis of Pediatric Pulmonary Invasive Mold Infections (DOMINIC, ClinicalTrials.gov: NCT 03827694), will provide insight into non-culture assays for the diagnosis of IFD in children. Additional testing platforms, including the T2Candida assay (T2 Biosystems), metabolomic breath analysis, and next-generation sequencing need to be evaluated in pediatric patients (de Heer, et al 2016, Hamula, et al 2016, Hong, et al 2018).
While many pediatric treatment recommendations are extrapolated from adult data, several ongoing clinical trials are aimed at identifying effective antifungal agents for various manifestations of IFD in children. The Pediatric Antifungal Comparative Effectiveness (PEACE) trial (ClinicalTrials.gov: NCT 01869829) will compare the efficacy of echinocandin versus amphotericin B or triazole antifungal therapy for pediatric invasive candidiasis. Additionally, there are active Phase 1 studies examining pharmacokinetic/pharmacodynamic properties of posaconazole and isavuconazole (ClinicalTrials.gov: NCT 02452034 and 03241550) in children. The role of antifungal prophylaxis requires better definition for nuanced immunocompromised pediatric populations, particularly regarding the choice of antifungal agent and appropriate dosing.
Finally, there is great variability in antifungal prescribing across institutions and geographic regions, and investigation into antifungal stewardship is also needed (Hamdy, et al 2017). The impact of antifungal use on clinical management and costs is not well defined, and further research is needed to identify the best metrics and methods for antifungal stewardship.
Acknowledgements
The authors declare no competing financial interests. A.M.G was supported by a grant from the National Institutes of Health, USA (CA212299). We thank members of the divisions of Infectious Diseases and Oncology at the Children’s Hospital of Philadelphia for insightful discussions and input, in particular Dr. Brian Fisher and Dr. Alix Seif for critical review of the manuscript.
References
- Afzal S, Ethier MC, Dupuis LL, Tang L, Punnett AS, Richardson SE, Allen U, Abla O & Sung L (2009) Risk factors for infection-related outcomes during induction therapy for childhood acute lymphoblastic leukemia. Pediatr Infect Dis J, 28, 1064–1068. [DOI] [PubMed] [Google Scholar]
- Arendrup MC, Cuenca-Estrella M, Lass-Florl C & Hope WW (2013) Breakpoints for antifungal agents: an update from EUCAST focussing on echinocandins against Candida spp. and triazoles against Aspergillus spp. Drug Resist Updat, 16, 81–95. [DOI] [PubMed] [Google Scholar]
- Arvanitis M, Anagnostou T, Fuchs BB, Caliendo AM & Mylonakis E (2014) Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin Microbiol Rev, 27, 490–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azik FM, Tezer H, Ozkaya-Parlakay A, Aksu T, Bayram C, Fettah A, Tavil B & Tunc B (2015) Secondary antifungal prophylaxis in pediatric hematopoietic stem cell transplants. J Pediatr Hematol Oncol, 37, e19–22. [DOI] [PubMed] [Google Scholar]
- Barg AA, Malkiel S, Bartuv M, Greenberg G, Toren A & Keller N (2018) Successful treatment of invasive mucormycosis with isavuconazole in pediatric patients. Pediatr Blood Cancer, 65, e27281. [DOI] [PubMed] [Google Scholar]
- Barnes PD & Marr KA (2007) Risks, diagnosis and outcomes of invasive fungal infections in haematopoietic stem cell transplant recipients. Br J Haematol, 139, 519–531. [DOI] [PubMed] [Google Scholar]
- Bartlett AW, Cann MP, Yeoh DK, Bernard A, Ryan AL, Blyth CC, Kotecha RS, McMullan BJ, Moore AS, Haeusler GM & Clark JE (2018) Epidemiology of invasive fungal infections in immunocompromised children; an Australian national 10-year review. Pediatr Blood Cancer, e27564. [DOI] [PubMed] [Google Scholar]
- Bochennek K, Hassler A, Perner C, Gilfert J, Schoning S, Klingebiel T, Reinhardt D, Creutzig U & Lehrnbecher T (2016) Infectious complications in children with acute myeloid leukemia: decreased mortality in multicenter trial AML-BFM 2004. Blood Cancer J, 6, e382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos A, Zaoutis TE, Dvorak CC, Hoffman JA, Knapp KM, Nania JJ, Prasad P & Steinbach WJ (2008) Pediatric invasive aspergillosis: a multicenter retrospective analysis of 139 contemporary cases. Pediatrics, 121, e1286–1294. [DOI] [PubMed] [Google Scholar]
- Campigotto A, Richardson SE, Sebert M, McElvania TeKippe E, Chakravarty A & Doern CD (2016) Low Utility of Pediatric Isolator Blood Culture System for Detection of Fungemia in Children: a 10-Year Review. J Clin Microbiol, 54, 2284–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnola E, Bagnasco F, Menoni S, Muraca M, Prete A, Belotti T, Iori AP, Barberi W, Severino A, Proia A, Raiola AM, Vacca A, Cudillo L, Rambaldi A & Girmenia C (2018) Risk factors associated with development and mortality by invasive fungal diseases in pediatric allogeneic stem cell transplantation. A pediatric subgroup analysis of data from a prospective study of the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Bone Marrow Transplant, 53, 1193–1197. [DOI] [PubMed] [Google Scholar]
- Castagnola E, Cesaro S, Giacchino M, Livadiotti S, Tucci F, Zanazzo G, Caselli D, Caviglia I, Parodi S, Rondelli R, Cornelli PE, Mura R, Santoro N, Russo G, De Santis R, Buffardi S, Viscoli C, Haupt R & Rossi MR (2006) Fungal infections in children with cancer: a prospective, multicenter surveillance study. Pediatr Infect Dis J, 25, 634–639. [DOI] [PubMed] [Google Scholar]
- Cesaro S, Pegoraro A, Tridello G, Pillon M, Cannata E, Faggin S & Cecchetto G (2014) The role of surgery in the treatment of invasive fungal infection in paediatric haematology patients: a retrospective single-centre survey. Mycoses, 57, 394–399. [DOI] [PubMed] [Google Scholar]
- Cesaro S, Tridello G, Castagnola E, Calore E, Carraro F, Mariotti I, Colombini A, Perruccio K, Decembrino N, Russo G, Maximova N, Baretta V & Caselli D (2017) Retrospective study on the incidence and outcome of proven and probable invasive fungal infections in high-risk pediatric onco-hematological patients. Eur J Haematol, 99, 240–248. [DOI] [PubMed] [Google Scholar]
- Chamilos G, Lewis RE & Kontoyiannis DP (2008) Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis, 47, 503–509. [DOI] [PubMed] [Google Scholar]
- Chang YC, Graf E & Green AM (2019) Invasive Curvularia Infection in Pediatric Patients With Hematologic Malignancy Identified by Fungal Sequencing. J Pediatric Infect Dis Soc, 8, 87–91. [DOI] [PubMed] [Google Scholar]
- Cordonnier C, Rovira M, Maertens J, Cornely OA, Ljungman P, Einsele H, Voriconazole for Secondary Prophylaxis of Invasive Fungal Infections in Patients With Allogeneic Stem Cell Transplants study, g., the Infectious Diseases Working Party of the European Group for, B. & Marrow T (2011) Voriconazole as secondary antifungal prophylaxis in stem cell transplant recipients. Haematologica, 96, e9–10; author reply e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada A, Akova M, Arendrup MC, Boekhout T, Chowdhary A, Cuenca-Estrella M, Freiberger T, Guinea J, Guarro J, de Hoog S, Hope W, Johnson E, Kathuria S, Lackner M, Lass-Florl C, Lortholary O, Meis JF, Meletiadis J, Munoz P, Richardson M, Roilides E, Tortorano AM, Ullmann AJ, van Diepeningen A, Verweij P, Petrikkos G, European Society of Clinical, M., Infectious Diseases Fungal Infection Study, G. & European Confederation of Medical, M. (2014a) ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect, 20 Suppl 3, 5–26. [DOI] [PubMed] [Google Scholar]
- Cornely OA, Cuenca-Estrella M, Meis JF & Ullmann AJ (2014b) European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Fungal Infection Study Group (EFISG) and European Confederation of Medical Mycology (ECMM) 2013 joint guidelines on diagnosis and management of rare and emerging fungal diseases. Clin Microbiol Infect, 20 Suppl 3, 1–4. [DOI] [PubMed] [Google Scholar]
- Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Ullmann AJ, Bouza E, Heussel CP, Lortholary O, Rieger C, Boehme A, Aoun M, Horst HA, Thiebaut A, Ruhnke M, Reichert D, Vianelli N, Krause SW, Olavarria E, Herbrecht R & AmBiLoad Trial Study G (2007a) Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis, 44, 1289–1297. [DOI] [PubMed] [Google Scholar]
- Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R & Angulo-Gonzalez D (2007b) Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med, 356, 348–359. [DOI] [PubMed] [Google Scholar]
- de Heer K, Vonk SI, Kok M, Kolader M, Zwinderman AH, van Oers MH, Sterk PJ & Visser CE (2016) eNose technology can detect and classify human pathogenic molds in vitro: a proof-of-concept study of Aspergillus fumigatus and Rhizopus oryzae. J Breath Res, 10, 036008. [DOI] [PubMed] [Google Scholar]
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for R, Treatment of Cancer/Invasive Fungal Infections Cooperative, G., National Institute of, A. & Infectious Diseases Mycoses Study Group Consensus G (2008) Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis, 46, 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekio F, Bhatti TR, Zhang SX & Sullivan KV (2015) Positive Impact of Fungal Histopathology on Immunocompromised Pediatric Patients With Histology-Proven Invasive Fungal Infection. Am J Clin Pathol, 144, 61–67. [DOI] [PubMed] [Google Scholar]
- Doring M, Muller C, Johann P, Erbacher A, Kimmig A, Schwarze CP, Lang P, Handgretinger R & Muller I (2012) Analysis of posaconazole as oral antifungal prophylaxis in pediatric patients under 12 years of age following allogeneic stem cell transplantation. BMC Infect Dis, 12, 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak CC, Steinbach WJ, Brown JM & Agarwal R (2005) Risks and outcomes of invasive fungal infections in pediatric patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant, 36, 621–629. [DOI] [PubMed] [Google Scholar]
- Estcourt LJ, Stanworth SJ, Hopewell S, Doree C, Trivella M & Massey E (2016) Granulocyte transfusions for treating infections in people with neutropenia or neutrophil dysfunction. Cochrane Database Syst Rev, 4, CD005339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier MC, Science M, Beyene J, Briel M, Lehrnbecher T & Sung L (2012) Mould-active compared with fluconazole prophylaxis to prevent invasive fungal diseases in cancer patients receiving chemotherapy or haematopoietic stem-cell transplantation: a systematic review and meta-analysis of randomised controlled trials. Br J Cancer, 106, 1626–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro JL, Prasad PA, Fisher BT, Gerber JS, Coffin SE, Walsh TJ & Zaoutis TE (2013) Ocular manifestations of candidemia in children. Pediatr Infect Dis J, 32, 84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher BT, Robinson PD, Lehrnbecher T, Steinbach WJ, Zaoutis TE, Phillips B & Sung L (2018) Risk Factors for Invasive Fungal Disease in Pediatric Cancer and Hematopoietic Stem Cell Transplantation: A Systematic Review. J Pediatric Infect Dis Soc, 7, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadou SP, Pongas G, Fitzgerald NE, Lewis RE, Rytting M, Marom EM & Kontoyiannis DP (2012) Invasive Mold Infections in Pediatric Cancer Patients Reflect Heterogeneity in Etiology, Presentation, and Outcome: A 10-Year, Single-Institution, Retrospective Study. J Pediatric Infect Dis Soc, 1, 125–135. [DOI] [PubMed] [Google Scholar]
- Gomez CA, Budvytiene I, Zemek AJ & Banaei N (2017) Performance of Targeted Fungal Sequencing for Culture-Independent Diagnosis of Invasive Fungal Disease. Clin Infect Dis, 65, 2035–2041. [DOI] [PubMed] [Google Scholar]
- Gomez SM, Caniza M, Fynn A, Vescina C, Ruiz C, Iglesias D, Sosa F & Sung L (2018) Fungal infections in hematopoietic stem cell transplantation in children at a pediatric children’s hospital in Argentina. Transplant Infectious Disease, 20, e12913–e12919. [DOI] [PubMed] [Google Scholar]
- Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, Shadduck RK, Shea TC, Stiff P, Friedman DJ & et al. (1992) A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med, 326, 845–851. [DOI] [PubMed] [Google Scholar]
- Greenberg RN, Mullane K, van Burik JA, Raad I, Abzug MJ, Anstead G, Herbrecht R, Langston A, Marr KA, Schiller G, Schuster M, Wingard JR, Gonzalez CE, Revankar SG, Corcoran G, Kryscio RJ & Hare R (2006) Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother, 50, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groll AH, Castagnola E, Cesaro S, Dalle J-H, Engelhard D, Hope W, Roilides E, Styczynski J, Warris A & Lehrnbecher T (2014) Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. The Lancet Oncology, 15, e327–e340. [DOI] [PubMed] [Google Scholar]
- Groppo ER, El-Sayed IH, Aiken AH & Glastonbury CM (2011) Computed tomography and magnetic resonance imaging characteristics of acute invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg, 137, 1005–1010. [DOI] [PubMed] [Google Scholar]
- Hahn T, Cummings KM, Michalek AM, Lipman BJ, Segal BH & McCarthy PL Jr. (2002) Efficacy of high-efficiency particulate air filtration in preventing aspergillosis in immunocompromised patients with hematologic malignancies. Infect Control Hosp Epidemiol, 23, 525–531. [DOI] [PubMed] [Google Scholar]
- Hale KA, Shaw PJ, Dalla-Pozza L, MacIntyre CR, Isaacs D & Sorrell TC (2010) Epidemiology of paediatric invasive fungal infections and a case-control study of risk factors in acute leukaemia or post stem cell transplant. Br J Haematol, 149, 263–272. [DOI] [PubMed] [Google Scholar]
- Hamdy RF, Zaoutis TE & Seo SK (2017) Antifungal stewardship considerations for adults and pediatrics. Virulence, 8, 658–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamula CL, Hughes K, Fisher BT, Zaoutis TE, Singh IR & Velegraki A (2016) T2Candida Provides Rapid and Accurate Species Identification in Pediatric Cases of Candidemia. Am J Clin Pathol, 145, 858–861. [DOI] [PubMed] [Google Scholar]
- Hazar V, Karasu GT, Uygun V, Ozturk G, Kilic SC, Kupesiz A, Daloglu H, Aksoylar S, Atay D, Ince EU, Karakukcu M, Ozbek N, Tayfun F, Kansoy S, Ozyurek E, Akcay A, Gursel O, Haskologlu S, Kaya Z, Yilmaz S, Tanyeli A & Yesilipek A (2019) Risks and outcomes of invasive fungal infections in pediatric allogeneic hematopoietic stem cell transplant recipients receiving fluconazole prophylaxis: a multicenter cohort study by the Turkish Pediatric Bone Marrow Transplantation Study Group. Med Mycol, 57, 161–170. [DOI] [PubMed] [Google Scholar]
- Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B, Invasive Fungal Infections Group of the European Organisation for, R., Treatment of, C. & the Global Aspergillus Study, G. (2002) Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med, 347, 408–415. [DOI] [PubMed] [Google Scholar]
- Hol JA, Wolfs TF, Bierings MB, Lindemans CA, Versluys AB, Wildt de A, Gerhardt CE & Boelens JJ (2014) Predictors of invasive fungal infection in pediatric allogeneic hematopoietic SCT recipients. Bone Marrow Transplant, 49, 95–101. [DOI] [PubMed] [Google Scholar]
- Hong DK, Blauwkamp TA, Kertesz M, Bercovici S, Truong C & Banaei N (2018) Liquid biopsy for infectious diseases: sequencing of cell-free plasma to detect pathogen DNA in patients with invasive fungal disease. Diagn Microbiol Infect Dis, 92, 210–213. [DOI] [PubMed] [Google Scholar]
- Hovi L, Saarinen-Pihkala UM, Vettenranta K & Saxen H (2000) Invasive fungal infections in pediatric bone marrow transplant recipients: single center experience of 10 years. Bone Marrow Transplant, 26, 999–1004. [DOI] [PubMed] [Google Scholar]
- Huppler AR, Fisher BT, Lehrnbecher T, Walsh TJ & Steinbach WJ (2017) Role of Molecular Biomarkers in the Diagnosis of Invasive Fungal Diseases in Children. J Pediatric Infect Dis Soc, 6, S32–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba H, Pei D, Wolf J, Howard SC, Hayden RT, Go M, Varechtchouk O, Hahn T, Buaboonnam J, Metzger ML, Rubnitz JE, Ribeiro RC, Sandlund JT, Jeha S, Cheng C, Evans WE, Relling MV & Pui CH (2017) Infection-related complications during treatment for childhood acute lymphoblastic leukemia. Ann Oncol, 28, 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston DL, Lewis V, Yanofsky R, Gillmeister B, Ethier MC, Mitchell D, Cellot S, Dix D, Portwine C, Price V, Silva M, Zelcer S, Michon B, Bowes L, Stobart K, Brossard J, Beyene J & Sung L (2013) Invasive fungal infections in paediatric acute myeloid leukaemia. Mycoses, 56, 482–487. [DOI] [PubMed] [Google Scholar]
- Kanamori H, Rutala WA, Sickbert-Bennett EE & Weber DJ (2015) Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis, 61, 433–444. [DOI] [PubMed] [Google Scholar]
- Kanda Y, Yamamoto R, Chizuka A, Hamaki T, Suguro M, Arai C, Matsuyama T, Takezako N, Miwa A, Kern W, Kami M, Akiyama H, Hirai H & Togawa A (2000) Prophylactic action of oral fluconazole against fungal infection in neutropenic patients. A meta-analysis of 16 randomized, controlled trials. Cancer, 89, 1611–1625. [PubMed] [Google Scholar]
- Katragkou A, Fisher BT, Groll AH, Roilides E & Walsh TJ (2017) Diagnostic Imaging and Invasive Fungal Diseases in Children. J Pediatric Infect Dis Soc, 6, S22–S31. [DOI] [PubMed] [Google Scholar]
- King J, Pana ZD, Lehrnbecher T, Steinbach WJ & Warris A (2017) Recognition and Clinical Presentation of Invasive Fungal Disease in Neonates and Children. J Pediatric Infect Dis Soc, 6, S12–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi R, Kaneda M, Sato T, Suzuki D, Ichikawa M & Ariga T (2007) Evaluation of risk factors for invasive fungal infection after allogeneic stem cell transplantation in pediatric patients. J Pediatr Hematol Oncol, 29, 786–791. [DOI] [PubMed] [Google Scholar]
- Kyvernitakis A, Torres HA, Jiang Y, Chamilos G, Lewis RE & Kontoyiannis DP (2016) Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: a propensity score analysis. Clin Microbiol Infect, 22, 811 e811–811 e818. [DOI] [PubMed] [Google Scholar]
- Lamoth F, Cruciani M, Mengoli C, Castagnola E, Lortholary O, Richardson M, Marchetti O & Third European Conference on Infections in, L. (2012) beta-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin Infect Dis, 54, 633–643. [DOI] [PubMed] [Google Scholar]
- Larone DH (ed.) (2011) Medically Important Fungi. American Society of Microbiology Press, Washington, D.C. [Google Scholar]
- Lehrnbecher T, Becker K & Groll AH (2017) Current Algorithms in Fungal Diagnosis in the Immunocompromised Host. Methods Mol Biol, 1508, 67–84. [DOI] [PubMed] [Google Scholar]
- Lehrnbecher T, Ethier MC, Zaoutis T, Creutzig U, Gamis A, Reinhardt D, Aplenc R & Sung L (2009) International variations in infection supportive care practices for paediatric patients with acute myeloid leukaemia. Br J Haematol, 147, 125–128. [DOI] [PubMed] [Google Scholar]
- Lehrnbecher T, Robinson PD, Fisher BT, Castagnola E, Groll AH, Steinbach WJ, Zaoutis TE, Negeri ZF, Beyene J, Phillips B & Sung L (2016) Galactomannan, beta-D-Glucan, and Polymerase Chain Reaction-Based Assays for the Diagnosis of Invasive Fungal Disease in Pediatric Cancer and Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Clin Infect Dis, 63, 1340–1348. [DOI] [PubMed] [Google Scholar]
- Lestrade PP, Bentvelsen RG, Schauwvlieghe A, Schalekamp S, van der Velden W, Kuiper EJ, van Paassen J, van der Hoven B, van der Lee HA, Melchers WJG, de Haan AF, van der Hoeven HL, Rijnders BJA, van der Beek MT & Verweij PE (2019) Voriconazole Resistance and Mortality in Invasive Aspergillosis: A Multicenter Retrospective Cohort Study. Clin Infect Dis, 68, 1463–1471. [DOI] [PubMed] [Google Scholar]
- Marks DI, Pagliuca A, Kibbler CC, Glasmacher A, Heussel CP, Kantecki M, Miller PJ, Ribaud P, Schlamm HT, Solano C, Cook G & Group IS (2011) Voriconazole versus itraconazole for antifungal prophylaxis following allogeneic haematopoietic stem-cell transplantation. Br J Haematol, 155, 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, Heinz WJ, Jagannatha S, Koh LP, Kontoyiannis DP, Lee DG, Nucci M, Pappas PG, Slavin MA, Queiroz-Telles F, Selleslag D, Walsh TJ, Wingard JR & Maertens JA (2015) Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med, 162, 81–89. [DOI] [PubMed] [Google Scholar]
- Marty FM, Ostrosky-Zeichner L, Cornely OA, Mullane KM, Perfect JR, Thompson GR 3rd, Alangaden GJ, Brown JM, Fredricks DN, Heinz WJ, Herbrecht R, Klimko N, Klyasova G, Maertens JA, Melinkeri SR, Oren I, Pappas PG, Racil Z, Rahav G, Santos R, Schwartz S, Vehreschild JJ, Young JH, Chetchotisakd P, Jaruratanasirikul S, Kanj SS, Engelhardt M, Kaufhold A, Ito M, Lee M, Sasse C, Maher RM, Zeiher B, Vehreschild M, Vital & FungiScope Mucormycosis, I. (2016) Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis, 16, 828–837. [DOI] [PubMed] [Google Scholar]
- Maziarz RT, Brazauskas R, Chen M, McLeod AA, Martino R, Wingard JR, Aljurf M, Battiwalla M, Dvorak CC, Geroge B, Guinan EC, Hale GA, Lazarus HM, Lee JW, Liesveld JL, Ramanathan M, Reddy V, Savani BN, Smith FO, Strasfeld L, Taplitz RA, Ustun C, Boeckh MJ, Gea-Banacloche J, Lindemans CA, Auletta JJ & Riches ML (2017) Pre-existing invasive fungal infection is not a contraindication for allogeneic HSCT for patients with hematologic malignancies: a CIBMTR study. Bone Marrow Transplant, 52, 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MW, Kalasauskas D, Petraitis V, Petraitiene R & Walsh TJ (2017) Fungal Infections of the Central Nervous System in Children. J Pediatric Infect Dis Soc, 6, e123–e133. [DOI] [PubMed] [Google Scholar]
- Miller TP, Li Y, Kavcic M, Troxel AB, Huang YS, Sung L, Alonzo TA, Gerbing R, Hall M, Daves MH, Horton TM, Pulsipher MA, Pollard JA, Bagatell R, Seif AE, Fisher BT, Luger S, Gamis AS, Adamson PC & Aplenc R (2016) Accuracy of Adverse Event Ascertainment in Clinical Trials for Pediatric Acute Myeloid Leukemia. J Clin Oncol, 34, 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor M, Gilad G, Kornreich L, Fisher S, Yaniv I & Levy I (2011) Invasive fungal infections in pediatric oncology. Pediatr Blood Cancer, 56, 1092–1097. [DOI] [PubMed] [Google Scholar]
- Müller F-M, Trusen A & Weig M (2002) Clinical manifestations and diagnosis of invasive aspergillosis in immunocompromised children. European Journal of Pediatrics, 161, 563–574. [DOI] [PubMed] [Google Scholar]
- Musial CE, Cockerill FR 3rd & Roberts GD (1988) Fungal infections of the immunocompromised host: clinical and laboratory aspects. Clin Microbiol Rev, 1, 349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucci M & Anaissie E (2001) Revisiting the source of candidemia: skin or gut? Clin Infect Dis, 33, 1959–1967. [DOI] [PubMed] [Google Scholar]
- Nucci M & Anaissie E (2007) Fusarium infections in immunocompromised patients. Clin Microbiol Rev, 20, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor D, Bate J, Wade R, Clack R, Dhir S, Hough R, Vora A, Goulden N & Samarasinghe S (2014) Infection-related mortality in children with acute lymphoblastic leukemia: an analysis of infectious deaths on UKALL2003. Blood, 124, 1056–1061. [DOI] [PubMed] [Google Scholar]
- Otto WR, Pahud BA & Yin DE (2019) Pediatric Mucormycosis: A 10-Year Systematic Review of Reported Cases and Review of the Literature. J Pediatric Infect Dis Soc. [DOI] [PubMed] [Google Scholar]
- Pagano L, Busca A, Candoni A, Cattaneo C, Cesaro S, Fanci R, Nadali G, Potenza L, Russo D, Tumbarello M, Nosari A, Aversa F, Group S & Other A (2017) Risk stratification for invasive fungal infections in patients with hematological malignancies: SEIFEM recommendations. Blood Rev, 31, 17–29. [DOI] [PubMed] [Google Scholar]
- Palazzi DL, Arrieta A, Castagnola E, Halasa N, Hubbard S, Brozovich AA, Fisher BT & Steinbach WJ (2014) Candida speciation, antifungal treatment and adverse events in pediatric invasive candidiasis: results from 441 infections in a prospective, multi-national study. Pediatr Infect Dis J, 33, 1294–1296. [DOI] [PubMed] [Google Scholar]
- Pana ZD, Roilides E, Warris A, Groll AH & Zaoutis T (2017) Epidemiology of Invasive Fungal Disease in Children. J Pediatric Infect Dis Soc, 6, S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE & Sobel JD (2016) Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis, 62, e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WB, Kim NH, Kim KH, Lee SH, Nam WS, Yoon SH, Song KH, Choe PG, Kim NJ, Jang IJ, Oh MD & Yu KS (2012) The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin Infect Dis, 55, 1080–1087. [DOI] [PubMed] [Google Scholar]
- Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA & Bennett JE (2016) Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis, 63, e1–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammaert B, Desjardins A & Lortholary O (2012) New insights into hepatosplenic candidosis, a manifestation of chronic disseminated candidosis. Mycoses, 55, e74–84. [DOI] [PubMed] [Google Scholar]
- Reboli AC, Rotstein C, Pappas PG, Chapman SW, Kett DH, Kumar D, Betts R, Wible M, Goldstein BP, Schranz J, Krause DS, Walsh TJ & Anidulafungin Study G (2007) Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med, 356, 2472–2482. [DOI] [PubMed] [Google Scholar]
- Reboli AC, Shorr AF, Rotstein C, Pappas PG, Kett DH, Schlamm HT, Reisman AL, Biswas P & Walsh TJ (2011) Anidulafungin compared with fluconazole for treatment of candidemia and other forms of invasive candidiasis caused by Candida albicans: a multivariate analysis of factors associated with improved outcome. BMC Infect Dis, 11, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickerts V, Mousset S, Lambrecht E, Tintelnot K, Schwerdtfeger R, Presterl E, Jacobi V, Just-Nubling G & Bialek R (2007) Comparison of histopathological analysis, culture, and polymerase chain reaction assays to detect invasive mold infections from biopsy specimens. Clin Infect Dis, 44, 1078–1083. [DOI] [PubMed] [Google Scholar]
- Rubnitz JE, Lensing S, Zhou Y, Sandlund JT, Razzouk BI, Ribeiro RC & Pui CH (2004) Death during induction therapy and first remission of acute leukemia in childhood: the St. Jude experience. Cancer, 101, 1677–1684. [DOI] [PubMed] [Google Scholar]
- Science M, Robinson PD, MacDonald T, Rassekh SR, Dupuis LL & Sung L (2014) Guideline for primary antifungal prophylaxis for pediatric patients with cancer or hematopoietic stem cell transplant recipients. Pediatr Blood Cancer, 61, 393–400. [DOI] [PubMed] [Google Scholar]
- Simms-Waldrip T, Rosen G, Nielsen-Saines K, Ikeda A, Brown B & Moore T (2015) Invasive fungal infections in pediatric hematopoietic stem cell transplant patients. Infect Dis (Lond), 47, 218–224. [DOI] [PubMed] [Google Scholar]
- Slavin MA, Osborne B, Adams R, Levenstein MJ, Schoch HG, Feldman AR, Meyers JD & Bowden RA (1995) Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation--a prospective, randomized, double-blind study. J Infect Dis, 171, 1545–1552. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Klieger SB, Sulieman SE, Berger E, Treat JR & Fisher BT (2018) Retrospective review of immunocompromised children undergoing skin biopsy for suspected invasive infection: Analysis of factors predictive of invasive mold. Pediatr Dermatol, 35, 104–111. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Wang C, Srivastava DK, Burnette K, Shenep JL, Leung W & Hayden RT (2013) Timeline, epidemiology, and risk factors for bacterial, fungal, and viral infections in children and adolescents after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant, 19, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach WJ, Roilides E, Berman D, Hoffman JA, Groll AH, Bin-Hussain I, Palazzi DL, Castagnola E, Halasa N, Velegraki A, Dvorak CC, Charkabarti A, Sung L, Danziger-Isakov L, Lachenauer C, Arrieta A, Knapp K, Abzug MJ, Ziebold C, Lehrnbecher T, Klingspor L, Warris A, Leckerman K, Martling T, Walsh TJ, Benjamin DK Jr., Zaoutis TE & International Pediatric Fungal N (2012) Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr Infect Dis J, 31, 1252–1257. [DOI] [PubMed] [Google Scholar]
- Sung L, Gamis A, Alonzo TA, Buxton A, Britton K, Deswarte-Wallace J & Woods WG (2009) Infections and association with different intensity of chemotherapy in children with acute myeloid leukemia. Cancer, 115, 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung L, Lange BJ, Gerbing RB, Alonzo TA & Feusner J (2007a) Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood, 110, 3532–3539. [DOI] [PubMed] [Google Scholar]
- Sung L, Nathan PC, Alibhai SM, Tomlinson GA & Beyene J (2007b) Meta-analysis: Effect of Prophylactic Hematopoietic Colony-Stimulating Factors on Mortality and Outcomes of Infection. Ann Intern Med, 147, 400–411. [DOI] [PubMed] [Google Scholar]
- Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Florl C, Lewis RE, Munoz P, Verweij PE, Warris A, Ader F, Akova M, Arendrup MC, Barnes RA, Beigelman-Aubry C, Blot S, Bouza E, Bruggemann RJM, Buchheidt D, Cadranel J, Castagnola E, Chakrabarti A, Cuenca-Estrella M, Dimopoulos G, Fortun J, Gangneux JP, Garbino J, Heinz WJ, Herbrecht R, Heussel CP, Kibbler CC, Klimko N, Kullberg BJ, Lange C, Lehrnbecher T, Loffler J, Lortholary O, Maertens J, Marchetti O, Meis JF, Pagano L, Ribaud P, Richardson M, Roilides E, Ruhnke M, Sanguinetti M, Sheppard DC, Sinko J, Skiada A, Vehreschild M, Viscoli C & Cornely OA (2018) Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect, 24 Suppl 1, e1–e38. [DOI] [PubMed] [Google Scholar]
- Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H & Durrant S (2007) Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med, 356, 335–347. [DOI] [PubMed] [Google Scholar]
- van Burik J, Hare R, Solomon H, Corrado M & Kontoyiannis D (2006) Posaconazole Is Effective as Salvage Therapy in Zygomycosis: A Retrospective Summary of 91 Cases. Clin Infect Dis, 42, e61–e65. [DOI] [PubMed] [Google Scholar]
- van Burik JA, Ratanatharathorn V, Stepan DE, Miller CB, Lipton JH, Vesole DH, Bunin N, Wall DA, Hiemenz JW, Satoi Y, Lee JM, Walsh TJ, National Institute of A & Infectious Diseases Mycoses Study, G. (2004) Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis, 39, 1407–1416. [DOI] [PubMed] [Google Scholar]
- Vazquez J, Reboli AC, Pappas PG, Patterson TF, Reinhardt J, Chin-Hong P, Tobin E, Kett DH, Biswas P & Swanson R (2014) Evaluation of an early step-down strategy from intravenous anidulafungin to oral azole therapy for the treatment of candidemia and other forms of invasive candidiasis: results from an open-label trial. BMC Infect Dis, 14, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Bruggemann RJ, Chowdhary A, Cornely OA, Denning DW, Groll AH, Izumikawa K, Kullberg BJ, Lagrou K, Maertens J, Meis JF, Newton P, Page I, Seyedmousavi S, Sheppard DC, Viscoli C, Warris A & Donnelly JP (2015) International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat, 21–22, 30–40. [DOI] [PubMed] [Google Scholar]
- Wattier RL, Dvorak CC, Hoffman JA, Brozovich AA, Bin-Hussain I, Groll AH, Castagnola E, Knapp KM, Zaoutis TE, Gustafsson B, Sung L, Berman D, Halasa NB, Abzug MJ, Velegraki A, Sharma TS, Fisher BT & Steinbach WJ (2015) A Prospective, International Cohort Study of Invasive Mold Infections in Children. J Pediatric Infect Dis Soc, 4, 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KA, Gea-Banacloche J, Stroncek D & Kadri SS (2017) Granulocyte transfusions in the management of invasive fungal infections. Br J Haematol, 177, 357–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JM, Seibel NL & Walsh TJ (2005) Efficacy and Safety of Amphotericin B Lipid Complex in 548 Children and Adolescents With Invasive Fungal Infections. The Pediatric Infectious Disease Journal, 24, 167–174. [DOI] [PubMed] [Google Scholar]
- Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ & Feudtner C (2005) The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis, 41, 1232–1239. [DOI] [PubMed] [Google Scholar]
- Zaoutis TE, Roilides E, Chiou CC, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Prasad PA, Chu JH & Walsh TJ (2007) Zygomycosis in children: a systematic review and analysis of reported cases. Pediatr Infect Dis J, 26, 723–727. [DOI] [PubMed] [Google Scholar]